Abstract
Objectives
Intrathecal drug delivery systems represent an important component of interventional strategies for refractory chronic pain syndromes. Continuous intrathecal administration of opioids results in higher subarachnoid drug concentrations, improved pain scores, and less frequent side effects when compared with systemic opioid administration. Substantial costs arise at the time of surgical implantation and at revision for battery depletion or treatment of a complication. Despite current widespread use, the real-world longevity and cost of implanted intrathecal pumps (ITP) has not been fully quantified.
Materials and Methods
Patients with an ITP implanted at Cleveland Clinic Pain Management Center between January 1998 and December 2012 were included. ITP longevity was calculated as the time between implant and explant for depletion of the system's battery. Using the 2013 fee schedule of the Centers for Medicare & Medicaid Services, the daily cost of having a functioning ITP was calculated. The costs of office visits for pump refills and the cost of intrathecal medications were not included, nor were the possible savings due to decreased utilization of alternate medical services.
Results
Three hundred sixty-five patients had 559 pumps implanted. Postlaminectomy syndrome was the most common indication (40%). The median system longevity for all pumps was 5.4 years (97.5% confidence interval: [5.0, 5.8]), including pumps extracted prematurely, as well as those that reached the elective replacement interval. The median ITP longevity was 5.9 years (95% confidence interval: [5.6, 6.1]) for pumps explanted for end of battery life. The median system cost per day was $10.46. The median cost per day of pumps explanted for end of battery life was $9.26, versus $44.59 for pumps explanted prematurely due to complications.
Conclusions
Overall, the cohort experienced an increased incidence of pump-related complications and a device longevity that was within the range of the manufacturer's anticipated lifespan. Increasing the lifespan of the ITP and improving patient selection have the potential to significantly improve the cost-effectiveness of intrathecal therapy.
Keywords: Adverse events, chronic pain, complications, cost of therapy, indications, intrathecal drug delivery, intrathecal pump, intrathecal pump longevity
Introduction
Chronic pain impacts the quality of multiple functional domains, including family life, workplace performance, social interactions, and sleep patterns. Intrathecal drug delivery forms an important component of interventional strategies for refractory cancer and non-cancer-related chronic pain syndromes. The intrathecal administration of opioid medications permits delivery of higher drug concentrations into the cerebrospinal fluid (CSF) along with lower concentrations reaching the systemic circulation, sparing the undesired secondary effects of these same medications when administered by other routes (1). Quality of life measures are improved while the overall health-care utilization costs are decreased in selected patients with intrathecal drug delivery systems when compared with conventional medical management (2–9).
An intrathecal drug delivery system is composed of a surgically implanted battery-operated pump and medication reservoir connected to an intrathecal catheter. The tip of the catheter is placed into the CSF. Placement of the system generates upfront costs associated with the implantation of pump and catheter components, maintaining surgical equipment, surgical staff support, operating room use, anesthetic administration, and postoperative monitoring. The SynchroMed EL and SynchroMed II programmable infusion pumps (Medtronic, Minneapolis, MN, USA), which were used in all patients in this cohort, are capable of noninvasive programming and continuous drug delivery via an internal peristaltic mechanism. The pump is powered by an internal battery, and the system can be refilled via percutaneous port access into the reservoir.
Substantial costs arise at the time of surgical implantation, as well as at the time of revision for expenditure of the system's battery, in the event of catheter displacement or fracture, device malfunction, infection, or other complication that requires surgical explantation or revision. The Medtronic SynchroMed II Implant Manual reports an estimated device longevity of 4–7 years in laboratory tests, with a linear decay in battery life when flow rates exceed 0.9 mL/day (10), while the SynchroMed EL is more flow-dependent, with an estimated battery life ranging from 3 to 7 years (11). In addition to flow rate, it is known that the pump longevity is affected by medication type, the number of medications present within the pump, and the presence of a patient-delivered bolus option (10).
While they are currently in widespread use, the real-world cost of implanting and maintaining functioning intrathecal drug delivery systems has not been quantified, especially in a long-term cohort. De Lissovoy et al. quantified the costs of implanting and maintaining an intrathecal drug delivery system in a modeled population where adverse event rates were drawn from published data supplemented by experts' estimates of complication rates (1997) (2). The cumulative cost incurred by one real-world cohort of patients in Saskatchewan was found to total 29,410 Canadian dollars when assessed over a 5-year period (7). This sum included not only the drug delivery system but also those costs associated with advanced spine imaging, pharmacotherapy, and adjunctive therapies such as physical therapy, chiropractic, and acupuncture, which may vary when compared with similar utilization costs in an American cohort.
The primary objective of our study was to summarize the longevity of the intrathecal pump (ITP): the time interval between implant of the ITP and elective replacement due to end of battery life in a real-world cohort (the latter generally occurs 60–90 days prior to the calculated end of life of the battery).
The secondary objectives were to summarize the indications for ITP placement; to assess the ITP system longevity, which is the time from implant to explant, revision, or replacement regardless of the cause (including premature extractions and complications owing to the catheter or the pump); and to characterize the cost of maintaining a functioning ITP averaged over the observed system lifespan.
Methods
With approval from the institutional review board, we queried both the hospital electronic medical record and the Cleveland Clinic Pain Management Center database and obtained a cohort of 365 patients implanted with intrathecal drug delivery systems at the Cleveland Clinic Pain Management Center between January 1998 and December 2012. Basic procedure information retrieved from the registries was augmented manually by additional clinical data obtained from patients' electronic medical records.
Participants
In an effort to best illustrate our real-life experience with intrathecal drug delivery patients in a tertiary referral center, all 365 patients implanted with the device throughout the study period were considered. Most (72%) of the implants utilized the SynchroMed II system, with the remainder employing the previous-generation SynchroMed ELITP. It should be noted that there was no difference in cost or reimbursement between SynchroMed EL and SynchroMed II systems (12).
Variables
Data were extracted from patients' electronic medical records, the operative reports, and the provider's electronic progress notes. We included age, gender, race, the ICD-9 diagnosis associated with the indication for the implant (postlaminectomy syndrome, complex regional pain syndrome, cancer pain, spinal stenosis, spinal cord injury, etc.), date of implantation, dates of the revisions or explants, and corresponding indications (elective replacement interval reached, infection, granuloma, lack of efficacy, or surgical complication related to the pump pocket, the catheter, or the device itself).
Follow-up data were obtained continuously through retrospective review of the patient's electronic medical records at the time of device refill, which occurred no less frequently than every 3 months. The most recent encounter with the Pain Management Department of Cleveland Clinic was conservatively specified as the endpoint in all longevity calculations.
The interval from placement to revision or replacement for any indication was obtained for each patient. System longevity was defined as the time between implant and pump explant due to either battery expenditure (elective replacement) or to device-related or surgical complications (premature explant).
Time to ITP replacement (ITP lifespan or ITP longevity) was defined as the time between implant and explant or revision due to the battery expenditure only (reaching the elective replacement interval) and was limited to those who had an uncomplicated ITP lifespan from placement to revision.
The professional and technical components of the 2013 Medicare reimbursement rates for implantation of intrathecal drug delivery systems were obtained from Medicare fee schedules. The reimbursements included implantation of a programmable pump, insertion or revision of the catheter, and removal of the pump and catheter if relevant. As we were interested only in costs attributable to the hardware, infusion drug costs were not included. An average cost per day was calculated per patient based upon the observed pump longevity. Elective replacement represents an interval that occurs 60–90 days prior to the calculated end of battery life, the point when the device motor stops and medication is no longer delivered.
Statistical Analysis
We summarized patients' demographic characteristics and initial diagnoses for an implant using standard univariate summary statistics such as mean ± standard deviation or N (%), as appropriate.
Primary Outcome
Complete data on ITP lifespan (ITP longevity) was not observed in those patients with a recent implant, those whose death occurred prior to encountering the elective replacement interval, or those lost to follow-up. Patients with incomplete data on battery survival were censored at the earliest of either death or premature revision or most recent follow-up visit to the Pain Management Center of Cleveland Clinic. To account for incomplete data, the distribution of ITP lifespan was summarized graphically using Kaplan–Meier survival density estimation. The median time to replacement due to battery expenditure along with 95% confidence limits were assessed based on the Kaplan–Meier survival density estimates. The confidence limits were adjusted for a possible correlation among the multiple ITP pumps within a patient. Confidence limits were estimated based on bootstrapped standard error with 10,000 bootstrap replications (13).
Secondary Outcomes
Kaplan–Meier analysis was similarly used to summarize system longevity. The median system longevity was adjusted for multiple pumps, and 95% confidence limits were evaluated. The complete data on system longevity were not available with recent implants, death, or loss of follow-up. These outcomes were censored at the date of death or last follow-up visit. Reasons for premature replacement or removal were tabulated, and the proportion for each of the indications was calculated.
The relationship between the diagnosis for an implant and longevity of the system was analyzed using a multivariate mixed-effects Cox regression model (14), which is appropriate for censored time-to-event outcomes and accounts for possible correlation between repeated pumps within a single patient. Diagnoses with 20 patients or fewer were combined into a category called “other” for modeling purposes. The following variables were entered into the Cox regression model: age, gender, race, and diagnosis. The hypotheses of relationship between diagnosis and system survival were evaluated by testing whether or not all hazard ratios corresponding to different initial diagnoses were equal to 1.0 (model-based Wald test).
The type I error rate was set at 5% for the primary outcome and used for the confidence interval estimation. Type I error rate for the secondary hypothesis also was set at 5%. SAS statistical software version 9.3 (SAS Institute, Cary, NC, USA) for 64-bit Microsoft Windows and R statistical software version 2.15.2 for the 64-bit Unix operating system (R Foundation for Statistical Computing, Vienna, Austria) were used for the analysis.
The cost per day of an ITP over the observed lifetime was calculated by dividing the total cost by the number of days the pump was functioning. Summaries of the costs per day were calculated based on different patient cohorts, including all implanted ITPs, ITPs with uncomplicated lifespans from implant to revision for elective replacement of battery, ITPs that were removed prior to reaching the elective replacement interval, first ITPs, and second or later ITPs.
Results
We obtained data for 365 patients, including 187 patients (51%) having one or more pumps with complete lifespan over the study period, and 178 patients (49%) who did not have a complete pump lifespan on file. These 178 patients either had a recent implant that was still functioning satisfactorily during the first device lifespan, had died, or were receiving interval follow-up care outside of our institution. Of 187 patients having a complete ITP lifespan during the study, 140 (75%) patients had more than one pump implanted throughout the study period. In total, 559 implanted pumps were considered, including 249 pumps with complete lifespan and 310 pumps that did not reach the end of lifespan due to recent implant, patient's death, or follow-up outside our institution. Regarding the 249 pumps removed, 194 new pumps were implanted to replace them (78%).
The demographic characteristics and initial diagnoses for implant are reported in Table 1. Most common indications for implant included postlaminectomy syndrome (145 patients; 40%), spasticity (52; 14%), spinal stenosis (49; 13%), and complex regional pain syndrome (48; 13%). The percentages of SynchroMed EL and SynchroMed II ITPs in different cohorts and the ratios between them are summarized in Table 2.
Table 1. Patient Demographics and Baseline (N = 365).
| Age (years), mean ± SD | 54.2 ± 14.1 |
| Female, N (%) | 193 (53) |
| Ethnicity, N (%) | |
| Caucasian | 336 (92) |
| African American | 20 (5) |
| Other | 9 (2) |
| Principal indication for intrathecal pump implant, N (%) | |
| Postlaminectomy syndrome | 145 (40) |
| Spasticity | 52 (14) |
| Spinal stenosis | 49 (13) |
| Complex regional pain syndrome | 48 (13) |
| Cancer pain | 20 (6) |
| Other* | 49 (14) |
A heterogeneous group of diagnoses (each representing <5%) including postherpetic neuralgia, peripheral neuropathy, spinal cord injury, poststroke pain, diabetic neuropathy, and intractable degenerative disk disease.
Table 2.
Summary of the Percentages of SynchroMed EL and SynchroMed II Pumps.
| 1st implant | 2nd implant | 3rd implant | 4th implant | 5th implant | 6th implant | Total | |
|---|---|---|---|---|---|---|---|
| Number of pumps | 365 | 140 | 39 | 9 | 3 | 3 | 559 |
| Number of SynchroMed EL pumps | 127 | 24 | 4 | 1 | 1 | 0 | 157 |
| Number of SynchroMed II pumps | 238 | 116 | 35 | 8 | 2 | 3 | 402 |
| Percentage of SynchroMed EL pumps | 34.8 | 17.1 | 10.2 | 11.1 | 33.3 | 0 | 28 |
| Percentage of SynchroMed II pumps | 65.2 | 82.9 | 89.7 | 88.9 | 66.7 | 100 | 72 |
Outcomes
Kaplan–Meier estimates of ITP longevity and overall system longevity are displayed in Figure 1. The estimated median ITP longevity, which is the median time (adjusted for intrasubject correlation) between implant of intrathecal drug delivery systems and expenditure of the ITP due to depletion of the battery, was 5.9 years (95% confidence interval: [5.6, 6.1]).
Figure 1.
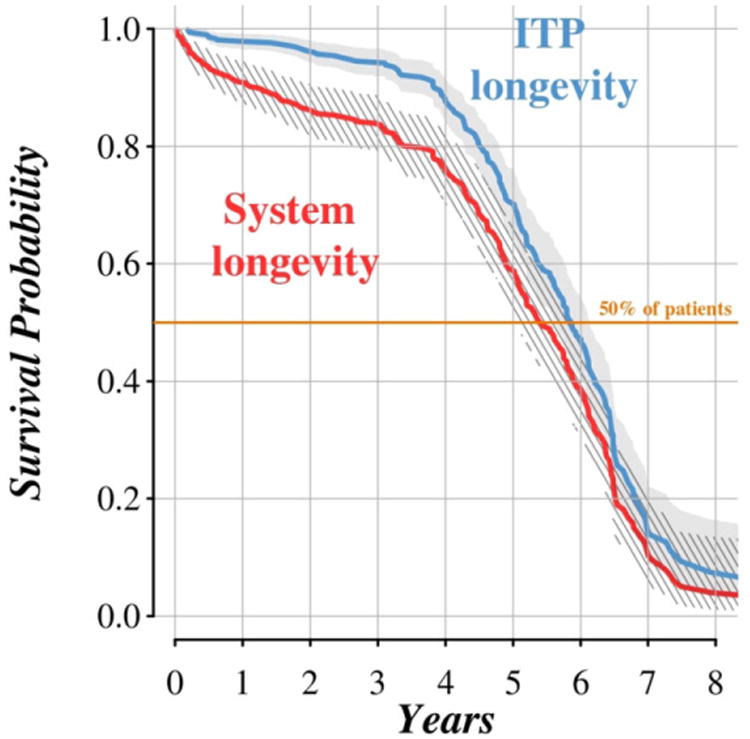
The Kaplan–Meier survival curves for system longevity and longevity of intrathecal pump device. Patients with incomplete data on battery survival were censored at the earliest of death, premature revision, or most recent follow-up visit to the Pain Management Center of Cleveland Clinic. System longevity was censored at the date of death or last follow-up visit.
Median system longevity (adjusted for intrasubject correlation) was estimated at 5.4 years (97.5% confidence interval: [5.0, 5.8]), owing to those patients who underwent explant or revision of the system prior to reaching the elective replacement interval.
Reasons for premature replacement or removal were infection (16%), lack of therapeutic efficacy (11%), surgical complications (3%), and granuloma formation (1%) (summarized in Table 3). The estimated median system longevities (with 95% confidence intervals) by reasons for premature replacement are presented in Table 4. The disposition of infection cases and culture data from ITPs suspected to be infected are reported in Tables 5 and 6. Data on infection in Tables 5 and 6 contain information on all cases of infections, including multiple repeated infections for a single patient.
Table 3.
Reasons for Pump Replacement or Removal (N = 559).
| Reason for extraction | Number of pumps (N = 559) | Proportion of pumps replaced after completing lifespan (N = 249) (%) | Proportion of pumps replaced overall (N = 559) |
|---|---|---|---|
| End of system lifespan | 171 | 69 | 31 |
| Premature extraction | 78 | 31 | 14 |
| Infection | 41 | 16 | 7 |
| Surgical complications | 7 | 3 | 1 |
| Pocket-related | 3 | 1 | 1 |
| Catheter-related | 2 | 1 | 0 |
| Pump-related | 2 | 1 | 0 |
| Granuloma | 2 | 1 | 0 |
| Lack of efficacy | 28 | ||
| Censored pumps* | 310 | — | 55 |
Censored due to recent implant, death, or loss of follow-up.
Table 4.
Estimated System Longevity for Premature Extraction.
| Infection | 0.3 [0.2, 0.8] |
| Surgical complications | 3.4 [0.04, 5.8] |
| Granuloma | 1.9 [1.8, 2.0] |
| Lack of efficacy | 2.2 [1.3, 4.9] |
| Overall | 1.1 [0.6; 1.6] |
Estimated median system longevity in years [95% confidence interval] for pumps extracted prematurely (note that above estimates were based only on patients with premature extraction events).
Table 5.
Disposition of Patients with Intrathecal Pump Explant Due to Infection (N = 38).
| Disposition | N (%) |
|---|---|
| Discharged on postoperative day 0 with oral antimicrobial therapy | 5 (13) |
| Discharged on postoperative day 0 with intravenous antimicrobial therapy* | 1 (3) |
| Admitted for inpatient oral antibiotic therapy | 3 (8) |
| Admitted for inpatient intravenous antibiotic therapy | 28 (74) |
One patient who was admitted to the hospital did not have recorded historical data on the route of antimicrobial administration.
This individual had a preexisting peripherally inserted central catheter line in situ.
Table 6.
Culture Data from Intrathecal Drug Delivery Systems Suspected to be Infected (N = 38).
| Organisms cultured | N (%) |
|---|---|
| None | 17 (45) |
| Staphylococcus spp. | 12 (32) |
| Enterococcus spp. | 4 (11) |
| Streptococcus spp. | 1 (3) |
| Pseudomonas spp. | 1 (3) |
| Yeast* | 1 (3) |
Two patients did not have recorded culture data.
All cultures revealed a single isolate except for this individual's culture, which also grew Staphylococcus species.
We did not find a significant association between initial indication for therapy and overall system longevity (Wald test p = 0.23; significance criterion: 0.025 = 0.05/2); that is, we did not find enough evidence to claim the hazard of experiencing a system failure was different comparing any two studied diagnoses at any time point.
In order to determine any difference in the longevity between the two models of ITP (SynchroMed EL, SynchroMed II), a log-rank test was used to compare system longevities. No significant difference was found (p = 0.17). We also evaluated if the system longevity was degraded when comparing first vs. second or later pumps. Again, using a log-rank test, no significant difference was found (p = 0.18).
For the cost analysis, only pumps where data on a complete lifespan were present were included in the analysis. Using the 249 complete pump lifespans observed throughout the study period and the 2013 Medicare reimbursement rates, the median cost among the cohort was $10.46 ($4.08–$2973.10) per day. When explants due to infection, therapeutic failure, surgical complications, or granuloma were excluded from this cohort, the number of pumps with complete lifespans totaled 171, and the median cost was $9.26 ($4.08–$248.05) per day. Conversely, when examining the cohort who underwent premature revision of the ITP (N = 78), the cost increased to $44.59 ($5.81, $2973.10) per day, indicating a majority of these premature revisions occurred early in the lifespan of the ITP.
When first pumps were analyzed, their median lifespan was longer at 1689 days versus 1596 days for second or later pumps. The cost per day for first pumps was similar to the all-pump cohort at $10.30 ($5.29–$2973.10) per day (N = 187). Second or later pumps' cost per day was slightly higher than that of first pumps at $10.73 ($4.08–$533.63) (n = 62). A full summary of the costs by cohort is broken down in Table 7.
Table 7.
Cost per Day (USD).
| Cost/day | All intrathecal pumps (N = 249) | Pumps excluding explants (N = 171) | Explanted pumps (N = 78) | First pumps (N = 187) | Second or later pumps (N = 62) |
|---|---|---|---|---|---|
| Median | 10.46 | 9.26 | 44.59 | 10.30 | 10.73 |
| Minimum | 4.08 | 4.08 | 5.81 | 5.29 | 4.08 |
| Maximum | 2973.10 | 248.05 | 2973.10 | 2973.10 | 533.63 |
| SD | 355.25 | 34.20 | 596.22 | 404.62 | 103.35 |
Discussion
This study was an evaluation of ITP longevity in a real-world clinical cohort in our institution. The accessibility of a longitudinal cohort enabled calculations based upon consecutive ITP implants within a single patient and permitted assessments based upon data obtained from multiple systems in the same patient. As we accounted for consecutive ITP implants, the confidence limits were adjusted for a possible correlation between sequential pumps.
When considering the hardware components of the device alone, we observed a median ITP longevity (time from ITP implant to reaching the elective replacement interval) of 5.9 years, in line with the manufacturer's stated longevity (10). This observed longevity represents a best-case scenario when premature revision rates and complications equal zero.
Median ITP system longevity in the entire cohort was observed to be 5.4 years, owing to the fact that about 31% of patients underwent premature revision throughout one or more of their ITP lifespans. The primary indications for the premature revisions were infection, lack of efficacy, and adverse surgical events. We did not find evidence for association between longevity of the ITP system and the initial indication for the implant (p = 0.23), though we hypothesized that neuropathic pain conditions, which often require relatively higher opioid doses, would be associated with shorter longevity owing to proportionally higher flow rates. The relatively small sample size of our study might be the factor limiting our chance to detect possible association between diagnosis at the time of implant and system longevity.
The cost of having an ITP did not differ greatly between the cohorts of all implanted ITPs (median $10.46, range $4.08–2973.10), ITPs with uncomplicated lifespans from implant to revision upon reaching the elective replacement interval (median $9.26, range $4.08–248.05), first ITPs (median $10.30, range $5.29–2973.10), and second or later ITPs (median $10.73, range $4.08–33.63). It was anticipated that the cost per day for second or later pumps would be less than that for first pumps, as therapeutic failures would have been excluded from the latter group. Surprisingly, the cost per day in the secondary-or-later cohort was actually higher than that in the first-ITP cohort ($10.73/day vs. $10.30). While the cost difference is not substantial, the higher cost per day in the later-ITP cohort could be explained by the need for drug flow rates to increase over time in opioid-tolerant patients, thus having an impact on the battery life of the ITP pump.
The most costly cohort included those individuals who underwent explant of the ITP prior to reaching the elective replacement interval. Reasons included therapeutic failure, infection, and non-compliance. Cost per day for this cohort was over four times higher than for all other cohorts (median $44.59, range $5.81–2973.10). This significant cost difference is further demonstrated when comparing the cost range among these groups. Among pumps that were explanted for reasons other than complications, the maximum cost per day was $248.05. For pumps that were explanted due to a complication, the maximum cost per day was over 10 times higher at $2973.10. Complications requiring premature removal of the system occur in a bimodal pattern, with postoperative infections and wound dehiscence occurring shortly after implant, and therapeutic failure occurring late once a decision is made that no dose or medication combination is providing clinical benefit.
Since the majority of the cost of intrathecal drug delivery systems is incurred at inception of the therapy, cost savings are primarily achieved by increasing the longevity of the system, such that the high initial costs are averaged over a long pump lifespan. In this cohort, we observed two factors opposing a long ITP lifespan: (1) early ITP explant without replacement due to complications or therapeutic failure and (2) early replacement due to surgical or infectious complications. In our practice, we seek to counteract these liabilities through strategies to most accurately determine the patients in whom the device will provide therapeutic benefit, averting early explants. Secondly, we continually seek to adopt strategies to reduce infectious complications, though the rate of infection is higher than has been reported elsewhere (15–18). We hypothesize this may be due to the comparatively high risk and increased comorbidities seen in our patient population. As illustrated in Tables 4 and 5, the development of infectious complications resulted in morbidity and additional costs attributable to the shortened ITP lifespan. The inpatient hospitalization costs and the costs of parenteral antimicrobial therapy likely further increase the cost per day in this subset by several multiples.
Overall, the cost per day of implanting and maintaining a pump is significant at over $10 per day. This cost is only for the hardware of the ITP and does not include medication costs, personnel costs for ITP refill or any associated costs generated by postoperative complications. However, while an ITP can be costly, studies have shown this method to be extremely effective, with marked improvements in quality of life (4). Furthermore, the use of an ITP can help avoid many of the complications of taking oral analgesics, and its cost can be counteracted by patients' returning to productive living or participating in society (19). While this study was able to demonstrate the cost per day of the hardware components of an intrathecal delivery system, the inability to include all relevant costs and quality-of-life outcomes was a limitation. Furthermore, we did not compare these costs and outcomes with other routes of opioid administration or chronic pain interventions.
In spite of efforts to avoid premature revisions, further advances in the development of ITPs with a longer battery life would result in the most substantial cost savings, as depletion of the system's battery remains the primary indication for patients to return to the operating room. Increasing the battery life by 1 year alone would reduce the ITP cost by $2 per patient per day. The benefits of a longer battery life would be compounded, as the number of postoperative infectious complications would proportionally decrease. Evolution in ITP longevity remains a useful area for innovation, and a longer-lasting ITP has the potential to gain market share, decrease health-care costs, and improve quality of life.
In conclusion, two factors may optimize the cost–benefit of intrathecal drug delivery systems: 1) minimizing premature pump explantation, which can be achieved by proper patient selection and proper surgical technique, which will reduce the incidence of therapeutic failures and early postoperative complications; and 2) improving technology to allow longer battery life. Increasing the battery life by 1 year alone would reduce ITP cost by $2 per patient per day.
Acknowledgments
Authorship Statement: Drs. Bolash, Udeh, and Mekhail designed and conducted the study, performed data collection and analysis, and drafted the manuscript. Dr. Saweris assisted with data collection and preparation of the manuscript. Dr. Guirguis assisted with data collection and analysis. Dr. Dalton and Ms. Makarova assisted with data analysis and preparation of the figures. The Evidence Based Pain Research Division at the Cleveland Clinic provided funding. The Division of Outcomes Research at the Cleveland Clinic provided statistical support. All authors had complete access to the study data and approved the final manuscript.
Footnotes
Conflict of Interest: The authors reported no conflict of interest.
For more information on author guidelines, an explanation of our peer review process, and conflict of interest informed consent policies, please go to http://www.wiley.com/bw/submit.asp?ref=1094-7159&site=1
References
- 1.Moore RA, McQuay HJ. Prevalence of opioid adverse events in chronic non-malignant pain: systematic review of randomised trials of oral opioids. Arthritis Res Ther. 2005;7:R1046–R1051. doi: 10.1186/ar1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.De Lissovoy G, Brown RE, Halpern M, Hassenbusch SJ, Ross E. Cost-effectiveness of long-term intrathecal morphine therapy for pain associated with failed back surgery syndrome. Clin Ther. 1997;19:96–112. doi: 10.1016/s0149-2918(97)80077-x. [DOI] [PubMed] [Google Scholar]
- 3.Winkelmuller M, Winkelmuller W. Long-term effects of continuous intrathecal opioid treatment in chronic pain of nonmalignant etiology. J Neurosurg. 1996;85:458–467. doi: 10.3171/jns.1996.85.3.0458. [DOI] [PubMed] [Google Scholar]
- 4.Mueller-Schwefe G, Hassenbusch SJ, Reig E. Cost effectiveness of intrathecal therapy for pain. Neuromodulation. 1999 Apr;2:77–87. doi: 10.1046/j.1525-1403.1999.00077.x. [DOI] [PubMed] [Google Scholar]
- 5.Roberts LJ, Finch PM, Goucke CR, Price LM. Outcome of intrathecal opioids in chronic non-cancer pain. Eur J Pain. 2001;5:353–361. doi: 10.1053/eujp.2001.0255. [DOI] [PubMed] [Google Scholar]
- 6.Deer TR, Caraway DL, Kim CK, Dempsey CD, Stewart CD, McNeil KF. Clinical experience with intrathecal bupivacaine in combination with opioid for the treatment of chronic pain related to failed back surgery syndrome and metastatic cancer pain of the spine. Spine J. 2002;2:274–278. doi: 10.1016/s1529-9430(02)00199-7. [DOI] [PubMed] [Google Scholar]
- 7.Kumar K, Hunter G, Demeria DD. Treatment of chronic pain by using intrathecal drug therapy compared with conventional pain therapies: a cost-effectiveness analysis. J Neurosurg. 2002;97:803–810. doi: 10.3171/jns.2002.97.4.0803. [DOI] [PubMed] [Google Scholar]
- 8.Thimineur MA, Kravitz E, Vodapally MS. Intrathecal opioid treatment for chronic non-malignant pain: a 3-year prospective study. Pain. 2004;109:242–249. doi: 10.1016/j.pain.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 9.Biggs SA, Duarte RV, Raphael JH, Ashford RL. Influence of a latent period in QALY analysis: pilot study of intrathecal drug delivery systems for chronic non-malignant pain. Br J Neurosurg. 2011;25:401–406. doi: 10.3109/02688697.2010.551676. [DOI] [PubMed] [Google Scholar]
- 10.Medtronic. SynchroMed II Implant Manual. 2003 http://manuals.medtronic.com/wcm/groups/mdtcom_sg/@emanuals/@era/@neuro/documents/documents/contrib_163045.pdf.
- 11.Medtronic. SynchroMed EL Programmable Infusion Pump for Intrathecal Baclofen Therapy. [accessed May 1, 2013];2013 http://professional.medtronic.com/pt/neuro/itb/prod/synchromedel/
- 12.Centers for Medicare & Medicaid Services. DMEPOS Fee Schedule. [accessed May 12, 2013];2013 http://www.cms.gov/Medicare/Medicare-Fee-for-ServicePayment/DMEPOSFeeSched/DMEPOS-Fee-Schedule.html.
- 13.Efron B, Tibshirani R. Monographs on Statistics and Applied Probability. New York: Chapman & Hall; 1993. An introduction to the bootstrap. [Google Scholar]
- 14.Cox DR. Regression models and life-tables. J Roy Stat Soc B. 1972;34:187–220. [Google Scholar]
- 15.Njee TB, Irthum B, Roussel P, Peragut JC. Intrathecal morphine infusion for chronic non-malignant pain: a multiple center retrospective survey. Neuromodulation. 2004;7:249–259. doi: 10.1111/j.1094-7159.2004.04210.x. [DOI] [PubMed] [Google Scholar]
- 16.Fjelstad AB, Hommelstad J, Sortenberg A. Infections related to intrathecal baclofen therapy in children and adults: frequency and risk factors. J Neurosurg Pediatr. 2009;4:487–493. doi: 10.3171/2009.6.PEDS0921. [DOI] [PubMed] [Google Scholar]
- 17.Taira T, Ueta T, Katayama Y, et al. Rate of complications among the recipients of intrathecal baclofen pump in Japan: a multicenter study. Neuromodulation. 2013;16:266–272. doi: 10.1111/ner.12010. [DOI] [PubMed] [Google Scholar]
- 18.Engle MP, Vinh BP, Harun N, Koyyalagunta D. Infectious complications related to intrathecal drug delivery system and spinal cord stimulator system implantations at a comprehensive cancer pain center. Pain Physician. 2013;16:251–257. [PubMed] [Google Scholar]
- 19.Benyamin R, Trescot A, Datta S, et al. Opioid complications and side effects. Pain Physician. 2008;11:S105–S120. [PubMed] [Google Scholar]


