Abstract
The cerebellum is connected to cerebral areas that subserve a range of sensory and motor functions. In this review, we summarize new literature demonstrating deficits in visual perception, proprioception, motor control, and motor learning performance following cerebellar damage. In particular, we highlight novel results that together suggest a general role of the cerebellum in estimating and predicting movement dynamics of the body and environmental stimuli. These findings agree with the hypothesized role of the cerebellum in the generation and calibration of predictive models for a variety of functions.
1. Introduction
The cerebellum is interconnected with many brain areas including those involved in motor, sensory and higher brain function (reviewed in [1]). This, coupled with the highly stereotyped anatomy of the cerebellum, suggests that it is performing the same type of computation for different cerebral functions. Yet, it is not understood how this connectivity and anatomy translates to normal function or dysfunction when the cerebellum is damaged. Here we review several recent studies that have shed light on the way in which cerebellar dysfunction affects perception in visual and somatosensory domains, as well as movement control. A general theme that is emerging is that cerebellar dysfunction impairs the ability to make predictions important for certain kinds of sensory function and movement control.
2. The cerebellum and sensory perception
Focal damage to the cerebellum does not seem to impair primary sensory function: clinical and laboratory tests of basic sensation (e.g. proprioception tested passively) often yield results similar to healthy controls (e.g. [2]). Rather cerebellar involvement seems to be important for more complex tasks that require sensory input to form perceptions of environmental stimuli and govern behavior in response to those perceptions. The roles of vision and somatosensation are perhaps two of the most well studied sensory modalities in these sensorimotor interactions. Here we review some of the work that demonstrates specific visual and somatosensory deficits that have been observed in individuals with cerebellar damage.
2.1 The cerebellum recalibrates visual perceptions of stimulus dynamics, but does not calibrate visual weighting
Cerebellar damage often causes oculomotor deficits, such as dysmetric saccades and nystagmus, which can indirectly affect visual perception [3]. Yet, deficits in visual perception have been noted in studies where eye movements and critical fixation periods were controlled between cerebellar patients and healthy participants [4–5]. One suggestion is that cerebellar activity is necessary for processing temporal information associated with visual stimulus motion (e.g. [6]); however, recent evidence suggests that the cerebellar contribution to visual motion perception may be in the estimation and prediction of stimulus dynamics [7*–8*].
Roth and colleagues (2013) studied people with cerebellar damage as they evaluated the temporal accuracy of a reappearing target, moving at constant velocity, whose trajectory was partially blocked by an occluder (Figure 1 [7*]). While cerebellar patients, both focal lesion and degenerative disease, showed similar performance to controls in baseline conditions, they exhibited impaired recalibration of their temporal estimates when a consistent delay was added to the time of target occlusion. Deluca et al. (2014) found that in addition to recalibration impairments, when the velocity of a partially occluded visual stimulus was decelerating, cerebellar patients showed deficits in estimating the baseline distance required for the stimulus to come to a complete stop [8*]. These deficits depended on lesion location such that individuals with focal lesions to the anterior cerebellum showed similar performance to controls, whereas those with lesions to the posterior cerebellum showed significant impairments. Of the individuals with degenerative disease, baseline performance deficits were seen only in those at an advanced stage of disease progression. Notably, deficient recalibration of distance estimates was seen in all cerebellar patients. That cerebellar patients exhibited intact estimation of constant velocity, but were impaired when estimating changing velocities suggests that the cerebellum’s role in visual perception may extend beyond the processing of temporal features of stimulus motion. Instead the cerebellum may be important for calibrating estimates of the visual object dynamics.
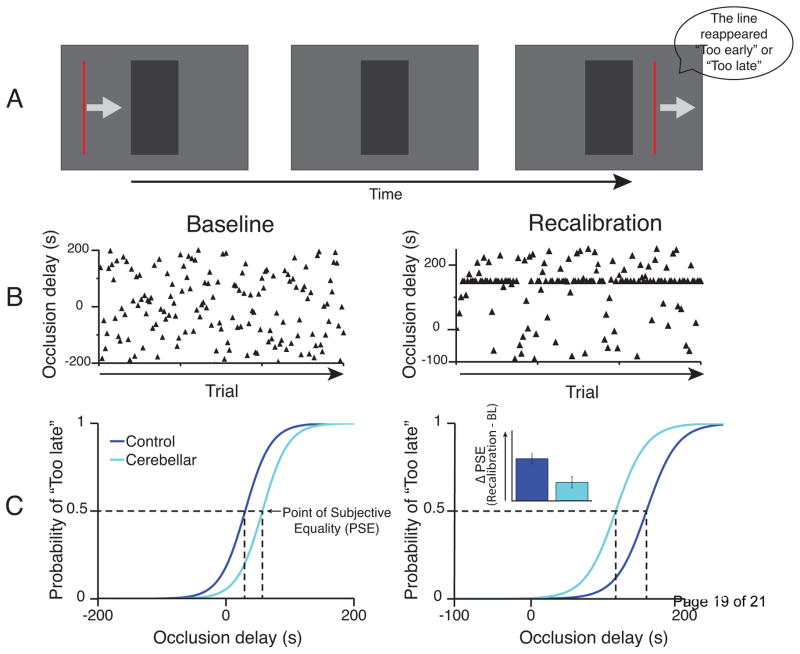
Figure 1.
The effect of cerebellar damage on perception of the temporal dynamics of visual stimulus motion. A. Schematic of a task requiring estimation of the velocity of a visual stimulus in order to predict the correct time of reappearance following a period occlusion. B. Schematic of two experimental conditions. In Baseline the levels of occlusion delay (i.e. the time the stimulus is occluded) are equally distributed across trials to determine the baseline point of subjective equality (PSE). At the PSE, no delay is perceived and subjects judge the velocity of the stimulus to have remained constant even when it was occluded. In Recalibration, half of trials shift the mean occlusion delay relative to the baseline PSE, while the other half involve delays that are randomly distributed to determine the new PSE. Exposing participants to a large proportion of trials with a fixed delay causes a recalibration of the perceived stimulus velocity while it is behind the occluder. In other words, participants judge that the velocity changes during the occlusion time. Accordingly, this recalibration is reflected in a shift in the PSE. C. The baseline task yields similar PSE values between control participants and cerebellar patients, but the recalibration experiment reveals reduced shift of PSE in cerebellar patients. Thus, cerebellar damage seems to impair recalibration of predictive estimates of stimulus dynamics. Adapted from Roth et al. (2013) [7*].
In contrast, recent work shows that cerebellar dysfunction does not affect the ability to recalibrate the relationship between two sensory modalities (e.g. vision and proprioception). Two studies showed that cerebellar patients could realign proprioceptive estimates of hand position relative to an offset visual target. One study demonstrated that patients could realign their ‘felt’ hand position (the hand was hidden from view) relative to a visual target, despite the fact that these same subjects were impaired in a visuomotor prism adaptation task [9**]. This dissociation is important because it demonstrates the specificity of the deficits—patients clearly have a cerebellar deficit on the visuomotor task but not the visual-proprioceptive recalibration. Henriques, Filippopulous, Straube and Eggert (2014) compared proprioceptive realignment following a reaching task where a cursor was gradually rotated relative to the unseen hand [10]. They studied visuomotor adaptation with unconstrained reaches and a visual-proprioceptive discrepancy task where the movement was externally constrained. In both conditions the cerebellar patients performed comparably to the controls—they adapted reach direction and showed changes in felt hand position (i.e. proprioceptive alignment) relative to the targets. While both of these studies show that cerebellar patients can realign proprioception relative to vision, the first showed a separate motor adaptation deficit, which has commonly been reported, whereas the second did not. Why was this the case? The tasks that were used in these studies were different, but not markedly so as they seem to capture the same type of information. Perhaps the crucial difference is in the patients that were studied: Block and Bastian (2012) studied individuals with cerebellar degeneration and moderate to severe ataxia, whereas Henriques et al. (2014) studied individuals with focal strokes with milder ataxia. Konczak et al. (2005) have shown that patients who have damage only to the cerebellar cortex (as is common in stroke) recover well relative to those with nuclear involvement (which is common in degenerative diseases) [11]; this may explain the differences observed here. Despite these issues, it seems likely that the cerebellum is not required for realigning vision and proprioception, a function that both the Block and Bastian (2012) and Henriques et al. (2014) studies suggest is subserved by the posterior parietal cortex.
Finally, one recent study asked whether the cerebellum might be important for a different kind of visual-proprioceptive integration. Christensen et al. (2014) tested how well people with and without cerebellar damage could visually detect biological motion in a visual display [12]. At times, subjects made movements synchronous with the visual display, and at other times asynchronous with the display. Control participants showed a clear effect of moving while judging biological motion-- synchronous movement facilitated motion detection and asynchronous movement inhibited it. In contrast, the cerebellar group showed no influence of simultaneous movement on biological motion perception. These findings add to literature suggesting a role for the cerebellum in the integration of visual information to form an estimate of stimulus dynamics, but open new questions about the contribution of motor action to this process.
2.2 The cerebellum contributes to somatosensation during active movement
The precise contribution of the cerebellum to somatosensation remains unclear. Historically, cerebellar dysfunction has not been associated with deficits in somatosensation [13–14] and more recent work has found that cerebellar patients have normal proprioception when tested passively [2]. Yet, human neuroimaging results have shown cerebellar activity during somatosensory processing [15] and some neurophysiological studies in animal models have observed cerebellar cortical activity to be better correlated with tactile inputs compared with movement (e.g. [16]). An early case study by Angel (1980) described an individual with hemiataxia due to damage in the right lateral cerebellar hemisphere [17]. When asked to lift objects with his ataxic arm, this individual had difficulty discriminating their weight (i.e. barognosis). Angel suggested that the perception of a load during movement might depend on corollary discharge of the motor command, which could be affected by cerebellar damage.
Bhanpuri, Okamura and Bastian (2012) studied a group of cerebellar patients performing active force and torque discrimination tasks, as well as a passive elbow position discrimination task [18*]. Here patients had deficits in active force and torque discrimination, but no deficits in the passive position discrimination, suggesting that the difficulty lies in active movements. More recently, the same authors explored active proprioception by comparing self-generated (i.e. active) proprioceptive discrimination to passive proprioceptive discrimination (Figure 2 [19**]). Relative to controls, cerebellar patients showed poorer discrimination during active movements, but not when the arm was moved passively. Additionally, when the dynamics of active movements by control subjects were perturbed with unpredictable forces, they showed proprioceptive discrimination deficits similar to cerebellar patients. One interpretation is that cerebellar damage disrupted predictions about self-generated movements, which could be used to help estimate the location of the limb. It is also possible that active muscle contraction normally enhances peripheral proprioceptive signals and that cerebellar damage disrupts this in some manner. We favor the first interpretation, which dovetails nicely with the hypothesized role of the cerebellum in using sensory information to generate internal models of the motor apparatus and environment [20–22].
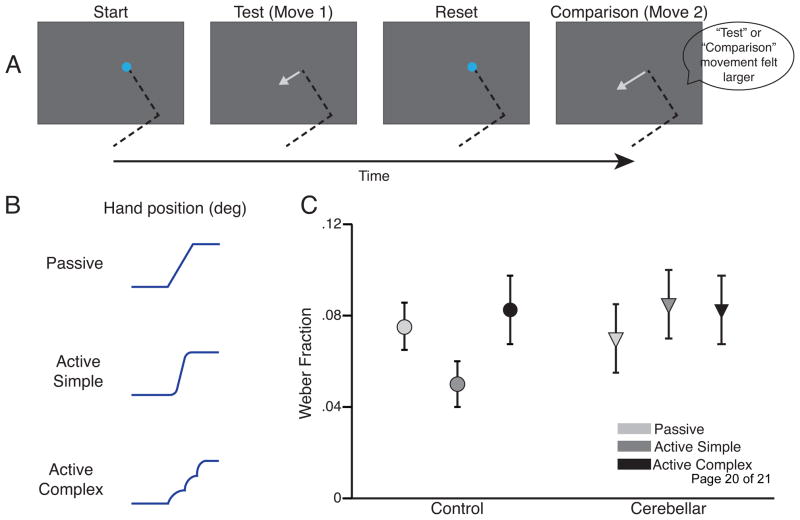
Figure 2.
The effect of cerebellar damage on proprioceptive discrimination performance. A. Schematic of a task requiring discrimination between the lengths of successive arm movements. The two movements are either performed actively by participants or passively with a robotic arm. B. Schematic of data from the 3 experimental conditions, illustrated as the change in elbow angle as a function of time: Passive – a robotic arm passively moves participants’ arms, Active Simple – participants actively move their arm, Active Complex – participants actively move their arms, but the robot applies unpredictable perturbing forces to impair internal predictions of limb dynamics. C. Proprioceptive discrimination thresholds for control participants and cerebellar patients. Control participants show improved discrimination when actively moving and reduced discrimination when the robot created unpredictable dynamics, but cerebellar patients showed no such effect of movement condition. Cerebellar damage may impair the prediction of limb dynamics and this may be important for estimating limb position. Adapted from Bhanpuri et al. (2013). [19**].
3. The cerebellum in motor control and learning
3.1 Deficient internal models can be related to motor control deficits following cerebellar damage
The cerebellum has been implicated in the calculation of internal models of the sensorimotor apparatus and surrounding environment for some time [21–22]. Previous work has posited links between the motor symptoms of cerebellar damage and impaired prediction of dynamics in multi-joint coordination [23]. Until recently though, direct links between disrupted internal models and impaired motor control in cerebellar patients had been lacking. Bhanpuri et al. (2014) has demonstrated that patient specific deficits in arm movements can be explained by a mismatch in internally modeled versus actual limb properties. Cerebellar patients were studied making single joint reaching movements between two targets. The direction of dysmetria (i.e. overshoot or undershoot) exhibited by each patient was systematic, and correlated with movement velocity early in the reach. Patients who typically overshot an endpoint position (i.e. hypermetria) consistently exhibited lower early movement velocities, and patients that undershot it (i.e. hypometria) exhibited higher early velocities. Notably, patients’ tendency to make hyper- or hypometric reaches persisted even when they returned over multiple days.
Patient specific deficits could be explained using a model with a biased estimation of limb inertia, and motor corrective movements driven by delayed sensory feedback (Figure 3 [24**]). In the case of hypermetria, patients produced low initial velocities due to an underestimation of limb inertia, and then made corrective movements that led to overshooting the target. Hypometria, could be modeled as an overestimation of limb inertia, with high initial velocities and corrective movements that undershot the desired endpoint position. Pure timing deficits could not explain these patterns of movement. Importantly, the authors found that altering the inertial properties of the patients’ upper limbs using a robotic arm compensated for these internal model biases and corrected their dysmetria on subsequent reaches. Furthermore, unpredictably changing the inertial properties of the arm in control participants produced behavior that resembled the dysmetric reaches of cerebellar patients. Together, these results suggest that cerebellar damage may induce systematic biases in internal model representations of limb dynamics and these biases may specifically affect limb inertia.
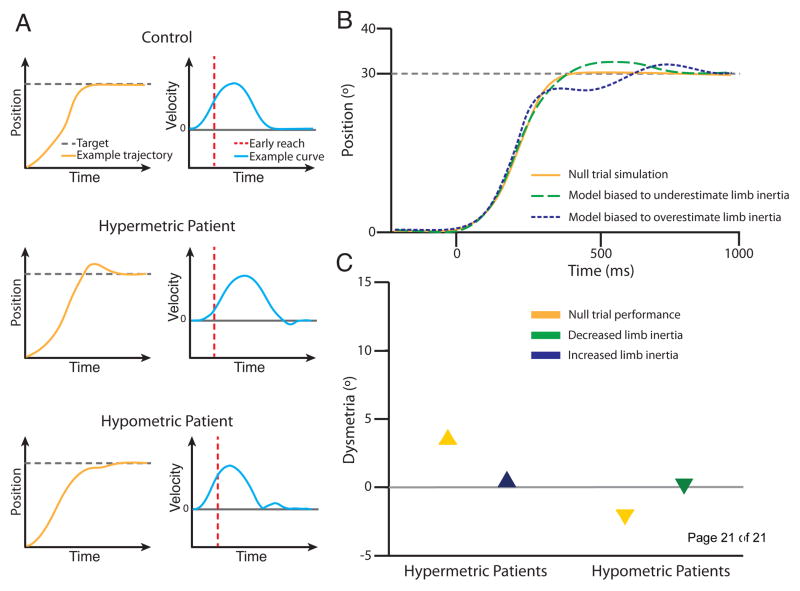
Figure 3.
Cerebellar dysmetria can be related to biased internal models of limb dynamics. A. Example reach trajectories and velocity profiles of a typical control subject, a cerebellar patient who exhibits hypermetria and a cerebellar patient who exhibits hypometria in null conditions. A hypermetric patient will have a slower initial movement velocity, and then make corrective movements that lead to target overshoot., A hypometric patient will show a faster initial movement velocity, and then make corrective movements that lead to target undershoot. These findings are consistent with biased internal models of limb inertia. B. Examples of simulated trajectories from a mathematical model biased to misestimate limb inertia. Hypermetria is simulated with a model that underestimates limb inertia, while hypometria is simulated with a model that overestimates limb inertia. C. Schematic of the dysmetria exhibited by cerebellar patients in a baseline condition (i.e. null trials) and two conditions where a robotic arm was used to alter limb inertia to match the hypothesized bias of their internal model. Reducing limb inertia in hypermetric patients and increasing it in hypometric patients can reduce the dysmetria exhibited following cerebellar damage. Adapted from Bhanpuri et al. (2014) [24**].
3.2 Cerebellar learning- gradual adaptation gives mixed results
It is thought that the cerebellum calibrates internal model estimates through an error-based learning process often referred to as adaptation. Adaptation deficits are ubiquitous in studies of cerebellar patients, including visuomotor adaptation [25–26] and force-field adaptation [27–28], as well as split-belt walking adaptation [29]. Yet some recent work has noted improved motor adaptation in cerebellar patients when force field perturbations are introduced gradually over many movements rather than abruptly in one step [30]. A follow-up study however, has shown that this preserved adaptation to small errors may be related to environmental dynamics rather than the gradual perturbation – patients could learn when a clockwise force field was applied but not when a counter-clockwise field was given [31**]. Note that in the Criscimagna-Hemminger, Bastian and Shadmehr (2010) study, the gradual group always received the clockwise field [30]. Gibo, Criscimagna-Hemminger, Okamura and Bastian (2013) explain this by showing that cerebellar patients had directional biases in baseline movement conditions [31**]. When the force field added to their bias, the patients never appeared to adapt. When the force field was in the opposite direction of their bias, the cerebellar patients appeared to learn to counter it. Thus, when cerebellar patients move within favorable environment dynamics, regardless of error size, they seem to be able to take advantage of other compensatory strategies to improve their movement.
Similarly, there are conflicting results in studies of visual rotation learning in reaching. This is interesting because environmental dynamics are not as relevant here—there are no forces applied to the subjects. Izawa, Chriscimagna-Hemminger and Shadmehr (2013) found that cerebellar patients shifted their reach direction in a gradual visuomotor rotation task to the same degree as controls, but did not shift the perceived location of their hand in a proprioceptive localization task following it [32]. As discussed earlier Henriques et al. 2014 also showed that cerebellar patients can learn a gradual visuomotor rotation, but in their study patients could realign their proprioceptive estimates of hand position. In contrast, Schlerf et al. (2013) showed that cerebellar patients have deficits in adapting their reaching movements to a gradual visuomotor rotation as well as an abrupt one [33].
The basis of these discrepancies is hard to understand. Both Izawa et al. (2013) and Schlerf et al. (2013) studied individuals with cerebellar degeneration, yet came up with very different results. While lesion type does not seem to explain these results, differences in the type of error feedback provided may help explain some of the contradictory findings. While Izawa et al. (2013) provided participants with cursor feedback throughout the reaching movement, Schlerf et al. (2013) only provided endpoint feedback of reaching errors. Learning with endpoint feedback may rely more heavily on updating of feedforward models of the correct reach angle. Thus, the results of Schlerf et al. (2013) may show that true feedforward adaptation is impaired in cerebellar patients, whether it occurs in one step or many. The finding that cerebellar patients can adapt to a gradual visual rotation when cursor feedback is provided may instead reflect an ability to use compensatory online feedback corrections to improve reach accuracy, rather than a preserved mechanism of feedforward adaptation. This would fall in line with Izawa et al.’s (2013) result that cerebellar patients showed no proprioceptive realignment following the adaptation task, suggesting that although they were able to follow the gradual perturbation they did so without any modification to feedforward estimates of reach direction. Overall, these findings raise the interesting question of whether learning deficits following cerebellar damage are absolute. Further studies are needed to understand whether compensatory strategies, such those described above and in [31], may be leveraged in cerebellar patients to yield any retention of the adapted movement. It will also be essential to understand if there are spared learning mechanisms that can be used by cerebellar patients and substituted where error-based adaptation is normally used.
4. Conclusions
Cerebellar damage clearly impairs both sensory and motor function, consistent with its widespread anatomical connections to different brain areas. We do not understand the function of the cerebellum to basal ganglia connection and this should be addressed in future work. However, in the sensory domain, we now know that cerebellar damage impairs visual perception of stimulus movement and proprioceptive perception during active movement. These deficits seem to be due to poor predictions of environment and body dynamics. In the motor domain, cerebellar damage disrupts internal estimates of limb inertia, which leads to characteristic patterns of dysmetria during arm movements. One possible function that can be applied across domains is the prediction of movement dynamics of the body or an external stimulus. This type of computation would require continual calibration for changing body and environmental properties. Cerebellum-dependent adaptation may be used to calibrate these predictions based on error feedback. Yet, there are discrepancies in the literature as to whether the size of the error affects the ability of cerebellar patients to learn—some studies show improved learning with small gradual errors whereas others show no difference. Conflicting results may actually be due to a different factor (e.g. the type of feedback provided), which may have enabled patients to use a different learning mechanism in some cases. Resolving these issues is important, since understanding what learning mechanisms are intact could impact our ability to provide effective motor training in rehabilitation.
Highlights.
Cerebellar damage impairs predictions of visual object motion.
Cerebellar damage impairs active proprioception during movement execution but not passive proprioception.
Motor deficits from cerebellar damage may be due to impaired predictions of limb inertia.
A general function of the cerebellum may be predicting visual, sensory and motor movements.
Acknowledgments
Supported by NIH R01 HD040289 to AJB.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as...
* of particular interest
** of outstanding interest
- 1.Bostan AC, Dum RP, Strick PL. Cerebellar networks with the cerebral cortex and basal ganglia. Trends Cogn Sci. 2013;17:241–54. doi: 10.1016/j.tics.2013.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maschke M, Gomez CM, Tuite PJ, Konczak J. Dysfunction of the basal ganglia, but not the cerebellum, impairs kinaesthesia. Brain. 2003;126:2312–2322. doi: 10.1093/brain/awg230. [DOI] [PubMed] [Google Scholar]
- 3.Leigh RJ, Zee DS, editors. The neurology of eye movements. 3. Oxford University Press; 1999. [Google Scholar]
- 4.Händel B, Thier P, Haarmeier T. Visual motion perception deficits due to cerebellar lesions are paralleled by specific changes in cerebro-cortical activity. J Neurosci. 2009;29:15126–15133. doi: 10.1523/JNEUROSCI.3972-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nawrot M, Rizzo M. Chronic motion perception deficits from midline cerebellar lesions in human. Vision Res. 1998;38:2219–2224. doi: 10.1016/s0042-6989(97)00297-6. [DOI] [PubMed] [Google Scholar]
- 6.O’Reilly JX, Mesulam MM, Nobre AC. The cerebellum predicts the timing of perceptual events. J Neurosci. 2008;28:2252–2260. doi: 10.1523/JNEUROSCI.2742-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7*.Roth MJ, Synofzik M, Lindner A. The cerebellum optimizes perceptual predictions about external sensory events. Curr Biol. 2013;23:930–935. doi: 10.1016/j.cub.2013.04.027. This study shows that cerebellar patients are impaired at recalibrating spatiotemporal predictions about the movement of a visual target. [DOI] [PubMed] [Google Scholar]
- 8*.Deluca C, Golzar A, Santandrea E, Moretto G, Lo Gerfo E, Estocinora J, Moretto G, Fiashi A, Panzeri M, Mariotti C, Tinazzi M, Chelazzi L. The cerebellum and visual perceptual learning : Evidence from a motion extrapolation task. Cortex. 2014;58:52–71. doi: 10.1016/j.cortex.2014.04.017. In this study cerebellar patients judged the final position of a decelerating target. Patients showed deficits in learning to judge the deceleration rate of the baseline stimulus. The extent of these deficits differed between focal lesion and degenerative disease patients. The authors concluded that visual perceptual learning might involve the cerebellum when judgments depend on predictive estimation of stimulus dynamics. [DOI] [PubMed] [Google Scholar]
- 9**.Block HJ, Bastian AJ. Cerebellar involvement in motor but not sensory adaptation. Neuropsychologia. 2012;50:1766–1775. doi: 10.1016/j.neuropsychologia.2012.03.034. This study tested whether an intact cerebellum is necessary for the realignment of sensory weighting. The authors found that although cerebellar patients were impaired in a prism adaptation task relative to controls, they showed intact visually-driven recalibration of proprioceptive estimates of target location. The authors suggest that the cerebellum may not be necessary for adjustments of the relationship between vision and proprioception. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Henriques DYP, Filippopulos F, Straube A, Eggert T. The cerebellum is not necessary for visually driven recalibration of hand proprioception. Neuropsychologia. 2014;64:195–120. doi: 10.1016/j.neuropsychologia.2014.09.029. [DOI] [PubMed] [Google Scholar]
- 11.Konczak J, Schoch B, Dimitrova A, Gizewski E, Timmann D. Functional recovery of children and adolescents after cerebellar tumour resection. Brain. 2005;128:1428–41. doi: 10.1093/brain/awh385. [DOI] [PubMed] [Google Scholar]
- 12.Christensen A, Giese MA, Sultan F, Mueller OM, Goericke SL, Ilg W, Timmann D. An intact action-perception coupling depends on the integrity of the cerebellum. J Neurosci. 2014;34:6707–6716. doi: 10.1523/JNEUROSCI.3276-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dow RE, Moruzzi G. The Physiology and Pathology of the Cerebellum. Minneapolis: University of Minnesota Press; 1958. [Google Scholar]
- 14.Holmes G. The symptoms of acute cerebellar injuries due to gunshot injuries. Brain. 1917;40:461–535. [Google Scholar]
- 15.Gao JH, Parsons LM, Bower JM, Xiong J, Li J, Fox PT. Cerebellum implicated in sensory acquisition and discrimination rather than motor control. Science. 1996;272:545–547. doi: 10.1126/science.272.5261.545. [DOI] [PubMed] [Google Scholar]
- 16.Hartmann MJ, Bower JM. Tactile responses in the granule cell layer of cerebellar folium crus IIa of freely behaving rats. J Neurosci. 2001;21:3549–3563. doi: 10.1523/JNEUROSCI.21-10-03549.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Angel RW. Barognosis in a patient with hemiataxia. Ann Neurol. 1980;7:73–77. doi: 10.1002/ana.410070113. [DOI] [PubMed] [Google Scholar]
- 18*.Bhanpuri NH, Okamura AM, Bastian AJ. Active force perception depends on cerebellar function. J Neurophysiol. 2012;107:1612–1620. doi: 10.1152/jn.00983.2011. This study tested the hypothesis that the cerebellum may have a selective role in forming percepts that involve concurrent movement production. The authors found that cerebellar patients were impaired at active, but not passive force perception. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19**.Bhanpuri NH, Okamura AM, Bastian AJ. Predictive modeling by the cerebellum improves proprioception. J Neurosci. 2013;33:14301–1436. doi: 10.1523/JNEUROSCI.0784-13.2013. This study showed that cerebellar patients showed impairments in a proprioceptive discrimination task when judgments required active movements, but not when a robot passively moved the arm between two positions. Matched control subjects displayed similar deficits as patients when unpredictable perturbations disrupted internal estimates of arm dynamics during active movements. The authors concluded that the role of the cerebellum in active movement perception is in the prediction of limb dynamics. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bastian AJ. Learning to predict the future: the cerebellum adapts feedforward movement control. Curr Opin Neurobiol. 2006;16:645–649. doi: 10.1016/j.conb.2006.08.016. [DOI] [PubMed] [Google Scholar]
- 21.Kawato M. Internal models for motor control and trajectory planning. Curr Opin Neurobiol. 1999;9:718–727. doi: 10.1016/s0959-4388(99)00028-8. [DOI] [PubMed] [Google Scholar]
- 22.Wolpert DM, Miall RC, Kawato M. Internal models in the cerebellum. Trends Cogn Sci. 1998;2:338–347. doi: 10.1016/s1364-6613(98)01221-2. [DOI] [PubMed] [Google Scholar]
- 23.Bastian AJ, Martin TA, Keating JG, Thach WT. Cerebellar ataxia: abnormal control of interaction torques across multiple joints. J Neurophysiol. 1996;76:492–509. doi: 10.1152/jn.1996.76.1.492. [DOI] [PubMed] [Google Scholar]
- 24**.Bhanpuri NH, Okamura AM, Bastian AJ. Predicting and correcting ataxia using a model of cerebellar function. Brain. 2014;137:1931–1944. doi: 10.1093/brain/awu115. This study was the first to link a damaged internal model of limb dynamics, hypothesized to result from cerebellar damage, to patient-specific movement deficits. In three experiments the authors show that the degree and direction of dysmetria exhibited by cerebellar patients can be attributed to a biased internal model of limb inertia. The authors were also able to correct patient dysmetria by externally altering limb inertia with an exoskeleton robot to match the biased internal model. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weiner MJ, Hallett M, Funkenstein HH. Adaptation to lateral displacement of vision in patients with lesions of the central nervous system. Neurology. 1983;33:766–772. doi: 10.1212/wnl.33.6.766. [DOI] [PubMed] [Google Scholar]
- 26.Tseng YW, Diedrichsen J, Krakauer JW, Shadmehr R, Bastian AJ. Sensory prediction errors drive cerebellum-dependent adaptation of reaching. J Neurophysiol. 2007;98:54–62. doi: 10.1152/jn.00266.2007. [DOI] [PubMed] [Google Scholar]
- 27.Maschke M, Gomez CM, Ebner TJ, Konczak J. Hereditary cerebellar ataxia progressively impairs force adaptation during goal-directed arm movements. J Neurophysiol. 2004;91:230–238. doi: 10.1152/jn.00557.2003. [DOI] [PubMed] [Google Scholar]
- 28.Smith MA, Shadmehr R. Intact ability to learn internal models of arm dynamics in Huntington’s disease but not cerebellar degeneration. J Neurophysiol. 2005;93:2809–2821. doi: 10.1152/jn.00943.2004. [DOI] [PubMed] [Google Scholar]
- 29.Morton SM, Bastian AJ. Cerebellar contributions to locomotor adaptations during splitbelt treadmill walking. J Neurosci. 2006;26:9107–9116. doi: 10.1523/JNEUROSCI.2622-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Criscimagna-Hemminger SE, Bastian AJ, Shadmehr R. Size of error affects cerebellar contributions to motor learning. J Neurophysiol. 2010;103:2275–2284. doi: 10.1152/jn.00822.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31**.Gibo TL, Criscimagna-Hemminger SE, Okamura AM, Bastian AJ. Cerebellar motor learning: are environment dynamics more important than error size? J Neurophysiol. 2013;110:322–333. doi: 10.1152/jn.00745.2012. This study showed that the improvement cerebellar patients displayed in the gradual force field adaptation task of Criscimagna-Hemminger et al. (2010) was due to a facilitation effect of the direction of force perturbations helping to counter movement biases patients exhibit in baseline conditions. Patients were impaired when the gradual perturbation required patients to adapt their movements in the opposite direction. The results indicate that environment dynamics impact how cerebellar patients can improve their movements more than error-size. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32*.Izawa J, Criscimagna-Hemminger SE, Shadmehr R. Cerebellar contributions to reach adaptation and learning sensory consequences of action. J Neurosci. 2012;32:4230–4239. doi: 10.1523/JNEUROSCI.6353-11.2012. In this study cerebellar patients adapted to a gradual visuomotor rotation similarly to matched control participants, but did not show any recalibration in a proprioceptive localization task following the adaptation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schlerf JE, Xu J, Klemfuss NM, Griffiths TL, Ivry RB. Individuals with cerebellar degeneration show similar adaptation deficits with large and small visuomotor errors. J Neurophysiol. 2013;109:1164–1173. doi: 10.1152/jn.00654.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]