Abstract
A model examining the effects of an increasing number of maltreatment subtypes experienced on antisocial behavior, as mediated by impulsivity and moderated by a polygenic index of dopaminergic genotypes, was investigated. An African American sample of children (N = 1012, M age = 10.07) with and without maltreatment histories participated. Indicators of aggression, delinquency, and disruptive peer behavior were obtained from peer and counselor rated measures to form a latent variable of antisocial behavior; impulsivity was assessed by counselor report. Five genotypes in four dopaminergic genes (DRD4, DRD2, DAT1, and COMT) conferring heightened environmental sensitivity were combined into one polygenic index. Using SEM, a first-stage, moderated-mediation model was evaluated. Age and sex were entered as covariates, both as main effects and in interaction with maltreatment and the gene index. The model had excellent fit: χ2(32, N =1012) = 86..51, p<0.001; CFI = 0.982; TLI = 0.977; RMSEA = 0.041; SRMR = 0.022. The effect of maltreatment subtypes on antisocial behavior was partially mediated by impulsivity (β= 0.173, p<0.001), and these relations were moderated by the number of differentiating dopaminergic genotypes. Specifically, a significant GxE interaction (b = 0.016, p = 0.013) indicated that the relation between maltreatment and impulsivity was stronger as children evinced more differentiating genotypes, thereby strengthening the mediational effect of impulsivity on antisocial behavior. These findings elucidate the manner by which maltreated children develop early signs of antisocial behavior, and the genetic mechanisms involved in greater vulnerability for maladaptation in impulse-control within context of child maltreatment.
Over 1.25 million children per year suffer from abuse and neglect, costing the US economy over $220 million a day (Prevent Child Abuse America, Gelles & Perlman, 2012). Child maltreatment is considered one of the most detrimental environmental pathogens, increasing the risk for disruptions to biological and psychological processes and physical-health across time (Cicchetti & Toth, 2015). Deprived of the crucial experience of positive parenting, maltreated children tend to follow a cascading developmental path of compromised adaptive behaviors (Cicchetti, 2013; Widom, 2014). Children lacking quality parental care are often subject to extremely disruptive parent-child interactions, inadequate co-regulation of behavioral and emotional responses, and unpredictable parent behavior, which can damage key biological systems (Egeland, Yates, Appleyard, & Van Dulmen, 2002; Pollak, Cicchetti, Hornung, & Reed, 2000; Rogosch, Cicchetti, Shields, & Toth, 1995; Rogosch, Dackis, & Cicchetti, 2011).
One of the most consistent findings in the literature is the association between child maltreatment and antisocial behavior - consisting of aggressive tendencies, ineffective and disruptive peer relations, social information processing deficits, and delinquent acts (e.g., Cicchetti & Rogosch, 2001; Cicchetti, Rogosch, & Thibodeau, 2012; Hong, Espelage, Grogan-Kaylor, & Allen-Meares, 2012; Jaffee, Caspi, Moffitt, & Taylor, 2004; McCrory, De Brito, & Viding, 2012; Teisl & Cicchetti, 2008). Using innovative twin-study designs, researchers have demonstrated that the effect of child abuse on antisocial behavior is partially environmentally mediated, despite shared genes (Jaffee et al., 2004; Moffitt, 2005). Antisocial behaviors impinge on the salubrious functioning of individuals, relationships, and the community at large; determining the precise manner by which these attributes arise in contexts of familial adversity has proven to be a difficult challenge for researchers (Jaffee, Strait, & Odgers, 2012).
One avenue in which antisocial tendencies may emerge in children with a history of maltreatment is through disruptions to a child's behavioral control systems, namely increased impulsivity. Impulsivity broadly refers to a 1) failure to adaptively inhibit pre-potent behaviors and urges, 2) tendency to under-weigh loss in the face of reward and, 3) lack of foresight and judgment while emphasizing quick and shortsighted behaviors. Inadequate impulse-control may contribute to antisociality in a number of ways, such as a diminished regard for the consequences of negative social behavior, difficulty regulating behavior in emotionally taxing circumstances, heightened responses to external threat, and failure to delay gratification when it would otherwise promote adaptive social functioning. Indeed, impulsivity is a known risk factor for antisociality (Derefinko, DeWall, Metze, Walsh, & Lynam, 2011; Lesch, & Merschdorf, 2000; Loeber et al., 2012; Luengo, Carrillo-De-La-Pena, Otero, & Romero, 1994; Neumann, Barker, Koot, & Maughan, 2010). Some forms of proactive aggression and deviancy are the result not of impulsivity per se, but of very deliberate acts often associated with callous-unemotional traits (Frick & White, 2008). However, maltreated children, especially those who are physically abused, are particularly prone to reactive forms of aggression, which are more closely related to impulsivity (Raine et al., 2006; Shields & Cicchetti, 1998).
Numerous studies demonstrate that abused and neglected children tend to display more impulsive-like behaviors than nonmaltreated children (e.g., Manly, Kim, Rogosch, & Cicchetti, 2001; Oshri, Rogosch, Burnette, & Cicchetti, 2011; Oshri, Sutton, Clay-Warner, & Miller, 2015; Sujan, Humphreys, Ray, & Lee, 2014; Wanklyn, Day, Hart, & Girard, 2012). Parents in maltreating families are less likely to scaffold and construct strategies for their children's regulation of behaviors and emotions during times of distress, which may contribute to deficits in impulse control (Kim, Cicchetti, Rogosch, & Manly, 2009). Moreover, children in maltreating circumstances tend to have heightened reactivity to anger and threat, a possible adaptive response in circumstances of abuse, but which nevertheless may have carry-over effects and contribute to impulsivity in non-threating situations (Pollak et al., 2000; Shields & Cicchetti, 1998). Additional deficits in higher-order cognitive processes observed in maltreated children, such as executive function and executive control networks, may also contribute to difficulties in regulating behavioral impulses (Cowell, Cicchetti, Rogosch, & Toth, 2015; Rogosch & Cicchetti, 2005).
Given relations among child maltreatment, impulsivity, and antisocial behavior, it is unsurprising that researchers have modeled all variables together. Oshri et al. (2015), in a sample of 361 emerging adults, found that the strongest indirect link between child abuse/neglect and risk taking behaviors, including antisocial behavior, was impulsivity. Brodsky et al. (2001) revealed in a study of adults with depression, that child maltreatment was strongly related to impulse control deficits, antisocial behavior, and suicide. Futhermore, consistent mediation effects have arisen using the specific concept of ego-control as measured by a Q card-sorting paradigm (Block & Block, 1969/1980; Block & Block, 2006). Ego-control is a personality-based, dimensional measure of impulse control behaviors, ranging from inhibited and constrained or ego-overcontrolled, to impulsive, unconstrained, and without delay of gratification or ego-undercontrolled. The dimension of ego-undercontrol is described as an explicit indicator of impulsivity (Block, Gjerde, & Block, 1986). Moreover, a factor analysis by White et al. (1994) reported ego-undercontrol to load highly onto a latent variable of behavioral impulsivity, which subseqently predicted delinquency. With a longitudinal, person-centered design, Oshri, Rogosch, and Cicchetti (2013) found that profiles of ego-undercontrol mediated the relations between child maltreatment and externalizing problem behaviors. Using ego-undercontrol, among other measures, to index behavioral dysregulation/impulsivity, Egeland et al. (2002) found it partially mediated the longitudinal relations between child abuse and antisocial behavior. Relations among maltreatment, ego-control, and antisociality may have cascading affects, compromising adaption in many areas of life. To support this concept, Oshri et al. (2011), demonstrated that ego-undercontrol directs the effect of maltreatment on antisocial behavior, which in turn potentiates the use and abuse of illicit substances in adolescence. These studies have revealed that antisocial behavior observed in maltreated children may be due, in part, to increased levels of impulsivity especially when indexed by ego-control.
Child maltreatment is a complex phenomenon with dimensions ranging from when in development maltreatment occurred, how often maltreatment is perpetrated, the severity of, and the specific type(s) of abuse/neglect. Experiencing multiple subtypes of maltreatment is a typical finding in maltreated populations (Bolger et al., 1998; Manly, Cicchetti, & Barnett, 1994; Manly et al., 2001) and is known to be especially deleterious to development. An increasing number of maltreatment subtypes experienced has been shown to be related heightened impulsive-like traits such as inappropriate affect, liability/negativity, reduced emotion regulation, (Shields & Cicchetti, 1998). Diminished emotion regulation has also been shown to mediate the relations between the number of maltreatment subtypes experienced and externalizing behaviors (Kim & Cicchetti, 2010). Finally, children who have experienced more subtypes of maltreatment are less likely to demonstrate resilient functioning (Cicchetti & Rogosch, 2012). Given related findings, a greater number of subtypes experienced may be particularly damaging to ego-control and adaptive social behavior.
The precise biological manner by which abuse and neglect contribute to impulsive-like traits and antisociality has yet to be studied in detail. Adverse and stressful experiences such as maladaptive parenting may overload the allostatic stress response system, impacting a range of neurobehavioral functioning important for the regulation of impulses. Chronic over-activation of limbic regions such as the amygdala, during times of stress, in conjunction with deficiencies in the orbitofrontal cortex may undermine key impulse regulatory processes (Dackis et al., 2012; De Brito et al., 2013; Lupien, McEwen, Gunnar, & Heim, 2009). In addition to corticolimbic pathways, child maltreatment has been shown to disrupt cortisol functioning, a major stress hormone implicated in the HPA axis (Cicchetti & Rogosch, 2001; Hart, Gunnar, & Cicchetti, 1996; Murray-Close et al., 2008; Ouellet-Morin et al., 2011; Tarullo & Gunnar, 2006; Trickett, Noll, Susman, Shenk, & Putnam, 2010). Atypical variability in cortisol levels has been associated with both increased impulsivity and aggression (e.g., Alink, Cicchetti, Kim, & Rogosch, 2012; Lovallo, 2013). One particular neurotransmitter system affected by stress-induced-alterations to the HPA axis is that of dopaminergic functioning; dopamine functioning is closely tied to variation in impulsivity. Cortisol secretion during times of acute stress stimulates the release of dopamine in the nucleus accumbens; however with chronic stress, this feedback loop can be slowed as a result of dampened cortisol reactivity. This dampening of the HPA axis results in a deficiency of dopamine at the n. accumbens and is thought to result in impulsive behavior and diminished feeling of reward (Lovallo, 2013). Understanding individual differences within these biological systems may be crucial for understanding patterns of risk and resilience in maltreated children.
A central tenant of developmental psychopathology is the basic but powerful concept of multifinality (Cicchetti & Rogosch, 1996), wherein individuals with similar risk exposures traverse divergent developmental pathways, leading to variation in adaptation and maladaptation. The use of molecular genetic markers in understanding this developmental heterogeneity has been a fast moving, provocative, and informative direction. Several frameworks of understanding the role of genetics in moderating behavioral outcomes in the face of adversity have emerged (e.g. Ellis, Boyce, Belsky, Bakermans-Kranenburg, & van IJzendoorn, 2011; Rosenthal, 1970; van Goozen, Fairchild, Snoek, & Harold, 2007). van Goozen et al. (2007) outlines a model whereby the effect of early childhood adversity on antisocial behavior problems is mediated by disinhibited behavior, driven by neurobiological deficits, and moderated throughout by variation in genotype. Given the role of dopamine dysregulation in impulsivity, genetic predisposition to inefficient dopaminergic functioning may increase children's vulnerability to the effects of maltreatment on impulse-control. Specifically, genotypes conferring particular inefficiencies in the release, reuptake transportation, and reception of dopamine may index a heightened sensitivity or susceptibility to the environment (Bakermans-Kranenburg & van IJzendoorn, 2011). Belsky and Beaver (2011) found that cumulative genetic sensitivity, including the presence of multiple-dopamine-inefficient-genotypes, predicted greater levels of adolescent behavioral dysregulation in the context of unsupportive parenting. In many cases, these interactions can have clinical implications for disorders in which impulsivity is a core feature. Maltreated girls homozygous for the 10-repeat allele of the dopamine transporter gene (DAT1) have been found to have more ADHD symptoms and to be 2.5 times more likely to be diagnosed with the combined type of ADHD (hyperactive and inattentive), as compared to those not homozygous (Li & Lee, 2012). Moreover, Bakermans-Kranenburg and van IJzendoorn (2011) conducted a meta-analysis and found robust evidence that genotypes conferring dopamine inefficiency increased susceptibility to the environment. Finally, in a very relevant example of moderated mediation, Davies, Cicchetti, and Hentges (2014) found that carriers of two DAT1 variants increased children's risk for uninhibited temperament and subsequent behavior problems at age two in families with high maternal unresponsiveness.
Growing evidence suggests that child maltreatment poses a severe threat to impulse control systems and subsequently the development of antisocial behavior. Children with more genotypes that confer dysfunction of the dopamine system may be particularly vulnerable to the effects of maltreatment on heightened ego-undercontrol (impulsivity). A large, developmental model, which examines these relationships collectively, with multiple genes, multiple-informants, a prospective measure of maltreatment, and appropriate covariate control (see Keller, 2014) is lacking in the literature. This study aims to address this research gap, and address criticisms of GxE methodology more generally. We aim to examine the effect of an increasing number of maltreatment subtypes on antisocial behavior as mediated by ego-control (impulsivity). Furthermore, we aim to derive a polygenic index of dopaminergic genotypes and test the moderating role this index has on the association between maltreatment subtypes experienced and impulsivity. Given a paucity of research in this area with African-American populations coupled with the need to study genetic effects with homogenous ancestral samples, we will utilize an all-African-American sample.
Based on an extensive literature review, seven variants across four dopamineric genes were chosen for further analysis and were used to form a polygenic index of differentiating genotypes. These variants were selected for the following two primary reasons 1) their relationship to dopamine inefficiency, and 2) their reliable interaction with maltreatment, or child adversity more generally, to predict impulsive-like behaviors. Two variants in DRD4 were chosen. The first is a well-studied, 48 base pair, variable number of tandem repeats (VNTR). Functional studies have shown that the 7-repeat allele is related to two-folds less efficient receptor function of inhibiting forskolin-stimulated cyclic AMP (cAMP) levels (Asghari et al., 1995). This variant has been shown, in a dominant manner, to interact with maltreatment to predict greater levels of impulsive-like traits such as externalizing behavior and poor self-regulation (Belsky & Beaver, 2011; Bakermans-Kranenburg & van IJzendoorn, 2011). Additionally, this was one variant included in the Bakermans-Kranenburg and van IJzendoorn (2011) meta-analysis, demonstrating reliable sensitivity effects. The second variant is a single nucleotide polymorphism (SNP), rs1800955, referred to as DRD4 −521 C/T. The T allele has been related to 40% less transcriptional activity of DRD4 (Okuyama, Ishiguro, Toru, & Arinami, 1999); however, mixed expression results exist, however (see Simpson, Vetuz, Wilson, Brookes, & Kent, 2010). Nevertheless, differences in resilient functioning among children with a varying number of maltreatment subtypes experienced appear to be greatest for those carrying the TT genotype, suggestive of enhanced sensitivity and differentiation (Cicchetti & Rogosch, 2012).
Next, two DRD2 variants were selected. The first is Taq1A, rs1800497, which is actually part of a gene cluster close to Ankyrin repeat and kinase domain containing 1 (ANKK1) gene. The T (A1) allele has been associated with reduced expression of the D2 receptor in the striatum (Thomson et al., 1997). Carrying at least one copy of the T allele is a reliable measure of differential susceptibility to the environment (Bakermans-Kranenburg & van IJzendoorn, 2011). Familial adversity has been shown to interact with this SNP to predict irritability, stress deregulation, executive control, and ADHD (Bakermans-Kranenburg & van IJzendoorn, 2011; Waldman, 2007; Wiebe et al., 2009); additionally the T allele associates directly with impulsivity (White, Morris, Lawford, & Young, 2008). The second DRD2 variant is rs1799732 (−141C Ins/Del). The deletion (del) allele has been associated with decreased protein expression (Arinami, Gao, Hamaguchi, & Toru, 1997) and appears to be additive in effect (see Ghosh, Pradhan, & Mittal, 2013; Sáiz et al., 2010) despite a number of studies using dominant models (e.g. Davis & Loxton, 2013). Presence of the del allele has been associated with addictive personality traits including impulsivity (Davis & Loxton, 2013), and the del/del genotype has been particularly associated with ADHD + Oppositional Defiant Disorder (Maitra et al., 2014).
Two variants were selected from the DAT1 gene. The dopamine transporter clears the synapse of dopamine via dopamine reuptake into the presynaptic neuron. The first variant is rs40184, the C/C genotype is thought to confer risk for ADHD like symptoms, including impulsivity (Caylak, 2012; Rommelse, 2008). This SNP was one of two DAT1 variants used in a similar design by Davies et al. (2014), wherein this variant and rs27072 moderated the mediation of maternal unresponsiveness on problem behaviors though increased uninhibited temperament. Next, we chose a 40-bp VNTR of DAT1 containing a 10-repeat allele known to confer sensitivity to the environment when homozygous (Bakermans-Kranenburg & van IJzendoorn, 2011). Children of maltreating families, and children of maternally insensitive mothers, carrying the 10R/10R genotype tend to show greater levels of ADHD symptoms and lower levels of self-control (Li & Lee, 2012; Wright, Schnupp, Beaver, Delisi, & Vaughn, 2012). Carriers of the 10R/10R genotype have also been shown to be more vulnerable to deficits in attention, planning, and cognitive flexibility, processes closely tied to impulse control (Cornish et al., 2005).
Finally, a SNP (rs4680,val158met) of the Catechol-O-methyltransferase (COMT) gene was selected. COMT breaks down catecholamine neurotransmitters, such as dopamine, and the val allele is 40% more effective in doing so (Chen et al., 2004; Kim & Lee, 2011). Down-regulated dopamine levels are strongly associated with impulsivity (Lovallo, 2013), in addition to conferring sensitivity to the environment (Bakermans-Kranenburg & van IJzendoorn, 2011). Perroud et al. (2010) found that, in the presence of sexual abuse, those with the greatest levels of anger traits tended to carry the val/val genotype of COMT; Wagner et al. (2010) found similar genotypic effects on impulsive aggression for sexually abused females with borderline personality disorder. In other forms of early adversity, such as low-socio-economic-status, val/val carriers appear to be at most risk for symptoms of ADHD, including impulsivity (Nobile et al., 2010).
The Current Study
The contribution of dopaminergic genetic variation in modulating the relations between cumulative child maltreatment, impulsivity, and early signs of antisocial behavior demands further attention and rigorous methodological approaches. To date, no research has examined the relations between all of these variables in a developmental sample. In this study, we attempt to build on previous GxE research by addressing many of the extant criticisms of this body of literature, including small sample size, improper control of covariates, weak environmental measurement, single-gene focus, and one level of analysis. Furthermore, we continue to build on research, which focuses solely on African-American populations, still under-represented in psychological literature. Through a multi-indicator, multi-informant approach, our study aims to examine the effects of a greater number of maltreatment subtypes experienced on antisocial behavior as mediated by the personality construct of ego-unercontrol (impulsivity). Additionally, we aim to understand multifinality by examining the moderating influence of cumulative-dopaminergic-genetic-sensitivity on the association between maltreatment subtypes experienced and impulsivity. This research framework is guided by three primary hypotheses:
A greater number of maltreatment subtypes experienced will be associated with an increase in antisocial behavior, above and beyond all covariate influence including genetic main effects and GxE effects.
Impulsivity will partially or fully mediate the association between maltreatment subtypes experienced and antisocial behavior. Specifically, a greater number of subtypes will be related to increased ego-undercontrol, which will in turn relate to increased antisociality.
The number of maltreatment subtypes will interact with a cumulative genetic index of differentiating genotypes to predict impulsivity (“a” path). Specifically, those with more differentiating dopaminergic genotypes will be most susceptible to the effects of a greater number of maltreatment subtypes on ego-undercontrol. and this GxE will remain significant with the inclusion of all covariate, covariate by maltreatment, and covariate by polygenic index terms.
Method
Participants
In this study children 6- to 13- years of age (N = 1012; M age = 10.07, SD = 1.60) were recruited to participate in a research-based, summer camp program developed for low-income, school-aged youth. Maltreated (n = 493) and nonmaltreated children (n = 519) comprised the complete sample of participants. Among the child participants, 500 were girls and 512 were boys. The sample was entirely African American as indexed by the Add Health system for coding race and ethnicity (http://www.cpc.unc.edu/projects/addhealth/data/code/race) (DeYoung, Cicchetti, Rogosch, Gray, Eastman, & Grigorenko, 2011). To verify an accurate degree of homogeneous ancestry, a SNP panel of 106 ancestral informative genetic markers (AIMS) was utilized to classify individuals into African, European, and Native American descent (Lai et al., 2009; Yaeger et al., 2008). This sample had a mean proportion of African-American ancestry of .93, corroborating genetic homogeneity; 16 samples were unable to be processed (1.6% of sample).
Recruitment procedures
Informed consent was obtained from all parents of all maltreated and nonmaltreated children; furthermore consent was given for examination of any Department of Human Services (DHS) records pertaining to the families. Maltreated children were identified by the county DHS as having experienced child abuse and/or neglect and were representative of youth receiving DHS services. To recruit maltreating families, a recruitment liaison from DHS contacted a random sample of eligible families and explained the study; if parents were interested, then they signed a release to have their names provided to the project team. Families were free to choose whether or not to participate in this study, as well as free to withdraw at any time. Detailed maltreatment information was obtained through comprehensive searches of DHS records and coded using operational criteria from maltreatment nosology specified by the Maltreatment Classification System (MCS: Barnett, Manly, & Cicchetti, 1993).
Maltreated children were largely from low socioeconomic status backgrounds, a finding consistent with the demographics of maltreating families nationwide (National Incidence Study – NIS-4; Sedlak et al., 2010). Nonmaltreated from socio-demographically comparable backgrounds were recruited from families receiving Temporary Assistance for Needy Families (TANF). In a similar manner, a DHS recruitment liaison reached eligible nonmaltreating families, described the research project, and if parents were interested, their names were provided to the project team for recruitment. Highly trained research assistants interviewed mothers of nonmaltreated children to ensure of a lack of DHS involvement and any prior maltreatment experiences by utilizing the Maternal Maltreatment Classification Interview (Cicchetti, Toth, & Manly, 2003). Additionally, DHS records were searched a year following camp attendance to confirm available information on maltreatment. Only children from families in which no history of documented neglect or abuse were retained in the nonmaltreatment group. Finally, families who received DHS preventive services due to concerns of risk for maltreatment were excluded, thereby minimizing any possible unidentified child abuse/neglect.
A series of regressions were conducted to examine whether or not participant characteristics associated with the number of maltreatment subtypes experienced (the maltreatment variable of interest to this study), ranging from 0 subtypes (nonmaltreated) to 4 subtypes experienced. Males were slightly more likely than females to experience increased subtypes (p = .02); all other associations were non-significant indicating characteristic comparability across the maltreatment variable (see Table 1).
Table 1.
Child Characteristics Across Number of Maltreatment Subtypes
| Zero Subtypes (Nonmaltreated) | One Subtype Of Maltreatment | Two Subtypes Of Maltreatment | Three Subtypes Of Maltreatment | Four Subtypes Of Maltreatment | ||
|---|---|---|---|---|---|---|
| M (SD) or % | M (SD) or % | M (SD) or % | M (SD) or % | M (SD) or % | p-value of Regression | |
| Age | 9.99 (1.59) | 10.32 (1.58) | 10.04 (1.59) | 9.99 (1.66) | 10.06 (2.25) | 0.69 |
| Gender (% male) | 47.59 | 52.80 | 50.26 | 65.79 | 50.00 | 0.02 |
| Maternal Marital Status | ||||||
| Married, living together | 30.91 | 29.23 | 29.73 | 27.14 | 44.44 | Reference |
| Single | 47.30 | 45.64 | 36.21 | 48.57 | 11.11 | 0.45 |
| No longer married | 21.78 | 25.13 | 34.05 | 24.29 | 44.44 | 0.06 |
| Family history of public assistance | 96.47 | 98.96 | 99.46 | 95.71 | 100.00 | 0.17 |
Maltreatment classification
The MCS (Barnett et al., 1993) has been shown to be an extremely reliable and valid measure for classifying child maltreatment typology (Bolger, Patterson, & Kupersmidt, 1998; English, Upadhyaya, Litrownik, Marshall, Runyan et al., 2005; Manly, 2005) that utilizes DHS records, specifying investigations and findings involving maltreatment in identified families over time. Rather than relying on case dispositions and official designations, the MCS codes all available information from DHS records, making independent determinations of maltreating environments. On the basis of operationalized criteria, the MCS designates all subtypes of experienced child maltreatment (i.e., emotional maltreatment, neglect, physical abuse, sexual abuse). DHS record coding was completed by trained research assistants, doctoral students, and clinical psychologists; coders were required to meet strict reliabilities with criterion standards prior to coding actual records for the study. Coders demonstrated acceptable reliability with the criterion (weighted κ's ranging from .86 to .98). Reliabilities (κ's) for the presence vs. absence of maltreatment subtypes ranged from .90 to 1.00.
With regards to subtypes of maltreatment, neglect encompasses failure to provide for the child's basic physical needs for adequate food, clothing, shelter, and medical treatment. In addition to inadequate attention to physical needs, forms of this subtype include lack of supervision, moral-legal neglect, and education neglect. Emotional maltreatment involves extreme thwarting of children's basic emotional needs for psychological safety and security, acceptance and self-esteem, and age-appropriate autonomy. Examples of emotional maltreatment of increasing severity include belittling and ridiculing the child, extreme negativity and hostility, exposure to severe marital violence, abandoning the child, and suicidal or homicidal threats. Physical abuse involves the non-accidental infliction of physical injury on the child (e.g., bruises, welts, burns, choking, broken bones). Injuries range from minor and temporary to permanently disfiguring. Finally, sexual abuse involves attempted or actual sexual contact between the child and caregiver for purposes of the caregiver's sexual satisfaction and/or financial benefit. Events range from exposure to pornography or adult sexual activity, to sexual touching and fondling, to forced intercourse with the child.
Children in the maltreatment group all had documented histories of abuse and/or neglect occurring in their families according to DHS records. However, DHS record information was not complete enough to code maltreatment subtype information for 1 (0.1%) of the maltreated children. Among the remaining maltreated children, 81.7% had experienced neglect, 56.4% had experienced emotional maltreatment, 30.8% had experienced physical abuse, and 7.1% had experienced sexual abuse. 56.6% of the maltreated children had experienced two or more maltreatment subtypes.
As a continuous variable of abuse and/or neglect, children were scored on the number of maltreatment subtypes experienced, ranging from 0 (nonmaltreated) to 4 (having documented experience of all forms of child maltreatment at least once in their childhood).
Procedure
Maltreated and nonmaltreated children attended a week-long day camp and participated in research assessments. At the camp, children were assigned to groups of eight children of the same age and sex; half of the children assigned to each group were maltreated. Three trained camp counselors conducted each group and were unaware of the maltreatment status of the children as well as the hypotheses of the study. The camp lasted 7hrs/day for five days, providing 35 hours of child-counselor and child-peer interactions. After assent, in addition to recreational activities, children participated in a variety of research assessments (see Cicchetti & Manly, 1990, for detailed descriptions of camp procedures) and provided salivary DNA samples. Additionally, trained research assistants, unaware of study hypotheses and maltreatment status, conducted individual research assessments with children, wherein questionnaires and other research measures were administered. Clinical consultation and intervention was provided if any concerns over danger to self or others emerged during the research sessions. All children completed sociometric ratings of their peers at the end of the week. The counselors, who had been trained extensively for two weeks prior to the camp, also completed assessment measures on individual children. These were based on their observations and interactions with children in their respective camp groups.
Measures
The measures described below comprise only a subset of assessments conducted during the research camp. The context of the camp and measurement battery used provided a multi-informant, mulit-perspective evaluation of child functioning including indicators of impulsivity and early signs of antisocial behavior. Antisocial measures include peer evaluations and counselor-report assessments of individual children; impulsivity was measured using Q-Set methodology.
Indicators of antisocial behaviors
Peer ratings
After interacting with their peers during the week of summer camp, children evaluated the characteristics of their camp group peers via a sociometric peer ratings method on the last day of camp (cf., Bukowski, Sippola, Hoza, & Newcomb, 2000; Coie & Dodge, 1983). Counselors guided the sociometric assessment with individual children. For each peer in the camp group, children were given six behavioral descriptors characterizing different types of social behavior. Children were asked to rate each peer on how characteristic the behavioral descriptor was for that peer on a three-point scale. In the current study ratings from peers for physically aggressive behavior and disruptiveness were used. All ratings from peers on each child for each of the two social behavioral descriptors were averaged. The correlation between the descriptors was .78.
Teacher Report Form
(TRF; Achenbach, 1991). Behavioral symptomatology was evaluated at the end of each week by counselors' completion of the TRF. The TRF is a validated, reliable, and widely used instrument to assess behavioral functioning from the perspective of teachers, and the measure was used in the present study, because camp counselors are able to observe children in a similar manner as teachers. The TRF, contains 118 items rated for frequency, assesses two broadband dimensions of child symptomatology, externalizing and internalizing, as well as total behavior problems. In the present study, we examined the Rule Breaking or Delinquent Behavior Problems subscale and the Aggressive Behavior problem subscale; the counselors' scores for each child were averaged to obtain individual child scores for the two subscales. Interrater reliability was estimated for the dimensions of externalizing and internalizing based on average intraclass correlations among pairs of raters ranged from .78 to .88 (M = .83) for externalizing and from .56 to .84 (M = .68) for internalizing.
Assessment of impulsivity
California Child Q-Set
As an index of variation in impulsivity as a personality construct, the ego-control criterion-sort of the California Child Q-Set was utilized (CCQ; Block & Block, 1969/1980). At the end of each camp week, two counselors completed the CCQ independently on the children in their group. The CCQ is comprised of 100 varied items of social, cognitive, and personality characteristics. Camp counselors rated each child's behavioral profiles by sorting the individual items printed on cards into nine piles with a fixed distribution ranging from least characteristic (scored 1) to most characteristic (scored 9). Scores were averaged across raters to derive one Q-set per child. This Q-set methodology has numerous psychometric strengths including observer-evaluations as opposed to self-reporting, continuous form as opposed to dichotomous data, and finally, the use of fixed distributions reduces intra- and inter-judge differences (Block, 1961).
The CCQ has been used to derive a number of Q-criterion sorts to assess a wide range of specific psychological constructs, in which prototypical Q-sets developed from expert raters are correlated to individual child's sort profile to derive criterion score correlations ranging from −1 to 1. One such Q-criterion sort utilized in this study measures Block's dynamic construct of ego control, defined by Funder and Block (1989) as “the individual's generalized disposition or capacity to modulate and contain impulses, feelings, and desires; to inhibit action; and to be insulated from environmental distractions”. The ego control dimension ranges from high ego-undercontrol (with scores closer to 1) to high ego-overcontrol (with scores closer to −1). Children with profiles characteristic of ego-undercontrol (more positive ego control congruence scores) are described as spontaneous, often unable to delay gratification, rebellious, and unpredictable; such traits are consistent with current conceptualizations of, and factor analyses of impulsivity (Letzring, Block, & Funder, 2005; Niv, Tuvblad, Raine, Wang, & Baker, 2012; White et al., 1994). Thus, the ego control Q-criterion scores derived from each child's averaged Q-sort profile were utilized to assess variation in impulsivity. Average intraclass correlations for the ego-control dimension was .80.
DNA collection, extraction, and genotyping
Trained research assistants obtained DNA samples from participants by collecting buccal cells using the Epicentre Catch-All Collection Swabs or by collecting saliva using the Oragene DNA Self-Collection kits. For buccal cells, DNA was extracted and prepared for polymerase chain reaction (PCR) amplification using the Epicentre BuccalAmp DNA Extraction Kit (Epicentre, Cat. No. BQ090155C). For saliva samples, DNA was purified from 0.5 ml of Oragene-DNA solution using the DNAgenotek protocol for manual sample purification using prepIT-L2P. Sample concentrations were determined using the Quant-iT PicoGreen dsDNA Assay Kit (P7589, Invitrogen). Genotyping was preformed following previously published protocols. First, DNA was whole-genome amplified using the Repli-g kit (Qiagen, Catalogue No. 150043) per the kit instructions to preserve the availability of data over the long-term for this valuable sample. Then, amplified samples were diluted to a working concentration.
The DRD4 exon 3 VNTR length was determined by PCR amplifying DNA with primers DRD4 F3 (5'CGGCCTGCAGCGCTGGGA3') and DRD4 R2 D4 (5'CCTGCGGGTCTGCGGTGGAGT3') on a MasterCycler Gradient (Eppendorf, Inc). The Using a CEQ8000 (Beckman Coulter, Inc.), the resulting products were analyzed for length. The DAT1 VNTR was genotyped using the previously reported primers TGTGGTGTAGGGAACGGCCTGAG and CTTCCTGGAGGTCACGGCTCAAGG (Barr et al., 2001; Vandenbergh et al., 1992); the fragments were then analyzed on a 3130xl Genetic Analyzer (Applied Biosystems). The DRD4 −521C/T polymorphism (rs1800955) was analyzed using a Taq Man SNP assay from Applied Biosystems, Inc. Allelic determinations were made using Taq Man Genotyping Master Mix (Applied Biosystems, Catalog No. 4371357) with amplification on an ABI 9700 thermal cycler and analyzing the endpoint fluorescence using a Tecan M200 with JMP 8.0 (SAS, Inc.). The genotyping procedures for DAT1 rs40184, DRD2 rs1800497, DRD2 rs1799732 and COMT rs4680 were similar to those of rs1800955.
For any genotype that could not be determined after the first run, the assay was repeated up to four times and if the null result endured, then a genotype was not assigned to that individual and was treated as missing. DNA samples were genotyped in duplicate for quality control; futhermore, human DNA from cell lines was purchased from Coriell Cell Repositories for all representative genotypes in duplicate and genotypes confirmed by sequencing using DTC& chemistry on an ABI 3130x1. These and a negative template control were run alongside study samples representing 9% of the total data output. Any samples that were not able to be genotyped to a 95% or greater confidence level were repeated under the same conditions.
Results
Hardy-Weinberg and rGE Tests
All genotypes of interest were tested for Hardy-Weinberg equilibrium using the statistical software R version 2.15.2, with package `genetics' version 1.3.8 (R Core Team, 2012; Warnes, 2012). Additionally, before inclusion in the modeling process, each individual genetic variant and the polygenic index of differentiating genotypes were stringently tested for any evidence of confounding gene-environment correlations (rGE). First, a series of chi-square tests was performed to test for associations between each genotype of interest and maltreatment status. Second, a series of linear regressions was run to test associations between each genotype and the number of maltreatment subtypes experienced. Any genotypes, which failed either of the two rGE tests, were excluded from the final index of differentiating genotypes. Finally, to rule out that the final index of differentiating genotypes did not associate with either maltreatment status or the number of maltreatment subtypes experienced, two series of two regression analyses were run. The first was a logistic regression of maltreatment status regressed onto the gene index, and the second was a linear regression with the number of maltreatment subtypes experienced regressed onto the gene index. If the gene index passed these final tests, then it was deemed acceptable for inclusion into the primary modeling process.
Genotype distributions for six of the seven polymorphisms did not differ significantly from Hardy-Weinberg equilibrium (see table 3, DAT1 VNTR, χ2 (9, N = 1003) = 17.88, p = .99; rs40184, χ2 (1, N = 1003) = .95, p = .34; rs1800955, χ2 (1, N = 1003) = .36, p = .60; rs4680, χ2 (1, N = 1003) = 2.50, p = .12; rs1799732, χ2 (1, N = 1003) = .03, p = .84; rs1800497, χ2 (1, N = 1003) = 1.31, p = .25.) except for the DRD4 VNTR variant, χ2 (44, N = 1003) = 255.57, p = .02. Given the large number of repeat alleles (9), with 45 possible genotypes, a departure from HWE with a large sample size is not unusual. Deviation from HWE can result from the violation of HWE principles such as nonrandom mating, migration, selection, and mutation or genotyping errors (Xu, Turner, Little, Bleecker, & Meyers, 2002). Deviation from HWE is acceptable for our analysis as long as genotyping error is not a contributing factor. Genotyping error is very unlikely given our strict quality controls and thus, we did not exclude DRD4 from our polygenic index.
Table 3.
Genotype Frequencies, Call Rate, and Hardy-Weinberg Equilibrium for selected genetic variants
| Gene | Call Rate | Major Allele | Minor Allele | HWE | p-value | |
|---|---|---|---|---|---|---|
| Homozygote N | Heterozygote N | Homozygote N | χ 2 | |||
| DRD4 | 99.11 | 0 Copies | 1 Copies | 2 Copies | 255.57 | .02 |
| 7-Repeat | 706 | 248 | 49 | |||
| VNTR | ||||||
| DRD4 | 99.50 | TT | TC | CC | .36 | .60 |
| rs1800955 | 376 | 486 | 145 | |||
| COMT | 99.80 | GG | GA | AA | 2.50 | .12 |
| rs4680 | 502 | 405 | 103 | |||
| DRD2 | 99.40 | GG | GA | AA | 1.31 | .25 |
| rs1800497 | 420 | 447 | 139 | |||
| DRD2 | 99.40 | AA | AC | CC | .03 | .84 |
| rs1799732 | 269 | 505 | 232 | |||
| DAT1 | 99.30 | TT | TC | CC | .95 | .34 |
| rs40184 | 264 | 487 | 254 | |||
| DAT1 | 98.80 | 0 Copies | 1 Copies | 2 Copies | 17.88 | .99 |
| 10-Repeat | 548 | 395 | 57 | |||
| VNTR |
Using chi-square tests for each of the seven polymorphisms, only the DRD2 rs1800497 variant slightly associated with maltreatment status, χ2 (1, N = 1006) = 3.94, p = .047. All other variants did not show significant associations (DAT1 VNTR, χ2 (1, N = 1006) = .002, p = .96; DRD4 VNTR, χ2 (1, N = 1006) = 1.32, p = .25; rs40184, χ2 (1, N = 1006) = 1.49, p = .22; rs1800955, χ2 (1, N = 1006) = .001, p = .99; rs4680, χ2 (1, N = 1006) = .07, p = .80; rs1799732, χ2 (1, N = 1006) = .55, p = .46.). Because of a possible rGE confound, we excluded DRD2 rs1800497 from our polygenic index. With the remaining six polymorphisms, we conducted linear regressions between each genotype and number of maltreatment subtypes. Only the DAT1 rs40184 variant predicted the number of maltreatment subtypes (β = 0.074, t(1003) = 2.36, p = .019), all other linear relationships were non-significant (data not presented). We also excluded DAT1 rs40184 from our final polygenic index over concerns of a rGE confound. The remaining five variants were combined to form one polygenic index indicating the total number of differentiating genotypes present. We tested whether this polygenic index predicted either maltreatment status or the number of maltreatment subtypes via logistic regression and linear regression, respectively. Both tests were non-significant (data not presented) and a final genetic index of five variants was retained for modeling. Each individual was scored according to the 1) presence of at least one 7-repeat copy of the DRD4 VNTR allele, 2) presence of the 10-repeate homozygote genotype of DAT1 VNTR, 3) presence of T/T genotype of DRD4 −521C/T rs1800955, 4) presence of G/G (Val/Val) of COMT rs4680, and 5) presence of Del/Del genotype of DRD2 rs1799732, for a total score ranging from 0 to 5.
Data Analytic Strategy
Structural equation modeling (SEM) was conducted in Mplus version 7.2 data analysis package (Muthén & Muthén, 1998–2012). Rates of missingness averaged 0.4% for the antisocial behavior indicators, 0.3% for the ego control index of impulsivity, and 2% for the polygenic index of differentiating genotypes. These data were assumed to be at least missing-at-random (MAR); however, this could not be explicitly tested due to lack of longitudinal data. Furthermore, descriptive data suggested that the four antisocial indicators were skewed. To address issues of both missingingness and skew, a robust maximum likelihood estimator was utilized (MLR), which by default models data under the missing data theory using all available data via Full Information Maximum Likelihood (Muthén & Muthén, 1998–2012). Any missing data from exogenous data was modeled by explicitly bringing the respective covariates into the model by estimating their variances.
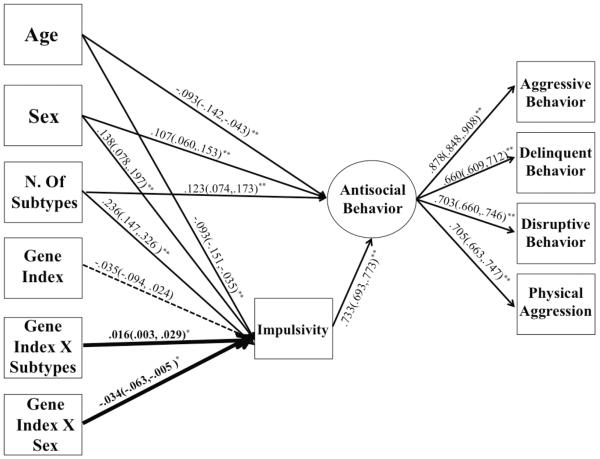
The primary SEM model (Model 1) was comprised of one latent factor referred to as antisocial behavior with four indicators, one observed mediator of impulsivity indexed by ego control, and nine covariate predictors including age, gender, number of maltreatment subtypes experienced, polygenic index of differentiating genotypes (Wray et al., 2014), and five interaction terms. Following the recommendations of Cohen, Cohen, West, and Aiken (2003), age, number of maltreatment subtypes, and the polygenic index were grand-mean centered because of their inclusion as interaction terms as continuous variables. Five interaction terms were created including the primary term of interest: Polygenic Index X Number of Maltreatment Subtypes along with Polygenic Index X Gender, Polygenic Index X Age, Number of Maltreatment Subtypes X Gender, and Number of Maltreatment Subtypes X Age. The inclusion of all covariate by environment and covariate by gene interaction terms is in line with the recommendations of Keller (2014), ensuring proper control for possible multiplicative, confounding effects.
For the measurement component of Model 1, the antisocial latent factor was loaded onto the four antisocial indicators of Rule Breaking, Delinquent behavior, Physical Aggression, and Disruptive Behavior as measured by the TRF and sociometric ratings, respectively. Residual covariances were estimated between the two TRF indictors and the two sociometric ratings; as the scales came from the same respective measures and reporters, their errors were not assumed to be independent. For identification purposes, at least one predictor had to be modeled with the in order to obtain fit indices for the measurement model. The measurement model with one predictor had good absolute and incremental fit: χ2 (3, N =1012) = 32.00, p<0.000; CFI = 0.987; TLI = 0.956; RMSEA = 0.098; SRMR = 0.017. With good initial, approximate fit, this measurement model was included in a larger structural model. Specifically impulsivity was regressed onto the nine covariate predictors. Furthermore, the antisocial latent factor was also regressed onto the nine covariate predictors, in line with a full-partial use of covariates.
For purposes of interpreting the Polygenic Index X Number of Maltreatment Subtypes effect, three additional SEM models were run which varied from Model 1 in terms of where the polygenic index variable was centered (Models 2,3,&,4). Total, direct, and mediation (indirect) effects, of the number of maltreatment subtypes on antisocial behavior via impulsivity were estimated for each of these three secondary models. These estimates illustrate the manner by which the total and mediation effects vary as a function of differing polygenic index levels. Confidence intervals for the mediation effects were estimated in four alternative ways using the statistical software R version 2.15.2, with package `RMediation' version 1.1.3 (R Core Team, 2012; Tofighi & MacKinnon, 2011). Specifically, two distribution-of-product approaches (PRODCLIN, MacKinnon et al., 2007; RDOP, Tofighi, & MacKinnon, 2011), the Monte Carlo method (Tofighi & MacKinnon, 2011), and the asymptotic normal distribution approach (Tofighi & MacKinnon, 2011) were estimated.
Bivariate Correlation Analyses
Table 2 summarizes the Pearson bivariate correlations for the variables included in the structural equation modeling. These results demonstrate statistically significant relations between the number of maltreatment subtypes, impulsivity, and the latent construct of antisocial behavior. Age and sex were associated with impulsivity and antisocial behavior but not number of maltreatment subtypes. Finally, the polygenic index of differentiating alleles did not show any significant associations with any variables.
Table 2.
Pearson Correlation Between Modeled Variables
| 1 | 2 | 3 | 4 | 5 | 6 | |
|---|---|---|---|---|---|---|
| 1. N. of maltreatment subtypes | 1.00 | |||||
| 2. Age | .01 | 1.00 | ||||
| 3. Sex | .07 | −.02 | 1.00 | |||
| 4. Polygenic Index | .00 | −.03 | .05 | 1.00 | ||
| 5. Impulsivity | .17** | -.09** | .15** | −.02 | 1.00 | |
| 6. Latent Antisocial Behavior | .13** | −.10** | .11** | −.03 | .76** | 1.00 |
Note.
p<.01.
Primary Structural Equation Model
The primary moderated mediation model (Model 1) tested whether impulsivity mediated the association between the number of maltreatment subtypes and antisocial behavior, as moderated by a polygenic index of differentiating genotypes with all relevant main effect and interactive covariates controlled for in a full-partial fashion. This initial model had good absolute and incremental fit: χ2 (30, N =1012) = 87.006, p<0.000; CFI = 0.981; TLI = 0.966; RMSEA = 0.044; SRMR = 0.016. While the Chi-Square Test of Model Fit was significant, this is expected given such a large sample size. The number of maltreatment subtypes was significantly, positively related to both impulsivity and antisocial behavior (β = 0.237, one-tailed p<0.000, β = 0.091, one-tailed p = 0.004; respectively). As hypothesized, the interaction between number of maltreatment subtypes and the polygenic index predicting impulsivity was significant (“a” path moderation) and in the expected direction (b = 0.016, p = 0.013); all other interactions with the polygenic gene index were non-significant. This primary GxE effect remained significant even when correcting for the false discovery rate (Benjamini & Hochberg, 1995) of five covariate interaction terms predicting impulsivity (p = .040). Additionally, all Number of Maltreatment Subtypes X Covariate interactions were non-significant except Maltreatment Subtype X Sex predicting impulsivity (b = −0.034, p = 0.020).
For lack of specific hypotheses and rationale (absence of correlations among respective variables) to include all Covariate X Maltreatment and Covariate X Gene Index interaction terms and because most were found to be non-significant predictors in the preliminary model, we trimmed all non-significant interaction terms from Model 1. This trimmed model generally had better absolute and incremental fit than the full model: χ2 (32, N =1012) = 86.511, p<0.000; CFI = 0.982; TLI = 0.977; RMSEA = 0.041; SRMR = 0.022. Furthermore the coefficient and significance level of the number of Maltreatment Subtypes X Polygenic Index predicting impulsivity effect remained the same. To insure this nested model (H0) fit the data as well or better than the full model (H1), a scaled chi-square difference test was preformed (Satorra, 2000). This test found the nested model to not fit significantly better or worse than the full model (X2 (9, N = 1012) = 11.11, p = .268). Thus, because the trimmed model fit as well as the full model and is more parsimonious, it was chosen as our final model (Model 1). Model 1 results are depicted in Figure 1. The predictors and covariates explain 64.2% of the variance in antisocial behavior.
Figure 1.
Model 1 N. of Maltreatment Subtypes on Antisocial Behavior as Mediated By Impulsivity and Moderated By Dopaminergic Genes. Standardized estimates and standardized confidence intervals reported for main effects, unstandardized interaction effects are bolded. Non-significant path is dashed. *p<.05.**p<.01.
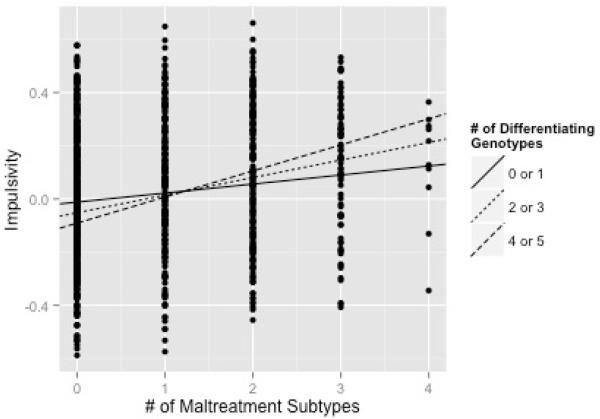
Mediation Effects Differ Across Polygenic Index Levels
In order to interpret the effect of the polygenic index X number of maltreatment subtypes interaction, on the overall partial-mediation of number of maltreatment subtypes on antisocial behavior via impulsivity, three secondary models were performed. Each subsequent model was identical to Model 1 (with all covariate control); however, the polygenic index variable was collapsed into a smaller continuous variable and centered specifically at 0 or 1 genotypes present, 2 or 3 genotypes present, and 4 or 5 genotypes present for Model 2, Model 3, and Model 4, respectively. By collapsing and centering the polygenic variable in this manner, there was a more equitable distribution of participants at each level of the polygenic index variable, and it is straightforward to examine how the mediation effect varies depending on genotypes carried. Table 4 summarizes the total, mediation (indirect) effects, and alternative confidence intervals from each secondary model. Across the three models, the direct effect of maltreatment subtypes on antisocial behavior, as expected, remained the same (β = 0.123, p<0.001). In each model there was a significant, partial, mediation. However, the mediation effect increased as the number of maltreatment subtypes increased. Consequently, the effect of the number of maltreatment subtypes experienced on antisocial behavior as mediated by impulsivity is more apparent in youth who carry more differentiating genotypes. The Polygenic Index X Number of Maltreatment Subtypes interaction is depicted in Figure 2, with three simple slopes corresponding to each varying effect of maltreatment on impulsivity.
Table 4.
Summaries of Secondary Model Effects
| Mediation Effect | Total Effect | PRODCLIN | RDOP | Monte Carlo | Asymptotic Normal | |
|---|---|---|---|---|---|---|
| Unstandardized (Standardized) Beta | Unstandardized (Standardized) Beta | Unstandardized 95% CI of Mediation Effect | Unstandardized 95% CI of Mediation Effect | Unstandardized 95% CI of Mediation Effect | Unstandardized 95% CI of Mediation Effect | |
| 0 or 1 Genotypes (Model2) | .791(.101)* | 1.76(.225)* | .108 – 1.50 | .482 – 1.50 | .105 – 1.50 | .105 – 1.50 |
| 2 or 3 Genotypes (Model3) | 1.55(.198)** | 2.52(.322)** | 1.04 – 2.08 | 1.48 – 2.08 | 1.04 – 2.08 | 1.03 – 2.07 |
| 4 or 5 Genotypes (Model4) | 2.31(.295)** | 3.28(.419)** | 1.51 – 3.12 | 2.18 – 3.12 | 1.52 – 3.12 | 1.50 – 3.12 |
Note.
p<.05.
p <.01.
Figure 2.
Polygenic Index x Number of Maltreatment Subtypes Interaction Predicting Impulsivity. Simple slopes corrected for all covariate effects.
Discussion
Consistent evidence demonstrates that maltreated children are disproportionally at-risk for developing antisocial behaviors such as aggression, delinquency, and conflictual peer relations (see Cicchetti & Rogosch, 2001; Cicchetti et al., 2012; Hong, Espelage, Grogan-Kaylor, & Allen-Meares, 2012; Jaffee, Caspi, Moffitt, & Taylor, 2004). Moreover, early adversity, can lead to uninhibited behavior, a desire for more immediate rewards, and a lack of thoughtful planning, indicative of diminished dopamine functioning (Lovallo, 2013). Such difficulties in adequately controlling impulses may, in part, explain the prevalence of antisociality in maltreated children (see Egeland, Yates, Appleyard, & Van Dulmen, 2002; Oshri et al., 2015). Development, nevertheless, is extraordinarily heterogeneous, and not all maltreated children exhibit deficiencies in social behavior; individual differences in genetic variation may shed light on divergent paths of functioning (Cicchetti et al., 2012). Maltreated children who carry more genotypes that confer inefficiencies in the dopamine system may be particularly sensitive or vulnerable to developing impulse control deficits, and consequently antisocial behavior.
It was hypothesized that an increasing number of maltreatment subtypes would predict early signs of antisocial behavior, and that higher levels of ego-undercontrol, an index of impulsivity, would partially or fully mediate this effect. Additionally, we postulated that the number of maltreatment subtypes experienced would interact with a polygenic index of differentiating, dopaminergic genotypes. Specifically, carriers of more differentiating genotypes would show a larger increase in ego-undercontrol as the number of maltreatment subtypes increased. However, we expected that the main effect of maltreatment on antisocial behavior would remain even in the context of a GxE interaction. Indeed, after controlling for nine covariate effects including covariate by gene index, and covariate by maltreatment terms, the effect of maltreatment subtypes on antisocial behavior remained significant (β = 0.123, p<0.001). Ego-undercontrol did partially mediate this association, and the mediation was informed by a GxE effect. Carriers of more differentiating genotypes appeared to be more sensitive to the effects of a greater number of maltreatment subtypes on ego-undercontrol. That is, the effect of maltreatment on impulsivity increased as the number of differentiating genotypes carried increased. Likewise, the mediation (indirect) effect of maltreatment subtypes on antisocial behavior via impulsivity increased as the number of differentiating genotypes carried increased.
This is the first study to test such a moderated mediation effect, bringing together genetic, social, personality, and family levels-of-analysis into one model. The findings presented here closely resemble the data of previous studies. Davies et al. (2014) formed a polygenic index from two dopaminergic genes, and found that index to moderate a similar mediation between maternal unresponsiveness, uninhibited temperament, and child behavior problems. Using three of the same dopamine genetic variants as this study, Belsky and Beaver (2011), demonstrated greater effects of unsupportive parenting on adolescent self-regulation for those with more differentiating genogytypes. Although at first glance, the interaction plot in Figure 2 may appear to depict differential susceptibility effects (Belsky & Pluess, 2009), this cannot be definitively tested. Although statistical tests exist that differentiate interaction effects such as differential susceptibility, diathesis-stress, and vantage sensitivity (see Roisman et al., 2012; Widaman et al., 2012), the environmental range in this study is too limited to do so. Differential susceptibility theory suggests that some children are disproportionally sensitive to both positive and negative environments, for better or for worse (Belsky & Pluess, 2009). In this study, our environmental range is constrained to the negative end of the spectrum, as both maltreated and nonmaltreated children come from disadvantaged, socio-economic backgrounds. Without a comparison group residing in a more promotive family environment, inferences regarding differential susceptibility effects are speculative at best. Furthermore, we made no specific hypotheses regarding particular environmental sensitivity effects (Pluess, 2015), other than carriers of more differentiating genotypes would show the largest effects of maltreatment on impulsivity.
There were no specific hypotheses pertaining to whether or not the polygenic index of differentiating genotypes would have a main effect on impulsivity or antisocial behavior. There were no main effects of the polygenic index on either outcome when controlling for all covariate influence, including the GxE. A number of studies have shown main effects of the genes comprising the polygenic index on impulsivity-like traits (e.g. Cornish et al., 2005; Gizer, & Waldman, 2012; White et al., 2008). These discrepant findings could result for a number of reasons. Firstly, this study used an all African-American sample; most genetic association studies with these particular dopaminergic variants have focused on primarily Caucasian or mixed race samples. At least for African-American children aged 6–13, an increase in the number of genotypes conferring dopamine inefficiency may not directly predict ego-undercontrol or antisocial behavior. Moreover, these variants were chosen specifically because of their moderating influences rather than their direct influences on behavior. Instead of conferring risk per se, it may be more appropriate to consider these variants as conferring sensitivity or plasticity (Belsky et al., 2009). Namely, the risk imposed by the cumulative effect of dopaminergic, inefficient genotypes on developing impulsivity depends entirely on how many maltreatment subtypes were experienced.
The GxE interaction effect was relatively small (b = 0.016, p = 0.013); however, this is to be expected as genetic moderation effects tend to be small (Duncan & Keller, 2011). Futhermore, this interaction effect contributed to nontrivial increases in the mediation of maltreatment subtypes on antisocial behavior via impulsivity (see Table 4). The reporting of possibly biased or unsubstantiated GxE effects has been criticized (see Duncan & Keller, 2011; Keller, 2014) based on underpowered analyses, improper use of covariates, publication bias, and inadequate measurement of the environment among other reasons. Addressing these extant criticisms is a major strength of this study. A large sample size (N = 1012) with homogenous ancestry is unique among gene-environment interaction studies, ensuring proper control for possible ancestral confounds. Multiple informants provided measurement of multiple, related forms of antisocial behavior, ensuring a more solid latent construct. A multi-genic approach was utilized as opposed to a one-gene/one-variant methodology. By examining multiple genes across distinctive dopaminergic functions (reuptake transportation, metabolism, etc.), a more complete cumulative index of dopamine inefficiency is generated. Maltreatment, as an environmental variable was measured prospectively and objectively using the MCS (Barnett et al., 1993). Finally, each covariate by maltreatment, and covariate by gene index interaction term was added to the model, a practice recommended by Keller (2014). Covariates which correlate with the environmental variable and/or the genetic variable may have confounding multiplicative effects in addition to any confounding main effects. We only found evidence of a confounding interaction between maltreatment subtypes experienced and sex in the prediction of impulsivity. Given the lack of specific hypotheses on covariate interaction effects and for reasons of parsimony, we trimmed all non-significant interaction term paths. This nested model fit the data as well as the full model, thereby ensuring that trimming did not negatively impact on model fit (X2 (9, N = 1012) = 11.11, p = .268).
Another major strength of this investigation was a focus on dopamine genetic moderation. Most GxE studies on antisocial behavior examine only serotoninergic genotypes (e.g. Capsi et al., 2002; Cicchetti et al., 2012, Li & Lee, 2010; Weder et al., 2009). Despite the literature focus on serotonergic processes, dopamine is no less important of a contributing factor in the development of antisocial behavior (Lovallo, 2013). Impulse-control appears to be particularly linked with individual differences in dopaminergic functioning (Buckholtz et al., 2010), more so than serotonin. However, more work needs to be done to elucidate the various independent or dependent ways in which both dopamine and serotonin genes modulate the effects of child maltreatment on antisociality, as they are indeed, interrelated neurotransmitter systems (Seo, Patrick, & Kennealy, 2008).
Despite the strengths of this investigation, some limitations do exist. First and foremost, this is a cross-sectional, correlational design. In order to demonstrate true mediation, the order of effects and causality need to be determined (MacKinnon, & Fairchild, 2009) requiring longitudinal data and an experimental design. It is possible that antisocial behavior and impulsivity are developing concurrently, rather than impulsivity arising first and subsequently influencing antisocial traits. However, previous longitudinal studies using ego-control as a mediator between maltreatment and antisocial behavior corroborate the flow of our model design (see Egeland et al., 2002; Oshri, Rogosch, & Cicchetti 2013). In addition, as expected, the GxE term predicted impulsivity but not antisocial behavior. If impulsivity were indeed the mediator (closer along the causal chain to the genotype), then the strength of the GxE effects should be stronger for impulsivity than for the distal outcomes of antisocial behavior. It is also possible that impulsivity and antisocial behavior are the result of shared genes between parents and children; however, this is unlikely given twin-study designs demonstrating substantial environmental mediation above and beyond the influence of shared genes (see Jaffee et al., 2004; Moffitt, 2005). Only one measure of impulsivity was utilized, and antisocial behavior was treated as one large construct; both constructs are broad, and multi-faceted. Although this study aimed to understand basic associations between these variables, more work should be done to examine precise subtypes of antisocial behavior and impulsivity. For example, impulsivity may better predict reactive and physical forms of antisocial behavior as opposed to proactive and covert forms. Finally, this study may have benefited by the inclusion of biological endophenotypes (Gottesman & Gould, 2003; Lenzenweger, 2015). Although genotypic data are available in our sample, underlying disruptions to neurobiology in these maltreated children are undetermined. A model that incorporates neuroimaging and epigenetic analyses, may be able to better elucidate the precise manner by which child maltreatment, dopamine genotypes, and impulsivity influence the development of antisocial behavior.
In summary, the results of this current investigation emphasize the need for more comprehensive efforts to reduce the occurrence of child maltreatment and mitigate its sequelae. Antisocial behavior is extremely costly to individuals, families, and society at large; early identification and intervention is key to preventing a life-course persistent trajectory (Moffitt, 2006). The finding that more subtypes of maltreatment experienced predicts increasing impulsivity and antisocial behavior is cause for concern, given the fact that 56.6% of maltreated children in this study experienced multiple subtypes. Such cumulative abuse and neglect is likely to overwhelm the body's efforts to maintain healthy homeostasis in critical biological systems (allostatic over-load). As these data suggest, maltreated individuals may be at greatest risk for social maladaptation when they are prone to inefficient dopaminergic functioning. Identifying genetic markers of differential sensitivity to child maltreatment has a number of crucial implications. Genetic information is merely a proxy for the underlying structure and function of biological systems; as such, GxE effects may shed light on critical neurobiological processes important for influencing divergent paths of development (Cicchetti, 2013). Based on the findings herein, genotypes conferring more efficient dopaminergic functioning may be prime candidates in the search for factors important for resilient functioning. It is unlikely that dopaminergic genotypes alone will contribute to multi-domain competence; however, when they are coupled with a host of other neurobiological, familial, and social factors, they may have an appreciable impact on resilient functioning (Cicchetti, 2013; Luthar, Cicchetti, & Becker, 2000; Masten, 2001; Rutter, 2012). Nevertheless, the findings from this study reveal that children residing in similar maltreating families exhibit differential paths of adaptation, some of which can be explained by a greater number of dopaminergic genotypes conferring sensitivity to the environment.
Acknowledgments
Funding received from the National Institute on Drug Abuse (R01DA17741), the National Institute of Mental Health (R01MH083979), and the Spunk Fund, Inc. supported this research.
References
- Achenbach TM. Manual for the Child Behavior Checklist/4–18 and 1991 Profile. University of Vermont, Department of Psychiatry; Burlington, VT: 1991. [Google Scholar]
- Alink LR, Cicchetti D, Kim J, Rogosch FA. Longitudinal associations among child maltreatment, social functioning, and cortisol regulation. Developmental Psychology. 2012;48(1):224. doi: 10.1037/a0024892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arinami T, Gao M, Hamaguchi H, Toru M. A functional polymorphism in the promoter region of the dopamine D2 receptor gene is associated with schizophrenia. Human Molecular Genetics. 1997;6(4):577–582. doi: 10.1093/hmg/6.4.577. [DOI] [PubMed] [Google Scholar]
- Asghari V, Sanyal S, Buchwaldt S, Paterson A, Jovanovic V, Van Tol HH. Modulation of intracellular cyclic AMP levels by different human dopamine D4 receptor variants. Journal of Neurochemistry. 1995;65(3):1157–1165. doi: 10.1046/j.1471-4159.1995.65031157.x. [DOI] [PubMed] [Google Scholar]
- Bakermans-Kranenburg MJ, Van IJzendoorn MH. Gene-environment interaction of the dopamine D4 receptor (DRD4) and observed maternal insensitivity predicting externalizing behavior in preschoolers. Developmental Psychobiology. 2006;48(5):406–409. doi: 10.1002/dev.20152. [DOI] [PubMed] [Google Scholar]
- Bakermans-Kranenburg MJ, van IJzendoorn MH. Differential susceptibility to rearing environment depending on dopamine-related genes: New evidence and a meta-analysis. Development and Psychopathology. 2011;23(01):39–52. doi: 10.1017/S0954579410000635. [DOI] [PubMed] [Google Scholar]
- Barnett D, Manly JT, Cicchetti D. Defining child maltreatment: The interface between policy and research. In: Cicchetti D, Toth SL, editors. Child abuse, Child development, and Social Policy. Ablex; Norwood, NJ: 1993. pp. 7–74. [Google Scholar]
- Barr CL, Xu C, Kroft J, Feng Y, Wigg K, Zai G, Tannock R, Schachar R, Malone M, Roberts W, Nothen MM, Grunhage F, Vandenbergh DJ, Uhl D, Sunohara G, King N, Kennedy JL. Haplotype study of three polymorphisms at the dopamine transporter locus confirm linkage to attention-deficit/hyperactivity disorder. Biological Psychiatry. 2001;49(4):333–339. doi: 10.1016/s0006-3223(00)01053-2. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society. Series B (Methodological) 1995:289–300. [Google Scholar]
- Belsky J, Beaver KM. Cumulative genetic plasticity, parenting and adolescent self-regulation. Journal of Child Psychology and Psychiatry. 2011;52(5):619–626. doi: 10.1111/j.1469-7610.2010.02327.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belsky J, Jonassaint C, Pluess M, Stanton M, Brummett B, Williams R. Vulnerability genes or plasticity genes & quest. Molecular Psychiatry. 2009;14(8):746–754. doi: 10.1038/mp.2009.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belsky J, Pluess M. Beyond diathesis stress: differential susceptibility to environmental influences. Psychological Bulletin. 2009;135(6):885. doi: 10.1037/a0017376. [DOI] [PubMed] [Google Scholar]
- Block J. The Q-sort method in personality assessment and psychiatric research. Charles C. Thomas; Springfield, IL: 1961. [Google Scholar]
- Block J, Block JH. Venturing a 30-year longitudinal study. American Psychologist. 2006;61(4):315. doi: 10.1037/0003-066X.61.4.315. [DOI] [PubMed] [Google Scholar]
- Block JH, Block J. The California Child Q Set. Consulting Psychologists Press; Palo Alto, CA: 1969, 1980. In J. Block, J. H. k, & Keyes, S. (1988). Longitudinally foretelling drug usage in adolescence: Early childhood personality and environmental precursors, Child Development, 59, 336–355. [Google Scholar]
- Block J, Gjerde PF, Block JH. More misgivings about the Matching Familiar Figures Test as a measure of reflection-impulsivity: Absence of construct validity in preadolescence. Developmental Psychology. 1986;22(6):820. [Google Scholar]
- Bolger KE, Patterson CJ, Kupersmidt JB. Peer relationships and self-esteem among children who have been maltreated. Child Development. 1998;69:1171–1197. [PubMed] [Google Scholar]
- Brodsky BS, Oquendo M, Ellis SP, Haas GL, Malone KM, Mann JJ. The relationship of childhood abuse to impulsivity and suicidal behavior in adults with major depression. American Journal of Psychiatry. 2001;158(11):1871–1877. doi: 10.1176/appi.ajp.158.11.1871. [DOI] [PubMed] [Google Scholar]
- Buckholtz JW, Treadway MT, Cowan RL, Woodward ND, Li R, Ansari MS, et al. Dopaminergic network differences in human impulsivity. Science. 2010;329(5991):532–532. doi: 10.1126/science.1185778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukowski WM, Sippola L, Hoza B, Newcomb AF. Pages from a sociometric notebook: An analysis of nomination and rating scale measures of acceptance, rejection, and social preference. In: Cillessen AHN, Bukowski WM, editors. New Directions for Child and Adolescent Development: Vol. 88. Recent Advances in the Measurement of Acceptance and Rejection in the Peer System. Jossey-Bass; San Francisco: 2000. pp. 11–26. [DOI] [PubMed] [Google Scholar]
- Caspi A, McClay J, Moffitt TE, Mill J, Martin J, Craig IW, et al. Role of genotype in the cycle of violence in maltreated children. Science. 2002;297(5582):851–854. doi: 10.1126/science.1072290. [DOI] [PubMed] [Google Scholar]
- Caylak E. Biochemical and genetic analyses of childhood attention deficit/hyperactivity disorder. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics. 2012;159(6):613–627. doi: 10.1002/ajmg.b.32077. [DOI] [PubMed] [Google Scholar]
- Chen J, Lipska BK, Halim N, Ma QD, Matsumoto M, Melhem S, Kolachana BS, Hyde TM, Herman MM, Apud J, Egan MF, Kleinman JE, Weinberger DR. Functional Analysis of Genetic Variation in Catechol-O-Methyltransferase (COMT): Effects on mRNA, Protein, and Enzyme Activity in Postmortem Human Brain. The American Journal of Human Genetics. 2004;75(5):807–821. doi: 10.1086/425589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J, Cohen P, West SG, Aiken LS. Applied multiple regression/correlation analysis for the behavioral sciences. Routledge; New York, NY: 2003. [Google Scholar]
- Cicchetti D. Annual research review: resilient functioning in maltreated children—past, present, and future perspectives. Journal of Child Psychology and Psychiatry. 2013;54(4):402–422. doi: 10.1111/j.1469-7610.2012.02608.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicchetti D, Manly JT. A personal perspective on conducting research with maltreating families: Problems and solutions. In: Brody G, Sigel I, editors. Methods of Family Research: Families at risk. Vol. 2. Lawrence Erlbaum Associates; Hillsdale, NJ: 1990. pp. 87–133. [Google Scholar]
- Cicchetti D, Rogosch FA. Equifinality and multifinality in developmental psychopathology. Development and Psychopathology. 1996;8(04):597–600. [Google Scholar]
- Cicchetti D, Rogosch FA. The impact of child maltreatment and psychopathology on neuroendocrine functioning. Development and Psychopathology. 2001;13(04):783–804. [PubMed] [Google Scholar]
- Cicchetti D, Rogosch FA. Gene× Environment interaction and resilience: Effects of child maltreatment and serotonin, corticotropin releasing hormone, dopamine, and oxytocin genes. Development and Psychopathology. 2012;24(02):411–427. doi: 10.1017/S0954579412000077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicchetti D, Rogosch FA, Thibodeau EL. The effects of child maltreatment on early signs of antisocial behavior: Genetic moderation by tryptophan hydroxylase, serotonin transporter, and monoamine oxidase A genes. Development and Psychopathology. 2012;24(03):907–928. doi: 10.1017/S0954579412000442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicchetti D, Toth SL. A multilevel perspective on child maltreatment. In: Lamb M, Garcia Coll C, editors. Handbook of child psychology and developmental science, 7th ed., Vol. 3: Socioemotional Process. Wiley; New York: 2015. [Google Scholar]
- Cicchetti D, Toth SL, Manly JT. Maternal Maltreatment Interview. Unpublished manuscript; Rochester, NY: 2003. [Google Scholar]
- Coccaro EF, Sripada CS, Yanowitch RN, Phan KL. Corticolimbic function in impulsive aggressive behavior. Biological Psychiatry. 2011;69(12):1153–1159. doi: 10.1016/j.biopsych.2011.02.032. [DOI] [PubMed] [Google Scholar]
- Cornish KM, Manly T, Savage R, Swanson J, Morisano D, Butler N, Grant C, Cross G, Bentley L, Hollis CP. Association of the dopamine transporter (DAT1) 10/10-repeat genotype with ADHD symptoms and response inhibition in a general population sample. Molecular Psychiatry. 2005;10(7):686–698. doi: 10.1038/sj.mp.4001641. [DOI] [PubMed] [Google Scholar]
- Coie JD, Dodge KA. Continuities and changes in children's social status: A five-year longitudinal study. Merrill-Palmer Quarterly. 1983;29:261–282. [Google Scholar]
- Cowell RA, Cicchetti D, Rogosch FA, Toth SL. Childhood maltreatment and its effect on neurocognitive functioning: Timing and chronicity matter. Development and Psychopathology. 2015;27(02):521–533. doi: 10.1017/S0954579415000139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuomo C, Sarchiapone M, Di Giannantonio M, Mancini M, Roy A. Aggression, impulsivity, personality traits, and childhood trauma of prisoners with substance abuse and addiction. The American Journal of Drug and Alcohol Abuse. 2008;34(3):339–345. doi: 10.1080/00952990802010884. [DOI] [PubMed] [Google Scholar]
- Davis C, Loxton NJ. Addictive behaviors and addiction-prone personality traits: Associations with a dopamine multilocus genetic profile. Addictive Behaviors. 2013;38(7):2306–2312. doi: 10.1016/j.addbeh.2013.02.012. [DOI] [PubMed] [Google Scholar]
- De Brito SA, Viding E, Sebastian CL, Kelly PA, Mechelli A, Maris H, McCrory EJ. Reduced orbitofrontal and temporal grey matter in a community sample of maltreated children. Journal of Child Psychology and Psychiatry. 2013;54(1):105–112. doi: 10.1111/j.1469-7610.2012.02597.x. [DOI] [PubMed] [Google Scholar]
- Derefinko K, DeWall CN, Metze AV, Walsh EC, Lynam DR. Do different facets of impulsivity predict different types of aggression? Aggressive Behavior. 2011;37(3):223–233. doi: 10.1002/ab.20387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeYoung C, Cicchetti D, Rogosch FA, Gray J, Eastman M, Grigorenko E. Sources of cognitive exploration: Genetic variation in the prefrontal dopamine system predicts Openness/Intellect. Journal of Research in Personality. 2011;45:364–371. doi: 10.1016/j.jrp.2011.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan LE, Keller MC. A critical review of the first 10 years of candidate gene-by-environment interaction research in psychiatry. American Journal of Psychiatry. 2011;168(10):1041–1049. doi: 10.1176/appi.ajp.2011.11020191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egeland B, Yates T, Appleyard K, Van Dulmen M. The long-term consequences of maltreatment in the early years: A developmental pathway model to antisocial behavior. Children's services: Social policy, Research, and Practice. 2002;5(4):249–260. [Google Scholar]
- Ellis BJ, Boyce WT, Belsky J, Bakermans-Kranenburg MJ, Van IJzendoorn MH. Differential susceptibility to the environment: An evolutionary–neurodevelopmental theory. Development and Psychopathology. 2011;23(01):7–28. doi: 10.1017/S0954579410000611. [DOI] [PubMed] [Google Scholar]
- English DJ, Upadhyaya MP, Litrownik AJ, Marshall JM, Runyan DK, Graham JC, et al. Maltreatment's wake: The relationship of maltreatment dimensions to child outcomes. Child Abuse & Neglect. 2005;29(5):597–619. doi: 10.1016/j.chiabu.2004.12.008. [DOI] [PubMed] [Google Scholar]
- Frick PJ, White SF. Research review: The importance of callous‐ unemotional traits for developmental models of aggressive and antisocial behavior. Journal of Child Psychology and Psychiatry. 2008;49(4):359–375. doi: 10.1111/j.1469-7610.2007.01862.x. [DOI] [PubMed] [Google Scholar]
- Funder DC, Block J. The role of ego-control, ego-resiliency, and IQ in delay of gratification in adolescence. Journal of Personality and Social Psychology. 1989;57(6):1041. doi: 10.1037//0022-3514.57.6.1041. [DOI] [PubMed] [Google Scholar]
- Gelles Richard J., Perlman Staci. Estimated annual cost of child abuse and neglect. Prevent Child Abuse America; Chicago, IL: 2012. [Google Scholar]
- Ghosh J, Pradhan S, Mittal B. Identification of a novel ANKK1 and other dopaminergic (DRD2 and DBH) gene variants in migraine susceptibility. Neuromolecular Medicine. 2013;15(1):61–73. doi: 10.1007/s12017-012-8195-9. [DOI] [PubMed] [Google Scholar]
- Gizer IR, Waldman ID. Double dissociation between lab measures of inattention and impulsivity and the dopamine transporter gene (DAT) and dopamine D4 receptor gene (DRD4) Journal of Abnormal Psychology. 2012;121(4):1011. doi: 10.1037/a0028225. [DOI] [PubMed] [Google Scholar]
- Gottesman II, Gould TD. The endophenotype concept in psychiatry: etymology and strategic intentions. American Journal of Psychiatry. 2003;160(4):636–645. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- Hart J, Gunnar M, Cicchetti D. Altered neuroendocrine activity in maltreated children related to symptoms of depression. Development and Psychopathology. 1996;8(01):201–214. [Google Scholar]
- Hong JS, Espelage DL, Grogan-Kaylor A, Allen-Meares P. Identifying potential mediators and moderators of the association between child maltreatment and bullying perpetration and victimization in school. Educational Psychology Review. 2012;24(2):167–186. [Google Scholar]
- Jaffee SR, Caspi A, Moffitt TE, Taylor A. Physical maltreatment victim to antisocial child: evidence of an environmentally mediated process. Journal of Abnormal Psychology. 2004;113(1):44. doi: 10.1037/0021-843X.113.1.44. [DOI] [PubMed] [Google Scholar]
- Jaffee SR, Strait LB, Odgers CL. From correlates to causes: can quasi-experimental studies and statistical innovations bring us closer to identifying the causes of antisocial behavior? Psychological Bulletin. 2012;138(2):272. doi: 10.1037/a0026020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller MC. Gene × environment interaction studies have not properly controlled for potential confounders: the problem and the (simple) solution. Biological Psychiatry. 2014;75(1):18–24. doi: 10.1016/j.biopsych.2013.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Cicchetti D, Rogosch FA, Manly JT. Child maltreatment and trajectories of personality and behavioral functioning: Implications for the development of personality disorder. Development and Psychopathology. 2009;21(03):889–912. doi: 10.1017/S0954579409000480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Lee D. Prefrontal cortex and impulsive decision making. Biological Psychiatry. 2011;69(12):1140–1146. doi: 10.1016/j.biopsych.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai CQ, Tucker KL, Choudhry S, Parnell LD, Mattei J, Garcia-Bailo B, et al. Population admixture associated with disease prevalence in the Boston Puerto Rican health study. Human Genetics. 2009;125:199–205. doi: 10.1007/s00439-008-0612-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesch KP, Merschdorf U. Impulsivity, aggression, and serotonin: a molecular psychobiological perspective. Behavioral Sciences & the Law. 2000;18(5):581–604. doi: 10.1002/1099-0798(200010)18:5<581::aid-bsl411>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Letzring TD, Block J, Funder DC. Ego-control and ego-resiliency: Generalization of self-report scales based on personality descriptions from acquaintances, clinicians, and the self. Journal of Research in Personality. 2005;39(4):395–422. [Google Scholar]
- Lenzenweger MF. Endophenotype, intermediate phenotype, biomarker: Definitions, concept comparisons, clarifications. Depression and Anxiety. 2013;30(3):185–189. doi: 10.1002/da.22042. [DOI] [PubMed] [Google Scholar]
- Li JJ, Lee SS. Latent class analysis of antisocial behavior: interaction of serotonin transporter genotype and maltreatment. Journal of Abnormal Child Psychology. 2010;38(6):789–801. doi: 10.1007/s10802-010-9409-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JJ, Lee SS. Interaction of dopamine transporter (DAT1) genotype and maltreatment for ADHD: a latent class analysis. Journal of Child Psychology and Psychiatry. 2012;53(9):997–1005. doi: 10.1111/j.1469-7610.2012.02563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeber R, Menting B, Lynam DR, Moffitt TE, Stouthamer-Loeber M, Stallings R, Farrington DP, Pardini D. Findings from the Pittsburgh Youth Study: Cognitive impulsivity and intelligence as predictors of the age–crime curve. Journal of the American Academy of Child & Adolescent Psychiatry. 2012;51(11):1136–1149. doi: 10.1016/j.jaac.2012.08.019. [DOI] [PubMed] [Google Scholar]
- Lovallo WR. Early life adversity reduces stress reactivity and enhances impulsive behavior: Implications for health behaviors. International Journal of Psychophysiology. 2013;90(1):8–16. doi: 10.1016/j.ijpsycho.2012.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luengo MA, Carrillo-De-La-Pena MT, Otero JM, Romero E. A short-term longitudinal study of impulsivity and antisocial behavior. Journal of Personality and Social Psychology. 1994;66(3):542. doi: 10.1037//0022-3514.66.3.542. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nature Reviews Neuroscience. 2009;10(6):434–445. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- Luthar SS, Cicchetti D, Becker B. The construct of resilience: A critical evaluation and guidelines for future work. Child Development. 2000;71:543–562. doi: 10.1111/1467-8624.00164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKinnon DP, Fritz MS, Williams J, Lockwood CM. Distribution of the product confidence limits for the indirect effect: Program PRODCLIN. Behavior Research Methods. 2007;39(3):384–389. doi: 10.3758/bf03193007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKinnon DP, Fairchild AJ. Current directions in mediation analysis. Current Directions in Psychological Science. 2009;18(1):16–20. doi: 10.1111/j.1467-8721.2009.01598.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manly JT. Advances in research definitions of child maltreatment. Child Abuse & Neglect. 2005;29(5):425–439. doi: 10.1016/j.chiabu.2005.04.001. [DOI] [PubMed] [Google Scholar]
- Manly JT, Cicchetti D, Barnett D. The impact of subtype, frequency, chronicity, and severity of child maltreatment on social competence and behavior problems. Development and Psychopathology. 1994;6:121–143. [Google Scholar]
- Manly JT, Kim JE, Rogosch FA, Cicchetti D. Dimensions of child maltreatment and children's adjustment: Contributions of developmental timing and subtype. Development and Psychopathology. 2001;13:759–782. [PubMed] [Google Scholar]
- Masten AS. Ordinary magic: Resilience processes in development. American Psychologist. 2001;56:227–238. doi: 10.1037//0003-066x.56.3.227. [DOI] [PubMed] [Google Scholar]
- Maitra S, Sarkar K, Ghosh P, Karmakar A, Bhattacharjee A, Sinha S, Mukhopadhyay K. Potential Contribution of Dopaminergic Gene Variants in ADHD Core Traits and Co-Morbidity: A Study on Eastern Indian Probands. Cellular and Molecular Neurobiology. 2014;34(4):549–564. doi: 10.1007/s10571-014-0038-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrory E, De Brito SA, Viding E. The link between child abuse and psychopathology: a review of neurobiological and genetic research. Journal of the Royal Society of Medicine. 2012;105(4):151–156. doi: 10.1258/jrsm.2011.110222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell C, Hobcraft J, McLanahan SS, Siegel SR, Berg A, Brooks-Gunn J, Garfinkel I, Notterman D. Social disadvantage, genetic sensitivity, and children's telomere length. Proceedings of the National Academy of Sciences. 2014;111(16):5944–5949. doi: 10.1073/pnas.1404293111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffitt TE. The new look of behavioral genetics in developmental psychopathology: gene-environment interplay in antisocial behaviors. Psychological Bulletin. 2005;131(4):533. doi: 10.1037/0033-2909.131.4.533. [DOI] [PubMed] [Google Scholar]
- Moffitt TE. Life-course-persistent versus adolescence-limited antisocial behavior. In: Cicchetti D, Cohen D, editors. Developmental Psychopathology (2nd ed.), Vol. 3: Risk, Disorder, and Adaptation. Wiley; New York: 2006. pp. 570–598. [Google Scholar]
- Murray-Close D, Han G, Cicchetti D, Crick NR, Rogosch FA. Neuroendocrine regulation and physical and relational aggression: the moderating roles of child maltreatment and gender. Developmental Psychology. 2008;44(4):1160. doi: 10.1037/a0012564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthén LK, Muthén BO. Mplus User's Guide. Seventh Edition Muthén & Muthén; Los Angeles, CA: 1998–2012. [Google Scholar]
- Neumann A, Barker ED, Koot HM, Maughan B. The role of contextual risk, impulsivity, and parental knowledge in the development of adolescent antisocial behavior. Journal of Abnormal Psychology. 2010;119(3):534. doi: 10.1037/a0019860. [DOI] [PubMed] [Google Scholar]
- Niv S, Tuvblad C, Raine A, Wang P, Baker LA. Heritability and longitudinal stability of impulsivity in adolescence. Behavior Genetics. 2012;42(3):378–392. doi: 10.1007/s10519-011-9518-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobile M, Rusconi M, Bellina M, Marino C, Giorda R, Carlet O, Vanzin L, Molteni M, Battaglia M. COMT Val158Met polymorphism and socioeconomic status interact to predict attention deficit/hyperactivity problems in children aged 10–14. European Child & Adolescent psychiatry. 2010;19(7):549–557. doi: 10.1007/s00787-009-0080-1. [DOI] [PubMed] [Google Scholar]
- Okuyama Y, Ishiguro H, Toru M, Arinami T. A genetic polymorphism in the promoter region of DRD4 associated with expression and schizophrenia. Biochemical and Biophysical Research Communications. 1999;258(2):292–295. doi: 10.1006/bbrc.1999.0630. [DOI] [PubMed] [Google Scholar]
- Oshri A, Rogosch FA, Burnette ML, Cicchetti D. Developmental pathways to adolescent cannabis abuse and dependence: Child maltreatment, emerging personality, and internalizing versus externalizing psychopathology. Psychology of Addictive Behaviors. 2011;25(4):634. doi: 10.1037/a0023151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshri A, Rogosch FA, Cicchetti D. Child maltreatment and mediating influences of childhood personality types on the development of adolescent psychopathology. Journal of Clinical Child & Adolescent Psychology. 2013;42(3):287–301. doi: 10.1080/15374416.2012.715366. [DOI] [PubMed] [Google Scholar]
- Oshri A, Sutton TE, Clay-Warner J, Miller JD. Child maltreatment types and risk behaviors: Associations with attachment style and emotion regulation dimensions. Personality and Individual Differences. 2015;73:127–133. [Google Scholar]
- Ouellet-Morin I, Odgers CL, Danese A, Bowes L, Shakoor S, Papadopoulos A, Caspi A, Moffitt TE, Arseneault L. Blunted cortisol responses to stress signal social and behavioral problems among maltreated/bullied 12-year-old children. Biological Psychiatry. 2011;70(11):1016–1023. doi: 10.1016/j.biopsych.2011.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perroud N, Jaussent I, Guillaume S, Bellivier F, Baud P, Jollant F, Leboyer M, Lewis CM, Malafosse A, Courtet P. COMT but not serotonin‐ related genes modulates the influence of childhood abuse on anger traits. Genes, Brain and Behavior. 2010;9(2):193–202. doi: 10.1111/j.1601-183X.2009.00547.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pluess M. Child Development Perspectives. 2015. Individual Differences in Environmental Sensitivity. [Google Scholar]
- Pollak SD, Cicchetti D, Hornung K, Reed A. Recognizing emotion in faces: developmental effects of child abuse and neglect. Developmental Psychology. 2000;36(5):679. doi: 10.1037/0012-1649.36.5.679. [DOI] [PubMed] [Google Scholar]
- R Core Team . R Foundation for Statistical Computing. Vienna, Austria: 2013. R: A language and environment for statistical computing. URL http://www.R-project.org/ [Google Scholar]
- Rogosch FA, Cicchetti D. Child maltreatment, attention networks, and potential precursors to borderline personality disorder. Development and Psychopathology. 2005;17(04):1071–1089. doi: 10.1017/s0954579405050509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogosch FA, Cicchetti D, Shields A, Toth SL. Parenting dysfunction in child maltreatment. In: Bornstein MH, editor. Handbook of Parenting. Lawrence Erlbaum Associates, Inc; Hillsdale, NJ: 1995. pp. 127–159. [Google Scholar]
- Rogosch FA, Dackis MN, Cicchetti D. Child maltreatment and allostatic load: Consequences for physical and mental health in children from low-income families. Development and Psychopathology. 2011;23(04):1107–1124. doi: 10.1017/S0954579411000587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roisman GI, Newman DA, Fraley RC, Haltigan JD, Groh AM, Haydon KC. Distinguishing differential susceptibility from diathesis–stress: Recommendations for evaluating interaction effects. Development and Psychopathology. 2012;24(02):389–409. doi: 10.1017/S0954579412000065. [DOI] [PubMed] [Google Scholar]
- Rommelse NN, Altink ME, Arias- Vásquez A, Buschgens CJ, Fliers E, Faraone SV, Buitelaar JK, Sergeant JA, Franke B, Oosterlaan J. A review and analysis of the relationship between neuropsychological measures and DAT1 in ADHD. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics. 2008;147(8):1536–1546. doi: 10.1002/ajmg.b.30848. [DOI] [PubMed] [Google Scholar]
- Rosenthal D. Genetic Theory and Abnormal Behavior. McGraw-Hill; New York, NY: 1970. [Google Scholar]
- Rutter M. Resilience as a dynamic concept. Development and Psychopathology. 2012;24:335–344. doi: 10.1017/S0954579412000028. [DOI] [PubMed] [Google Scholar]
- Satorra A. Scaled and adjusted restricted tests in multi-sample analysis of moment structures. In: Heijmans RDH, Pollock DSG, Satorra A, editors. Innovations in multivariate statistical analysis. Kluwer Academic Publishers; London: 2000. pp. 233–247. A Festschrift for Heinz Neudecker. [Google Scholar]
- Sáiz PA, García-Portilla MP, Arango C, Morales B, Arias B, Corcoran P, Fernandez JM, Alvarez V, Coto E, Bascaran M, Bousono M, Fananas L, Bobes J. Genetic polymorphisms in the dopamine-2 receptor (DRD2), dopamine-3 receptor (DRD3), and dopamine transporter (SLC6A3) genes in schizophrenia: Data from an association study. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2010;34(1):26–31. doi: 10.1016/j.pnpbp.2009.09.008. [DOI] [PubMed] [Google Scholar]
- Sedlak AJ, Mettenburg J, Basena M, Petta I, McPherson K, Greene A, Li S. Fourth National Incidence Study of Child Abuse and Neglect (NIS–4): Report to Congress, Executive Summary. U.S. Department of Health and Human Services, Administration for Children and Families; Washington, DC: 2010. [Google Scholar]
- Shields A, Cicchetti D. Reactive aggression among maltreated children: The contributions of attention and emotion dysregulation. Journal of Clinical Child Psychology. 1998;27(4):381–395. doi: 10.1207/s15374424jccp2704_2. [DOI] [PubMed] [Google Scholar]
- Seo D, Patrick CJ, Kennealy PJ. Role of serotonin and dopamine system interactions in the neurobiology of impulsive aggression and its comorbidity with other clinical disorders. Aggression and Violent Behavior. 2008;13(5):383–395. doi: 10.1016/j.avb.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson J, Vetuz G, Wilson M, Brookes KJ, Kent L. The DRD4 receptor Exon 3 VNTR and 5′ SNP variants and mRNA expression in human post- mortem brain tissue. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics. 2010;153(6):1228–1233. doi: 10.1002/ajmg.b.31084. [DOI] [PubMed] [Google Scholar]
- Sujan AC, Humphreys KL, Ray LA, Lee SS. Differential association of child abuse with self-reported versus laboratory-based impulsivity and risk-taking in young adulthood. Child Maltreatment. 2014 doi: 10.1177/1077559514543827. 1077559514543827. [DOI] [PubMed] [Google Scholar]
- Teisl M, Cicchetti D. Physical abuse, cognitive and emotional processes, and aggressive/disruptive behavior problems. Social Development. 2008;17(1):1–23. [Google Scholar]
- Thompson J, Thomas N, Singleton A, Piggot M, Lloyd S, Perry EK, Morris CM, Perry RH, Ferrier IN, Court JA. D2 dopamine receptor gene (DRD2) Taql A polymorphism: reduced dopamine D2 receptor binding in the human striatum associated with the A1 allele. Pharmacogenetics and Genomics. 1997;7(6):479–484. doi: 10.1097/00008571-199712000-00006. [DOI] [PubMed] [Google Scholar]
- Tofighi D, MacKinnon DP. RMediation: An R package for mediation analysis confidence intervals. Behavior Research Methods. 2011;43(3):692–700. doi: 10.3758/s13428-011-0076-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenbergh DJ, Persico AM, Hawkins AL, Griffin CA, Li X, Jabs EW, Uhl GR. Human dopamine transporter gene (DAT1) maps to chromosome 5p15. 3 and displays a VNTR. Genomics. 1992;14(4):1104–1106. doi: 10.1016/s0888-7543(05)80138-7. [DOI] [PubMed] [Google Scholar]
- Wagner S, Baskaya Ö, Anicker NJ, Dahmen N, Lieb K, Tadić A. The catechol o- methyltransferase (COMT) val158met polymorphism modulates the association of serious life events (SLE) and impulsive aggression in female patients with borderline personality disorder (BPD) Acta Psychiatrica Scandinavica. 2010;122(2):110–117. doi: 10.1111/j.1600-0447.2009.01501.x. [DOI] [PubMed] [Google Scholar]
- Waldman ID. Gene–environment interactions reexamined: Does mother's marital stability interact with the dopamine D2 gene in the etiology of childhood attention-deficit/hyperactivity disorder? Development and Psychopathology. 2007;19:1117–1128. doi: 10.1017/S0954579407000570. [DOI] [PubMed] [Google Scholar]
- Wanklyn SG, Day DM, Hart TA, Girard TA. Cumulative Childhood Maltreatment and Depression Among Incarcerated Youth Impulsivity and Hopelessness as Potential Intervening Variables. Child Maltreatment. 2012;17(4):306–317. doi: 10.1177/1077559512466956. [DOI] [PubMed] [Google Scholar]
- Warnes G, Gorjanc G, Leisch F, Man M. genetics: Population Genetics. R package version 1.3.8. 2012 http://CRAN.R-project.org/package=genetics.
- Weder N, Yang BZ, Douglas-Palumberi H, Massey J, Krystal JH, Gelernter J, Kaufman J. MAOA Genotype, Maltreatment, and Aggressive Behavior: The Changing Impact of Genotype at Varying Levels of Trauma. Biological Psychiatry. 2009;65(5):417–424. doi: 10.1016/j.biopsych.2008.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White JL, Moffitt TE, Caspi A, Bartusch DJ, Needles DJ, Stouthamer-Loeber M. Measuring impulsivity and examining its relationship to delinquency. Journal of Abnormal Psychology. 1994;103(2):192. doi: 10.1037//0021-843x.103.2.192. [DOI] [PubMed] [Google Scholar]
- White MJ, Morris CP, Lawford BR, Young RM. Behavioral phenotypes of impulsivity related to the ANKK1 gene are independent of an acute stressor. Behavioral and Brain Functions. 2008;4:54. doi: 10.1186/1744-9081-4-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiebe SA, Espy KA, Stopp C, Respass J, Stewart P, Jameson TR, et al. Gene–environment interactions across development: Exploring DRD2 genotype and prenatal smoking effects on self-regulation. Developmental Psychology. 2009;45:31–44. doi: 10.1037/a0014550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widaman KF, Helm JL, Castro-Schilo L, Pluess M, Stallings MC, Belsky J. Distinguishing ordinal and disordinal interactions. Psychological Methods. 2012;17(4):615. doi: 10.1037/a0030003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widom CS. Handbook of Child Maltreatment. Springer; Netherlands: 2014. Longterm Consequences of Child Maltreatment; pp. 225–247. [Google Scholar]
- Wray NR, Lee SH, Mehta D, Vinkhuyzen AA, Dudbridge F, Middeldorp CM. Research Review: Polygenic methods and their application to psychiatric traits. Journal of Child Psychology and Psychiatry. 2014;55(10):1068–1087. doi: 10.1111/jcpp.12295. [DOI] [PubMed] [Google Scholar]
- Wright JP, Schnupp R, Beaver KM, Delisi M, Vaughn M. Youth Violence and Juvenile Justice. 2012. Genes, maternal negativity, and self-control: Evidence of a gene× environment interaction. 1541204011429315. [Google Scholar]
- Xu J, Turner A, Little J, Bleecker ER, Meyers DA. Positive results in association studies are associated with departure from Hardy-Weinberg equilibrium: hint for genotyping error? Human Genetics. 2002;111(6):573–574. doi: 10.1007/s00439-002-0819-y. [DOI] [PubMed] [Google Scholar]
- Yaeger R, Alvial-Bront A, Abdul K, Nolan PC, Grann VR, Birchette MG, et al. Comparing genetic ancestry and self-described race in African Americans born in the United States and in Africa. Cancer Epidemiology, Biomarkers & Prevention. 2008;17:1329–1338. doi: 10.1158/1055-9965.EPI-07-2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Goozen SH, Fairchild G, Snoek H, Harold GT. The evidence for a neurobiological model of childhood antisocial behavior. Psychological Bulletin. 2007;133(1):149. doi: 10.1037/0033-2909.133.1.149. [DOI] [PubMed] [Google Scholar]