Abstract
Lumbar spinal stenosis (LSS) may result from degenerative changes of the spine, which lead to neural ischemia, neurogenic claudication, and a significant decrease in quality of life. Treatments for LSS range from conservative management including epidural steroid injections (ESI) to laminectomy surgery. Treatments vary greatly in cost and success. ESI is the least costly treatment may be successful for early stages of LSS but often must be repeated frequently. Laminectomy surgery is more costly and has higher complication rates. Minimally invasive lumbar decompression (mild®) is an alternative. Using a decision-analytic model from the Medicare perspective, a cost-effectiveness analysis was performed comparing mild® to ESI or laminectomy surgery. The analysis population included patients with LSS who have moderate to severe symptoms and have failed conservative therapy. Costs included initial procedure, complications, and repeat/revision or alternate procedure after failure. Effects measured as change in quality-adjusted life years (QALY) from preprocedure to 2 years postprocedure. Incremental cost-effectiveness ratios were determined, and sensitivity analysis conducted. The mild® strategy appears to be the most cost-effective ($43,760/QALY), with ESI the next best alternative at an additional $37,758/QALY. Laminectomy surgery was the least cost-effective ($125,985/QALY).
Keywords: lumbar spinal stenosis, mild procedure, lower back pain, surgical procedures, economics, quality-adjusted life years, cost-effectiveness
BACKGROUND
Lumbar spinal stenosis (LSS) often results from age related degenerative changes in the lumbar spine. The degenerative changes may manifest as disk bulge, which compromise the size of spinal canal anteriorly, hypertrophy and buckling of the ligamentum flavum, which encroach on the size of the spinal canal posteriorly and by hypertrophy of the lumbar facet joints, which compromise the lateral recesses of the spinal canal.1,2 LSS patients experience pain with any excessive walking or standing, leading to serious physical limitation. Pain and physical limitations result in significant decrease in the quality of life.1 These limitations can have great physical, psychological, and financial negative impact on the individual patient and more importantly on the healthcare system and on the society as a whole.
Treatments for LSS range from conservative management, such as medications, physical therapy with or without epidural steroid injections (ESI) to major surgical decompression of the lumbar spine with or without fusion/instrumentation. Treatments have varying degrees of success, due to symptom levels, severity of the stenosis, the presence or the absence of neurologic deficits, the associated comorbidities as well the time of intervention in relation to the progress of the disease process. Furthermore, these treatments vary great in cost. Conservative treatment on 1 end is the least costly and often successful for early stages of LSS or in patients with minimal symptoms. Treatment, however, must be repeated frequently as its effect is time limited. Surgical decompression on the other hand, if successful, can be 1-time intervention. However, it is much more costly, an option only for moderate or severe symptom sufferers, and is associated with a considerably higher rate of complications, which increase the cost of care. Lastly, there are a considerable number of elderly patients, based on their degree of symptoms or disability, who have failed conservative therapy and either refuse surgery or considered “high risk” of surgery. This leaves these patients untreated or continuing to receive short-term relief with repeated ESI. The latter may lead to steroid-related complications as osteoporosis, worsening of diabetes, or hypertension, as well as increased cost over the long term.
For LSS sufferers, who have failed conservative therapy or received minimal benefit from serial epidural steroid injections, or who are not candidates for surgery, another option exists. The minimally invasive lumbar decompression procedure (mild®; Vertos Medical, Aliso Viejo, CA, U.S.A) allows, under fluoroscopic guidance, lumbar decompression to be performed through a small port without compromising the integrity of the spine. The advantages of this novel technique are that the procedure is minimally invasive, requires minimal anesthesia/deep sedation, is associated with shorter recovery and may have a lower risk of postoperative complications. These advantages may also result in lower overall costs compared with surgical decompression. In addition, it may provide a much greater and more durable level of clinical benefit compared with ESI for patients not eligible for surgery or for those with moderate to severe symptoms. However, it should be emphasized that the mild® procedure is only suitable for patients with symptomatic neurogenic claudication where the thickened ligamentum flavum is the main reason for LSS.
Recent trials have thus far shown durability of treatment effectiveness compared with conservative treatment including serial ESI for suitable patients with a moderate to severe symptoms.3–7 To date, studies have not examined the benefits of ESI or surgery for LSS suffers who have failed conservative therapy in terms of costs or quality of life. Furthermore, no research has included mild® as a possible treatment option for this patient group treatment option from an economic evaluation standpoint. Therefore, the aim of this study is to evaluate the cost-effectiveness of 3 possible options to treat LSS patients who failed the conservative treatment; namely, ESI, mild® procedure and decompression laminectomy.
METHODS
This study was conducted using a cost-utility decision-analytic model. Medicare payer perspective using 2013 fee schedule was adopted to insure fair reliable comparison of the cost to render such treatment. This perspective was also chosen as the majority of patients suffering LSS are at or over Medicare eligible age. While the duration of pain from LSS can last a patient’s lifetime without treatment, there was limited clinical trial data for the mild® procedure beyond this time frame; thus, costs over a 2-year time frame were considered for the model.
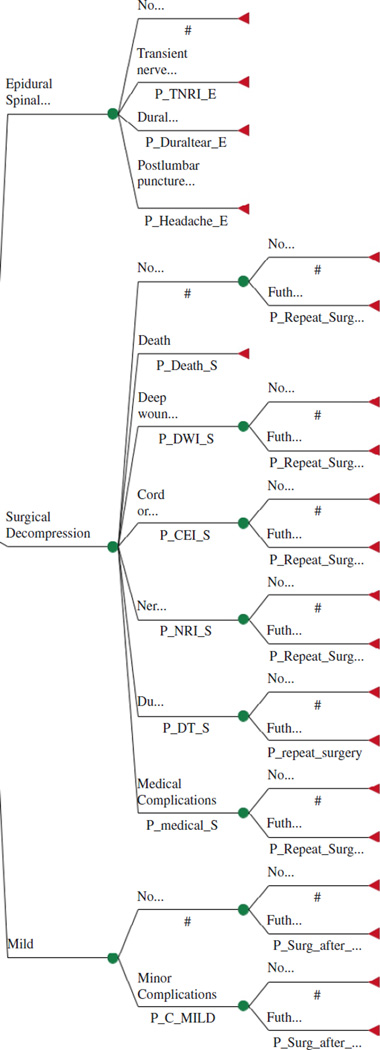
The health benefits considered for this analysis were the life years gained in a quality context, that is, quality-adjusted life years (QALY). The expected incremental costs and health benefits were compared with the standard treatment options for patients with symptomatic lumbar spinal stenosis after failure of conservative therapy. Current treatment options for such patients include serial ESI, mild® procedure, or surgical decompression with or without fusion. These treatment options are listed in Figure 1 and included in the model at the decision node. The uncertain events incorporated into the model included minor or major complications, further treatment, and death. All future costs and benefits were discounted at a rate of 3% in line with current recommendations.8 All cost and benefit data were collected and adjusted where necessary to Medicare fee schedule for the base year 2013 in U.S. dollars.
Figure 1.
Decision tree.
Ethics approval from this study was obtained from the Cleveland Clinic Institutional Review Board.
Economic Model Structure, Description, and Assumptions
To examine the cost-effectiveness of mild® vs. serial ESI or surgical lumbar decompression, a decision tree was constructed using TreeAge Pro® 2012 (TreeAge Software, Inc., Williamstown, MA, U.S.A.). The patient population used was restricted to only those patients who have symptomatic LSS who failed the conservative treatment and would fulfill the clinical criteria for the mild® procedure. For this population, the model presents 3 possible treatment scenarios and is presented in Figure 1. It includes all reasonably possible and mutually exclusive health states throughout the treatment of their LSS with the likelihood of a patients moving to this health state determined by probabilities at the decision node. Such health states include the type of epidural received, any complications they may have from the treatment, and whether further treatment is required within the 2-year time frame.
The terminal node, represented by a triangle, indicates the end of possible health states. At this terminal node, the costs and benefits of a patient reaching this point are input into the model. Based on the terminal node inputs and the probabilities of each health state occurring, the model rolls back the data and analyzes the cost and benefit of each treatment scenario. The cost and benefits of each strategy are compared to determine the optimal strategy choice.
For each intervention, certain treatment path assumptions were made. If no or minimal relief or symptoms returned anytime in the 2 years after the mild® procedure, the procedure was considered failed treatment. It was assumed that these patients would proceed to the surgical option. For all surgical intervention patients who have no or minimal relief or their symptoms return in the 2 years after their surgical procedure, the procedure was also considered a failed treatment. It was assumed that these patients would have a repeat surgical intervention. No further treatment option was considered for serial epidural patients as the population considered for this study already assumes failed conservative therapy. As serial epidural injection is considered part of the conservative therapy regime, we assumed these patients would receive only minimal relief from epidural injections, but that it was the best treatment option available to them.
Discounting
In line with the recommendations of the panel on cost-effectiveness, all future costs and benefits were discounted at an annual rate of 3%.8 This is based on the theory that current costs and benefits are valued higher than those to be gained in the future and are in line with currently published cost-effectiveness analyses.8–10
Willingness to Pay
The willingness to pay is a proposed monetary value at which it is believed society is willing to pay for a year of life in full health (ie, 1 QALY). This value is now widely accepted to be 2 to 2.5 times the GDP per capita of the country in question. In the United States, this value is approximately $100,000 per QALY gained and has been used in numerous cost-effectiveness studies.9,11,12 Thus, any QALY gain that costs less than $100,000 is generally regarded to be a good use of healthcare resources.
Incremental Costs
For each intervention, all relevant costs from the Medicare perspective were included. The incremental costs of implementing each intervention included the cost of the initial intervention, the cost or any repeat or revision procedure, and the cost of any alternate treatment if the initial treatment failed within the 2-year time frame. Because under the Medicare reimbursement model, the majority of complications are not reimbursable, within 90 days of the intervention, additional costs for complications were not included in the model.
At our tertiary referral center, spine surgery statistics indicated 70% of the symptomatic neurogenic claudication patients will undergo 3-level laminectomy, with 20% and 10% undergoing 2 levels and 1-level laminectomy, respectively. To ensure our analysis remained conservative in its parameter values, our model assumed that 75% of the patients would have a 2-level laminectomy with the remaining 25% receiving a 1-level laminectomy.13 No fusion or instrumentation surgeries were included as they are only considered if there is structural compromise or instability. The average length of stay after laminectomy decompression surgery was 3 days. For the epidural option, again based on actual utilization of ESI for patients with LSS in our program, it was assumed these patients would continue with serial ESI annually and that 80% would have the ESI through the lumbar interlaminar approach and the other 20% through the caudal approach. Based on our utilization data, the LSS patient receives an average of 6 to 8 injections per year. A conservative 6 injections per year was chosen for the model, which is in line with CMS reimbursement guidelines. For the mild® intervention, the number of levels or the bilaterality was not considered, as the CMS reimbursement is a fixed fee regardless of the bilaterality or the number of levels treated.
Incremental Benefits (Outcome)
Incremental benefits were measured in QALY. This measure of benefit considers the quantity of life years gained and the desirability of being in that health state. For each intervention, the QALY gain was calculated for each available period to provide and year 1 and year 2 total. QALY reductions were calculated for any complication and subtracted from the total QALY gain based on the severity and duration of the complication attributable to the intervention.
For ESI, the QALY gains were abstracted from the published literature.14 ESI is considered a form of conservative treatment. As our study population has already “failed” or had very little benefit from conservative therapy, the QALY gains abstracted from the literature were reduced by 25% for our model. We feel this adjustment was justified as our model population has moderate to severe symptoms and has previously failed conservative therapy. Therefore, the expected QALY gains after steroid injection would not be as great as symptoms would not be relieved to the same level or last for as long as the published literature population.
Similar to ESI, QALY gains from surgery were abstracted from the published literature.9,15 As our study population is not considered to be at a level of LSS severity that requires surgery, QALY gains abstracted from published literature were reduced by 25%. We feel this adjustment was justified while the level of symptom relief would be similar between our study population and the literature populations, the patients reported in the literature would be starting from a much lower quality of life prior to surgery. This lower quality of life before surgery would result in a greater QALY gain after surgery, a gain greater than would be expected for our study population (Table 1).
Table 1.
Cost Variables
| Variable | Baseline $US 2013 |
Range $US 2013 |
Source $US 2013 |
|---|---|---|---|
| Epidural steroid injection (Interlaminar) – 1 level | 679.09 | 509.32, 848.86 | 27 |
| Epidural Steroid Injection (caudal) – 1 level | 620.35 | 465.26, 775.44 | 27 |
| Mild | 4470.33 | 3352.74, 5587.91 | 27 |
| Decompression surgery – 1 level | 10,018.28 | 7513.71, 12,522,50 | 24, 25, 27 |
| Decompression surgery – 2 levels | 13,844.73 | 10,383.55, 17,305.91 | 24, 25, 27 |
| Discount rate | 3% | 0%, 5% | 8 |
The mild® QALY gains were calculated from the combined data of 4 mild clinical trials conducted in 2009 to 2012. There were a total of 301 trial participants. For each time period, participants were included if complete data were available at that time point and each point prior. Table 2 indicates the number of valid participant data available for each measurement period. To determine the gain in QALY’s attributable to a procedure, the preference-based instrument SF-6D or the EQ-5D is recommended. However, the mild® trials chose to focus on disease-specific outcomes rather than more general outcomes and used the Oswestry Disability Index (ODI). The ODI is a disease-specific tool that provides a numeric rating scale for back and leg pain. An algorithm developed to convert ODI scores to the more general SF-6D was used to calculate any QALY gains in the mild® trials.16 This conversion methodology has been adopted in many analyses similar to this study.9 Using the algorithm, a SF-6D score was calculated for each trial outcome measurement period: baseline, 1 week, 1 month, 6 months, 12 months, 24 months. For each patient, the difference in SF-6D scores from the measurement time to baseline was calculated and multiplied by the length of time as the previous measurement period. These differences were added together to get a final 24-month SF-6D score, equating to the final QALY gain. This methodology is in line with current guidelines for calculating QALY’s as it takes into account that the gain in QALY’s over time is not linear and that QALY gains can often be limited in the beginning until a full recovery is made.17,18
Table 2.
Mild Quality-adjusted Life Years (QALY) Gain Statistics
| Number of Valid Responses | Mean | Median | Min | Max | Standard Deviation | |
|---|---|---|---|---|---|---|
| 1-week QALY | 260 | 0.0012 | 0.0010 | −0.0028 | 0.0066 | 0.0017 |
| 3-month QALY | 260 | 0.0210 | 0.0171 | −0.0291 | 0.0851 | 0.0277 |
| 6-month QALY | 246 | 0.0205 | 0.0181 | −0.0311 | 0.0907 | 0.0240 |
| 12-month QALY | 123 | 0.0350 | 0.0337 | −0.0725 | 0.1554 | 0.0476 |
| 24-month QALY | 84 | 0.0532 | 0.0414 | −0.1761 | 0.3004 | 0.0906 |
| 12-month QALY Total | 0.0777 | 0.0716 | −0.1355 | 0.3378 | 0.101 | |
| 24-month QALY Total | 0.1309 | 0.1113 | −0.3116 | 0.6382 | 0.1916 |
Uncertainty and Sensitivity Analysis
In Tables 1–5, each variable, its low and high value and its source are presented. Analyses were performed around the value range for each variable to determine the robustness of the model to changes in the values. The model was first tested using the unadjusted QALY gains for surgery and epidural steroid injections. Further variables for which the model was most sensitive to were identified through a series of one-way sensitivity analyses. This involves varying the value of variable, 1 variable at a time. The variables with highly sensitive values were rigorously tested using a threshold and 2-way sensitivity analyses. The previous analyses test the uncertainty amongst the variables. To test the variability within a population, microsimulation analysis was used. This analysis involves replicating 1 patient running through the model with the outcomes based on the underlying probabilities of model events. A microsimulation analysis of 1,000 simulations was conducted with the costs, effects, and net monetary benefits calculated for each simulation for each strategy.
Table 5.
Complication, Revision and Failure Rates, Treatment Choice by Treatment Option
| Baseline % | Range | Source | |
|---|---|---|---|
| Mild | |||
| Death | 0.0 | 0.0, 0.05 | 28 |
| Deep wound infection | 0.0 | 0.0, 0.05 | 28 |
| Cord or cauda equine injury | 0.0 | 0.0, 0.05 | 28 |
| Nerve root injury | 0.0 | 0.0, 0.05 | 28 |
| Dural tear | 0.0 | 0.0, 0.05 | 28 |
| Medical (POMI, PE etc.) | 0.0 | 0.0, 0.05 | 28 |
| Epidural-Interlaminar | |||
| Transient nerve root irritation | 0.28 | 0.21, 0.35 | 29 |
| Dural puncture | 0.8 | 0.6, 1.0 | 29 |
| Postlumbar puncture headache | 0.07 | 0.05, 0.09 | 29 |
| Epidural-Caudal | |||
| Transient nerve root irritation | 0.0 | 0.0, 0.05 | 29 |
| Dural puncture | 0.0 | 0.0, 0.05 | 29 |
| Postlumbar puncture headache | 0.0 | 0.0, 0.05 | 29 |
| Percent receiving caudal epidural | 20% | 0.0%, 50% | 30 |
| Number epidurals 2 years | 12 | 6, 16 | 30 |
| Laminectomy surgery | |||
| Death | 0.8 | 0.6, 1.0 | 31 |
| Deep wound infection | 1.0 | 0.75, 1.25 | 17 |
| Cord or cauda equine injury | 0.1 | 0.08, 0.125 | 17 |
| Nerve root injury | 1.0 | 0.75, 1.25 | 17 |
| Dural tear | 5.7 | 4.28, 7.13 | 17 |
| Medical (POMI, PE etc.) | 2.2 | 1.65, 2.75 | 17 |
| Percent receiving 1 level surgery | 25% | 0.0%, 50% | 13 |
| Percent revision surgery | 8.9% | 0.0%, 13.4% | 24, 32 |
Incremental Cost-Effectiveness Ratio
The incremental cost-effectiveness ratio (ICER) is a common methodology that allows interventions across different disease states to be compared. An ICER examines the incremental costs and incremental benefits of 1 intervention over another, with results presented as a ratio. Depending on the number of interventions evaluated, many ICERS may result with each representing the incremental cost and incremental benefit of an intervention over the next most cost-effective option. To calculate an ICER, the equation ICER = (C1–C2)/(E1–E2) with C1 and E1 being the costs and effectiveness (benefits) of the intervention in question, and C2 and E2 being the costs and effectiveness (benefits) of next most cost-effective option.18
Net Monetary Benefits
A cost-effectiveness analysis only tells us the optimal choice of strategy. It does not consider the cost of the strategy in relation to society’s willingness to pay (WTP) for a QALY. The net monetary benefit is a way of incorporating the willingness to pay to determine whether a strategy should be accepted or rejected. Net monetary benefits (NMB) are calculated by multiplying the QALY gained by the willingness to pay for a QALY, then subtracting the costs of the intervention.19,20 If the NMB are positive, the intervention should be implemented as an interventions value exceeds its cost. If the NMB are negative, the intervention should be rejected as the value of the intervention is less than the cost of the intervention.
RESULTS
Baseline Analysis
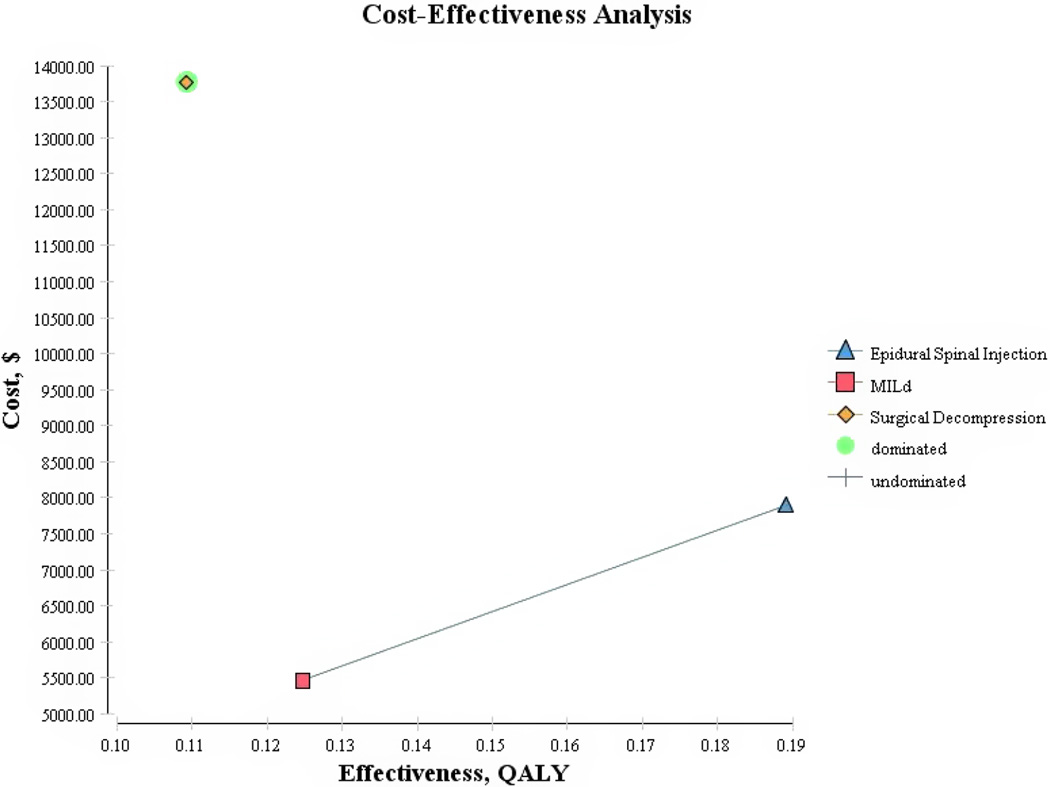
With the model variables set to baseline values, all 3 interventions generated health benefits for an additional cost. The 2-year costs, benefits and ICERs are listed in Table 6 and in graphical form in Figure 2. The optimal choice in terms of costs and benefits was the mild® strategy at $43,760 per QALY. The next best alternative was ESI at an additional $37,758 per QALY. Laminectomy surgery was dominated by both mild® and ESI’s as it would be both more costly and less effective according to the baseline analysis ($125,985 per QALY).
Table 6.
Two-year Average and Incremental Cost and Effect Outcomes (Adjusted)
| Intervention | Cost ($) | Incremental Cost ($) | Effect (QALY) | Incremental Effect (QALY) | ICER | Average CER |
|---|---|---|---|---|---|---|
| Mild | 5457.92 | 0.12 | 43,760 | |||
| Epidural Spinal Injection | 7887.98 | 2430.06 | 0.19 | 0.06 | 37,758 | 41,717 |
| Surgery | 13770.72 | 5882.74 | 0.11 | −0.08 | −73,739 | 125,985 (Dominated) |
QALY, quality-adjusted life years; ICER, incremental cost-effectiveness ratio.
Figure 2.
Cost per quality-adjusted life years (QALY) for lumbar spinal stenosis (Adjusted QALY).
Sensitivity Analysis
Sensitivity analysis was used to test the robustness of mild® being the choice strategy to changes within the model parameters. One-way sensitivity analysis demonstrated that the choice strategy was robust to most model variables. The variables with influence on the cost per QALY included QALY gain, intervention costs and surgical revision rate. Only one parameter tested changed the choice of optimal strategy. It is only if the LSS patients require 3 or fewer ESI’s per year, the choice strategy would change from mild® to ESI. Further one-way sensitivity analysis showed that if the QALY gain for surgery exceeded 0.218, mild® would remain the choice strategy; however, surgery would no longer be dominated by mild®. Laminectomy surgery for an additional cost would become a cost-effective option and would be plotted on the cost-effectiveness frontier. The results of the microsimulation, testing population variability, demonstrated results similar to the baseline analysis. Mild® remained the choice strategy with ESI cost-effective at an additional $34,716 per QALY. Laminectomy surgery remained dominated. To test mild® in a very conservative or worst-case scenario, the analysis was performed with the QALY gains for surgery and ESI abstracted from the published literature for were not reduced by 25% (unadjusted). Even under this skeptical scenario, mild® remained the choice strategy (Figure 3).
Figure 3.
Cost per quality-adjusted life years (QALY) for lumbar spinal stenosis (Unadjusted QALY).
Net Monetary Benefits
When ESI was compared with mild®, if the WTP threshold was $40,500 or greater, the incremental NMB of ESI remained positive, while mild procedure remained the same. Because surgery was dominated by both strategies, the incremental NMB were not calculated.
DISCUSSION
In this economic climate, our aging population lives longer, with multiple healthcare issues, however, they strive for a higher quality of life. This has an increasing demand on our healthcare system that is already suffering from decreasing resources. As such, there is a need more than ever to ensure that available healthcare resources are used efficiently and effectively. Owing to this climate, much focus has been around cost-effectiveness analysis and measuring the value of specific healthcare interventions.8 The gold standard of these analyses is to not just consider costs, but to consider them in the context of quality of life, and the comparative cost per quality-adjusted life year gained for comparable interventions.
Further to the economic climate, our aging population is increasing not only in size but also in the complexity of their healthcare issues as well as in expectations. Also with many advances in healthcare technology, the life expectancy is longer, and therefore, the duration in which our aging population require healthcare is becoming longer. With this, much of the healthcare resource utilization analysis has focused on costly healthcare conditions and chronic health conditions. LSS is one such chronic condition. It has a great negative impact on quality of life and available treatments vary greatly in terms of costs, degree and duration of clinical benefit as well as in the span of the complications associated with such treatments. To date, much of the research has focused on 2 treatment options: serial epidural steroid injection and decompression laminectomy surgery.9,15,21–23 While studies have shown these options are cost-effective, the result is highly dependent on the level of severity of symptoms.9,14,17,22,24,25 Serial ESI’s are cost-effective in early cases and laminectomy surgery cost-effective for late cases those with severe symptoms. However, studies have not evaluated either intervention for moderate symptom sufferers. Furthermore, no studies have analyzed the cost-effectiveness of a third treatment option, mild® in relation to these intervention options.
This analysis aimed to evaluate the cost-effectiveness of the 3 possible options, ESI, mild® procedure, and laminectomy surgery for patients who failed the conservative approach. The results demonstrated that for these patients, when quality of life and the need for repeat treatment are considered over a 2-year period, mild® is a cost-effective option and is the optimal intervention of choice. The laminectomy surgery intervention was not the preferred option as it both costs more and has a lower QALY gain then either mild or ESI’s. ESI’s were a cost-effective option after mild®, but for additional $37,758 per QALY. Sensitivity analysis demonstrated that mild® would not be the choice strategy under only one scenario, if the number of ESI’s in a 2-year period was reduced to 6 or fewer. However, such patients represent a minority of LSS patients that are seen in clinical practice. Most probably, they are at earlier stages of the disease and should not be compared with those who have severe symptoms or failed conservative treatment. Of course, these patients should continue to be treated with ESI until their symptoms become severe or fail to respond to ESI. At such time, the mild® treatment becomes the cost-effective choice.
Our results may be affected by 2 key limitations: The first limitation of short time horizon was due to mild® only being a relatively new treatment with limited longer-term follow-up of patients. As such, we were only able to extend our time horizon to 2 years, including only the costs and benefits within this time frame. LSS is a chronic condition with symptoms that can last a lifetime without treatment. Because of this, there is a great opportunity to have even very expensive treatments be cost-effective because of the potential for large QALY gains when the lifetime is considered. By limiting our study to just 2 years, the results might be weighted toward less costly interventions where the cost per QALY gained can be small. ESI’s are the least costly of all 3 options and have relatively limited risks; however, the duration their effectiveness is very short.
While ESI has shown to be cost-effective in previous studies,14 those patients were early cases with less than moderate/severe symptoms and may have seen QALY gains for a longer period before requiring an additional injection. For patients with moderate to severe symptoms however, more frequent injections may be required and may not result in the same level of relief (and therefore QALY gained) per injection. If we were to forecast beyond the 2 years, moderate to severe sufferers would continue to require frequent injections at a cost per treatment with only a small QALY gain per treatment. Additionally, frequent ESI might be associated with increased morbidities especially in patients at risk of osteoporosis, diabetic, or hypertensive patients.26 The mild® and surgery options in contrast, while they have a much higher upfront cost, both interventions would continue to accrue QALY’s at no additional cost when we forecast beyond 2 years. As more long-term outcome data become available for mild®, we strongly recommended that this analysis is repeated. As it took several decades of life to get the ligamentum flavum thickened and buckled enough to cause significant LSS, we hypothesize that patients who successfully receive mild® will continue to gain relief and gain quality of life for several years to come. With this hypothesis, mild® would continue to have a lower cost per QALY each subsequent year, while epidural steroid injections cost per QALY would increase due to the costs associated continued repeat therapy and the inclusion of costs of complications such as osteoporosis, worsening diabetes, and worsening hypertension associated with the long-term use of epidural steroid injections.26 We further hypothesize these additional costs would make epidural steroid injections strongly dominated by mild® and no longer a cost-effective option for patients with moderate to severe symptoms. On the other hand, we hypothesize that the cost of laminectomy surgery per QALY will reduce each year the time horizon is extended. However, owing to surgery’s much higher upfront cost as compared to mild®, its higher complication rate, and the revision rate, we do not predict its cost per QALY to be less than that of mild®, at least in the near future. For all the above issues, it is important to repeat our analysis in the future when long-term data on mild® effectiveness become available.
The second major limitation of this study was the lack of quality of life data for LSS patients with moderate to severe symptoms after epidural steroid injection or surgery. Quality of life gain after epidural steroid injection was available for those with early “mild” symptoms. Quality of life after surgery was available for severe symptom suffers. No studies examined the gain in quality of life for either intervention for a patient with moderate/severe symptoms. Because quality of life gain attributable to a procedure is the difference in quality of life before the procedure to the quality of life in time periods after the procedure, gains for early “mild” or severe symptom sufferers do not translate to moderate sufferers as quality of life before the procedure is different for all 3 levels. Furthermore, the distinction between early “mild”, moderate, and severe symptom sufferers is arbitrary.
At 2 years for patients with LSS who have failed conservative therapy, have moderate to severe symptoms, and are considered suitable candidates, mild® is a cost-effective option in terms of QALY gains and costs. As longer-term quality of life data becomes available, we anticipate the cost per QALY for mild® to reduce as life continues to accrue with high quality at little to no additional cost. Subsequently, we anticipate that epidural steroid injections might eventually be dominated by mild® as additional QALY gains are only achieved with repeat treatment and additional cost under the former treatment. As further quality of life data becomes available, expanding this analysis to include patients with severe symptoms who are not eligible for surgery, and even eligible surgery patients will be warranted, as the potential impact on cost and quality of life for these patients is significant.
CONCLUSIONS
This study demonstrates that for LSS patients who have moderate to severe symptoms of neurogenic claudications and have failed conservative therapy, that mild® procedure is a cost-effective alternative for appropriate patients rather than continuing with conservative treatment and repeated serial ESI or proceeding to laminectomy surgery.
Table 3.
Two-year Unadjusted Quality-adjusted Life Years Gains by Treatment Option
| Baseline | Range | Source | |
|---|---|---|---|
| Mild | 0.131 | 0, 0.323 | 16, 28 |
| Epidural | 0.252 (0.21 × 12) | 0, 0.315 | 14 |
| Surgery | 0.17 | 0.12, 0.26 | 9, 15, 17, 25 |
| Discount rate | 3% | 0%, 5% | 8 |
Table 4.
Quality-adjusted Life Years Loss by Complication
| Baseline | Range | Source | |
|---|---|---|---|
| Baseline QOL | 0.97 | 17 | |
| Death | 0 | 0 | 17 |
| Deep wound infection | 0.33 for 2 weeks | 0.25, 0.41 | 17 |
| Postlumbar puncture headache | 0.33 for 2 days | 0.25, 0.41 | 17, 29 |
| Nerve root irritation | 0.33 for 2 days | 0.25, 0.41 | 17, 29 |
| Cord or cauda equina injury | 0.39 | 0.29, 0.49 | 17 |
| Nerve root injury | 0.79 | 0.59, 0.99 | 17 |
| Dural tear | 0.33 for 2 days | 0.25, 0.41 | 17 |
| Medical | 0.33 for 7 days | 0.25, 0.41 | 17 |
Acknowledgments
The study was internally funded by the Outcomes Research Department of Cleveland Clinic; no external funds were received.
Footnotes
Disclosures: The authors have no conflict of interest to report.
REFERENCES
- 1.Katz JN, Harris MB. Lumbar spinal stenosis. N Engl J Med. 2008;358:818–825. doi: 10.1056/NEJMcp0708097. [DOI] [PubMed] [Google Scholar]
- 2.Kalichman L, Cole R, Kim DH. Spinal stenosis prevalence and association with symptoms: the Framingham study. Spine J. 2009;9:545–550. doi: 10.1016/j.spinee.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Deer TR, Kapural L. New image-guided ultra minimally invasive lumbar decompression method: the mild procedure. Pain Physician. 2010;13:35–41. [PubMed] [Google Scholar]
- 4.Mekhail N, Costandi S, Abraham B, Samuel SW. Functional and patient-reported outcomes in symptomatic lumbar spinal stenosis following percutaneous decompression. Pain Pract. 2012;12:417–425. doi: 10.1111/j.1533-2500.2012.00565.x. [DOI] [PubMed] [Google Scholar]
- 5.Mekhail N, Vallejo R, Coleman MH, Benyamin RM. Long-term results of percutaneous lumbar decompression mild (R) for spinal stenosis. Pain Pract. 2012;12:184–193. doi: 10.1111/j.1533-2500.2011.00481.x. [DOI] [PubMed] [Google Scholar]
- 6.Lingreen R, Grider JS. Retrospective review of patient self-reported improvement and post-procedure findings for mild. Pain Physician. 2010;13:555–560. [PubMed] [Google Scholar]
- 7.Brown LL. A double-blind, randomized, prospective study of epidural steroid injection vs. the mild (R) procedure in patients with symptomatic lumbar spinal stenosis. Pain Pract. 2012;12:333–341. doi: 10.1111/j.1533-2500.2011.00518.x. [DOI] [PubMed] [Google Scholar]
- 8.Weinstein MC, Siegel JE, Gold MR, Kamlet MS, Russell LB. Recommendations of the panel on cost-effectiveness in health and medicine. JAMA. 1996;276:1253–1258. [PubMed] [Google Scholar]
- 9.Glassman SD, Polly DW, Dimar JR, Carreon LY. The cost effectiveness of single-level instrumented posterolateral lumbar fusion at 5 years after surgery. Spine (Phila Pa 1976) 2012;37:769–774. doi: 10.1097/BRS.0b013e3181e03099. [DOI] [PubMed] [Google Scholar]
- 10.Harrington AR, Armstrong EP, Nolan PE, Jr, Malone DC. Cost-effectiveness of apixaban, dabigatran, rivaroxaban, and warfarin for stroke prevention in atrial fibrillation. Stroke. 2013;44:1676–1681. doi: 10.1161/STROKEAHA.111.000402. [DOI] [PubMed] [Google Scholar]
- 11.Hirth RA, Chernew ME, Miller E, Fendrick AM, Weissert WG. Willingness to pay for a quality-adjusted life year: in search of a standard. Med Decis Making. 2000;20:332–342. doi: 10.1177/0272989X0002000310. [DOI] [PubMed] [Google Scholar]
- 12.McCabe C, Claxton K, Culyer A. The NICE cost-effectiveness threshold: what it is and what that means. Pharmacoeconomics. 2008;26:733–744. doi: 10.2165/00019053-200826090-00004. [DOI] [PubMed] [Google Scholar]
- 13.Cleveland Clinic. Spine Surgery Statistics. Personal Communication; 2012. Annual Report. [Google Scholar]
- 14.Whynes DK, McCahon RA, Ravenscroft A, Hardman J. Cost effectiveness of epidural steroid injections to manage chronic lower back pain. BMC Anesthesiol. 2012;12:26–32. doi: 10.1186/1471-2253-12-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tosteson AN, Tosteson TD, Lurie JD, et al. Comparative effectiveness evidence from the spine patient outcomes research trial surgical versus nonoperative care for spinal stenosis, degenerative spondylolisthesis, and intervertebral disc herniation. Spine (Phila Pa 1976) 2011;36:2061–2068. doi: 10.1097/BRS.0b013e318235457b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carreon LY, Glassman SD, McDonough CM, Rampersaud R, Berven S, Shainline M. Predicting SF-6D utility scores from the oswestry disability index and numeric rating scales for back and leg pain. Spine (Phila Pa 1976) 2009;34:2085–2089. doi: 10.1097/BRS.0b013e3181a93ea6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuntz KM, Snider RK, Weinstein JN, Pope MH, Katz JN. Cost-effectiveness of fusion with and without instrumentation for patients with degenerative spondylolisthesis and spinal stenosis. Spine (Phila Pa 1976) 2000;25:1132–1139. doi: 10.1097/00007632-200005010-00015. [DOI] [PubMed] [Google Scholar]
- 18.Gray Alistair M, Clarke Philip M, Wolstenholme Jane, Wordsworth Sarah. Applied Methods of Cost-Effectiveness Analysis in Health Care. Handbooks in Health Economic Evaluation. Oxford, U.K: Oxford University Press; 2011. [Google Scholar]
- 19.Hoch JS, Briggs AH, Wilan AR. Something old, something new, something borrowed, something blue: a framework for the marriage of health econometrics and cost-effectiveness analysis. Health Econ. 2002;11:415–430. doi: 10.1002/hec.678. [DOI] [PubMed] [Google Scholar]
- 20.Briggs AH, O’Brien BJ, Blackhouse G. Thinking outside the box: recent advances in the analysis and presentation of uncertainty in cost-effectiveness studies. Annu Rev Public Health. 2002;23:377–401. doi: 10.1146/annurev.publhealth.23.100901.140534. [DOI] [PubMed] [Google Scholar]
- 21.Radcliff K, Kepler C, Hilibrand A, et al. Epidural steroid injections are associated with less improvement in patients with lumbar spinal stenosis: a subgroup analysis of the spine patient outcomes research trial. Spine (Phila Pa 1976) 2013;38:279–291. doi: 10.1097/BRS.0b013e31827ec51f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Burnett MG, Stein SC, Bartels RHMA. Cost-effectiveness of current treatment strategies for lumbar spinal stenosis: nonsurgical care, laminectomy, and X-STOP clinical article. J Neurosurg Spine. 2010;13:39–46. doi: 10.3171/2010.3.SPINE09552. [DOI] [PubMed] [Google Scholar]
- 23.Parker SL, Fulchiero EC, Davis BJ, et al. Cost-effectiveness of multilevel hemilaminectomy for lumbar stenosis-associated radiculopathy. Spine J. 2011;11:705–711. doi: 10.1016/j.spinee.2011.04.024. [DOI] [PubMed] [Google Scholar]
- 24.Kim S, Mortaz Hedjri S, Coyte PC, Rampersaud YR. Cost-utility of lumbar decompression with or without fusion for patients with symptomatic degenerative lumbar spondylolisthesis. Spine J. 2012;12:44–54. doi: 10.1016/j.spinee.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 25.Tosteson AN, Lurie JD, Tosteson TD, et al. Surgical treatment of spinal stenosis with and without degenerative spondylolisthesis: cost-effectiveness after 2 years. Ann Intern Med. 2008;149:845–853. doi: 10.7326/0003-4819-149-12-200812160-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huntoon M. Steroid Complications. In: Rathmall NJ, editor. Complications in Regional Anesthesia and Pain Medicine. New York, NY: Elsevier; 2006. pp. 331–336. [Google Scholar]
- 27.Centers for Medicare and Medicaid. [accessed January 15, 2013];Reimbursement Schedule. 2013 http://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/PhysicianFeeSched/index.html.
- 28.Vertos Data Set. Aliso Viejo, CA: Vertos Medical; 2012. [Google Scholar]
- 29.Manchikanti L, Malla Y, Wargo BW, Cash KA, Pampati V, Fellows B. A prospective evaluation of complications of 10,000 fluoroscopically directed epidural injections. Pain Physician. 2012;15:131–140. [PubMed] [Google Scholar]
- 30.Cleveland Clinic. Pain Management Statistics. Personal Communication; 2012. Annual Report. [Google Scholar]
- 31.Deyo RA, Mirza SK, Martin BI. Trends, major medical complications, and charges associated with surgery for lumbar spinal stenosis in older adults. J Am Med Assoc. 2010;303:1259–1265. doi: 10.1001/jama.2010.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Malter AD, McNeney B, Loeser JD. 5-year reintervention rates after different types of lumbar spine surgery. Spine (Phila Pa 1976) 1998;23:814–820. doi: 10.1097/00007632-199804010-00015. [DOI] [PubMed] [Google Scholar]