Abstract
Background
There is a growing body of evidence linking micronutrient deficiencies and malaria incidence arising mostly from P. falciparum endemic areas. We assessed the impact of micronutrient deficiencies on malaria incidence and vice versa in the Brazilian state of Amazonas.
Methodology/Principal Findings
We evaluated children <10 years old living in rural communities in the state of Amazonas, Brazil, from May 2010 to May 2011. All children were assessed for sociodemographic, anthropometric and laboratory parameters, including vitamin A, beta-carotene, zinc and iron serum levels at the beginning of the study (May 2010) and one year later (May 2011). Children were followed in between using passive surveillance for detection of symptomatic malaria. Those living in the study area at the completion of the observation period were reassessed for micronutrient levels. Univariate Cox-proportional Hazards models were used to assess whether micronutrient deficiencies had an impact on time to first P. vivax malaria episode. We included 95 children median age 4.8 years (interquartile range [IQR]: 2.3–6.6), mostly males (60.0%) and with high maternal illiteracy (72.6%). Vitamin A deficiencies were found in 36% of children, beta-carotene deficiency in 63%, zinc deficiency in 61% and iron deficiency in 51%. Most children (80%) had at least one intestinal parasite. During follow-up, 16 cases of vivax malaria were diagnosed amongst 13 individuals. Micronutrient deficiencies were not associated with increased malaria incidence: vitamin A deficiency [Hazard ratio (HR): 1.51; P-value: 0.45]; beta-carotene [HR: 0.47; P-value: 0.19]; zinc [HR: 1.41; P-value: 0.57] and iron [HR: 2.31; P-value: 0.16]). Upon reevaluation, children with al least one episode of malaria did not present significant changes in micronutrient levels.
Conclusion
Micronutrient serum levels were not associated with a higher malaria incidence nor the malaria episode influenced micronutrient levels. Future studies targeting larger populations to assess micronutrients levels in P. vivax endemic areas are warranted in order to validate these results.
Introduction
Malaria is one of the most serious public health problems in the world, with 3.3 billion people at risk of contracting the disease and almost one million deaths annually, primarily caused by Plasmodium falciparum in African children under five years of age [1]. In Latin America most cases occur in the Brazilian Amazon with nearly 150,000 cases reported yearly, the vast majority caused by P. vivax (87.8%) [2].
Micronutrient deficiencies are bidirectionally associated with several infections due to specific micronutrients playing key roles in the function of the immune system. Certain deficiencies may also predispose to malaria incidence [3–6], while malaria itself has been associated with malnutrition [7–9] and possibly micronutrient deficiencies [10]. The relationship between micronutrient deficiencies and malaria comes mostly from animal [11–16] and cross sectional studies [17] predominantly in P. falciparum malaria. These data prompted several authors to propose micronutrient supplementation, i.e. vitamin A and zinc to prevent malaria incidence [5, 17, 18], severity [19] and mortality [4–6, 20, 21], an approach that was shown to be generally successful. The association between iron deficiency and malaria incidence is still controversial. Some studies have shown that iron supplementation may increase the risk of malaria incidence [22–25].
Despite the growing body of evidence associating micronutrient deficiency and P. falciparum malaria, there is still a lack of studies addressing this possible association in P. vivax. Plasmodium vivax is different from P. falciparum as it can lead to relapses and persist as an asymptomatic infection. Therefore, P. vivax could be associated with chronic inflammation, similar to other infections [26–31], which may have a detrimental effect on micronutrient levels as has been shown for chronic infections such as HIV [28, 31] and tuberculosis [32].
Previous studies in the Brazilian Amazon show insufficient micronutrient intake in native populations in spite of the high micronutrient content of local fruits and fish [33, 34]. We set up a prospective study to assess whether vitamin A, beta-carotene, zinc, or iron serum levels were associated with an increased risk of vivax malaria incidence, and whether having malaria affected the serum levels of these micronutrients.
Methods
Ethics statement
This study was approved by the Ethics Committee Board of the Fundação de Medicina Tropical Dr. Heitor Vieira Dourado (1899/2008 and 918/2010 approvals). Each participant and his/her parents or legal guardians signed written informed consent forms, after they were clear about the purpose and the study protocol.
Study area and population
The study was undertaken in two rural communities located in recently colonized areas devoted to agriculture (Panelão and Castanho Sítio Communities), located in the Municipality of Careiro in the Amazonas State, from May 2010 to May 2011. The municipality has an area of 6,124,300 km2 and has 31,063 inhabitants. The climate is tropical and humid, with rainfalls ranging from 2,100 to 2,400 mm per annum. The municipality is connected to the capital of the state, Manaus, through a federal road (112 km of distance). Malaria is endemic in this area. The major economic activities are family farming, hunting and fishing. Drinking water comes from rainwater reservoirs or creeks. Garbage collection and sanitation are absent. Two health agents in each community are responsible for health care. According to a census performed in 2008 the total population of both communities was 790 people, including 300 children ranging from 1 month to 14 years of age [2, 7].
Study design
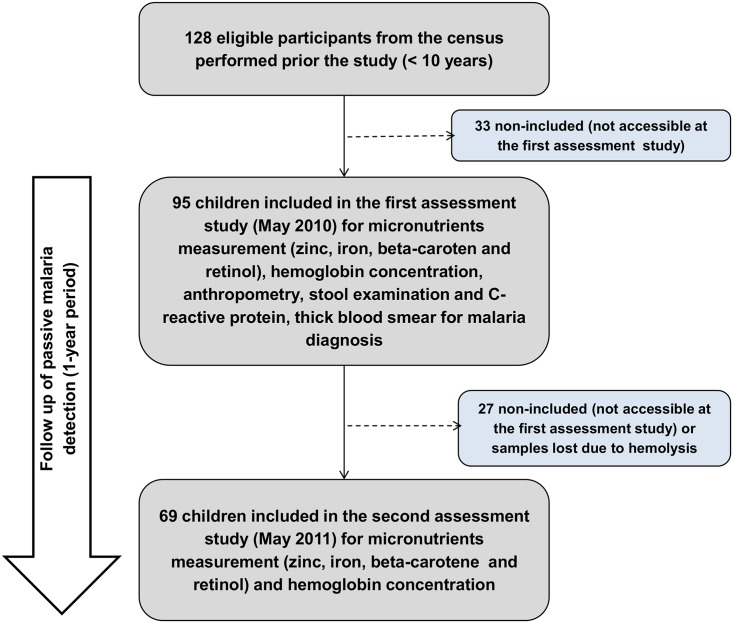
All children <10 years residing in the study area in May 2010 (Baseline) were eligible for the study. Socio-demographic information, including health history, maternal education and housing conditions were collected. Each child underwent an anthropometric nutritional status assessment (weight and height, brachial circumference [7]), laboratory analyses for hemoglobin, micronutrient serum levels (vitamin A, beta-carotene, zinc and iron), C reactive protein, parasitological stool analyses of feces and testing for malaria (thick smear and molecular testing). After this initial evaluation, we captured information on malaria incidence via passive surveillance for 1 year, in which all febrile episodes were tested for Plasmodium infection. Children present in the community by May 2011 were reevaluated for the abovementioned parameters in order to assess the effects of malaria on serum micronutrient levels (Reevaluation) (Fig 1).
Fig 1. Study flow chart.
From 128 eligible participants from the 2008 census, 95 children were included in the baseline assessment (2010) and 69 completed the follow up (2011).
Nutritional status assessment
Anthropometric measurements were performed at baseline and at reevaluation, with minimal clothing and no shoes [35]. Weight and height were obtained by internationally recommended methods [36]. Weight was measured using a digital weight scale for children above 2 years of age and a mechanical pediatric scale for younger children, both in kilograms, while height was assessed by a single observer with the use of a portable anthropometer stadiometer with lateral scale in centimeters. All instruments were adjusted and verified after each measurement and fully calibrated every three months. The measurement techniques were harmonized according to the mentioned procedures [35–37], with all team members following the same standardized protocol. Body mass index (BMI) was calculated using the ANTHRO and ANTHRO PLUS softwares [38]. Body mass index for age Z-scores below -2 were defined as undernutrition. The classification of each individual as having adequate or inadequate BMI was made according to the World Health organization (WHO) standards for each age [39].
Micronutrient assessment
A sample of 5 ml of blood was collected from each child and that was placed on filter paper for vitamin A and beta-carotene assessments and in a tube with heparin for zinc and iron measurements. All samples were transferred to the laboratory in cold conditions and protected from light; samples were centrifuged at 15,000 rpm for 30 seconds to obtain plasma. Vitamin A and beta-carotene were assessed using high performance liquid chromatography in plasma. Erythrocytic zinc and serum iron levels were assessed by atomic chromatography [40]. The following cut-off points were used to define micronutrient deficiency: ≤10 μg/dL for vitamin A, ≤20 μg/dL for beta-carotene, ≤70 μg/dL for zinc and ≤70 μg/dL for iron [41–45].
Malaria diagnosis
Thick blood smear (TBS) were prepared as recommended by the Walker technique [46] and evaluated by an experienced microscopist. Microscopy results were confirmed by polymerase chain reaction, using the Kit QIAmp DNA Blood Mini Kit (Quiagen, Hilden, Germany) and amplified DNA using the Applied Biosystems 7500 Fast System. A previous study by our group showed a 97.8% concordance between TBS and PCR [7]. A malaria case was documented when the child presented with fever and a positive TBS.
Hemoglobin concentration
Hemoglobin concentration was measured in venous blood using a portable photometer (HemoCue, Anglholm, Sweden) at the beginning and at reevaluation. Anemia was defined as hemoglobin <12 g/dL [47].
Stool examination
Stool samples were taken at baseline (May 2010) and on revaluation (May 2011) to examine the association between helminth infection and micronutrient indicators. Samples were stored in flasks containing 10% formalin as preservative. Flasks were labeled with the patient’s name and date of collection and were kept at room temperature until stool examination at the end of the day of collection. Spontaneous sedimentation [48] and centrifugal flotation in zinc sulfate solution [49] methods were applied before the samples were analyzed with a microscope for detection of helminthes and protozoan parasites using the direct observation, Kato-Katz, and the Faust and Hoffman methods. All tests were performed and evaluated by a single experienced examiner.
Statistical analyses
Descriptive statistics were employed to evaluate overall demographic, anthropometric and laboratory characteristics to ensure that distributional assumptions for statistical tests were met. Continuous variables were reported as median [first quartile-third quartile] and categorical variables as frequencies and percentages. Univariate Cox proportional hazard models were used to assess the impact of baseline characteristics on time to malaria incidence over the one year follow up. Chi-2 or Fisher’s exact test were performed to assess the effects of malaria incidence on the delta of micronutrients serum levels (Δ = reevaluation minus baseline serum levels). Migration from the area was documented and follow-up time adjusted. All analyses were performed using STATA 13 (College Station, TX).
Results
Using results from the 2008 census, 128 children would be eligible (<10 years old) by the time of the baseline assessment (May 2010). As 33 children had moved out of the area, ninety-five children were included in the study (Table 1). Median age was 4.8 years (interquartile range (IQR) [2.3–6.6]) and most were male (60.0%). A total of 33 (34.7%) of the children had previous malaria. Height-for-age (Z score <-1) was recorded in 42.1%, height-for-age (Z score <-2) in 11.6%, BMI-for-age (Z score <-1) in 17.9% and BMI-for-age (Z score <-2) in 3,2% of the children. Mean hemoglobin was 11.5 g/dL IQR [11–12.2] and anemia prevalence was 57.4%. Vitamin A deficiency was observed in 35.9%, beta-carotene deficiency in 63.0%, zinc deficiency in 60.9% and iron deficiency in 51.1% of the children. A total of 76 (80.0%) of the children had one ore more intestinal parasite. The most common intestinal parasite found was Ascaris lumbricoides, in 44.2% of the children.
Table 1. Baseline characteristics of 95 children evaluated for nutritional and micronutrient status in Careiro, Brazil, May 2010.
| Baseline characteristics | Median (IQR) or N (%) |
|---|---|
| Age | 4.8 IQR [2.3–6.6] |
| Males | 57 (60.0%) |
| Maternal educational level | |
| Illiterate | 69 (72.6%) |
| Basic education | 26 (27.4%) |
| Previous malaria | 33 (34.7%) |
| Nutritional status | |
| Height-for-Age (Z score <-1) | 40 (42.1%) |
| Height-for-Age (Z score <-2) | 11(11.6%) |
| BMI-for-age (Z score <-1) | 17 (17.9%) |
| BMI-for-age (Z score <-2) | 3 (3,2%) |
| Hemoglobin (g/dL) | 11.5 IQR [11–12.2] |
| Anemia | 52 (54.7%) |
| C-reactive protein | 4 IQR [3–5] |
| Micronutrient deficiencies | |
| Vitamin A | 33 (35.9%) |
| Beta-carotene | 58 (63.0%) |
| Zinc | 56 (60.9%) |
| Iron | 47 (51.1%) |
| Intestinal parasitosis | 76(80.0%) |
| Ascaris lumbricoides | 42(44.2%) |
| Giardia lamblia | 37(39.0%) |
| Entamoeba histolytica/dispar | 18(19.0%) |
| Trichuris trichiura | 17(17.9%) |
| Hookworms | 3 (3.2%) |
| Enterobius vermicularis | 3 (3.2%) |
Abbreviations: IQR: Inter-quartile rage; N: number; BMI: Body mass index.
Cut-off points for micronutrient deficiency: ≤10 μg/dL for vitamin A, ≤20 μg/dL for beta-carotene, ≤70 μg/dL for zinc and ≤70 μg/dL for iron. Anemia was defined as hemoglobin <12 g/dL (47).
Impact of micronutrient deficiencies on malaria incidence
We followed 95 children through passive surveillance for a year and detected 17 cases of malaria, 16 by P. vivax and 1 by P. falciparum. Three patients had P. vivax malaria twice during the observation period. In the time to P. vivax incidence analyses, baseline vitamin A serum levels were not associated with the outcome (Hazard ratio [HR]: 1.51; 95%confidence interval [CI]: 0.51–4.52), nor did beta-carotene (HR: 0.47; 95%CI: 0.16–1.42), or zinc (HR: 1.41; 95%CI: 0.43–4.57) (Table 2 and Fig 2). We found a slight trend towards an association of iron deficiency and malaria incidence (HR: 2.31; 95%CI: 0.71–7.51) and also found a similar trend for maternal illiteracy (HR: 4.81; 95%CI: 0.62–36.97), having had malaria (HR: 2.33; 95%CI: 0.78–6.94), and the number of intestinal parasites detected during the baseline evaluation (HR: 1.59; 95%CI: 0.89–2.85). Association strengths remained similar to crude results after adjustment for potential confounders (sex, age, maternal illiteracy and intestinal parasites).
Table 2. Univariate Cox Proportional Hazard analyses of baseline characteristics associated with time to malaria incidence in Careiro, Brazil, May 2010.
| Variables | First year (P. vivax cases = 13) | ||
|---|---|---|---|
| Hazard ratio | p-value | 95% CI | |
| Age (years) | 1.08 | 0.48 | 0.87–1.33 |
| Male | 0.76 | 0.62 | 0.25–2.25 |
| Maternal Illiteracy | 4.81 | 0.13 | 0.62–36.97 |
| Previous malaria | 2.33 | 0.13 | 0.78–6.94 |
| Height-for-Age (Z score <-1) | 1.17 | 0.78 | 0.39–3.48 |
| BMI-for-age (Z score <-1) | 1.42 | 0.59 | 0.39–5.17 |
| Hemoglobin <12 mg/dL | 0.87 | 0.83 | 0.26–2.90 |
| Vitamin A deficiency | 1.51 | 0.45 | 0.51–4.52 |
| Beta-carotene deficiency | 0.47 | 0.19 | 0.16–1.42 |
| Zinc deficiency | 1.41 | 0.57 | 0.43–4.57 |
| Iron deficiency | 2.31 | 0.16 | 0.71–7.51 |
| Number of intestinal parasites | 1.59 | 0.12 | 0.89–2.85 |
Abbreviations: P. vivax: Plasmodium vivax, CI: Confidence interval. BMI: Body mass index.
Cut-off points for micronutrient deficiency: ≤10 μg/dL for vitamin A, ≤20 μg/dL for beta-carotene, ≤70 μg/dL for zinc and ≤70 μg/dL for iron.
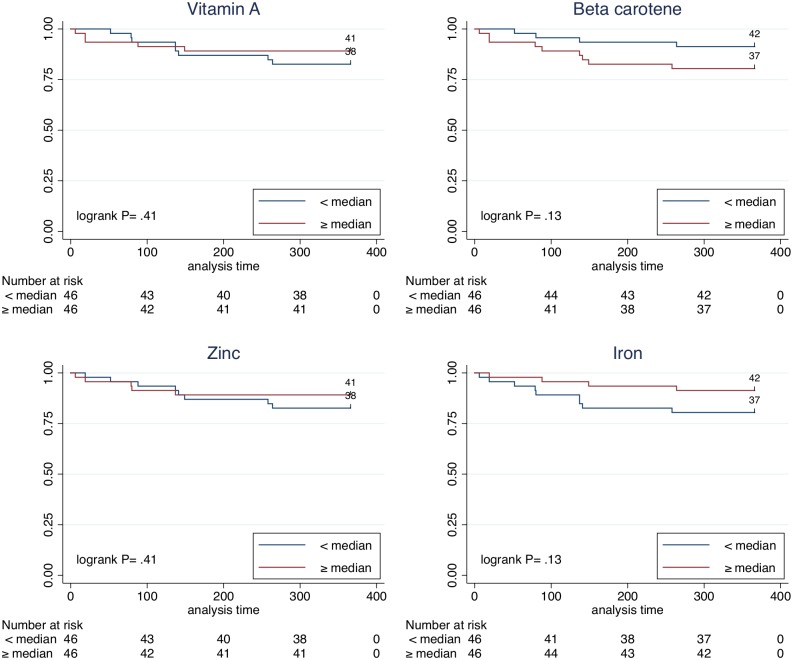
Fig 2. Survival analysis of time to first Plasmodium vivax malaria episode.
Time to the first malaria episode was similar between participants with micronutrient levels above or below the median, for vitamin A, beta-carotene, zinc and iron.
Impact of malaria in micronutrients
The impact of having had malaria on micronutrient levels was assessed based on reevaluation conducted 1 year after the baseline assessment. Because 27 children had moved out of the study area by reevaluation time, the remaining 68 children were reassessed for micronutrient levels and these results were compared with baseline assessments to obtain the Δ for each micronutrient. Having had malaria was not associated with a Δ of any of the assessed micronutrients (Table 3). Adjustment for potential confounders (sex, age, maternal illiteracy and intestinal parasites) did not change these results substantially.
Table 3. Association between malaria during the observation period (May 2010–May 2011) and less than median micronutrient serum levels during the same period.
| Below median | 2010 median (μg/dL) | 2011 median (μg/dL) | Malaria (N = 56) | No malaria (N = 12) | p-value |
|---|---|---|---|---|---|
| Delta (Δ) Vitamin A | 21.0 | 20.0 | 29 (52.7%) | 5 (38.4%) | 0.54 |
| Δ Beta-caroten* | 25.8 | 19.3 | 25 (46.3%) | 8 (66.7%) | 0.34 |
| Δ Iron* | 70.0 | 69.8 | 27 (50.0%) | 6 (46.2%) | 0.80 |
| Δ Zinc* | 67.6 | 69.8 | 27 (50.9%) | 6 (46.2%) | 0.76 |
P-values calculated by Chi2 or Fisher’s exact test.
Cases that were evaluated both in 2010 and 2011 are shown.
*2 children lacked data for Δ serum level of beta-carotene and zinc, and 1 child for Δ serum level of iron.
Discussion
We evaluated the bidirectional association of vivax malaria and micronutrient deficiencies and found no association of deficiencies of vitamin A, beta-carotene, zinc, or iron with malaria incidence. Incident malaria evaluated throughout a year was not associated with worsening of micronutrient deficiencies either. We reviewed the extant literature to compare our results with previous findings, mostly from P. falciparum endemic areas.
Vitamin A deficiency is found in 17.4% of children in Brazil [50, 51]. In our study, more than one third of children were vitamin A deficient and about two thirds had deficiency of the vitamin A precursor beta-carotene. Such results highlight the profound differences between the underdeveloped areas in the Brazilian Amazon and the rest of to the country. In addition, the data shows a nutritional paradox reflected in this area with abundance of fruits rich in precursors of vitamin A where inhabitants present important deficiencies, especially children from a lower socio-economical status [52–54]. Most children had also deficiencies in zinc (61%) and iron (51%) at proportions comparable to the sub-Saharan region [55]. These findings should be considered by the Ministry of Health to implement educational strategies to increase the intake of regional foods rich in vitamin A precursors and minerals, such as zinc and iron.
Our results did not support an association of vitamin A levels at baseline and incidence of P. vivax malaria in the Brazilian Amazon. Previous studies, mostly in P. falciparum had shown an association of vitamin A levels with severity [17, 56] and mortality [57, 58], prompting several randomized controlled trials using vitamin A supplementation as a means to improve malaria outcomes [59, 60]. However most studies showing an association between micronutrients and malaria involved cases of P. falciparum malaria. One published study in an area where P. vivax is predominant assessed hemoglobin levels by day 30 and found no difference between vitamin A supplementation and the control group [18], in agreement with our findings
We did not find an association between zinc deficiency and malaria incidence. Interestingly that studies have addressed the role of zinc supplementation in malaria outcomes have shown dissimilar results. Some studies found a protective effect of zinc supplementation on the number of malaria related clinic visits in Gambia [61] and rates of malaria incidence in Papua New Guinea [4], while a study in Burkina Faso showed no association [62]. All these studies had a predominance of P. falciparum malaria and results may differ in P. vivax endemic areas. Our results are similar to a study that found no association of zinc supplementation and P. vivax outcomes in Peru where P. vivax also predominates [63].
We found a slight trend towards an association of iron deficiency and malaria incidence and a similar trend for maternal illiteracy, having had malaria and the number of intestinal parasites detected during the baseline evaluation. These parameters are likely interrelated and may serve as markers of a lower socio-economical status: living in areas with the poorest hygienic conditions may result in an increased risk of acquiring malaria. This trend, although not statistically significant, may go against previous studies that found a possible protective effect of lower iron levels on P. falciparum incidence [23]. An association between iron supplementation and malaria mortality was reported in Zanzibar [64]. In a study in the Peruvian Amazon with similar epidemiologic characteristics to our study group [63], the authors detected an increased risk of malaria incidence after iron supplementation. A previous study by our group assessed 168 children in the same study location and found low levels of meat, fish or poultry consumption [54, 65]. These results explain the high prevalence of zinc and Iron deficiencies in our study population as these micronutrients come mostly from the consumption of animal products. Children are not offered these products as a result of these being considered “reimosos”, a tightly held food taboo in the Amazon region [54, 66–68].
We also assessed whether patients having malaria during the 1 year follow up would have a downtrend in their micronutrient serum levels by comparing the measurements from 2010 and 2011. We did not find a statistically significant association of malaria incidence and Δ of any of the micronutrients. Previous studies have shown that malaria incidence may reduce the ingestion of micronutrients [69] and their absorption [70]. These studies were conducted in P. falciparum endemic areas. Our study does not support the same effect by P. vivax.
Our findings should be interpreted in the context of some limitations. We report the experience of one municipality in the Brazilian Amazon and our findings may not be generalizable to other settings. Our sample size and, especially, the low number of events, could have prevented us from detecting existing associations a larger population would be able to. Unmeasured patient, socio-economical and administrative factors may affect malaria incidence and micronutrient levels. We were not able to assess dietary patterns, and although there is evidence of homogeneous habits within the study population [7, 65], there may be important individual variations that may have an impact on the nutritional status of children. We performed passive surveillance for malaria incidence, and possibly more cases and asymptomatic ones could have been detected if we had implemented an active surveillance system. Despite these limitations, our study advances the knowledge of nutritional status and P. vivax malaria in several ways. We found a high prevalence of micronutrient deficiencies and intestinal parasitosis in the study area.
In conclusion, we were not able to observe an association between serum micronutrient levels and malaria incidence, although we found a trend towards an association between iron deficiency and malaria incidence. Iron deficiency may be a marker of poorer socio-economical status and higher malaria risk as a result. Further studies assessing micronutrient levels in P. vivax endemic areas in larger populations are needed to validate our results.
Acknowledgments
We would like to thank Mrs. Raimunda Ericilda Araujo for assistance collecting the samples. We thank the Municipality of Careiro, and Tatiana Melo Lopes at Instituto Nacional de Pesquisas da Amazônia (INPA) for micronutrient laboratory assessments.
Data Availability
Due to ethical restrictions regarding patient privacy, data are available upon request. Requests for the data may be sent to the corresponding author.
Funding Statement
This study was funded by CNPq, FAPEAM (Fundação de Amparo à Pesquisa do Estado do Amazonas) and Fundació Cellex (grant 483758/2009-4; G64334048/2007). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.World Health Organization. 2013 World Malaria Report. Geneva: World Health Organization; 2014. [Google Scholar]
- 2.Brazilian Ministry of Health. Situação Epidemiológica da Malária no Brasil, 2000 a 2011. Brasília: Brazilian Ministry of Health; 2013. [Google Scholar]
- 3.dos Santos-Valente EC, da Silva R, de Moraes-Pinto MI, Sarni RO, Costa-Carvalho BT. Assessment of nutritional status: vitamin A and zinc in patients with common variable immunodeficiency. J Investig Allergol Clin Immunol. 2012;22: 427–431. [PubMed] [Google Scholar]
- 4.Shankar AH, Genton B, Baisor M, Paino J, Tamja S, Adiguma T, et al. The influence of zinc supplementation on morbidity due to Plasmodium falciparum: a randomized trial in preschool children in Papua New Guinea. Am J Trop Med Hyg. 2000;62: 663–669. [DOI] [PubMed] [Google Scholar]
- 5.Shankar AH, Genton B, Semba RD, Baisor M, Paino J, Tamja S, et al. Effect of vitamin A supplementation on morbidity due to Plasmodium falciparum in young children in Papua New Guinea: a randomised trial. Lancet. 1999;354: 203–209. [DOI] [PubMed] [Google Scholar]
- 6.Shankar AH, Prasad AS. Zinc and immune function: the biological basis of altered resistance to infection. Am J Clin Nutr. 1998;68: 447S–463S. [DOI] [PubMed] [Google Scholar]
- 7.Alexandre MA, Benzecry SG, Siqueira AM, Vitor-Silva S, Melo GC, Monteiro WM, et al. The Association between Nutritional Status and Malaria in Children from a Rural Community in the Amazonian Region: A Longitudinal Study. PLoS Negl Trop Dis. 2015;9: e0003743 10.1371/journal.pntd.0003743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tanner M, Burnier E, Mayombana C, Betschart B, de Savigny D, Marti HP, et al. Longitudinal study on the health status of children in a rural Tanzanian community: parasitoses and nutrition following control measures against intestinal parasites. Acta Trop. 1987;44: 137–174. [PubMed] [Google Scholar]
- 9.Nyakeriga AM, Troye-Blomberg M, Chemtai AK, Marsh K, Williams TN. Malaria and nutritional status in children living on the coast of Kenya. Am J Clin Nutr. 2004;80: 1604–1610. [DOI] [PubMed] [Google Scholar]
- 10.Frosch AE, Ondigo BN, Ayodo GA, Vulule JM, John CC, Cusick SE. Decline in childhood iron deficiency after interruption of malaria transmission in highland Kenya. Am J Clin Nutr. 2014;100: 968–973. 10.3945/ajcn.114.087114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roos A, Hegsted DM, Stare FJ. Nutritional studies with the duck; the effect of vitamin deficiencies on the course of P. lophurae infection in the duck and the chick. J Nutr. 1946;32: 473–484. [DOI] [PubMed] [Google Scholar]
- 12.Krishnan S, Krishnan AD, Mustafa AS, Talwar GP, Ramalingaswami V. Effect of vitamin A and undernutrition on the susceptibility of rodents to a malarial parasite Plasmodium berghei. J Nutr. 1976;106: 784–791. [DOI] [PubMed] [Google Scholar]
- 13.Foote SJ, Burt RA, Baldwin TM, Presente A, Roberts AW, Laural YL, et al. Mouse loci for malaria-induced mortality and the control of parasitaemia. Nat Genet. 1997;17: 380–381. [DOI] [PubMed] [Google Scholar]
- 14.Arif AJ, Mathur PD, Chandra S, Singh C, Sen AB. Effect of zinc diet on xanthine oxidase activity of liver of mice infected with Plasmodium berghei. Indian J Malariol. 1987;24: 59–63. [PubMed] [Google Scholar]
- 15.Harvey PW, Bell RG, Nesheim MC. Iron deficiency protects inbred mice against infection with Plasmodium chabaudi. Infect Immun. 1985;50: 932–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cardoso MA, Colli C, Garcia PB, Ferreira MU, Penteado MV, Andrade Junior HF. Effect of dietary iron on the course of Plasmodium berghei malaria in young rats. Braz J Med Biol Res. 1993;26: 1297–1303. [PubMed] [Google Scholar]
- 17.Das BS, Thurnham DI, Das DB. Plasma alpha-tocopherol, retinol, and carotenoids in children with falciparum malaria. Am J Clin Nutr. 1996;64: 94–100. [DOI] [PubMed] [Google Scholar]
- 18.Uscategui RM, Correa AM, Carmona-Fonseca J. Changes in retinol, hemoglobin and ferritin concentrations in Colombian children with malaria. Biomedica. 2009;29: 270–281. [PubMed] [Google Scholar]
- 19.Mayo-Wilson E, Junior JA, Imdad A, Dean S, Chan XH, Chan ES, et al. Zinc supplementation for preventing mortality, morbidity, and growth failure in children aged 6 months to 12 years of age. Cochrane Database Syst Rev. 2014;5: CD009384 10.1002/14651858.CD009384.pub2 [DOI] [PubMed] [Google Scholar]
- 20.Sazawal S, Black RE, Ramsan M, Chwaya HM, Dutta A, Dhingra U, et al. Effect of zinc supplementation on mortality in children aged 1–48 months: a community-based randomised placebo-controlled trial. Lancet. 2007;369: 927–934. [DOI] [PubMed] [Google Scholar]
- 21.White NJ, Pukrittayakamee S, Hien TT, Faiz MA, Mokuolu OA, Dondorp AM. Malaria. Lancet. 2014;383: 723–735. 10.1016/S0140-6736(13)60024-0 [DOI] [PubMed] [Google Scholar]
- 22.Zlotkin S, Newton S, Aimone AM, Azindow I, Amenga-Etego S, Tchum K, et al. Effect of iron fortification on malaria incidence in infants and young children in Ghana: a randomized trial. JAMA. 2013;310: 938–947. 10.1001/jama.2013.277129 [DOI] [PubMed] [Google Scholar]
- 23.Jonker FA, Calis JC, van Hensbroek MB, Phiri K, Geskus RB, Brabin BJ, et al. Iron status predicts malaria risk in Malawian preschool children. PLoS One. 2012;7: e42670 10.1371/journal.pone.0042670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Athuman M, Kabanywanyi AM, Rohwer AC. Intermittent preventive antimalarial treatment for children with anaemia. Cochrane Database Syst Rev. 2015;1: CD010767 10.1002/14651858.CD010767.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sazawal S, Black RE, Kabole I, Dutta A, Dhingra U, Ramsan M. Effect of iron/folic acid supplementation on the outcome of malaria episodes treated with sulfadoxine-pyrimethamine. Malar Res Treat. 2014;2014: 625905 10.1155/2014/625905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nasi M, Pinti M, Mussini C, Cossarizza A. Persistent inflammation in HIV infection: established concepts, new perspectives. Immunol Lett. 2014;161: 184–188. 10.1016/j.imlet.2014.01.008 [DOI] [PubMed] [Google Scholar]
- 27.Womack JA, Chang CC, So-Armah KA, Alcorn C, Baker JV, Brown ST, et al. HIV infection and cardiovascular disease in women. J Am Heart Assoc. 2014;3: e001035 10.1161/JAHA.114.001035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Battistini TR, Sarni RO, de Souza FI, Pitta TS, Fernandes AP, Hix S, et al. Lipodystrophy, lipid profile changes, and low serum retinol and carotenoid levels in children and adolescents with acquired immunodeficiency syndrome. Nutrition. 2010;26: 612–616. 10.1016/j.nut.2009.06.024 [DOI] [PubMed] [Google Scholar]
- 29.Desvignes L, Weidinger C, Shaw P, Vaeth M, Ribierre T, Liu M, et al. STIM1 controls T cell-mediated immune regulation and inflammation in chronic infection. J Clin Invest. 2015;125: 2347–2362. 10.1172/JCI80273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kaufmann SH, Dorhoi A. Inflammation in tuberculosis: interactions, imbalances and interventions. Curr Opin Immunol. 2013;25: 441–449. 10.1016/j.coi.2013.05.005 [DOI] [PubMed] [Google Scholar]
- 31.Gillis J, Smieja M, Cescon A, Rourke SB, Burchell AN, Cooper C, et al. Risk of cardiovascular disease associated with HCV and HBV coinfection among antiretroviral-treated HIV-infected individuals. Antivir Ther. 2014;19: 309–317. 10.3851/IMP2724 [DOI] [PubMed] [Google Scholar]
- 32.Amare B, Moges B, Mulu A, Yifru S, Kassu A. Quadruple burden of HIV/AIDS, tuberculosis, chronic intestinal parasitoses, and multiple micronutrient deficiency in ethiopia: a summary of available findings. Biomed Res Int. 2015;2015: 598605 10.1155/2015/598605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yuyama LK, Cozzolino SM. Interaction of zinc and vitamin A in rats receiving a regional diet of Manaus, Amazonas, Brazil. Effect of supplementation with vitamin A, zinc and zinc and vitamin A. Arch Latinoam Nutr. 1996;46: 216–220. [PubMed] [Google Scholar]
- 34.Tavares BM, Veiga VG, Yuyama LKO, Bueno MB, Fisberg RM, Fisberg M. Nutritional status and energy and nutrients intake of children attending day-care centers in the city of Manaus, Amazonas, Brazil: are there differences between public and private day-care centers? Rev Paul Pediatr. 2012;30: 42–50. [Google Scholar]
- 35.Brazilian Ministry of Health. Anthropometry: how to measure and weigh. Brasília: Brazilian Ministry of Health; 2004. [Google Scholar]
- 36.Yang H, Onis M. Algorithms for converting estimates of child malnutrition based on the NCHS reference into estimates based on the WHO child growth standards. BMC Pediatr. 2008;8: 19 10.1186/1471-2431-8-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.World Health Organization. WHO Expert Committee on Physical Status: the Use and Interpretation of Anthropometry. Geneva: World Health Organization; 1995. [PubMed] [Google Scholar]
- 38.World Health Organization. Child growth standards. Geneva: World Health Organization; 2007. [Google Scholar]
- 39.De Onis M. WHO child growth standards: growth velocity based on weight, length and head circumference: methods and development. Geneva: World Health Organization; 2009. [Google Scholar]
- 40.Whitehouse RR, Prasad AS, Rabbani PI, Cossack ZT. Zinc in plasma, neutrophilis, lymphocytes, and erythrocytes as determined by flameless atomic absorption spectrophotometry. Clin Chem. 1982;28: 475–480. [PubMed] [Google Scholar]
- 41.Davis TM, Binh TQ, Danh PT, Dyer JR, St John A, Garcia-Webb P, et al. Serum vitamin A and E concentrations in acute falciparum malaria: modulators or markers of severity? Clin Sci (Lond). 1994;87: 505–511. [DOI] [PubMed] [Google Scholar]
- 42.Ramalho RA, Flores H, Saunders C. Hipovitaminose A no Brasil: um problema de saúde pública. Pan Am J Public Health. 2002;12: 117–123. [DOI] [PubMed] [Google Scholar]
- 43.Duggan C, MacLeod WB, Krebs NF, Westcott JL, Fawzi WW, Premji ZG, et al. Plasma zinc concentrations are depressed during the acute phase response in children with falciparum malaria. J Nutr. 2005;135: 802–807. [DOI] [PubMed] [Google Scholar]
- 44.Sandström B. Bioavailability of zinc. Eur J Clin Nutr. 1997;51: S17–S9. [PubMed] [Google Scholar]
- 45.Santos HG, Sardinha FAA, Colli C. Zinco eritrocitário (validação de um método de análise) e zinco dietético na avaliação do estado nutricional de mulheres adultas. Rev Bras Cienc Farm. 2005;41: 205–213. [Google Scholar]
- 46.World Health Organization. Microscopic diagnosis of Malaria. Geneva: World Health Organization; 2006. [Google Scholar]
- 47.World Health Organization. Iron deficiency anaemia, assessment, prevention, and control a guide for programme managers. Geneva: World Health Organization; 2001. [Google Scholar]
- 48.Lutz A. Schistosoma mansoni e a schistosomose, segundo observações feitas no Brasil. Mem Inst Oswaldo Cruz. 1919;11: 121–155. [Google Scholar]
- 49.Faust EC, Sawitz W, Tobie J. Comparative efficiency of various techniques for the diagnosis of protozoa and helminths in feces. J Parasitol. 1939;25: 241–262. [Google Scholar]
- 50.Ramalho RA, Flores H, Saunders C. Hypovitaminosis A in Brazil: a public health problem. Rev Panam Salud Publica. 2002;12: 117–122. [DOI] [PubMed] [Google Scholar]
- 51.Brazilian Ministry of Health. Pesquisa nacional de demografia e saúde da criança e da mulher. Brasília: Brazilian Ministry of Health; 2009. [Google Scholar]
- 52.Augusto RA, Cobayashi F, Cardoso MA, Team AS. Associations between low consumption of fruits and vegetables and nutritional deficiencies in Brazilian schoolchildren. Public Health Nutr. 2015;18: 927–935. 10.1017/S1368980014001244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cobayashi F, Augusto RA, Lourenco BH, Muniz PT, Cardoso MA. Factors associated with stunting and overweight in Amazonian children: a population-based, cross-sectional study. Public Health Nutr. 2014;17: 551–560. 10.1017/S1368980013000190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Marinho HA, Roncada MJ. Ingestão e hábitos alimentares de pré-escolares de três capitais da Amazônia Ocidental Brasileira: um enfoque especial à ingestão de vitamina A. Acta Amaz 2002; 33: 263–274. [Google Scholar]
- 55.Okebe JU, Yahav D, Shbita R, Paul M. Oral iron supplements for children in malaria-endemic areas. Cochrane Database Syst Rev. 2011: CD006589 10.1002/14651858.CD006589.pub3 [DOI] [PubMed] [Google Scholar]
- 56.Mwanga-Amumpaire J, Ndeezi G, Tumwine JK. Effect of vitamin A adjunct therapy for cerebral malaria in children admitted to Mulago hospital: a randomized controlled trial. Afr Health Sci. 2012;12: 90–97. 10.4314/ahs.v12i2.3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mfonkeu JB, Gouado I, Kuate HF, Zambou O, Combes V, Grau GE, et al. Biochemical markers of nutritional status and childhood malaria severity in Cameroon. Br J Nutr. 2010;104: 886–892. 10.1017/S0007114510001510 [DOI] [PubMed] [Google Scholar]
- 58.Vitamin A supplementation in northern Ghana: effects on clinic attendances, hospital admissions, and child mortality. Ghana VAST Study Team. Lancet. 1993;342: 7–12. [PubMed] [Google Scholar]
- 59.Olofin IO, Spiegelman D, Aboud S, Duggan C, Danaei G, Fawzi WW. Supplementation with multivitamins and vitamin A and incidence of malaria among HIV-infected Tanzanian women. J Acquir Immune Defic Syndr. 2014;67: S173–S178. 10.1097/QAI.0000000000000375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Manning L, Laman M, Rosanas-Urgell A, Michon P, Aipit S, Bona C, et al. Severe anemia in Papua New Guinean children from a malaria-endemic area: a case-control etiologic study. PLoS Negl Trop Dis. 2012;6: e1972 10.1371/journal.pntd.0001972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bates CJ, Evans PH, Dardenne M, Prentice A, Lunn PG, Northrop-Clewes CA, et al. A trial of zinc supplementation in young rural Gambian children. Br J Nutr. 1993;69: 243–255. [DOI] [PubMed] [Google Scholar]
- 62.Muller O, Becher H, van Zweeden AB, Ye Y, Diallo DA, Konate AT, et al. Effect of zinc supplementation on malaria and other causes of morbidity in west African children: randomised double blind placebo controlled trial. BMJ. 2001;322: 1567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Richard SA, Zavaleta N, Caulfield LE, Black RE, Witzig RS, Shankar AH. Zinc and iron supplementation and malaria, diarrhea, and respiratory infections in children in the Peruvian Amazon. Am J Trop Med Hyg. 2006;75: 126–132. [DOI] [PubMed] [Google Scholar]
- 64.Sazawal S, Black RE, Ramsan M, Chwaya HM, Stoltzfus RJ, Dutta A, et al. Effects of routine prophylactic supplementation with iron and folic acid on admission to hospital and mortality in preschool children in a high malaria transmission setting: community-based, randomised, placebo-controlled trial. Lancet. 2006;367: 133–143. [DOI] [PubMed] [Google Scholar]
- 65.Macedo RS, Benzecry SG, Araújo L, Lacerda MV. Avaliação de consumo alimentar e nutricional de escolares de 5 a 15 anos de uma área endêmica de malária do Amazonas. Efdeports (Rev Dig Buenos Aires). 2010;142: 1. [Google Scholar]
- 66.Murrieta RSS. O dilema do papa-chibé: consumo alimentar, nutrição e práticas de intervenção na Ilha de Ituqui, Baixo Amazonas, Pará. Rev Antropol. 1998;1: 41. [Google Scholar]
- 67.Murrieta RS. Dialética do sabor: alimentação, ecologia e vida cotidiana em comunidades ribeirinhas da Ilha de Ituqui, Baixo Amazonas, Pará. Rev Antropol. 2001;2: 44. [Google Scholar]
- 68.Rodrigues AG. Buscando raízes. Horiz Antrop. 2001; 16: 131–144. [Google Scholar]
- 69.Bresnahan KA, Chileshe J, Tanumihardjo SA. Quantification of food and nutrient intakes in Zambian children with and without malaria under controlled feeding conditions. Exp Biol Med (Maywood). 2014;239: 45–51. [DOI] [PubMed] [Google Scholar]
- 70.Glinz D, Hurrell RF, Righetti AA, Zeder C, Adiossan LG, Tjalsma H, et al. In Ivorian school-age children, infection with hookworm does not reduce dietary iron absorption or systemic iron utilization, whereas afebrile Plasmodium falciparum infection reduces iron absorption by half. Am J Clin Nutr. 2015;101: 462–470. 10.3945/ajcn.114.090175 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Due to ethical restrictions regarding patient privacy, data are available upon request. Requests for the data may be sent to the corresponding author.