Abstract
The HIV-1 plague continues unabatedly across sub-Saharan Africa. In Botswana and Swaziland, nearly 40% of the entire adult population is already infected. No current program is capable of slowing the advancing tide. An effective vaccine and widespread treatment are years, if not, decades away. In this most urgent situation, I propose that pre-exposure chemoprophylaxis be studied as a means to reduce the spread of HIV-1 among at-risk individuals.
In sub-Saharan Africa, the human immunodeficiency virus type 1 (HIV-1) epidemic has reached staggering proportions with infection rates in several urban areas exceeding 35% of the adult population [1]. While there is no question of the need for interventional strategies to stem the overwhelming tide of new infections, there is also no clear consensus as to what approaches might be the most effective. Considerable attention has been focused on the development of an immunoprophylactic vaccine [2] particularly for application in developing countries. Unfortunately, the first phase 3 studies of a candidate HIV vaccine failed to provide protection from infection [3-5] and none of the remaining vaccine candidates currently in clinical trials appear likely to induce potent protective immunity [6]. Given HIV's propensity to escape cellular and humoral responses[7,8], there exists no clear approach or viral target for vaccine development. The World Health Organization (WHO) is committed to developing centers to treat those infected with HIV-1 in areas hardest hit by the epidemic [9]. While these efforts may ease the suffering of those already affected, it is not clear they will have a broad impact on the HIV-1 epidemic in the immediate future. Herein, I propose a novel interventional approach with the potential for immediate impact: that is the administration of chemoprophylaxis to uninfected individuals who live in areas with a high prevalence of HIV-1.
In Europe and the United States, chemoprophylaxis is recommended for health-care workers (HCW) exposed to blood or body fluids from an HIV-infected patient through percutaneous injury or mucous membrane exposure. The risk of HIV-1 transmission from a blood-contaminated needle stick is estimated to be about 0.3%. One study of HCW exposed to HIV-1 found that azidothymidine (AZT) reduced infections by 81% (95% CI = 43%–94%) [10]. In animal studies, chronic infection with simian immunodeficiency virus (SIV) was eliminated by 4-week administration of a single agent, tenofovir, even when treatment was delayed up to 24 hours after viral inoculation [11,12].
Chemoprophylaxis for HIV has also been shown to be effective in cases of vertical transmission. The results of the first large-scale clinical trial of mother-to-child transmission (MTCT), PACTG 076, showed that a regimen based on AZT alone effectively reduced infant infection by 66% (22.6% of infants in the placebo group became infected compared to 7.6% of the AZT group) [13]. Mothers were placed on oral AZT beginning at 14 to 34 weeks of gestation and given intravenous AZT during labor. Each newborn then received oral AZT for 6 weeks. The protective effect was not entirely attributable to AZT's modest effect on maternal HIV-1 RNA viral load. This and other studies confirm that chemoprophylaxis with a single drug can potently reduce the rate of HIV-1 transmission [14] and suggest that the efficacy with currently recommended therapeutic regimens (two- and three-drug combinations) may be even higher.
Clearly, there are many differences between these scenarios and the requirements inherent in the prophylactic treatment of a large population at continuous risk for HIV exposure. A HCW or newborn (in the absence of breastfeeding) is exposed only once to the virus and chemoprophylaxis is needed on an individual basis for 4 to 6 weeks. Chemoprophylaxis of a large population would be widespread and ongoing, and would require treatment of many individuals, but would carry the added benefit of conferring protection on their sexual contacts. The success of post-exposure prophylaxis is supported by studies of HCW and MTCT and while the large-scale efficacy of pre-exposure prophylaxis in large population is unknown, it is reasonable to assume a similar degree of protection might be afforded.
Of the 40 million people living with HIV-1 infection throughout the world, more than 60% are in sub-Saharan Africa [1]. Further, 30% of all HIV-1-infected individuals reside within southern Africa, although this region has less than 2% of the world's population. In two countries, Botswana and Swaziland, HIV-1 prevalence for the entire adult population is approaching 40%. If there is no decline in prevalence or incidence rates of infection in these countries, then the risk of acquiring HIV-1 among those currently uninfected is enormous. Based on data from a cohort of HIV discordant couples in Uganda, it is estimated that the rate of male-to-female transmission is 0.0009 per act, while the rate of female-to-male transmission is 0.0013, with an overall HIV-1 heterosexual transmission rate of 0.0011 per coital act [15]. If chemoprophylaxis can reduce these rates by 80–90%, then the rate of heterosexual transmission would fall to approximately 1 in 10,000 per act. Over time, the prevalence of HIV-1 would decrease as fewer new cases occurred, leading to a further reduction in the incidence of infection.
Through collaborative efforts of pharmaceutical companies and scientists from the United States and Europe, small numbers of HIV-infected individuals in developing countries are now being given antiretroviral therapy. While the drugs are being supplied at reduced cost, treatment remains prohibitively expensive for the majority of infected individuals. Moreover, administration of complex HIV therapeutic regimens requires frequent clinical monitoring, and physicians must be trained in the interpretation of laboratory values and the proper actions to be taken. While these treatment efforts represent an important first step, it is perhaps overly optimistic to assume that the WHO will reach its goal to have 3 million HIV-infected people (<10% of all cases) in developing countries under treatment by the end of 2005 [16]. More likely this outcome will take many years, if not decades, for the countries of southern Africa to develop the infrastructure to treat the majority of HIV-infected people.
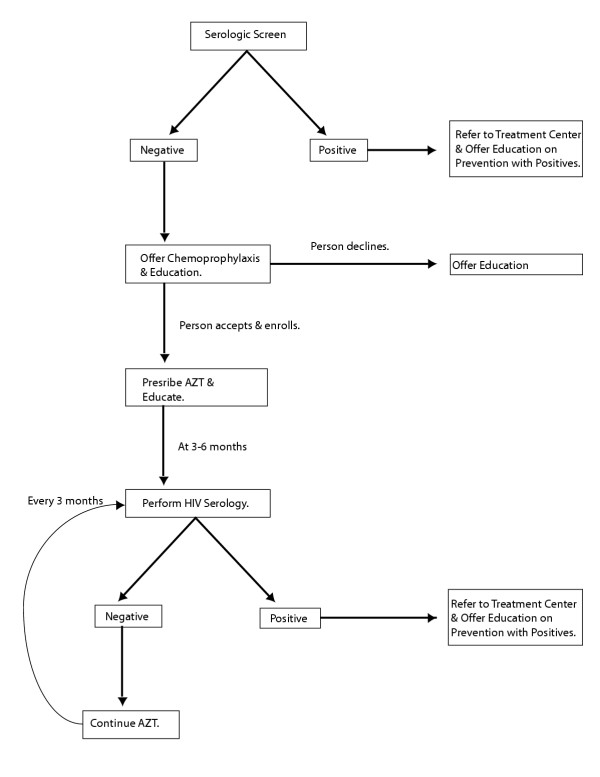
Pre-exposure chemoprophylaxis, on the other hand, can be administered at a lower cost and the regimens simplified. In one of many possible scenarios, HIV-uninfected individuals could be identified by serologic screening and prescribed AZT (Figure 1). Once on AZT, individuals would periodically (every 3 to 6 months) be tested for HIV-1 seroconversion. Concurrently, they would be educated on the risks of infection even while on prophylactic treatment. HIV infection that occurs while an individual is on chemoprophylaxis would necessitate the provision of combination therapy.
Figure 1.
Outline of one possible, chemoprophylaxis scenario. High-risk individuals would be screened by serology for HIV-1 infection. Positive individuals would be referred to a treatment center, if available. Negative individuals would be offered enrollment in the chemoprophylaxis program. Once enrolled, individuals would receive education on HIV prevention and be prescribed AZT. Every 3–6 months, these participants would be serologically re-tested. If positive, the person would be removed from the program, offered education on prevention for positives, and referred to the treatment center. If negative, AZT would be continued. Those who decline repeat testing would be taken off AZT. In this way, only those who are uninfected or recently infected would be on AZT.
There are many potential drawbacks to this strategy. While the effect of incomplete adherence to treatment in the setting of chronic HIV-1 infection is well documented, it is not known whether the same outcome will result from prophylactic HIV therapy. Drug-resistant virus will undoubtedly develop in those who become HIV-infected and continue to take only AZT (or any single drug). Viral resistance could reduce the chances of treatment success in that individual, and over time, the resistant virus could replace the wild-type virus in the population at large. With this in mind, drugs like nevirapine, which promote viral resistance in chronically infected individuals after a single dose [17,18], should be avoided in a prophylactic setting. A more reasonable choice would be one of the nucleoside analogue reverse transcriptase inhibitors (NRTIs), reserving other drugs with little cross-resistance to the selected NRTI as the mainstay of treatment.
Behavior modifications may also result if people interpret chemoprophylaxis as potent protection against HIV and engage more frequently in high-risk activities. This concern has been raised in the context of HIV vaccine trials and has thus far not been substantiated. In fact participants in a recent HIV vaccine trial did not have an increase in high-risk behavior, as some anticipated, but rather had a decrease in intravenous drug use and needle sharing [19]. Similarly, educational programs aimed at increasing awareness of the purpose and limitations of chemoprophylaxis may have a positive impact on high-risk behavior.
Pre-exposure prophylaxis, as suggested here, would be administered to uninfected individuals for years to decades until the epidemic is contained or eliminated. Consequently, strong consideration must be given to the long-term toxicity of the drug(s) used. Newer NRTIs have a low incidence of side effect profiles [20]. The currently recommended dose of AZT, 600 mg per day, is much safer than the dose, 1500 mg per day, used in the initial monotherapy studies [21,22]. Further, many anti-HIV-1 compounds appear to be safe for use in pregnancy and do not cause significant fetal harm [23]. While safety concerns must be considered, lifelong chemoprophylaxis would certainly be less toxic than lifelong combination therapy. In short, for those with a great chance of becoming infected, the risks of pre-exposure chemoprophylaxis do not, a priori, outweigh the potential benefits.
One population that may be best suited for examination of this hypothesis is female commercial sex workers (FSW) in southern Africa. Behavior intervention programs, which include education and safer sex recommendations, have had a dramatic effect on HIV-1 incidence in Thailand and other areas [24,25]. Similar programs in Uganda had no effect on HIV-1 transmission [26]. The HIV-1 prevalence rate in FSW is >68% in some cities in Africa [27]. Further, some hypothesize that this group is the major node or linchpin for maintaining the epidemic [28]. Several cohorts have previously been studied and clearly, those FSW who are uninfected are at enormous risk for HIV-1 infection. Chemoprophylaxis should be studied in the context of traditional prevention programs.
It is worth recalling that chemoprophylaxis is currently used to protect individuals and groups in many settings [29]. Anti-influenza agents are often used to stop flu outbreaks in nursing homes. Travelers to areas with endemic malaria are given mefloquine to prevent infection with Plasmodium spp. Individuals with a history of rheumatic fever are maintained on penicillin for many years to prevent re-infection with Group A streptococcus. Routine practice already dictates that HIV-infected patients with acquired immunodeficiency syndrome (AIDS) be given antibiotics to prevent Pneumocystis jiroveci pneumonia, toxoplasmic encephalitis, and Mycobacterium avium complex infections. No precedent exists for administering ongoing chemoprophylaxis to large populations of at-risk individuals. However, the urgency of the HIV epidemic and the absence of any effective measures for curbing new infections mandate that novel strategies be considered. With respect to all infectious diseases, the proverb "a ounce of prevention is worth a pound of cure" certainly holds true. In the case of HIV infection, the stakes are much higher.
References
- AIDS epidemic update December 2003. Geneva, UNAIDS/WHO; 2003. [Google Scholar]
- Desrosiers RC. Prospects for an AIDS vaccine. Nat Med. 2004;10:221–223. doi: 10.1038/nm0304-221. [DOI] [PubMed] [Google Scholar]
- Cohen J. Public health. AIDS vaccine still alive as booster after second failure in Thailand. Science. 2003;302:1309–1310. doi: 10.1126/science.302.5649.1309a. [DOI] [PubMed] [Google Scholar]
- Cohen J. HIV/AIDS. Vaccine results lose significance under scrutiny. Science. 2003;299:1495. doi: 10.1126/science.299.5612.1495. [DOI] [PubMed] [Google Scholar]
- Pitisutithum P. 11th Conference on Retroviruses and Opportunistic Infections. San Francisco; 2004. Efficacy of AIDSVAX B/E Vaccines in Injecting Drug Use. [Google Scholar]
- Burton DR, Desrosiers RC, Doms RW, Feinberg MB, Gallo RC, Hahn B, Hoxie JA, Hunter E, Korber B, Landay A, Lederman MM, Lieberman J, McCune JM, Moore JP, Nathanson N, Picker L, Richman D, Rinaldo C, Stevenson M, Watkins DI, Wolinksky SM, Zack JA. Public health. A sound rationale needed for phase III HIV-1 vaccine trials. Science. 2004;303:316. doi: 10.1126/science.1094620. [DOI] [PubMed] [Google Scholar]
- Burton DR, Desrosiers RC, Doms RW, Koff WC, Kwong PD, Moore JP, Nabel GJ, Sodroski J, Wilson IA, Wyatt RT. HIV vaccine design and the neutralizing antibody problem. Nat Immunol. 2004;5:233–236. doi: 10.1038/ni0304-233. [DOI] [PubMed] [Google Scholar]
- Smith SM. HIV CTL escape: at what cost? Retrovirology. 2004;1:8. doi: 10.1186/1742-4690-1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The 3 by 5 Initiative. Geneva, World Health Organization; 2004. [Google Scholar]
- Cardo DM, Culver DH, Ciesielski CA, Srivastava PU, Marcus R, Abiteboul D, Heptonstall J, Ippolito G, Lot F, McKibben PS, Bell DM. A case-control study of HIV seroconversion in health care workers after percutaneous exposure. Centers for Disease Control and Prevention Needlestick Surveillance Group. N Engl J Med. 1997;337:1485–1490. doi: 10.1056/NEJM199711203372101. [DOI] [PubMed] [Google Scholar]
- Tsai CC, Follis KE, Sabo A, Beck TW, Grant RF, Bischofberger N, Benveniste RE, Black R. Prevention of SIV infection in macaques by (R)-9-(2-phosphonylmethoxypropyl)adenine. Science. 1995;270:1197–1199. doi: 10.1126/science.270.5239.1197. [DOI] [PubMed] [Google Scholar]
- Tsai CC, Emau P, Follis KE, Beck TW, Benveniste RE, Bischofberger N, Lifson JD, Morton WR. Effectiveness of postinoculation (R)-9-(2-phosphonylmethoxypropyl) adenine treatment for prevention of persistent simian immunodeficiency virus SIVmne infection depends critically on timing of initiation and duration of treatment. J Virol. 1998;72:4265–4273. doi: 10.1128/jvi.72.5.4265-4273.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor EM, Sperling RS, Gelber R, Kiselev P, Scott G, O'Sullivan MJ, VanDyke R, Bey M, Shearer W, Jacobson RL, et al. Reduction of maternal-infant transmission of human immunodeficiency virus type 1 with zidovudine treatment. Pediatric AIDS Clinical Trials Group Protocol 076 Study Group. N Engl J Med. 1994;331:1173–1180. doi: 10.1056/NEJM199411033311801. [DOI] [PubMed] [Google Scholar]
- Paul SM, Burr CK, DiFerdinando GT. Updated recommendations for reducing vertical HIV transmission. N J Med. 2003;100:27–31; quiz 69-70. [PubMed] [Google Scholar]
- Gray RH, Wawer MJ, Brookmeyer R, Sewankambo NK, Serwadda D, Wabwire-Mangen F, Lutalo T, Li X, vanCott T, Quinn TC. Probability of HIV-1 transmission per coital act in monogamous, heterosexual, HIV-1-discordant couples in Rakai, Uganda. Lancet. 2001;357:1149–1153. doi: 10.1016/S0140-6736(00)04331-2. [DOI] [PubMed] [Google Scholar]
- Fleck F. WHO admits its target on AIDS drugs may be unrealistic. Bmj. 2004;328:1151. doi: 10.1136/bmj.328.7449.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eshleman SH, Mracna M, Guay LA, Deseyve M, Cunningham S, Mirochnick M, Musoke P, Fleming T, Glenn Fowler M, Mofenson LM, Mmiro F, Jackson JB. Selection and fading of resistance mutations in women and infants receiving nevirapine to prevent HIV-1 vertical transmission (HIVNET 012) Aids. 2001;15:1951–1957. doi: 10.1097/00002030-200110190-00006. [DOI] [PubMed] [Google Scholar]
- Jackson JB, Becker-Pergola G, Guay LA, Musoke P, Mracna M, Fowler MG, Mofenson LM, Mirochnick M, Mmiro F, Eshleman SH. Identification of the K103N resistance mutation in Ugandan women receiving nevirapine to prevent HIV-1 vertical transmission. Aids. 2000;14:F111–5. doi: 10.1097/00002030-200007280-00001. [DOI] [PubMed] [Google Scholar]
- van Griensvan F, Keawkungwal J, Tappero JW, Sangkum U, Pitisuttithum P, Vanichseni S, Suntharasamai P, Orelind K, Gee C, Choopanya K. Lack of increased HIV risk behavior among injection drug users participating in the AIDSVAX B/E HIV vaccine trial in Bangkok, Thailand. Aids. 2004;18:295–301. doi: 10.1097/00002030-200401230-00020. [DOI] [PubMed] [Google Scholar]
- Montessori V, Press N, Harris M, Akagi L, Montaner JS. Adverse effects of antiretroviral therapy for HIV infection. Cmaj. 2004;170:229–238. [PMC free article] [PubMed] [Google Scholar]
- McLeod GX, Hammer SM. Zidovudine: five years later. Ann Intern Med. 1992;117:487–501. doi: 10.7326/0003-4819-117-6-487. [DOI] [PubMed] [Google Scholar]
- Abu-ata O, Slim J, Perez G, Smith SM. HIV therapeutics: past, present, and future. Adv Pharmacol. 2000;49:1–40. doi: 10.1080/000187300243381. [DOI] [PubMed] [Google Scholar]
- Recommendations for Use of Antiretroviral Drugs in Pregnant HIV-1-Infected Women for Maternal Health and Interventions to Reduce Perinatal HIV-1 Transmission in the United States. Washington, Department of Health and Human Services and the Henry J. Kaiser Foundation; 2004. [Google Scholar]
- Rojanapithayakorn W, Hanenberg R. The 100% condom program in Thailand. Aids. 1996;10:1–7. doi: 10.1097/00002030-199601000-00001. [DOI] [PubMed] [Google Scholar]
- Valdiserri RO, Ogden LL, McCray E. Accomplishments in HIV prevention science: implications for stemming the epidemic. Nat Med. 2003;9:881–886. doi: 10.1038/nm0703-881. [DOI] [PubMed] [Google Scholar]
- Kamali A, Quigley M, Nakiyingi J, Kinsman J, Kengeya-Kayondo J, Gopal R, Ojwiya A, Hughes P, Carpenter LM, Whitworth J. Syndromic management of sexually-transmitted infections and behaviour change interventions on transmission of HIV-1 in rural Uganda: a community randomised trial. Lancet. 2003;361:645–652. doi: 10.1016/S0140-6736(03)12598-6. [DOI] [PubMed] [Google Scholar]
- Morison L, Weiss HA, Buve A, Carael M, Abega SC, Kaona F, Kanhonou L, Chege J, Hayes RJ. Commercial sex and the spread of HIV in four cities in sub-Saharan Africa. Aids. 2001;15 Suppl 4:S61–9. doi: 10.1097/00002030-200108004-00007. [DOI] [PubMed] [Google Scholar]
- Jha P, Nagelkerke NJD, Ngugi EN, Prasada Rao JVR, Willbond B, Moses S, Plummer FA. PUBLIC HEALTH: Reducing HIV Transmission in Developing Countries. Science. 2001;292:224–225. doi: 10.1126/science.1058187. [DOI] [PubMed] [Google Scholar]
- Mandell GL, Bennett JE, Dolin R. Principles and Practices of Infectious Diseases. 5th. Philadelphia, Churchill Livingstone; 2000. [Google Scholar]