Abstract
Background
gC1qR is a multifunctional cellular protein that has been linked to inflammation and cancer. gC1qR is highly upregulated in adenocarcinomas as compared to normal tissue counterparts, and soluble gC1qR (sgC1qR) has been detected in vitro in the pericellular milieu of proliferating malignant cells.
Aim
The present study explored the tissue expression of gC1qR in pancreatic cancer by immunohistochemistry, and the presence of sgC1qR in vivo, by examining blood and malignant effusions from patients with metastatic pancreatic adenocarcinoma.
Methods
Tissue expression of gC1qR by pancreatic adenocarcinoma was visualized by immunohistochemistry. SgC1qR was quantified in serum from healthy volunteers (n=20) and pancreatic cancer patients (n=34), as well as in malignant pleural (n=23) and peritoneal effusions (n=27), using a newly developed, sensitive immunocapture sandwich ELISA.
Results
Overexpression of gC1qR was confirmed in pancreatic adenocarcinoma compared to nonmalignant pancreatic tissue. Moreover, increased serum levels of sgC1qR (0.29 ± 0.22 ng/ml) were noted in patients with metastatic pancreatic cancer compared to healthy controls (0.15 ± 0.10 ng/ml) (mean ± S.D.) (p=0.035). In 11 of 16 patients for whom sequential samples were available, serum sgC1qR levels rose with disease progression, and paralleled changes in tumor biomarkers, CEA and CA19.9. In addition to blood, sgC1qR was detected in malignant pleural (0.55 ± 0.47 ng/ml) and peritoneal effusions (0.57 ± 0.38 ng/ml).
Conclusion
This study provides the first evidence for the presence of sgC1qR in vivo. The ability to detect sgC1qR in blood and body fluids will enable further studies to elucidate its pathophysiology in malignancy.
Keywords: gC1qR, Blood, Pancreatic cancer, Peritoneal fluid, Complement, ELISA
Introduction
gC1qR is a multifunctional cellular protein of 33 kDa that has been implicated in inflammation [1] and malignancy [2]. It was initially identified as a receptor for the globular heads of C1q [3–5]. gC1qR is localized in several cellular compartments, including mitochondria [6–9], endoplasmic reticulum and nucleus, as well as the cell surface, where it appears to be associated with lipid rafts [5,10–15].
The ability of gC1qR to modulate the activity of ligands both inside and outside of the cell is increasingly recognized. Several binding partners have been identified, including C1q [3,16], vitronectin [17], and high molecular weight kininogen [18–20]. These interactions lead to classical complement pathway activation with generation of inflammatory cytokines [21,22], and activation of the kinin system with production of bradykinin and ensuing vascular permeability [22]. gC1qR is also important for the maintenance of oxidative phosphorylation [6], and stable knockdown of gC1qR has been shown to shift metabolism from oxidative phosphorylation to glycolysis [23]. In addition, gC1qR has been implicated as a potential regulator of cell proliferation, adhesion, migration, and invasion [15,24].
We hypothesize that gC1qR may play a role in carcinogenesis. Indeed, differential gC1qR expression has been reported in a variety of tumors, particularly epithelial tumors compared to their nonmalignant histologic counterparts [25,26]. gC1qR expression on the cell membrane has been shown to promote tumor cell migration and invasion in vitro [27], and recent evidence suggests that gC1qR overexpression in primary ovarian carcinomas is associated with decreased overall survival and decreased progression free survival [28]. The observation that increased gC1qR expression occurs also in benign inflammatory and proliferative lesions [26], suggests that increased gC1qR expression may be a marker of cell proliferation, with potential diagnostic and therapeutic applications.
In addition to cellular gC1qR expression, soluble gC1qR (sgC1qR) has been observed in vitro [21]. sgC1qR may exert potent physiologic effects including activation of complement, coagulation, and kinin systems. Moreover, sgC1qR was shown to bind to cultured endothelial cells and to induce the bradykinin/B1 receptor [29], which promotes angiogenensis [30].
The presence of sgC1qR in vivo has not been established. The present study developed a reliable, high-sensitivity ELISA to screen blood and body fluids as proof of principle for the existence of sgC1qR in vivo. Serum samples from healthy volunteers and patients with pancreatic cancer were tested. In addition, malignant pleural and peritoneal effusions were evaluated. The data provide clear evidence of the presence of sgC1qR in blood and malignant body fluids, and suggest that sgC1qR levels may change with disease progression. The ability to quantify sgC1qR levels will enable further research into gC1qR biology in health and disease.
Methods
Biological samples
Histologic sections of pancreatic adenocarcinoma were obtained from the archives of the Department of Pathology at Mount Sinai School of Medicine, New York, NY, according to Institutional Review Board-approved protocols. Immunohistochemical analysis was performed on archived paraffin-embedded tissue sections (4m thick), as previously described [26].
Residual patient serum (n=34) as well as peritoneal (n=27) and pleural (n=23) fluid samples were obtained from the Clinical Laboratories at Memorial Sloan Kettering Cancer Center (MSKCC), New York, NY, after all diagnostic testing had been completed. Serum from healthy volunteers was provided from the Clinical Laboratory serum repository. Samples of serum and malignant pleural or peritoneal effusions were from patients with advanced (Stage IV) pancreatic cancer. All blood and body fluid samples were de-identified before inclusion in the study. This study was approved by the MSKCC Institutional Review Board for the Protection of Human Subjects.
sgC1qR analysis
sgC1qR was detected with a new in vitro assay developed for the quantitative determination of sgC1qR in plasma, serum, culture supernatants, and body fluids (Hycult Biotech, Uden, The Netherlands). The assay was performed according to manufacturer instructions. Briefly, ELISA plates/wells (Nunc, Thermo Scientific, Netherlands) coated with anti-gC1qR antibody (HM2014) and blocked for nonspecific binding with bovine serum albumin were washed, and incubated with recombinant gC1qR standards or samples, diluted in dilution buffer (PBS/0.1%BSA), for 1 hour at room temperature. After 4 washes, the wells were reacted with a biotinylated polyclonal anti-gC1qR antibody in dilution buffer for 1 hour at room temperature. After washing, the conjugate was allowed to react with streptavidin-conjugated Horse Radish Peroxidase (HRP). Color development was performed by addition of Tetramethylbenzidine (TMB) substrate. The reaction was stopped using an acidic buffer. The absorbance at 450nm was measured with a microplate reader. sgC1qR quantitation was achieved by comparing the cleavage of the TMB substrate by patient or control samples relative to a set of recombinant gC1qR standards ranging in concentration from 0–20 ng/ml.
Measurement of cancer biomarkers, CEA and CA19.9
Serum CEA and CA19.9 levels were determined in the Clinical Biochemistry Laboratory at MSKCC using a fluorometric enzyme immunoassay on the Tosoh AIA 2000 (Tosoh USA, Grove City, OH), as part of the patient’s clinical evaluation. Reference ranges for CEA (0–5 ng/ml) and CA19.9 (0–40 ng/ml) were established by the performing laboratory.
Data analysis
Mean and standard deviation were calculated for individual data sets. Statistical significance between data sets was analyzed by Mann-Whitney U test for unpaired data, or Student t-test for paired data. Significance was defined as p<0.05.
Results
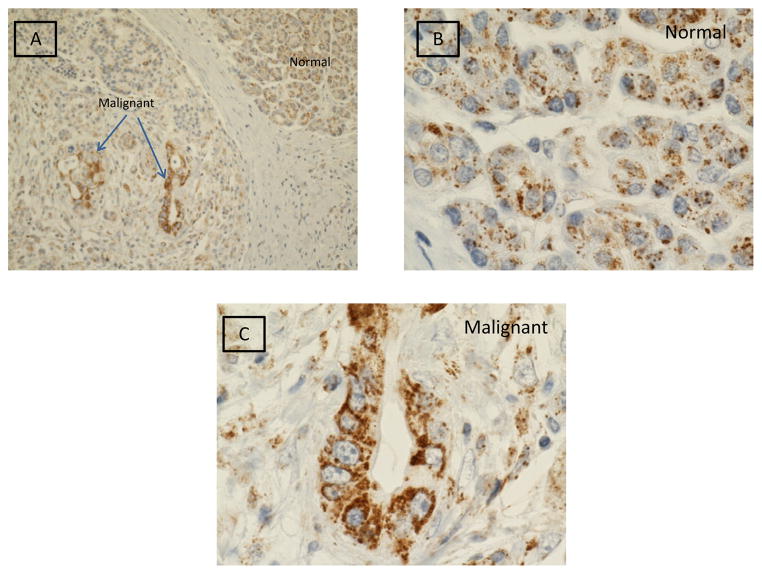
Figure 1 illustrates gC1qR expression in malignant and benign pancreatic tissue. Marked gC1qR expression is noted in malignant cells. Relative to benign cells, dense cytoplasmic gC1qR inclusions are seen by light microscopy in malignant cells of the exocrine pancreas. Both low (20x) and high (100x oil immersion) magnification views are shown.
Figure 1.
Immunohistochemical staining of gC1qR (brown stain) in malignant and normal cells of the exocrine pancreas. Light microscopic images obtained at low power (20x) (Panel A) and high power (100x oil immersion) (Panels B and C) are shown.
sgC1qR ELISA
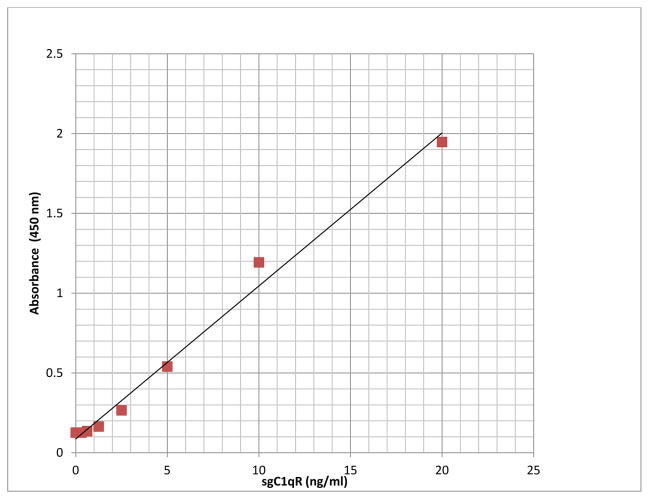
A newly developed sandwich ELISA assay was used to measure sgC1qR. The optimum combination of the capture (murine monoclonal antibody) and detection antibody (polyclonal rabbit anti-human gC1qR) was reached based on most optimal signal/noise ratio (data not shown). No signals were obtained in absence of capture or detection antibody (data not shown). The assay was linear between 0–20 ng/ml. Over this concentration range, the relationship between absorbance and sgC1qR concentration yielded a correlation coefficient of 0.99 (Figure 2). Using serum samples or normal plasma, anticoagulated with either EDTA or sodium citrate (blood collection tubes from Becton Dickinson, Franklin Lakes, NJ), to which known concentrations of recombinant gC1qR were added (spiked samples), gC1qR recovery was >95% with an intra- assay variability of 17 + 3%. Anticoagulation of blood with heparin produced false elevations in gC1qR quantitation and is therefore not recommended (data not shown). To minimize matrix effects, plasma/serum and body fluid samples were diluted 1:20 in the assay.
Figure 2.
Representative results of sgC1qR ELISA standard curve showing linear response between absorbance and sgC1qR concentration from 0–20 ng/ml.
sgC1qR levels in serum from healthy volunteers and patients with pancreatic cancer
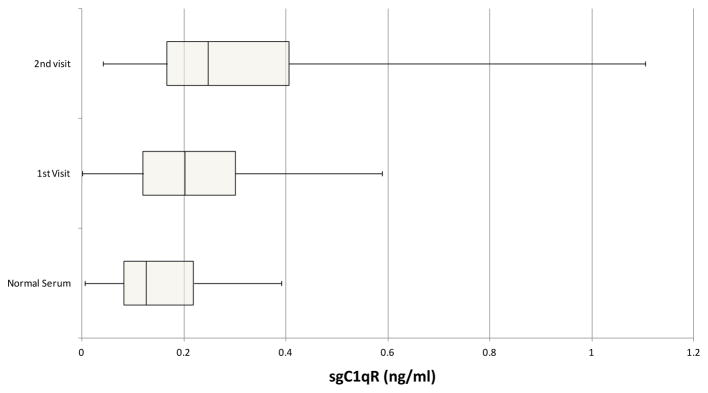
Serum samples for healthy volunteers and patients with stage IV pancreatic cancer were tested for sgC1qR. Patients (n=34) (male=19, female =15) ranged in age from 40–83 years. Healthy volunteers (n=20) (male=10, female =10) ranged in age from 35–70 years. sgC1qR was detected in serum of healthy volunteers, and increased levels (p =0.035) were found in patients with pancreatic cancer, although significant overlap was appreciated (Figure 3). Further increases in sgC1qR levels were noted with disease progression (p=0.005), in samples from patients obtained during a second visit, 3–12 months later.
Figure 3.
Comparison of sgC1qR in normal serum and serum from patients with pancreatic cancer obtained at two different visits 3–12 months apart, designated visit 1 and 2. Data are presented as box and whisker plots showing median sgC1qR levels and the interquartile range, representing the middle 50% of sgC1qR levels, framed by the box. The error bars (whiskers) represent sgC1qR values in the upper (25%) and lower (25%) quartiles.
To further evaluate the relationship between sgC1qR levels and progression of disease, we examined changes in the relationship between sgC1qR and tumor markers CA19.9 and CEA using serum sample pairs obtained 3–12 months apart. Although direct correlations between gC1qR and CA19.9 or CEA were not statistically significant, (r= 0.1056 and 0.1692, respectively), the relationship between changes (increase/decrease) over time between sgC1qR and tumor markers showed agreement for 75% of serum samples (12 of 16 serum sample pairs). Increases in sgC1qR levels, CEA, and CA19.9 levels were seen in 11 of 16 samples over time, whereas decreases in sgC1qR, CEA, and CA19.9 were noted for one serum pair. In the remaining 25% of sample pairs (4 of 16), tumor markers increased and sgC1qR levels decreased. The reason for the discordance is currently unknown.
sgC1qR in malignant effusions
sgC1qR levels were measurable also in malignant pleural and peritoneal paracentesis fluids (Table 1). Benign effusions were not available for comparison. Interestingly, sgC1qR levels appeared to be higher in malignant effusions than in serum.
Table 1.
Summary of sgC1qR levels in serum and body fluids of patients with pancreatic cancer
| Specimen Type | n | sgC1qR (ng/ml) (mean ± S.D.) |
|---|---|---|
| Normal Serum | 20 | 0.15 ± 0.10 |
| Patient Serum | 34 | 0.29 ± 0.22 |
| Pleural Fluid | 23 | 0.55 ± 0.44 |
| Peritoneal Fluid | 27 | 0.57 ± 0.38 |
Discussion
Inflammation, complement, and cancer are tightly linked, with complement playing both antagonistic and supportive roles in carcinogenesis [2]. gC1qR has been reported to contribute to inflammation by direct activation of the complement, kinin, and coagulation cascades [1]. Moreover, overexpression of gC1qR has been demonstrated in adenocarcinomas and in a range of epithelial cancers [25,26]. Data from several sources suggest a role for gC1qR as a potential therapeutic and diagnostic target [28,31–34]. For example, in a mouse model, changes in cellular gC1qR expression levels appear to coincide with specific stages of tumor progression [32], and in a study of 63 patients with breast cancer, gC1qR mRNA expression levels correlated with axillary lymph node metastasis and poor patient survival [34]. Similar observations were reported in patients with ovarian cancer, in whom gC1qR overexpression correlated with poor outcome [28].
Since sgC1qR has been reported in vitro in culture supernatants [21], and may play a role in angiogenesis and modulation of inflammation [1,2], the present study used a newly developed quantitative assay to measure sgC1qR in blood and body fluids. These results provide proof of principle for the presence of quantifiable sgC1qR in vivo.
Measurable sgC1qR levels were detected in the serum of healthy volunteers, and increased levels were found in the serum of patients with pancreatic cancer. Interestingly, changes in patient sgC1qR levels were observed with progression of disease, many of which paralleled changes in known tumor markers, such as CEA and CA19.9. In addition, sgC1qR was detected in malignant pleural and peritoneal effusions. Although the source of sgC1qR is not identified, these findings would support secretion of sgC1qR from cancer cells, as observed in in vitro studies [21]. However, sgC1qR secretion from inflammatory cells or apoptotic cells cannot be ruled out.
Whereas the current study provides proof of principle for the presence of sgC1qR in vivo in blood and body fluids, further studies are required to characterize sgC1qR expression in patients with cancer, and to understand its pathobiology. Limitations of the present study relate to the small sample size, and an inability to evaluate benign/infectious or inflammatory blood and body fluids. In addition, patient and control groups matched for comorbidities are required to determine the specificity of sgC1qR as a marker of malignancy or inflammation, particularly as in vitro evidence suggests gC1qR upregulation by inflammatory cytokines [35]. Results of the present study encourage further investigation of sgC1qR in vitro and in vivo. Since gC1qR antibodies used in the ELISA cross react with mouse and rat gC1qR, it is conceivable that the assay may be adapted for studies of sgC1qR in rodent models.
Acknowledgments
The authors are grateful to Adrienne Jarocki and Kajal Kothadia, Memorial Sloan Kettering Cancer Center and Jan van Groningen, Hycult Biotech, for technical assistance.
This work was supported in part by grants from the National Institutes of Health (RO1 AI 060866 and RO1 AI-084178)
References
- 1.Peerschke EI, Ghebrehiwet B. The contribution of gC1qR/p33 in infection and inflammation. Immunobiology. 2007;212:333–342. doi: 10.1016/j.imbio.2006.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Peerschke EI, Ghebrehiwet B. cC1qR/CR and gC1qR/p33: observations in cancer. Mol Immunol. 2014;61:100–109. doi: 10.1016/j.molimm.2014.06.011. [DOI] [PubMed] [Google Scholar]
- 3.Ghebrehiwet B, Lim BL, Peerschke EIB, Willis AC, Reid KB. Isolation, cDNA cloning, and overexpression of a 33-kDA cell surface glycoprotein that binds to the globular heads of C1q. J Exp Med. 1994;179:1809–1821. doi: 10.1084/jem.179.6.1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ghebrehiwet B, Peerschke EIB. Structure and function of gC1q-R a multiligand binding membrane protein. Immunobiol. 1998;199:225–238. doi: 10.1016/S0171-2985(98)80029-6. [DOI] [PubMed] [Google Scholar]
- 5.Ghebrehiwet B, Lim BL, Kumar R, Feng X, Peerschke EI. gC1qR/p33, a member of a new class of multifunctional and multicompartmental cellular proteins is involved in inflammation and infection. Immunol Rev. 2001;180:65–77. doi: 10.1034/j.1600-065x.2001.1800106.x. [DOI] [PubMed] [Google Scholar]
- 6.Muta T, Kang D, Kitajima S, Fujiwara T, Hamasaki N. p32 protein, a splicing factor 2-associated protein, is localized in mitochondrial matrix and is functionally important in maintaining oxidative phosphorylation. J Biol Chem. 1997;272:24363–24370. doi: 10.1074/jbc.272.39.24363. [DOI] [PubMed] [Google Scholar]
- 7.Dedio J, Jahnen-Dechent W, Bachmann M, Muller-Esterl W. The multiligand-binding protein gC1qR, putative C1q receptor, is a mitochondrial protein. J Immunol. 1998;160:3534–3542. [PubMed] [Google Scholar]
- 8.Matthews DA, Russell WC. Adenovirus core protein V interacts with p32-a protein which is associated with both the mitochondria and the nucleus. J Gen Virol. 1998;79:1677–1685. doi: 10.1099/0022-1317-79-7-1677. [DOI] [PubMed] [Google Scholar]
- 9.Majumdar M, Meenakshi J, Goswami SK, Datta K. Hyaluronan binding protein 1 (HABP1)/C1QBP/p32 is an endogenous substrate for MAP kinase and is translocated to the nucleus upon mitogenic stimulation. Biochem Biophys Res Commun. 2002;291:829–837. doi: 10.1006/bbrc.2002.6491. [DOI] [PubMed] [Google Scholar]
- 10.Braun L, Ghebrehiwet B, Cossart P. gC1qR/p32, a C1q binding protein is a novel receptor for Listeria monocytogenes. EMBO J. 2000;19:1458–1466. doi: 10.1093/emboj/19.7.1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kittelson DJ, Chianese-Bullock KA, Yao ZQ, Braciale TJ, Hahn YS. Interaction between complement receptor gC1qR and hepatitis C virus core protein inhibits T-lymphocyte proliferation. J Clin Invest. 2000;106:1239–1249. doi: 10.1172/JCI10323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Soltys BJ, Kang D, Gupta RS. Localization of p32 protein (gC1q-R) in mitochondria and at specific extramitochondrial locations in normal tissue. Histochem Cell Biol. 2000;114:245–255. doi: 10.1007/s004180000191. [DOI] [PubMed] [Google Scholar]
- 13.Mahdi F, Madar ZS, Figueroa CD, Schmaier AH. Factor XII interacts with multiprotein assembly of urokinase plasminogen activator receptor, gC1qR and cytokeratin 1 on endothelial cell membranes. Blood. 2002;99:3585–3596. doi: 10.1182/blood.v99.10.3585. [DOI] [PubMed] [Google Scholar]
- 14.Mahdi F, Shariat-Madar Z, Todd RF, III, Figueroa CD, Schmaier AH. Expression and localization of cytokeratin 1 and urokinase plasminogen activator receptor on endothelial cells. Blood. 2001;97:2342–2350. doi: 10.1182/blood.v97.8.2342. [DOI] [PubMed] [Google Scholar]
- 15.Kim KB, Yi JS, Nguyen N, Lee JH, Kwon YC, et al. Cell surface receptor for complement component C1q (gC1qR) is a key regulator for lamellipodia formation and cancer metastasis. J Biol Chem. 2011;286:23093–23101. doi: 10.1074/jbc.M111.233304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lynch NJ, Reid KB, van den Berg RH, Daha MR, Leigh LEA, et al. The murine homologues of gC1qBP, a 33 kDA protein that binds to the globular “heads” of C1q. FEBS Lett. 1997;418:111–114. doi: 10.1016/s0014-5793(97)01348-3. [DOI] [PubMed] [Google Scholar]
- 17.Lim BL, Reid KB, Ghebrehiwet B, Peerschke EI, Leigh LA, et al. The binding proteins for globular “heads” of complement C1q, gC1qR: Functional expression and characterization as a novel vitronectin binding factor. J Biol Chem. 1996;271:26739–26744. doi: 10.1074/jbc.271.43.26739. [DOI] [PubMed] [Google Scholar]
- 18.Herwald H, Dedio J, Kellner R, Loos M, Mueller-Esterl W. Isolation and characterization of the kininogen-binding protein p33 from endothelial cells. Identity with gC1q receptor. J Biol Chem. 1996;271:13040–13047. doi: 10.1074/jbc.271.22.13040. [DOI] [PubMed] [Google Scholar]
- 19.Joseph K, Ghebrehiwet B, Kaplan AP. Cytokeratin 1 and gC1qR mediate high molecular weight kininogen binding to endothelial cells. Clin Immunol. 1999;92:246–255. doi: 10.1006/clim.1999.4753. [DOI] [PubMed] [Google Scholar]
- 20.Joseph K, Ghebrehiwet B, Peerschke EI, Reid KB, Kaplan AP. Identification of the zinc-dependent endothelial cell binding protein for high molecular weight kininogen and factor XII: identify with the receptor which binds to the globular “heads” of C1q (gC1q-R) Proc Natl Acad Sci USA. 1996;93:8552–8557. doi: 10.1073/pnas.93.16.8552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peterson KL, Zhang W, Lu PD, Keilbaugh SA, Peerschke EI, et al. The C1q-binding membrane proteins cC1qR and gC1qR are released from activated cells. Subcellular localization and immunochemical characterization. Clin Immunol Immunopathol. 1997;84:17–26. doi: 10.1006/clin.1997.4374. [DOI] [PubMed] [Google Scholar]
- 22.Ghebrehiwet B, Cebada-Mora C, Tantral L, Jesty J, Peerschke EI. gC1qR/p33 serves as a molecular bridge between the complement and contact systems and is an important catalyst for inflammation. Adv Exp Med Biol. 2006;586:95–105. doi: 10.1007/0-387-34134-X_7. [DOI] [PubMed] [Google Scholar]
- 23.Fogal V, Richardson AD, Karmali PP, Scheffler IE, Smith JW, et al. Mitochondrial p32 protein is a critical regulator of tumor metabolism via maintenance of oxidative phosphorylation. Mol Cell Biol. 2010;30:1303–1318. doi: 10.1128/MCB.01101-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Feng X, Tonnesen MG, Peerschke EI, Ghebrehiwet B. Cooperation of C1q receptors and integrins in C1q-mediated endothelial cell adhesion and spreading. J Immunol. 2002;168:2441–2448. doi: 10.4049/jimmunol.168.5.2441. [DOI] [PubMed] [Google Scholar]
- 25.Rubinstein DB, Stortchevoi A, Boosalis M, Ashfaq R, Ghebrehiwet B, et al. Receptor for the globular heads of C1q (gC1q-R, p33, hyaluronan-binding protein) is preferentially expressed by adenocarcinoma cells. Int J Cancer. 2004;110:741–750. doi: 10.1002/ijc.20105. [DOI] [PubMed] [Google Scholar]
- 26.Dembitzer FR, Kinoshita Y, Burstein D, Phelps RG, Beasley MB, et al. gC1qR expression in normal and pathologic human tissues: differential expression in tissues of epithelial and mesenchymal origin. J Histochem Cytochem. 2012;60:467–474. doi: 10.1369/0022155412440882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Prakash M, Kale S, Ghosh I, Kundu GC, Datta K. Hyaluronan-binding protein I (HBPI/p32/gC1qR) induces melanoma cell migration and tumor growth in NF-kappa B dependent MMP-2 activation through integrin α(v)β(3) interaction. Cell Signal. 2011;23:1563–1577. doi: 10.1016/j.cellsig.2011.04.009. [DOI] [PubMed] [Google Scholar]
- 28.Yu G, Wang J. Significance of hyaluronan binding protein (HABP1/P32/gC1qR) expression in advanced serous ovarian cancer patients. Exp Mol Pathol. 2013;94:210–215. doi: 10.1016/j.yexmp.2012.06.007. [DOI] [PubMed] [Google Scholar]
- 29.Ghebrehiwet B, Ji Y, Valentino A, Pednekar L, Ramadass M, et al. Soluble gC1qR is an autocrine signal that induces B1R expression on endothelial cells. J Immunol. 2014;192:377–384. doi: 10.4049/jimmunol.1302031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Parenti A, Morbidelli L, Ledda F, Granger HJ, Ziche M. The bradykinin/B1 receptor promotes angiogenesis by up-regulation of endogenous FGF-2 in endothelium via the nitric oxide synthase pathway. FASEB J. 2001;15:1487–1489. [PubMed] [Google Scholar]
- 31.Fogal V, Shang L, Krajewski S, Ruoslahti E. Mitochondrial/cell surface protein p32/gC1qR as a molecular target in tumor cells and tumor stroma. Cancer Res. 2008;68:7210–7218. doi: 10.1158/0008-5472.CAN-07-6752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sanchez-Martin D, Cuesta AM, Fogal V, Ruoslahti E, Alvarex-Vallina L. The multicompartmental p32/gC1qR as a new target for antibody-based tumor targeting strategies. J Biol Chem. 2011;286:5197–5203. doi: 10.1074/jbc.M110.161927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ghosh I, Chowdhury AR, Rajeswari MR, Datta K. Differential expression of hyaluronic acid binding protein 1 (HABP1)/P32/C1QBP during progression of epidermal carcinoma. Mol Cell Biochem. 2004;267:133–139. doi: 10.1023/b:mcbi.0000049362.04033.ea. [DOI] [PubMed] [Google Scholar]
- 34.Chem YB, Jiang CT, Shang GQ, Wang JS, Pang D. Increased expression of hyaluronic acid binding protein 1 is correlated with poor prognosis in patients with breast cancer. J Surg Oncol. 2009;100:382–386. doi: 10.1002/jso.21329. [DOI] [PubMed] [Google Scholar]
- 35.Guo WX, Ghebrehiwet B, Weksler B, Schweitzer K, Peerschke EIB. Up-regulation of endothelial cell binding proteins/receptors for complement component C1q by inflammatory cytokines. J Lab Clin Med. 1999;133:541–550. doi: 10.1016/s0022-2143(99)90183-x. [DOI] [PubMed] [Google Scholar]