Abstract
The fat mass and obesity associated (FTO) gene plays an important role in adipogenesis. However, its function during porcine intramuscular preadipocyte proliferation and differentiation remains poorly understood. In this study, we prepared the antiserum against porcine FTO (pFTO), which was used to determine its subcellular localization and tissue distribution. Our data indicated that pFTO was localized predominantly in the nucleus. Real-time quantitative PCR and western blot analysis showed that pFTO was highly expressed in the lung and subcutaneous adipose tissue. Overexpression of pFTO in porcine intramuscular preadipocytes significantly promoted cell proliferation and lipid deposition. Furthermore, overexpression of pFTO in differentiating porcine intramuscular preadipocytes also significantly increased the mRNA levels of adipocyte differentiation transcription factors peroxisome proliferators-activated receptor γ (PPARγ), CCAAT/enhancer binding protein α (C/EBPα), lipoprotein lipase (LPL) and fatty acid synthase (FAS). Our findings provide the first functional evidence to reveal a role of pFTO in porcine intramuscular preadipocyte proliferation and differentiation.
Introduction
Pork quality is becoming a major concern for the swine industry due to the development of export markets and increased consumer demands [1]. Intramuscular fat (IMF) is one of the important factors influencing meat quality, which shows a positive correlation with the meat quality traits such as the flavor, tenderness, juiciness and water holding capacity [2, 3]. IMF content could be modulated by some key genes [4]. Thus, it is necessary to identify candidate genes related to IMF content in the pig.
In 2007, the fat mass and obesity associated (FTO) gene was discovered and found to be significantly associated with both children and adults obesity [5]. A genome-wide study showed that the FTO gene was located on procine chromosome 6 [6], which probably hosts genes related to meat quality traits including IMF content [7, 8]. In addition, the porcine FTO (pFTO) gene has been widely reported to be associated with some fat-related traits [9–17], including IMF deposition, IMF content or total lipid percentage in muscle in pigs.
In this study, we prepared the polyclonal antibody against pFTO and detected its tissue distribution and localization. We also investigated the role of pFTO in porcine intramuscular predipocytes proliferation and differentiation.
Materials and Methods
Ethics statement
This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of Sichuan Agricultural University. The protocol was approved by the Animal Care Advisory Committee of Sichuan Agricultural University under permit No. YYS130125.
Antibody production and identification
Polyclonal antibody against pFTO was prepared as described previously [18]. Initially, 300 μg of the purified recombinant pFTO protein [19] was multi-point injected (subcutaneous) into the Kunming mouse after being emulsified with complete Freund’s complete adjuvant. After 2 weeks, the booster injection was administered with the same amount of antigen emulsified incomplete Freund’s adjuvant. The booster injections were repeated three times in a 10-day interval, and the antiserum was collected at 7 days after the final booster injection. ELISA analysis was performed according to methods described by [18].
Tissue sample collection
Three 10-week-old female Duroc × Yorkshire × Landrace (DLY) pigs (31–31.6 kg body weight) were slaughtered in a humane manner according to protocols approved by the Animal Care Advisory Committee of Sichuan Agricultural University (Permit Number: YYS130125). The tissue samples of liver, heart, skeletal muscle, lung, kidney, spleen, and subcutaneous adipose were removed and immediately snap-frozen in liquid nitrogen before being stored at -80°C until use.
Plasmid construction
The DNA fragment containing the entire open reading frame of pFTO was amplified using primers FTO-PCDNA3.1(+)-Hin-F (5’- CCCAAGCTTATGAAGCGAACCCCAACCGCCG-3’) and FTO-PCDNA3.1(+)-Xho-R (5’-CCGCTCGAGCTAGGGTTTGGCTTCCAGAAGC-3’) and the plasmid pET30a(+)-pFTO [19] as a template. The FTO-PCDNA3.1(+)-Hin-F and FTO-PCDNA3.1(+)-Xho-R primers introduced HindIII and XhoI restriction sites (underlined), respectively. The purified PCR product was digested with HindIII and XhoI and ligated into pcDNA3.1(+) digested with the same restriction enzymes. The recombinant construct was transformed into E. coli DH5a strain and kanamycin resistant colonies were selected. Proper construction was identified by colony PCR and DNA sequencing and was designated as pcDNA3.1(+)-pFTO.
Cell isolation and culture
Porcine intramuscular preadipocytes were isolated from longissimus lumborum muscle of DLY piglets less than 3 days old. The pig obtained from Sichuan Zhengyuan swine industry Co., Ltd. (Chengdu, Sichuan, China) was slaughtered according to protocols approved by the Animal Care Advisory Committee of Sichuan Agricultural University. Briefly, approximately 1 g of the muscle was removed aseptically and washed in phosphate buffered saline (PBS) under sterile conditions. The muscle tissue was cut into pieces approximately 1 mm3 in size using ophthalmic scissors, transferred to 50 mL centrifuge tubes, and digested with 0.2% collagenase II (Sigma) for 2 h at 37°C in a water bath. The digested solution was filtered through 200 and 400-mesh cell sieves. The filtered solution was collected and centrifuged at 1,000 rpm for 10 min. The pellet was resuspended in DMEM/F12 medium supplemented with 15% fetal bovine serum (FBS) (Gibco), 100 U/mL penicillin, and 100 μg/mL streptomycin. Cells were maintained at 37°C under 5% CO2 in a humidified incubator. The medium containing unattached cells was discarded after 6 h and fresh culture medium was added. The culture medium was renewed every 2 days. The cells (passages 1–3) were identified by immunofluorescence with anti-DLK1/Pref-1 antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA).
Cell differentiation was initiated as before [20]. Briefly, two days after contact inhibition, the cells were induced to differentiation with the hormone cocktail [0.5 mM 3-isobutyl-1-methylxanthine (IBMX), 1 μM dexamethasone (DEX), 10 μg/mL insulin] for 2 days. The medium was then shifted to medium containing 10% FBS and 10 μg/mL insulin for 2 days, followed by replaced with DMEM/F12 supplemented with 10% FBS for the remaining culture period. The medium was replaced every 2 days.
pFTO overexpression
Porcine intramuscular preadipocytes were seeded in 24-well plates and transfected with 0.5 μg of the plasmid pcDNA3.1(+)-pFTO or the empty vector pcDNA3.1(+) using Lipofectamine 2000 (Invitrogen) according to the manufacturer’s instruction.
RNA isolation and reverse transcription
Total RNA was isolated from the adherent cultured porcine intramuscular preadipocytes and collected tissue samples using RNAiso Plus reagent (TaKaRa, Dalian, China). The concentrations of RNA were determined using a Beckman DU-800 spectrophotometer (Beckman Coulter, Fullerton, CA, USA). One microgram of total RNA was transcribed into single-stranded cDNA using PrimeScript® RT reagent Kit with gDNA Eraser (TaKaRa).
Real-time quantitative PCR
Real-time quantitative PCR was performed using an ABI 7900HT Real-time PCR system (384-cell standard block). The gene specific primers (Sangon Biotech, Shanghai, China) used are listed in Table 1. The PCR cycling conditions used were: 45 cycles at 95°C for 15 s and 60°C for 30 s. Data analysis was performed using the comparative Ct method [21] with β-actin as an endogenous control.
Table 1. List of genes, primer sequences, GenBank accession numbers, and product sizes in this study.
| Gene name | Primer | Sequence | GenBank accession no. | Product size (bp) |
|---|---|---|---|---|
| FTO | Forward | 5'-ACCTGAAAGAGGAGCCCTAC-3' | KM232950 | 170 |
| Reverse | 5'-CCACACATCGGGATCTCTG-3' | |||
| PPARγ | Forward | 5'-AATTAGATGACAGCGACCTGGCGA-3' | NM_214379 | 102 |
| Reverse | 5'-TGTCTTGAATGTCCTCGATGGGCT-3' | |||
| C/EBPα | Forward | 5'-CGTGGAGACTCAACAGAAGG-3' | AF103944 | 95 |
| Reverse | 5'-GCAGCGTGTCCAGTTCGCGG-3' | |||
| LPL | Forward | 5'-ACCGTTGCAACAACTTGGGCTATG-3' | NM_214286 | 98 |
| Reverse | 5'-ACTTTGTAGGGCATCTGAGCACGA-3' | |||
| β-actin | Forward | 5'-CCACGAAACTACCTTCAACTCC-3' | DQ845171 | 132 |
| Reverse | 5'-GTGATCTCCTTCTGCATCCTGT-3' |
Western blot analysis
Western blot analysis was performed as described by [22]. Briefly, proteins were transferred to a nitrocellulose membrane (Beyotime) after electrophoresis, and then the membrane were blocked with 3% non-fat milk in TBST. The membrane was incubated with the prepared mouse anti-pFTO polyclonal antibody followed by horseradish peroxidase (HRP)-conjugated goat anti-mouse IgG. The signals were visualized by using SuperSignal West Pico Chemiluminescent Substrate (Pierce) according to the manufacturer’s instructions.
Immunofluorescence
Porcine intramuscular preadipocytes were cultured in 24-well plates. After washing twice with PBS buffer, cells were fixed with 4% paraformaldehyde in PBS for 15 min. Cells were then washed three times with PBS and permeabilized with 0.5% Triton X-100 in PBS for 20 min. After washing three times with PBS, cells were incubated with immunol staining block buffer (Beyotime) for 30 min and incubated at 4°C overnight with the anti-DLK1/Pref-1 antibody (1:100) or the prepared anti-pFTO polyclonal antibody (1:100). Cells were washed three times with PBS and subsequently incubated with the FITC-conjugated secondary antibody (1:200, Santa Cruz) in the dark for 1 h at 37°C. To detect the cell nucleus, specimens were further incubated with 4’,6’-diamidino-2-phenylindole (DAPI) (Beyotime) for 10 min. Background staining was removed by washing three times with PBS. Images were collected on a Nikon Eclipse TS100 inverted fluorescence microscope.
EdU proliferation assay
EdU (5-ethynyl-2'-deoxyuridine), a nucleoside analog of thymidine, is incorporated into DNA during active DNA synthesis only by proliferating cells. Proliferating porcine intramuscular preadipocytes were determined by using the Click-iT EdU Alexa Fluor 594 imaging kit (Invitrogen) according to the manufacturer’s instruction. Briefly, porcine intramuscular preadipocytes were incubated with 10 μM EdU for 6 h before fixation, permeabilization, and EdU staining. Cell nuclei were stained with Hoechst 33342 (Invitrogen) at a concentration of 5 μg/mL for 30 min.
Oil red O staining
On day 8 of differentiation, the lipid droplets present in porcine intramuscular preadipocytes were stained with the Oil red O staining kit (GENMED Scientifics INC. USA) as before [20]. Briefly, cells were washed with PBS and fixed with 4% formaldehyde in PBS for 1 h. Then the cells were washed with 60% propan-1-ol and stained with Oil red O solution for 20 min, followed by rinsed with distilled water before being destained in 100% propan-1-ol for 15 min.
Triglyceride content Assay
Triglyceride (TG) content was analyzed using triacylglycerol detection kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) according to the manufacturer’s protocol. Briefly, the differentiated porcine intramuscular preadipocytes were washed with PBS and subjected to sonication for rupture. BCA protein assay kit (Pierce) was used to measure the protein concentration of disrupted cells. The TG content was expressed in mmol/g protein.
Statistical analysis
All data were expressed as mean ± SE (standard error). Data analyses were performed using SPSS11.5 software. Group differences were analyzed by One-way ANOVA followed by Tukey test. P < 0.05 was considered statistically significant.
Results
Titre and specificity of polyclonal antibody
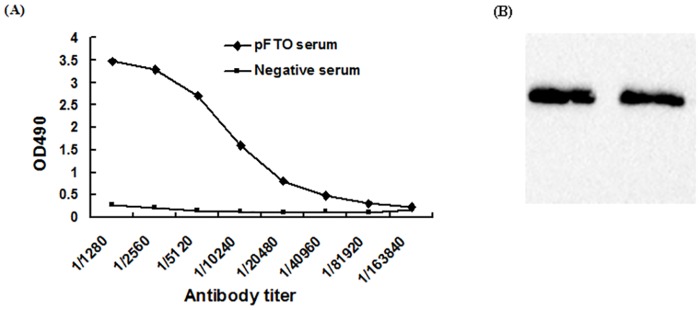
FTO antiserum was diluted to different concentrations (1:1280–1:163840) and was detected by ELISA. The titre of the antiserum in ELISA test was 1:128,000 (Fig 1A), which was 2.1-fold greater than pre-immunization serum [23]. Western blot analysis revealed the antiserum obtained was specific to its immunogen, recombinant pFTO (Fig 1B).
Fig 1. Identification of the polyclonal antibody against pFTO.
(A) ELISA of the antiserum against pFTO; (B) Western blot analysis of anti-FTO antibody. Lanes 1 and 2: the purified pFTO protein detected by polyclonal antibody against pFTO.
Tissue distribution of pFTO
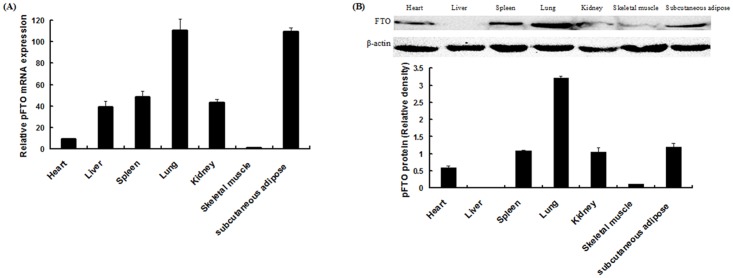
Expression of pFTO was assessed by real-time quantitative PCR and western blot analysis in various porcine tissues. As shown in Fig 2A, pFTO mRNA was most abundant in the lung and subcutaneous adipose, followed by the spleen, kidney, liver, heart and skeletal muscle. As shown in Fig 2B, pFTO protein was most abundant in the lung, followed by the subcutaneous adipose, spleen, heart and kidney, and to a lesser extent in the skeletal muscle. No pFTO protein was detected in the liver (Fig 2B).
Fig 2. Tissue distributions of pFTO.
(A) Relative expression levels of pFTO mRNA in different tissues. (B) Western blot analysis of pFTO protein levels in different tissues. The amount of pFTO was normalized to the amount of β-actin. Bars presented the means ± SE (n = 3).
Identification of porcine intramuscular preadipocytes
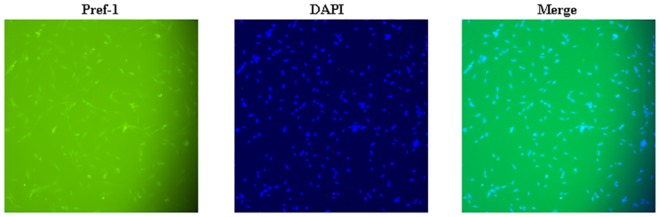
Immunofluorescence staining with an anti-Pref-1 antibody showed that the cells shared about 100% positive reaction for Pref-1 (Fig 3), suggesting that the isolated cells were indeed porcine intramuscular preadipocytes.
Fig 3. Identification of porcine intramuscular preadipocytes.
Porcine intramuscular preadipocytes were characterized by Pref-1 immunofluorescent staining (100×).
Subcellular localization analysis of pFTO
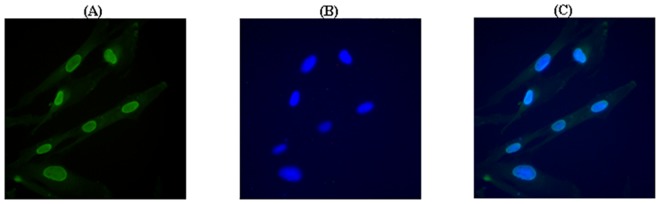
We used the polyclonal pFTO antiserum to investigate its subcellular localization in porcine intramuscular preadipocytes by indirect fluorescent immunocytochemistry. Cell nuclei were visualized by DAPI staining. As shown in Fig 4, the pFTO protein was localized predominantly in the nucleus.
Fig 4. Subcellular localization of pFTO in porcine intramuscular preadipocytes using an indirect fluorescent immunocytochemical technique.
(A) Endogenous pFTO protein in porcine intramuscular preadipocytes stained first with the anti-pFTO polyclonal antibody and then with FITC-conjugated goat anti-mouse IgG (green). (B) The nucleus marked with DAPI (blue). (C) The merged image.
Effect of pFTO on porcine intramuscular predipocytes proliferation
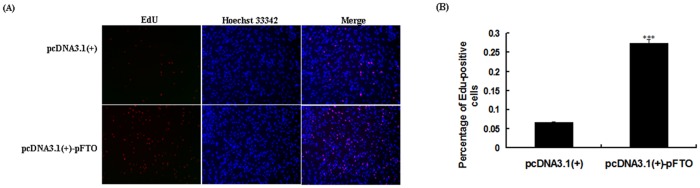
To examine the effect of pFTO on porcine intramuscular preadipocytes proliferation, the pcDNA3.1(+)-pFTO plasmid was transfected into porcine intramuscular preadipocytes. As shown in Fig 5, compared with the control group, overexpression of pFTO significantly accelerated proliferation of porcine intramuscular preadipocytes (P < 0.001).
Fig 5. Effect of pFTO on the proliferation of porcine intramuscular preadipocytes.
Porcine intramuscular preadipocytes were seeded in a 24-well plate at a density of 1 × 104 cells/well. When the cells reached about 50% confluence, 0.5 μg of the plasmid pcDNA3.1(+)-pFTO or the empty vector pcDNA3.1(+) was transfected. (A) Cell proliferation was evaluated by EdU proliferation assay after 24 h of transfection. The Click-it reaction revealed EdU staining (red). The cell nuclei were stained with Hoechst 33342 (blue). (B) The percentage of EdU-positive cells was quantified. Results are presented as mean ± SE (n = 8). ***P < 0.001 as compared with the control group.
Expression profile of pFTO mRNA in differentiating porcine intramuscular preadipocytes
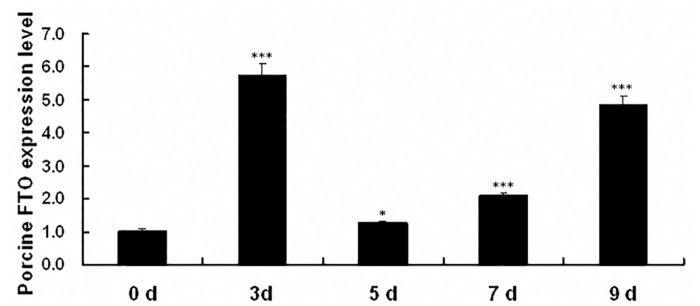
To investigate the role of pFTO in fat deposit, its transcription profile during adipogenic differentiation was first analyzed using porcine intramuscular preadipocytes. Primary porcine intramuscular preadipocytes were induced to differentiate for 9 days. As shown in Fig 6, the mRNA expression of pFTO was initial upregulated with the highest fold upregulation at day 3. The pFTO mRNA expression then declined, but was observed to be upregulated subsequently at day 7 and persisted for 9 days.
Fig 6. Expression pattern of pFTO mRNA during porcine intramuscular preadipocytes differentiation.
RNA was extracted from the differentiating porcine intramuscular preadipocytes on the days 0, 3, 5, 7 and 9. pFTO mRNA expression was analyzed by real-time quantitative PCR. Data were the mean and SE from three independent experiments. *P < 0.05, ***P < 0.001.
Effect of pFTO on differentiation of porcine intramuscular preadipocytes
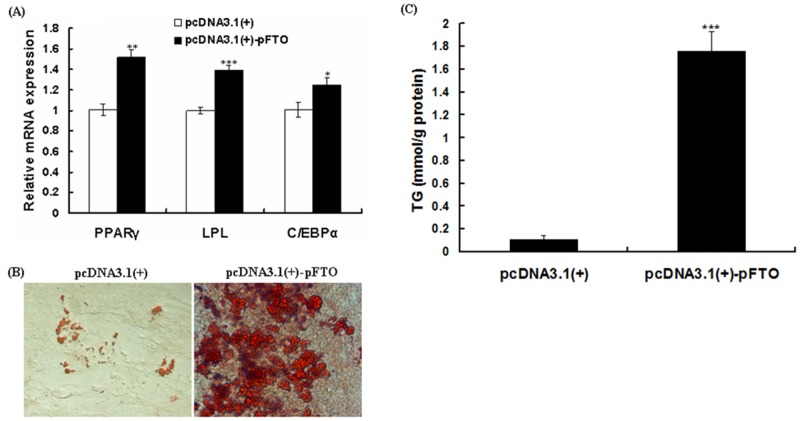
To test if pFTO regulates adipogenic differentiation of porcine intramuscular preadipocytes, pFTO was overexpressed by transfection of pcDNA3.1(+)-pFTO plasmid and then the cells were induced to differentiate for 8 days. Overexpression of pFTO increased the expression of the peroxisome proliferators-activated receptor γ (PPARγ), CCAAT/enhancer binding protein α (C/EBPα), fatty acid synthase (FAS) and lipoprotein lipase (LPL) (Fig 7A), suggesting that upregulation of pFTO was necessary for adipogenic differentiation. Meanwhile, Oil red O staining (Fig 7B) and TG content analyses (Fig 7C) showed that overexpression of pFTO significantly increased the lipid droplets numbers and larger droplet sizes.
Fig 7. Overexpression of pFTO promotes adipogenesis and lipid accumulation.
Porcine intramuscular preadipocytes were transfected with 0.5 μg of the recombinant plasmid pcDNA3.1(+)-pFTO or the empty vector pcDNA3.1(+) at the density of 90%. Cell differentiation was initiated as described in materials and methods section. (A) mRNA expression levels of PPARγ, C/EBPα, LPL and FAS were detected by real-time quantitative PCR at day 8 of differentiation. The results are represented as mean ± SE (n = 3). *P < 0.05, **P < 0.01, ***P < 0.001. (B) Intracellular lipid was stained with Oil red O at day 8 of differentiation (×400). (C) TG content was determined at day 8 of differentiation. Values are mean ± SE (n = 3). ***P < 0.001.
Discussion
In this study, we used the purified recombinant pFTO protein to immune Kunming mice and produced anti-FTO antiserum. The polyclonal antibody against the pFTO protein binded specifically to pFTO, which indicated that the antibody was specific and sensitive as a tool for further study the subcellular localization and physiological function of pFTO. Using indirect fluorescent immunocytochemistry, here we found that the pFTO protein was localized predominantly to the nucleus of porcine intramuscular preadipocytes, consistent with previous reports [24–26]. FTO is ubiquitously expressed in different tissue of mammals, with highest levels in brain and hypothalamus [24]. Although much attention has been paid to the role of FTO in brain/hypothalamus [24, 27], studies on its other functions are emerging [28, 29]. In this study, we examined the FTO expression at the mRNA and protein levels in porcine different tissues. We found that the mRNA and protein levels of FTO were most abundant in the lung and subcutaneous adipose of DLY pigs. Lung is an important site for priming immune protection. The high level of FTO expression found in lung suggested that FTO might play a role in protective immunity. Not surprisingly, there is a study has confirmed the relationship between FTO and immune-related infectious diseases [29]. Adipose tissue is regarded as a principle site for lipid storage and also functions as an endocrine organ with an important role in adipogenesis [26, 30–33]. In Suzhong pigs, FTO mRNA and protein expression in backfat was significantly higher than that in other tissue [14, 15]. Together, these studies indicated that FTO might play an important role in fat deposition and would be one of the associate genes affecting meat quality traits [11].
In this study, the expression profile of pFTO mRNA in differentiating porcine intramuscular preadipocytes was examined. We found that the mRNA expression of pFTO was significantly upregulated during porcine intramuscular preadipocytes differentiation. The dramatic upregulation of pFTO mRNA expression in differentiating porcine intramuscular preadipocytes was biphasic. Our result was not consistent with previous findings in 3T3-L1 preadipocytes [26, 31]. Zhao et al. found that the FTO protein expression decreased during 3T3-L1 preadipocytes differentiation [31]. Zhang et al. showed that the FTO mRNA expression was not significant changed during 3T3-L1 preadipocytes differentiation [26].
IMF is the last fat to be deposited within the animal. Intramuscular (IM) adipocytes proliferation and differentiation display specific biological features. The lipid metabolism and secretory function of IM adipocytes differ from that of nonmuscular adipocytes [34]. Therefore, this study was undertaken to investigate the effect of porcine FTO on the differentiation of IM adipocytes isolated from porcine skeletal muscle. In this study, we demonstrated that pFTO can enhance porcine preadipocyte proliferation. Adipogenesis is well-controlled by the sequential expression of various transcription factors [35]. PPARγ and C/EBPα have been considered as hallmarks of adipogenesis [36, 37]. Moreover, LPL, secreted by adipocytes and other tissues, is a key enzyme that plays a pivotal role in lipid metabolism by regulating lipid accumulation [38]. In the present study, pFTO overexpression significantly increased mRNA levels of PPARγ, C/EBPα and LPL in differentiating porcine intramuscular preadipocytes, and promoted the adipogenesis as demonstrated by Oil Red O staining and triglyceride content measurements. These results are consistent with the findings of previous in vitro and in vivo studies [26, 31–33]. In vitro studies using FTO depletion or FTO overexpression in 3T3-L1 preadipocytes or mouse embryonic fibroblasts have revealed that FTO was associated with the mRNA levels of adipogenesis related genes such as PPARγ, C/EBPα [26, 32, 33]. In vivo studies using FTO overexpression have also observed the same result [32]. Furthermore, knockdown of FTO significantly impaired adipogenic capacity as demonstrated by Oil Red O staining and triglyceride content measurements [26, 31–33]. Taken together, these results suggested that pFTO promoted porcine intramuscular preadipocytes proliferation and differentiation.
In summary, we report the production of highly specific anti-pFTO polyclonal antibody and the nuclear localization of endogenous pFTO in porcine intramuscular preadipocytes. Further, we analyzed the expression patterns of the pFTO in various porcine tissues by real-time quantitative PCR and western blot analysis. We also provide the first evidence that pFTO can enhance the porcine intramuscular preadipocytes proliferation and differentiation. Therefore, pFTO may be an important and indispensable regulator target to control the body fat deposition in pigs or to treat/prevent obesity-related disorders in humans. Additional research needs to be conducted to explore signaling pathways of pFTO that regulates adipogenic differentiation in porcine intramuscular preadipocytes.
Acknowledgments
We would like to thank the staff at our laboratory for the assistance they have provided in this study.
Data Availability
All relevant data are within the paper.
Funding Statement
This work was supported by the National Natural Science Foundation of China (No. 31201811) (XC) and the Specific Research Supporting Program for Discipline Construction at Sichuan Agricultural University (ZH). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.van Wijk HJ, Dibbits B, Baron EE, Brings AD, Harlizius B, Groenen MA, et al. Identification of quantitative trait loci for carcass composition and pork quality traits in a commercial finishing cross. J Anim Sci. 2006;84: 789–799. [DOI] [PubMed] [Google Scholar]
- 2.Fernandez X, Monin G, Talmant A, Mourot J, Lebret B. Influence of intramuscular fat content on the quality of pig meat-1. Composition of the lipid fraction and sensory characteristics of m. longissimus lumborum. Meat Sci. 1999;53: 59–65. [DOI] [PubMed] [Google Scholar]
- 3.Gerbens F, Verburg FJ, Van Moerkerk HT, Engel B, Buist W, Veerkamp JH, et al. Associations of heart and adipocyte fatty acid binding protein gene expression with intramuscular fat content in pigs. J Anim Sci. 2001;79: 347–354. [DOI] [PubMed] [Google Scholar]
- 4.Janss LLG, Van Arendonk JAM, Brascamp EW. Bayesian statistical analyses for presence of single genes affecting meat quality traits in a crossed pig population. Genetics. 1997;145: 395–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Frayling TM, Timpson NJ, Weedon MN, Zeggini E, Freathy RM, Lindgren CM, et al. A common variant in the FTO gene is associated with body mass index and predisposes to childhood and adult obesity. Science. 2007;316: 889–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Du ZQ, Fan B, Zhao X, Amoako R, Rothschild MF. Association analyses between type 2 diabetes genes and obesity traits in pigs. Obesity. 2009;17: 323–329. 10.1038/oby.2008.557 [DOI] [PubMed] [Google Scholar]
- 7.Ovilo C, Pérez-Enciso M, Barragán C, Clop A, Rodríguez C, Oliver MA, et al. A QTL for intramuscular fat and backfat thickness is located on porcine chromosome 6. Mamm Genome. 2000;11: 344–346. [DOI] [PubMed] [Google Scholar]
- 8.Hu ZL, Reecy JM. Animal QTLdb: beyond a repository. Mamm Genome. 2007;18: 1–4. [DOI] [PubMed] [Google Scholar]
- 9.Fan B, Du ZQ, Rothschild MF. The fat mass and obesity-associated (FTO) gene is associated with intramuscular fat content and growth rate in the pig. Anim Biotechnol. 2009;20: 58–70. 10.1080/10495390902800792 [DOI] [PubMed] [Google Scholar]
- 10.Zhang LF, Miao XT, Hua XC, Jiang XL, Lu YP, Xu NY. Polymorphism in 5' regulatory region of the porcine fat mass and obesity associated (FTO) gene is associated with intramuscular fat content in a Jinhua × Pietrain F2 reference population. J Anim Vet Adv. 2009;8: 2329–2334. [Google Scholar]
- 11.Fontanesi L, Scotti E, Buttazzoni L, Davoli R, Russo V. The porcine fat mass and obesity associated (FTO) gene is associated with fat deposition in Italian Duroc pigs. Anim Genet. 2009;40: 90–93. 10.1111/j.1365-2052.2008.01777.x [DOI] [PubMed] [Google Scholar]
- 12.Dvořáková V, Bartenschlager H, Stratil A, Horák P, Stupka R, Čítek J, et al. Association between polymorphism in the FTO gene and growth and carcass traits in pig crosses. Genet Sel Evol. 2012;44: 13 10.1186/1297-9686-44-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Szydlowski M, Salamon S, Grzes M, Switonski M. SNP in the 5' flanking region of the pig FTO gene is associated with fatness in Polish Landrace. Livest Sci. 2012;150: 397–400. [Google Scholar]
- 14.Fu YF, Li L, Ren SW. Effect of FTO expression and polymorphism on fat deposition in Suzhong pigs. Asian Australas J Anim Sci. 2013a;26: 1365–1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fu YF, Li L, Wang XM, Li BX, Fang XM, Zhao F, et al. The mRNA expression, cloning and sequencing of FTO gene and bioinformatics analysis in Suzhong pig. Acta Agriculturae Boreali-Sinica. 2013b;28: 128–134. [Google Scholar]
- 16.Tao X, Men XM, Deng B, Xu ZW. Effects of breed, postnatal development, and nutrition on mRNA expression of the FTO gene in porcine muscle and its relationship with intramuscular fat deposition. Czech J Anim Sci. 2013;58: 381–388. [Google Scholar]
- 17.Dina C, Meyre D, Gallian S, Durand E, Körner A, Jacobson P, et al. Variation in FTO contributes to childhood obesity and severe adult obesity. Nat Genet. 2007;39: 724–726. [DOI] [PubMed] [Google Scholar]
- 18.Chen XL, Wang H, Huang ZQ, Zhou B, Jia G, Liu GM, et al. Fusion expression of porcine phosphotyrosine interaction domain containing 1 gene and preparation, characterization of its polyclonal antibody. Chinese J Anim Nutr. 2014;26: 3349–3355. [Google Scholar]
- 19.Zhou B, Chen XL, Huang ZQ, Jia G, Liu GM. Heterologous expression and purification of porcine fat mass and obesity-associated gene in Escherichia coli. Turk J Biol. 2015;39: 217–222. [Google Scholar]
- 20.Chen XL, Huang ZQ, Wang H, Jia G, Liu GM, Zhao H, et al. Porcine phosphotyrosine interaction domain containing 1 modulates 3T3-L1 preadipocyte proliferation and differentiation. Biologia. 2013;68: 1010–1014. [Google Scholar]
- 21.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2–ΔΔCt method. Methods. 2001;25: 402–408. [DOI] [PubMed] [Google Scholar]
- 22.Wang H, Chen XL, Huang ZQ, Zhou B, Jia G, Liu GM, et al. Expression and purification of porcine PID1 gene in Escherichia coli. Turk J Biol. 2014;38: 523–527. [Google Scholar]
- 23.Liu XJ, Wu Y, Li MC, Chen S, Zhou YC. Production of polyclonal antibody against interleukin-33 and assessment to its distribution in murine liver and lung. J Biomed Biotechnol. 2009;2009: 729197 10.1155/2009/729197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gerken T, Girard CA, Tung YCL, Webby CJ, Saudek V, Hewitson KS, et al. The obesity-associated FTO gene encodes a 2-oxoglutarate-dependent nucleic acid demethylase. Science. 2007;318: 1469–1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gulati P, Cheung MK, Antrobus R, Church CD, Harding HP, Tung YC, et al. Role for the obesity-related FTO gene in the cellular sensing of amino acids. Proc Natl Acad Sci USA. 2013;110: 2557–2562. 10.1073/pnas.1222796110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang M, Zhang Y, Ma J, Guo F, Cao Q, Zhang Y, et al. The demethylase activity of fto (fat mass and obesity associated protein) is required for preadipocyte differentiation. PLoS One. 2015;10: e0133788 10.1371/journal.pone.0133788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McTaggart JS, Lee S, Iberl M, Church C, Cox RD, Ashcroft FM. FTO is expressed in neurones throughout the brain and its expression is unaltered by fasting. PLoS One. 2011;6: e27968 10.1371/journal.pone.0027968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gan XT, Zhao G, Huang CX, Rowe AC, Purdham DM, Karmazyn M. Identification of fat mass and obesity associated (FTO) protein expression in cardiomyocytes: regulation by leptin and its contribution to leptin-induced hypertrophy. PLoS One. 2013;8: e74235 10.1371/journal.pone.0074235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Feng Y, Wang FL, Pan HQ, Qiu SS, Lü JQ, Wu L, et al. Obesity- associated gene FTO rs9939609 polymorphism in relation to the risk of tuberculosis. BMC Infect Dis. 2014;14: 592 10.1186/s12879-014-0592-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kershaw EE, Flier JS. Adipose tissue as an endocrine organ. J Clin Endocrinol Metab. 2004;89: 2548–2556. [DOI] [PubMed] [Google Scholar]
- 31.Zhao X, Yang Y, Sun BF, Shi Y, Yang X, Xiao W, et al. FTO-dependent demethylation of N6-methyladenosine regulates mRNA splicing and is required for adipogenesis. Cell Res. 2014;24: 1403–1419. 10.1038/cr.2014.151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Merkestein M, Laber S, McMurray FF, Andrew D, Sachse G, Sanderson J, et al. FTO influences adipogenesis by regulating mitoticclonal expansion. Nat Commun. 2015;6: 6792 10.1038/ncomms7792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang XX, Zhu LN, Chen JQ, Wang YZ. mRNA m6A methylation downregulates adipogenesis in porcine adipocytes. Biochem Biophys Res Commun. 2015;459: 201–207. 10.1016/j.bbrc.2015.02.048 [DOI] [PubMed] [Google Scholar]
- 34.Gardan D, Gondret F, Louveau I. Lipid metabolism and secretory function of porcine intramuscular adipocytes compared with subcutaneous and perirenal adipocytes. Am J Physiol Endocrinol Metab. 2006;291: 372–380. [DOI] [PubMed] [Google Scholar]
- 35.Rosen ED, Walkey CJ, Puigserver P, Spiegelman BM. Transcriptional regulation of adipogenesis. Genes Dev. 2000;14: 1293–1470. [PubMed] [Google Scholar]
- 36.Cowherd RM, Lyle RE, McGehee RE. Molecular regulation of adipocyte differentiation. Semin Cell Dev Biol. 1999;10: 3–10. [DOI] [PubMed] [Google Scholar]
- 37.Rosen ED, Scarraf P, Troy AE, Bradwin G, Moore K, Milstone DS, et al. PPARγ is required for the differentiation of adipose tissue in vivo and in vitro. Mol Cell. 1999;4: 611–617. [DOI] [PubMed] [Google Scholar]
- 38.Bouraoui L, Cruz-Garcia L, Gutiérrez J, Capilla E, Navarro I. Regulation of lipoprotein lipase gene expression by insulin and troglitazone in rainbow trout (Oncorhynchus mykiss) adipocyte cells in culture. Comp Biochem Physiol. 2012;161: 83–88. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.