Abstract
Background
Renal ischemia-reperfusion injury (IRI) is a major cause of kidney damage after e.g. renal surgery and transplantation. Ischemic postconditioning (IPoC) is a promising treatment strategy for renal IRI, but early clinical trials have not yet replicated the promising results found in animal studies.
Method
We present a systematic review, quality assessment and meta-analysis of the preclinical evidence for renal IPoC, and identify factors which modify its efficacy.
Results
We identified 39 publications studying >250 control animals undergoing renal IRI only and >290 animals undergoing renal IRI and IPoC. Healthy, male rats undergoing warm ischemia were used in the vast majority of studies. Four studies applied remote IPoC, all others used local IPoC. Meta-analysis showed that both local and remote IPoC ameliorated renal damage after IRI for the outcome measures serum creatinine, blood urea nitrogen and renal histology. Subgroup analysis indicated that IPoC efficacy increased with the duration of index ischemia. Measures to reduce bias were insufficiently reported.
Conclusion
High efficacy of IPoC is observed in animal models, but factors pertaining to the internal and external validity of these studies may hamper the translation of IPoC to the clinical setting. The external validity of future animal studies should be increased by including females, comorbid animals, and transplantation models, in order to better inform clinical trial design. The severity of renal damage should be taken into account in the design and analysis of future clinical trials.
Introduction
Renal ischemia and reperfusion injury (IRI) is a major cause of acute kidney injury (AKI) after e.g. renal surgery, coronary artery bypass grafting and abdominal aortic aneurysm repair, which results in increased morbidity and mortality[1]. Renal IRI is also considered an important cause of delayed graft function after renal transplantation and is associated with prolonged hospital stay and acute rejection[2,3].
Ischemic postconditioning (IPoC) is a protective strategy in which (repeated) brief, intermittent periods of ischemia and reperfusion are applied in the early phase of reperfusion after a prolonged ischemic episode. Since its discovery in 2003 in the dog heart[4], IPoC has been shown to attenuate IRI in various organs and a variety of animal species, and is effective when applied to either the target organ, or a remote organ or tissue[5,6]. Thus, IPoC poses a promising treatment strategy for IRI in patients.
Following the promising results obtained in animal studies, the feasibility and efficacy of renal IPoC in patients has been investigated in two clinical trials[7,8]. Although application of local IPoC seemed feasible and safe in patients undergoing donation-after-circulatory-death kidney transplantation, it had no effect on delayed graft function incidence or renal function in a paired kidney analysis[7]. Remote IPoC (RIPoC) appeared to hasten the early recovery of graft function in patients undergoing living donor kidney transplantation, but did not affect graft function >24 hours post-operatively[8]. In addition, clinical trials investigating the effect of IPoC on the myocardium have also yielded conflicting results (reviewed in[9,10]). Thus, the question arises why the replication of the promising results found in animals has been limited in patients, and how the translation of IPoC from animal studies to patients may be improved.
Previously, meta-analysis and systematic review of preclinical studies have proven useful in optimizing the design of both preclinical and clinical studies[11–13]. Although an overview of experimental studies in this field exists[14], a systematic review of the preclinical evidence for renal IPoC is lacking. It remains unclear if and how factors pertaining to the IPoC protocol (e.g. timing and duration) and the animals under investigation (e.g. sex, comorbidities) influence IPoC efficacy. As a result, the IPoC stimulus could have been suboptimal or incorrectly applied in clinical trials, or unsuitable for the patient population. We therefore conducted a systematic review and meta-analysis of evidence on the protective effect of IPoC in animal models of renal IRI. This approach allowed us to analyze the influence of variables such as IPoC timing, IPoC duration, sex and comorbidity on treatment efficacy. We also assessed the extent to which the preclinical data might be at risk of bias, either through publication bias, or through factors relating to experimental design.
Materials and Methods
For an extended version, see S1 Text. The review methodology was predefined and documented in a protocol[15], published online on February 12th 2015. The review question was: what is the effect of local or remote IPoC on renal function in animal models of renal IRI?
Amendments to the review protocol
After study selection, we found that the timing and duration of the IPoC protocol depended strongly on the site of postconditioning. We therefore decided to perform separate meta-analyses of studies using local, remote, and local+remote postconditioning, to avoid collinearity.
For serum creatinine and blood urea nitrogen (BUN), all data could be expressed in the same unit of measurement, but differences in baseline measurements between studies were observed. We therefore performed meta-analysis of the normalised mean difference (NMD) instead of the standardized mean difference (SMD). For renal histology, we expressed all scores as a percentage on the grading scale used, and performed meta-analysis of the mean difference (MD), instead of the SMD. This allowed us to include studies reporting the histology score as a percentage on the grading scale used.
Study identification
A systematic, computerized search in the databases Medline (via PubMed) and EMBASE (S1 Table) was performed on February 4th 2015, using the search components ‘kidney’, ‘ischemic postconditioning’ and an animal search filter for either PubMed[16] or EMBASE[17]. To identify additional relevant studies, the reference lists of included studies and relevant reviews were hand searched. No language restrictions were applied.
Selection of studies
After removal of duplicates, all references were screened for inclusion based on their title and abstract. The following inclusion criteria were applied: the study 1) is an original article presenting unique data with a control group, 2) is performed in vivo in animals with or without comorbidities, but without genetic modifications, 3) reports on renal ischemia-reperfusion injury and outcome measures related to kidney injury or function, and 4) examined the effect of remote and/or local ischemic postconditioning. Subsequently, the full-text manuscripts of eligible studies were reviewed for inclusion. Studies involving co-medication other than anaesthetics or analgesics, or a co-intervention other than collateral nephrectomy were excluded. Studies performed in a renal transplantation model were excluded from the present dataset, but labelled for future reference. In both phases, references were independently assessed for inclusion by two reviewers (KW and SJ).
Study characteristics and data extraction
Study characteristics were extracted by one reviewer (SJ) and checked for inconsistencies by a second reviewer (TM). We selected the following outcome measures for analysis: serum creatinine, BUN and renal histology scores (Jablonski[18] or comparable). Data was collected as mean and standard deviation (SD). For serum creatinine and BUN, all data was recalculated to the same unit of measurement (respectively umol/L and mmol/L). For renal histology, scores were expressed as a percentage on the grading scale used. If an outcome was measured at several time-points, data was extracted for the time-point of greatest efficacy. If a study reported data from several experimental groups, it was extracted as separate comparisons and the number of animals in the control group was corrected (number of animals divided by number of comparisons).
Risk of bias and study quality
Two reviewers (SJ and TM) independently assessed the risk of bias and study quality of each included study. In case of discrepancies, consensus was reached by discussion with a third reviewer (KW). Risk of bias was assessed using SYRCLE’s Risk of Bias tool[19]. Reporting bias (item #9) was not assessed, since none of the studies reported the use of a study protocol predefining primary and secondary outcomes. When assessing selection bias, groups within a study were considered similar at baseline if sex and baseline serum creatinine did not significantly differ between groups (or, if baseline creatinine was unavailable, body weight). To assess whether studies were free of other risks of bias, addition of animals to groups during the experiment and a possible conflict of interest were taken into account. We also assessed reporting of the following study quality items: any randomization, any blinding, regulation of body temperature within 3°C variation and sample size calculation.
Data analysis
Data was analyzed using Stata/SE (StataCorp, Texas, USA). For the outcome measures serum creatinine and BUN, meta-analysis was performed on the NMD, which allows us to correct for baseline kidney injury by relating the magnitude of the effect of treatment to a baseline measured in untreated animals[20]. For histology, the MD was used. A random effects model was used to account for expected between-study heterogeneity. To assess heterogeneity, the I2 and adjusted R2 statistics were determined. To examine potential sources of heterogeneity, predefined subgroup analyses were performed on subgroups containing data from at least three studies. For the duration of IPoC ischemia, studies were categorized using increments of 0.7 log, which resulted in categories of 26–125, 126–630 and 631–3162 seconds of ischemia. For the duration of index ischemia, studies were categorized using increments of 15 minutes, resulting in categories of 16–30, 31–45, 46–60, 61–75 (no studies) and 76–90 minutes. Differences between subgroups were determined by calculating the difference in NMD and MD respectively and the 95% confidence intervals (CI) of the difference. Results are reported as a NMD or MD [95%-CI], unless stated otherwise. For each outcome measure, the significance level for subgroup analyses was adjusted for the number of analyses using the Bonferroni-Holm method[21].
Publication bias was assessed for each outcome measure by visual evaluation of funnel plots, Duval and Tweedie’s trim and fill analysis and by performing Egger’s test for small study effects. Sensitivity analyses were carried out for creatinine and BUN using a fixed time point of outcome assessment (24 hrs). For histology, a sensitivity analysis was performed using only Jablonski histology scores.
Results
Study identification and selection
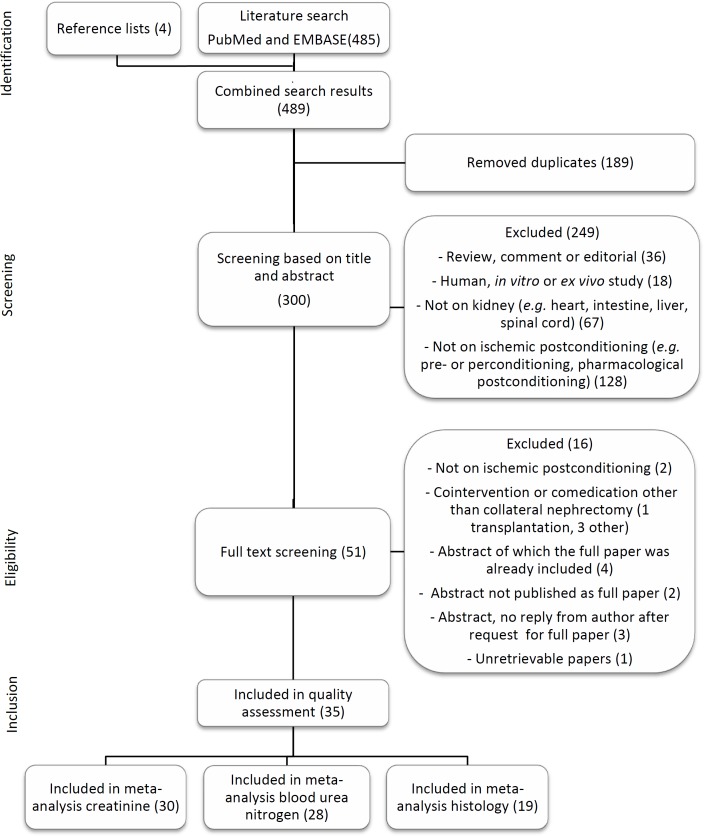
A flow chart of the study selection process is shown in Fig 1. The computerized search retrieved 213 references from PubMed and 272 from EMBASE. Four additional references were added after hand searching reference lists of included studies and relevant reviews. After duplicate removal, 300 references were screened based on title and abstract and 51 studies continued to the eligibility phase. Two letters to the editor[22,23] were included since they presented unique data and sufficient methodological detail. One study investigating IPoC in a canine model of renal transplantation with cold ischemia was excluded, because of the differences in pathophysiology compared to warm ischemia. Finally, 35 studies were included in the risk of bias assessment, all but one of which reported on one or more of the selected outcome measures.
Fig 1. Flow chart of study selection.
The number of studies in each phase are shown between brackets.
Study characteristics
The study characteristics are summarized in Table 1. Out of the 35 included studies, 31 were performed in rats, one in dogs and three in mice. Male animals were used in all but three studies. There were only three studies investigating the effect of RIPoC, all of which used the hind limb as remote tissue. The most commonly used durations of index ischemia were 45 and 60 min. The IPoC protocol varied between studies, however, local application of 6 cycles of 10/10 seconds of reperfusion/ischemia was most commonly used.
Table 1. Study characteristics.
Duration of index ischemia is given in minutes, IPoC protocol timing and duration in seconds, time of outcome measurement in hours.
| Study | Species/ strain | Sex | Index isch | # IPoC cycles | IPoC isch | IPoC rep | IPoC delay | Coll ntx? | Site of IPoC | Time of OM (hrs) | OM |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Chen 2008 [41] | R/W | M | 45 | 6 | 10 | 10 | 0 | Y | LIPoC | 24/48/72 | Cr, BUN, H(J) |
| Chen 2011 [42] | R/SD | M | 60 | 6 | 10 | 10 | 0 | Y | LIPoC | 4 | Cr, BUN, H(D) |
| Chen 2014 [43] | R/W | M | 45 | 6 | 10 | 10 | 0 | Y | LIPoC | 24 | Cr, BUN, H(J) |
| Chen 2015 [44] | R/W | M | 45 | 6 | 10 | 10 | 0 | Y | LIPoC | 24 | Cr, BUN, H(J) |
| Eldaif 2010 [45] | R/SD | M | 45 | 4 | 45 | 45 | 0 | Y | LIPoC | 24 | Cr, BUN, H(O) |
| Fan 2009 [46] | R/SD | M | 60 | 6 | 10 | 10 | 0 | ? | LIPoC | 6 | Cr, BUN, H(D) |
| Guo 2014 [47] | R/SD | M | 45 | 3 | 10 | 10 | 0 | N | LIPoC | 0/1/3/6/12/24/48 | Cr, BUN |
| Ji 2012 [48] | R/SD | M | 45 | 6 | 10 | 10 | 0 | Y | LIPoC | 6/12/24/48/72 | Cr, BUN, H(D) |
| 15 | 15 | ||||||||||
| Jiang 2010 [49] | D | M | 60 | 6 | 30 | 30 | 0 | Y | LIPoC | 72 | Cr, BUN, H(J) |
| 60 | 60 | ||||||||||
| Jiang 2014 [50] | R/SD | M | 60 | 4 | 300 | 300 | 0 | Y | RIPoC† | 24 | Cr, BUN, H(J) |
| Kadkhodaee 2011 [22] | R/SD | M | 45 | 4 | 300 | 300 | 0 | Y | RIPoC† | 24 | Cr, BUN |
| Kadkhodaee 2014 [51] | R/SD | M | 45 | 4 | 10/300 | 10/300 | 0 | Y | LIPoC/RIPoC† | 24 | Cr, BUN, H(D) |
| Lemoine 2015 [52] | M/C57BL6 | M | 30 | 3 | 30 | 30 | 0 | Y | LIPoC | 24 | Cr, BUN, H(O) |
| Li 2010 [53] | R/SD | M | 45 | 10 | 20 | 20 | 0 | N | LIPoC | 24 | Cr, BUN, H(J) |
| Li 2012 [54] | R/SD | F | 45 | 10 | 20 | 20 | 0 | Y | LIPoC | 1/3/6/12/24 | Cr, BUN |
| Liu 2007 [55] | R/W | M | 45 | 6 | 10 | 10 | 0 | Y | LIPoC | 24 | Cr, BUN, H(J) |
| Mahfoudh-Boussaid 2012 [24] | R/W | M | 60 | 6 | 10 | 10 | 0 | N | LIPoC | 2 | Cr, H(J) |
| Mahmoudi 2014 [35] | R/SD | m/f | 45 | 4 | 10 | 10 | 0 | Y | LIPoC | 24 | Cr, BUN, H(O) |
| Miklós 2012 [26] | R/W | M | 45 | 4 | 15 | 15 | 0 | N | LIPoC | 2 | Cr, BUN, H(J) |
| Serviddio 2008 [27] | R/W | M | 90 | 3 | 300 | 180-360-720* | 0 | Y | LIPoC | 0/0.6/24/48 | Cr, BUN, H(O) |
| Shokeir 2012 [56] | R/SD | M | 45 | 3 | 300 | 180-360-720* | 0 | Y | LIPoC | 2/24/48 | Cr, BUN, H(O) |
| Shokeir 2014 [57] | R/SD | M | 45 | 3 | 300 | 180-360-720* | 0 | Y | LIPoC | 2/24/48 | Cr, BUN |
| Szwarc 2007 [58] | M/Swiss | F | 30 | 3 | 30 | 30 | 0 | Y | LIPoC | 0–192 | Cr |
| Tan 2013 [59] | R/SD | M | 45 | 3 | 30 | 30 | 420 | Y | LIPoC | 1/48/168 | Cr, H(D) |
| Tang 2008 [60] | R/W | M | 60 | 6 | 10 | 10 | 0 | Y | LIPoC | 24 | Other |
| Tao 2012 [61] | R/SD | m | 45 | 6 | 10 | 10 | 0 | Y | LIPoC | 24 | Cr, BUN, H(D) |
| Wang 2010 [62] | R/W | m | 60 | 6 | 10 | 10 | 0 | Y | LIPoC | 24 | Cr, BUN, H(J) |
| Weng 2012 [28] | R/SD | m | 45 | 6 | 10 | 10 | 0 | Y | LIPoC | 2016 | Cr, BUN, H(O) |
| 6 | 8 | 8 | LIPoC | ||||||||
| Wever 2012 [23] | R/SD | m | 25 | 3 | 300 | 300 | 0 | Y | RIPoC† | 48 | Cr, BUN, H(O) |
| 9 | 308 | 308 | Both† | ||||||||
| Xia 2014 [25] | R/SD | m | 60 | 6 | 10 | 10 | 0 | Y | LIPoC | 1/3/6/24 | Cr, BUN, H(O) |
| Yun 2009 A [63] | R/SD | m | 45 | 6 | 10 | 10 | 0 | Y | LIPoC | 1/3/6/12/24 | Cr, BUN, H(J) |
| Yun 2009 B [64] | R/SD | m | 45 | 6 | 10 | 10 | 0 | Y | LIPoC | 24 | Cr, BUN, H(J) |
| Zhang 2011 [65] | R/SD | m | 45 | 3 | 10 | 10 | 0 | N | LIPoC | 6 | Cr, BUN, H(D) |
| Zhu 2008 [66] | R/W | m | 60 | 6 | 10 | 10 | 0 | Y | LIPoC | 24 | H(D) |
| Zhuang 2009 [67] | M/C57 | m | 26 | 3 | 30 | 30 | 0/600 | Y | LIPoC | 48 | Cr, BUN, H(O) |
*the duration of reperfusion elongated in each cycle
†remote tissue was the hind limb. Index isch = index ischemia in minutes, IPoC isch = duration of ischemic phase in IPoC protocol, IPoC rep = duration of reperfusion phase in IPoC protocol, IPoC delay = delay between end of index ischemia and start of IPoC, Coll ntx = collateral nephrectomy, OM = outcome measure, R = rat, D = dog, M = mouse, SD = Sprague-Dawley, W = Wistar, m = male, f = female, Y = yes, N = no,? = unknown, LIPoC = local ischemic postconditioning, RIPoC = remote ischemic postonditioning, Cr = serum creatinine, BUN = blood urea nitrogen, H(J) = renal histology assessed by Jablonski score, H(O) = renal histology assessed by other scoring system, H(D) = descriptive reporting of renal histology.
Risk of bias and study quality
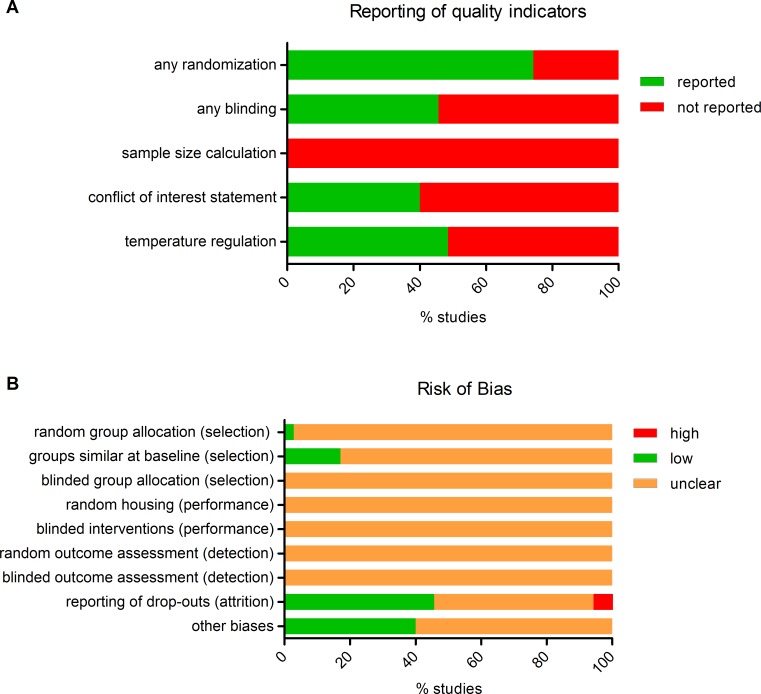
The results of the study quality and risk of bias assessment are shown in Fig 2 and S2 Table. Randomization and blinding are essential measures to reduce bias, but are infrequently reported. Seventy-four percent of the included studies reported random allocation of the animals, however, only one study adequately specified the method of randomisation. Studies that reported blinding (46%), only did so for the outcome assessment of histology. None of the studies reported a sample size calculation. As a consequence of insufficient reporting, the risk of bias was unclear for most items of the risk of bias tool.
Fig 2. Risk of bias and study quality assessment.
Top: Reporting of five key study quality indicators was found to be poor in many cases. Bottom: Using SYRCLE's risk of bias tool, the risk of selection, performance, detection, attrition and other biases was assessed. Lack of (adequate) reporting of measures to reduce bias resulted in a high percentage of unclear risk of bias for most items.
Meta-analyses
Studies investigating local, remote, or local+remote IPoC were analyzed separately. Only the local IPoC group contained enough studies to perform subgroup analysis for any of the outcome measures. One study reporting creatinine clearance[24] was excluded from analysis because serum creatinine data could not be obtained. Data from two studies[25,26] was excluded because serum creatinine or BUN levels were the same in the experimental group and the sham group, indicating that the experimental group did not sustain a sufficient amount of renal IRI. For renal histology, two studies were excluded due to incomplete outcome data[26], [27].
Serum creatinine
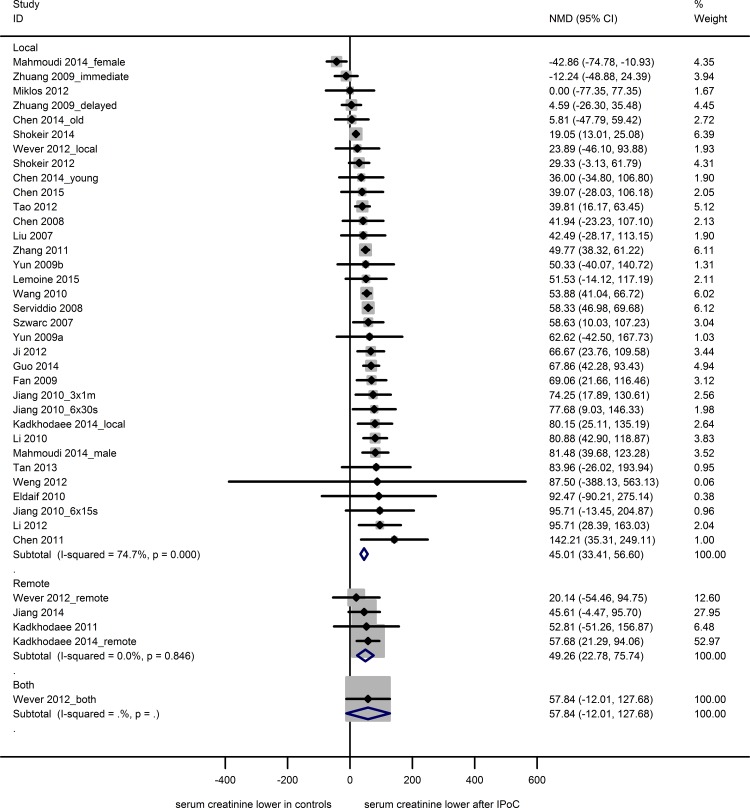
Thirty-one studies reported serum creatinine data from 39 experiments, using 258 sham animals, 247 control animals undergoing renal IRI only and 298 experimental animals undergoing both IRI and IPoC. Both the control and experimental groups contained 3 to 12 animals (median n = 8). The IRI-induced rise in serum creatinine was reduced by both LIPoC (34 experiments; NMD 45.0 [33.4, 56.6]) and RIPoC (4 experiments; NMD 49.3 [22.8, 75.7]; Fig 3). One study investigating the combination of LIPoC and RIPoC showed no effect (NMD 57.84 [-12.0, 127.7]).
Fig 3. Meta-analysis creatinine.
The summary effects show a decrease in serum creatinine after local or remote IPoC. One study investigating the combination of local and remote IPoC showed no effect. Data are presented as NMD and 95% CI. Within subgroup weights from random effects analysis are shown.
Subgroup analysis results for the LIPoC studies are shown in S3 Table. LIPoC had a beneficial effect on creatinine in all subgroups, except for mouse, female, 4 cycles of LIPoC and 16–30 minutes of index ischemia. Overall heterogeneity was high (I2 74.7%), but none of the subgroup variables accounted for a significant proportion of the observed heterogeneity.
BUN
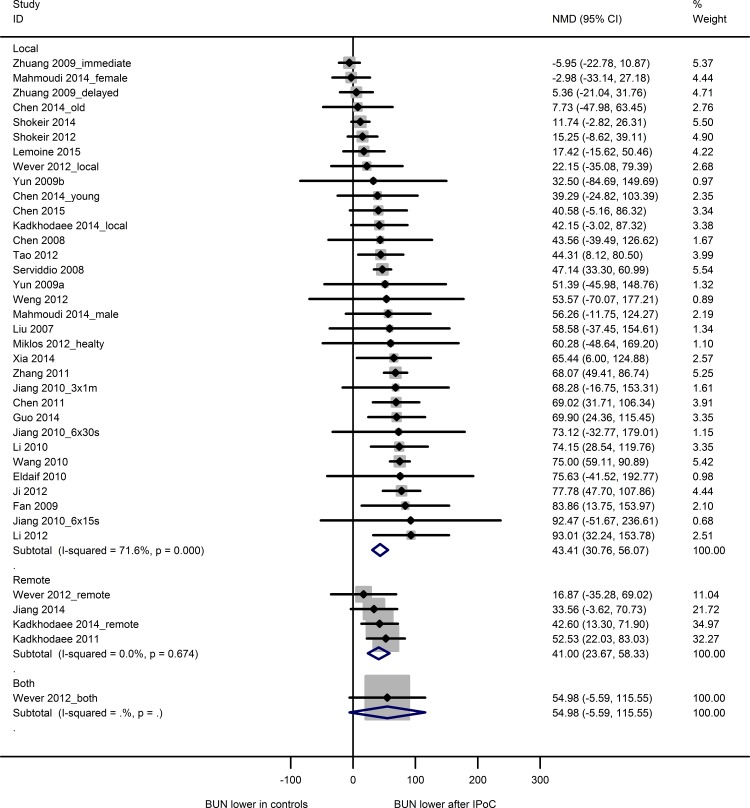
Twenty-eight studies reported BUN data from 36 experiments, using 226 sham animals, 222 control animals and 269 IPoC-treated animals. Both the control and experimental groups contained 3 to 12 animals (median n = 8). The IRI-induced rise in BUN was reduced by both LIPoC (33 experiments; NMD 43.4 [30.8, 56.1]) and RIPoC (4 experiments; NMD 41.0 [23.7, 58.3]; Fig 4). One study investigating the combination of LIPoC and RIPoC showed no effect (NMD 55.0 [-5.6, 115.6]).
Fig 4. Meta-analysis blood urea nitrogen.
The summary effects show a decrease in blood urea nitrogen after local or remote IPoC. One study investigating the combination of local and remote IPoC showed no effect. Data are presented as NMD and 95% CI. Within subgroup weights from random effects analysis are shown.
Subgroup analysis results for the LIPoC studies are shown in S4 Table. The effect of species and sex on LIPoC efficacy could not be analyzed due to insufficient data. LIPoC had a beneficial effect on BUN in all subgroups, except for mouse, female, 4 cycles of IPoC, 631–3162 seconds of IPoC ischemia and 16–30 minutes of index ischemia. Overall heterogeneity was high (I2 71.6%). A significant proportion of heterogeneity was explained by the duration of index ischemia (adjusted R2 44.5%; p<0.007), indicating that the efficacy of postconditioning increased with the duration of index ischemia. None of the other subgroup variables accounted for a significant proportion of heterogeneity.
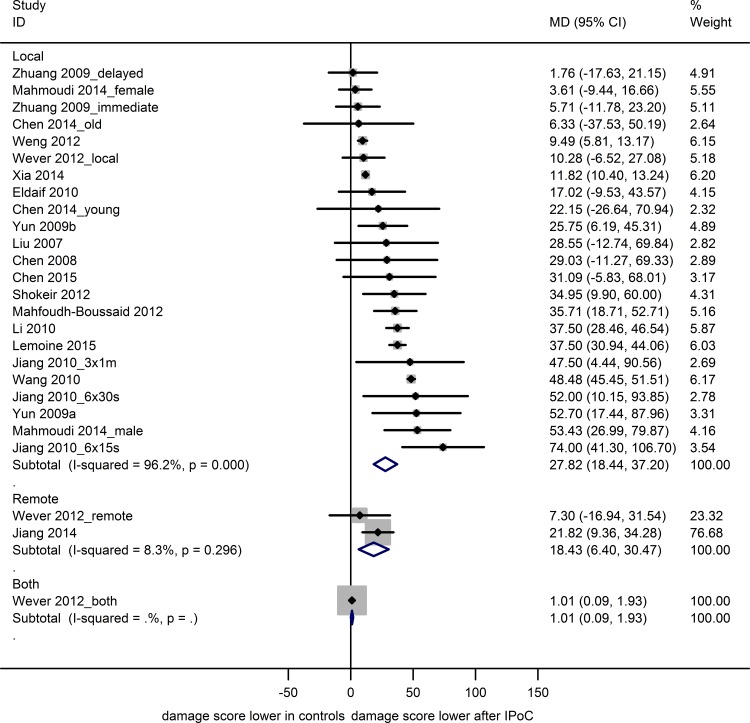
Renal histology
Nineteen studies reported data on renal histology from 26 experiments, using 149 sham, 152 control and 191 IPoC-treated animals. Both the control and experimental groups contained 4 to 10 animals (median n = 8). Renal histology scores were reduced after renal IRI in animals treated with LIPoC (23 experiments; MD 27.8 [18.4, 37.2] or RIPoC (2 experiments; MD 18.4 [6.4, 30.5]) or the combination of the two (1 experiment; MD 1.0 [0.1, 1.93]; Fig 5).
Fig 5. Meta-analysis renal histology.
The summary effects show a decrease renal damage score after local or remote IPoC, and the combination of the two. Data are presented as MD and 95% CI. Within subgroup weights from random effects analysis are shown.
A positive effect of LIPoC on histology scores (S5 Table) was observed in most subgroups, similar to the results obtained for serum creatinine. The effect of species, sex and site of postconditioning on IPoC efficacy could not be analyzed due to insufficient data. Overall heterogeneity was very high (I2 96.2%), but could not be attributed to any of the subgroup variables.
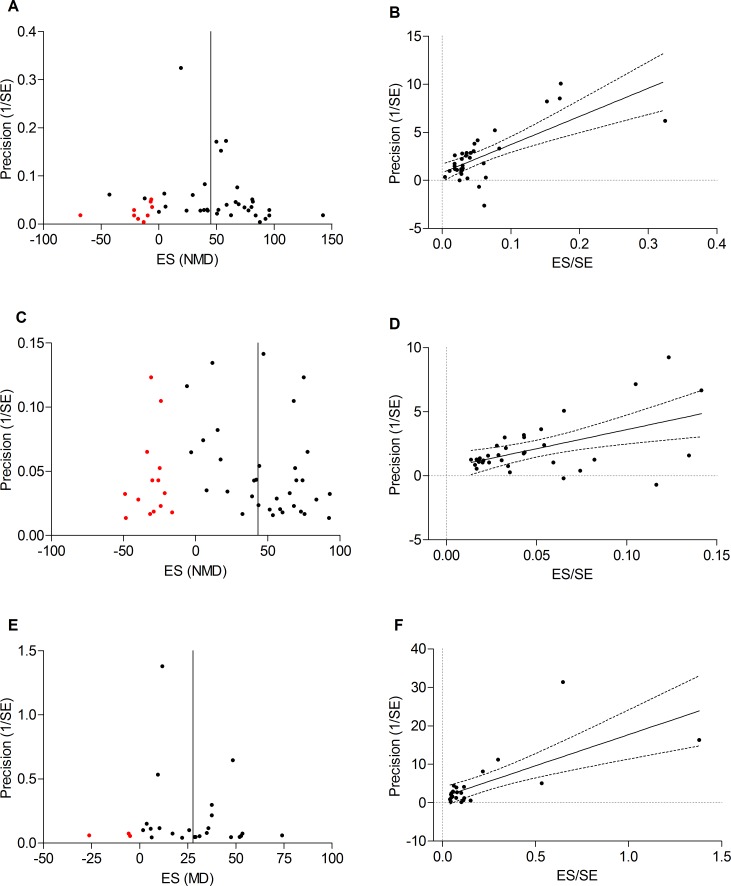
Publication bias
Publication bias could be assessed for LIPoC only, due to insufficient data for RIPoC. Possible publication bias was observed for all outcome measures when visually evaluating funnel plots for asymmetry. Duval and Tweedie’s trim and fill analysis resulted in filled data points for all outcome measures (Fig 6A, 6C and 6E), indicating that small, negative studies were underrepresented. However, Egger’s test indicated that no small study effects were present (Fig 6B, 6D and 6F).
Fig 6. Publication bias.
Trim and fill analysis for studies on local IPoC indicates funnel plot asymmetry for respectively creatinine (A), BUN (C) and renal histology (E). The 95% confidence interval of Egger’s regression line (dashed lines) does not include the origin of the graph, indicating no small study effects for creatinine (B), BUN (D) and renal histology (F).
Sensitivity analyses
Sensitivity analyses were performed to assess the robustness of our findings. For serum creatinine and BUN, a fixed time point of 24 hrs for outcome assessment was chosen instead of the time point of the greatest efficacy. The analyses contained 24 studies for both creatinine and BUN. The summary effect found in the sensitivity analysis did not differ from the original analysis for either creatinine (NMD 43.3 [30.7, 55.9] versus 45.0 [33.4, 56.6]) or BUN (NMD 37.3 [24.7, 50.8] versus 43.4 [30.8, 56.1]). The overall heterogeneity was slightly lower in the sensitivity analyses (I2 64.3% versus 74.7% for creatinine and 69.3% versus 71.6% for BUN).
For renal histology, a sensitivity analysis was performed by excluding all studies which did not use the Jablonski grading scale. The summary effect found in the sensitivity analysis on the remaining 13 studies did not differ from the original analysis (MD 40.2 [32.5, 47.8] versus 27.8 [18.4, 37.2]). However, heterogeneity was considerably lower in the sensitivity analysis (I2 41.5% vs. 96.2%). This was surprising, since all scales roughly scored the same features of tubular damage (e.g. cellular vacuolization, loss of brush border, cast formation). However, since the overall effect of IPoC was robust, we feel that our decision to pool all scoring systems is justified.
One study[28] measured serum creatinine and BUN twelve weeks after renal IRI. At this time-point, values were similar in all groups, which resulted in extremely large confidence intervals in our NMD meta-analysis. However, omitting this study had no effect on meta-analysis outcomes.
Discussion
IPoC efficacy and sources of heterogeneity
This systematic review and meta-analysis provides a quantitative summary of all preclinical in vivo evidence on IPoC against renal IRI. Our review shows a protective effect of both LIPoC and RIPoC on renal function and histology, based on a reduction in serum creatinine, BUN and renal histology scores. The high between-study heterogeneity was partially explained by the duration of index ischemia, i.e. LIPoC efficacy appeared to increase as the duration of index ischemia increased. The other study characteristics under investigation did not account for significant proportions of heterogeneity, or could not be analysed due to insufficient data (especially for RIPoC). For LIPoC, the remaining heterogeneity is high, especially for renal histology. Importantly, differences in the risk of bias between studies may represent a significant source of unexplained heterogeneity, but insufficient reporting currently prevents us from testing this hypothesis (see below).
Methodological quality
Adequate reporting of methodological details is crucial to determine the risk of bias in primary studies and to assess the quality of a body of evidence. Insufficient reporting of preclinical research methodology occurs in many fields and is often associated with an overestimation of treatment effects e.g.[29–31]. We show that details on key measures to reduce bias (such as randomisation and blinding) and other study quality indicators were missing from many studies included in our review. The risk of bias in most studies therefore remains unclear. Consequently, some studies may have overestimated the effect of IPoC, which may have influenced the outcome of our meta-analysis.
The number of animals per group was very low in a number of studies. This is a matter of concern, since underpowered studies have an increased risk of finding false positive results. Systematic reviews have suggested that underpowering of in vivo studies is common, and that this greatly contributes to translational failure[31,32]. Since none of the included studies reported a sample size calculation, we cannot exclude the possibility of an effect of underpowering on our meta-analysis.
Study characteristics
The present review points out several apparent differences between the experimental design of current clinical trials on renal IPoC, and the preceding animal studies. Firstly, we show that 99% of the preclinical evidence was obtained from animals undergoing warm renal ischemia. In contrast, the two published clinical trials on IPoC[7,8], as well as a third trial in progress (ISRCTN66437627), all study the effect of IPoC after renal transplantation. To our knowledge, these trials were predominantly based on results obtained in animal models of warm IRI. Only one animal study investigating IPoC after renal transplantation[33] was retrieved by our search (which was not limited to a specific model of renal IRI). This study does show a protective effect of local IPoC after transplantation, however, there are substantial differences between these models (e.g. warm versus cold ischemia and renal denervation), and animal models using warm ischemia may not optimally predict outcomes in the clinical transplantation setting.
Secondly, we show that 90% of the animal studies investigated LIPoC, even though RIPoC is generally considered to be more applicable in clinical practice. Thus far, one clinical trial investigated LIPoC[7], and two applied RIPoC ([8] and ISRCTN66437627). Regarding the LIPoC protocol, our meta-analysis did not identify any factors related to timing or duration which influence its efficacy. Since nearly all evidence was obtained in rats, it remains unclear whether the same timing and duration is effective in all species (including humans). Only two studies have used larger animals, whose metabolic rate is more comparable to humans. Of note, Van den Akker et al [7] adjusted their clinical IPoC protocol to fit the metabolic rate in humans, but found no beneficial effect. Furthermore, there is not enough preclinical evidence to assess if timing and/or duration of the protocol influences the efficacy of RIPoC. We suggest that the optimal timing and duration of the postconditioning protocols should be determined separately for LIPoC and RIPoC.
Concerning the population under investigation, nearly all preclinical studies used male animals, whereas the clinical trials included both men and women. This sex bias (which is widespread in preclinical studies) is reason for concern, considering the evidence that females react differently to both IRI[34] and IPoC[35]. Secondly, we found no studies using animals with relevant comorbidities such as hypertension or diabetes mellitus, which are often present in patients undergoing renal surgery or transplantation. The absence of comorbidities in experimental animals has previously been described as a possible explanation for the translational failure of conditioning strategies [36–38].
Publication bias
Visual inspection of funnel plots, as well as trim and fill analysis, indicate a possible presence of publication bias in this field. The direction of effect did not change after trim and fill, but neutral and negative studies were underrepresented. On the other hand, Egger’s test did not indicate any small-study effects, and Funnel plot asymmetry may be explained by other factors such as true heterogeneity, study quality or chance[39]. Based on this analysis we assess the risk of publication bias to be mild (histology and creatinine) to moderate (BUN). This should be kept in mind when interpreting our results, since data from a range of animal studies strongly suggested that publication bias is associated with a substantial overestimation of treatment effects[40].
Clinical implications and future perspective
This review is the first systematic overview of preclinical evidence for the efficacy of IPoC in animal models of renal IRI. It provides useful insights in the variables influencing IPoC efficacy, within the limitations inherent to combining data from different experiments. Sensitivity analyses showed that the observed overall efficacy is robust for all outcome measures. Our finding that IPoC efficacy may increase with the duration of renal ischemia suggests that IPoC is less effective when kidney injury is mild. The severity of renal IRI in patients varies with the type of surgery they receive, their co-morbidities and additional measures which can be taken to reduce IRI. Thus, IPoC may not be equally potent in all patients, and this should be taken into account when including patients in clinical trials and analyzing clinical and preclinical results.
We also find that the body of evidence on which clinical trials are presently based is narrow, and its quality unclear. In particular, indirectness and risk of bias are reasons to interpret the preclinical findings with care. The present review points out a number of opportunities for improvement and future research, in order to increase clinical relevance of the preclinical studies and provide sufficient validity to guide clinical trial design. Preclinical studies should use both sexes, animals with relevant comorbidities, and it should be investigated whether the results obtained thus far can be replicated in transplantation models. Larger animal species may be used to better resemble the metabolic rate in humans. Importantly, to avoid effects of insufficient reporting, underpowering and publication bias in systematic reviews, it is of the utmost importance that the design, execution and reporting of animal studies is improved, for instance through the use of the GSPC and ARRIVE guidelines by authors and journals. Only then, preclinical evidence can be used to its full extent.
Supporting Information
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
Acknowledgments
The authors thank information specialist A. Tillema for her help in optimizing the search strategy, and J. Liao for her help in translating Chinese articles.
Abbreviations
- AKI
acute kidney injury
- BUN
blood urea nitrogen
- CI
confidence interval
- IPoC
ischemic postconditioning
- IRI
ischemia-reperfusion injury
- LIPoC
local ischemic postconditioning
- MD
raw mean difference
- NMD
normalised mean difference
- RIPoC
remote ischemic postconditioning
- SMD
standardised mean difference
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The authors have no support or funding to report.
References
- 1.Dasta JF, Kane-Gill SL, Durtschi AJ, Pathak DS, Kellum JA (2008) Costs and outcomes of acute kidney injury (AKI) following cardiac surgery. Nephrol Dial Transplant 23: 1970–1974. 10.1093/ndt/gfm908 [DOI] [PubMed] [Google Scholar]
- 2.Perico N, Cattaneo D, Sayegh MH, Remuzzi G (2004) Delayed graft function in kidney transplantation. Lancet 364: 1814–1827. [DOI] [PubMed] [Google Scholar]
- 3.Roodnat JI, Mulder PG, Van Riemsdijk IC, JN IJ, van Gelder T, Weimar W (2003) Ischemia times and donor serum creatinine in relation to renal graft failure. Transplantation 75: 799–804. [DOI] [PubMed] [Google Scholar]
- 4.Zhao ZQ, Corvera JS, Halkos ME, Kerendi F, Wang NP, Guyton RA, et al. (2003) Inhibition of myocardial injury by ischemic postconditioning during reperfusion: comparison with ischemic preconditioning. Am J Physiol Heart Circ Physiol 285: H579–588. [DOI] [PubMed] [Google Scholar]
- 5.Kerendi F, Kin H, Halkos ME, Jiang R, Zatta AJ, Zhao ZQ, et al. (2005) Remote postconditioning. Brief renal ischemia and reperfusion applied before coronary artery reperfusion reduces myocardial infarct size via endogenous activation of adenosine receptors. Basic Res Cardiol 100: 404–412. [DOI] [PubMed] [Google Scholar]
- 6.Zhao ZQ (2010) Postconditioning in reperfusion injury: a status report. Cardiovasc Drugs Ther 24: 265–279. 10.1007/s10557-010-6240-1 [DOI] [PubMed] [Google Scholar]
- 7.van den Akker EK, Hesselink DA, Manintveld OC, Lafranca JA, de Bruin RW, Weimar W, et al. (2014) Ischemic postconditioning in human DCD kidney transplantation is feasible and appears safe. Transpl Int 27: 226–234. 10.1111/tri.12242 [DOI] [PubMed] [Google Scholar]
- 8.Kim WH, Lee JH, Kim GS, Sim HY, Kim SJ (2014) The effect of remote ischemic postconditioning on graft function in patients undergoing living donor kidney transplantation. Transplantation 98: 529–536. 10.1097/TP.0000000000000098 [DOI] [PubMed] [Google Scholar]
- 9.Abdelnoor M, Sandven I, Limalanathan S, Eritsland J (2014) Postconditioning in ST-elevation myocardial infarction: a systematic review, critical appraisal, and meta-analysis of randomized clinical trials. Vasc Health Risk Manag 10: 477–491. 10.2147/VHRM.S67154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khan AR, Binabdulhak AA, Alastal Y, Khan S, Faricy-Beredo BM, Luni FK, et al. (2014) Cardioprotective role of ischemic postconditioning in acute myocardial infarction: a systematic review and meta-analysis. Am Heart J 168: 512–521.e514. 10.1016/j.ahj.2014.06.021 [DOI] [PubMed] [Google Scholar]
- 11.Pound P, Ebrahim S, Sandercock P, Bracken MB, Roberts I (2004) Where is the evidence that animal research benefits humans? Bmj 328: 514–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van der Worp HB, Macleod MR, Kollmar R (2010) Therapeutic hypothermia for acute ischemic stroke: ready to start large randomized trials? J Cereb Blood Flow Metab 30: 1079–1093. 10.1038/jcbfm.2010.44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wever KE, Menting TP, Rovers M, van der Vliet JA, Rongen GA, Masereeuw R, et al. (2012) Ischemic preconditioning in the animal kidney, a systematic review and meta-analysis. PLoS One 7: e32296 10.1371/journal.pone.0032296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van den Akker EK, Manintveld OC, Hesselink DA, de Bruin RW, Ijzermans JN, Dor FJ (2013) Protection against renal ischemia-reperfusion injury by ischemic postconditioning. Transplantation 95: 1299–1305. 10.1097/TP.0b013e318281b934 [DOI] [PubMed] [Google Scholar]
- 15.Jonker SJ, Wever KE (2015) Systematic review protocol for animal intervention studies: Ischemic postconditioning of the kidney—a systematic review of animal studies. https://www.radboudumc.nl/Research/Organisationofresearch/Departments/cdl/SYRCLE/Documents/SR%20protocol%20renal%20%20IPoC_SJKW.pdf: SYRCLE. pp. 1–7.
- 16.Hooijmans CR, Tillema A, Leenaars M, Ritskes-Hoitinga M (2010) Enhancing search efficiency by means of a search filter for finding all studies on animal experimentation in PubMed. Lab Anim 44: 170–175. 10.1258/la.2010.009117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Vries RB, Hooijmans CR, Tillema A, Leenaars M, Ritskes-Hoitinga M (2014) Updated version of the Embase search filter for animal studies. Lab Anim 48: 88 10.1177/0023677213494374 [DOI] [PubMed] [Google Scholar]
- 18.Jablonski P, Howden BO, Rae DA, Birrell CS, Marshall VC, Tange J (1983) An experimental model for assessment of renal recovery from warm ischemia. Transplantation 35: 198–204. [DOI] [PubMed] [Google Scholar]
- 19.Hooijmans CR, Rovers MM, de Vries RB, Leenaars M, Ritskes-Hoitinga M, Langendam MW (2014) SYRCLE's risk of bias tool for animal studies. BMC Med Res Methodol 14: 43 10.1186/1471-2288-14-43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vesterinen HM, Sena ES, Egan KJ, Hirst TC, Churolov L, Currie GL, et al. (2014) Meta-analysis of data from animal studies: a practical guide. J Neurosci Methods 221: 92–102. 10.1016/j.jneumeth.2013.09.010 [DOI] [PubMed] [Google Scholar]
- 21.Holm S (1979) A simple sequential rejective multiple test procedure. Scandinavian Journal of Statistic 6: 65–70. [Google Scholar]
- 22.Kadkhodaee M, Seifi B, Najafi A, Sedaghat Z (2011) First report of the protective effects of remote per- and postconditioning on ischemia/reperfusion-induced renal injury. Transplantation 92: e55 10.1097/TP.0b013e31823411f8 [DOI] [PubMed] [Google Scholar]
- 23.Wever KE, Menting T, Masereeuw R, van der Vliet JA, Rongen GA, Warle MC (2012) Local and remote ischemic postconditionings have synergistic protective effects on renal ischemia-reperfusion injury. Transplantation 94: e1–2. 10.1097/TP.0b013e318257ad76 [DOI] [PubMed] [Google Scholar]
- 24.Mahfoudh-Boussaid A, Zaouali MA, Hauet T, Hadj-Ayed K, Miled AH, Ghoul-Mazgar S, et al. (2012) Attenuation of endoplasmic reticulum stress and mitochondrial injury in kidney with ischemic postconditioning application and trimetazidine treatment. J Biomed Sci 19: 71 10.1186/1423-0127-19-71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xia A, Li Y, Li N, Xue Z, Xia J, Wei T, et al. (2014) Roles of MAPKAPK-2 and HSP27 in the reduction of renal ischemia-reperfusion injury by ischemic postconditioning in rats. Int Urol Nephrol 46: 1455–1464. 10.1007/s11255-014-0748-4 [DOI] [PubMed] [Google Scholar]
- 26.Miklos Z, Kurthy M, Degrell P, Ranczinger E, Vida M, Lantos J, et al. (2012) Ischaemic postconditioning reduces serum and tubular TNF-alpha expression in ischaemic-reperfused kidney in healthy rats. Clin Hemorheol Microcirc 50: 167–178. 10.3233/CH-2011-1414 [DOI] [PubMed] [Google Scholar]
- 27.Serviddio G, Romano AD, Gesualdo L, Tamborra R, Di Palma AM, Rollo T, et al. (2008) Postconditioning is an effective strategy to reduce renal ischaemia/reperfusion injury. Nephrol Dial Transplant 23: 1504–1512. 10.1093/ndt/gfm779 [DOI] [PubMed] [Google Scholar]
- 28.Weng X, Shen H, Kuang Y, Liu X, Chen Z, Zhu H, et al. (2012) Ischemic postconditioning inhibits the renal fibrosis induced by ischemia-reperfusion injury in rats. Urology 80: 484 e481–487. [DOI] [PubMed] [Google Scholar]
- 29.Currie GL, Delaney A, Bennett MI, Dickenson AH, Egan KJ, Vesterinen HM, et al. (2013) Animal models of bone cancer pain: systematic review and meta-analyses. Pain 154: 917–926. 10.1016/j.pain.2013.02.033 [DOI] [PubMed] [Google Scholar]
- 30.Macleod MR, van der Worp HB, Sena ES, Howells DW, Dirnagl U, Donnan GA (2008) Evidence for the efficacy of NXY-059 in experimental focal cerebral ischaemia is confounded by study quality. Stroke 39: 2824–2829. 10.1161/STROKEAHA.108.515957 [DOI] [PubMed] [Google Scholar]
- 31.Vesterinen HM, Sena ES, ffrench-Constant C, Williams A, Chandran S, Macleod MR (2010) Improving the translational hit of experimental treatments in multiple sclerosis. Mult Scler 16: 1044–1055. 10.1177/1352458510379612 [DOI] [PubMed] [Google Scholar]
- 32.Scott S, Kranz JE, Cole J, Lincecum JM, Thompson K, Kelly N, et al. (2008) Design, power, and interpretation of studies in the standard murine model of ALS. Amyotroph Lateral Scler 9: 4–15. 10.1080/17482960701856300 [DOI] [PubMed] [Google Scholar]
- 33.Jiang B, Chen Q, Liu X, Kong D, Kuang Y, Weng X, et al. (2012) Ischemic postconditioning protects renal function after 24 hours of cold preservation in a canine autotransplantation model. Transplant Proc 44: 1776–1781. 10.1016/j.transproceed.2012.05.040 [DOI] [PubMed] [Google Scholar]
- 34.Robert R, Ghazali DA, Favreau F, Mauco G, Hauet T, Goujon JM (2011) Gender difference and sex hormone production in rodent renal ischemia reperfusion injury and repair. J Inflamm (Lond) 8: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mahmoudi A, Kadkhodaee M, Golab F, Najafi A, Sedaghat Z (2014) Postconditioning is protective in renal reperfusion injury only in male rats. A gender difference study. Acta Physiol Hung: 1–10. [DOI] [PubMed] [Google Scholar]
- 36.McCafferty K, Forbes S, Thiemermann C, Yaqoob MM (2014) The challenge of translating ischemic conditioning from animal models to humans: the role of comorbidities. Dis Model Mech 7: 1321–1333. 10.1242/dmm.016741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bice JS, Baxter GF (2015) Postconditioning signalling in the heart: mechanisms and translatability. Br J Pharmacol 172: 1933–1946. 10.1111/bph.12976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Przyklenk K (2011) Efficacy of cardioprotective 'conditioning' strategies in aging and diabetic cohorts: the co-morbidity conundrum. Drugs Aging 28: 331–343. 10.2165/11587190-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 39.Mavridis D, Salanti G (2014) Exploring and accounting for publication bias in mental health: a brief overview of methods. Evid Based Ment Health 17: 11–15. 10.1136/eb-2013-101700 [DOI] [PubMed] [Google Scholar]
- 40.Tsilidis KK, Panagiotou OA, Sena ES, Aretouli E, Evangelou E, Howells DW, et al. (2013) Evaluation of excess significance bias in animal studies of neurological diseases. PLoS Biol 11: e1001609 10.1371/journal.pbio.1001609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen H, Xing B, Liu X, Zhan B, Zhou J, Zhu H, et al. (2008) Ischemic postconditioning inhibits apoptosis after renal ischemia/reperfusion injury in rat. Transpl Int 21: 364–371. [DOI] [PubMed] [Google Scholar]
- 42.Chen LN, Wang XH, Yu L, Wei X, Yang F, Wang M, et al. (2011) The protective effects of ischemic postconditioning on renal ischemia-reperfusion injury in rats. [Chinese]. Journal of Xi'an Jiaotong University (Medical Sciences) 32: 684–686+721. [Google Scholar]
- 43.Chen H, Xing B, Wang L, Weng X, Chen Z, Liu X (2014) Aged kidneys are refractory to ischemic postconditioning in a rat model. Ren Fail 36: 1575–1580. 10.3109/0886022X.2014.949769 [DOI] [PubMed] [Google Scholar]
- 44.Chen H, Xing B, Wang L, Weng X, Chen Z, Liu X (2015) Toll-like Receptor 4 Is Involved in Renoprotective Effect of Ischemic Postconditioning After Renal Ischemia/Reperfusion Injury in Rats. Urology 85: 483 e481–487. [DOI] [PubMed] [Google Scholar]
- 45.Eldaif SM, Deneve JA, Wang NP, Jiang R, Mosunjac M, Mutrie CJ, et al. (2010) Attenuation of renal ischemia-reperfusion injury by postconditioning involves adenosine receptor and protein kinase C activation. Transpl Int 23: 217–226. 10.1111/j.1432-2277.2009.00949.x [DOI] [PubMed] [Google Scholar]
- 46.Fan ZL, Du X, Li WP, Zhang T, Yu Y, Ju HW (2009) Effect of ischemic postconditionging on ichemia-reperfusion injury and expression of NF-kappaB in rat kidney. [Chinese]. Journal of Dalian Medical University 31: 524–528. [Google Scholar]
- 47.Guo Q, Du X, Zhao Y, Zhang D, Yue L, Wang Z (2014) Ischemic postconditioning prevents renal ischemia reperfusion injury through the induction of heat shock proteins in rats. Mol Med Rep 10: 2875–2881. 10.3892/mmr.2014.2641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ji X, Ma LL, Lu J, Zhang SD, Huang Y, Hou XF, et al. (2012) [Expression of kidney injury molecule-1 in renal ischemic postconditioning and its protective effect against renal ischemia-reperfusion injury in rats]. Beijing da xue xue bao 44: 511–517. [PubMed] [Google Scholar]
- 49.Jiang B, Liu X, Chen H, Liu D, Kuang Y, Xing B, et al. (2010) Ischemic postconditioning attenuates renal ischemic/reperfusion injury in mongrel dogs. Urology 76: 1519 e1511–1517. [DOI] [PubMed] [Google Scholar]
- 50.Jiang H, Chen R, Xue S, Zhu H, Sun X, Sun X (2014) Protective effects of three remote ischemic conditioning procedures against renal ischemic/reperfusion injury in rat kidneys: a comparative study. Ir J Med Sci. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kadkhodaee M, Najafi A, Seifi B (2014) Classical and remote post-conditioning effects on ischemia/reperfusion-induced acute oxidant kidney injury. Int J Surg 12: 1162–1166. 10.1016/j.ijsu.2014.09.007 [DOI] [PubMed] [Google Scholar]
- 52.Lemoine S, Pillot B, Rognant N, Augeul L, Rayberin M, Varennes A, et al. (2015) Postconditioning with cyclosporine a reduces early renal dysfunction by inhibiting mitochondrial permeability transition. Transplantation 99: 717–723. 10.1097/TP.0000000000000530 [DOI] [PubMed] [Google Scholar]
- 53.Li Y, Xia AZ, Xing SH (2010) Protective effect of edaravone against renal ischemia/reperfusion injury and compared with ischemic postconditioning in rats. Yao Xue Xue Bao 45: 840–848. [PubMed] [Google Scholar]
- 54.Li M, Wang HM, Li ST, Liu GH (2012) Effects of ischemic postconditioning on renal function and Ur-NAG enzyme in rats. [Chinese]. Journal of Xi'an Jiaotong University (Medical Sciences) 33: 644–646. [Google Scholar]
- 55.Liu X, Chen H, Zhan B, Xing B, Zhou J, Zhu H, et al. (2007) Attenuation of reperfusion injury by renal ischemic postconditioning: the role of NO. Biochem Biophys Res Commun 359: 628–634. [DOI] [PubMed] [Google Scholar]
- 56.Shokeir AA, Hussein AM, Awadalla A, Samy A, Abdelaziz A, Khater S, et al. (2012) Protection against renal ischaemia/reperfusion injury: A comparative experimental study of the effect of ischaemic preconditioning vs. postconditioning. Arab Journal of Urology 10: 418–424. 10.1016/j.aju.2012.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shokeir AA, Hussein AM, Barakat N, Abdelaziz A, Elgarba M, Awadalla A (2014) Activation of nuclear factor erythroid 2-related factor 2 (Nrf2) and Nrf-2-dependent genes by ischaemic pre-conditioning and post-conditioning: New adaptive endogenous protective responses against renal ischaemia/reperfusion injury. Acta Physiologica 210: 342–353. 10.1111/apha.12164 [DOI] [PubMed] [Google Scholar]
- 58.Szwarc I, Soullier S, Gayrard N, Mejean C, Mourad G, Argiles A (2007) Ischemic postconditioning prevents ischemic acute renal failure. Transplant Proc 39: 2554–2556. [DOI] [PubMed] [Google Scholar]
- 59.Tan X, Zhang L, Jiang Y, Yang Y, Zhang W, Li Y, et al. (2013) Postconditioning ameliorates mitochondrial DNA damage and deletion after renal ischemic injury. Nephrol Dial Transplant 28: 2754–2765. 10.1093/ndt/gft278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tang TC S.; Zhou L.; Zhang Y.; Lu Y. (2008) Effect of ischemic post-conditioning on bcl-2 and bax mRNA expression in rat kidney after acute hot ischemic-reperfusion Chinese Journal of Blood Transfusion: 502–505. [Google Scholar]
- 61.Tao JY, Mi H, Mo ZN, Huang WH, Chen K, Liang YY, et al. (2012) Protective effect of curcumin preconditioning and ischemic postconditioning against renal ischemia-reperfusion injury in rats. [Chinese]. Chinese Journal of Tissue Engineering Research 16: 10015–10020. [Google Scholar]
- 62.Wang W, Tang T, Zhang P, Bu H (2010) Postconditioning attenuates renal ischemia-reperfusion injury by preventing DAF down-regulation. J Urol 183: 2424–2431. 10.1016/j.juro.2010.01.066 [DOI] [PubMed] [Google Scholar]
- 63.Yun Y, Duan WG, Chen P, Wu HX, Shen ZQ, Qian ZY, et al. (2009) Ischemic postconditioning modified renal oxidative stress and lipid peroxidation caused by ischemic reperfusion injury in rats. Transplant Proc 41: 3597–3602. 10.1016/j.transproceed.2009.06.203 [DOI] [PubMed] [Google Scholar]
- 64.Yun Y, Duan WG, Chen P, Wu HX, Shen ZQ, Qian ZY, et al. (2009) Down-regulation of cyclooxygenase-2 is involved in ischemic postconditioning protection against renal ischemia reperfusion injury in rats. Transplant Proc 41: 3585–3589. 10.1016/j.transproceed.2009.06.209 [DOI] [PubMed] [Google Scholar]
- 65.Zhang WL, Zhao YL, Liu XM, Chen J, Zhang D (2011) Protective role of mitochondrial K-ATP channel and mitochondrial membrane transport pore in rat kidney ischemic postconditioning. Chin Med J (Engl) 124: 2191–2195. [PubMed] [Google Scholar]
- 66.Zhu YC, Tang TL, Cui S, Zhou B, Wang W, Zeng GJ, et al. (2008) The effect of ischemic postconditioning on apoptosis induced by acute hot renal ischemic-reperfusion. [Chinese]. Journal of Sichuan University (Medical Science Edition) 39: 921–924. [PubMed] [Google Scholar]
- 67.Zhuang SL B.; Pang M. (2009) Postconditioning Does Not Improve Renal Function or Attenuate Tubular Damage in Ischemia/Reperfusion-Induced Acute Kidney Injury in Mice. The Open Pathology Journal 3: 18–21. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.