Advanced cancer patients frequently experience debilitating symptoms that occur in clusters. The results of this preliminary study suggest significant correlation over time and improvement in the fatigue/anorexia-cachexia/depression cluster at day 8 and day 15 after treatment with dexamethasone. These findings suggest that fatigue, anorexia-cachexia, and depression may share a common pathophysiologic basis.
Keywords: Symptom clusters, Interventions, Dexamethasone, Principal component analysis, Cytokines
Abstract
Objective.
Advanced cancer patients frequently experience debilitating symptoms that occur in clusters, but few pharmacological studies have targeted symptom clusters. Our objective was to examine the effects of dexamethasone on symptom clusters in patients with advanced cancer.
Methods.
We reviewed the data from a previous randomized clinical trial to determine the effects of dexamethasone on cancer symptoms. Symptom clusters were identified according to baseline symptoms by using principal component analysis. Correlations and change in the severity of symptom clusters were analyzed after study treatment.
Results.
A total of 114 participants were included in this study. Three clusters were identified: fatigue/anorexia-cachexia/depression (FAD), sleep/anxiety/drowsiness (SAD), and pain/dyspnea (PD). Changes in severity of FAD and PD significantly correlated over time (at baseline, day 8, and day 15). The FAD cluster was associated with significant improvement in severity at day 8 and day 15, whereas no significant change was observed with the SAD cluster or PD cluster after dexamethasone treatment.
Conclusion.
The results of this preliminary study suggest significant correlation over time and improvement in the FAD cluster at day 8 and day 15 after treatment with dexamethasone. These findings suggest that fatigue, anorexia-cachexia, and depression may share a common pathophysiologic basis. Further studies are needed to investigate this cluster and target anti-inflammatory therapies.
Implications for Practice:
Results of this preliminary study suggest that fatigue-anorexia/cachexia/depression cluster scores significantly improved in the dexamethasone treatment group as compared with placebo. These findings indicate that symptoms in the fatigue/anorexia-cachexia/depression cluster might share a common causative mechanism. Further studies are needed to validate these findings.
Introduction
Patients with advanced cancer frequently have severe physical and psychological symptoms [1, 2]. These symptoms usually present in clusters rather than in isolation [3, 4]. Dodd et al. defined a symptom cluster as three or more symptoms that co-occur, whether they share an etiology [3].
Pain, fatigue, sleep disturbance, drowsiness, dyspnea, anorexia/cachexia, and depression are the most common and debilitating symptoms in patients with advanced cancer and are associated with overall survival time [1, 5, 6]. When uncontrolled negatively, these symptoms affect a patient’s physical, psychosocial, and functional well-being [1, 7]. These symptoms usually co-occur and constitute clinically relevant clusters in patients with advanced cancer [8–10]. Unfortunately, these symptoms, as well as clusters, are often inadequately treated in cases of advanced cancer [11–14].
Few pharmacological studies have targeted symptom clusters in advanced cancer [15] because of limited understanding of the symptoms’ causative mechanisms. However, for the symptoms mentioned in the preceding paragraph, evolving evidence points to the central role of proinflammatory cytokines in their causation and worsening [16–24]. Yet several studies suggest only a modest association of proinflammatory cytokines with cancer-related pain, fatigue, anorexia, and depression [21, 25–31]. These cytokines are presumed to be secreted by the tumor and host in response to the disease and its treatments, including radiation therapy and chemotherapy. These cytokines have been implicated in the pathophysiology of these symptoms by acting at multiple levels, including brain-hypothalamic-pituitary-adrenal axis alteration, sleep disturbance, and psychological factors, muscle mass, strength, immunity (cellular and humoral), and metabolic status [20, 21].
Prior studies by our team [32, 33] and others [12, 34–36] support the role of steroids’ potent anti-inflammatory effects to alleviate cancer-related symptoms. However, these studies have not assessed the effect of steroids on symptom clusters using validated outcome measures. Therefore, in this secondary analysis of data from a previously reported study [37], we examined the effects of a pharmacological treatment (dexamethasone) on symptom clusters in patients with advanced cancer.
Methods
The study design and patient recruitment method have been described previously [37]. Briefly, we accessed the original data from a previously conducted placebo-controlled, double-blind, randomized clinical trial to determine the effects of dexamethasone and placebo on symptom burden (NCT00489307).
Analysis of Intervention on Symptom Cluster
Participants
As previously described, patients were recruited from the outpatient clinics for palliative care, pain management, and oncology at the MD Anderson Cancer Center, Houston, TX; from the population of outpatients at Lyndon B. Johnson General Hospital, Houston, TX; or from the Four Seasons Hospice, Flat Rock, NC. Inclusion criteria included a diagnosis of advanced cancer and the presence of at least three symptoms during the previous 24 hours (pain, fatigue, chronic nausea and anorexia/cachexia, sleep problems, depression, or poor appetite) with an average intensity of ≥4 on the Edmonton Symptom Assessment Scale (ESAS; a 0–10 scale). Other important inclusion criteria were as follows: normal cognition, no current infections, hemoglobin level ≥9 g/L within 1 week of enrollment, life expectancy of at least 4 weeks, no history of acquired immunodeficiency syndrome, absolute neutrophil count ≥750 cells/mm3 within 1 week of enrollment in the study, and no diabetes or surgery in the last 2 weeks of enrollment in the study.
Intervention
Eligible patients who agreed to participate were randomly assigned to receive 4 mg of dexamethasone or placebo orally, twice a day, for 14 days. We reviewed the patients’ demographic information and their scores on the Brief Pain Inventory (BPI), Functional Assessment of Chronic Illness Therapy-Fatigue (FACIT-F), Functional Assessment of Anorexia/Cachexia Therapy (FAACT), and Hospital Anxiety and Depression Scale (HADS). The data for the all symptom assessment instruments were collected at baseline, day 8, and day 15 (primary endpoint) of the previous study. For this analysis, we included all participants in the trial who had received study drug (dexamethasone or placebo) in accordance with the study protocol. The following assessment instruments were administered as part of the original protocol.
BPI
The BPI asks patients to rate their pain for the last week on 0–10 scales at its “worst,” “least,” “average,” and as it is “now.” The scales are presented in a 10-cm line, with each number equidistant from the next. Each scale is bounded by the words “no pain” at the 0 end and “pain as bad as you can imagine” at the 10 end. Issues of the validity and reliability of the BPI have been examined in detail [38, 39]. For this study we used the “pain at its worst in the last 24 hours” item as the outcome measure for pain [40].
FACIT-F
The FACIT-F is a well-validated quality-of-life instrument widely used to assess cancer-related fatigue in clinical trials [41]. FACIT-F allows patients to rate the intensity of their fatigue and its related symptoms on a scale of 0–4 (0 = not at all, 4 = very much). This scale has strong internal consistency (α = 0.93–0.95). It has a sensitivity of 0.92 and specificity of 0.69 [42]. For this study, we used the 13-item FACIT-F fatigue subscale as the outcome measure for fatigue.
FAACT
The FAACT-Anorexia/Cachexia subscale is a 12-item symptom-specific subscale designed to measure patients’ concerns about their anorexia/cachexia during the previous 7 days. The FAACT has internal consistency and a reliability coefficient (Cronbach α) of 0.88 for the 12 components. Patients rate the intensity of anorexia/cachexia and its related symptoms on a 0–4 scale like that used in the FACIT-F.
HADS
Depression and anxiety were measured by using the HADS scale. This 14-item questionnaire has been validated in many clinical situations and has been widely used in patients with advanced disease [43, 44].
Ethical Considerations
We received approval from The University of Texas MD Anderson Cancer Center’s institutional review board to conduct the present analysis. All patients had provided written informed consent for such a use of their data at the time of their enrollment in the trial. Due diligence was taken to protect the patients’ confidentiality.
Statistical Analysis
Our objective was to examine the effects of dexamethasone on the symptom clusters in patients with advanced cancer. To compare patient characteristics, we used Wilcoxon rank sum test and the chi-square test (or the Fisher exact test for variables with expected cell frequencies of ≤5).
Principal component analysis with varimax rotation (PCA) was performed on the baseline ESAS items to derive the symptom clusters. PCA is statistical technique used in prior studies in patients with advanced cancer to examine the symptoms that correlate with each other to form a distinct and stable pattern, which is known as a component in the PCA model [45–48]. In 114 evaluable patients, the following three clusters accounted for 63% of the total variance: the fatigue/anorexia-cachexia/depression (FAD) cluster, the sleep/anxiety/drowsiness (SAD) cluster, and the pain/dyspnea (PD) cluster. Cluster scores of the clusters identified by using PCA were computed by adding each scale, divided by the maximum value for the scale: FAD = (fatigue subscale/52 + anorexia-cachexia subscale/48 + recoded HADS-Depression/21); SAD = (recoded ESAS-Sleep/10 + recoded HADS-Anxiety/21 + recoded ESAS-Drowsiness/10); PD = (recoded BPI/10 + recoded ESAS-Dyspnea/10). Clusters range from 0 to 3.0 or 4.0 depending on the number of symptoms in the cluster; a higher number indicates better quality-of-life scores. Spearman ρ correlation coefficients were used to evaluate associations of cluster scores at baseline, day 8, and day 15 after treatment of dexamethasone. Changes in FAD, SAD, and PD cluster scores from baseline to day 8 and day 15 between the dexamethasone and placebo groups were compared by using an independent-sample Mann-Whitney U test.
We analyzed all data for all patients who received at least one dose of study medication at each time point; hence, no imputation of missing data was applied for the analysis of data in this study. The normality assumption was tested by using the Shapiro-Wilk W statistic [49]. A p value of ≤.05 was considered to represent a statistically significant difference. Because this was a preliminary study, the p values were not corrected for multiple comparisons. Analyses were performed by using SAS software, version 9.3 (SAS Institute, Inc., Cary, NC, https://www.sas.com) and IBM SPSS Statistics, version 19 (IBM, Armonk, NY, http://www-01.ibm.com).
Results
Patient Characteristics
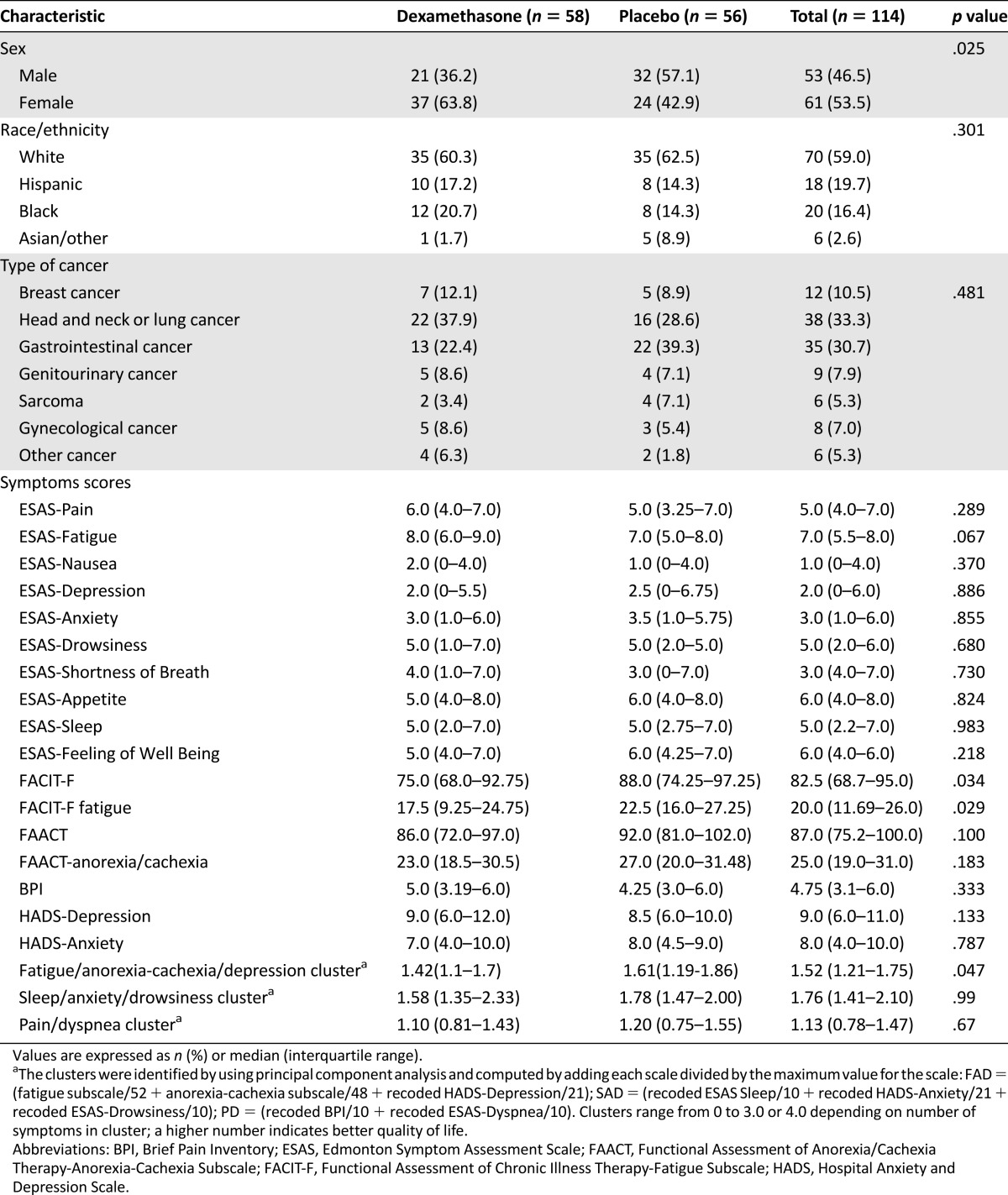
A total of 114 participants were included in this study. At baseline, we found no difference in patient characteristics between the dexamethasone and placebo groups except that dexamethasone group had higher proportion of woman and more severe FAD cluster scores (Table 1).
Table 1.
Patient characteristics at baseline
Correlations
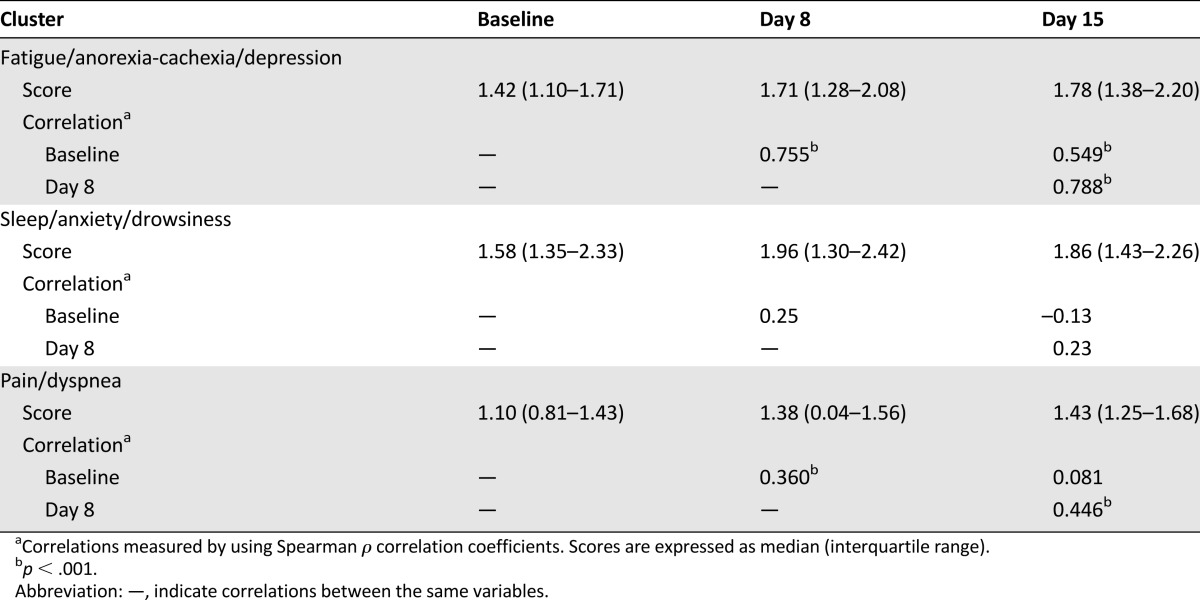
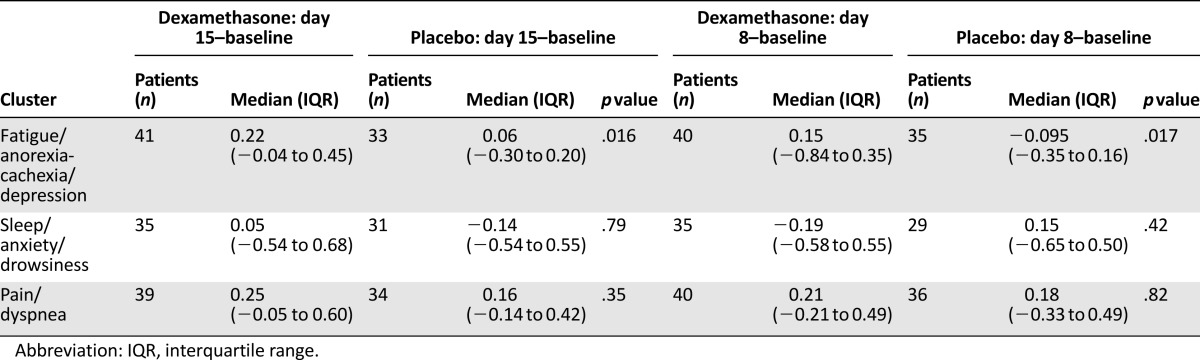
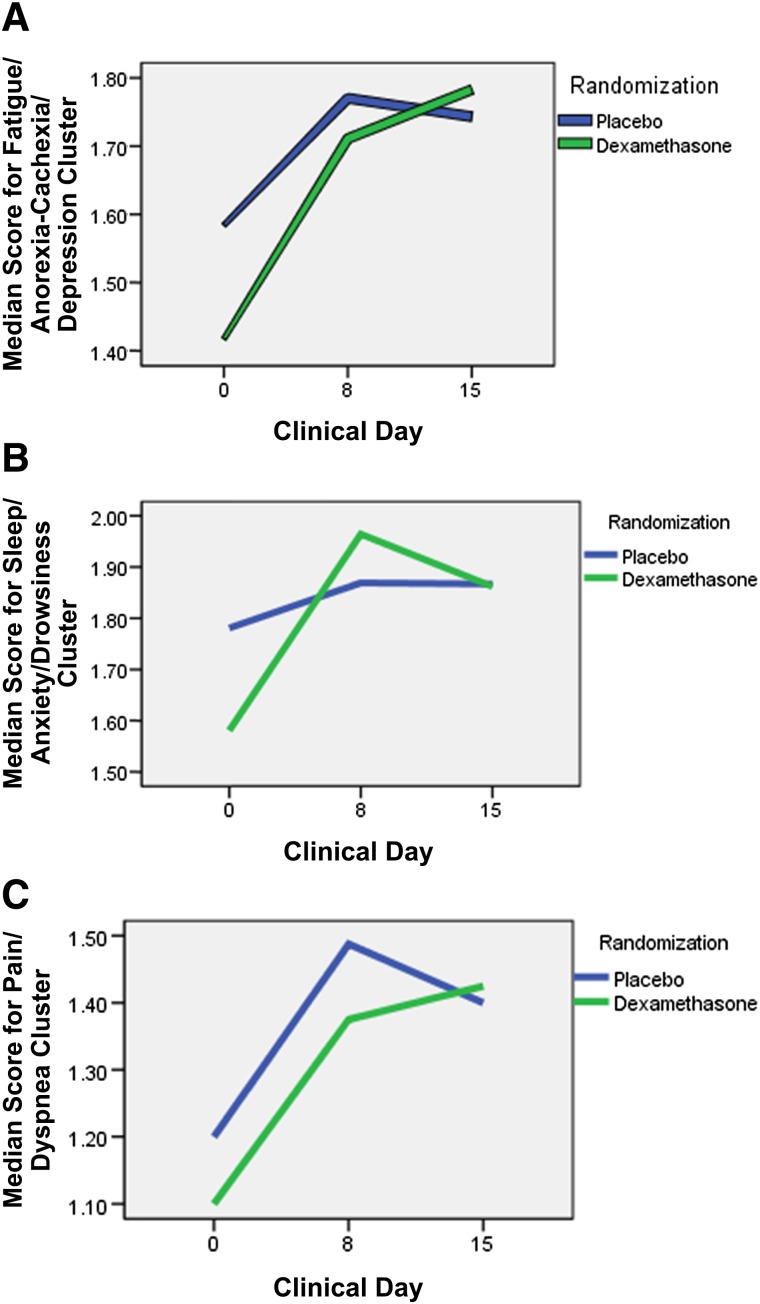
FAD and FAD cluster scores were significantly correlated with one another at all three time points (baseline, day 8, and day 15) (Table 2). Median (interquartile range) improvement in the FAD cluster at day 15 and day 8 was significantly higher in the dexamethasone group than in the placebo group (0.22 [−0.04 to 0.45] vs. 0.06 [−0.30–0.20], p = .016, and 0.15 [−0.84 to 0.35] vs. −0.095 [−0.35 to 0.16], p = .017), respectively (Table 3). Figure 1 shows the median symptom cluster scores at baseline, day 8, and day 15 in the FAD, SAD, and PD clusters.
Table 2.
Association between cluster scores at baseline, day 8, and day 15
Table 3.
Median changes in cluster scores at day 8 and day 15
Figure 1.
Median cluster scores at baseline, day 8, and day 15 by treatment group. (A): Fatigue/anorexia-cachexia/depression cluster. (B): Sleep/anxiety/drowsiness cluster. (C): Pain/dyspnea cluster.
Discussion
In this preliminary study, we examined for the first time the effects of a pharmacological intervention on a symptom clusters in patients with advanced cancer. Of the three clusters identified by principal component analysis, we found significant improvement in the FAD cluster scores compared with placebo at day 8 and day 15 of treatment.
Prior studies on the use of steroids showed short-term beneficial effects on cancer-related fatigue and anorexia [50–52]. However, no studies have investigated the use of steroids for the treatment of depression in advanced cancer patients. The response in the same direction under treatment with dexamethasone in the FAD cluster may suggest that clustered symptoms have the same pathophysiology and thus respond to the same treatment. Because cytokine dysregulation is significantly associated with the pathophysiology of cancer-related fatigue, anorexia/cachexia, and depression [20, 21, 25, 31, 53, 54], the beneficial effects of dexamethasone on the FAD cluster are probably due to the ability of the drug to inhibit these mediators. However, this hypothesis remains to be tested.
The results also suggest that dexamethasone is not effective against SAD and PD clusters. This result is consistent with findings of prior steroid studies in which pain was the primary outcome [52, 55]. This lack of significant improvement in these symptoms may be due to a lack of significant short-term efficacy or to inadequate dosing for the treatment of SAD and PD clusters; there was no deterioration of the symptom cluster scores or significant differences in the adverse events between groups [32]. It is important to remember, however, that dose and time are important factors in treating patients with advanced cancer. The administration of high doses of dexamethasone over a long period may not be practical because of the high frequency of adverse effects [56]. Furthermore, follow-up data for patients with advanced cancer are limited because of disease progression, and logistic issues are frequent problems in this cohort. These factors underscore the need for interventions that result in early improvement of clusters of symptoms. Future studies are needed to test the possibility of combined treatments (multimodal therapies) that might have synergistic effects and thereby promptly improve symptom cluster.
The results of this study are important for various reasons. First, although most patients with advanced cancer present with a cluster of physical and psychological symptoms, most intervention studies have focused on single symptoms. Therefore, these studies have not considered the potential effects (beneficial or deleterious) of the interventions on other symptoms that may affect the patient’s quality of life. Second, even in intervention studies that have targeted a symptom cluster, most have used nonpharmacological interventions [15, 57]. The current study is the first pharmacological study [15, 58, 59] to target a symptom cluster in advanced cancer. Third, our study is unique in that it tested the effects of a drug (dexamethasone) known to affect the common pathway of causation (inflammation [15, 21]) of all the symptoms in the cluster. The positive response of fatigue and anorexia/cachexia to dexamethasone further substantiates the close association of these symptoms and the need to treat both symptoms together rather than individually. Finally, an important positive aspect of this study is that we used validated tools and a novel method to analyze the effects of the intervention on the symptom cluster.
Despite the strengths of this analysis, its multiple limitations should also be mentioned. First, this analysis was the secondary objective of the previously published original study and therefore was not adequately powered to obtain any definitive conclusions. Second, our failure to detect a treatment response to dexamethasone in the SAD and PD clusters may have resulted from the small size of that group, not a true lack of effect. Hence, larger studies are needed to validate our findings. Third, the science of symptom clusters is evolving, and what constitutes a clinically significant improvement in a symptom cluster remains to be defined.
Conclusion
The results of this preliminary study suggest that FAD cluster scores significantly improved in the dexamethasone group as compared with placebo. These findings suggest that the symptoms of the FAD cluster (fatigue/anorexia-cachexia/depression) may share a common pathophysiologic basis. Further well-designed studies are needed to target the FAD cluster using anti-inflammatory therapies.
This article is available for continuing medical education credit at CME.TheOncologist.com.
Acknowledgments
We thank Kathryn B. Carnes for manuscript review. This study was supported by the American Cancer Society, Mentored Research Scholar Grant (MRSG-07-001-01-CCE). Preparation of this manuscript was supported in part by the MD Anderson Cancer Center support grant CA 016672; American Cancer Society (RSG-11-170-01-PCSM) (S.Y.).
Footnotes
For Further Reading: Hye Sook Han, Ji Chan Park, Suk Young Park et al. A Prospective Multicenter Study Evaluating Secondary Adrenal Suppression After Antiemetic Dexamethasone Therapy in Cancer Patients Receiving Chemotherapy: A Korean South West Oncology Group Study. The Oncologist 2015;20:1432–1439.
Implications for Practice: This large prospective multicenter study indicates that approximately 15% of cancer patients receiving chemotherapy with a normal adrenal response show secondary adrenal suppression after antiemetic dexamethasone therapy. Adrenal suppression was particularly significant for patients cotreated with megestrol acetate. Clinicians need increased awareness of the potential for adrenal insufficiency secondary to antiemetic dexamethasone therapy in cancer patients receiving chemotherapy. These findings should help encourage prospective studies designed to determine the adequate doses and durations of antiemetic dexamethasone therapy required to reduce dexamethasone-related adverse effects while controlling chemotherapy-induced nausea and vomiting.
Author Contributions
Conception and design: Sriram Yennurajalingam, Eduardo Bruera
Provision of study material or patients: Sriram Yennurajalingam, Janet L. Williams, Gary Chisholm, Eduardo Bruera
Collection and/or assembly of data: Sriram Yennurajalingam, Janet L. Williams, Gary Chisholm, Eduardo Bruera
Data analysis and interpretation: Sriram Yennurajalingam, Gary Chisholm, Eduardo Bruera
Manuscript writing: Sriram Yennurajalingam, Janet L. Williams, Gary Chisholm, Eduardo Bruera
Final approval of manuscript: Sriram Yennurajalingam, Janet L. Williams, Gary Chisholm, Eduardo Bruera
Disclosures
The authors indicated no financial relationships.
References
- 1.Teunissen SCCM, Wesker W, Kruitwagen C, et al. Symptom prevalence in patients with incurable cancer: A systematic review. J Pain Symptom Manage. 2007;34:94–104. doi: 10.1016/j.jpainsymman.2006.10.015. [DOI] [PubMed] [Google Scholar]
- 2.Yennurajalingam S, Urbauer DL, Casper KLB, et al. Impact of a palliative care consultation team on cancer-related symptoms in advanced cancer patients referred to an outpatient supportive care clinic. J Pain Symptom Manage. 2011;41:49–56. doi: 10.1016/j.jpainsymman.2010.03.017. [DOI] [PubMed] [Google Scholar]
- 3.Dodd MJ, Miaskowski C, Paul SM. Symptom clusters and their effect on the functional status of patients with cancer. Oncol Nurs Forum. 2001;28:465–470. [PubMed] [Google Scholar]
- 4.Dodd M, Janson S, Facione N, et al. Advancing the science of symptom management. J Adv Nurs. 2001;33:668–676. doi: 10.1046/j.1365-2648.2001.01697.x. [DOI] [PubMed] [Google Scholar]
- 5.Walsh D, Rybicki L, Nelson KA, et al. Symptoms and prognosis in advanced cancer. Support Care Cancer. 2002;10:385–388. doi: 10.1007/s00520-001-0318-z. [DOI] [PubMed] [Google Scholar]
- 6.Trajkovic-Vidakovic M, de Graeff A, Voest EE, et al. Symptoms tell it all: A systematic review of the value of symptom assessment to predict survival in advanced cancer patients. Crit Rev Oncol Hematol. 2012;84:130–148. doi: 10.1016/j.critrevonc.2012.02.011. [DOI] [PubMed] [Google Scholar]
- 7.Butt Z, Rosenbloom SK, Abernethy AP, et al. Fatigue is the most important symptom for advanced cancer patients who have had chemotherapy. J Natl Compr Canc Netw. 2008;6:448–455. doi: 10.6004/jnccn.2008.0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu L, Fiorentino L, Natarajan L, et al. Pre-treatment symptom cluster in breast cancer patients is associated with worse sleep, fatigue and depression during chemotherapy. Psychooncology. 2009;18:187–194. doi: 10.1002/pon.1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Laird BJA, Scott AC, Colvin LA, et al. Pain, depression, and fatigue as a symptom cluster in advanced cancer. J Pain Symptom Manage. 2011;42:1–11. doi: 10.1016/j.jpainsymman.2010.10.261. [DOI] [PubMed] [Google Scholar]
- 10.Dong ST, Costa DSJ, Butow PN, et al. Symptom clusters in advanced cancer patients: An empirical comparison of statistical methods and the impact on quality of life. J Pain Symptom Manage. 2016;51:88–98. doi: 10.1016/j.jpainsymman.2015.07.013. [DOI] [PubMed] [Google Scholar]
- 11.Fisch MJ, Lee J-W, Weiss M, et al. Prospective, observational study of pain and analgesic prescribing in medical oncology outpatients with breast, colorectal, lung, or prostate cancer. J Clin Oncol. 2012;30:1980–1988. doi: 10.1200/JCO.2011.39.2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yennurajalingam S, Bruera E. Palliative management of fatigue at the close of life: “It feels like my body is just worn out.”. JAMA. 2007;297:295–304. doi: 10.1001/jama.297.3.295. [DOI] [PubMed] [Google Scholar]
- 13.Macciò A, Madeddu C, Mantovani G. Current pharmacotherapy options for cancer anorexia and cachexia. Expert Opin Pharmacother. 2012;13:2453–2472. doi: 10.1517/14656566.2012.734297. [DOI] [PubMed] [Google Scholar]
- 14.Irving G, Lloyd-Williams M. Depression in advanced cancer. Eur J Oncol Nurs. 2010;14:395–399. doi: 10.1016/j.ejon.2010.01.026. [DOI] [PubMed] [Google Scholar]
- 15.Berger AMYS, Yennu S, Million R. Update on interventions focused on symptom clusters: What has been tried and what have we learned? Curr Opin Support Palliat Care. 2013;7:60–66. doi: 10.1097/SPC.0b013e32835c7d88. [DOI] [PubMed] [Google Scholar]
- 16.Hutchinson MR, Coats BD, Lewis SS, et al. Proinflammatory cytokines oppose opioid-induced acute and chronic analgesia. Brain Behav Immun. 2008;22:1178–1189. doi: 10.1016/j.bbi.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cleeland CS, Bennett GJ, Dantzer R, et al. Are the symptoms of cancer and cancer treatment due to a shared biologic mechanism? A cytokine-immunologic model of cancer symptoms. Cancer. 2003;97:2919–2925. doi: 10.1002/cncr.11382. [DOI] [PubMed] [Google Scholar]
- 18.Miaskowski C, Aouizerat BE. Is there a biological basis for the clustering of symptoms? Semin Oncol Nurs. 2007;23:99–105. doi: 10.1016/j.soncn.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 19.Bower JE, Ganz PA, Irwin MR, et al. Inflammation and behavioral symptoms after breast cancer treatment: do fatigue, depression, and sleep disturbance share a common underlying mechanism? J Clin Oncol. 2011;29:3517–3522. doi: 10.1200/JCO.2011.36.1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miller AH, Ancoli-Israel S, Bower JE, et al. Neuroendocrine-immune mechanisms of behavioral comorbidities in patients with cancer. J Clin Oncol. 2008;26:971–982. doi: 10.1200/JCO.2007.10.7805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Seruga B, Zhang H, Bernstein LJ, et al. Cytokines and their relationship to the symptoms and outcome of cancer. Nat Rev Cancer. 2008;8:887–899. doi: 10.1038/nrc2507. [DOI] [PubMed] [Google Scholar]
- 22.Miller DB, O’Callaghan JP. Depression, cytokines, and glial function. Metabolism. 2005;54(suppl 1):33–38. doi: 10.1016/j.metabol.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 23.Austin PJ, Moalem-Taylor G. The neuro-immune balance in neuropathic pain: Involvement of inflammatory immune cells, immune-like glial cells and cytokines. J Neuroimmunol. 2010;229:26–50. doi: 10.1016/j.jneuroim.2010.08.013. [DOI] [PubMed] [Google Scholar]
- 24.Watkins LR, Maier SF. Implications of immune-to-brain communication for sickness and pain. Proc Natl Acad Sci USA. 1999;96:7710–7713. doi: 10.1073/pnas.96.14.7710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fearon KC, Glass DJ, Guttridge DC. Cancer cachexia: Mediators, signaling, and metabolic pathways. Cell Metab. 2012;16:153–166. doi: 10.1016/j.cmet.2012.06.011. [DOI] [PubMed] [Google Scholar]
- 26.Raison CL, Borisov AS, Woolwine BJ, et al. Interferon-alpha effects on diurnal hypothalamic-pituitary-adrenal axis activity: Relationship with proinflammatory cytokines and behavior. Mol Psychiatry. 2010;15:535–547. doi: 10.1038/mp.2008.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dantzer R, Kelley KW. Twenty years of research on cytokine-induced sickness behavior. Brain Behav Immun. 2007;21:153–160. doi: 10.1016/j.bbi.2006.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sommer C, Kress M. Recent findings on how proinflammatory cytokines cause pain: peripheral mechanisms in inflammatory and neuropathic hyperalgesia. Neurosci Lett. 2004;361:184–187. doi: 10.1016/j.neulet.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 29.de Raaf PJ, Sleijfer S, Lamers CHJ, et al. Inflammation and fatigue dimensions in advanced cancer patients and cancer survivors: an explorative study. Cancer. 2012;118:6005–6011. doi: 10.1002/cncr.27613. [DOI] [PubMed] [Google Scholar]
- 30.Laird BJ, McMillan DC, Fayers P, et al. The systemic inflammatory response and its relationship to pain and other symptoms in advanced cancer. The Oncologist. 2013;18:1050–1055. doi: 10.1634/theoncologist.2013-0120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saligan LN, Olson K, Filler K, et al. The biology of cancer-related fatigue: A review of the literature. Support Care Cancer. 2015;23:2461–2478. doi: 10.1007/s00520-015-2763-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bruera E, Roca E, Cedaro L, et al. Action of oral methylprednisolone in terminal cancer patients: A prospective randomized double-blind study. Cancer Treat Rep. 1985;69:751–754. [PubMed] [Google Scholar]
- 33.Yennurajalingam S, Frisbee-Hume S, Palmer JL, et al. Reduction of cancer-related fatigue with dexamethasone: A double-blind, randomized, placebo-controlled trial in patients with advanced cancer. J Clin Oncol. 2013;31:3076–3082. doi: 10.1200/JCO.2012.44.4661. [DOI] [PubMed] [Google Scholar]
- 34.Moertel CG, Schutt AJ, Reitemeier RJ, et al. Corticosteroid therapy of preterminal gastrointestinal cancer. Cancer. 1974;33:1607–1609. doi: 10.1002/1097-0142(197406)33:6<1607::aid-cncr2820330620>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 35.Della Cuna GRPA, Pellegrini A, Piazzi M. Effect of methylprednisolone sodium succinate on quality of life in preterminal cancer patients: A placebo-controlled, multicenter study. The Methylprednisolone Preterminal Cancer Study Group. Eur J Cancer Clin Oncol. 1989;25:1817–1821. doi: 10.1016/0277-5379(89)90353-2. [DOI] [PubMed] [Google Scholar]
- 36.Lundström SH, Fürst CJ. The use of corticosteroids in Swedish palliative care. Acta Oncol. 2006;45:430–437. doi: 10.1080/02841860500401167. [DOI] [PubMed] [Google Scholar]
- 37.Yennurajalingam S, Frisbee-Hume F, Delgado-Guay MO, Bull J, et al. Dexamethasone (DM) for cancer-related fatigue: A double-blinded, randomized, placebo-controlled trial. J Clin Oncol. 2012;(suppl):9002a. doi: 10.1200/JCO.2012.44.4661. [DOI] [PubMed] [Google Scholar]
- 38.Daut RL, Cleeland CS, Flanery RC. Development of the Wisconsin Brief Pain Questionnaire to assess pain in cancer and other diseases. Pain. 1983;17:197–210. doi: 10.1016/0304-3959(83)90143-4. [DOI] [PubMed] [Google Scholar]
- 39.Atkinson TM, Rosenfeld BD, Sit L, et al. Using confirmatory factor analysis to evaluate construct validity of the Brief Pain Inventory (BPI) J Pain Symptom Manage. 2011;41:558–565. doi: 10.1016/j.jpainsymman.2010.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Atkinson TM, Mendoza TR, Sit L, et al. The Brief Pain Inventory and its “pain at its worst in the last 24 hours” item: clinical trial endpoint considerations. Pain Med. 2010;11:337–346. doi: 10.1111/j.1526-4637.2009.00774.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cella DF, Tulsky DS, Gray G, et al. The Functional Assessment of Cancer Therapy scale: Development and validation of the general measure. J Clin Oncol. 1993;11:570–579. doi: 10.1200/JCO.1993.11.3.570. [DOI] [PubMed] [Google Scholar]
- 42.Cella D, Eton DT, Lai J-S, et al. Combining anchor and distribution-based methods to derive minimal clinically important differences on the Functional Assessment of Cancer Therapy (FACT) anemia and fatigue scales. J Pain Symptom Manage. 2002;24:547–561. doi: 10.1016/s0885-3924(02)00529-8. [DOI] [PubMed] [Google Scholar]
- 43.Johnston M, Pollard B, Hennessey P. Construct validation of the hospital anxiety and depression scale with clinical populations. J Psychosom Res. 2000;48:579–584. doi: 10.1016/s0022-3999(00)00102-1. [DOI] [PubMed] [Google Scholar]
- 44.Carroll BT, Kathol RG, Noyes R, Jr, et al. Screening for depression and anxiety in cancer patients using the Hospital Anxiety and Depression Scale. Gen Hosp Psychiatry. 1993;15:69–74. doi: 10.1016/0163-8343(93)90099-a. [DOI] [PubMed] [Google Scholar]
- 45.Barsevick AM, Whitmer K, Nail LM, et al. Symptom cluster research: conceptual, design, measurement, and analysis issues. J Pain Symptom Manage. 2006;31:85–95. doi: 10.1016/j.jpainsymman.2005.05.015. [DOI] [PubMed] [Google Scholar]
- 46.Cheung WY, Le LW, Zimmermann C. Symptom clusters in patients with advanced cancers. Support Care Cancer. 2009;17:1223–1230. doi: 10.1007/s00520-009-0577-7. [DOI] [PubMed] [Google Scholar]
- 47.Yennurajalingam S, Kwon JH, Urbauer DL, et al. Consistency of symptom clusters among advanced cancer patients seen at an outpatient supportive care clinic in a tertiary cancer center. Palliat Support Care. 2013;11:473–480. doi: 10.1017/S1478951512000879. [DOI] [PubMed] [Google Scholar]
- 48.Chow E, Fan G, Hadi S, et al. Symptom clusters in cancer patients with bone metastases. Support Care Cancer. 2007;15:1035–1043. doi: 10.1007/s00520-007-0241-z. [DOI] [PubMed] [Google Scholar]
- 49.Shapiro SS, Wilk MB. An analysis of variance test for normality (complete samples) Biometrika. 1965;52:591–611. [Google Scholar]
- 50.Loprinzi CL, Kugler JW, Sloan JA, et al. Randomized comparison of megestrol acetate versus dexamethasone versus fluoxymesterone for the treatment of cancer anorexia/cachexia. J Clin Oncol. 1999;17:3299–3306. doi: 10.1200/JCO.1999.17.10.3299. [DOI] [PubMed] [Google Scholar]
- 51.Wilcox JC, Corr J, Shaw J, et al. Prednisolone as an appetite stimulant in patients with cancer. Br Med J (Clin Res Ed) 1984;288:27. doi: 10.1136/bmj.288.6410.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Paulsen O, Klepstad P, Rosland JH, et al. Efficacy of methylprednisolone on pain, fatigue, and appetite loss in patients with advanced cancer using opioids: A randomized, placebo-controlled, double-blind trial. J Clin Oncol. 2014;32:3221–3228. doi: 10.1200/JCO.2013.54.3926. [DOI] [PubMed] [Google Scholar]
- 53.Breitbart W, Rosenfeld B, Tobias K, et al. Depression, cytokines, and pancreatic cancer. Psychooncology. 2014;23:339–345. doi: 10.1002/pon.3422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lutgendorf SK, Weinrib AZ, Penedo F, et al. Interleukin-6, cortisol, and depressive symptoms in ovarian cancer patients. J Clin Oncol. 2008;26:4820–4827. doi: 10.1200/JCO.2007.14.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Paulsen Ø, Aass N, Kaasa S, et al. Do corticosteroids provide analgesic effects in cancer patients? A systematic literature review. J Pain Symptom Manage. 2013;46:96–105. doi: 10.1016/j.jpainsymman.2012.06.019. [DOI] [PubMed] [Google Scholar]
- 56.Hempen C, Weiss E, Hess CF. Dexamethasone treatment in patients with brain metastases and primary brain tumors: Do the benefits outweigh the side-effects? Support Care Cancer. 2002;10:322–328. doi: 10.1007/s00520-001-0333-0. [DOI] [PubMed] [Google Scholar]
- 57.Chan CWH, Richardson A, Richardson J. Managing symptoms in patients with advanced lung cancer during radiotherapy: Results of a psychoeducational randomized controlled trial. J Pain Symptom Manage. 2011;41:347–357. doi: 10.1016/j.jpainsymman.2010.04.024. [DOI] [PubMed] [Google Scholar]
- 58.Kerr CW, Drake J, Milch RA, et al. Effects of methylphenidate on fatigue and depression: A randomized, double-blind, placebo-controlled trial. J Pain Symptom Manage. 2012;43:68–77. doi: 10.1016/j.jpainsymman.2011.03.026. [DOI] [PubMed] [Google Scholar]
- 59.Yennurajalingam S, Willey JS, Palmer JL, et al. The role of thalidomide and placebo for the treatment of cancer-related anorexia-cachexia symptoms: Results of a double-blind placebo-controlled randomized study. J Palliat Med. 2012;15:1059–1064. doi: 10.1089/jpm.2012.0146. [DOI] [PMC free article] [PubMed] [Google Scholar]