Although there were important regional variations, patients and physicians were generally aware of the advances in precision medicine and showed willingness to participate in associated biomarker testing. However, considerable opportunity remains for physicians and support groups to better educate patients about the ability of biomarker testing to inform the most effective, personalized treatment strategy, tailored to the patient’s own tumor.
Keywords: Biomarkers, Colorectal cancer, Individualized medicine, Personalized medicine, Surveys
Abstract
Background.
Two separate multinational surveys of oncologists and patients with cancer were conducted to assess the awareness and use of biomarkers in clinical practice. These data explore the self-reported and physician-assessed levels of patient cancer literacy and factors affecting physicians’ choice to use biomarkers in treatment decisions.
Patients and Methods.
Interviews were conducted via telephone with patients and online with physicians. Physicians had 3–35 years of experience; were treating more than 15 patients/month; and specialized in breast, lung, or colorectal cancer. Patients had received treatment for breast, lung, or colorectal cancer within the previous 5 years.
Results.
Interviews with 895 physicians and 811 patients were completed. Most patients and physicians reported that patients understood that a tumor could be tested to determine what treatment would be most effective (78% and 73%, respectively) and that patients would be willing to participate in a personalized treatment plan. Whereas 85% of patients felt that they understood their treatment when it was explained to them, only 23% of doctors felt that their patients were always fully informed. Most physicians (90%) reported using biomarkers; among the 10% not performing biomarker analysis, the most cited obstacles were local availability, speed of obtaining results, and cost.
Conclusion.
These data demonstrate wide global use of biomarker testing but with regional variations reflecting cultural and local practice. Self-reported and physician-assessed cancer literacy, although generally high, highlighted important regional variations and the need to provide patients with additional information.
Implications for Practice:
Two surveys were conducted to evaluate the global use of biomarkers in clinical practice and the largely unreported patient experience of precision medicine. These findings are especially relevant because they address both self-reported and physician-assessed levels of patients’ “cancer literacy.” This unique opportunity allowed for identification of areas where patients and physicians are communicating effectively, and also where there is a teachable gap in patient education. Furthermore, surveying physicians about the advantages and roadblocks they experience with biomarker testing provided valuable information on ways to improve the delivery of precision medicine to provide personalized care and ultimately enhance patient care.
Introduction
Recent initiatives to encourage the development of useful and accurate biomarkers have highlighted the role of personalized care as an increasingly important tool in modern medicine [1]. Identification of tumor-specific alterations, such as DNA point mutations, amplifications, and translocations, can help determine a patient’s prognosis or predict how well the patient will respond to certain treatments [2, 3]. The presence of biomarkers can serve as a positive predictor, which identifies patients likely to respond to a particular treatment, or a negative predictor, which can detect groups of patients unlikely to derive a clinically meaningful benefit from a given therapy.
Several biomarkers are already in routine clinical use. In metastatic colorectal cancer (mCRC), mutation of RAS is used as a predictive biomarker to select patients most likely to benefit from epidermal growth factor receptor (EGFR) antibody therapy [4–10]. For patients with breast cancer—in addition to the long-standing use of hormone receptor testing—the expression of human epidermal growth factor receptor 2 (HER2) serves as a biomarker for response to HER2 antibody therapy [11–13], and, in non-small cell lung cancer (NSCLC), EGFR mutation and ALK gene rearrangement serve as biomarkers for response to EGFR tyrosine kinase inhibitor [14] and crizotinib therapy [15], respectively.
Over the past few years, biomarker testing and personalized care have been active areas of research, and guidance to clinicians has changed to reflect new information. For example, as early as 2009, the National Comprehensive Cancer Network (NCCN) recommended that patients with mCRC be tested for mutations in exon 2 of the KRAS gene, on the basis of evidence supporting an important prognostic and predictive role for KRAS in mCRC [16, 17]. With recent advances in detection tools and an improved understanding of the effect of RAS pathway mutations on appropriate patient selection, the NCCN recommendations have been updated to include testing of KRAS exons 3 and 4 and NRAS exons 2, 3, and 4 [18]. Similarly, the NCCN guidelines for NSCLC have recently been updated to account for our increased knowledge regarding the importance of biomarker testing for personalized care: ALK testing is now recommended—in addition to EGFR testing—before the initiation of first-line therapy [19]. Thus, both CRC and NSCLC have now joined breast cancer—a tumor type for which, for years, the NCCN guidelines have recommended hormone receptor and HER2 testing [20]—as tumor types for which treatment decisions utterly rely on upfront biomarker testing.
As summarized earlier in the text, customized patient care provided on the basis of biomarker status is an important component of precision medicine, a care model whereby medical decisions and treatments are tailored to the individual patient’s genetics, environment, and lifestyle. Biomarkers have become part of modern clinical practice, but an in-depth understanding of how patients experience personalized care and how physicians implement it in their routine practice is not yet available. Providing timely information to patients to fully inform them about their treatment and biomarker screening options will allow them to appreciate the value of personalized treatment options. Whereas it is assumed that physicians endeavor to provide their patients with an understanding of biomarkers and how the results of biomarker testing could improve their therapeutic options, physicians are often unable to assess how well their patients have assimilated the complex information. The willingness of patients to undergo additional diagnostic procedures and manage consequent delays in the initiation of treatment may also be a key factor in the success of such approaches. Furthermore, with the ongoing process of biomarker discovery, it is important that patients understand the value of biomarker testing because this may influence their willingness to allow their tumor tissue to be used for future studies. Improved understanding of the challenges that physicians and patients face regarding biomarker testing may enable physicians to better align their treatment plans with practice guidelines.
This paper discusses the results of two surveys—one directed to physicians and the other directed to patients—and compares patient-reported and physician-assessed patient cancer literacy. Global uptake of and barriers to biomarker testing are also discussed.
Methods
Patient Survey
Between September and December 2011, interviews of patients with breast cancer, NSCLC, and mCRC were conducted via 15-minute telephone surveys. Interviews were conducted in Argentina, China, France, Germany, Italy, Spain, and the U.K. by a maximum of five interviewers per region. Ipsos (Paris, France, http://www.ipsos.com) identified patients by their physicians and patient associations; in Spain, patients were contacted only through patient associations. To help identify appropriate patients, physicians were given a questionnaire describing the type of patients sought for the survey. Qualified patients were asked whether they wanted to participate in the survey, and those who agreed were given information on how to contact the fieldwork team. To facilitate patient recruitment through patient associations, contacts were given a description of the target respondents, which was distributed along with information on how to contact the fieldwork team. The survey was then conducted in the following order: screening questions, awareness of cancer types and screening, attitudes toward personalized treatments, and sources of information. The full list of survey questions can be found in the supplemental online Appendix.
Physician Survey
Interviews with physicians were conducted via 10-minute online interviews between October and December 2013. Countries included in the survey were as follows: Argentina, Brazil, China, France, Germany, Italy, Japan, Russia, Saudi Arabia, Spain, Turkey, and the U.K. Physicians were recruited via Ipsos. Eligible physicians had 3–35 years of experience in their current specialty, were involved in the day-to-day management of patients with cancer, and saw at least 15 patients per month. Physicians were assigned to a quota on the basis of the most frequent type of cancer they treated in their practice. Soft quotas were placed such that 25% of physicians were assigned to the stage IV breast cancer and stage IIIB/IV NSCLC quotas and 50% were assigned to the stage IV mCRC quota. The survey was conducted in the following order: Physicians were asked to evaluate how much their patients understood about their care, what concerns patients may have about their treatment, and how patients would describe their experience with personalized care. Physicians were then asked about their own use of biomarkers in their practice and factors that contributed to their decision to use biomarkers. The full list of survey questions can be found in the supplemental online Appendix.
Results
Characteristics of Surveyed Physicians and Patients
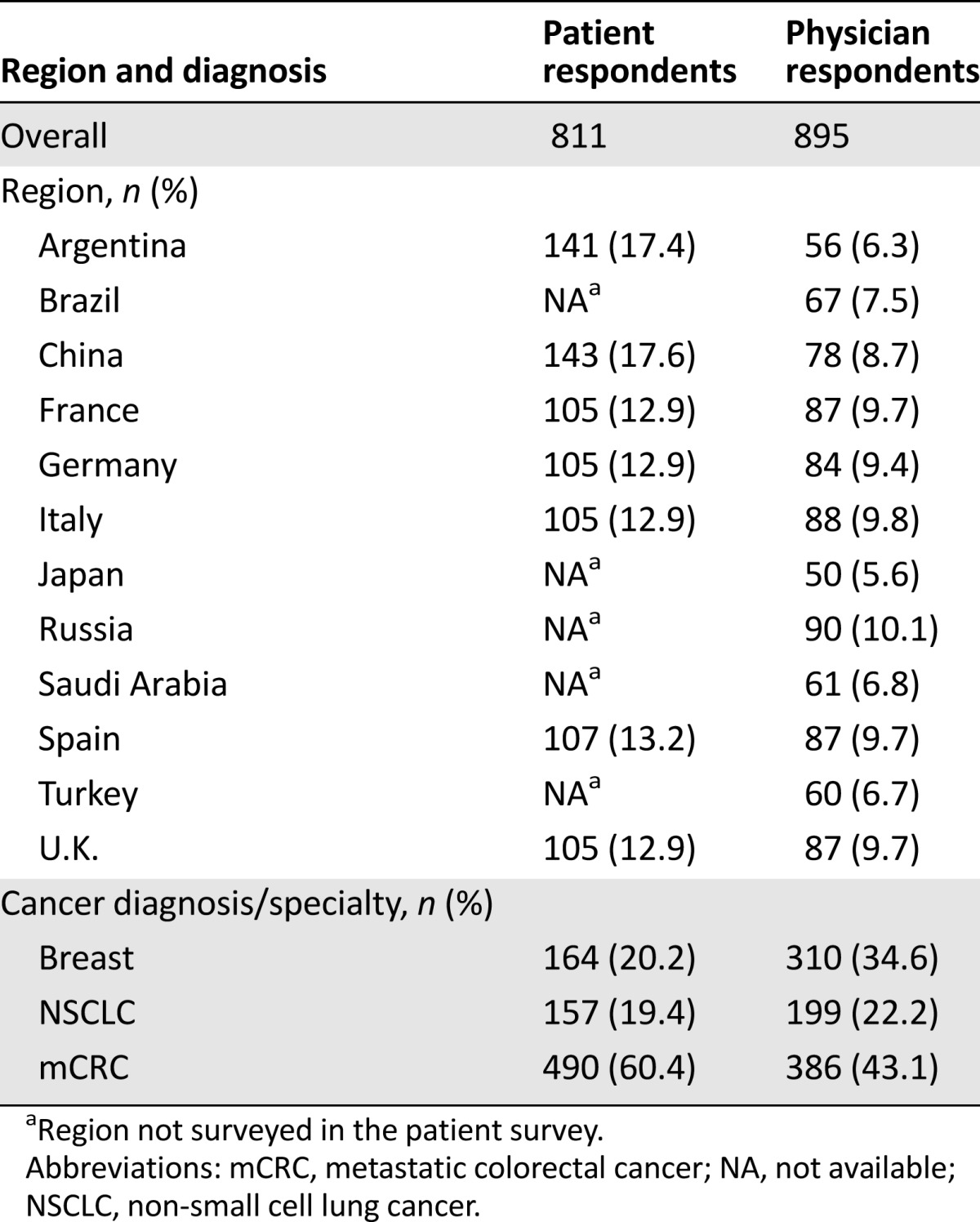
As stated earlier, patients and physicians from Argentina, China, France, Germany, Italy, Spain, and the U.K. were surveyed. In addition, physicians from Brazil, Japan, Russia, Saudi Arabia, and Turkey were interviewed (Table 1). Patients had been diagnosed with NSCLC (19.4%), breast cancer (20.2%), or mCRC (60.4%; Table 1). Physicians were categorized as specialists in NSCLC (22.2%), breast cancer (34.6%), or mCRC (43.1%) according to their highest patient caseload (Table 1). Physicians described themselves as clinical oncologists (17.7%), medical oncologists (49.1%), clinical and medical oncologists (20.1%), oncologists (6.6%), gastroenterologists (4.1%), breast cancer specialists (1.0%), lung cancer specialists (1.0%), or oncology surgeons (0.4%). Among the surveyed patients, 2% were aged 25–35 years, 13% were aged 36–45 years, 27% were aged 46–55 years, and 58% were aged >55 years; 46% of the responding patients were male and 54% were female.
Table 1.
Region and diagnosis/specialty of patients and physicians
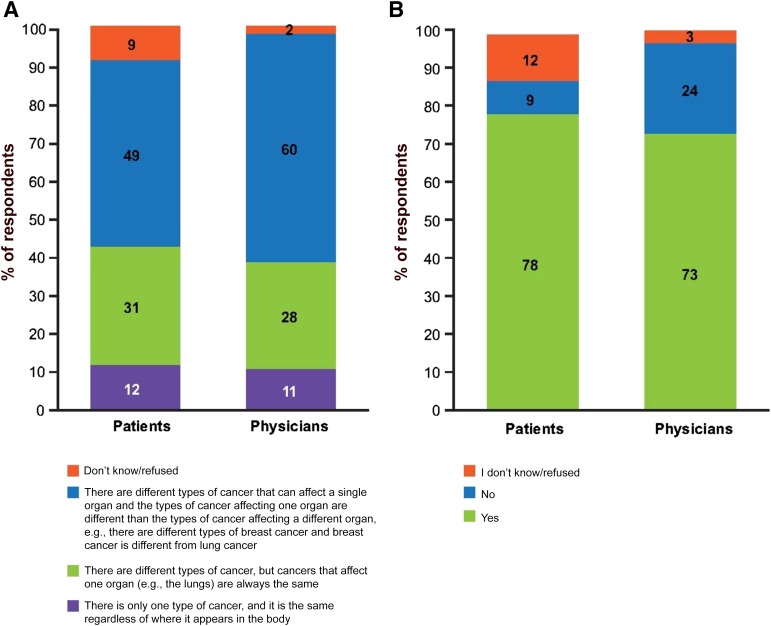
Patients’ Understanding of Tumor Biology and the Principle of Precision Medicine
Patients were asked questions to assess their understanding of cancer biology and precision medicine. Physicians were asked to evaluate how they believed their patients would answer the same or similar questions. Nearly half (49%) of the surveyed patients correctly identified that different types of cancer can affect a single organ and that the types of cancer affecting one organ are different from those affecting other organs. Similarly, most physicians believed that patients understood this concept (Fig. 1A). In contrast, physicians underestimated the percentage of patients who realized that all cancer drugs target both healthy and cancer cells at 49%, whereas 66% of patients reported that they knew this was the case (data not shown).
Figure 1.
Self-reported and physician-assessed patient understanding of tumor biology and personalized care. (A): Patient and physician responses, respectively, to the questions “Which one of the following statements do you believe to be correct?” and “Which one of the following statements do you believe describes most of your cancer patients’ understanding of types of cancer?” (B): Patient and physician responses, respectively, to the questions “Do you think it’s possible in some circumstances for a person to have their tumor tested to help their doctor decide which treatment(s) to give?” and “Do you think your cancer patients understand that it's possible, in some circumstances, for a person to have their tumor tested to help their doctor decide which treatment(s) to give?” The values do not add up to 100% due to rounding.
Most patients (78%) understood that a tumor can be tested to help inform a doctor’s decision-making, which was accurately predicted by physicians (73%; Fig. 1B). This was largely consistent across regions. However, China was a notable exception; 97% of patients and 91% of physicians reported unusually high patient awareness of biomarker testing (supplemental online Fig. 1 and data not shown).
Patient Willingness to Consider Biomarker Testing
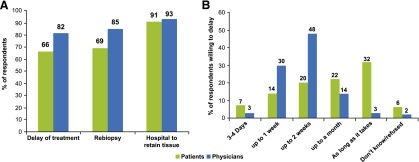
More than half of patients (66%) reported willingness to delay treatment to allow for additional tumor testing, but this was overestimated by physicians, 82% of whom believed that their patients would be willing to delay (Fig. 2A). Interestingly, physicians underestimated the amount of time that patients would be willing to delay treatment to undergo testing, with 22% of patients reporting that they would wait as long as a month and 32% reporting that they would delay as long as it takes. In contrast, only 14% and 3% of physicians, respectively, believed that patients would be willing to wait this long (Fig. 2B).
Figure 2.
Self-reported and physician-assessed patient willingness to participate in a personalized care plan. (A): Patient responses to the questions “Would you be prepared to delay your treatment for additional tumor testing if there was a chance you could receive a drug that might work better for you?,” “Would you be prepared for the doctor to perform a tumor re-biopsy if there was a chance you could receive a drug that might work better for you?,” and “Would you allow a hospital to keep a sample of your cancer for future research?” Physician responses to the questions “In general, do you think your cancer patients would be prepared to delay their treatment for additional tumor testing if there was a chance they could receive a drug that might work better for them?,” “Do you think your cancer patients would be prepared for you to perform a tumor re-biopsy if there was a chance they could receive a drug that might work better for them?,” and “Do you think your cancer patients would allow a hospital to keep a sample of their cancer for future research?” (B): Patient and physician responses, respectively, to the questions “How long would you be willing to delay it for?” and “How long do you think they would be willing to delay their treatment for additional tumor testing?”
Most patients (69%) and physicians (85%) reported that patients would willingly undergo rebiopsy to improve their treatment options (Fig. 2A). However, this trend was not seen in China, where most patients (76%) indicated that they would not be willing to undergo rebiopsy (supplemental online Fig. 2). Regarding whether patients would allow the hospital to retain a sample of their tumor tissue, more than 90% of both patients and physicians reported yes (Fig. 2A).
Patient Desire for Knowledge About Cancer and Their Treatment Options
Most patients (85%) felt that they understood their treatment when it was explained to them by their doctor, but only 23% of doctors (Fig. 3A) felt that their patients were always fully informed. Complementary to this, 78% of oncologists thought that patients needed more information to help them understand the different types of cancers and how they progress. Despite this difference in perceived understanding, 89% of both patients and physicians agreed that patients wanted all their treatment options explained to them. The most common reasons for wanting all treatment options explained were so that patients could be involved in decisions about their treatment (63% and 75%, respectively) and aware of potential adverse effects (63% and 59%, respectively; Fig. 3B). However, about half of patients and physicians indicated that although patients desire information for their own reference, they still want their doctor to make all major decisions about their treatment (50% and 48%, respectively; Fig. 3B).
Figure 3.
Self-reported and physician-assessed patient desire for knowledge about their cancer and treatment options. (A): Physician responses to the question “Do you think your cancer patients feel fully informed and understand their treatment when it is explained by you?” (B): Responses of patients and physicians who answered yes, respectively, to “Would you want your doctor to give you information about all of the available treatment options for your cancer?” or “In general, do you think your cancer patients would want you to give them information about all of the available treatment options for their cancer?” when asked “If yes, why would that be?” (C): Responses of patients and physicians, respectively, when asked “What sources were most useful to you in providing clear information about your cancer and treatment options?" or “What sources of information, if any, are available to your cancer patients to support them in making treatment decisions about their cancer?”
Patient-reported and physician-assessed sources of information about diagnosis and treatment differed considerably. Although both physicians and patients were asked to indicate all sources of information that were available or useful to patients, respectively—from a list of family/friends, nurses, Internet sites, newspaper or magazine articles, and support groups—physicians were also given the option of hospital patient information leaflets and patients were given the option of their doctor. Most patients (91%) identified their doctor as the most helpful source of information (data not shown). Interestingly, physicians believed that the most common sources available to patients were Internet sites (73%); however, only 24% of patients indicated that these were a helpful source of information (Fig. 3C). After their physician, patients indicated that family/friends and their nurses were the most helpful sources of information (38% and 27%, respectively; Fig. 3C). However, only 45% of physicians believed that their patients had access to these nurses (Fig. 3C). This varied dramatically by region; in Russia, only 19% of physicians believed that their patients had access to nurses compared with 83% of physicians in the U.K. (data not shown).
Physicians on the Role of Multidisciplinary Teams
This survey also assessed physicians’ opinion of the role of a multidisciplinary team (MDT) composed of oncologists, radiologists, nurses, and surgeons in planning patient care. Globally, 82% of physicians believed that the treatment decision is a shared decision-making process among the doctor, MDT, and patient. Although this was consistent across specialties, there were considerable regional variations in the response. In Saudi Arabia, only 21% of physicians held this belief, which is in stark contrast to the more than 97% of respondents in Brazil, China, and Turkey who approved of the MDT approach. In Spain, Argentina, and Russia, there was also a comparatively low consensus that the treatment decision was a shared process (72%, 77%, and 70%, respectively).
Physicians’ Use of Biomarker Testing
Most physicians (90%) indicated that they currently use biomarker testing. In contrast, whereas only 9% of physicians indicated that they do not use biomarker testing, 24% of patients reported that their tumors were not tested. According to the physicians, the most commonly used tests were the KRAS mutation test (87%), hormone receptor test (84%), and HER2 expression test (80%).
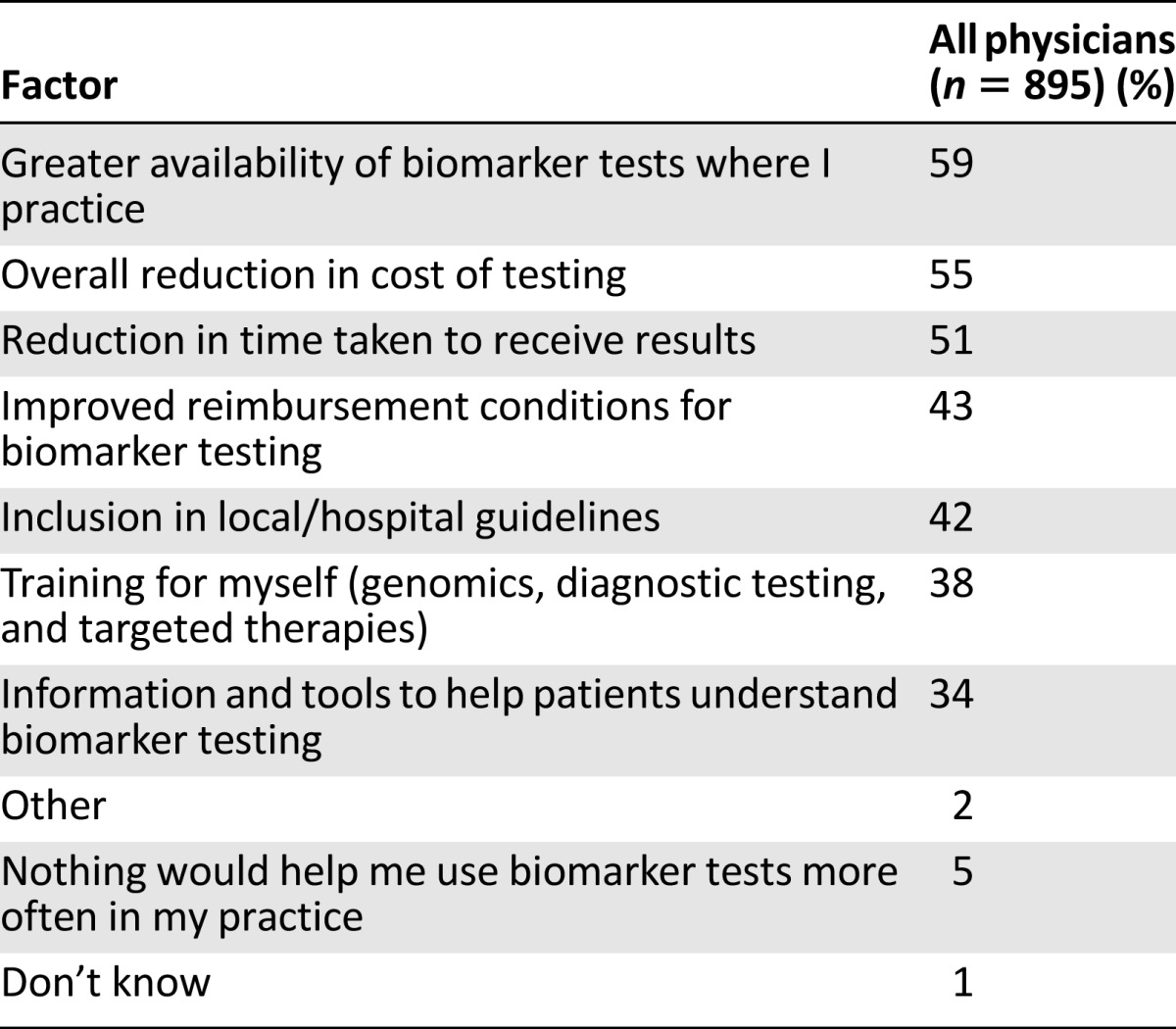
The most common reasons that physicians used biomarker tests were because they influenced treatment options (72%) or were part of treatment guidelines (63%; Table 2). In contrast, the most common reasons that physicians did not use biomarker tests were because they were too expensive or not reimbursed (55%) or were not available or part of treatment guidelines where the doctors practice (47% and 35%, respectively; Table 3). Interestingly, 10% of physicians said that their patients did not want to delay treatment for testing or did not understand the benefits of testing (Table 3). More than half of physicians indicated that greater availability of biomarker tests where they practice (59%), reduction in cost (55%), and reduction in time to get results (51%) would encourage them to use biomarker testing more routinely (Table 4).
Table 2.
Factors affecting physicians’ decision to perform biomarker tests

Table 3.
Factors affecting physicians’ decision not to perform biomarker tests
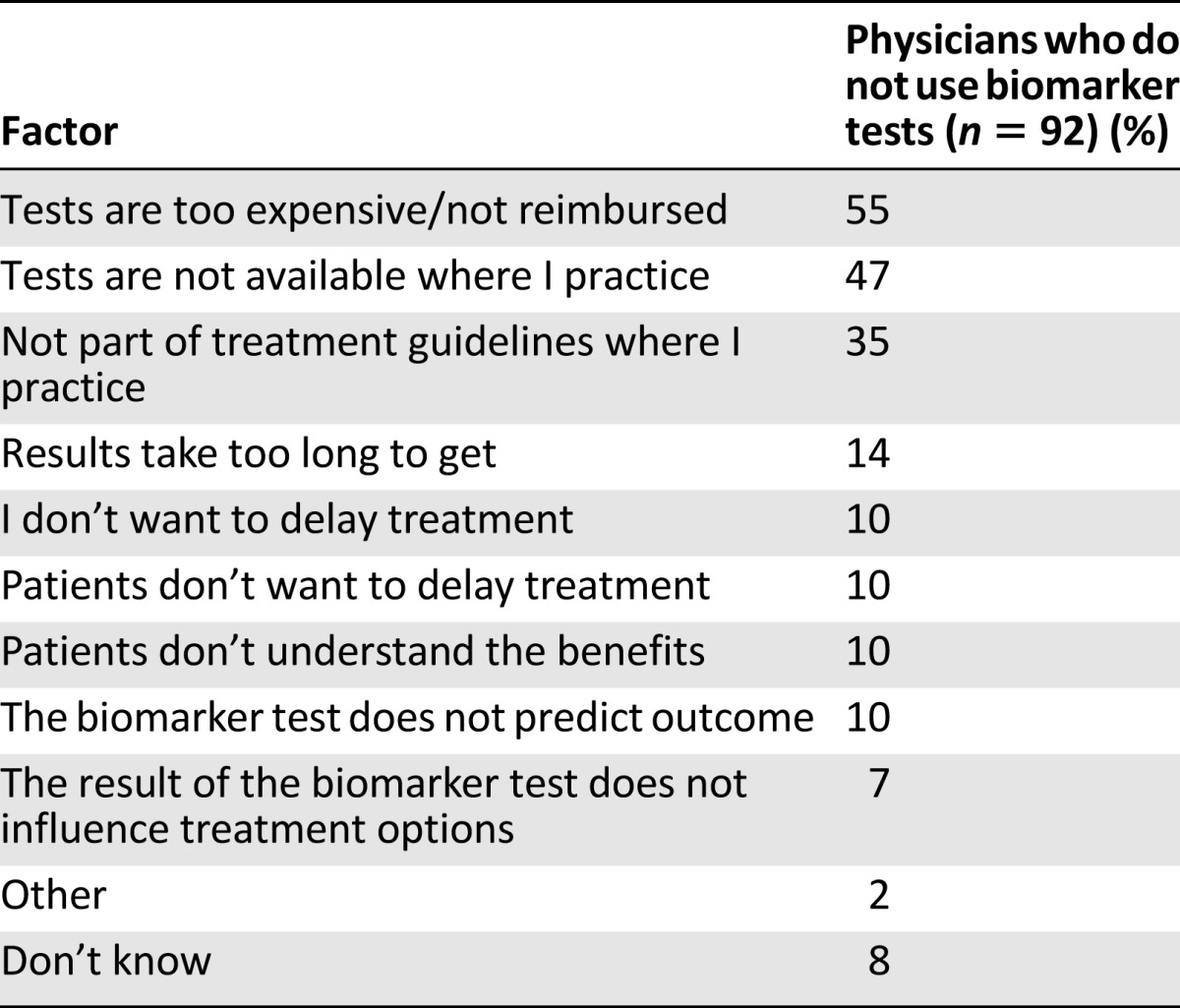
Table 4.
Factors that would encourage physicians to use biomarker tests
Discussion
Progress in the development and establishment of molecular tests to inform the personalization of anticancer therapy is intrinsically linked to the analysis of human tissue samples. Therefore, for testing to begin, patients with cancer must understand the nature of the research and provide their consent for their tissues and clinical data to be used. Furthermore, for patients to engage in a personalized approach to cancer treatment decisions—including any additional testing that may be required—they must have at least a basic understanding of tumor biology. The current surveys were therefore designed to assess the level of knowledge in a multinational cohort of patients with cancer and physicians, as well as the factors affecting use of biomarker testing.
These data must be interpreted in light of several important factors and limitations. First, we cannot exclude the possibility that the responding patients are not representative of the total pool of patients with CRC, NSCLC, and breast cancer; for example, it is plausible that those who responded were self-selected to be more knowledgeable about the subject matter in question. Second, it is conceivable that the survey questions were not adequately sensitive to important differences in the biology and clinical management of CRC, NSCLC, and breast cancer; for example, biopsy testing in the three tumor types is different, which may have affected willingness to undergo rebiopsy. Third, the survey encompassed only 12 countries, and therefore not all major regions that use precision medicine were assayed; accordingly, it is possible that additional regional differences are not captured here.
Fourth, these surveys were conducted in 2011 and 2013, before certain important advances in biomarker testing; for example, this survey predates the appreciation that expanded RAS testing is a clinically useful evaluation tool in CRC and the results therefore reflect the thoughts and opinions of physicians and patients at a time when only KRAS testing was a part of clinical practice. Fifth, the patient survey was performed 2 years earlier than the physician survey; therefore, differences in patient and physician responses may indicate how the understanding of precision medicine and personalized care evolved between 2011 and 2013. Sixth, in this setting, respondents were asked to select from provided options, which limited the possibility to provide further specific information in some cases; for example, although respondents were asked whether patients would undergo rebiopsy, they were not asked to elaborate on what led to their decision.
Seventh, we acknowledge a fundamental difference between established biomarkers and biomarkers of uncertain utility; we cannot exclude the possibility that our survey could have been affected by ambiguities introduced by these two categories of biomarkers. Finally, while patients were categorized as having CRC, NSCLC, or breast cancer, their disease stage was not recorded; hence, this survey did not address potential differences in the responses of patients with curable versus incurable cancer.
Globally, patient and physician responses indicated that patients were aware of basic tumor biology and treatments. Although patients’ willingness to participate in personalized care was slightly overestimated by their physicians, in 2011 most patients reported that they would participate in personalized medicine. Most patients globally were willing to undergo tumor rebiopsy, but there was an interesting regional exception in China, where, despite a high knowledge of biomarker testing, 76% of patients indicated that they would not be willing to undergo rebiopsy. This may represent an opportunity to educate patients in this region about how the information gained by rebiopsy could improve their therapeutic options, thereby increasing their willingness to participate in personalized care.
Physicians underestimated the time that patients would be willing to delay treatment; 32% of patients were willing to delay treatment for biomarker testing for “as long as it takes,” but only 3% of physicians believed that their patients would wait this long. This suggests that patients who are open to biomarker testing may be willing to delay treatment to receive more effective, personalized care. It is of interest that physicians misjudged patient responses, and this may reflect an opportunity to improve the dialogue between patients and physicians.
Also notable is the high percentage of patients (91%) who indicated that they would allow a hospital to keep a sample of their tumor for future research purposes. This general willingness of patients to allow researchers to perform unspecified research on their tissues may demonstrate their altruism, the trust they place in their health care providers to use such material appropriately, or possibly the desire to help develop medicine for future patients. Therefore, it is important that legislation and local regulatory systems do not create inappropriate barriers that would prevent the use of donated patient tissues in research programs that might further facilitate the personalization of patient care. In the future, the development of blood-based rather than tissue-based methods for the detection of solid tumor biomarkers may help increase the willingness of patients to participate in personalized care even further. With much less invasive and more convenient testing procedures, both doctors and their patients may participate more fully in personalized care, without such obstacles as rebiopsy, long-term storage of tumor specimens, or treatment delays.
Despite a global willingness of patients with cancer to participate in precision medicine, 78% of oncologists think that these patients need more information to help them understand the different types of cancers and how they progress. Therefore, there is still a clear need for patients to have access to additional sources of information that will allow them to more fully understand their treatment options and better engage in decision-making. Furthermore, patients with a better understanding of their disease, available treatment options, and ongoing research may be more likely to participate in clinical trials, thus paving the way for future identification of additional biomarkers, more effective treatment options, and more cost-effective therapies.
MDTs are emerging as an important component for treatment decisions and patient care. Globally, most physicians reported that the MDT played a role in making treatment decisions, but in several countries this was not the case. Most notably, most physicians in Saudi Arabia did not believe that an MDT should be part of the decision-making process. Differences in the perceived importance of the MDT may reflect the ongoing evolution of the role that it plays during the patient journey. In many hospitals, treatment of patients with mCRC with liver metastases must be discussed by an MDT, which includes a liver surgeon, and in some countries laws require patients to be presented to an MDT [21]. Studies have indicated that the involvement of an MDT contributes positively to the treatment of patients with cancer, including increased survival in patients with colorectal, breast, and lung cancer [22]. Therefore, educating physicians in regions where the involvement of an MDT is lower than average may improve patient care and outcomes.
Use of biomarker testing was high globally but displayed regional variations that may reflect cultural and local practice. Although only 9% of physicians indicated that they did not use biomarker testing, 24% of patients indicated that their tumors were not tested. This discrepancy may be due to several factors. There may be a subset of patients who were tested without realizing or understanding the effect that the test would have on their treatment. Alternatively, this may be due to the timing of the surveys. The patient survey was administered in 2011, 2 years before the physician survey, and the prevalence of biomarker testing may have increased during that time. Nevertheless, this represents an opportunity to help patients and physicians communicate effectively during the treatment process and educate patients about their care. For example, to improve the ability of patients to retain information from their office visits, it might be useful for physicians to assess the take-home materials that patients are provided with (e.g., listing the tests that were performed) and describe how the information will be used.
Physicians who did not use biomarker tests indicated that cost and availability were the main roadblocks to use. Complementary to this, all physicians, regardless of whether they currently use biomarker testing, indicated that greater availability of biomarker tests where they practice and an overall reduction in the cost of testing would encourage them to use biomarker testing more frequently. These findings show that efforts to increase access and control costs may encourage doctors to participate in testing more effectively compared with efforts targeted toward patient or physician education. Interestingly, 10% of physicians did not use biomarker testing because they believed that their patients would not want to delay treatment for testing or understand the benefits of testing. However, most patients indicated that they understood the value of biomarker testing and would be willing to delay treatment to undergo testing, so the results may suggest that some physicians are underestimating the willingness of patients to participate. This may represent another opportunity to improve communication between physicians and patients, who may be more educated and willing to participate in a personalized care plan than their physicians realize.
Physicians reported that the test they most commonly used was the KRAS mutation test. This may be because 43.1% of physicians were classified as CRC specialists, where KRAS testing is most relevant. However, it is important to consider that these specialist categories were defined by the diagnosis of the majority of the physicians’ patients. Therefore, even physicians categorized as breast cancer specialists may also treat patients with mCRC and therefore commonly use KRAS testing.
Conclusion
Patients and physicians were generally aware of the advances in precision medicine and showed willingness to participate in associated biomarker testing. However, considerable opportunity remains for physicians and support groups to better educate patients about the ability of biomarker testing to inform the most effective, personalized treatment strategy, tailored to the patient’s own tumor.
See http://www.TheOncologist.com for supplemental material available online.
Supplementary Material
Acknowledgments
We acknowledge medical writing assistance provided by Meghan Sullivan of ClinicalThinking, Hamilton, New Jersey, which was funded by Merck KGaA.
Author Contributions
Conception/Design: Fortunato Ciardiello, Richard Adams, Josep Tabernero, Thomas Seufferlein, Julien Taieb, Vladimir Moiseyenko, Brigette Ma, Gustavo Lopez, Johan F. Vansteenkiste, Sabine Tejpar
Provision of study material or patients: Fortunato Ciardiello, Richard Adams, Josep Tabernero, Thomas Seufferlein, Julien Taieb, Vladimir Moiseyenko, Brigette Ma, Gustavo Lopez, Johan F. Vansteenkiste, Sabine Tejpar
Collection and/or assembly of data: Regina Esser
Data analysis and interpretation: Fortunato Ciardiello, Richard Adams, Josep Tabernero, Thomas Seufferlein, Julien Taieb, Vladimir Moiseyenko, Brigette Ma, Gustavo Lopez, Johan F. Vansteenkiste, Regina Esser, Sabine Tejpar
Manuscript writing: Fortunato Ciardiello, Richard Adams, Josep Tabernero, Thomas Seufferlein, Julien Taieb, Vladimir Moiseyenko, Brigette Ma, Gustavo Lopez, Johan F. Vansteenkiste, Regina Esser, Sabine Tejpar
Final approval of manuscript: Fortunato Ciardiello, Richard Adams, Josep Tabernero, Thomas Seufferlein, Julien Taieb, Vladimir Moiseyenko, Brigette Ma, Gustavo Lopez, Johan F. Vansteenkiste, Regina Esser, Sabine Tejpar
Disclosures
Fortunato Ciardiello: Merck KGaA, Bayer, Roche, Astellas, Lilly (C/A), Bayer, Astra Zeneca (RF); Richard Adams: Merck Serono (C/A, ET); Josep Tabernero: Amgen, Boehringer, Celgene, Chugai, Imclone, Lilly, Merck, Merck Serono, Millennium, Novartis, Roche, Sanofi, Symphogen, Taiho (C/A); Thomas Seufferlein: Merck Serono, Roche, Amgen (C/A, H); Julien Taieb: Roche, Merck Serono, Amgen, Celgene, Lilly (C/A); Brigette Ma: Novartis, Boehringer Ingelheim (C/A), Merck Serono, Sanofi, Roche, Novartis (H), Merck Serono, Sanofi Aventis, Roche, Novartis (RF); Johan F. Vansteenkiste: Merck Serono (C/A); Regina Esser: Merck KGaA Germany (E, OI); Sabine Tejpar: Merck KGaA (C/A, RF, H). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.Ashley EA. The precision medicine initiative: A new national effort. JAMA. 2015;313:2119–2120. doi: 10.1001/jama.2015.3595. [DOI] [PubMed] [Google Scholar]
- 2.Greaves M, Maley CC. Clonal evolution in cancer. Nature. 2012;481:306–313. doi: 10.1038/nature10762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tran B, Dancey JE, Kamel-Reid S, et al. Cancer genomics: Technology, discovery, and translation. J Clin Oncol. 2012;30:647–660. doi: 10.1200/JCO.2011.39.2316. [DOI] [PubMed] [Google Scholar]
- 4.Karapetis CS, Khambata-Ford S, Jonker DJ, et al. K-ras mutations and benefit from cetuximab in advanced colorectal cancer. N Engl J Med. 2008;359:1757–1765. doi: 10.1056/NEJMoa0804385. [DOI] [PubMed] [Google Scholar]
- 5.Bokemeyer C, Bondarenko I, Hartmann JT, et al. Efficacy according to biomarker status of cetuximab plus FOLFOX-4 as first-line treatment for metastatic colorectal cancer: The OPUS study. Ann Oncol. 2011;22:1535–1546. doi: 10.1093/annonc/mdq632. [DOI] [PubMed] [Google Scholar]
- 6.Douillard JY, Siena S, Cassidy J, et al. Randomized, phase III trial of panitumumab with infusional fluorouracil, leucovorin, and oxaliplatin (FOLFOX4) versus FOLFOX4 alone as first-line treatment in patients with previously untreated metastatic colorectal cancer: The PRIME study. J Clin Oncol. 2010;28:4697–4705. doi: 10.1200/JCO.2009.27.4860. [DOI] [PubMed] [Google Scholar]
- 7.Van Cutsem E, Köhne CH, Láng I, et al. Cetuximab plus irinotecan, fluorouracil, and leucovorin as first-line treatment for metastatic colorectal cancer: Updated analysis of overall survival according to tumor KRAS and BRAF mutation status. J Clin Oncol. 2011;29:2011–2019. doi: 10.1200/JCO.2010.33.5091. [DOI] [PubMed] [Google Scholar]
- 8.Amado RG, Wolf M, Peeters M, et al. Wild-type KRAS is required for panitumumab efficacy in patients with metastatic colorectal cancer. J Clin Oncol. 2008;26:1626–1634. doi: 10.1200/JCO.2007.14.7116. [DOI] [PubMed] [Google Scholar]
- 9.Bokemeyer C, Köhne CH, Ciardiello F, et al. FOLFOX4 plus cetuximab treatment and RAS mutations in colorectal cancer. Eur J Cancer. 2015;51:1243–1252. doi: 10.1016/j.ejca.2015.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Van Cutsem E, Lenz HJ, Köhne CH, et al. Fluorouracil, leucovorin, and irinotecan plus cetuximab treatment and RAS mutations in colorectal cancer. J Clin Oncol. 2015;33:692–700. doi: 10.1200/JCO.2014.59.4812. [DOI] [PubMed] [Google Scholar]
- 11.Marty M, Cognetti F, Maraninchi D, et al. Randomized phase II trial of the efficacy and safety of trastuzumab combined with docetaxel in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer administered as first-line treatment: The M77001 study group. J Clin Oncol. 2005;23:4265–4274. doi: 10.1200/JCO.2005.04.173. [DOI] [PubMed] [Google Scholar]
- 12.Romond EH, Perez EA, Bryant J, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353:1673–1684. doi: 10.1056/NEJMoa052122. [DOI] [PubMed] [Google Scholar]
- 13.Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–792. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 14.Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361:947–957. doi: 10.1056/NEJMoa0810699. [DOI] [PubMed] [Google Scholar]
- 15.Ou SH. Crizotinib: a novel and first-in-class multitargeted tyrosine kinase inhibitor for the treatment of anaplastic lymphoma kinase rearranged non-small cell lung cancer and beyond. Drug Des Devel Ther. 2011;5:471–485. doi: 10.2147/DDDT.S19045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Engstrom PF, Arnoletti JP, Benson AB, 3rd, et al. NCCN clinical practice guidelines in oncology: Colon cancer. J Natl Compr Canc Netw. 2009;7:778–831. doi: 10.6004/jnccn.2009.0056. [DOI] [PubMed] [Google Scholar]
- 17.Allegra CJ, Jessup JM, Somerfield MR, et al. American Society of Clinical Oncology provisional clinical opinion: Testing for KRAS gene mutations in patients with metastatic colorectal carcinoma to predict response to anti-epidermal growth factor receptor monoclonal antibody therapy. J Clin Oncol. 2009;27:2091–2096. doi: 10.1200/JCO.2009.21.9170. [DOI] [PubMed] [Google Scholar]
- 18.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology. Colon Cancer. V2.2015. Available at http://www.nccn.org/professionals/physician_gls/pdf/colon.pdf. Accessed March 3, 2015. [DOI] [PubMed]
- 19.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology. Non-Small Cell Lung Cancer. V7.2015. Available at http://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf. Accessed September 30, 2015.
- 20.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology. Breast Cancer. V3.2015. Available at http://www.nccn.org/professionals/physician_gls/pdf/breast.pdf. Accessed September 30, 2015.
- 21.Generalitat de Catalunya. Departament de Salut. OncoGuía de colon y recto. Actualización 2008. Available at http://www.guiasalud.es/GPC/GPC_498_oncog_colon_2008_esp.pdf. Accessed March 17, 2015.
- 22.Prades J, Remue E, van Hoof E, et al. Is it worth reorganising cancer services on the basis of multidisciplinary teams (MDTs)? A systematic review of the objectives and organisation of MDTs and their impact on patient outcomes. Health Policy. 2015;119:464–74. doi: 10.1016/j.healthpol.2014.09.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.