Among men with prostate cancer metastatic to bone, experiencing ≥1 skeletal-related event is associated with poorer survival, increased health care resource utilization, and increased costs. These negative effects emphasize the importance of preventing SREs in this population.
Keywords: Prostate Cancer, Cancer of the prostate, Prostate neoplasms, Neoplasm metastases, Metastasis, Bone, Bone fractures, Extramedullary spinal cord compression
Abstract
Background.
Approximately 40% of men diagnosed with metastatic prostate cancer experience one or more skeletal-related events (SREs), defined as a pathological fracture, spinal cord compression, or surgery or radiotherapy to the bone. Accurate assessment of their effect on survival, health care resource utilization (HCRU), and cost may elucidate the value of interventions to prevent SREs.
Materials and Methods.
Men older than age 65 years with prostate cancer and bone metastasis diagnosed between 2004 and 2009 were identified from linked Surveillance Epidemiology and End Results–Medicare records. Hazard ratios (HRs) and 95% confidence intervals (CIs) for the risk for death associated with SREs were calculated by using Cox regression. HCRU and costs (in 2013 U.S. dollars) were evaluated in a propensity score-matched cohort by using Poisson regression and Kaplan-Meier sample average estimators, respectively.
Results.
Among 3,297 men with prostate cancer metastatic to bone, 40% experienced ≥1 SRE (median follow-up, 19 months). Compared with men who remained SRE-free, men with ≥1 SRE had a twofold higher risk for death (HR, 2.29; 95% CI, 2.09–2.51). Pathological fracture was associated with the highest risk for death (HR, 2.77; 95% CI, 2.38–3.23). Among men with ≥1 SRE, emergency department visits were twice as frequent (95% CI, 1.77–2.28) and hospitalizations were nearly four times as frequent (95% CI, 3.20–4.40). The attributable cost of ≥1 SRE was $21,191 (≥1 SRE: $72,454 [95% CI, $67,362–$76,958]; SRE-free: $51,263 [95% CI, $45,439–$56,100]).
Conclusion.
Among men with prostate cancer metastatic to bone, experiencing ≥1 SRE is associated with poorer survival, increased HCRU, and increased costs. These negative effects emphasize the importance of SRE prevention in this population.
Implications for Practice:
This study confirms the substantial adverse clinical and economic effects of skeletal-related events (SREs) in men with prostate cancer. Compared with men who have prostate cancer metastatic to the bones and no SREs, men with prostate cancer metastatic to the bones experiencing ≥1 SRE had a twofold increase in the risk for death, a twofold increase in the number of emergency department visits, and a fourfold increase in the number of hospitalizations; they also incurred an additional $21,000 in direct medical costs attributed to SREs. Strategies to prevent SREs are potentially of high value in this patient population.
Introduction
Metastatic prostate cancer represents a small, but clinically significant, proportion of all prostate cancer cases. Although only 4% of the approximately 220,800 prostate cancer cases diagnosed in 2015 will be metastatic [1], these individuals have a 5-year overall survival of 28%, compared with nearly 100% for individuals diagnosed with local or regional disease [2]. Bone is the most common site for prostate cancer metastasis, accounting for as many as 80% of all metastasis [3, 4]. Approximately 40%–50% of men with prostate cancer that has metastasized to bone will experience a skeletal-related event (SRE) [5–10]. SREs typically consist of high-morbidity clinical episodes involving bone metastasis, including pathological fractures, spinal cord compression, bone metastasis that requires surgical stabilization, or radiotherapy for pain relief or prevention of fractures.
SREs represent a substantial challenge in the management of metastatic prostate cancer given their association with high morbidity [11], reduced quality of life [12, 13], high health care resource utilization (HCRU) and costs [5, 6, 14, 15], and lower survival [10]. In an analysis of Surveillance Epidemiology and End Results (SEER)–Medicare linked records of 126,978 men with prostate cancer, Sathiakumar et al. reported that patients with bone metastasis who experienced an SRE had a 10-fold higher risk for death than patients without bone metastasis or an SRE (adjusted hazard ratio [HR] for mortality, 10.2; 95% confidence interval [CI], 9.8–10.7) [10]. Previous studies have estimated the costs associated with SREs among men with metastatic prostate cancer to be between $7,553 to $88,838 per episode [16]. However, the methods used by previous studies have not specifically addressed the question of differences in survival, resource use, and costs attributable to SREs. This study aims to refine the estimates of survival, HCRU, and costs by using methods that account for the occurrence of SREs over time after diagnosis of bone metastasis and for censoring in a contemporary cohort of patients with metastatic prostate cancer.
The objective of this study was to estimate the effect of experiencing one or more SREs on survival, HCRU, and costs in a population-based cohort of Medicare-enrolled men with prostate cancer and bone metastasis. Quantifying the effect of SREs on these clinical and economic outcomes among men with metastatic prostate cancer may inform the potential value of medical interventions to prevent SREs.
Materials and Methods
Study Design and Data Source
We performed a retrospective cohort study using the SEER-Medicare linked database. SEER is a nationally representative program of cancer registries collecting clinical, demographic, and cause-of-death data on all cancer patients diagnosed within the regional catchment areas. Medicare Parts A and B insurance claims were linked to SEER records by using a probabilistic matching algorithm. Medicare claims data were obtained for complete years 2003 through 2010.
Study methods were reviewed by the Fred Hutchinson institutional review board (#8108) and met the requirements for “Exempt” research requiring no further institutional review board review.
Study Population
Men diagnosed with incident distant-stage prostate cancer with confirmed bone metastasis between January 1, 2004, and December 31, 2009, in 1 of the 17 SEER cancer registry catchment areas were eligible for inclusion in this study. Cohort follow-up time extended until December 31, 2010, with vital status recorded by SEER. A complete list of inclusion and exclusion criteria is given in supplemental online Appendix A.
Definition and Ascertainment of SREs
Medicare Parts A and B claims were used to ascertain diagnosis and procedural codes for defining an SRE event (codes listed in supplemental online Appendix B). Because Medicare claims do not specify the target of radiation (i.e., bone versus another site), only radiotherapy claims preceded by a claim for bone pain (International Classification of Diseases, Ninth Revision [ICD-9], code 733.90) or bone metastasis (ICD-9 code 198.5) in the 2 weeks prior were classified as an SRE.
SREs frequently occur as a collection of related events; for example, radiation or surgery may be used to treat a pathologic fracture. In observational studies using administrative claims data, enumerating SREs requires that claims occurring close together in time are grouped into a single SRE episode and a defined time window between claims to define separate episodes. Prior studies have used time windows of 30–90 days to define unique SRE episodes [5, 17]. We defined unique SRE episodes using a 60-day gap between claims with any of the diagnosis or procedural SRE-defining codes listed in supplemental online Appendix B. After consulting with experts in the field of prostate cancer oncology, we considered a 60-day time window sufficient to reasonably accommodate for the time spent in establishing the diagnosis and treating SREs with surgery and/or radiation. In addition, a sensitivity analysis enumerating claims using a 90-day time window was performed. Similar to previous studies, when multiple SRE claims occurred within the defined time window, a hierarchy based on clinical severity was used to classify the type of SRE as follows: (a) spinal cord compression, (b) pathological fracture, (c) surgery to the bone, and (d) radiotherapy to bone metastasis [5, 14, 15]. A single date marking the SRE episode was created on the basis of the date of the first claim for the SRE event used to define the SRE episode type.
Outcomes
SEER links to the National Death Registry to ascertain vital status, and the date of death is provided with SEER records. HCRU was identified from procedural codes in Medicare Parts A and B claims data and was categorized into emergency department (ED) visits, hospitalizations, ambulatory visits, imaging tests, use of bone-sparing agents, and chemotherapy administration. The total reimbursement amount from all Medicare Parts A and B claims in the follow-up period for each patient were used in the calculation of costs. Costs were converted to 2013 U.S. dollars by using the medical care component of the Consumer Price Index [18]; costs occurring more than 1 year after the date of the first SRE episode were discounted by 3% [19].
Covariates
Age at diagnosis of prostate cancer with bone metastasis, race, area-level sociodemographic characteristics, and SEER registry were ascertained from SEER records. ICD-9 codes appearing in Medicare Parts A and B claims in the 12-month period before diagnosis of prostate cancer with bone metastasis were used to calculate the Klabunde comorbidity index [20]. Treatment for prostate cancer, but not treatment for SREs, was defined by using procedural and diagnostic codes listed in supplemental online Appendix C. To ensure that we were adjusting only for treatment that occurred before the date of a first SRE episode, codes occurring in supplemental online Appendix C were not included in the measurement of previous treatment after the date of a first SRE episode among patients who experienced ≥1 SRE.
Statistical Analysis
Survival
Adjusted Cox proportional hazards regression modeling was used to estimate the association between the occurrence of an SRE and the risk for death, expressed by an HR and 95% CI, in the entire cohort of patients with prostate cancer metastatic to bones identified from SEER-Medicare records. Survival time was calculated from the date of prostate cancer diagnosis with bone metastasis (t0) to the date of death from SEER records. Survival times were censored if patients disenrolled from Medicare or added a Medicare Advantage plan because we could no longer track all claims for such patients or if patients were still alive at the data cutoff of December 31, 2010. The occurrence of an SRE was treated as a time-dependent exposure, so that all patients entered the cohort at t0 and contributed survival time to the SRE-free group until the date of a first SRE episode, at which time a patient’s remaining survival time contributed to the group with ≥1 SRE or continued to contribute to the SRE-free group if no SREs occurred. In a separate model, we assessed the effect of subsequent SRE episodes using numerical exposure categories: one, two or three or more episodes. Finally, a model for the type of first SRE episode compared the survival experience of patients whose first SRE episode was classified as radiation to the bone, bone surgery, pathological fracture, or spinal cord compression to that of patients who remained SRE-free.
All models were adjusted for age at and year of diagnosis of prostate cancer with bone metastasis, race, Klabunde comorbidity index score, and prostate cancer treatment received before the date of a first SRE episode.
Propensity Score Matching
Propensity score matching was performed to identify and match individuals who experienced ≥1 SRE to those who remained SRE-free but had a similar likelihood of experiencing ≥1 SRE on the basis of observed baseline characteristics. This approach reduces the effect of selection bias in observational studies [21]. We obtained propensity scores using logistic regression to model the predicted probability of experiencing ≥1 SRE for each patient based on the same variables used for adjustment in the survival analysis: age at and year of diagnosis of prostate cancer with bone metastasis, race, Klabunde comorbidity index score, and prostate cancer treatment received before the date of a first SRE episode. The propensity score resulting from the logistic regression model was then used to match patients who remained SRE-free for the observed follow-up period to patients who experienced ≥1 SRE by using caliper matching with replacement, with a caliper equal to two times the standard deviation of the estimated propensity score. SRE-free patients were then assigned an index date corresponding to the date of the first SRE episode of their matched exposed patient. If the SRE-free patient died or was censored before his assigned index date, he was not an eligible match for that exposed patient. Given that this constraint could not be evaluated until the propensity matching had been carried out, three potential unexposed patients were identified for each exposed. When more than one of the unexposed patients in the 3:1 match met the criteria that the index date came before death or censoring, the unexposed patient with the closest propensity score to the matched exposed patient was chosen, to come up with a 1:1 matched cohort.
Economic Evaluation
To evaluate the additional HCRU and cost attributable to SREs, we estimated the difference in the rate of HCRU and the difference in cost between patients who experienced ≥1 SRE and those who remained SRE-free within the propensity-matched cohort.
For each patient, all Medicare claims for HCRU categories of interest (ED visits, hospitalizations, ambulatory visits, imaging tests, use of bone-sparing agents, and use of chemotherapy) occurring between the date of a first SRE episode or the index date for matched unexposed patients and the date of death or censoring were counted and included in the analysis of resource use. Poisson regression models were used to estimate the incidence rate ratio (IRR) and 95% CI corresponding to the additional resource utilization associated with SRE episodes. We fit models comparing the incidence rate of resource utilization between the no-SRE group and individuals experiencing ≥1 SRE of any type (dichotomous), each additional SRE episode (continuous), and a first SRE episode of each of the four types (i.e., spinal cord compression, pathological fracture, surgery to the bone, and radiotherapy). Robust variance estimation was used, and all models included person-years as an independent variable to adjust for the different lengths of follow-up across patients.
Direct medical care costs, ascertained from the reimbursement amount for all Medicare claims (not limited to the HCRU categories described above) occurring between the date of a first SRE episode or the index date for matched unexposed patients to the date of death or censoring were included in the analysis of cost. To compare total cost estimates for the group with one or more SREs and the SRE-free group, the Kaplan-Meier sample average (KMSA) technique was used [22, 23]. This nonparametric method takes into account differences in survival time and censoring between patients with ≥1 SRE and SRE-free patients, on average, by weighting the costs in each group by the group-specific survival probabilities over 30-day time intervals. A technical explanation of this method is provided in supplemental online Appendix D. The resulting KMSA estimates of total cost were subtracted to arrive at the average difference in costs. A secondary lifetime cost analysis was performed to calculate costs from the time of diagnosis of prostate cancer with bone metastasis to death or censoring. The expanded time horizon of the lifetime cost analysis may capture some claims leading up to the diagnosis of an SRE for diagnosis or treatment of underlying symptoms.
Sensitivity Analysis
Given that several similar observational studies have used a 90-day time window for defining unique SRE episodes, we performed a sensitivity analysis in which we expanded our time window from 60 to 90 days and recounted the number of SRE episodes identified in our sample. The Cox regression models for survival estimating the risk for death with subsequent numbers of SREs compared with a first SRE was rerun using this 90-day window using the same adjustment variables.
All statistical analyses were conducted in Stata software, version 14 (Stata Corp., College Station, TX, http://www.stata.com). Statistical significance was assessed using a p value <.05.
Results
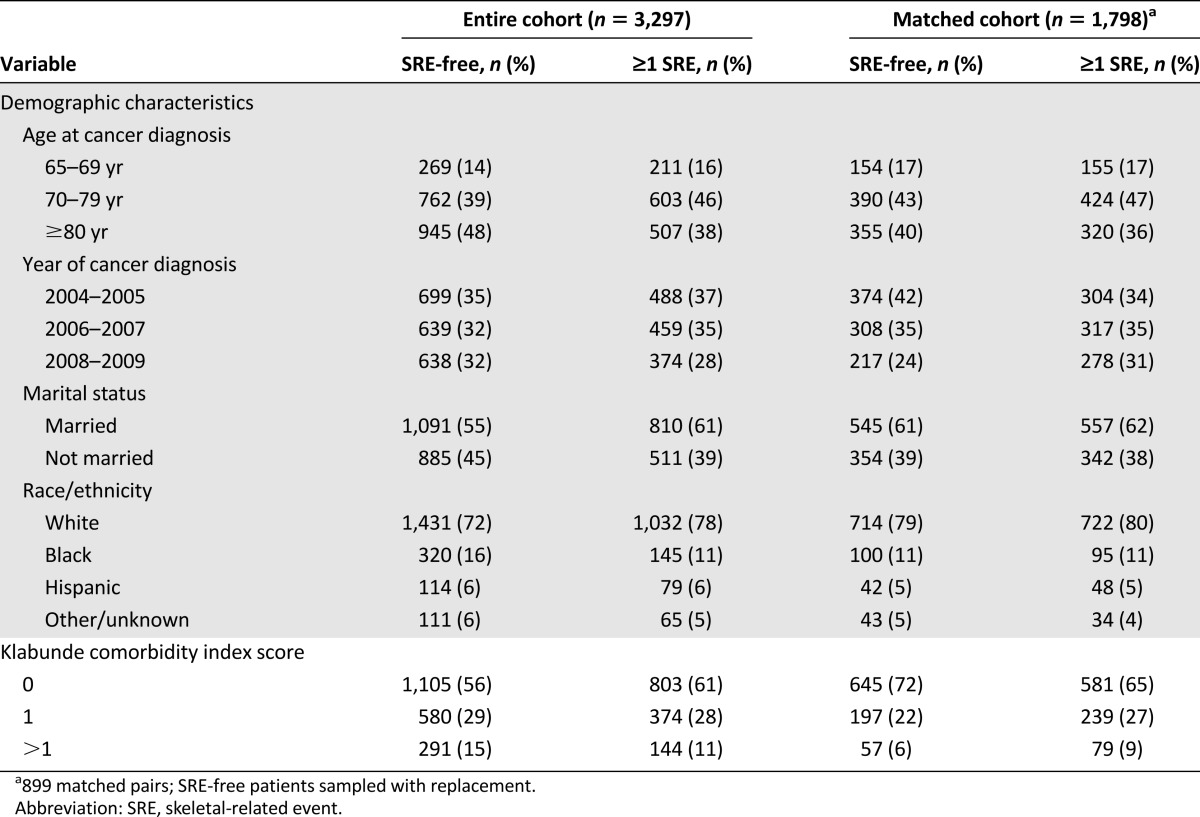
A total of 3,297 patients who had newly diagnosed prostate cancer with metastasis to bone were identified from the SEER registry. Forty percent of patients experienced ≥1 SRE (n = 1,321) during the follow-up period. In the entire cohort, SRE patients were more likely to be married and had fewer comorbidities (Table 1). The propensity score matching resulted in 899 pairs of patients with ≥1 SRE matched to patients who remained SRE-free. The propensity score-matched cohort was balanced on all characteristics except for year of diagnosis because of the condition that the SRE-free patients had to live at least until the date of their matched patient’s first SRE (Table 1).
Table 1.
Comparison of baseline characteristics and survival experience between the entire cohort of eligible individuals and the propensity score-matched cohort
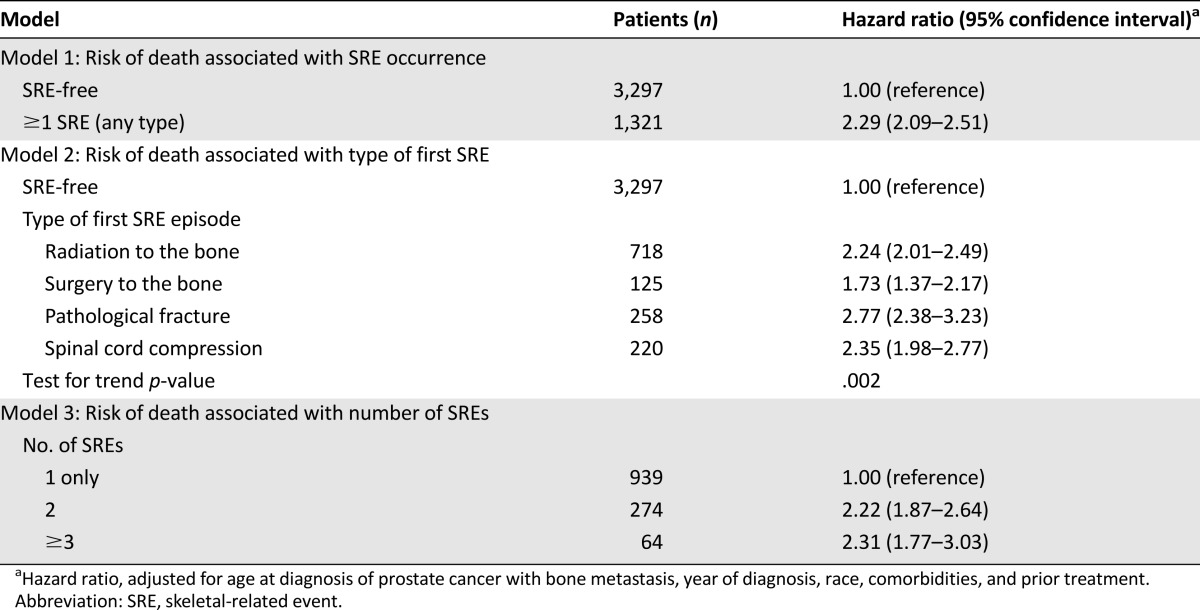
Compared with patients who remained SRE-free, patients with ≥1 SRE were more than twice as likely to die at any given time (HR, 2.29; 95% CI, 2.09–2.51) (Table 2). The risk for death was significantly elevated for all types of SREs compared with patients who remained SRE-free, although risk varied considerably by the type of first SRE episode (test of heterogeneity, p = .002). Patients whose first SRE episode was a pathologic fracture had nearly 3 times the risk for death as patients who remained SRE-free (HR, 2.77; 95% CI, 2.38–3.23), and the risk for death associated with surgery to the bone was 73% higher than that in patients who remained SRE-free. Compared with the 939 patients who experienced only one SRE episode, subsequent SRE episodes were associated with twice the risk for death. We could not detect a difference between the risk for death in patients who experienced two SRE episodes (HR, 2.22; 95% CI, 1.87–2.64) and those who experienced three or more (HR, 2.31; 95% CI, 1.77–3.03).
Table 2.
Risk for death associated with occurrence of skeletal-related event, type of first skeletal-related event, and number of skeletal-related events (n = 3,297)
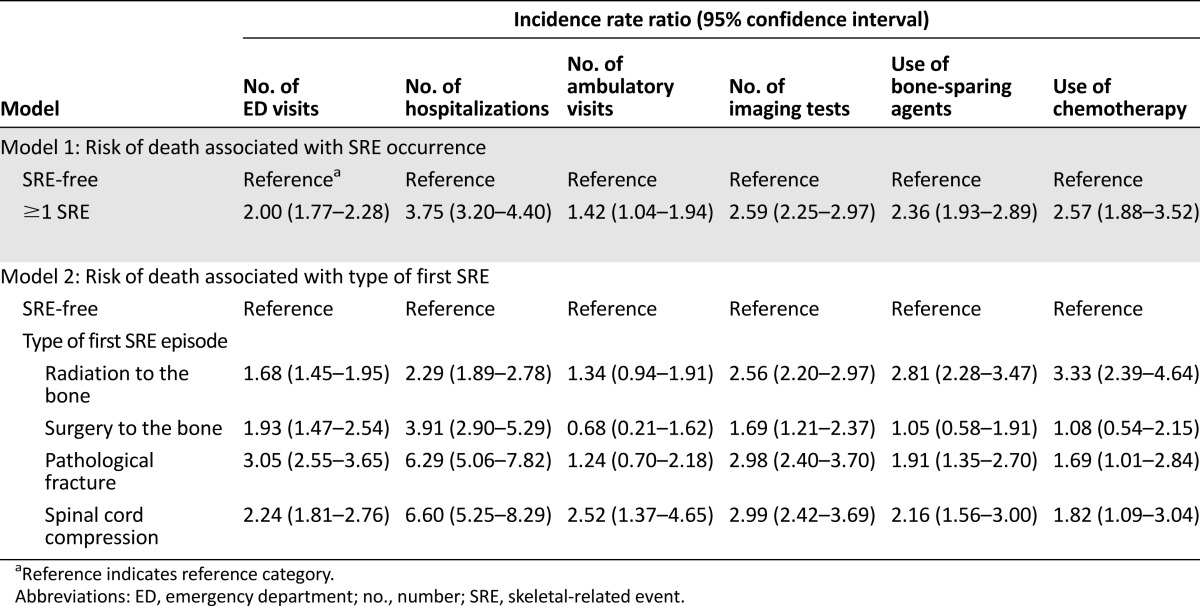
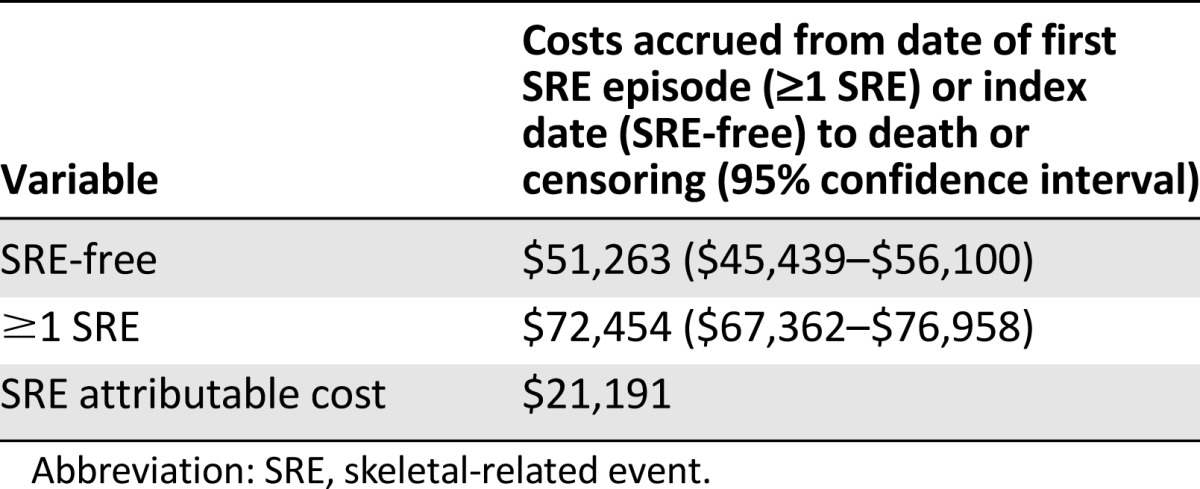
Among men with prostate cancer and bone metastasis, those who experienced ≥1 SRE used substantially more health care resources than men who remained SRE-free (Table 3). The rate of ED visits was twice as high (IRR, 2.00; 95% CI, 1.77–2.28) and the rate of hospitalizations was nearly 4 times as high (IRR, 3.75; 95% CI, 3.20–4.40) in patients who experienced ≥1 SRE compared with patients who remained SRE-free. Patients who experienced ≥1 SRE also had higher rates of ambulatory visits, imaging, surgical procedures, use of bone-sparing agents, and chemotherapy. The rate of ED visits was particularly high for patients whose first SRE episode was a pathologic fracture (IRR, 3.05; 95% CI, 2.55–3.65), compared with matched patients who remained SRE-free. In addition, patients whose first SRE episode was a pathologic fracture (IRR, 6.29; 95% CI, 5.06–7.82) or spinal cord compression (IRR, 6.60; 95% CI, 5.25–8.29) had more than 6 times the rate of hospitalizations than patients who remained SRE-free. The mean per-patient cost attributable to experiencing ≥1 SRE was estimated to be $21,191 (≥1 SRE: $72,454 [95% CI, $67,362–$76,958]; SRE-free: $51,263 [95% CI, $45,439–$56,100]) (Table 4). When the reimbursement amounts for all Medicare claims between the diagnosis of prostate cancer with bone metastasis and death or censoring were included in the analysis, the per-patient average lifetime cost for patients experiencing ≥1 SRE was $27,425 higher than the cost of patients who remained SRE-free.
Table 3.
Health care resource utilization for a propensity score-matched cohort of men older than 65 years of age, diagnosed with prostate cancer and bone metastasis (n = 899 matched pairs)
Table 4.
Kaplan-Meier sample average estimated mean per-patient cost for a propensity-matched cohort of men older than 65 years of age diagnosed with prostate cancer and bone metastasis (n = 899 matched pairs)
Sensitivity Analysis
Changing the time window used to define unique SRE episodes from 90 days to 60 days resulted in 107 more SRE episodes (90-day window: 1,773 episodes; 60-day window: 1,880 episodes) among the 1,321 patients who experienced ≥1 SRE. When the Cox regression model comparing the survival experience among men who experienced only one SRE to those who experienced two SREs or three or more SREs was performed by using the 1,880 SRE episodes identified with the 60-day definition, no differences in the overall results were observed (data not shown).
Discussion
To better understand how SREs affect men with advanced prostate cancer and the costs of caring for this disease, we estimated the association between the occurrence of ≥1 SRE and survival, HCRU, and costs in a population-based cohort of Medicare-enrolled men with prostate cancer and bone metastasis. Using an episode-based approach to defining SREs from Medicare claims data, we observed a cumulative SRE episode incidence of 40% among 3,297 men during a median follow-up of 19 months. Among men who experienced ≥1 SRE, the risk for death was more than double that of men who remained SRE-free, the rates of HCRU were substantially higher, and the per-patient average cost was increased by more than $21,000.
This study differs from previous studies investigating the consequences of SREs among men with prostate cancer, adding to existing literature in several ways. The method of time-dependent exposure modeling used to compare the survival experience of men who experienced ≥1 SRE with that of men who remained SRE-free addresses the question of the effect of the SRE itself and avoids attributing survival time occurring before an SRE to the survival experience. Previous studies may have underestimated the association between SREs and the risk for death by comparing the entire survival experience from diagnosis of metastatic prostate cancer to death in men with and without SREs, as was done in a study by Onukwugha et al. Those authors reported a 14% higher risk for death associated with SREs [24]. DePuy et al. used propensity score matching and found a 30% higher risk for death associated with SREs by comparing only survival times calculated after the date of a first SRE or matched index date [25].
Our approach improves upon that of previous studies by using survival data from diagnosis of prostate cancer with bone metastasis through death and attributing survival time to the pre- and post-SRE periods to estimate the risk for death associated with SREs. In addition, this study adds additional information about the incremental risk for death associated with an SRE to the work of Sathiakumar et al. That study used similar statistical methods to investigate a related scientific question about the risk for death associated with bone metastasis and SRE together compared with the risk for death in prostate cancer patients without bone metastasis or SREs [10]. Similar to previous studies, our study found that patients with SREs had a higher rate of HCRU and costs than their matched SRE-free counterparts [5, 6, 14, 15]. Previous studies demonstrated that the main driver of costs is the high rate of hospitalizations among SRE patients [5, 14]. In this study, the costs attributable to SREs resulted from a higher rate of utilization of expensive health care services and resources, including ED visits, hospitalizations, use of bone-sparing agents, and use of chemotherapy.
The results of these analyses should be interpreted with an understanding of the study limitations. Our use of SEER-Medicare, although allowing for a large population-based sample of prostate cancer with bone metastasis patients, did not provide the clinical granularity of medical records or prospectively collected data. Incomplete control of confounding by unmeasured factors, such as performance status or other measures of the underlying disease process that contribute both to the likelihood of experiencing an SRE and to the clinical and economic outcomes, may result in biased estimates of the effect of SREs. In addition, the use of administrative claims data for the identification and enumeration of SREs required several subjective decisions. Although our use of a hierarchy is similar to the approach in previous studies [5, 14, 15], we used a 60-day time window to define unique SRE episodes, whereas other studies have used 30-day and 90-day time windows. Our sensitivity analysis suggests that a 60-day window does not substantially affect the number of SREs counted from claims data. However, as pointed out in a recent review of the clinical and economic burden of bone metastasis and SREs in prostate cancer, the lack of a standard method for defining SREs limits comparison across studies [16].
Another important limitation concerns the generalizability of the study results. We estimated the clinical and economic effects of SREs among men with newly diagnosed prostate cancer with bone metastasis, a group that makes up 4% of all prostate cancer cases. It is possible that men diagnosed with incident prostate cancer that has already metastasized to bone have an inherently more aggressive disease than men diagnosed at early stages who go on to develop bone metastasis, making the generalizability of our results to the larger population of men who develop metastatic prostate cancer uncertain. Despite these limitations, this study uses appropriate statistical methods to demonstrate the substantial negative effect of SREs on survival among men with prostate cancer and bone metastasis.
Several drugs have recently been approved to prevent SREs in men with metastatic prostate cancer. In the Alpharadin in Symptomatic Prostate Cancer trial, radium-223 improved overall survival and increased the time to a symptomatic SRE, as compared with best supportive care plus placebo, among men with castration-resistant prostate cancer with multiple bone metastases [26, 27]. Zoledronic acid also decreases the frequency of SREs and increases the time to a first SRE among men with hormone-refractory metastatic prostate cancer [8]. In a phase III trial comparing the use of denosumab, a fully humanized monoclonal antibody, to zoledronic acid among men with castration-resistant prostate cancer and at least one bone metastasis, time to a first SRE was more than 3 months longer with denosumab, although overall survival was not significantly different [28]. The observed increased HCRU and resulting costs attributable to SREs indicate the potential high value of developing and implementing strategies to prevent or delay SREs in this population.
Conclusion
The substantial clinical and economic burden of SREs among men with prostate cancer supports the need for real-world studies investigating the value of their prevention.
See http://www.TheOncologist.com for supplemental material available online.
Supplementary Material
Acknowledgments
We acknowledge Debbie Delaney for her assistance with study management and organization. Research support was provided by Bayer Healthcare Inc.
Author Contributions
Conception/Design: Jean A. McDougall, Aasthaa Bansal, Bernardo H.L. Goulart, Jeannine S. McCunne, Adriana Valderrama, Sean D. Sullivan, Scott D. Ramsey
Collection and/or assembly of data: Jean A. McDougall, Bernardo H.L. Goulart, Jeannine S. McCunne, Andy Karnopp, Catherine Fedorenko, Stuart Greenlee, Scott D. Ramsey
Data analysis and interpretation: Jean A. McDougall, Aasthaa Bansal, Bernardo H.L. Goulart, Jeannine S. McCunne, Andy Karnopp, Catherine Fedorenko, Stuart Greenlee, Adriana Valderrama, Sean D. Sullivan, Scott D. Ramsey
Manuscript writing: Jean A. McDougall, Aasthaa Bansal, Bernardo H.L. Goulart, Jeannine S. McCunne, Adriana Valderrama, Sean D. Sullivan, Scott D. Ramsey
Final approval of manuscript: Jean A. McDougall, Aasthaa Bansal, Bernardo H.L. Goulart, Jeannine S. McCunne, Adriana Valderrama, Sean D. Sullivan, Scott D. Ramsey
Disclosures
Jean A. McDougall: Bayer Inc. (RF); Adriana Valderrama: Bayer Healthcare Inc. (E); Scott D. Ramsey: Bayer Pharmaceuticals (C/A, RF). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.American Cancer Society. Survival rates for prostate cancer. 2015. Available at http://www.cancer.org/cancer/prostatecancer/detailedguide/prostate-cancer-survival-rates Accessed July 7, 2015.
- 2.Howlader NNA, Krapcho M, Garshell J, et al. SEER Cancer Statistics Review, 1975-2010. Bethesda, MD: National Cancer Institute, 2013 [Google Scholar]
- 3.Bubendorf L, Schöpfer A, Wagner U, et al. Metastatic patterns of prostate cancer: An autopsy study of 1,589 patients. Hum Pathol. 2000;31:578–583. doi: 10.1053/hp.2000.6698. [DOI] [PubMed] [Google Scholar]
- 4.Yuen KK, Shelley M, Sze WM, et al. Bisphosphonates for advanced prostate cancer. Cochrane Database Syst Rev. 2006;(4):CD006250. doi: 10.1002/14651858.CD006250. [DOI] [PubMed] [Google Scholar]
- 5.Hagiwara M, Delea TE, Saville MW, et al. Healthcare utilization and costs associated with skeletal-related events in prostate cancer patients with bone metastases. Prostate Cancer Prostatic Dis. 2013;16:23–27. doi: 10.1038/pcan.2012.42. [DOI] [PubMed] [Google Scholar]
- 6.Lage MJ, Barber BL, Harrison DJ, et al. The cost of treating skeletal-related events in patients with prostate cancer. Am J Manag Care. 2008;14:317–322. [PubMed] [Google Scholar]
- 7.Saad F, Gleason DM, Murray R, et al. A randomized, placebo-controlled trial of zoledronic acid in patients with hormone-refractory metastatic prostate carcinoma. J Natl Cancer Inst. 2002;94:1458–1468. doi: 10.1093/jnci/94.19.1458. [DOI] [PubMed] [Google Scholar]
- 8.Saad F, Gleason DM, Murray R. Long-term efficacy of zoledronic acid for the prevention of skeletal complications in patients with metastatic hormone-refractory prostate cancer. J Natl Cancer Inst. 2004;96:879–882. doi: 10.1093/jnci/djh141. [DOI] [PubMed] [Google Scholar]
- 9.Smith MR, Saad F, Coleman R, et al. Denosumab and bone-metastasis-free survival in men with castration-resistant prostate cancer: results of a phase 3, randomised, placebo-controlled trial. Lancet. 2012;379:39–46. doi: 10.1016/S0140-6736(11)61226-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sathiakumar N, Delzell E, Morrisey MA, et al. Mortality following bone metastasis and skeletal-related events among men with prostate cancer: a population-based analysis of US Medicare beneficiaries, 1999-2006. Prostate Cancer Prostatic Dis. 2011;14:177–183. doi: 10.1038/pcan.2011.7. [DOI] [PubMed] [Google Scholar]
- 11.Coleman RE. Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin Cancer Res 2006;12:6243s–6249s. [DOI] [PubMed]
- 12.Costa L, Badia X, Chow E, Lipton A, Wardley A. Impact of skeletal complications on patients' quality of life, mobility, and functional independence. Support Care Cancer 2008;16:879–889. [DOI] [PubMed]
- 13.Weinfurt KP, Li Y, Castel LD, et al. The significance of skeletal-related events for the health-related quality of life of patients with metastatic prostate cancer. Ann Oncol. 2005;16:579–584. doi: 10.1093/annonc/mdi122. [DOI] [PubMed] [Google Scholar]
- 14.Barlev A, Song X, Ivanov B, et al. Payer costs for inpatient treatment of pathologic fracture, surgery to bone, and spinal cord compression among patients with multiple myeloma or bone metastasis secondary to prostate or breast cancer. J Manag Care Pharm. 2010;16:693–702. doi: 10.18553/jmcp.2010.16.9.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jayasekera J, Onukwugha E, Bikov K, et al. The economic burden of skeletal-related events among elderly men with metastatic prostate cancer. Pharmacoeconomics. 2014;32:173–191. doi: 10.1007/s40273-013-0121-y. [DOI] [PubMed] [Google Scholar]
- 16.Yong C, Onukwugha E, Mullins CD. Clinical and economic burden of bone metastasis and skeletal-related events in prostate cancer. Curr Opin Oncol. 2014;26:274–283. doi: 10.1097/CCO.0000000000000071. [DOI] [PubMed] [Google Scholar]
- 17.Hess G, Barlev A, Chung K, et al. Cost of palliative radiation to the bone for patients with bone metastases secondary to breast or prostate cancer. Radiat Oncol. 2012;7:168. doi: 10.1186/1748-717X-7-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bureau of Labor Statistics. Consumer Price Index: Measuring price change for medical care in the CPI. 2015. Available at http://www.bls.gov/cpi/cpifact4.htm Accessed July 10, 2014.
- 19.Drummond MF, Sculpher MJ, Torrance GW, et al. Methods for the Economic Evaluation of Health Care Programmes. 3rd ed. Oxford, United Kingdom: Oxford University Press; 2005. [Google Scholar]
- 20.Klabunde CN, Potosky AL, Legler JM, et al. Development of a comorbidity index using physician claims data. J Clin Epidemiol. 2000;53:1258–1267. doi: 10.1016/s0895-4356(00)00256-0. [DOI] [PubMed] [Google Scholar]
- 21.Rosenbaum PR, Rubin DB. Constructing a control-group using multivariate matched sampling methods that incorporate the propensity score. Am Stat. 1985;39:33–38. [Google Scholar]
- 22.Etzioni R, Urban N, Baker M. Estimating the costs attributable to a disease with application to ovarian cancer. J Clin Epidemiol. 1996;49:95–103. doi: 10.1016/0895-4356(96)89259-6. [DOI] [PubMed] [Google Scholar]
- 23.Etzioni RD, Feuer EJ, Sullivan SD, et al. On the use of survival analysis techniques to estimate medical care costs. J Health Econ. 1999;18:365–380. doi: 10.1016/s0167-6296(98)00056-3. [DOI] [PubMed] [Google Scholar]
- 24.Onukwugha E, Yong C, Mullins CD, et al. Skeletal-related events and mortality among older men with advanced prostate cancer. J Geriatr Oncol. 2014;5:281–289. doi: 10.1016/j.jgo.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 25.DePuy V, Anstrom KJ, Castel LD, Schulman KA, Weinfurt KP, Saad F. Effects of skeletal morbidities on longitudinal patient-reported outcomes and survival in patients with metastatic prostate cancer. Support Care Cancer 2007;15:869–876. [DOI] [PubMed]
- 26.Parker C, Nilsson S, Heinrich D, et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med. 2013;369:213–223. doi: 10.1056/NEJMoa1213755. [DOI] [PubMed] [Google Scholar]
- 27.Sartor O, Coleman R, Nilsson S, et al. Effect of radium-223 dichloride on symptomatic skeletal events in patients with castration-resistant prostate cancer and bone metastases: Results from a phase 3, double-blind, randomised trial. Lancet Oncol. 2014;15:738–746. doi: 10.1016/S1470-2045(14)70183-4. [DOI] [PubMed] [Google Scholar]
- 28.Fizazi K, Carducci M, Smith M, et al. Denosumab versus zoledronic acid for treatment of bone metastases in men with castration-resistant prostate cancer: a randomised, double-blind study. Lancet. 2011;377:813–822. doi: 10.1016/S0140-6736(10)62344-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.