Among patients aged ≥65 years who had distant-stage neuroendocrine tumors, three doses of octreotide long-acting repeatable, administered every 28 days and within 12 months of diagnosis, were compared (low: <20 mg; medium: 21–30 mg; and high: >30 mg) in terms of 5-year survival. Compared with a medium octreotide LAR dosage, a low dosage was associated with significantly worse survival, whereas a high initial dosage did not show additional survival benefits.
Keywords: Neuroendocrine tumors, Malignant carcinoid syndrome, Somatostatin analogue, SEER-Medicare
Abstract
Introduction.
Octreotide long-acting repeatable (LAR) is approved for the management of carcinoid syndromes and may improve progression-free survival of patients with well-differentiated neuroendocrine tumors (NETs). It is unknown whether the dosage of octreotide LAR affects survival. This paper evaluates the association between initial octreotide LAR dosage and overall survival of elderly patients with NETs.
Patients and Methods.
Patients with distant-stage NET diagnosed between January 1999 and December 2009 who received octreotide LAR treatment within 12 months of diagnosis were identified from the Surveillance, Epidemiology, and End Results (SEER)-Medicare database. Those under age 65 years, enrolled in health maintenance organizations, or without continuous enrollment in Medicare Parts A and B were excluded. We compared the 5-year survival of patients with NET based on dose per 28 days averaged over the initial 3 months: low (≤20 mg); medium (21–30 mg); high (>30 mg). Kaplan-Meier estimations and Cox proportional hazard modeling were used to examine the association between octreotide LAR dose and survival.
Results.
Among 222 patients with distant-stage NET who received octreotide LAR treatment, 81 (36%) received a low dosage, 82 (37%) received a medium dosage, and only 59 (27%) received a high dosage. Multivariate analysis showed that compared with a medium octreotide LAR dose, a low dosage was associated with significantly worse survival (hazard ratio [HR]: 2.00; p = .001), whereas a high initial dosage (HR: 1.09; p = .719) did not show additional survival benefits over that observed with a medium dosage.
Conclusion.
This population-based study suggests potential survival benefits for octreotide LAR provided within 12 months of diagnosis at a dosage of 21–30 mg among elderly patients with distant-stage NET.
Implications for Practice:
This population-based study examined the association between octreotide long-acting repeatable (LAR) dosage and the survival of elderly patients with neuroendocrine tumor (NET). Of the 222 patients, 36% received less than or equal to 20 mg per 28 days, 37% received 21–30 mg per 28 days, and 27% received more than 30 mg per 28 days. The multivariate analyses suggest that the initial dose of octreotide LAR in elderly patients with NET should be at least 21–30 mg every 28 days. It is unclear if higher doses improve survival further beyond medium dosage.
Introduction
Incidence of neuroendocrine tumors (NETs) has increased more than fivefold from 1973 (1.09 per 100,000) to 2004 (5.25 per 100,000) with improvement in diagnostic techniques, and current NET prevalence in the United States is estimated to be more than 100,000 [1]. NETs develop from the neuroendocrine cells distributed throughout the body and may be classified by anatomic location or by embryonic origin. More than one-third arise from the embryonic foregut (thymus, lungs, stomach, and proximal duodenum), whereas the rest arise from the midgut (distal duodenum, jejunum, ileum, appendix, and proximal large bowel) or hind gut (distal large bowel and rectum) [2]. Midgut NETs are classically associated with carcinoid syndrome due to secretion of serotonin and other bioactive substances, although they may be occasionally associated with NETs arising from other areas as well. In addition, about 15%–30% of pancreatic neuroendocrine tumors may cause symptoms due to excess production of hormones such as insulin, glucagon, or gastrin [3].
NETs are characterized by the presence of G-protein-coupled somatostatin cell surface receptors, subtypes 1–5, that, when stimulated, have inhibitory effects on secretory and growth pathways [4]. Given the very short half-life of native somatostatin, multiple synthetic somatostatin analogs with longer half-lives have been developed, of which octreotide, lanreotide, and their corresponding, longer-acting depot forms, octreotide long-acting repeatable (LAR) and lanreotide autogel, are approved by the U.S. Food and Drug Administration for treatment of NET. Octreotide was initially approved in 1988 for subcutaneous administration and octreotide LAR was later approved in 1998 for monthly intramuscular injection for control of several syndromes related to hormonal excess in NETs [5]. The phase III PROMID (Placebo-Controlled Prospective Randomized Study on the Antiproliferative Efficacy of Octreotide Acetate LAR in Patients with Metastatic Neuroendocrine Midgut Tumors) trial randomized patients with advanced, midgut carcinoids to octreotide LAR versus placebo and showed significant improvement in the primary endpoint of progression-free survival (PFS) [6]. The more recent phase III CLARINET (Study of Lanreotide Autogel in Non-functioning Entero-pancreatic Endocrine Tumors) trial randomized patients with gastroenteropancreatic NETs to placebo versus lanreotide and similarly showed an improvement in PFS, thus extending the use of somatostatin analogs to tumor control of all gastroenteropancreatic NETs [7]. Together, these data show that somatostatin analogs provide effective tumor and symptom control in NETs. However, the appropriate initial doses for these drugs are yet unknown and there are no ongoing prospective trials addressing this deficiency in knowledge.
The primary objective of this study was to evaluate the association between initial octreotide LAR dosage and survival of elderly patients with NETs, using retrospective, population-based data. Secondary objectives included evaluation of effects of tumor characteristics, other treatments, and sociodemographic characteristics of patients on survival.
Materials and Methods
Data Source
The Surveillance, Epidemiology, and End Results (SEER) registry data from the National Cancer Institute linked with Medicare claims data were used in this population-based study. The SEER registries collect clinical, demographic, and cause-of-death information for persons with cancer; the registries cover approximately 28% of the U.S. population. The Medicare claims data provide information on claims for covered health-care services from the time of a person's Medicare eligibility until death. Linking these two data sources makes available cancer patients’ date of death, and allows the ascertainment of treatments received by patients as well as their comorbidity through the use of International Classification of Disease 9th Revision (ICD-9), Current Procedural Terminology, and Healthcare Common Procedure Coding System (HCPCS) codes in Medicare claims. The unique quality of SEER-Medicare data makes it a widely accepted data source for health-services research on cancer and it is considered demographically representative [8].
Study Cohort
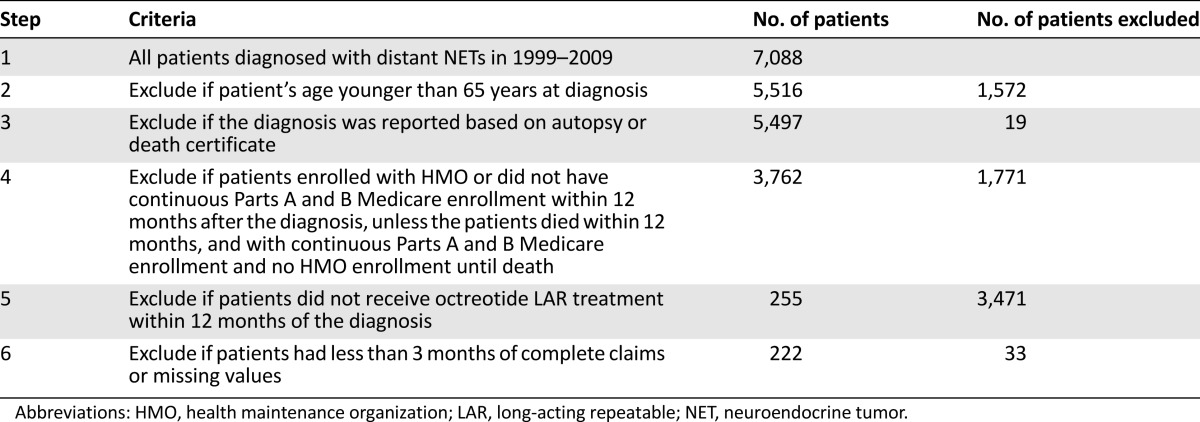
Our study cohort included 222 elderly patients with distant-stage NET who received octreotide LAR treatment within 12 months of the diagnosis. They were diagnosed with NET between July 1, 1999, and December 31, 2009, and followed up until December 31, 2011, in terms of survival information. The latest claims date available in the SEER-Medicare data at the time of this research was December 31, 2010. The patients with NET were identified based on International Classification of Diseases for Oncology, third edition (ICD-O-3) codes: 8150, 8151, 8152, 8153, 8154, 8155, 8156, 8157, 8240, 8241, 8242, 8243, 8244, 8245, 8246, and 8249. HCPCS codes (J-2353, J-2352) and National Drug Code (NDC) codes (00078034061, 00078034084, 00078034161, 00078034184, 00078034261, 00078034284) were used to identify octreotide LAR treatment within 12 months of diagnosis. We excluded patients who enrolled in health maintenance organizations (HMOs) or did not have continuous Medicare Parts A and B enrollment during the 12 months after diagnosis, unless the patients died within 12 months and had continuous Part A and B enrollment and no HMO enrollment until death. We also excluded patients with less than 3 months of complete claims, to ensure the completeness of medical claims to identify octreotide LAR use and dosage, and other treatments received by patients. Patients with missing values for covariates or younger than 65 years old at diagnosis were also excluded. Table 1 provides a detailed flowchart illustrating the inclusion and exclusion criteria of our study cohort.
Table 1.
Flowchart for study sample construction
Explanatory Variables
The main variable of interest was the dosage level received in the first 3 months of therapy. We calculated the total dosage received over the initial 3 months since diagnosis, based on the HCPCS codes and NDC codes, as explained in the study cohort section. Then we averaged it into the dosage level per 28 days and categorized it into 3 groups: low-dosage group (≤20 mg), medium-dosage group (21–30 mg), and high-dosage group (>30 mg). In our multivariate analyses, we also controlled for clinical and demographic characteristics and comorbidities.
The clinical factors considered in the analyses included tumor size, histologic grade, primary cancer site, presence of carcinoid syndromes, and other treatments received. Tumor size was classified into three groups: ≤2 cm, >2 cm, and unknown. The histologic grade had three categories: well differentiated, moderately or poorly differentiated, and not determined or unknown or mixed. Primary cancer sites included the following six categories: small intestine; cecum and appendix; colon; larynx, bronchus, lung, trachea, and other respiratory organs; pancreas; and all others. The presence of carcinoid syndrome was defined based on ICD-9 codes as having at least two claims for flushing (782.62), diarrhea (564.5, 787.91), or carcinoid syndrome (259.2), with the earliest one occurring before the start of octreotide LAR treatment. Other treatments included 4 binary variables (yes/no) indicating whether a patient received resection of primary tumor within 6 months of cancer diagnosis, resection of liver metastases, radiation, and chemotherapy within 12 months of diagnosis.
The demographic information included age (65–69, 70–74, 75+ years), sex (male vs. female), and race/ethnicity (non-Hispanic white vs. all others). We used the Deyo-Romano modified Charlson comorbidity score, which is a commonly adopted measure for comorbidities in studies using claims data, such as the SEER-Medicare data [9–12]. The comorbidity score was derived from Medicare Provider Analysis and Review outpatient and carriers’ claims files during the 12 months preceding diagnosis and categorized into 2 groups: 0 and ≥1.
Statistical Analyses
We used both Kaplan-Meier estimation and Cox proportional hazard model to evaluate the association between octreotide LAR dosage and 5-year survival. We report the median survival with the corresponding 95% confidence interval for each dose group, the log-rank test, hazard ratios (HRs) from multivariate analyses, and corresponding 95% confidence intervals (CIs) for the 5-year survival.
All statistical analyses were conducted in SAS 9.3 (SAS Institute, Cary, NC, http://www.sas.com). The institutional review board at The University of Texas MD Anderson Cancer Center exempted this study for approval because all patients in the database had been de-identified.
Results
Table 2 presents the descriptive statistics of the study cohort by dosage level. We masked some numbers and percentages in this table because of data confidentiality requirement by the SEER-Medicare database. Of the 222 elderly patients with distant-stage NET who received octreotide LAR within 12 months of diagnosis, 81 (36%) received low dosages (≤20 mg per 28 days), 82 (37%) received medium dosages (21–30 mg per 28 days), and only 59 (27%) received high dosages (>30 mg per 28 days). The bivariate analysis found that older patients (>75 years of age) were more likely to get low dosages. No other statistically significant differences in the dosage of octreotide LAR were detected by sex, race/ethnicity, comorbidity, and all the clinical factors.
Table 2.
Description of the study sample by dosage level
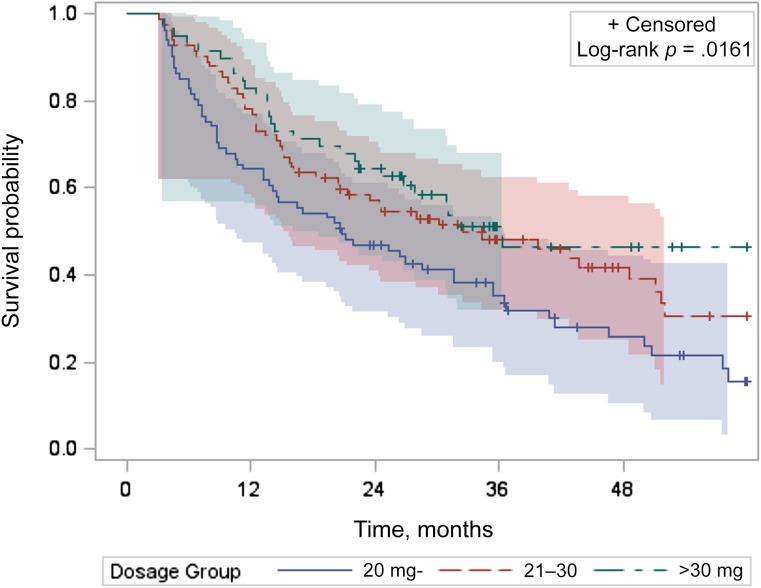
Figure 1 provides the unadjusted Kaplan-Meier curves for 5-year survival. The data suggest that there were significant overall survival differences across the three octreotide LAR dosage groups by log-rank test (p = .016); however, the curves crossed for the two higher dosage groups. The log-rank test comparing the medium-dosage and high-dosage groups yielded a p-value of .356, showing no significant survival difference. The median survival of patients who received low dosages was 20.8 months (95% CI: 13.2–31.5), compared with 32.6 months (95% CI: 20.5–51.1) and 36.3 months (95% CI: 24.98 to not applicable) for patients who received medium and high dosages, respectively.
Figure 1.
Unadjusted Kaplan-Meier curves with 95% Hall-Wellner bands for 5-year survival for patients with distant-stage neuroendocrine tumor by dosage group (dosage: <20 mg, 81 patients; 21–30 mg, 82 patients; >30 mg, 59 patients).
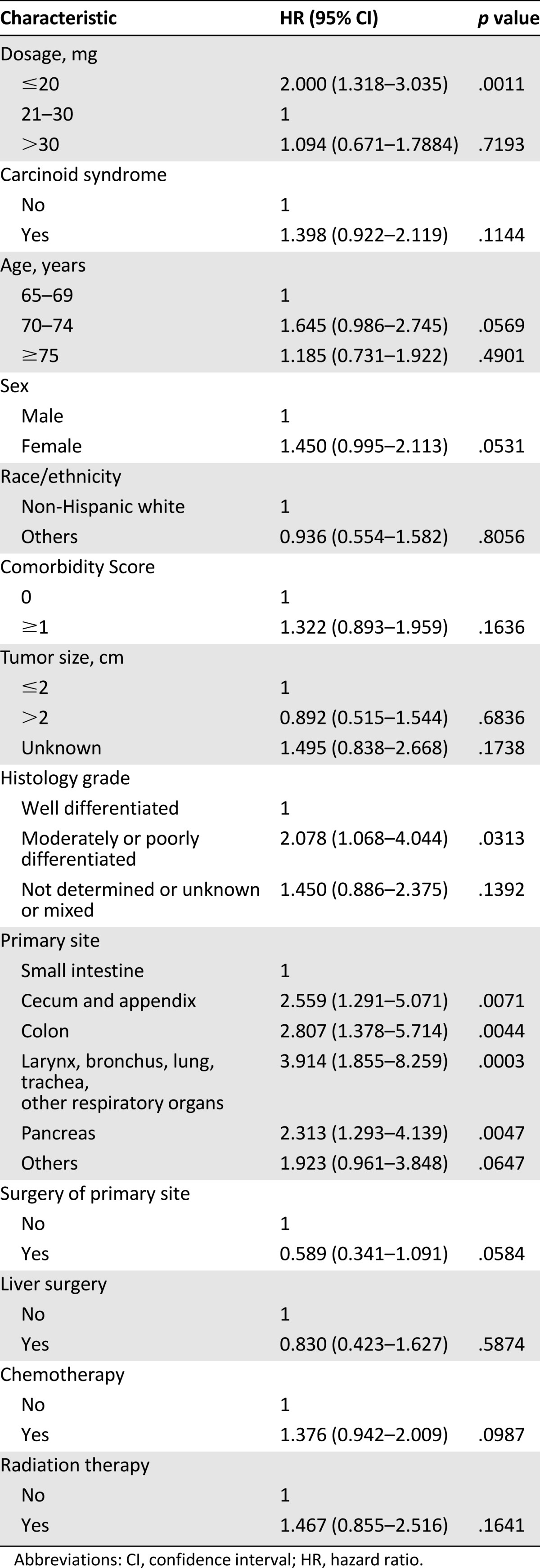
Table 3 shows the results of the multivariate Cox proportional hazard model for 5-year survival. It suggested that low octreotide LAR dosage was significantly negatively associated with survival (HR: 2.00; p = .001) compared with medium dosage. However, it also suggested that there is no statistically significant survival benefit with high dosage compared with medium dosage (HR: 1.09; p = .719). Patients with moderately or poorly differentiated histology had worse survival outcomes (HR: 2.08; p = .031) compared with patients with well-differentiated histology, Patients having the primary cancer site in the cecum and appendix (HR: 2.56; p = .007); colon (HR: 2.81; p = .004); and larynx, bronchus, lung, trachea, and other respiratory organs (HR: 3.91; p < .001) or pancreas (HR: 2.31; p = .005) had worse survival outcomes compared with those whose primary cancer site was in the small intestine.
Table 3.
Cox proportional hazard model for survival
Discussion
Well-differentiated NETs are often treated with somatostatin analogs such as octreotide LAR for symptom and tumor control. Using the SEER-Medicare database, we had previously shown that therapy with octreotide LAR in elderly patients is associated with improved survival but that approximately half of the elderly patients with functional carcinoid tumors had a delay of >6 months after diagnosis in initiation of octreotide LAR therapy [13]. As follow-up to our earlier studies, we aimed to determine if there is a relationship between initial dosage of octreotide LAR and 5-year survival outcomes among elderly patients with NET. In our cohort, there was a wide range of initial octreotide LAR dosage (Table 1), which reflects the current clinical practice. Octreotide LAR is currently available in 10-, 20-, and 30-mg formulations. While the PROMID trial evaluated octreotide LAR 30 mg in comparison with placebo, the National Comprehensive Cancer Network guidelines recommend either 20 mg or 30 mg of octreotide LAR as reasonable choices for initial dose [2, 6]. Other retrospective studies have shown that there is even larger variability in current clinical practice, with doses as low as 10 mg being used [14]. This is particularly important because our study showed significant differences in survival according to octreotide LAR dosage levels. Compared with the medium dosage (20 mg per 28 days) group, the low dosage (<20 mg per 28 days) group had inferior overall survival (HR: 2; 95% CI: 1.318–3.035). Overall, these results suggest that the optimal initial dosage of octreotide LAR should be at least 20 mg every 28 days. In line with our previous observations, older patients were at a higher risk of receiving lower doses, perhaps related to patient or physician preferences or to logistic issues such as difficulties accessing health-care facilities for administration of octreotide LAR intramuscularly on a monthly basis.
These differences in survival noted in our study could potentially be related to better pharmacokinetics of octreotide at higher doses. A prospective study evaluating plasma octreotide levels at different dosage levels of octreotide LAR showed that although therapeutic levels were reached in all 3 groups tested (10, 20, and 30 mg), there were differences in pharmacokinetics, including delay in reaching steady-state levels and lower trough concentrations within the 10-mg group compared with higher doses [15]. Furthermore, the 10-mg dose was least effective in suppression of urinary 5-hydroxyindoleacetic acid levels, a surrogate measure of symptom control in carcinoid syndrome. Another study also showed that there were significant changes in mean plasma levels of octreotide between the 20- and 30-mg doses of octreotide LAR [15]. Furthermore, this study also showed that in addition to the dose, other factors such as body mass index and sex also have a significant impact on plasma levels of octreotide. Although this study did not evaluate the 10-mg dose, it underscores the complex pharmacokinetics of octreotide and variations of plasma levels between the available formulations.
Differences in survival could also be related to dose-dependent antitumor effects. The antitumor effect of somatostatin analogs in NET are thought to be multifactorial because of direct effects on the tumor cells (e.g., induction of apoptosis, inhibition of cell proliferation and growth) and effects on tumor microenvironment (e.g., inhibition of angiogenesis, inhibition of trophic hormone release) [16]. Some studies have also suggested additional effects such as modulation of the immune system for somatostatin analogs [16]. It is conceivable that some of these antitumor effects maybe dose dependent and more pronounced at doses higher than 10 mg.
In our study, increases in dosage beyond 30 mg, as in the high-dose group (>30 mg per 28 days) did not appear to improve survival further compared with medium dosage. This is in contrast to retrospective studies that have shown that patients with refractory carcinoid syndrome achieve symptom control by increasing dose of octreotide LAR beyond 30 mg [14, 17]. These data are in line with consensus guidelines recommending titration of octreotide LAR dose beyond 20–30 mg as required for symptom control. However, few studies have evaluated the effects of higher doses of octreotide LAR on survival and are also limited by their small size and heterogeneity, although some have suggested better tumor control by increasing octreotide dose at time of progression [17]. First, increasing the octreotide dose could have differential effects on hormone versus tumor control. Second, while most of these studies looked at increasing the dose at progression, our study attempted to discern the optimal initial dosage. Finally, it is also possible that a survival benefit may have been missed by the small size of our study or by its design that combined all doses >30 mg into a single group, with 14 (23.7%) of the 59 patients having a dosage <40 mg. Multivariate analyses suggested significantly worse survival for patients with higher-grade tumors and with primary sites outside of the small intestine, including the pancreas. It should be noted that because this dataset was limited to patients diagnosed up to 2009, it may not fully reflect the improvements in overall survival of patients with pancreatic neuroendocrine tumors, because of subsequent FDA approval of multiple drugs, including everolimus and suninitib in May 2011 and lanreotide in December 2014.
This study is also subject to other limitations similar to any retrospective, population-based observation study. For example, patient characteristics and medical events can be inaccurate or not fully captured in Medicare claims and there may be other miscoding and inaccuracies in the claims. Furthermore, there may be unobserved confounding issues in our analyses. Although the three groups were well balanced for most demographic factors, there were significant differences in age, with a higher proportion of older patients in the low-dose group. This may have led to worse survival in the low-dose group. It is also possible that there are other unobserved factors driving both the lower dosage and worse survival. While prior studies have also shown that body mass index and method of intramuscular administration have a significant effect on octreotide levels, these variables were not captured in our dataset [15, 18].
Conclusion
To our knowledge, this is the first population-based study about the association between octreotide LAR dosage and the survival of elderly patients with NET. We found that an initial dosage level of 21–30 mg per 28 days starting within 12 months of diagnosis was significantly associated with better survival compared with lower dosage level (≤20 mg per 28 days). We did not identify statistically significant survival differences between the higher dosage (>30 mg per 28 days) and the medium dosage (21–30 mg per 28 days) groups. These results suggest potential survival benefits for octreotide LAR provided within 12 months of diagnosis at a level of 21–30 mg per 28 days as an initial dosage among elderly patients with distant-stage NET. Further studies examining the dose-dependent effects of octreotide LAR on tumor control are warranted.
Acknowledgments
This study was supported in part by Novartis Oncology. The funder sponsored the purchase of SEER-Medicare data and provided funding for analytical support. All authors had unrestricted access to the final study data on request. This study used the linked SEER-Medicare database. The interpretation and reporting of these data are the sole responsibility of the authors. We acknowledge the efforts of the Applied Research Program, National Cancer Institute, the Office of Research, Development and Information, Centers for Medicare & Medicaid Services, Information Management Services Inc., and the SEER Program tumor registries in the creation of the SEER-Medicare database.
Author Contributions
Conception/Design: Chan Shen, Ya-Chen Tina Shih, James C. Yao
Provision of study material or patients: James C. Yao
Collection and/or assembly of data: Chan Shen, Ying Xu, James C. Yao
Data analysis and interpretation: Chan Shen, Ying Xu, Arvind Dasari, Ya-Chen Tina Shih, James C. Yao
Manuscript writing: Chan Shen, Ying Xu, Arvind Dasari, Ya-Chen Tina Shih, James C. Yao
Final approval of manuscript: Chan Shen, Ying Xu, Arvind Dasari, Ya-Chen Tina Shih, James C. Yao
Disclosures
Chan Shen: Ipsen (RF); James C. Yao: Ipsen, Novartis (C/A), Novartis (RF); Ya-Chen Tina Shih: Novartis (RF). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.Yao JC, Hassan M, Phan A, et al. One hundred years after “carcinoid”: Epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol. 2008;26:3063–3072. doi: 10.1200/JCO.2007.15.4377. [DOI] [PubMed] [Google Scholar]
- 2.Kulke MH, Shah MH, Benson AB, 3rd, et al. Neuroendocrine tumors, version 1.2015. J Natl Compr Canc Netw. 2015;13:78–108. doi: 10.6004/jnccn.2015.0011. [DOI] [PubMed] [Google Scholar]
- 3.Halfdanarson TR, Rabe KG, Rubin J, et al. Pancreatic neuroendocrine tumors (PNETs): Incidence, prognosis and recent trend toward improved survival. Ann Oncol. 2008;19:1727–1733. doi: 10.1093/annonc/mdn351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rai U, Thrimawithana TR, Valery C, et al. Therapeutic uses of somatostatin and its analogues: Current view and potential applications. Pharmacol Ther. 2015;152:98–110. doi: 10.1016/j.pharmthera.2015.05.007. [DOI] [PubMed] [Google Scholar]
- 5.Rubin J, Ajani J, Schirmer W, et al. Octreotide acetate long-acting formulation versus open-label subcutaneous octreotide acetate in malignant carcinoid syndrome. J Clin Oncol. 1999;17:600–606. doi: 10.1200/JCO.1999.17.2.600. [DOI] [PubMed] [Google Scholar]
- 6.Rinke A, Müller HH, Schade-Brittinger C, et al. Placebo-controlled, double-blind, prospective, randomized study on the effect of octreotide LAR in the control of tumor growth in patients with metastatic neuroendocrine midgut tumors: Q report from the PROMID Study Group. J Clin Oncol. 2009;27:4656–4663. doi: 10.1200/JCO.2009.22.8510. [DOI] [PubMed] [Google Scholar]
- 7.Caplin ME, Pavel M, Ćwikła JB, et al. Lanreotide in metastatic enteropancreatic neuroendocrine tumors. N Engl J Med. 2014;371:224–233. doi: 10.1056/NEJMoa1316158. [DOI] [PubMed] [Google Scholar]
- 8.Warren JL, Klabunde CN, Schrag D, et al. Overview of the SEER-Medicare data: content, research applications, and generalizability to the United States elderly population. Med Care. 2002;40(suppl):IV-3–18. doi: 10.1097/01.MLR.0000020942.47004.03. [DOI] [PubMed] [Google Scholar]
- 9.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 10.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45:613–619. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 11.Romano PS, Roos LL, Jollis JG. Adapting a clinical comorbidity index for use with ICD-9-CM administrative data: Differing perspectives. J Clin Epidemiol. 1993;46:1075–1079; discussion 1081–1090. doi: 10.1016/0895-4356(93)90103-8. [DOI] [PubMed] [Google Scholar]
- 12.Klabunde CN, Potosky AL, Legler JM, et al. Development of a comorbidity index using physician claims data. J Clin Epidemiol. 2000;53:1258–1267. doi: 10.1016/s0895-4356(00)00256-0. [DOI] [PubMed] [Google Scholar]
- 13.Shen C, Shih YC, Xu Y, et al. Octreotide long-acting repeatable use among elderly patients with carcinoid syndrome and survival outcomes: A population-based analysis. Cancer. 2014;120:2039–2049. doi: 10.1002/cncr.28653. [DOI] [PubMed] [Google Scholar]
- 14.Strosberg JR, Benson AB, Huynh L, et al. Clinical benefits of above-standard dose of octreotide LAR in patients with neuroendocrine tumors for control of carcinoid syndrome symptoms: A multicenter retrospective chart review study. The Oncologist. 2014;19:930–936. doi: 10.1634/theoncologist.2014-0120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Joseph S, Li G, Lindholm E, et al. A prospective trial on the effect of body mass index and sex on plasma octreotide levels in patients undergoing long-term octreotide LAR therapy. Pancreas. 2010;39:964–966. doi: 10.1097/MPA.0b013e3181db01a8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anthony L, Freda PU. From somatostatin to octreotide LAR: Evolution of a somatostatin analogue. Curr Med Res Opin. 2009;25:2989–2999. doi: 10.1185/03007990903328959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Broder MS, Beenhouwer D, Strosberg JR, et al. Gastrointestinal neuroendocrine tumors treated with high dose octreotide-LAR: A systematic literature review. World J Gastroenterol. 2015;21:1945–1955. doi: 10.3748/wjg.v21.i6.1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boyd AE, DeFord LL, Mares JE, et al. Improving the success rate of gluteal intramuscular injections. Pancreas. 2013;42:878–882. doi: 10.1097/MPA.0b013e318279d552. [DOI] [PubMed] [Google Scholar]