Abstract
Background
In the PARTNER randomized controlled trial (RCT), which represented the first exposure to transapical transcatheter aortic valve replacement (TA-TAVR) for many clinical sites, high risk patients undergoing TA-TAVR derived similar health-related quality-of-life (HRQoL) outcomes when compared with surgical AVR (SAVR). With increasing experience, it is possible that HRQoL outcomes of TA-TAVR may have improved.
Methods and Results
We evaluated HRQoL outcomes at 1-, 6-, and 12-month follow-up among 875 patients undergoing TA-TAVR in the PARTNER non-randomized continued access (NRCA) registry, and compared these outcomes with those of the TA-TAVR and SAVR patients in the PARTNER RCT. HRQoL was assessed with the Kansas City Cardiomyopathy Questionnaire (KCCQ), the Medical Outcomes Study Short Form-12, and the EuroQoL-5D, with the KCCQ overall summary score serving as the primary endpoint. The NRCA TA-TAVR and RCT TA-TAVR and SAVR groups were generally similar. The primary outcome, the KCCQ summary score, did not differ between the NRCA TA-TAVR and the RCT TA-TAVR group at any follow-up timepoints, although there were small differences in favor of the NRCA cohort on several KCCQ subscales at 1 month. There were no significant differences in follow-up HRQOL between the NRCA-TAVR and the RCT SAVR cohorts on the KCCQ overall summary scale or any of the disease-specific or generic subscales.
Conclusions
Despite greater experience with TA-TAVR in the NRCA registry, HRQoL outcomes remained similar to those of TA-TAVR in the original RCT cohort and no better than those with SAVR. These findings have important implications for patient selection for TAVR when transfemoral access is not an option.
Clinical Trial Registration
Placement of AoRTic TraNscathetER Valve [PARTNER] trial; NCT00530894; http://clinicaltrials.gov/show/NCT00530894
Keywords: transcatheter aortic valve replacement, transapical, quality of life
Transapical (TA) access for transcatheter aortic valve replacement (TAVR) with the Edwards-SAPIEN valve is an accepted approach for high-risk patients with severe aortic stenosis (AS) in whom vascular anatomy precludes safe transfemoral (TF) access. Although the TA approach avoids potential access-site complications of the iliac and femoral vessels, TA access has its own limitations, including pain related to an anterior lateral thoracotomy and an increased risk of respiratory complications due to splinting and left lung atelectasis.1–3 Given the more invasive nature of the TA compared with the TF approach, whether TA-TAVR maintains the health-related quality-of-life (HRQoL) advantages of TF-TAVR over traditional surgical aortic valve replacement (SAVR) remains uncertain.
One of the main advantages of TAVR versus SAVR is the more rapid recovery from TAVR, which resulted in improved early HRQoL in the randomized Placement of AoRTic TraNscathetER Valve (PARTNER) A trial.4 However, this benefit of TAVR differed according to access site. In contrast to the TF approach, which was associated with significant early improvements in HRQoL compared with SAVR, patients who required TA access had no HRQoL benefit over SAVR at any time point following the procedure.4 Moreover, there was a statistically significant difference in Kansas City Cardiomyopathy Questionnaire (KCCQ) summary score in favor of SAVR at 6 months as well as trends in other HRQoL metrics favoring SAVR at the 1-month timepoint. Since the patients enrolled in the high-risk cohort of the PARTNER trial represented some of the first TA-TAVR procedures for many study sites, however, it is possible that TA results have improved with greater experience.5 We therefore sought to examine HRQoL outcomes after TA-TAVR in the larger and more contemporary non-randomized continued access registry (NRCA) of the PARTNER trial. We compared these outcomes with those of patients who underwent either TA-TAVR or SAVR within the TA cohort of the randomized PARTNER trial.
METHODS
Study design
The design of the PARTNER trial, along with its inclusion and exclusion criteria, is detailed in previous publications.6, 7 Patients deemed eligible for trial entry had severe AS, as defined by an aortic valve area of <0.8 cm2 and either a mean valve gradient of ≥40 mmHg or peak velocity of ≥4.0 m/sec. For Cohort A, all patients were required to be operable, but at high surgical risk with an expected risk of perioperative mortality >15% as determined by 2 surgeons. All patients had New York Heart Association class II, III, or IV heart failure symptoms. Prior to randomization, patients underwent assessment of aortic and iliofemoral anatomy to determine suitability for a TF approach with the SAPIEN heart valve system (Edwards Lifesciences, Irvine, CA). Those found suitable for TF access were randomized to TF-TAVR or SAVR. Those whose anatomy was prohibitive for the TF approach were randomized to TA-TAVR or SAVR. After enrollment of the randomized controlled trial (RCT) was completed with 699 patients, a prespecified NRCA registry provided treatment for 2068 additional patients, of whom 977 underwent TA-TAVR, with the remainder receiving TF-TAVR. The study was approved by the Institutional Review Board at each participating site and all patients provided written informed consent.
Measurement of HRQoL
Health-related quality of life was assessed at baseline and at 1, 6, and 12 month follow-up using 3 validated instruments: the Kansas City Cardiomyopathy Questionnaire (KCCQ), the Medical Outcomes Study 12-item Short Form (SF-12), and the EuroQOL-5D (EQ-5D). The KCCQ is a 23-item questionnaire addressing specific health domains pertaining to heart failure, including physical limitation, symptom frequency and burden, self-efficacy, and social limitation8 and has been shown to be reliable and valid in the assessment of HRQoL in patients with severe, symptomatic aortic stenosis.9 The KCCQ also provides an overall summary score, which ranges from 0 to 100 (with higher scores indicating improved HRQoL). The KCCQ overall summary score has been shown to correspond with New York Heart Association functional classification, with score ranges of 76–100, 61–75, 45–60, and 0–44 corresponding to New York Heart Association classes I, II, III, and IV, respectively.9 Small, moderate, and large clinical improvements in health status correspond to increases in KCCQ scores of approximately 5, 10, and 20 points, respectively.10
The SF-12 is a generic health status instrument that was derived from the original SF-36 health survey, one of the most extensively validated generic health status measures.11 The SF-12 physical and mental summary scores have been shown to correlate closely with the physical and mental component scores of the SF-36 and are scaled to overall population norms of 50 ± 10, with higher scores representing better health status. Minimum clinically important differences on the physical and mental summary scores are roughly 2 to 2.5 points.12 The EQ-5D is a health state classification system that is defined by self-ratings in 5 dimensions (self-care, mobility usual activities, pain/discomfort, anxiety/depression).13 For the purposes of this study, the EQ-5D domains were converted to utilities according to an algorithm developed for the US population.14 Utilities represent measures of an individual’s strength of preference for his or her current state of health on a scale ranging from 0 to 1, where 0 represents death and 1 represents perfect health.
Statistical analysis
Given the importance of adjusting follow-up HRQoL assessments for baseline values, patients with missing baseline scores were excluded from the analysis. Summary scores for the KCCQ, EQ-5D, and SF-12 were generated according to the scoring algorithms published by their developers.8, 11, 14 The pre-specified primary endpoint was the KCCQ overall summary score. All other subscales of the KCCQ along with the SF-12 and EQ-5D were considered secondary endpoints.
Separate 2-way comparisons were performed between the NRCA TA-TAVR group and the RCT TA-TAVR and RCT SAVR groups. Baseline differences in clinical characteristics and HRQoL scores were compared between groups using 2-sample Student t tests for continuous variables and chi-square tests for categorical variables. Within group changes from baseline were assessed using paired t tests. Longitudinal mixed effect models were used to examine the between group differences over time. Variables included in the models were time (1, 6, and 12 months), treatment group, baseline HRQoL, age, gender, chronic obstructive pulmonary disease, and the interaction between time and treatment group. The mixed models used all available HRQoL data from all follow-up time points, and accommodate missing data under the missing at random assumption.
All analyses were performed on an as-treated basis, and a 2-tailed p value of <0.05 was considered statistically significant for all comparisons. All analyses were performed using SAS for Windows version 9.2 (SAS Institute, Inc, Cary, NC) by an independent statistician in the Health Economics and Technology Assessment Group at Saint Luke’s Mid America Heart Institute.
RESULTS
Patient population and comparison to randomized PARTNER patients
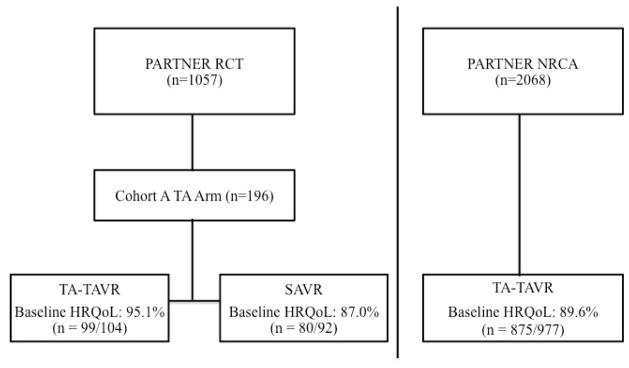
In the NRCA TA-TAVR group, 89.6% (875/977) had baseline HRQoL data. Of the patients randomized to TA-TAVR or SAVR in PARTNER Cohort A, 95.1% (99/104) of the TA-TAVR and 87.0% (80/92) of the SAVR patients had baseline HRQoL data (Figure 1). Among the NRCA TA-TAVR group, patients with baseline HRQoL data were generally similar to those without such data (Supplementary Appendix Table 1).
Figure 1.
PARTNER - Cohort A randomized control trial (RCT) transapical (TA) arm (transcatheter aortic valve replacement (TAVR) and surgical aortic valve replacement (SAVR)) and non-randomized continued access (NRCA) registry TA arm designs, including percentages with baseline health-related quality-of-life (HRQoL) data.
The baseline characteristics and HRQoL scores of the NRCA TA-TAVR, RCT TA-TAVR, and RCT SAVR patients are summarized in Table 1. Patients in the NRCA TA-TAVR group were slightly older than either the RCT TA-TAVR or RCT SAVR groups. In addition, patients in the NRCA TA-TAVR group were more likely to have undergone percutaneous coronary intervention and balloon aortic valvuloplasty prior to their aortic valve treatment. Patients in the RCT TA-TAVR group were more likely to have a history of cerebrovascular disease than those in the NRCA TA-TAVR group. Otherwise, the baseline characteristics of the 3 groups were similar.
Table 1.
Baseline characteristics and health-related quality-of-life scores
| NRCA TA-TAVR (n=875) | RCT TA-TAVR (n=99) | p-value* | RCT SAVR (n=80) | p-value* | |
|---|---|---|---|---|---|
| Demographic and clinical characteristics | |||||
| Age (years) | 84.6 ± 6.3 | 82.6 ± 6.9 | <0.01 | 83.4 ± 5.5 | 0.02 |
| Male (%) | 47.1 | 51.5 | 0.40 | 58.8 | 0.05 |
| STS Score | 12.0 ± 4.2 | 11.8 ± 3.7 | 0.64 | 11.8 ± 3.1 | 0.70 |
| Previous MI (%) | 29.4 | 28.3 | 0.82 | 36.3 | 0.20 |
| Prior CABG (%) | 51.1 | 50.5 | 0.91 | 57.5 | 0.27 |
| Prior PCI (%) | 46.6 | 32.3 | <0.01 | 43.0 | 0.55 |
| Prior BAV (%) | 28.6 | 12.1 | <0.01 | 12.5 | <0.01 |
| LV ejection fraction (%) | 52.2 ± 12.6 | 53.1 ± 12.3 | 0.50 | 53.3 ± 10.8 | 0.44 |
| Cerebrovascular Disease (%) | 29.9 | 41.8 | 0.02 | 33.3 | 0.54 |
| Peripheral Vascular Disease (%) | 61.7 | 62.2 | 0.91 | 63.3 | 0.78 |
| Diabetes Mellitus (%) | 35.0 | 40.4 | 0.28 | 48.8 | 0.01 |
| Renal Disease (Cr ≥ 2 mg/dL)(%) | 16.7 | 13.1 | 0.36 | 22.5 | 0.19 |
| Liver Disease (%) | 2.7 | 2.0 | 0.67 | 0.0 | 0.13 |
| COPD (Oxygen dependent) (%) | 8.7 | 12.1 | 0.26 | 8.8 | 0.98 |
| Major arrhythmia (%) | 48.5 | 53.5 | 0.34 | 47.5 | 0.86 |
| Permanent pacemaker (%) | 21.3 | 20.2 | 0.80 | 21.3 | 0.99 |
| Pulmonary hypertension (%) | 36.8 | 46.5 | 0.06 | 29.1 | 0.17 |
| Quality-of-life scores | |||||
| KCCQ Summary | 44.2 ± 21.5 | 40.6 ± 22.1 | 0.11 | 45.4 ± 19.7 | 0.64 |
| KCCQ Physical Limitation | 41.6 ± 24.5 | 41.2 ± 24.3 | 0.89 | 48.1 ± 23.4 | 0.03 |
| KCCQ Total Symptoms | 55.7 ± 22.8 | 49.6 ± 23.3 | 0.01 | 54.3 ± 21.6 | 0.61 |
| KCCQ Quality of Life | 40.2 ± 23.6 | 35.0 ± 26.6 | 0.04 | 39.7 ± 22.2 | 0.85 |
| KCCQ Social Limitation | 37.4 ± 28.2 | 35.3 ± 30.1 | 0.51 | 39.4 ± 26.9 | 0.54 |
| SF-12 Physical | 31.4 ± 8.1 | 29.4 ± 7.4 | 0.02 | 31.8 ± 8.6 | 0.69 |
| SF-12 Mental | 48.2 ± 11.2 | 46.8 ± 11.4 | 0.24 | 48.4 ± 9.6 | 0.86 |
| EQ-5D Utilities | 0.68 ± 0.19 | 0.67 ± 0.19 | 0.61 | 0.72 ± 0.17 | 0.12 |
p-values relate to the comparison with NRCA TA-TAVR
Values are mean ± SD or %.
BAV = balloon aortic valvuloplasty; CABG = coronary artery bypass grafting; COPD = chronic obstructive pulmonary disease; EQ-5D = EuroQol 5D; KCCQ = Kansas City Cardiomyopathy Questionnaire; LV = left ventricular; MI = myocardial infarction; NRCA = non-randomized continued access registry; PCI = percutaneous coronary intervention; SAVR = surgical aortic valve replacement; SF-12 = Medical Outcomes Study Short-Form 12; STS = Society of Thoracic Surgeons; TA = transapical; TAVR = transcatheter aortic valve replacement.
At baseline, there were no differences in HRQoL among the 3 groups with respect to the KCCQ summary score, the SF-12 mental score, and EQ-5D utility. There were small but statistically significant differences between the NRCA TA-TAVR group and the RCT TA-TAVR group with respect to the KCCQ total symptoms and quality of life scales as well as the SF-12 physical scale, each of which tended to be higher in the NRCA cohort. In addition, the score on the KCCQ physical limitations scale was higher in the RCT SAVR group as compared with the NRCA TA-TAVR group.
Within-group changes
Within-group changes in each HRQoL scale from baseline to 1-, 6-, and 12-month follow-up are shown in Table 2. For the NRCA TA-TAVR cohort, there were statistically significant and clinically meaningful improvements from baseline across all of the disease-specific and generic health status measures (with the exception of the SF-12 mental scale) beginning at the 1-month timepoint. These changes tended to increase further between 1 and 6 months, beyond which point there were no further consistent improvements. Based on published standards,10 the extent of improvement on the KCCQ summary scale was “moderately large” at 1 month (12.7 points) and “large” at 6 and 12 months (25.9 and 25.2 points, respectively). Qualitatively similar changes were seen for the RCT TA-TAVR and SAVR groups although the 1-month improvement was not significant for the KCCQ social limitations scale for the RCT TA-TAVR group and for both the physical limitations scale and social limitations scales in the RCT SAVR group.
Table 2.
Within-group comparisons of HRQoL compared with baseline
| NRCA TA-TAVR | RCT TA-TAVR | RCT SAVR | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Scale/Timepoint | n | Mean Δ vs. Baseline (95% CI) | p-value | n | Mean Δ vs. Baseline (95% CI) | p-value | n | Mean Δ vs. Baseline (95% CI) | p-value |
| KCCQ Summary | |||||||||
| 1 month | 711 | 12.7 (10.7, 14.7) | <0.01 | 78 | 12.4 (6.0, 18.8) | <0.01 | 61 | 12.5 (5.5, 19.5) | <0.01 |
| 6 months | 611 | 25.9 (23.9, 28.0) | <0.01 | 72 | 24.0 (16.7, 31.2) | <0.01 | 55 | 27.7 (21.3, 34.1) | <0.01 |
| 12 months | 506 | 25.2 (23.0, 27.4) | <0.01 | 67 | 29.5 (23.1, 35.9) | <0.01 | 58 | 22.2 (14.3, 30.0) | <0.01 |
| KCCQ Physical Limitation | |||||||||
| 1 month | 637 | 7.3 (4.7, 9.8) | <0.01 | 64 | 1.3 (−6.1, 8.8) | 0.72 | 51 | 1.7 (−7.7, 11.1) | 0.72 |
| 6 months | 540 | 18.8 (16.3, 21.4) | <0.01 | 62 | 11.5 (3.3, 19.7) | <0.01 | 51 | 17.6 (9.8, 25.4) | <0.01 |
| 12 months | 455 | 16.2 (13.5, 18.9) | <0.01 | 56 | 14.7 (6.7, 22.8) | <0.01 | 53 | 12.7 (4.2, 21.2) | <0.01 |
| KCCQ Total Symptoms | |||||||||
| 1 month | 703 | 10.2 (8.2, 12.2) | <0.01 | 78 | 13.2 (6.5, 19.8) | <0.01 | 60 | 12.1 (5.6, 18.5) | <0.01 |
| 6 months | 601 | 19.3 (17.2, 21.3) | <0.01 | 71 | 17.5 (10.3, 24.6) | <0.01 | 54 | 25.9 (19.4, 32.4) | <0.01 |
| 12 months | 498 | 18.6 (16.4, 20.8) | <0.01 | 65 | 23.3 (16.5, 30.1) | <0.01 | 58 | 19.4 (11.9, 27.0) | <0.01 |
| KCCQ Quality of Life | |||||||||
| 1 month | 699 | 20.0 (17.6, 22.4) | <0.01 | 78 | 22.3 (14.0, 30.6) | <0.01 | 61 | 20.9 (13.1, 28.7) | <0.01 |
| 6 months | 600 | 33.5 (31.2, 35.9) | <0.01 | 72 | 33.2 (24.8, 41.5) | <0.01 | 55 | 35.5 (28.0, 42.9) | <0.01 |
| 12 months | 499 | 35.1 (32.6, 37.6) | <0.01 | 66 | 41.9 (33.5, 50.3) | <0.01 | 57 | 29.7 (20.8, 38.6) | <0.01 |
| KCCQ Social Limitation | |||||||||
| 1 month | 584 | 11.5 (8.5, 14.6) | <0.01 | 61 | 6.9 (−3.2, 17.0) | 0.18 | 46 | 2.8 (−7.9, 13.4) | 0.60 |
| 6 months | 511 | 30.8 (27.8, 33.8) | <0.01 | 59 | 27.2 (16.2, 38.2) | <0.01 | 47 | 29.0 (19.4, 38.7) | <0.01 |
| 12 months | 438 | 28.5 (25.3, 31.8) | <0.01 | 50 | 34.2 (24.0, 44.5) | <0.01 | 47 | 22.8 (11.0, 34.7) | <0.01 |
| SF-12 Physical | |||||||||
| 1 month | 602 | 2.4 (1.7, 3.2) | <0.01 | 77 | 2.7 (0.5, 4.9) | 0.02 | 61 | 0.5 (−2.1, 3.0) | 0.71 |
| 6 months | 528 | 6.6 (5.7, 7.6) | <0.01 | 71 | 5.1 (2.5, 7.7) | <0.01 | 56 | 6.9 (4.2, 9.5) | <0.01 |
| 12 months | 423 | 5.4 (4.4, 6.5) | <0.01 | 67 | 7.0 (4.3, 9.6) | <0.01 | 57 | 4.7 (1.3, 8.0) | <0.01 |
| SF-12 Mental | |||||||||
| 1 month | 602 | 0.2 (−0.8, 1.2) | 0.69 | 77 | −0.7 (−3.6, 2.2) | 0.64 | 61 | 1.7 (−1.4, 4.8) | 0.27 |
| 6 months | 528 | 4.0 (2.9, 5.0) | <0.01 | 71 | 3.4 (0.3, 6.5) | 0.03 | 56 | 3.9 (1.2, 6.6) | <0.01 |
| 12 months | 423 | 3.1 (1.9, 4.4) | <0.01 | 67 | 3.3 (−0.1, 6.7) | 0.06 | 57 | 4.3 (1.1, 7.6) | 0.01 |
| EQ-5D Utilities | |||||||||
| 1 month | 665 | 0.02 (0.00, 0.04) | 0.02 | 75 | −0.03 (−0.08, 0.03) | 0.35 | 58 | 0.01 (−0.04, 0.06) | 0.74 |
| 6 months | 578 | 0.08 (0.06, 0.10) | <0.01 | 67 | 0.04 (−0.03, 0.10) | 0.25 | 51 | 0.07 (0.01, 0.12) | 0.03 |
| 12 months | 474 | 0.06 (0.04, 0.08) | <0.01 | 62 | 0.06 (0.01, 0.12) | 0.03 | 53 | 0.05 (−0.01, 0.12) | 0.11 |
HRQoL = health-related quality of life; EQ-5D = EuroQol 5D; KCCQ = Kansas City Cardiomyopathy Questionnaire; NRCA = non-randomized continued access registry; SAVR = surgical aortic valve replacement; SF-12 = Medical Outcomes Study Short-Form 12; TA = transapical; TAVR = transcatheter aortic valve replacement.
Longitudinal assessment and between-group comparisons
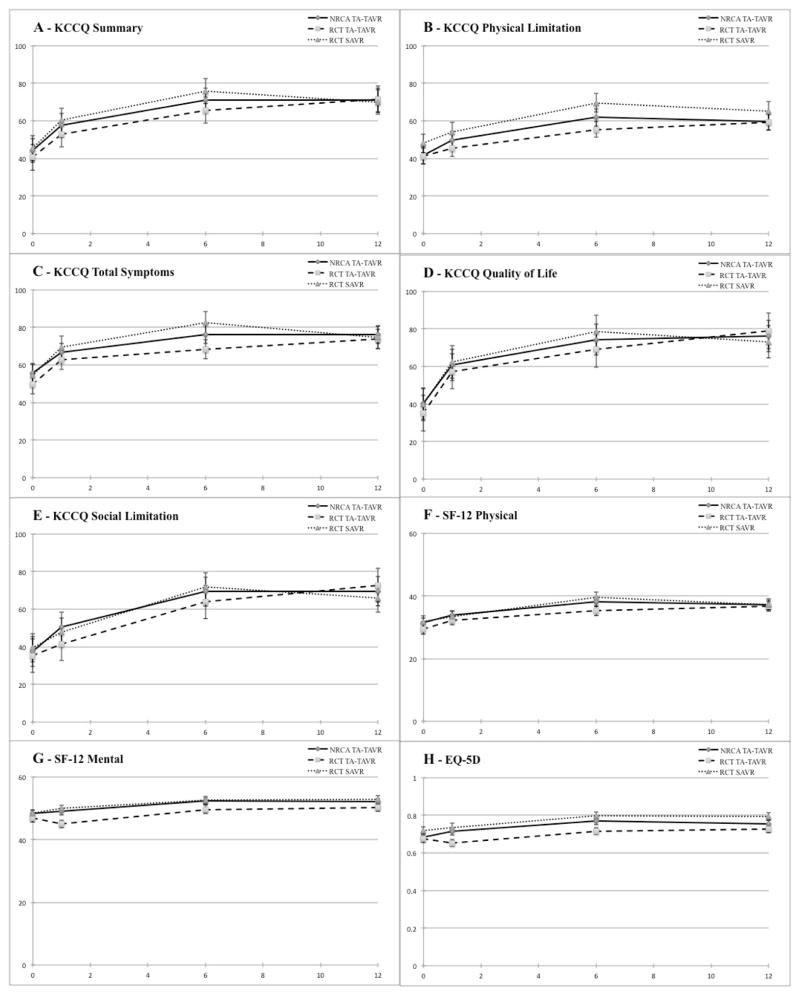
Adjusted mean scores by treatment group for each of the HRQoL domains and follow-up timepoints are shown in Figure 2, and adjusted between-group differences according to the longitudinal mixed effects model are summarized in Table 3. There was no difference in the KCCQ summary score between the NRCA TA-TAVR and RCT TA-TAVR groups at any follow-up timepoint. However, there were small but statistically significant differences in health status favoring the NRCA TA-TAVR group at either 1 or 6 month follow-up for several of the KCCQ subscales including physical limitations, quality of life, and social limitation as well as the SF-12 mental component and the EQ-5D utilities. There were no significant differences in 12-month health status between the NRCA and RCT TA-TAVR groups on any of the subscales.
Figure 2.
Adjusted mean scores derived from longitudinal growth curve models for Kansas City Cardiomyopathy Questionnaire (KCCQ) summary score (A), KCCQ subscales (B–E), the Short-Form 12 (SF-12) physical (F) and mental (G) scores, and EuroQol-5D (EQ-5D) utilities (H).
Table 3.
Adjusted between group differences according to longitudinal mixed effects models
| NRCA TA-TAVR vs RCT TA-TAVR | NRCA TA-TAVR vs RCT SAVR | |||
|---|---|---|---|---|
| Scale/Timepoint | Predicted Mean Difference**: NRCA - RCT (95% CI) | p-value | Predicted Mean Difference**: NRCA - RCT (95% CI) | p-value |
| KCCQ Summary | ||||
| 1 month | 4.2 (−0.9, 9.3) | 0.11 | −3.8 (−9.6,2.0) | 0.20 |
| 6 months | 2.2 (−2.1,6.4) | 0.32 | −2.3 (−7.0,2.5) | 0.35 |
| 12 months | −0.3 (−5.5,4.9) | 0.91 | −0.4 (−6.2,5.3) | 0.88 |
| KCCQ Physical Limitations | ||||
| 1 month | 6.2 (−0.4,12.8) | 0.07 | −2.1 (−9.6,5.4) | 0.58 |
| 6 months | 4.8 (−0.6,10.3) | 0.08 | −2.2 (−8.3,3.8) | 0.47 |
| 12 months | 3.1 (−3.6,9.9) | 0.36 | −2.4 (−9.6,4.8) | 0.52 |
| KCCQ Total Symptoms | ||||
| 1 month | 1.7 (−3.5,6.9) | 0.51 | −3.2 (−9.0,2.5) | 0.27 |
| 6 months | 5.5 (−0.5,10.5) | 0.03 | −7.4 (−12.8, −1.9) | 0.01 |
| 12 months | 0.9 (−4.2,6.0) | 0.73 | −0.3 (−5.8,5.2) | 0.91 |
| KCCQ Quality of Life | ||||
| 1 month | 3.4 (−2.4,9.3) | 0.25 | −3.9 (−10.3,2.6) | 0.24 |
| 6 months | 0.9 (−3.7,5.6) | 0.70 | −1.5 (−6.6,3.6) | 0.56 |
| 12 months | −2.1 (−8.2,4.0) | 0.50 | 1.3 (−5.3,7.9) | 0.70 |
| KCCQ Social Limitation | ||||
| 1 month | 8.5 (−1.0,16.1) | 0.03 | 0.8 (−7.7,9.3) | 0.86 |
| 6 months | 2.4 (−3.5,8.3) | 0.42 | 0.4 (−6.2,7.0) | 0.90 |
| 12 months | −5.0 (−13.0,3.0) | 0.22 | 0.0 (−8.5,8.4) | 1.00 |
| SF-12 Physical | ||||
| 1 month | 1.4 (−0.3,3.2) | 0.11 | 1.1 (−1.0,3.3) | 0.30 |
| 6 months | 1.3 (−0.4,2.9) | 0.15 | −1.4 (−3.9,1.1) | 0.26 |
| 12 months | 1.0 (−1.5,3.5) | 0.42 | 0.4 (−2.4,3.2) | 0.79 |
| SF-12 Mental | ||||
| 1 month | 2.7 (0.4,5.0) | 0.02 | −0.9 (−3.5,1.7) | 0.48 |
| 6 months | 2.1 (0.3,3.9) | 0.02 | −0.9 (−2.8,1.1) | 0.39 |
| 12 months | 1.5 (−1.1,4.0) | 0.26 | −0.8 (−3.5,1.9) | 0.56 |
| EQ-5D Utilities | ||||
| 1 month | 0.06 (0.01,0.11) | 0.02 | 0.00 (−0.06, 0.05) | 0.88 |
| 6 months | 0.04 (0.00,0.08) | 0.04 | −0.02 (−0.06, 0.03) | 0.46 |
| 12 months | 0.02 (−0.03,0.07) | 0.41 | −0.03 (−0.09, 0.03) | 0.28 |
Longitudinal mixed effects models adjusted for age, gender, COPD, and baseline HRQoL
Positive values indicate better HRQoL with NRCA TA-TAVR, whereas negative values indicate worse HRQOL with NRCA TA—TAVR.
COPD = chronic obstructive pulmonary disease; EQ-5D = EuroQol 5D; HRQoL = health-related quality-of-life; KCCQ = Kansas City Cardiomyopathy Questionnaire; NRCA = non-randomized continued access registry; SAVR = surgical aortic valve replacement; SF-12 = Medical Outcomes Study Short-Form 12; TA = transapical; TAVR = transcatheter aortic valve replacement.
When the NRCA TA-TAVR group was compared with the RCT SAVR group, there were no differences in the KCCQ summary score at any follow-up timepoint (Table 3). Moreover, there were no significant between group differences in any of the KCCQ subscales or the generic health status measures at any timepoint.
DISCUSSION
In this study, we have systematically examined health-related quality of life both early and late after TA-TAVR and SAVR using a battery of well-validated instruments. The principal findings of this analysis are: 1) Among patients undergoing TA-TAVR in the PARTNER continued access registry, there were substantial improvements in both disease specific and generic health status that were comparable to those observed in the randomized PARTNER trial; and 2) Although there was a suggestion of modest early in HRQoL benefit for patients treated with TA-TAVR in the NRCA registry compared with the RCT, the magnitude of these differences was small, and there remained no evidence of either early or late HRQoL improvement with TA-TAVR compared with SAVR.
In a previous study of the PARTNER RCT, Reynolds and colleagues found that HRQoL was better with TF-TAVR when compared with SAVR.4 However, in patients deemed unsuitable for a TF approach (who were therefore treated with TA-TAVR), there were no HRQoL benefits of TAVR compared with SAVR, and there were trends favoring SAVR at the 1 and 6 month timepoints. Since the PARTNER RCT represented the earliest experience with TA-TAVR for the vast majority of sites, however, it is possible that the lack of QOL benefit with TA-TAVR related mainly to the inexperience of the treating sites rather than any inherent limitations of the TA-TAVR technique. Indeed in the PARTNER RCT, the median number of TA-TAVR procedures was 4 (range, 1–20; Supplementary Appendix Figure 1). Recent studies have reported that the number of cases required to overcome the learning curve ranges from 18–100,15–17 a level that far exceeded the experience of virtually all of the PARTNER centers at the time of the RCT. The current analysis was therefore performed to determine whether the HRQoL outcomes of TA-TAVR have improved with increasing operator and center experience. Although we did find some evidence that early HRQoL outcomes have improved with greater experience, these differences were modest at best, and there remained no evidence of improved HRQoL compared with SAVR in either the short or the long-term.
Numerous previous studies have demonstrated that TAVR results in substantial HRQoL improvement compared with baseline.4, 18–20 Most recently, meta-analysis of 62 TAVR studies demonstrated that, TAVR generally results in improved functional status and quality of life21—findings that are similar to those with TA-TAVR in the PARTNER trial and NRCA. However, few studies to date have compared patient-reported outcomes of TAVR by access site22 or compared with a surgical control group.4
The lack of early HRQoL benefit with TA-TAVR compared with SAVR, even with greater experience in the NRCA registry, is likely related to both clinical factors and technical aspects of the procedure. The PARTNER trial adopted a “TF-first” mentality, thus relegating TA-TAVR to patients who did not qualify for the TF approach due to anatomical considerations. This strategy may have resulted in higher risk patients undergoing TA-TAVR than would be expected in a real-world clinical setting and could have contributed to worse HRQoL outcomes in this subgroup. Nonetheless, it is important to recognize that randomization in PARTNER was stratified by access site; as a result, those patients who were randomized to SAVR in the TA stratum were similarly high risk.
Previous studies have demonstrated that thoracotomy results in greater postoperative pain than median sternotomy, due to rib spreading and respiratory motion.23 This discomfort could have contributed to short-term HRQoL trends favoring SAVR as well. Procedural modification and localized administration of analgesia may provide some HRQoL benefit in patients undergoing TA-TAVR.24 However, these maneuvers do not eliminate the apical puncture and repair inherent to the TA approach, which could adversely impact left ventricular function and cause subsequent functional limitation.25
Whether other non-femoral approaches to TAVR, such as the transaortic approach, can overcome these issues and result in HRQoL benefits compared with either TA-TAVR or SAVR is currently unknown. In non-randomized studies, the transaortic approach has been shown to result in similar clinical outcomes when compared with the TA approach.26, 27 Potential advantages of the transaortic approach are avoidance of a thoracotomy and injury to the myocardium and apex, as well as potential for direct visualization of the aorta. In patients with specific high-risk comorbidities such as chronic obstructive pulmonary disease and left ventricular systolic dysfunction, these advantages of the transaortic approach may result in superior HRQoL outcomes. To date, however, no rigorous studies have compared HRQoL outcomes between the TA and transaortic approaches to TAVR. Studies comparing HRQoL outcomes of TA and alternative accesses would aid in prioritizing TAVR access options.
In addition to providing a sobering reminder that “less invasive” treatments do not always result in improved patient-centered outcomes, the lack of HRQoL benefit with TA-TAVR compared with SAVR has important economic ramifications. In order for TAVR to be economically attractive from a societal perspective, it must either have lower costs or improved health outcomes compared with the available alternatives.28 In the PARTNER A cost-effectiveness study, initial and 1-year costs were substantially higher with TA-TAVR compared with SAVR.29 In the case of TAVR for high risk surgical candidates, improved health outcomes can be interpreted as either improved long-term survival or better HRQoL. In the randomized PARTNER trial, however, there was no difference in survival between patients treated with TA-TAVR vs. SAVR.7 Although 1-year survival was improved with TA-TAVR in the continued access registry,5 whether this finding reflects improved technique or better patient selection (or both) is unknown. In the absence of definitive evidence of better long-term survival, evidence of improved HRQoL (in either the short or long-term) is therefore critical in order to justify the higher up-front cost of TAVR.
Study limitations
Our findings should be considered in light of a number of important limitations. Most importantly, although the original comparison of TA-TAVR and SAVR in the PARTNER A trial was randomized, the comparison of these 2 cohorts with patients from the NRCA was non-randomized and therefore subject to both measured and unmeasured confounding. The 3 cohorts were all enrolled using the same inclusion and exclusion criteria, however, and there were relatively few differences in observed baseline characteristics between the NRCA and RCT populations. Of note, patients in the RCT TA-TAVR group had a higher prevalence of cerebrovascular disease compared with the NRCA TA-TAVR group. If this difference translated into greater disability in the RCT group, it would have been expected to bias our results toward improved follow-up health status in the NRCA group. Since we did not find such a benefit, however, this baseline imbalance would appear to be an unlikely explanation for our findings. Follow-up health status data were missing on a modest proportion of patients, upwards of 30% in the NRCA cohort at 12-month follow-up, which could have biased our results. Although it is not possible to prove that follow-up data were truly missing at random, comparison of baseline characteristics between the patients with vs. without missing HRQoL data demonstrated no major differences (Supplementary Appendix Table 1). We also analyzed NRCA TA-TAVR patients with and without HRQoL data at 30-day follow-up in order to further investigate the reasonableness of the missing at random assumption. These groups also exhibited no major differences with regards to demographics and characteristics (Supplementary Appendix Table 2). In addition, the clinical outcomes of these patients are not significantly different with regards to mortality, stroke, and rehospitalization rates. However, the rates of vascular complications and bleeding events were higher in the group of patients without HRQoL data (Supplementary Appendix Table 3). Our primary analytic approach (longitudinal mixed effects models) was chosen in order to minimize the impact of missing data at any specific timepoint. Full details regarding completeness of HRQOL data are summarized in Supplementary Appendix Table 4. Finally, there were many fewer patients enrolled and randomized within the TA cohort of PARTNER A than the TF cohort. Thus, it is possible that the lack of a significant benefit of TA-TAVR compared with SAVR in the RCT was driven, in part, by reduced statistical power. Reduced power is less relevant to the NRCA cohort, however, since it was ~10 times larger than the RCT cohort.
Conclusions
In this systematic evaluation of HRQoL outcomes among patients from both the PARTNER RCT and NRCA registry, we found that TA-TAVR resulted in substantial HRQoL benefits compared with baseline that were evident within 1 month and sustained or enhanced at 1 year follow-up. Although there was a suggestion of modest early improvement in HRQoL in patients treated with TA-TAVR in the NRCA registry compared with the RCT, the magnitude of these differences was small and there remained no evidence of either early or late HRQoL improvement with TA-TAVR compared with SAVR. These findings have important implications for access site selection for patients undergoing TAVR, and further study is warranted to determine whether results are similar with other forms of non-femoral access.
Footnotes
DISCLOSURES
Dr. Cohen has received grant support from Edwards Lifesciences, Medtronic, Boston Scientific, Abbott Vascular, MedRad, Merck/Schering-Plough, and Eli Lilly-Daiichi Sankyo, consulting fees from Schering-Plough, Eli Lilly, Medtronic, and Cordis, and speaking honoraria from Eli Lilly, The Medicines Company, and St. Jude Medical. Dr. Reynolds has received research support from Edwards Lifesciences and Medtronic, and consulting fees from Medtronic. Dr. Williams has received consulting fees from Edwards Lifesciences. Dr. Kodali has received consulting fees from Edwards Lifesciences and serves on the advisory board for the Thubrikar Aortic Valve. Dr. Leon has been an unpaid member of the PARTNER Trial Executive Committee. Dr. Thourani has received research support from Edwards Lifesciences and Sorin Medical, consulting fees from DirectFlow Medical, St. Jude, and Sorin Medical, and royalties/intellectual property rights from Apica. Dr. Szeto has received consulting fees from MicroInterventional Devices. All other co-authors have no relevant disclosures.
References
- 1.Gotoda Y, Kambara N, Sakai T, Kishi Y, Kodama K, Koyama T. The morbidity, time course and predictive factors for persistent post-thoracotomy pain. Eur J Pain. 2001;5:89–96. doi: 10.1053/eujp.2001.0225. [DOI] [PubMed] [Google Scholar]
- 2.Katz J, Jackson M, Kavanagh BP, Sandler AN. Acute pain after thoracic surgery predicts long-term post-thoracotomy pain. Clin J Pain. 1996;12:50–5. doi: 10.1097/00002508-199603000-00009. [DOI] [PubMed] [Google Scholar]
- 3.Richardson J, Sabanathan S, Shah R. Post-thoracotomy spirometric lung function: the effect of analgesia. A review. J Cardiovasc Surg (Torino) 1999;40:445–56. [PubMed] [Google Scholar]
- 4.Reynolds MR, Magnuson EA, Wang K, Thourani VH, Williams M, Zajarias A, Rihal CS, Brown DL, Smith CR, Leon MB, Cohen DJ, Investigators PT. Health-related quality of life after transcatheter or surgical aortic valve replacement in high-risk patients with severe aortic stenosis: results from the PARTNER (Placement of AoRTic TraNscathetER Valve) Trial (Cohort A) J Am Coll Cardiol. 2012;60:548–58. doi: 10.1016/j.jacc.2012.03.075. [DOI] [PubMed] [Google Scholar]
- 5.Dewey TM, Bowers B, Thourani VH, Babaliaros V, Smith CR, Leon MB, Svensson LG, Tuzcu EM, Miller DC, Teirstein PS, Tyner J, Brown DL, Fontana GP, Makkar RR, Williams MR, George I, Kirtane AJ, Bavaria JE, Mack MJ. Transapical aortic valve replacement for severe aortic stenosis: results from the nonrandomized continued access cohort of the PARTNER trial. Ann Thorac Surg. 2013;96:2083–9. doi: 10.1016/j.athoracsur.2013.05.093. [DOI] [PubMed] [Google Scholar]
- 6.Leon MB, Smith CR, Mack M, Miller DC, Moses JW, Svensson LG, Tuzcu EM, Webb JG, Fontana GP, Makkar RR, Brown DL, Block PC, Guyton RA, Pichard AD, Bavaria JE, Herrmann HC, Douglas PS, Petersen JL, Akin JJ, Anderson WN, Wang D, Pocock S, Investigators PT. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med. 2010;363:1597–607. doi: 10.1056/NEJMoa1008232. [DOI] [PubMed] [Google Scholar]
- 7.Smith CR, Leon MB, Mack MJ, Miller DC, Moses JW, Svensson LG, Tuzcu EM, Webb JG, Fontana GP, Makkar RR, Williams M, Dewey T, Kapadia S, Babaliaros V, Thourani VH, Corso P, Pichard AD, Bavaria JE, Herrmann HC, Akin JJ, Anderson WN, Wang D, Pocock SJ, Investigators PT. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med. 2011;364:2187–98. doi: 10.1056/NEJMoa1103510. [DOI] [PubMed] [Google Scholar]
- 8.Green CP, Porter CB, Bresnahan DR, Spertus JA. Development and evaluation of the Kansas City Cardiomyopathy Questionnaire: a new health status measure for heart failure. J Am Coll Cardiol. 2000;35:1245–55. doi: 10.1016/s0735-1097(00)00531-3. [DOI] [PubMed] [Google Scholar]
- 9.Arnold SV, Spertus JA, Lei Y, Allen KB, Chhatriwalla AK, Leon MB, Smith CR, Reynolds MR, Webb JG, Svensson LG, Cohen DJ. Use of the Kansas City Cardiomyopathy Questionnaire for monitoring health status in patients with aortic stenosis. Circ Heart Fail. 2013;6:61–7. doi: 10.1161/CIRCHEARTFAILURE.112.970053. [DOI] [PubMed] [Google Scholar]
- 10.Spertus J, Peterson E, Conard MW, Heidenreich PA, Krumholz HM, Jones P, McCullough PA, Pina I, Tooley J, Weintraub WS, Rumsfeld JS Cardiovascular Outcomes Research C. Monitoring clinical changes in patients with heart failure: a comparison of methods. Am Heart J. 2005;150:707–15. doi: 10.1016/j.ahj.2004.12.010. [DOI] [PubMed] [Google Scholar]
- 11.Ware J, Jr, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34:220–33. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 12.Wyrwich KW, Spertus JA, Kroenke K, Tierney WM, Babu AN, Wolinsky FD Heart Disease Expert P. Clinically important differences in health status for patients with heart disease: an expert consensus panel report. Am Heart J. 2004;147:615–22. doi: 10.1016/j.ahj.2003.10.039. [DOI] [PubMed] [Google Scholar]
- 13.EuroQol G. EuroQol--a new facility for the measurement of health-related quality of life. Health Policy. 1990;16:199–208. doi: 10.1016/0168-8510(90)90421-9. [DOI] [PubMed] [Google Scholar]
- 14.Shaw JW, Johnson JA, Coons SJ. US valuation of the EQ-5D health states: development and testing of the D1 valuation model. Med Care. 2005;43:203–20. doi: 10.1097/00005650-200503000-00003. [DOI] [PubMed] [Google Scholar]
- 15.Alli OO, Booker JD, Lennon RJ, Greason KL, Rihal CS, Holmes DR., Jr Transcatheter aortic valve implantation: assessing the learning curve. JACC Cardiovasc Interv. 2012;5:72–9. doi: 10.1016/j.jcin.2011.09.014. [DOI] [PubMed] [Google Scholar]
- 16.D’Ancona G, Pasic M, Unbehaun A, Dreysse S, Drews T, Buz S, Kukucka M, Mladenow A, Hetzer R, Seifert B. Transapical aortic valve implantation: learning curve with reduced operating time and radiation exposure. Ann Thorac Surg. 2014;97:43–7. doi: 10.1016/j.athoracsur.2013.07.070. [DOI] [PubMed] [Google Scholar]
- 17.Lichtenstein KM, Kim JM, Gao M, Soon JL, Cheung A, Wood D, Webb JG, Ye J. Surgical risk algorithm as a measure of successful adoption of transapical transcatheter aortic valve implantation. J Thorac Cardiovasc Surg. 2014;147:1524–8. doi: 10.1016/j.jtcvs.2013.05.033. [DOI] [PubMed] [Google Scholar]
- 18.Arnold SV, Reynolds MR, Lei Y, Magnuson EA, Kirtane AJ, Kodali SK, Zajarias A, Thourani VH, Green P, Rodes-Cabau J, Beohar N, Mack MJ, Leon MB, Cohen DJ, Investigators P. Predictors of Poor Outcomes After Transcatheter Aortic Valve Replacement: Results From the PARTNER (Placement of Aortic Transcatheter Valve) Trial. Circulation. 2014;129:2682–90. doi: 10.1161/CIRCULATIONAHA.113.007477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arnold SV, Spertus JA, Lei Y, Green P, Kirtane AJ, Kapadia S, Thourani VH, Herrmann HC, Beohar N, Zajarias A, Mack MJ, Leon MB, Cohen DJ. How to define a poor outcome after transcatheter aortic valve replacement: conceptual framework and empirical observations from the placement of aortic transcatheter valve (PARTNER) trial. Circ Cardiovasc Qual Outcomes. 2013;6:591–7. doi: 10.1161/CIRCOUTCOMES.113.000354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reynolds MR, Magnuson EA, Lei Y, Leon MB, Smith CR, Svensson LG, Webb JG, Babaliaros VC, Bowers BS, Fearon WF, Herrmann HC, Kapadia S, Kodali SK, Makkar RR, Pichard AD, Cohen DJ Placement of Aortic Transcatheter Valves I. Health-related quality of life after transcatheter aortic valve replacement in inoperable patients with severe aortic stenosis. Circulation. 2011;124:1964–72. doi: 10.1161/CIRCULATIONAHA.111.040022. [DOI] [PubMed] [Google Scholar]
- 21.Kim CA, Rasania SP, Afilalo J, Popma JJ, Lipsitz LA, Kim DH. Functional status and quality of life after transcatheter aortic valve replacement: a systematic review. Ann Intern Med. 2014:160. doi: 10.7326/M13-1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bona V, Khawaja MZ, Bapat V, Young C, Hancock J, Redwood S, Fusari M, Thomas M. Early and late changes in quality of life following transcatheter aortic valve implantation using the transfemoral and transapical approaches. EuroIntervention. 2014 doi: 10.4244/EIJV11I2A41. [DOI] [PubMed] [Google Scholar]
- 23.Grossi EA, Zakow PK, Ribakove G, Kallenbach K, Ursomanno P, Gradek CE, Baumann FG, Colvin SB, Galloway AC. Comparison of post-operative pain, stress response, and quality of life in port access vs. standard sternotomy coronary bypass patients. Eur J Cardiothorac Surg. 1999;16(Suppl 2):S39–42. [PubMed] [Google Scholar]
- 24.Amat-Santos IJ, Dumont E, Villeneuve J, Doyle D, Rheault M, Lavigne D, Lemieux J, St-Pierre A, Mok M, Urena M, Nombela-Franco L, Blackburn S, Simon M, Bourgault C, Carrasco JL, Pibarot P, Cote M, Delarochelliere R, Cohen DJ, Rodes-Cabau J. Effect of thoracic epidural analgesia on clinical outcomes following transapical transcatheter aortic valve implantation. Heart. 2012;98:1583–90. doi: 10.1136/heartjnl-2012-302185. [DOI] [PubMed] [Google Scholar]
- 25.Meyer CG, Frick M, Lotfi S, Altiok E, Koos R, Kirschfink A, Lehrke M, Autschbach R, Hoffmann R. Regional left ventricular function after transapical vs. transfemoral transcatheter aortic valve implantation analysed by cardiac magnetic resonance feature tracking. Eur Heart J Cardiovasc Imaging. 2014 doi: 10.1093/ehjci/jeu103. [DOI] [PubMed] [Google Scholar]
- 26.Lardizabal JA, O’Neill BP, Desai HV, Macon CJ, Rodriguez AP, Martinez CA, Alfonso CE, Bilsker MS, Carillo RG, Cohen MG, Heldman AW, O’Neill WW, Williams DB. The transaortic approach for transcatheter aortic valve replacement: initial clinical experience in the United States. J Am Coll Cardiol. 2013;61:2341–5. doi: 10.1016/j.jacc.2013.02.076. [DOI] [PubMed] [Google Scholar]
- 27.Thourani VH, Gunter RL, Neravetla S, Block P, Guyton RA, Kilgo P, Lerakis S, Devireddy C, Leshnower B, Mavromatis K, Stewart J, Simone A, Keegan P, Nguyen TC, Merlino J, Babaliaros V. Use of transaortic, transapical, and transcarotid transcatheter aortic valve replacement in inoperable patients. Ann Thorac Surg. 2013;96:1349–57. doi: 10.1016/j.athoracsur.2013.05.068. [DOI] [PubMed] [Google Scholar]
- 28.Cohen DJ, Reynolds MR. Interpreting the results of cost-effectiveness studies. J Am Coll Cardiol. 2008;52:2119–26. doi: 10.1016/j.jacc.2008.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reynolds MR, Magnuson EA, Lei Y, Wang K, Vilain K, Li H, Walczak J, Pinto DS, Thourani VH, Svensson LG, Mack MJ, Miller DC, Satler LE, Bavaria J, Smith CR, Leon MB, Cohen DJ, Investigators P. Cost-effectiveness of transcatheter aortic valve replacement compared with surgical aortic valve replacement in high-risk patients with severe aortic stenosis: results of the PARTNER (Placement of Aortic Transcatheter Valves) trial (Cohort A) J Am Coll Cardiol. 2012;60:2683–92. doi: 10.1016/j.jacc.2012.09.018. [DOI] [PubMed] [Google Scholar]