Abstract
Diarrhoeal disease remains a major health burden worldwide. Secretory diarrhoeas are caused by certain bacterial and viral infections, inflammatory processes, drugs and genetic disorders. Fluid secretion across the intestinal epithelium in secretory diarrhoeas involves multiple ion and solute transporters, as well as activation of cyclic nucleotide and Ca2+ signalling pathways. In many secretory diarrhoeas, activation of Cl− channels in the apical membrane of enterocytes, including the cystic fibrosis transmembrane conductance regulator and Ca2+-activated Cl− channels, increases fluid secretion, while inhibition of Na+ transport reduces fluid absorption. Current treatment of diarrhoea includes replacement of fluid and electrolyte losses using oral rehydration solutions, and drugs targeting intestinal motility or fluid secretion. Therapeutics in the development pipeline target intestinal ion channels and transporters, regulatory proteins and cell surface receptors. This Review describes pathogenic mechanisms of secretory diarrhoea, current and emerging therapeutics, and the challenges in developing antidiarrhoeal therapeutics.
Introduction
Diarrhoeal diseases have been a major health problem throughout history.1 In the past, diarrhoeal diseases were often fatal and disease outbreaks spread quickly, affecting large populations. Today, despite the success of interventions such as oral and intravenous rehydration therapy, secretory diarrhoea remains a substantial cause of mortality and morbidity worldwide, particularly in children and the elderly. In 2015, it is estimated that worldwide 577,000 children aged <5 years and 502,000 adults aged >70 years will die from diarrhoeal diseases.2 For these vulnerable populations, the mortality risk due to diarrhoeal disease is often further increased by associated risk factors such as malnutrition and pre-existing enteric infections.3 In addition to the mortality risk, repeated diarrhoeal episodes are associated with long-term impairment of physical and mental development, with an estimated global loss of ~1,400 years of healthy life due to disability per 100,000 population.2,4
The prevalence of diarrhoeal disease, as for other important global causes of childhood mortality such as pneumonia and malaria, is correlated closely with climate and economic development. The most severely affected regions include developing countries in sub-Saharan Africa and South Asia.2 The major causes of diarrhoeal diseases in developing countries are infectious, including enterotoxin-producing bacteria, such as Vibrio cholerae and enterotoxigenic Escherichia coli; viruses, such as rotavirus; enteroinvasive bacteria, such as Shigella and Salmonella; and parasites, such as Entamoeba histolytica and Cryptosporidium parvum.5 The most common cause of severe diarrhoea worldwide is rotavirus (28% of diarrhoeal episodes), with V. cholerae producing at least 1–2% of cases.6 Severe diarrhoea outbreaks that rapidly affect large populations are often associated with complex humanitarian emergencies such as the displacement of people into refugee camps, natural disasters such as earthquakes, and armed conflict, leading to the loss of health and sanitation infrastructure. Examples include the refugee crisis in Rwanda in 1994, the conflict in Zimbabwe in 2008, and the earthquake in Haiti in 2010.
Many noninfectious causes of diarrhoea are prominent in developed countries. Diarrhoea is associated with adverse effects of drugs, particularly certain cancer and HIV therapeutics.7,8 Up to 28% of patients with HIV treated with protease inhibitors report more than four loose or watery stools per day.7 Intestinal inflammatory and autoimmune conditions, such as ulcerative colitis, Crohn’s disease and coeliac disease, can have a substantial diarrhoeal component.9,10 IBS is prevalent in developed countries; 10–20% of the adult population in the US are estimated to have IBS, of which around one-third suffer from chronic diarrhoea.11 Severe secretory diarrhoea is also caused by rare congenital disorders, such as microvillus inclusion disease, familial diarrhoea syndrome and tufting enteropathy, as well as by peptide-secreting neuroendocrine tumours.12–15 However, infectious causes of diarrhoea still represent a large proportion of the disease burden in developed countries. The incidence of diarrhoea caused by rota-viruses has fallen dramatically over the past 5 years with the widespread administration of the rotavirus vaccine, although the incidence of diarrhoea caused by noroviruses has increased and become the leading cause of disease outbreaks from contaminated food in the US.16 The main bacterial causes of food-related diarrhoeal disease in the US are Salmonella enterica (19,000 hospitalizations per year) and Campylobacter jejuni (8,000 hospitalizations per year),16 and there have been annual enterohaemorrhagic E. coli outbreaks since the early 1990s.
In this Review, we describe the major pathogenic mechanisms of secretory diarrhoea, discuss currently available pharmacological therapies and therapies that are being developed, and examine the major challenges in the development of diarrhoeal therapeutics.
Mechanisms of diarrhoeal disease
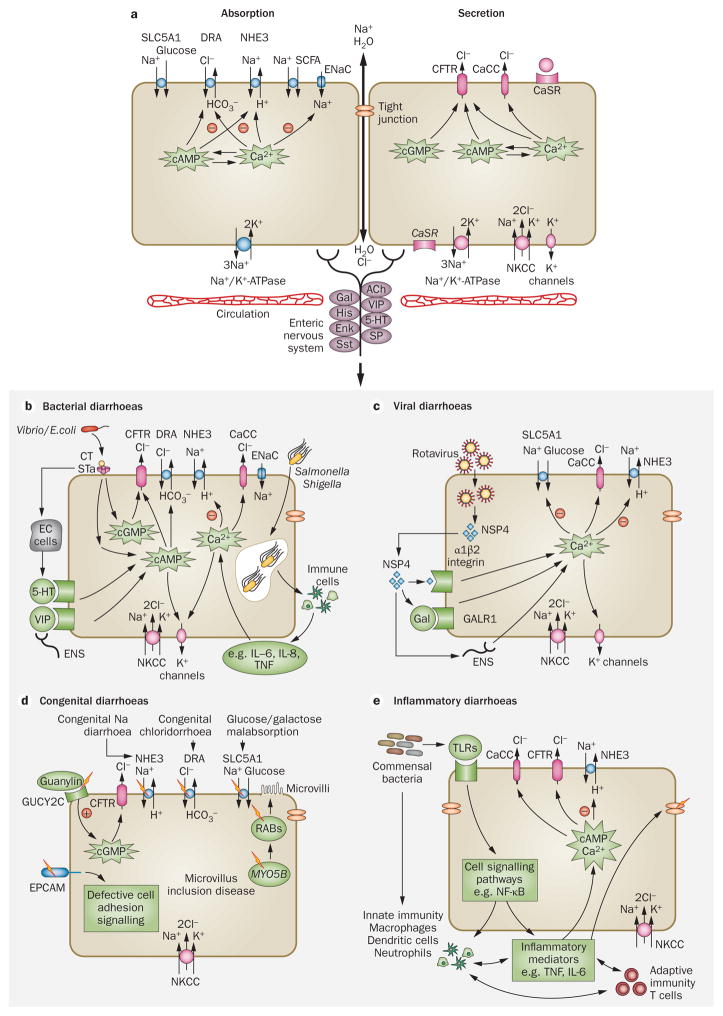
Diarrhoea results from excessive secretion and/or impaired absorption of fluid and electrolytes across the intestinal epithelium (Figure 1). The movement of fluid between the intestinal lumen and blood is driven by the active transport of ions, mainly Na+, Cl−, HCO3−, and K+, and solutes, mainly glucose. Fluid absorption or secretion involves the coordinated activity of membrane transporters located on the apical (lumen-facing) and basolateral (circulation-facing) epithelial membranes.17 The intestinal epithelium is structurally configured into long, finger-like projections (villi) and glandular, tube-like structures (crypts), with the relative villus-to-crypt ratio differing along the intestine. Functionally, both absorption and secretion can occur in the same epithelial cells, although secretory processes predominate in crypts and absorptive processes in villi.
Figure 1.
Mechanisms of intestinal fluid absorption and secretion in secretory diarrhoeas. a | Luminal and basolateral membrane transporters and intracellular signalling mechanisms are involved in intestinal fluid absorption and secretion by enterocytes. b | Some bacteria secrete enterotoxins that increase intracellular cyclic nucleotides, resulting in Cl− secretion and inhibition of NHE3 and Na+ absorption. Invasive bacteria cause a tissue inflammatory response involving recruitment of immune cells and release of cytokines, resulting in intracellular Ca2+ signalling. c | The rotaviral protein NSP4 causes elevation of cytoplasmic Ca2+ concentration by binding to integrin-α1β2, galanin and/or by activation of enteric nerves. Rotaviral NSP4 also inhibits NHE3 and SLC5A1. d | Congenital diarrhoeas result from rare inherited genetic mutations in several intestinal proteins. e | Activation of inflammatory signalling pathways such as NF-κB result in Ca2+ or cyclic nucleotide signalling and stimulation of Cl− secretion or inhibition of Na+ absorption. The release of inflammatory mediators such as TNF and IL-6 by activated T cells and neutrophils can also stimulate Cl− secretion. Abbreviations: CaCC, calcium-activated chloride channel; CaSR, calcium-sensing receptor; CFTR, cystic fibrosis transmembrane conductance regulator; CT, cholera toxin; DRA, down regulated in adenoma Cl−/HCO3− exchanger; EC, enterochromaffin; ENaC, epithelial Na+ channel; ENS, enteric nervous system; EPCAM, epithelial cell adhesion molecule; Gal, galanin; GALR1, galanin receptor 1; GUCY2C, guanylate cyclase C (heat stable enterotoxin receptor); 5-HT, 5-hydroxytryptamine; MYO5B, unconventional myosin-5b; NHE, sodium/hydrogen exchanger; NKCC, Na/K/Cl symporter; NSP4, nonstructural protein 4; RABs, Ras-related proteins; SCFA, short-chain fatty acid; SLC5A1, sodium/glucose cotransporter; STa, heat-stable toxin; TLR, toll-like receptor; VIP, vasoactive intestinal peptide.
Fluid absorption is driven by the active transport of Na+ across the epithelium with parallel Cl− or HCO3− absorption. The electrochemical driving force for this process is the basolateral Na+/K+-ATPase. In the small intestine, fluid absorption is facilitated by the sodium/hydrogen exchanger 3 (NHE3, also known as SLC9A3), sodium/glucose cotransporter 1 (SLC5A1), and Cl−/HCO3− exchanger (DRA [SLC26A3] and PAT1 [SLC26A6]).18–20 Electroneutral fluid absorption is carried out by the coordinated activity of NHE3 with Cl−/HCO3− exchangers (PAT1 for HCO3− absorption and DRA for Cl− absorption in the jejunum and colon).21,22 Substrate-specific transporters such as SLC5A1 facilitate cotransport of Na+ across the apical membrane together with D-glucose (or D-galactose), with the electroneutral glucose transporter SLC2A2 facilitating glucose exit across the basolateral membrane.23,24 In the colon, in addition to electroneutral Na+ transport by Na+/H+ exchange (proximal colon), absorption is facilitated by the epithelial Na+ channel and short-chain fatty acid transporters.25,26 Intracellular messengers, including Ca2+ and cyclic nucleotides such as cAMP and cGMP, inhibit the activity of apical Na+ transporters.27–30 There is considerable cross-activation of the intestinal epithelial second messengers cAMP and Ca2+.31,32
Intestinal fluid secretion is driven by transepithelial Cl− secretion through basolateral and apical Cl− channels and transporters. Cl− is transported into the cell at the basolateral membrane by a Na/K/Cl symporter (NKCC1, also known as SLC12A2), which is driven by the Na+ concentration gradient produced by the Na+/K+-ATPase.33 Basolateral K+ channels (KCNQ1/KNE3 and KCNN4) provide the electrochemical driving force for apical Cl− exit across Cl− channels,34 primarily the cyclic-nucleotide-activated cystic fibrosis transmembrane conductance regulator (CFTR) and Ca2+-activated Cl− channels (CaCCs).33 Enteric nerves and cell surface receptors such as the calcium-sensing receptor (CaSR) are thought to modulate intracellular signalling pathways and hence electrolyte absorption and secretion.35,36
Bacterial diarrhoeas
Bacteria such as V. cholerae and enterotoxigenic E. coli secrete specific enterotoxins (for example cholera toxin and heat-stable enterotoxin, respectively) that increase levels of intracellular cyclic nucleotides, resulting in activation of apical CFTR Cl− channels and hence Cl− secretion (Figure 1b).37,38 Data from immortalized and primary human intestinal cells show that elevation of cAMP, cGMP and Ca2+ concentrations by bacterial enterotoxins also inhibits NHE3.39,40 Bacteria can also increase various humoral agonists, neurotransmitters or neuropeptide receptors such as 5-hydroxytryptamine, VIP peptides and the galanin receptor type 1,41 thus activating Cl− secretion and inhibiting Na+ absorption.42 Invasive bacteria such as Salmonella and Shigella cause a tissue inflammatory response involving recruitment of immune cells and release of cytokines, resulting in intra-cellular Ca2+ signalling.43 Enteropathogenic and invasive bacteria also result in alterations in transport protein expression, with several studies showing evidence of impaired Na+ and Cl− absorption.40,44,45
Viral diarrhoeas
Enteric rotavirus infection causes fluid secretion as well as structural changes in the intestinal epithelium,46 producing an age-related secretory diarrhoea.47,48 An elaborated rotaviral protein (NSP4) is thought to act as an enterotoxin causing elevation of cytoplasmic Ca2+ concentration by binding to a membrane receptor (integrin α1β2), the neuropeptide galanin and/or by activation of enteric nerves (Figure 1c).49–51 Rotaviral NSP4 also inhibits NHE3 and SLC5A1.52 The molecular mechanisms by which other enteric viruses such as norovirus cause diarrhoea is not known at present.53 Multiple pathogenic mechanisms for drug-induced diarrhoeas are likely to exist, but there is evidence that certain drugs such as HIV protease inhibitors and chemotherapy agents induce diarrhoea through intra-cellular Ca2+-dependent mechanisms similar to those in rotaviral diarrhoea.54,55
Congenital diarrhoeas
Congenital diarrhoeas can result from rare inherited genetic mutations in several intestinal proteins (Figure 1d). The target genes in many of these familial enteropathies have been discovered with the advent of low-cost gene sequencing. Mutations in the Na+ transporter SLC5A1 underlie glucose and galactose mal-absorption, and mutations in the Cl−/HCO3− exchanger DRA result in congenital Cl− diarrhoea.56,57 A mutation in the guanylin receptor GUCY2C, which causes familial diarrhoea syndrome, results in constitutive cGMP activation with consequent CFTR-mediated Cl− secretion and NHE3 inhibition.14 Alterations in the integrity of intestinal structure caused by mutations in proteins such as MYO5B and the epithelial cell adhesion molecule also result in altered signalling with putative activation of electrolyte secretion.58,59 Additional details about congenital diarrhoeas can be found elsewhere.60,61
Inflammatory diarrhoea
Intestinal inflammation is seen in autoimmune diseases such as IBD and coeliac disease. Although the predominant feature of these diseases is chronic tissue damage secondary to inappropriate immune cell activation, several overlapping signalling pathways affect intestinal fluid transport homeostasis.62 Activation of epithelial inflammatory signalling pathways such as NF-κB result in Ca2+ or cyclic nucleotide signalling and stimulation of Cl− secretion or inhibition of Na+ absorption (Figure 1e). The release of inflammatory mediators such as TNF and IL-6 by activated T cells and neutrophils, which causes degranulation of mucosal mast cells and release of histamine and prostaglandins, can also stimulate Cl− secretion.63,64 Epithelial Na+ absorption is also probably affected by inflammation, with studies showing down-regulation of NHE1 (also known as SLC9A1) and NHE3 in IBD, and defective NHE3 function and regulation by adaptor proteins in an IL-10-deficient mouse model of colitis.65–67 Predominantly inflammatory diarrhoea is seen in response to some bacterial pathogens, such as Clostridium difficile, which is the most common cause of nosocomial antibiotic-associated diarrhoea. C. difficile-associated diarrhoea and colitis occurs secondary to elaborated toxins that activate epithelial inflammatory signalling pathways and recruit immune cells.68
Current management and treatment
The acute treatment of infectious diarrhoeal diseases centres around prompt and complete amelioration of dehydration. In hospitals, particularly in developed countries, this treatment is often achieved through intravenous fluid replacement.69 In developing countries and non-hospital settings oral rehydration solution (ORS) is the mainstay of treatment.70 Although ORS effectively treats dehydration when administered appropriately, it does not change fluid losses, diarrhoeal output or duration of illness. Most of the current and historical therapeutics used for symptomatic treatment are classed as antimotility agents or antisecretory agents based on their mechanism of action (Table 1). For diarrhoeas caused by enteric infections, various antibiotics are also used depending on the pathogenic organism.71 Antibiotics shorten illness duration and are used particularly for dysentery (bloody diarrhoea), but selection of appropriate antibiotics requires laboratory diagnosis of the pathogenic organism, which often is not available. Although antibiotics are effective in reducing symptoms and the duration of infectious diarrhoeas, their delayed onset of action means they do not prevent immediate dehydration. Also, concerns about antibiotic resistance and the fact that they are contra-indicated in specific enteric infections have prevented their recommendation for widespread use.72,73
Table 1.
Current antidiarrhoeal therapies
| Therapy (dose, administration) [Brand name] | Mechanism of action | Indications | Notes and adverse effects | References |
|---|---|---|---|---|
| Alosetron (1–2 mg daily, oral) [Lotronex®, Prometheus Laboratories, USA] | 5-hydroxytryptamine3receptor antagonist; antimotility, antisecretory | Refractory diarrhoea- predominant IBS | Contraindicated for patients with constipation; intestinal ileus, obstruction, perforation, ischaemic colitis | Koch et al. (2004)135 |
| Cholestyramine resin (4–12 g daily, oral) Questran®, Bristol–Myers Squibb, USA] Colesevelam (4–12 g daily, oral) [Welchol®, Daiichi-Sankyo, USA; Cholestagel®, Genzyme, Netherlands] | Sequesters bile salts; antisecretory, proabsorption | Bile acid malabsorption diarrhoea;ileal resection | Unlabelled indication; impaired absorption of other drugs; malabsorption of fat-soluble vitamins | Koch et al. (2004)135 |
| Crofelemer (250 mg daily, oral) [Fulyzaq®, Salix Pharmaceuticals, USA] | CFTR and CaCC channel inhibitor; antisecretory | Diarrhoea associated with antiretroviral therapy for HIV/AIDS | Not approved for infectious or other diarrhoeas | Macarthur et al. (2013)89 |
| Diphenoxylate and atropine (5–20 mg daily, oral) [Lomotil®, AMCo, UK] | μ-opioid receptor agonist; antimotility | Acute nonspecic diarrhoea | Contraindicated in diarrhoea associated with enterotoxigenic bacteria, pseudomembranous colitis and ulcerative colitis; CNS depression, dizziness, paralytic ileus | Karim et al. (1972)83 |
| Loperamide (4–16 mg daily, oral) [Imodium®, Johnson & Johnson, USA] | μ-opioid receptor agonist; antimotility | Acute nonspeci c diarrhoea, chronic diarrhoea associated with IBD | Contraindicated in bacterial enterocolitis, pseudomembranous colitis and ulcerative colitis; constipation, nausea | DuPont et al. (1990)85; Koch et al. (2004)135;Ericsson et al. (1990)137 |
| Racecadotril (100–300 mg daily, oral) [Hidrasec®, Tiorfan®, Bioproject, France] | Enkephalinase inhibitor; antisecretory | Acute nonspeci c diarrhoea | Not approved in USA; headache | Hamza et al. (1999)87 |
| Bismuth subsalicylate (0.5–4 g daily, oral) [Pepto-Bismol®, Procter & Gamble, USA;Bismatrol] | Inhibition of prostaglandin synthesis; antisecretory; antimicrobial | Acute mild nonspecic diarrhoea | Faecal discoloration, tinnitus, CNS depression, dizziness, Reyes syndrome in children | DuPont et al. (1990)85 |
Abbreviations: CaCC, calcium-activated chloride channel; CFTR, cystic fibrosis transmembrane conductance regulator; CNS, central nervous system.
ORS
ORS is an orally ingested solution that stimulates intestinal Na+ absorption by SLC5A1 and Na+-coupled amino acid transporters. The current WHO-recommended ORS is hypo-osmolar (245 mOsm/l) to increase water absorption.74 The widespread use of ORS over the past four decades has remarkably reduced the mortality associated with diarrhoeal disease by ~70%.75 However, ORS administration in developing countries has stagnated since 1995, with ORS only used in 30–35% of children with diarrhoea aged <5 years.76,77 In the past decade, alternative forms of ORS have been developed. In one form, the substrate linked to Na+ absorption is nonabsorbable starch, which is relatively resistant to digestion by pancreatic amylase and is metabolized by bacteria to short-chain fatty acids in the colon where it stimulates Na+ absorption by NHE2 (also known as SLC9A2).74,78 In clinical trials, patients with cholera who receive nonabsorbable starch ORS have a shorter duration of diarrhoea and require less parenteral fluid support than patients who receive conventional ORS.79 Zinc has been shown to shorten the duration of acute diarrhoea and is part of the WHO-recommended ORS.80 The mechanism of this effect of zinc is unclear, and whether zinc deficiency plays a role has not been established. In vitro, zinc blocks Cl− secretion by inhibiting K+ channels in the basolateral membrane and stimulating NHE3.81,82
Antimotility agents
Drugs that inhibit intestinal motility have been used extensively to treat diarrhoea. The putative mechanism of action for antimotility drugs is increased Na+ and fluid absorption as a result of slow intestinal transit. Loperamide and diphenoxylate are μ-opioid agonists that are widely used for mild, nonspecific diarrhoea. They are not recommended in bacterial diarrhoeas primarily owing to the risk of paralytic ileus, and diphenoxylate also has substantial central opioid effects.83 5-hydroxytryptamine3 antagonists such as alosetron have shown efficacy in chronic diarrhoea related to IBS;84 however, their use has been limited by concerns of ileus and ischaemic colitis. Although loperamide is widely used and effective in mild acute diarrhoea,85 the potential serious adverse effects of antimotility drugs together with a narrow therapeutic index has limited their recommendation and use, particularly for infectious diarrhoeas.
Antisecretory agents
Reducing intestinal fluid secretion has been a relatively underexploited area for antidiarrhoeal therapeutics. Historically, bismuth subsalicylate was shown to have antidiarrhoeal efficacy and early mechanistic studies indicated that salicylates such as aspirin inhibit enterotoxin-induced Cl− secretion and promote Na+ absorption.86 Racecadotril, an encephalinase inhibitor, or its active metabolite thiorphan, initially showed promise as an antidiarrhoeal. Inhibition of the breakdown of endogenous encephalins could exert anti-secretory effects through encephalin-stimulated activation of epithelial μ-opioid receptors. Small clinical studies initially showed efficacy in cholera-toxin-mediated fluid secretion; however, a large double-blind, placebo-controlled trial showed no improvement in diarrhoeal output or duration.87,88 More recently, a natural-product antisecretory agent, crofelemer, has been approved for use in HIV-related diarrhoeas based on a clinical trial showing efficacy in improving chronic diarrhoea in patients with HIV.89 Crofelemer is a heterogeneous proanthocyanidin oligomer extracted from the bark latex of the South American tree Croton lechleri. The putative mechanism of action for crofelemer is inhibition of Cl− channels in the apical membrane. However, in vitro studies showed crofelemer to be a weak and partial antagonist of CFTR, although a relatively strong inhibitor of CaCCs.90 It is unclear whether this inhibition of CaCCs underlies the efficacy of crofelemer in HIV-related diarrhoea.
Probiotics
Several meta-analyses and clinical studies in developed countries suggest that probiotics prevent or reduce the duration of diarrhoea.91–93 However, these effects seem to be highly strain-dependent and dose-dependent. Studies using specific bacterial strains have shown effects on expression and/or function of DRA, CFTR and NKCC transporters, suggesting that probiotics affect mechanisms of Cl− secretion and electroneutral absorption.94–96 A double-blind, placebo-controlled study in children aged 1–6 years showed that a specific probiotic (Lactobacillus reuteri) significantly reduced the incidence of acute diarrhoea, particularly in children with poor nutritional status.97 Currently, probiotics are not routinely recommended in community settings in developing countries, although increasing evidence shows that they might be beneficial as adjunctive antidiarrhoeal therapies.
New antidiarrhoeal therapies
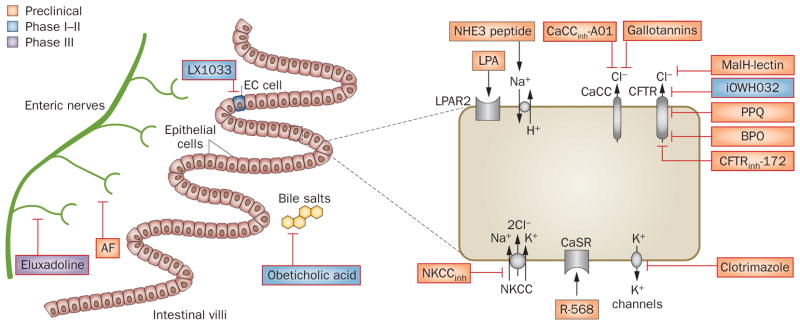
Most of the currently used antidiarrhoeal therapies are decades old and only in the last 5 years has there been renewed interest in development of antidiarrhoeal therapeutics,98 with several novel drug candidates targeting intestinal fluid transport (Table 2). Some of these new therapeutic candidates have emerged from greater understanding of the mechanisms of intestinal fluid secretion and the use of high-throughput screening technology. Figure 2 depicts the molecular targets for antidiarrhoeal therapeutics at various stages of development.
Table 2.
Potential antidiarrhoeal therapies
| Name/class | Target and mechanism | Potential indication | Development stage |
|---|---|---|---|
| Antisecretory factor | Inhibition of enteric nervous system; increased epithelial permeability | Acute secretory diarrhoea | Phase III |
| CaCCinh-A01 | Absorbable CaCC inhibitor | Acute secretory diarrhoea | Preclinical |
| iOWH032 | Extracellular-acting CFTR inhibitor | Acute secretory diarrhoea | Phase I |
| CFTRinh-172 | Absorbable CFTR inhibitor | Acute secretory diarrhoea | Preclinical |
| Clotrimazole | K+ channel inhibitor | Acute secretory diarrhoea | FDA-approved for other indication |
| Eluxadoline | μ-opioid receptor agonist | Diarrhoea- predominant IBD | Phase III |
| Gallotannins | CaCC inhibitors | Acute secretory diarrhoea | Preclinical Nutritional supplement |
| LX1033 | Tryptophan hydroxylase inhibitor; decreased 5-hydroxytryptamine synthesis | Diarrhoea- predominant IBD | Phase II |
| Lysophosphatidic acid | Lysophosphatidic acid receptor 2 antagonist | Acute secretory diarrhoea | Preclinical Nutritional supplement |
| MalH-lectin | Inhibitor of the external pore of CFTR | Acute secretory diarrhoea | Preclinical |
| NHE3 C-terminal peptide | NHE3 regulatory complex agonist | Acute secretory diarrhoea | Preclinical |
| Obeticholic acid | Semi-synthetic bile acid | Bile acid malabsorption diarrhoea | Phase II |
| PPQ/BPO | Absorbable CFTR inhibitors | Acute secretory diarrhoea | Preclinical |
| R-568 | Calcium-sensing receptor agonist | Acute secretory diarrhoea | Preclinical |
Abbreviations: BPO, benzopyrimido-pyrrolo-oxazinedione; CaCC, calcium-activated chloride channel; CFTR, cystic fibrosis transmembrane conductance regulator; NHE, sodium/hydrogen exchanger; PPQ, pyrimido-pyrrolo-quinoxalinedione.
Figure 2.
Potential therapies for secretory diarrhoeas at various stages of development and their molecular targets. Various molecular targets including intestinal ion channels and transporters, regulatory proteins and cell surface receptors are found on epithelial cells lining the intestine, enteric nerves and enterocytes. Novel therapeutics targeting these molecules are in the development pipeline. Abbreviations: AF, antisecretory factor; BPO, benzopyrimido-pyrrolo-oxazinedione; CaCC, calcium-activated chloride channel; CaSR, calcium-sensing receptor; CFTR, cystic fibrosis transmembrane conductance regulator; EC, enterochromaffin; LPA, lysophosphatidic acid; LPAR2, LPA receptor 2; NHE, sodium/hydrogen exchanger; NKCC, Na/K/Cl symporter; PPQ, pyrimido-pyrrolo-quinoxalinedione.
CFTR inhibitors
Activation of CFTR Cl− channels in the small intestine and colon occurs in secretory diarrhoeas caused by bacterial enterotoxins secreted in cholera and Traveller’s diarrhoea.99 Intestinal Cl− and fluid secretion are absent in CFTR-knockout mice and in patients with cystic fibrosis,100,101 and the use of CFTR inhibitors blocks colonic Cl− transport in human tissue.99 High-throughput screening has revealed three chemical classes of small-molecule CFTR inhibitors. The thiazolidinone CFTRinh-172 (Figure 3) inhibits CFTR by binding at or near Arg347 and stabilizes the channel in its closed state.102 Studies in mouse models of cholera and intestinal fluid secretion induced by heat-stable toxin have demonstrated efficacy of CFTRinh-172.99,103 Another class of CFTR inhibitors, which probably interfere with ATP gating, are the PPQ/BPO compounds;104,105 the most potent of these compounds, (R)-BPO-27, inhibits CFTR Cl− conductance with IC50~4 nM and has shown efficacy in models of polycystic kidney disease,104 but has not yet been tested in models of diarrhoea.
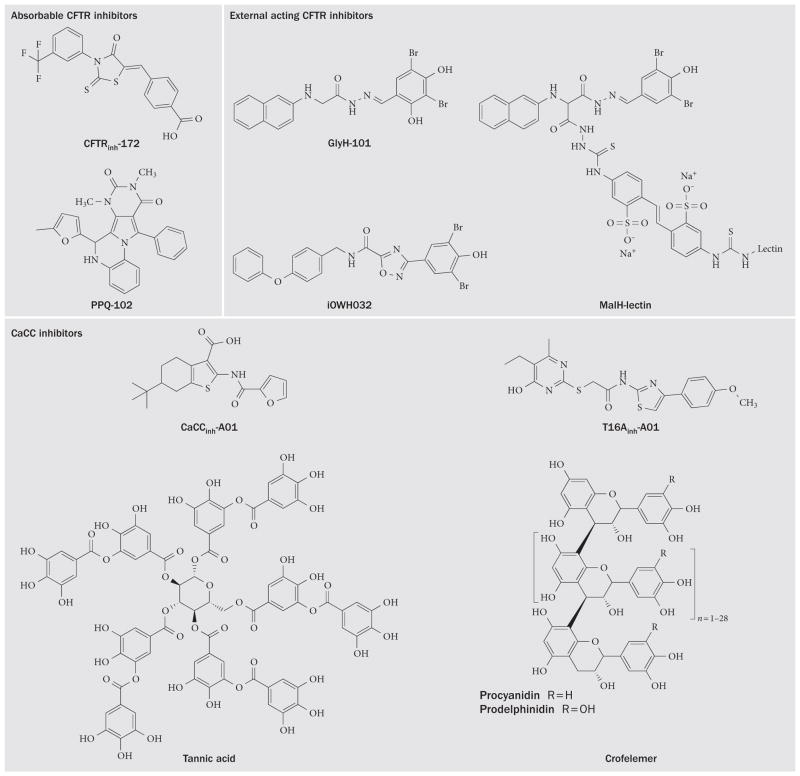
Figure 3.
Chemical structures of CFTR and CaCC inhibitors for secretory diarrhoeas. Abbreviations: CaCC, calcium-activated chloride channel; CFTR, cystic fibrosis transmembrane conductance regulator; PPQ, pyrimido-pyrrolo-quinoxalinedione.
Glycine hydrazides such as GlyH-101 are another class of CFTR inhibitors that bind to the CFTR pore on its extracellular surface as demonstrated by patch clamp, molecular modelling and efficacy of a membrane-impermeant polyethylene glycol-hydrazide conjugate.106 The GlyH-101 analogue iOWH032 has completed a safety clinical trial,107 although a planned phase II trial (NCT02111304) has been terminated before patient enrolment. iOWH032 is unlikely to be effective because it is a weak CFTR inhibitor and can undergo rapid convective washout in the intestine.108 To address the potency and washout limitations, non-absorbable macromolecular conjugates have been synthesized containing a malonic acid hydrazide (MalH) moiety that inhibits CFTR. One such conjugate, MalH- lectin, inhibited CFTR with an IC50 down to 50 nM, remained bound to CFTR for >6 h, and reduced mortality in a neonatal mouse model of cholera.109 The high potency of the MalH-lectin conjugate and its resistance to washout is possibly due to trapping in the glycocalyx on enterocytes. Multivalent MalH-polyethylene glycol conjugates have also been synthesized and shown to be CFTR inhibitiors with nanomolar potency,110 greater chemical stability and lower cost than lectin-containing CFTR inhibitor conjugates.
CaCC inhibitors
CaCCs provide a second route for Cl− secretion by enterocytes and might also be involved in enterotoxin-mediated secretory diarrhoeas by cross-talk in signalling mechanisms. CaCCs probably represent the primary pathway for apical membrane Cl− secretion in rotaviral and perhaps other viral diarrhoeas,48 as well as in secretory diarrhoeas induced by some HIV protease inhibitors54 and chemotherapeutics.55 The molecular identity of the major CaCC(s) in enterocytes remains unclear. The CaCC anoctamin-1 is expressed in interstitial cells of Cajal and has an important role in intestinal motility,111 but its role in Cl− conductance in enterocytes remains uncertain.112
Small-molecule and natural-product CaCC inhibitors have been identified. A phenotype-based screen in HT-29 cells identified several small-molecule CaCC inhibitors, the most potent being the 3-acyl-2-aminothiophene CaCCinh-A01.113 CaCCinh-A01 prevented watery diarrhoea in a neonatal mouse model of rotavirus.114 Screening of natural products to find potential CaCC inhibitors identified tannic acid, which motivated the discovery that polyphenolic gallotannins strongly inhibit CaCCs. Remarkably, oral administration of an alcohol-free red wine extract prevented rotaviral diarrhoea in neonatal mice, without effect on the rotaviral infection.114 The red wine extract did not inhibit CFTR or prevent cholera-toxin-induced intestinal fluid secretion. In another natural-product study, diarrhoea remedies from sources around the world were screened for inhibition of Cl− channels. A commonly used Thai herbal remedy fully inhibited both CFTR and CaCC Cl− conductance in vitro, and was efficacious in mouse models of cholera and rotaviral diarrhoea.115 Natural products represent inexpensive and readily available potential therapies for serious secretory diarrhoeas.
NKCC1 inhibitors
The electroneutral NKCC1 transporter is the major pathway for Cl− entry into enterocytes from the blood side and is therefore an attractive target for development of inhibitors that are predicted to block both CFTR-mediated and CaCC-mediated secretory diarrhoeas. NKCC inhibitors such as bumetanide are approved for use as diuretics, as the kidney expresses the NKCC2 isoform. Although novel NKCC1 inhibitors are emerging from screening efforts, challenges in their development for diarrhoeal therapy include obtaining high selectivity over NKCC2 (to prevent diuretic action) or confining their action to the intestine, and preventing potential off-target effects such as hearing impairment seen in NKCC1-knockout mice.116
CaSR agonists
The CaSR is a G-protein-coupled receptor expressed throughout the small intestine and colon.117 In the small intestine the CaSR is expressed on the basolateral membranes of crypt and villus epithelial cells and on the apical surface of villus cells,117 while in the colon it is present on the apical and basolateral membrane of crypt cells and on cells of the enteric nervous system and some entero-endocrine cells. Extracellular signals that activate the CaSR include divalent (Ca2+, Mg2+ and Cd2+) and trivalent (gadolinium3+ and La3+) cations, and some L-amino acids. Activation of the CaSR seems to have multiple actions on electrolyte transport, including decreasing enterotoxin-induced Cl− secretion and reversing inhibition of Na+ absorption.35 This effect may in part involve breakdown of cyclic nucleotides by phosphodiesterase activation35 and regulation of tight junction assembly.118 CaSR activation by calcimimetic drugs such as R-568 reduces fluid losses stimulated by bacterial enterotoxins and has been proposed as a potential antidiarrhoeal, although to date no clinical studies have investigated CaSR activation by calcimimetics or Ca2+ itself.
K+ channel inhibitors
Several studies have shown that inhibition of basolateral K+ channels reduces enterotoxin-mediated fluid secretion.119 The antifungal clotrimazole blocks both cAMP and Ca2+-sensitive K+ channels in enterocytes with IC50 ~5 μM and has emerged as a potential antidiarrhoeal drug candidate.120 Clotrimazole is approved by the FDA for other indications, with its primary adverse effects related to dose-dependent inhibition of cytochrome P450 enzymes. Other K+ channel inhibitors, including clotrimazole analogues121 and more potent new chemical entities, could be potential antisecretory development candidates.
NHE3 agonists
Studies in cell culture and rodent models suggest that NHE3 is inhibited in secretory diarrhoeas, which seems to be an exaggeration of the NHE3 inhibition that occurs physiologically early in the postprandial state.122 NHE3 activity in enterocytes is inhibited following acute elevations in cAMP, cGMP or Ca2+ concentrations, as produced by bacterial enterotoxins, ionophores or humoral agonists including 5-hydroxytryptamine and carba-chol.28,123,124 These mechanisms probably apply in human secretory diarrhoeas. NHE3 activity is reduced in congenital Na+ diarrhoea as seen in intestinal biopsies,125 with mutations in the transport domain of NHE3 accounting for this autosomal recessive condition (A. Janecke et al., unpublished data). Human enteroids generated from proximal small intestine of healthy individuals show NHE3 activity under basal conditions, which is acutely stimulated by dexamethasone and inhibited by cAMP, cGMP, Ca2+, bacterial enterotoxins and rotaviral infection.126 A peptide that mimics part of the NHE3 C-terminal domain and prevents NHE3 inhibition by cAMP, Ca2+ and cholera toxin has potential utility as a proabsorptive therapeutic.
Antisecretory factor
A secreted, 41 kilodalton protein named antisecretory factor released mainly from the pituitary gland 127 has been shown in rodent models to prevent intestinal fluid secretion induced by cholera toxin, heat-stable toxin, C. difficile toxin A, Campylobacter toxin and prosta-glandins.128 The active component of antisecretory factor consists of seven amino acids in its N-terminus. The antisecretory factor-16 peptide, consisting of the active N-terminal domain of the antisecretory factor, also blocks intestinal fluid secretion. The exact mechanism of action for antisecretory factor is unclear, but the antisecretory effect of antisecretory factor-16 was abolished by vagotomy, suggesting its action is on nerves and perhaps on capillary permeability. A modified egg-yolk product enriched with antisecretory factor (Salovum®, AS-Faktor AB, Sweden) reduced the duration of acute and prolonged (>7 days) diarrhoea in a randomized, placebo-controlled trial in children aged 7–60 months.129 Further clinical and mechanistic studies are needed for this promising drug.
Lysophosphatidic acid
Lysophosphatidic acid is a naturally occurring phospho-lipid present in many foods. Several studies have suggested that interaction of lysophosphatidic acid with its intestinal receptor (lysophosphatidic acid receptor 2) results in the formation of a macromolecular complex that modulates CFTR activity. Lysophosphatidic acid receptor agonists prevent enterotoxin-mediated fluid secretion in rodents, suggesting that LPA-based nutritional supplements could be used as adjunct therapy in diarrhoea.130 Lysophosphatidic acid also stimulates NHE3 in the small intestine through lysophosphatidic acid receptor 5 on Na+-absorptive cells.131
Other potential therapies
Several other potential antidiarrhoeal therapeutics with specific indications are currently in phase II or phase III clinical trials (Figure 2). Eluxadoline is a mixed μ-opioid and δ-opioid inhibitor with antimotility and analgesic properties developed primarily for diarrhoea-predominant IBS. Results from phase II trials showed some clinical improvement in stool consistency and abdominal pain, and little rebound constipation.132 Another compound aimed at diarrhoea-predominant IBS showing efficacy in phase II trials is LX1033, an inhibitor of tryptophan hydroxylase that affects intestinal motility by inhibition of 5-hydroxytryptamine synthesis.133 A semi-synthetic bile acid analogue, obeti-cholic acid, which activates the nuclear bile acid receptor (farnesoid X receptor), relieves symptoms in patients with bile acid diarrhoea, which has been suggested to contribute to diarrhoea in some patients with IBS.134 The action of obeticholic acid is thought to involve a feedback mechanism with an increase in the bile acid receptor, which results in increased intestinal production of fibroblast growth factor 19 and uptake into the portal system, thus reducing hepatic bile acid synthesis and intraluminal bile salt concentration.
Conclusions and future challenges
Although diarrhoeal diseases remain a major global health challenge, there has been a remarkable lack of effective new therapeutics. Part of the reason for the lack of progress is that global and regional health policy has rightly focused on approaches targeted at prevention, such as vaccines, improved sanitation, and better health delivery including the use of ORS. As described herein, there is now an emerging pipeline of potential antidiarrhoeal agents. In developing countries where diarrhoeas are primarily caused by infection, these new agents might be useful as adjuncts or additives to ORS, reducing diarrhoeal output and hence protecting against severe dehydration particularly in the early stages of disease, and secondarily promoting ORS usage. In complex medical emergencies, where availability of rehydration fluids might be limited, such therapeutics could provide life-saving alleviation of fluid loss. To be beneficial in developing countries the ideal therapeutic should have a wide therapeutic index, be stable in harsh environments and be extremely low-cost. Major challenges in moving forward include funding, logistics and design of informative clinical trials. The development of drugs that can be used safely in children and the elderly, where the disease burden is greatest, poses additional challenges in terms of drug adverse effects and patient recruitment for clinical trials. For indications such as diarrhoeas related to chronic inflammatory diseases, IBD or drug-induced diarrhoeas, the development pathway for antisecretory or proabsorptive compounds is clearer, as in the case of crofelemer. Progress towards new antisecretory agents has been accelerated with the elucidation of new regulatory pathways in intestinal fluid transport, raised awareness of natural-product remedies, and emergence of new tools such as ion-sensitive fluorescent proteins and human enteroid models for drug screening. The next and more challenging process will be translating these discoveries into safe, low-cost therapies to reduce the global health and economic burden of diarrhoeal diseases.
Key points.
Diarrhoeal disease remains a major global health burden
Secretory diarrhoea results from abnormal fluid and electrolyte absorption and/or secretion
Ion channels and transporters such as the cystic fibrosis transmembrane conductance regulator and sodium/hydrogen exchanger 3 are targets for antisecretory antidiarrhoeal drugs
New compounds targeting intestinal transporters are in development for antidiarrhoeal therapy, including small molecules, natural compounds and existing drugs
Antidiarrhoeal therapies might be useful as stand-alone therapy or together with oral rehydration solutions
Acknowledgments
J.R.T. has received a grant from the National Institute of Child Health and Human Development (HD00085), M.D. has received grants from the National Institute of Diabetes and Digestive and Kidney Diseases (DK089502, DK061765, DK026532, DK072084, DK099803) and a Gates Foundation Grand Challenges award, and A.S.V. has received grants from the National Institute of Diabetes and Digestive and Kidney Diseases (DK072517, DK035124, DK101373), the National Eye Institute (EY13574) and the National Institute of Biomedical Imaging and Bioengineering (EB000415).
Footnotes
Competing interests
A.S.V. is a named inventor on several CFTR and CaCC inhibitor patents owned by the University of California, San Francisco. J.R.T. and M.D. declare no competing interests.
Author contributions
The authors contributed equally to all aspects in the production of this article.
References
- 1.Kramer B, Kanof A. Diarrhea in children: a historical review. J Pediatr. 1960;57:769–783. doi: 10.1016/s0022-3476(60)80172-2. [DOI] [PubMed] [Google Scholar]
- 2.WHO. Global Health Observatory Data Repository [online] 2014 doi: 10.1080/02763869.2019.1693231. http://apps.who.int/gho/data/?theme=main. [DOI] [PubMed]
- 3.Walker CL, et al. Global burden of childhood pneumonia and diarrhoea. Lancet. 2013;381:1405–1416. doi: 10.1016/S0140-6736(13)60222-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moore SR, et al. Prolonged episodes of acute diarrhea reduce growth and increase risk of persistent diarrhea in children. Gastroenterology. 2010;139:1156–1164. doi: 10.1053/j.gastro.2010.05.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kotloff KL, et al. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): a prospective, case-control study. Lancet. 2013;382:209–222. doi: 10.1016/S0140-6736(13)60844-2. [DOI] [PubMed] [Google Scholar]
- 6.Rudan I, et al. Epidemiology and etiology of childhood pneumonia in 2010 estimates of incidence, severe morbidity, mortality, underlying risk factors and causative pathogens for 192 countries. J Glob Health. 2013;3:010401. doi: 10.7189/jogh.03.010401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clay PG, Crutchley RD. Noninfectious diarrhea in HIV seropositive individuals: a review of prevalence rates, etiology, and management in the era of combination antiretroviral therapy. Infect Dis Ther. 2014;3:103–122. doi: 10.1007/s40121-014-0047-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pessi MA, et al. Targeted therapy-induced diarrhea: A review of the literature. Crit Rev Oncol Hematol. 2014;90:165–179. doi: 10.1016/j.critrevonc.2013.11.008. [DOI] [PubMed] [Google Scholar]
- 9.Binder HJ. Mechanisms of diarrhea in inflammatory bowel diseases. Ann NY Acad Sci. 2009;1165:285–293. doi: 10.1111/j.1749-6632.2009.04039.x. [DOI] [PubMed] [Google Scholar]
- 10.Green PH, Jabri B. Celiac disease. Annu Rev Med. 2006;57:207–221. doi: 10.1146/annurev.med.57.051804.122404. [DOI] [PubMed] [Google Scholar]
- 11.Lovell RM, Ford AC. Global prevalence of and risk factors for irritable bowel syndrome: a meta-analysis. Clin Gastroenterol Hepatol. 2012;10:712–721.e4. doi: 10.1016/j.cgh.2012.02.029. [DOI] [PubMed] [Google Scholar]
- 12.Cutz E, et al. Microvillus inclusion disease: an inherited defect of brush-border assembly and differentiation. N Engl J Med. 1989;320:646–651. doi: 10.1056/NEJM198903093201006. [DOI] [PubMed] [Google Scholar]
- 13.Reifen RM, Cutz E, Griffiths AM, Ngan BY, Sherman PM. Tufting enteropathy: a newly recognized clinicopathological entity associated with refractory diarrhea in infants. J Pediatr Gastroenterol Nutr. 1994;18:379–385. [PubMed] [Google Scholar]
- 14.Fiskerstrand T, et al. Familial diarrhea syndrome caused by an activating GUCY2C mutation. N Engl J Med. 2012;366:1586–1595. doi: 10.1056/NEJMoa1110132. [DOI] [PubMed] [Google Scholar]
- 15.Krejs GJ. VIPoma syndrome. Am J Med. 1987;82:37–48. doi: 10.1016/0002-9343(87)90425-6. [DOI] [PubMed] [Google Scholar]
- 16.Centers for Disease Control and Prevention. CDC estimates of foodborne illness in the United States. http://www.cdc.gov/foodborneburden/ (2014)
- 17.Thiagarajah JR, Verkman AS. Water transport in the gastrointestinal tract. In: Johnson LR, Barrett KE, Ghishan FK, Merchant JL, Said HM, editors. Physiology of the Gastrointestinal Tract. 5. Elsevier Academic Press; 2012. [Google Scholar]
- 18.Gawenis LR, et al. cAMP inhibition of murine intestinal Na/H exchange requires CFTR-mediated cell shrinkage of villus epithelium. Gastroenterology. 2003;125:1148–1163. doi: 10.1016/s0016-5085(03)01212-5. [DOI] [PubMed] [Google Scholar]
- 19.Lin R, et al. D-glucose acts via sodium/glucose cotransporter 1 to increase NHE3 in mouse jejunal brush border by a Na+/H+ exchange regulatory factor 2-dependent process. Gastroenterology. 2011;140:560–571. doi: 10.1053/j.gastro.2010.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Walker NM, et al. Role of down-regulated in adenoma anion exchanger in HCO3− secretion across murine duodenum. Gastroenterology. 2009;136:893–901. doi: 10.1053/j.gastro.2008.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Seidler UE. Gastrointestinal HCO3− transport and epithelial protection in the gut: new techniques, transport pathways and regulatory pathways. Curr Opin Pharmacol. 2013;13:900–908. doi: 10.1016/j.coph.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 22.Xia W, et al. The distinct roles of anion transporters Slc26a3 (DRA) and Slc26a6 (PAT-1) in fluid and electrolyte absorption in the murine small intestine. Pflugers Arch. 2014;466:1541–1556. doi: 10.1007/s00424-013-1381-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wright EM, et al. The Na+/glucose cotransporter (SGLT1) Acta Physiol Scand Suppl. 1992;607:201–207. [PubMed] [Google Scholar]
- 24.Mueckler M. Facilitative glucose transporters. Eur J Biochem. 1994;219:713–725. doi: 10.1111/j.1432-1033.1994.tb18550.x. [DOI] [PubMed] [Google Scholar]
- 25.Canessa CM, Horisberger JD, Rossier BC. Epithelial sodium channel related to proteins involved in neurodegeneration. Nature. 1993;361:467–470. doi: 10.1038/361467a0. [DOI] [PubMed] [Google Scholar]
- 26.Krishnan S, Ramakrishna BS, Binder HJ. Stimulation of sodium chloride absorption from secreting rat colon by short-chain fatty acids. Dig Dis Sci. 1999;44:1924–1930. doi: 10.1023/a:1018871412748. [DOI] [PubMed] [Google Scholar]
- 27.Kurashima K, et al. Identification of sites required for down-regulation of Na+/H+ exchanger NHE3 activity by cAMP-dependent protein kinase. Phosphorylation-dependent and -independent mechanisms. J Biol Chem. 1997;272:28672–28679. doi: 10.1074/jbc.272.45.28672. [DOI] [PubMed] [Google Scholar]
- 28.Yun CH, et al. cAMP-mediated inhibition of the epithelial brush border Na+/H+ exchanger, NHE3, requires an associated regulatory protein. Proc Natl Acad Sci USA. 1997;94:3010–3015. doi: 10.1073/pnas.94.7.3010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen M, et al. Loss of PDZ-adaptor protein NHERF2 affects membrane localization and cGMP- and [Ca2+]- but not cAMP-dependent regulation of Na+/H+ exchanger 3 in murine intestine. J Physiol. 2010;588:5049–5063. doi: 10.1113/jphysiol.2010.198721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zachos NC, et al. Phospholipase C-γ binds directly to the Na+/H+ exchanger 3 and is required for calcium regulation of exchange activity. J Biol Chem. 2009;284:19437–19444. doi: 10.1074/jbc.M109.006098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hoque KM, et al. Epac1 mediates protein kinase A-independent mechanism of forskolin-activated intestinal chloride secretion. J Gen Physiol. 2010;135:43–58. doi: 10.1085/jgp.200910339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Namkung W, Finkbeiner WE, Verkman AS. CFTR-adenylyl cyclase I association responsible for UTP activation of CFTR in well-differentiated primary human bronchial cell cultures. Mol Biol Cell. 2010;21:2639–2648. doi: 10.1091/mbc.E09-12-1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barrett KE, Keely SJ. Chloride secretion by the intestinal epithelium: molecular basis and regulatory aspects. Annu Rev Physiol. 2000;62:535–572. doi: 10.1146/annurev.physiol.62.1.535. [DOI] [PubMed] [Google Scholar]
- 34.Matos JE, et al. Role of cholinergic-activated KCa1.1 (BK), KCa3.1 (SK4) and KV7.1 (KCNQ1) channels in mouse colonic Cl− secretion. Acta Physiol (Oxf) 2007;189:251–258. doi: 10.1111/j.1748-1716.2006.01646.x. [DOI] [PubMed] [Google Scholar]
- 35.Geibel J, et al. Calcium-sensing receptor abrogates secretagogue- induced increases in intestinal net fluid secretion by enhancing cyclic nucleotide destruction. Proc Natl Acad Sci USA. 2006;103:9390–9397. doi: 10.1073/pnas.0602996103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cooke HJ, Shonnard K, Wood JD. Effects of neuronal stimulation on mucosal transport in guinea pig ileum. Am J Physiol. 1983;245:G290–G296. doi: 10.1152/ajpgi.1983.245.2.G290. [DOI] [PubMed] [Google Scholar]
- 37.Field M, Fromm D, al-Awqati Q, Greenough WB. 3rd Effect of cholera enterotoxin on ion transport across isolated ileal mucosa. J Clin Invest. 1972;51:796–804. doi: 10.1172/JCI106874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rao MC, Guandalini S, Smith PL, Field M. Mode of action of heat-stable Escherichia coli enterotoxin. Tissue and subcellular specificities and role of cyclic GMP. Biochim Biophys Acta. 1980;632:35–46. doi: 10.1016/0304-4165(80)90247-0. [DOI] [PubMed] [Google Scholar]
- 39.Subramanya SB, et al. Differential regulation of cholera toxin-inhibited Na-H exchange isoforms by butyrate in rat ileum. Am J Physiol Gastrointest Liver Physiol. 2007;293:G857–G863. doi: 10.1152/ajpgi.00462.2006. [DOI] [PubMed] [Google Scholar]
- 40.Hecht G, et al. Differential regulation of Na+/H+ exchange isoform activities by enteropathogenic E. coli in human intestinal epithelial cells. Am J Physiol Gastrointest Liver Physiol. 2004;287:G370–G378. doi: 10.1152/ajpgi.00432.2003. [DOI] [PubMed] [Google Scholar]
- 41.Matkowskyj K, et al. Age-dependent differences in galanin-dependent colonic fluid secretion after infection with Salmonella typhimurium. Gut. 2009;58:1201–1206. doi: 10.1136/gut.2008.163832. [DOI] [PubMed] [Google Scholar]
- 42.Wapnir RA, Teichberg S. Regulation mechanisms of intestinal secretion: implications in nutrient absorption. J Nutr Biochem. 2002;13:190–199. doi: 10.1016/s0955-2863(02)00181-x. [DOI] [PubMed] [Google Scholar]
- 43.Berkes J, Viswanathan VK, Savkovic SD, Hecht G. Intestinal epithelial responses to enteric pathogens: effects on the tight junction barrier, ion transport, and inflammation. Gut. 2003;52:439–451. doi: 10.1136/gut.52.3.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gill RK, et al. Mechanism underlying inhibition of intestinal apical Cl/OH exchange following infection with enteropathogenic E. coli. J Clin Invest. 2007;117:428–437. doi: 10.1172/JCI29625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Marchelletta RR, et al. Altered expression and localization of ion transporters contribute to diarrhea in mice with Salmonella-induced enteritis. Gastroenterology. 2013;145:1358, 1368.e1–e4. doi: 10.1053/j.gastro.2013.08.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Greenberg HB, Estes MK. Rotaviruses: from pathogenesis to vaccination. Gastroenterology. 2009;136:1939–1951. doi: 10.1053/j.gastro.2009.02.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ball JM, Tian P, Zeng CQ, Morris AP, Estes MK. Age-dependent diarrhea induced by a rotaviral nonstructural glycoprotein. Science. 1996;272:101–104. doi: 10.1126/science.272.5258.101. [DOI] [PubMed] [Google Scholar]
- 48.Morris AP, et al. NSP4 elicits age-dependent diarrhea and Ca2+ mediated I− influx into intestinal crypts of CF mice. Am J Physiol. 1999;277:G431–G444. doi: 10.1152/ajpgi.1999.277.2.G431. [DOI] [PubMed] [Google Scholar]
- 49.Seo NS, et al. Integrins α1β1 and α2β1 are receptors for the rotavirus enterotoxin. Proc Natl Acad Sci USA. 2008;105:8811–8818. doi: 10.1073/pnas.0803934105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hempson SJ, et al. Rotavirus infection of murine small intestine causes colonic secretion via age restricted galanin-1 receptor expression. Gastroenterology. 2010;138:2410–2417. doi: 10.1053/j.gastro.2010.02.050. [DOI] [PubMed] [Google Scholar]
- 51.Lundgren O, et al. Role of the enteric nervous system in the fluid and electrolyte secretion of rotavirus diarrhea. Science. 2000;287:491–495. doi: 10.1126/science.287.5452.491. [DOI] [PubMed] [Google Scholar]
- 52.Halaihel N, et al. Direct inhibitory effect of rotavirus NSP4 (114–135) peptide on the Na+-D-glucose symporter of rabbit intestinal brush border membrane. J Virol. 2000;74:9464–9470. doi: 10.1128/jvi.74.20.9464-9470.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Payne DC, et al. Norovirus and medically attended gastroenteritis in, U. S children. N Engl J Med. 2013;368:1121–1130. doi: 10.1056/NEJMsa1206589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rufo PA, et al. Diarrhea-associated HIV-1 APIs potentiate muscarinic activation of Cl− secretion by T84 cells via prolongation of cytosolic Ca2+ signaling. Am J Physiol Cell Physiol. 2004;286:C998–C1008. doi: 10.1152/ajpcell.00357.2003. [DOI] [PubMed] [Google Scholar]
- 55.Andreyev J, et al. Guidance on the management of diarrhoea during cancer chemotherapy. Lancet Oncol. 2014;15:e447–e460. doi: 10.1016/S1470-2045(14)70006-3. [DOI] [PubMed] [Google Scholar]
- 56.Turk E, Zabel B, Mundlos S, Dyer J, Wright EM. Glucose/galactose malabsorption caused by a defect in the Na+/glucose cotransporter. Nature. 1991;350:354–356. doi: 10.1038/350354a0. [DOI] [PubMed] [Google Scholar]
- 57.Höglund P, et al. Mutations of the Down-regulated in adenoma (DRA) gene cause congenital chloride diarrhoea. Nat Genet. 1996;14:316–319. doi: 10.1038/ng1196-316. [DOI] [PubMed] [Google Scholar]
- 58.Kravtsov D, et al. Myosin 5b loss of function leads to defects in polarized signaling: implication for microvillus inclusion disease pathogenesis and treatment. Am J Physiol Gastrointest Liver Physiol. 2014;307:G992–G1001. doi: 10.1152/ajpgi.00180.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kozan PA, et al. Mutation of EpCAM leads to intestinal barrier and ion transport dysfunction. J Mol Med (Berl) 2015;93:535–545. doi: 10.1007/s00109-014-1239-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Canani RB, Terrin G. Recent progress in congenital diarrheal disorders. Curr Gastroenterol Rep. 2011;13:257–264. doi: 10.1007/s11894-011-0188-6. [DOI] [PubMed] [Google Scholar]
- 61.Pezzella V, et al. Investigation of chronic diarrhoea in infancy. Early Hum Dev. 2013;89:893–897. doi: 10.1016/j.earlhumdev.2013.08.007. [DOI] [PubMed] [Google Scholar]
- 62.Gareau MG, Barrett KE. Fluid and electrolyte secretion in the inflamed gut: novel targets for treatment of inflammation-induced diarrhea. Curr Opin Pharmacol. 2013;13:895–899. doi: 10.1016/j.coph.2013.08.014. [DOI] [PubMed] [Google Scholar]
- 63.Gelbmann CM, Schteingart CD, Thompson SM, Hofmann AF, Barrett KE. Mast cells and histamine contribute to bile acid-stimulated secretion in the mouse colon. J Clin Invest. 1995;95:2831–2839. doi: 10.1172/JCI117988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Medani M, et al. Prostaglandin D2 regulates human colonic ion transport via the DP1 receptor. Life Sci. 2015;122:87–91. doi: 10.1016/j.lfs.2014.12.017. [DOI] [PubMed] [Google Scholar]
- 65.Larmonier CB, et al. NHE3 modulates the severity of colitis in IL-10-deficient mice. Am J Physiol Gastrointest Liver Physiol. 2011;300:G998–G1009. doi: 10.1152/ajpgi.00073.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sullivan S, et al. Downregulation of sodium transporters and NHERF proteins in IBD patients and mouse colitis models: potential contributors to IBD-associated diarrhea. Inflamm Bowel Dis. 2009;15:261–274. doi: 10.1002/ibd.20743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yeruva S, et al. Preserved Na+/H+ exchanger isoform 3 expression and localization, but decreased NHE3 function indicate regulatory sodium transport defect in ulcerative colitis. Inflamm Bowel Dis. 2010;16:1149–1161. doi: 10.1002/ibd.21183. [DOI] [PubMed] [Google Scholar]
- 68.Sun X, Hirota SA. The roles of host and pathogen factors and the innate immune response in the pathogenesis of Clostridium difficile infection. Mol Immunol. 2015;63:193–202. doi: 10.1016/j.molimm.2014.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bruzzese E, Lo Vecchio A, Guarino A. Hospital management of children with acute gastroenteritis. Curr Opin Gastroenterol. 2013;29:23–30. doi: 10.1097/MOG.0b013e32835a352f. [DOI] [PubMed] [Google Scholar]
- 70.Sentongo TA. The use of oral rehydration solutions in children and adults. Curr Gastroenterol Rep. 2004;6:307–313. doi: 10.1007/s11894-004-0083-5. [DOI] [PubMed] [Google Scholar]
- 71.McMahan ZH, DuPont HL. Review article: the history of acute infectious diarrhoea management—from poorly focused empiricism to fluid therapy and modern pharmacotherapy. Aliment Pharmacol Ther. 2007;25:759–769. doi: 10.1111/j.1365-2036.2007.03261.x. [DOI] [PubMed] [Google Scholar]
- 72.Johnson JR, et al. Similarity between human and chicken Escherichia coli isolates in relation to ciprofloxacin resistance status. J Infect Dis. 2006;194:71–78. doi: 10.1086/504921. [DOI] [PubMed] [Google Scholar]
- 73.Gallay A, et al. Campylobacter antimicrobial drug resistance among humans, broiler chickens, and pigs, France. Emerg Infect Dis. 2007;13:259–266. doi: 10.3201/eid1302.060587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Binder HJ, Brown I, Ramakrishna BS, Young GP. Oral rehydration therapy in the second decade of the twenty-first century. Curr Gastroenterol Rep. 2014;16:376. doi: 10.1007/s11894-014-0376-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Das JK, Salam RA, Bhutta ZA. Global burden of childhood diarrhea and interventions. Curr Opin Infect Dis. 2014;27:451–458. doi: 10.1097/QCO.0000000000000096. [DOI] [PubMed] [Google Scholar]
- 76.Unger CC, et al. Treating diarrhoeal disease in children under five: the global picture. Arch Dis Child. 2014;99:273–278. doi: 10.1136/archdischild-2013-304765. [DOI] [PubMed] [Google Scholar]
- 77.Santosham M, et al. Progress and barriers for the control of diarrhoeal disease. Lancet. 2010;376:63–67. doi: 10.1016/S0140-6736(10)60356-X. [DOI] [PubMed] [Google Scholar]
- 78.Raghupathy P, et al. Amylase-resistant starch as adjunct to oral rehydration therapy in children with diarrhea. J Pediatr Gastroenterol Nutr. 2006;42:362–368. doi: 10.1097/01.mpg.0000214163.83316.41. [DOI] [PubMed] [Google Scholar]
- 79.Subramanya S, Ramakrishna BS, Binder HJ, Farthing MJ, Young GP. Evaluation of oral rehydration solution by whole-gut perfusion in rats: effect of osmolarity, sodium concentration and resistant starch. J Pediatr Gastroenterol Nutr. 2006;43:568–575. doi: 10.1097/01.mpg.0000239998.43141.b2. [DOI] [PubMed] [Google Scholar]
- 80.Lamberti LM, Walker CL, Chan KY, Jian WY, Black RE. Oral zinc supplementation for the treatment of acute diarrhea in children: a systematic review and meta-analysis. Nutrients. 2013;5:4715–4740. doi: 10.3390/nu5114715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hoque KM, Sarker R, Guggino SE, Tse CM. A new insight into pathophysiological mechanisms of zinc in diarrhea. Ann NY Acad Sci. 2009;1165:279–284. doi: 10.1111/j.1749-6632.2009.04442.x. [DOI] [PubMed] [Google Scholar]
- 82.Hoque KM, Rajendran VM, Binder HJ. Zinc inhibits cAMP-stimulated Cl secretion via basolateral K-channel blockade in rat ileum. Am J Physiol Gastrointest Liver Physiol. 2005;288:G956–G963. doi: 10.1152/ajpgi.00441.2004. [DOI] [PubMed] [Google Scholar]
- 83.Karim A, Ranney RE, Evensen KL, Clark ML. Pharmacokinetics and metabolism of diphenoxylate in man. Clin Pharmacol Ther. 1972;13:407–419. doi: 10.1002/cpt1972133407. [DOI] [PubMed] [Google Scholar]
- 84.Camilleri M, et al. Efficacy and safety of alosetron in women with irritable bowel syndrome: a randomised, placebo-controlled trial. Lancet. 2000;355:1035–1040. doi: 10.1016/S0140-6736(00)02033-X. [DOI] [PubMed] [Google Scholar]
- 85.DuPont HL, et al. Comparative efficacy of loperamide hydrochloride and bismuth subsalicylate in the management of acute diarrhea. Am J Med. 1990;88:15S–19S. doi: 10.1016/0002-9343(90)90270-n. [DOI] [PubMed] [Google Scholar]
- 86.Powell DW, Tapper EJ, Morris SM. Aspirin-stimulated intestinal electrolyte transport in rabbit ileum in vitro. Gastroenterology. 1979;76:1429–1437. [PubMed] [Google Scholar]
- 87.Hamza H, Ben Khalifa H, Baumer P, Berard H, Lecomte JM. Racecadotril versus placebo in the treatment of acute diarrhoea in adults. Aliment Pharmacol Ther. 1999;13(Suppl 6):15–19. doi: 10.1046/j.1365-2036.1999.00002.x-i1. [DOI] [PubMed] [Google Scholar]
- 88.Alam NH, Ashraf H, Khan WA, Karim MM, Fuchs GJ. Efficacy and tolerability of racecadotril in the treatment of cholera in adults: a double blind, randomised, controlled clinical trial. Gut. 2003;52:1419–1423. doi: 10.1136/gut.52.10.1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Macarthur RD, et al. Efficacy and safety of crofelemer for noninfectious diarrhea in HIV-seropositive individuals (ADVENT trial): a randomized, double-blind, placebo-controlled, two-stage study. HIV Clin Trials. 2013;14:261–273. doi: 10.1310/hct1406-261. [DOI] [PubMed] [Google Scholar]
- 90.Tradtrantip L, Namkung W, Verkman AS. Crofelemer, an antisecretory antidiarrheal proanthocyanidin oligomer extracted from Croton lechleri, targets two distinct intestinal chloride channels. Mol Pharmacol. 2010;77:69–78. doi: 10.1124/mol.109.061051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Szajewska H, Mrukowicz JZ. Probiotics in the treatment and prevention of acute infectious diarrhea in infants and children: a systematic review of published randomized, double-blind, placebo-controlled trials. J Pediatr Gastroenterol Nutr. 2001;33(Suppl 2):S17–S25. doi: 10.1097/00005176-200110002-00004. [DOI] [PubMed] [Google Scholar]
- 92.Sazawal S, et al. Efficacy of probiotics in prevention of acute diarrhoea: a meta-analysis of masked, randomised, placebo-controlled trials. Lancet Infect Dis. 2006;6:374–382. doi: 10.1016/S1473-3099(06)70495-9. [DOI] [PubMed] [Google Scholar]
- 93.Allen SJ, Martinez EG, Gregorio GV, Dans LF. Probiotics for treating acute infectious diarrhoea. Cochrane Database of Systematic Reviews. (11):Art. No.: CD003048. doi: 10.1002/14651858.CD003048.pub3. http://dx.doi.org/10.1002/14651858.CD003048.pub3. [DOI] [PMC free article] [PubMed]
- 94.Borthakur A, et al. The probiotic Lactobacillus acidophilus stimulates chloride/hydroxyl exchange activity in human intestinal epithelial cells. J Nutr. 2008;138:1355–1359. doi: 10.1093/jn/138.7.1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Raheja G, et al. Lactobacillus acidophilus stimulates the expression of SLC26A3 via a transcriptional mechanism. Am J Physiol Gastrointest Liver Physiol. 2010;298:G395–G401. doi: 10.1152/ajpgi.00465.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Resta-Lenert S, Barrett KE. Probiotics and commensals reverse TNF-α- and IFN-γ-induced dysfunction in human intestinal epithelial cells. Gastroenterology. 2006;130:731–746. doi: 10.1053/j.gastro.2005.12.015. [DOI] [PubMed] [Google Scholar]
- 97.Agustina R, et al. Randomized trial of probiotics and calcium on diarrhea and respiratory tract infections in Indonesian children. Pediatrics. 2012;129:e1155–e1164. doi: 10.1542/peds.2011-1379. [DOI] [PubMed] [Google Scholar]
- 98.Donowitz M, et al. Translational approaches for pharmacotherapy development for acute diarrhea. Gastroenterology. 2012;142:e1–e9. doi: 10.1053/j.gastro.2012.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Thiagarajah JR, Broadbent T, Hsieh E, Verkman AS. Prevention of toxin-induced intestinal ion and fluid secretion by a small-molecule CFTR inhibitor. Gastroenterology. 2004;126:511–519. doi: 10.1053/j.gastro.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 100.Grubb BR. Ion transport across the jejunum in normal and cystic fibrosis mice. Am J Physiol. 1995;268:G505– G513. doi: 10.1152/ajpgi.1995.268.3.G505. [DOI] [PubMed] [Google Scholar]
- 101.O’Loughlin EV, et al. Abnormal epithelial transport in cystic fibrosis jejunum. Am J Physiol. 1991;260:G758–G763. doi: 10.1152/ajpgi.1991.260.5.G758. [DOI] [PubMed] [Google Scholar]
- 102.Caci E, et al. Evidence for direct CFTR inhibition by CFTRinh-172 based on Arg347 mutagenesis. Biochem J. 2008;413:135–142. doi: 10.1042/BJ20080029. [DOI] [PubMed] [Google Scholar]
- 103.Ma T, et al. Thiazolidinone CFTR inhibitor identified by high-throughput screening blocks cholera toxin-induced intestinal fluid secretion. J Clin Invest. 2002;110:1651–1658. doi: 10.1172/JCI16112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Snyder DS, Tradtrantip L, Yao C, Kurth MJ, Verkman AS. Potent, metabolically stable benzopyrimido-pyrrolo-oxazinedione (BPO) CFTR inhibitors for polycystic kidney disease. J Med Chem. 2011;54:5468–5477. doi: 10.1021/jm200505e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Tradtrantip L, Sonawane ND, Namkung W, Verkman AS. Nanomolar potency pyrimido-pyrrolo-quinoxalinedione CFTR inhibitor reduces cyst size in a polycystic kidney disease model. J Med Chem. 2009;52:6447–6455. doi: 10.1021/jm9009873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Muanprasat C, et al. Discovery of glycine hydrazide pore-occluding CFTR inhibitors: mechanism, structure-activity analysis, and in vivo efficacy. J Gen Physiol. 2004;124:125–137. doi: 10.1085/jgp.200409059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.de Hostos EL, Choy RK, Nguyen T. Developing novel antisecretory drugs to treat infectious diarrhea. Future Med Chem. 2011;3:1317–1325. doi: 10.4155/fmc.11.87. [DOI] [PubMed] [Google Scholar]
- 108.Jin BJ, Thiagarajah JR, Verkman AS. Convective washout reduces the antidiarrheal efficacy of enterocyte surface-targeted antisecretory drugs. J Gen Physiol. 2013;141:261–272. doi: 10.1085/jgp.201210885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sonawane ND, Zhao D, Zegarra-Moran O, Galietta LJ, Verkman AS. Lectin conjugates as potent, nonabsorbable CFTR inhibitors for reducing intestinal fluid secretion in cholera. Gastroenterology. 2007;132:1234–1244. doi: 10.1053/j.gastro.2007.02.018. [DOI] [PubMed] [Google Scholar]
- 110.Sonawane ND, Zhao D, Zegarra-Moran O, Galietta LJ, Verkman AS. Nanomolar CFTR inhibition by pore-occluding divalent polyethylene glycol-malonic acid hydrazides. Chem Biol. 2008;15:718–728. doi: 10.1016/j.chembiol.2008.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Singh RD, et al. Ano1, a Ca2+-activated Cl− channel, coordinates contractility in mouse intestine by Ca2+ transient coordination between interstitial cells of Cajal. J Physiol. 2014;592:4051–4068. doi: 10.1113/jphysiol.2014.277152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Namkung W, Phuan PW, Verkman AS. TMEM16A inhibitors reveal TMEM16A as a minor component of calcium-activated chloride channel conductance in airway and intestinal epithelial cells. J Biol Chem. 2011;286:2365–2374. doi: 10.1074/jbc.M110.175109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.De La Fuente R, Namkung W, Mills A, Verkman AS. Small-molecule screen identifies inhibitors of a human intestinal calcium-activated chloride channel. Mol Pharmacol. 2008;73:758–768. doi: 10.1124/mol.107.043208. [DOI] [PubMed] [Google Scholar]
- 114.Ko EA, et al. Chloride channel inhibition by a red wine extract and a synthetic small molecule prevents rotaviral secretory diarrhoea in neonatal mice. Gut. 2014;63:1120–1129. doi: 10.1136/gutjnl-2013-305663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Tradtrantip L, Ko EA, Verkman AS. Antidiarrheal efficacy and cellular mechanisms of a Thai herbal remedy. PLoS Negl Trop Dis. 2014;8:e2674. doi: 10.1371/journal.pntd.0002674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Delpire E, Lu J, England R, Dull C, Thorne T. Deafness and imbalance associated with inactivation of the secretory Na-K-2Cl co-transporter. Nat Genet. 1999;22:192–195. doi: 10.1038/9713. [DOI] [PubMed] [Google Scholar]
- 117.Chattopadhyay N, et al. Identification and localization of extracellular Ca2+-sensing receptor in rat intestine. Am J Physiol. 1998;274:G122–G130. doi: 10.1152/ajpgi.1998.274.1.G122. [DOI] [PubMed] [Google Scholar]
- 118.Jouret F, et al. Activation of the Ca2+-sensing receptor induces deposition of tight junction components to the epithelial cell plasma membrane. J Cell Sci. 2013;126:5132–5142. doi: 10.1242/jcs.127555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.MacVinish LJ, Hickman ME, Mufti DA, Durrington HJ, Cuthbert AW. Importance of basolateral K+ conductance in maintaining Cl− secretion in murine nasal and colonic epithelia. J Physiol. 1998;510:237–247. doi: 10.1111/j.1469-7793.1998.237bz.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Rufo PA, et al. The antifungal antibiotic, clotrimazole, inhibits chloride secretion by human intestinal T84 cells via blockade of distinct basolateral K+ conductances. Demonstration of efficacy in intact rabbit colon and in an in vivo mouse model of cholera. J Clin Invest. 1997;100:3111–3120. doi: 10.1172/JCI119866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wulff H, Castle NA. Therapeutic potential of KCa3.1 blockers: recent advances and promising trends. Expert Rev Clin Pharmacol. 2010;3:385–396. doi: 10.1586/ecp.10.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Yeo CJ, Barry K, Gontarek JD, Donowitz M. Na+/H+ exchange mediates meal-stimulated ileal absorption. Surgery. 1994;116:388–394. [PubMed] [Google Scholar]
- 123.Gill RK, et al. Serotonin inhibits Na+/H+ exchange activity via 5-HT4 receptors and activation of PKCα in human intestinal epithelial cells. Gastroenterology. 2005;128:962–974. doi: 10.1053/j.gastro.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 124.Li X, et al. Carbachol regulation of rabbit ileal brush border Na+-H+ exchanger 3 (NHE3) occurs through changes in NHE3 trafficking and complex formation and is Src dependent. J Physiol. 2004;556:791–804. doi: 10.1113/jphysiol.2004.060921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Booth IW, Stange G, Murer H, Fenton TR, Milla PJ. Defective jejunal brush-border Na+/H+ exchange: a cause of congenital secretory diarrhoea. Lancet. 1985;1:1066–1069. doi: 10.1016/s0140-6736(85)92369-4. [DOI] [PubMed] [Google Scholar]
- 126.Foulke-Abel J, et al. Human enteroids as an ex-vivo model of host-pathogen interactions in the gastrointestinal tract. Exp Biol Med (Maywood) 2014;239:1124–1134. doi: 10.1177/1535370214529398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Johansson E, et al. Molecular cloning and expression of a pituitary gland protein modulating intestinal fluid secretion. J Biol Chem. 1995;270:20615–20620. doi: 10.1074/jbc.270.35.20615. [DOI] [PubMed] [Google Scholar]
- 128.Lange S, Lönnroth I. The antisecretory factor: synthesis, anatomical and cellular distribution, and biological action in experimental and clinical studies. Int Rev Cytol. 2001;210:39–75. doi: 10.1016/s0074-7696(01)10003-3. [DOI] [PubMed] [Google Scholar]
- 129.Zaman S, et al. Antisecretory factor effectively and safely stops childhood diarrhoea: a placebo-controlled, randomised study. Acta Paediatr Oslo Nor. 2014;103:659–664. doi: 10.1111/apa.12581. 1992. [DOI] [PubMed] [Google Scholar]
- 130.Li C, et al. Lysophosphatidic acid inhibits cholera toxin-induced secretory diarrhea through CFTR-dependent protein interactions. J Exp Med. 2005;202:975–986. doi: 10.1084/jem.20050421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Murtazina R, et al. NHERF2 is necessary for basal activity, second messenger inhibition, and LPA stimulation of NHE3 in mouse distal ileum. Am J Physiol Cell Physiol. 2011;301:C126–C136. doi: 10.1152/ajpcell.00311.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Dove LS, et al. Eluxadoline benefits patients with irritable bowel syndrome with diarrhea in a phase 2 study. Gastroenterology. 2013;145:329–338.e1. doi: 10.1053/j.gastro.2013.04.006. [DOI] [PubMed] [Google Scholar]
- 133.Brown PM, et al. The tryptophan hydroxylase inhibitor LX1031 shows clinical benefit in patients with nonconstipating irritable bowel syndrome. Gastroenterology. 2011;141:507–516. doi: 10.1053/j.gastro.2011.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Walters JR, et al. The response of patients with bile acid diarrhoea to the farnesoid X receptor agonist obeticholic acid. Aliment Pharmacol Ther. 2015;41:54–64. doi: 10.1111/apt.12999. [DOI] [PubMed] [Google Scholar]
- 135.Koch KM, et al. Alosetron repeat dose pharmacokinetics, effects on enzyme activities, and influence of demographic factors. Aliment Pharmacol Ther. 2004;20:223–230. doi: 10.1111/j.1365-2036.2004.02031.x. [DOI] [PubMed] [Google Scholar]
- 136.Hofmann AF, Poley JR. Cholestyramine treatment of diarrhea associated with ileal resection. N Engl J Med. 1969;281:397–402. doi: 10.1056/NEJM196908212810801. [DOI] [PubMed] [Google Scholar]
- 137.Ericsson CD, Johnson PC. Safety and efficacy of loperamide. Am J Med. 1990;88:10S–14S. doi: 10.1016/0002-9343(90)90269-j. [DOI] [PubMed] [Google Scholar]