Abstract
Background
Risk factors for initial Pseudomonas aeruginosa (Pa) acquisition, particularly environmental exposures, are poorly understood. We aimed to identify such risk factors in order to inform prevention strategies and identify high-risk populations.
Methods
The study cohort included all participants in the U.S. EPIC Observational Study who had no prior Pa-positive respiratory cultures (N=889). Cox proportional hazard models were used to test the effects of factors on age at first Pa-positive respiratory culture.
Results
Cystic fibrosis (CF) genotype functional class had an important effect on age at initial Pa acquisition (hazard ratio (HR) comparing minimal to residual CFTR function 2.87 (95% CI 1.88, 4.39)). None of the modifiable risk factors evaluated, including cigarette smoke, hot tub use, breastfeeding, or daycare, was associated with age at Pa acquisition. Similarly, newborn screening was not associated with age at Pa acquisition (HR 0.85, 95% CI 0.66, 1.09). Key associations were validated in a CF Foundation National Patient Registry replication cohort.
Conclusions
Given the ubiquitous presence of Pa in the environment, it may be that many imposed lifestyle changes will have less impact on age at initial Pa acquisition than genetic determinants.
Keywords: Cystic fibrosis, Pseudomonas, Risk factors, Epidemiology, Exposure, Environment
1. Introduction
The primary cause of morbidity and mortality in patients with cystic fibrosis (CF) is progressive obstructive pulmonary disease and bronchiectasis due to chronic endobronchial infection. Pseudomonas aeruginosa (Pa), a ubiquitous environmental bacterium, is a key pathogen in CF lung disease. Pa infection can begin very early in life, and the prevalence of Pa in respiratory cultures increases with age [2]. Chronic Pa infection is clearly associated with worse outcomes, including survival, lung function, chest radiograph scores, pulmonary exacerbations and nutritional status [3–5]. In general, CF patients progress from initial, often transient, Pa infection amenable to eradication to chronic infection with mucoid Pa that is virtually impossible to eradicate [6,7]. Current guidelines of care emphasize identification and eradication of initial Pa infection in an effort to delay or prevent chronic infection [8–12].
Our current understanding of risk factors for initial Pa acquisition is limited. While CF patients are frequently counseled to avoid hot tubs or swimming pools, there has been no evaluation of home environmental exposures as risk factors for initial Pa acquisition. Three small studies evaluated risk factors for age at initial Pa acquisition, yielding some concordant and some conflicting results [13–15]. The only characteristic consistently associated with age at Pa acquisition has been cystic fibrosis transmembrane conductance regulator (CFTR) genotype [14,16–19]. Despite early concerns that newborn screening might be associated with earlier acquisition of Pa [15], more recent studies have not implicated it as a risk factor [20–22].
The EPIC Observational Study (EPIC OBS) [23,24] is a U.S. national prospective study specifically designed to better define risk factors for age at first isolation of Pa from respiratory cultures, particularly modifiable exposures such as daycare attendance, breastfeeding or environmental tobacco smoke. Such risk factors have not to our knowledge been previously evaluated. Identification of these risk factors could inform prevention strategies and identify high-risk populations requiring closer monitoring. We also evaluated the generalizability of key findings in a separate sample from the U.S. Cystic Fibrosis Foundation National Patient Registry (CFNPR).
2. Methods
2.1. EPIC OBS study participants
The design of the EPIC OBS Study has been reported [23,24]. Children with an established diagnosis of CF [25] ≤12 years of age were enrolled at 59 accredited U.S. CF care centers between 2004 and 2006. The current analysis cohort is composed of all EPIC OBS participants who had no isolation of Pa from respiratory cultures both prior to and within 120 days after enrollment (as such individuals likely actually acquired Pa prior to enrollment). Written informed consent was obtained from the family of each participant and the study was approved by the Institutional Review Board at each participating site.
2.2. EPIC OBS data
Study data were collected at each clinical encounter via the CFNPR and study-specific forms, as previously reported [23]. Respiratory cultures were obtained and processed at local site labs as per clinical protocol, and results reported in the CFNPR. A questionnaire was administered to families at enrollment and annually, assessing exposure to a wide range of potential risk factors for Pa acquisition (see Appendix 2, online supplement (OLS)). The data cut-off date for the current analysis was December 31, 2009.
2.3. CFNPR replication cohort
In order to assess the generalizability of our results, a replication cohort was assembled from the U.S. CFNPR. Eligibility criteria mimicked those of the EPIC OBS cohort. See OLS for details. Data for the replication cohort was limited to that available in the CFNPR and thus does not include data from the annual family survey such as exposures or socioeconomic status or encounter-based data regarding therapies.
2.4. Analysis
The primary endpoint for this analysis was age at initial Pa acquisition, defined as the age at first isolation of Pa from a clinically-collected respiratory culture (oropharyngeal, sputum or bronchoalveolar lavage). Categorization of CFTR function was based on CFTR mutation functional class [19,24,26,27]: “minimal,” both alleles with mutations in class 1, 2 or 3; “residual,” at least one allele with a mutation class 4 or 5. See OLS for further details of CFTR functional classification.
Cox proportional hazards regression models were used to compute hazard ratios (HR) and 95% confidence intervals (CIs), testing the effect of fixed and time-varying covariates on age at Pa acquisition. Participants entered the analysis at their age at study entry. Participants who did not acquire Pa were censored at the age of their latest recorded respiratory culture. For time-varying covariates, Cox model estimates were derived using information about the participants at risk at each failure time.
3. Results
3.1. Participant characteristics at enrollment
A total of 1032 EPIC OBS Study participants had no isolation of Pa from a respiratory culture prior to enrollment, among which 143 were excluded due to Pa acquisition or loss to follow up during the first 120 days after enrollment. Thus, 889 participants comprise the EPIC OBS cohort for the current analysis. In the CFNPR, there were 10,577 CF patients born between 1992 and 2006, not enrolled in the EPIC OBS Study and alive in 2004. A total of 3,649 of these subjects had at least one encounter between November 2004 and October 2006, of which one encounter was chosen at random as the entry date, and had no Pa-positive cultures prior to that date. Nine hundred thirty seven were excluded due to Pa acquisition or loss to follow up during the first 120 days after study entry. Thus, 2712 subjects comprise the CFNPR replication cohort.
Demographic and clinical characteristics of the EPIC OBS cohort are shown in Table 1. As participants were enrolled prior to widespread CF newborn screening in the U.S., only 20% were identified by screening. Approximately 80% of participants received pancreatic enzyme replacement therapy and almost half used dornase alfa at enrollment. The mean (SD) length of follow up was 3.9 (0.7) years.
Table 1.
Characteristics at enrollment.
| Demographics | EPIC OBS cohort (N=889)
|
CFNPR replication cohort (N=2712)
|
|||
|---|---|---|---|---|---|
| N | % | N | % | ||
| Gender | Female | 446 | 50.2 | 1281 | 47.2 |
| Race | Caucasian | 856 | 96.3 | 2510 | 92.6 |
| Ethnicity | Not Hispanic | 816 | 91.8 | 2443 | 90.1 |
| Hispanic | 36 | 4.0 | 269 | 9.9 | |
| CFTR mutations | ΔF508 homozygous | 398 | 44.8 | 1163 | 42.9 |
| ΔF508 heterozygous | 348 | 39.1 | 977 | 36.0 | |
| Other | 105 | 11.8 | 426 | 15.7 | |
| CFTR functional classa | Minimal | 633 | 71.2 | 1697 | 62.6 |
| Residual | 113 | 12.7 | 262 | 9.7 | |
| Unclassified | 105 | 11.8 | 607 | 22.4 | |
| Meconium ileus | 176 | 19.8 | 514 | 19.0 | |
| Diagnosis by screeningb | 192 | 21.6 | 473 | 17.4 | |
| Mean | SD | Mean | SD | ||
|
| |||||
| Age at diagnosis (years) | 1.2 | 2.2 | 1.4 | 2.4 | |
| Age at enrollment/entry (years) | 4.6 | 3.5 | 5.0 | 3.9 | |
| Cultures per year while on study | 4.2 | 1.3 | 3.5 | 1.5 | |
| Weight percentile | 40.5 | 28.9 | 37.3 | 29.1 | |
| Height percentile | 36.6 | 27.4 | 34.6 | 27.5 | |
| BMI percentilec | 54.5 | 27.2 | 51.1 | 28.4 | |
| FEV1 percent predictedd | 98.8 | 17.5 | 94.8 | 17.8 | |
| Treatments | |||||
| Pancreatic enzymes | 704 | 79.2 | 2059 | 75.9 | |
| DNase | 407 | 45.8 | 505 | 18.6 | |
| High dose ibuprofen | 2 | 0.2 | 9 | 0.3 | |
| Inhaled hypertonic saline | 6 | 0.7 | 76 | 2.8 | |
| Inhaled antibiotics | 37 | 4.2 | 128 | 4.7 | |
| Organisms isolated from respiratory culturese | |||||
| S. aureus | 443 | 52.2 | 1140 | 42.0 | |
| Methicillin resistant S. aureus | 48 | 5.7 | 199 | 7.3 | |
| H. influenzae | 220 | 25.9 | 503 | 18.5 | |
| S. maltophilia | 40 | 4.7 | 115 | 4.2 | |
| A. xylosoxidans | 5 | 0.6 | 25 | 0.9 | |
| B. cepacia | 1 | 0.1 | 14 | 0.5 | |
Within each characteristic, column totals may not add up to 100% due to missing data.
Minimal, both alleles with mutations resulting in minimal CFTR function (class 1, 2, or 3), includes ΔF508; Residual, at least one allele with a mutation resulting in partial CFTR function (class 4 or 5); Unclassified, both alleles with unknown functional class, or 1 allele with minimal CFTR function and the second with unknown functional class.
Diagnosis by prenatal or newborn screening.
BMI, body mass index. Children age 2 years or older.
Children age 6 years or older. Percent predicted based on reference equations of Wang et al. [40] as per CF Foundation recommendations since all participants <12 years at enrollment.
Among children with culture results within 120 days following enrollment or entry date; not mutually exclusive.
The characteristics of the CFNPR replication cohort were generally similar (Table 1), except that a greater proportion could not be assigned a CFTR functional class and a lower percentage reported dornase alfa use, likely reflecting differential data collection methods in the two studies. In the EPIC OBS study, data on dornase alfa use was recorded in study-specific encounter forms as well as through the CFNPR encounter forms. The reported rate of dornase alfa use among EPIC OBS participants based only on CFNPR data was 13.7%, comparable to that reported in the CFNPR replication cohort.
3.2. Initial acquisition of Pa
Of the 889 EPIC OBS participants, 412 (46%) acquired Pa during 2611 person-years. Thus the rate of Pa acquisition was 16 cases (95% CI 14, 17) per 100 person-years. Among those acquiring Pa, the median age at acquisition was 3.25 years (95% CI 2.77, 4.03). In comparison, among all U.S. CF patients in the CFNPR born during the same years as the EPIC OBS participants (N=10,921), the median age at initial acquisition for the registry cohort was 4.00 years (95% CI 3.88, 4.16). Fig. 1a displays the Kaplan–Meier survival curve for time to initial Pa acquisition. The source of the initial Pa isolate was an oropharyngeal culture in 335 (81%), sputum in 66 (16%), bronchoalveolar lavage in 9 (2%), and unknown in 2 (0.5%). The initial isolate was non-mucoid in 297 (72%), mucoid in 42 (10%), and of unknown status in 73 (18%).
Fig. 1.
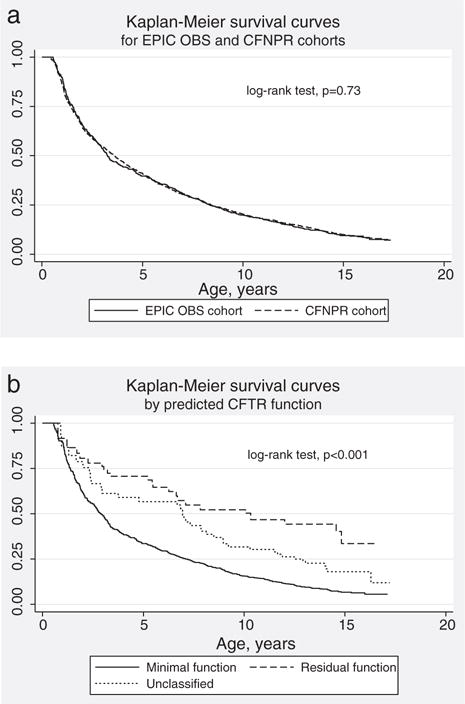
Kaplan–Meier survival curves for age at initial acquisition of Pseudomonas aeruginosa. a: EPIC observational cohort vs CFNPR replication cohort. b: Among EPIC Observational study participants, by CFTR functional class.
The Pa acquisition rate was similar in the CFNPR replication cohort: 16 cases (95% CI 15, 17) per 100 person-years. Median age at acquisition among those acquiring Pa was also similar: 3.63 (95% CI 3.16, 4.05) years, as was time to acquisition (Fig. 1a, p=0.73). The source of the initial Pa isolate was an oropharyngeal culture in 69%, sputum in 29% and bronchoscopy in 2%. Seventy percent were non-mucoid, 13% mucoid and 17% were of unknown status, similar to the EPIC OBS cohort.
3.3. Risk factors for age at initial Pa acquisition
The effects of demographic characteristics on age at Pa acquisition are shown in Table 2. In the EPIC OBS cohort, minimal CFTR function was associated with earlier Pa acquisition compared to residual CFTR function: HR 2.87 (95% CI 1.90, 4.35, p<0.0001). Kaplan–Meier survival curves for CFTR functional groups are displayed in Fig. 1b; median age at Pa acquisition was 2.9 years among participants with minimal CFTR function vs. 10.3 years for those with residual CFTR function. No other significant effects of demographic characteristics on age at Pa acquisition were detected. Diagnosis by screening was not associated with earlier Pa acquisition (HR 0.85, 95% CI 0.66, 1.08, p=0.18).
Table 2.
Univariate Cox models for demographic characteristicsa.
| Risk factor | EPIC OBS cohort
|
CFNPR replication cohort
|
||||||
|---|---|---|---|---|---|---|---|---|
| N in model | Hazard ratio | 95% CI | P value | N in model | Hazard ratio | 95% CI | P value | |
| Female | 889 | 1.08 | 0.89, 1.30 | 0.44 | 2712 | 1.12 | 1.00, 1.26 | 0.05 |
| Non-white race | 887 | 0.99 | 0.64, 1.54 | 0.96 | 2696 | 0.83 | 0.65, 1.06 | 0.14 |
| Hispanic ethnicity | 852 | 0.95 | 0.57, 1.58 | 0.83 | 2712 | 1.19 | 0.99, 1.43 | 0.06 |
| CFTR function: minimal | 851 | 2.87 | 1.90, 4.35 | <0.0001 | 2566 | 2.65 | 2.02, 3.47 | <0.0001 |
| CFTR function: unclassified | 1.80 | 1.08, 2.99 | 0.02 | 2566 | 1.61 | 1.19, 2.16 | 0.002 | |
| Genotype: both alleles ΔF508 | 851 | 1.39 | 0.98, 1.99 | 0.07 | 2566 | 1.86 | 1.53, 2.26 | <0.0001 |
| Genotype: one allele ΔF508 | 1.06 | 0.74, 1.53 | 0.75 | 2566 | 1.35 | 1.10, 1.65 | 0.004 | |
| Diagnosis age (years) | 889 | 1.00 | 0.95, 1.05 | 0.96 | 2697 | 0.97 | 0.94, 0.99 | 0.02 |
| Meconium ileus | 853 | 1.09 | 0.87, 1.37 | 0.46 | 2685 | 1.20 | 1.04, 1.37 | 0.01 |
| Diagnosis by screeningb | 738 | 0.85 | 0.66, 1.08 | 0.18 | 2271 | 0.74 | 0.62, 0.87 | 0.0003 |
Separate Cox models were developed for each characteristic. The reference groups are: male sex, white race, non-Hispanic ethnicity, residual CFTR function, neither allele deltaF508, no history of meconium ileus, and not diagnosed by prenatal or newborn screening. The number of children contributing observations to each model varied due to missing data.
Diagnosis by prenatal or newborn screening: the reference group excludes children with a family history of CF.
In the CFNPR replication cohort, the effects of demographic characteristics on age at initial Pa acquisition were similar in magnitude and direction of to those observed in the EPIC OBS cohort (Table 2). Due to the larger size of the replication cohort, more associations were statistically significant. Female gender was associated with a significantly increased risk of Pa acquisition (HR 1.12, 95% CI 1.00, 1.26, p=0.05). Minimal CFTR function and homozygous delta F508 genotype were associated with significantly increased risk of Pa acquisition, as was a history of meconium ileus. Later age at diagnosis (HR 0.97, 95% CI 0.94, 0.99 for each integer year of age, p=0.02) and identification by newborn screening (HR 0.74, 95% CI 0.62, 0.87, p=0.0003) were protective. After adjustment for the potential confounding effects of gender, race, ethnicity and CFTR functional class, diagnosis age and meconium ileus were no longer significant predictors of age at Pa acquisition (data not shown). Diagnosis after newborn screening remained protective (adjusted HR 0.81, 95% CI 0.68, 0.96, p=0.02).
Table 3 displays the results of Cox models for the effect of clinical characteristics at enrollment on age at Pa acquisition, adjusted for CFTR functional class, race, ethnicity and identification by newborn screening. In the EPIC OBS cohort, use of pancreatic enzymes was associated with earlier age at Pa acquisition even after adjustment for CFTR functional class (HR 1.78, 95% CI 1.03, 3.09). There were no significant associations of Dornase alfa, use of any inhaled therapy, weight percentile, BMI percentile, percent predicted FEV1 or respiratory culture results with age at Pa acquisition. Similarly, persistent infection (a time-varying covariate defined as the proportion of cultures since enrollment positive for the organism of interest) was not associated with age at Pa acquisition (data not shown).
Table 3.
Multivariate Cox models for baseline clinical characteristicsa.
| Risk factor | EPIC OBS cohort
|
CFNPR replication cohort
|
||||||
|---|---|---|---|---|---|---|---|---|
| N in model | Hazard ratio | 95% CI | P value | N in model | Hazard ratio | 95% CI | P value | |
| Pancreatic enzymes | 627 | 1.78 | 1.03, 3.09 | 0.04 | 1910 | 2.52 | 1.78, 3.57 | <0.0001 |
| DNase | 677 | 1.03 | 0.83, 1.28 | 0.81 | 2152 | 1.07 | 0.91, 1.26 | 0.41 |
| Any inhaled medicationb | 677 | 0.96 | 0.77, 1.19 | 0.71 | – | – | – | – |
| Weight percentile | 630 | 0.998 | 0.994, 1.00 | 0.38 | 2057 | 0.995 | 0.993, 0.998 | 0.0001 |
| Height percentile | 624 | 0.998 | 0.994, 1.00 | 0.39 | 1992 | 0.996 | 0.994, 0.999 | 0.007 |
| BMI percentilec | 435 | 0.997 | 0.992, 1.00 | 0.35 | 1395 | 0.997 | 0.994, 1.00 | 0.08 |
| FEV1 percent predictedd | 194 | 0.998 | 0.988, 1.01 | 0.63 | 688 | 0.992 | 0.985, 0.998 | 0.01 |
| S. aureus | 654 | 1.05 | 0.84, 1.30 | 0.68 | 1798 | 1.23 | 1.07, 1.42 | 0.004 |
| MRSA | 654 | 0.88 | 0.53, 1.44 | 0.61 | 1798 | 1.08 | 0.85, 1.38 | 0.52 |
| H. influenzae | 654 | 0.96 | 0.76, 1.21 | 0.72 | 1798 | 1.14 | 0.96, 1.34 | 0.13 |
| S. maltophilia | 654 | 1.08 | 0.71, 1.66 | 0.71 | 1798 | 1.37 | 1.04, 1.81 | 0.03 |
| A. xylosoxidans | 654 | 1.11 | 0.48, 2.56 | 0.80 | 1798 | 1.91 | 1.08, 3.38 | 0.03 |
Each row in the table shows results for a separate Cox model. The number of children contributing observations to each model varied due to missing data. Each model was adjusted for CFTR risk group, race, ethnicity, gender, and diagnosis by screening.
Not evaluated in the replication cohort as data on inhaled bronchodilators or inhaled corticosteroids not available.
Children age 2 years or older at enrollment.
Children age 6 years or older at enrollment.
In the CFNPR cohort, the directions of the effects of baseline clinical characteristics on age at Pa acquisition were similar, but additional significant associations were observed (Table 3). Use of pancreatic enzymes was associated with earlier age at Pa acquisition (HR 2.53, 95% CI 1.78, 3.58, p>0.0001). Greater weight percentile (HR 0.995, 95% CI 0.993, 0.998, p=0.0001), height percentile (HR 0.996, 95% CI 0.994, 0.999, p=0.007) and percent predicted FEV1 (HR 0.992, 95% CI 0.985, 0.998) were protective. Isolation of Staphylococcus aureus, Stenotrophomonas maltophilia and Achromobacter xylosoxidans from the baseline respiratory culture were associated with significantly earlier age at Pa acquisition.
The prevalence of reported exposures from the questionnaire completed by families at enrollment into the EPIC OBS Study is described in Table 4. About 75% received the influenza vaccine in the previous season, and about 30% reported exposure to cigarette smoke in the household. About one third of children <6 were in daycare >9 hours per week and about a third of children <2.5 years received palivizumab passive immunization in the prior respiratory syncytial virus (RSV) season.
Table 4.
Exposures reported on family surveys at enrollment in EPIC observational cohort.
| Exposure in past year | N | %a |
|---|---|---|
| Total number of participants with enrollment survey completed | 866 | – |
| Influenza vaccine | 669 | 77.2 |
| Household member smoked cigarettes | 253 | 29.2 |
| Mother smoked during pregnancy | 120 | 13.9 |
| Wood-burning stove used in home | 82 | 9.5 |
| Hot-tub use | 194 | 22.4 |
| Swimming pool use | 613 | 70.8 |
| Social event with others with CF | 129 | 14.9 |
| Daycare or preschoolb | 214 | 37.7 |
| Breastfedc | 138 | 52.3 |
| Received palivuzimabd | 104 | 34.0 |
| Mother’s education | ||
| High school graduate or less | 237 | 27.4 |
| Some college or more | 602 | 69.5 |
| Annual household income | ||
| <$20,000 | 79 | 9.1 |
| $20,000–39,000 | 145 | 16.7 |
| $40,000–59,000 | 132 | 15.2 |
| $60,000–79,000 | 124 | 14.3 |
| $80,000–99,000 | 93 | 10.7 |
| ≥$100,000 | 152 | 17.5 |
| Don’t know or missing | 141 | 16.3 |
Denominator is 866, except as noted. For exposures other than annual household income, the number of surveys with missing data ranged from 6 to 50.
Children younger than 6 years (N=567).
Children younger than 2 years (N=264).
Children younger than 2.5 years (N=306).
The associations of exposures reported by parents annually on the family questionnaire (as time-varying risk factors) and age at Pa acquisition are shown in Table 5. Influenza vaccine in the past year appeared borderline protective (HR 0.77, 95% CI 0.59, 1.00, p=0.05). Adjustment for CFTR functional class did not affect these results (data not shown). Swimming pool use in the past year also appeared protective (HR 0.69, 95% CI 0.52, 0.90, p=0.007), possibly due to confounding by an unmeasured characteristic such as overall lung health and level of physical activity. No association was detected between exposure in the past year to cigarette smoke, wood burning stoves, hot tubs, attendance at social events with others with CF, daycare/preschool, breastfeeding, palivizumab or socioeconomic status (household income and maternal education), and age at Pa acquisition.
Table 5.
Univariate Cox models for time-varying risk factors reported on annual family surveys in EPIC observational cohort.
| Risk factora | N in model | Hazard ratio | 95% CI | P value |
|---|---|---|---|---|
| Influenza vaccine during past year | 864 | 0.77 | 0.59, 1.00 | 0.05 |
| Household member smokes cigarettes | 866 | 0.96 | 0.78, 1.18 | 0.70 |
| Wood-burning stove used in home | 864 | 0.78 | 0.55, 1.12 | 0.18 |
| Hot-tub use during past year | 866 | 0.82 | 0.65, 1.05 | 0.12 |
| Swimming pool use during past year | 866 | 0.69 | 0.52, 0.90 | 0.007 |
| Social event with others with CF during past year | 864 | 1.09 | 0.83, 1.42 | 0.54 |
| Daycare or preschool during past yearb | 551 | 0.90 | 0.70, 1.17 | 0.45 |
| Breastfed during past yearc | 261 | 0.85 | 0.60, 1.21 | 0.38 |
| Received palivuzimab during past yeard | 284 | 1.02 | 0.75, 1.40 | 0.89 |
| Maternal education beyond high schoole | 839 | 0.90 | 0.74, 1.11 | 0.33 |
| Annual household income: ≥$80,000e | 725 | 1.05 | 0.81, 1.35 | 0.73 |
| Annual household income: $40,000–$79,000e | 1.03 | 0.80, 1.33 | 0.81 |
From separate Cox model for each risk factor, modeled as a time-varying covariate. The number of children contributing observations to each model varied due to missing data.
Children younger than 6 years.
Children younger than 2 years.
Children younger than 2.5 years.
As reported at enrollment; the reference group for annual income is <$40,000.
To address concerns that older study participants might represent an unusual population by virtue of having survived without acquiring Pa up to the age at enrollment, we repeated our evaluation of risk factors among the participants who were enrolled below the median age of Pa acquisition in the replication cohort (3.6 years) and found very similar results (OLS Figure E1, Tables E1, E2 and E3).
4. Discussion
The EPIC Observational Study sheds light on risk factors for initial Pa acquisition. Ours is the first study to evaluate home environmental exposures as potential risk factors. None of the modifiable risk factors that we evaluated, including breastfeeding, daycare attendance, hot tubs or wood burning stoves, was associated with age at initial Pa acquisition. Given that families are frequently counseled to make lifestyle changes to minimize early Pa exposure, these results have important implications for counseling CF families. Instead, genetic factors had an important effect on age at Pa acquisition. CFTR mutations with minimal function (both mutations in functional class 1, 2 or 3) were associated with earlier Pa acquisition compared to mutations with residual function (at least one mutation in functional class 4 or 5); the median age at Pa acquisition was 2.9 years among participants with minimal CFTR function vs. 10.3 years for those with residual CFTR function. A recent study by Collaco et al. found that ambient temperature is associated with age at Pa acquisition; in two retrospective cohorts, age at Pa acquisition was 9 to 15 months earlier in the warmest temperature quartile compared to the coldest [28]. This effect appears relatively small compared to that of CFTR functional class. Given the ubiquitous presence of Pa in the environment, it may be that many imposed lifestyle changes will have less impact on age at initial Pa acquisition than genetic determinants.
Age at initial Pa acquisition is likely to be affected by a complex interplay between host and environmental factors. The consistent finding by us and others [14,16,17,19] that CFTR functional status is associated with age at Pa acquisition is an example of the role of host factors in Pa acquisition. Loss of CFTR function may predispose to endobronchial infection either by altering properties of airway surface liquid, thus impairing innate defenses and bacterial clearance [29], or because CFTR may bind directly to Pa [30]. Indeed, in the neonatal CF pig, the lack of CFTR function appears to globally impair bacterial eradication [31].
Concerns regarding earlier acquisition of Pa among infants identified by newborn screening have existed since the Wisconsin Neonatal Screening Trial, in which the benefits of newborn screening were offset by earlier acquisition of Pa at one site in the early years of the study [15]. Since that time, several studies have found no evidence of earlier Pa acquisition among newborn-screened infants [20–22]. Similarly, we found no evidence of earlier acquisition of Pa among EPIC OBS participants diagnosed by newborn screening. In fact, in the CFNPR replication cohort newborn screening was associated with a significant delay in age at Pa acquisition. However, it should be noted that newborn screening in the U.S. was limited to several states at the time of CF diagnosis in our two cohorts. It is not possible to separate the independent effects of newborn screening from those of the environment or monitoring and treatment practices in those states performing newborn screening.
The baseline characteristics and rates of Pa acquisition were very similar between the EPIC OBS and the CFNPR replication cohorts. In addition, the median age at acquisition of Pa among the EPIC OBS participants was very similar to that seen in all U.S. CF patients born during the same years in the CFNPR: 3.25 vs. 4.00 years. The effects of demographic and clinical risk factors on age at initial Pa acquisition were also in general similar between the EPIC OBS and CFNPR replication cohorts, though there were some discrepancies in the magnitude of the effects and in statistical significance, mostly due to the larger size of the replication cohort. Female gender and a history of meconium ileus were associated with earlier age at Pa acquisition in the CFNPR replication cohort, similar to findings in prior studies [13,14,32], though meconium ileus was no longer a significant risk factor after adjustment for CFTR functional class. In the EPIC OBS cohorts, while the hazard ratios were similar to those in the replication cohort, the associations were not statistically significant. Thus, the effect of gender on age at Pa acquisition remains unclear, and the effect of meconium ileus appears to be mediated through CFTR functional class. Interestingly, greater weight and height percentiles at enrollment were significantly protective in the replication cohort, as was reported recently by Ranganathan et al. [33]. The possible association of better nutritional status with delayed acquisition of Pa deserves further exploration, and may reflect underlying disease severity or a true protective effect of better nutrition.
Finally, isolation of S. aureus, S. maltophilia and A. xylosoxidans were each associated with significantly earlier age at Pa acquisition in the replication cohort. In prior studies, S. aureus was found to be a risk factor for early age at initial Pa acquisition in a newborn-screened cohort [15], while it was found to be protective against mucoid Pa infection in an older CF cohort [32]. Thus, the role of S. aureus in Pa infection remains unclear. The observed association of S. maltophilia and A. xylosoxidans with earlier Pa acquisition may reflect acquisition of these organisms with more intensive antibiotic treatment and thus more severe lung disease, or perhaps more effective ascertainment of these organisms at the same sites that most effectively culture Pa from respiratory cultures.
We found a borderline protective effect of the influenza vaccine on age at Pa acquisition. Infections with viruses such as influenza may trigger pulmonary exacerbations and contribute to structural airway damage [34], thereby increasing susceptibility to Pa. While aerosol medications were a risk factor for Pa acquisition in a prior small study [15], we found no association between use of any inhaled medications and age at Pa acquisition.
Assuming 412 Pa acquisition events, 80% power and alpha=0.05, we had an adequate sample size to detect a hazard ratio of 1.47 for a risk factor with 15% prevalence (such as participating in a social event with others with CF) and a hazard ratio of 1.35 for a risk factor with 30% prevalence (such as cigarette smoke exposure or breastfeeding). We would have missed associations with lower hazard ratios, though such associations might not be overly significant from a clinical or public health standpoint. In addition, we may have missed a true association due to our measurement methods (parent report rather than direct measurement) and limited frequency of assessment (annually). We were unable to assess the effect of these exposures in the replication cohort as the CFNPR does not contain data on these exposures. In addition, there were certainly potential risk factors that we did note evaluate, such as viral respiratory infections or cohorting practices. These potential risk factors deserve further investigation.
In our study, we used oropharyngeal (OP) cultures as the primary endpoint. In the U.S. OP swabs are the standard method for collecting respiratory cultures in non-expectorating CF patients. While they have reasonable specificity, OP cultures have limited sensitivity compared to simultaneous lower airway cultures [35]. Thus, our conclusions regarding risk factors may be more relevant for upper than lower airway Pa acquisition. In addition, our eligibility criteria, including enrolling children up to 12 years of age, were designed to be concordant with the EPIC Clinical Trial [36], a randomized, controlled trial of early Pa eradication regimens nested in the EPIC Observational Study. To address concerns that the older participants in the EPIC OBS cohort might represent an unusual population since by definition they had not acquired Pa by the age at enrollment, we repeated our analyses among the participants who were enrolled before 3.6 years, the median age of Pa acquisition in the replication cohort, and found similar results.
What are the implications of our results for CF clinics? We did not evaluate the effects of cohorting practices on risk of Pa acquisition, as this has previously been evaluated [37,38]. There is no evidence that the risk of patient-to-patient transmission is less among those with residual CFTR function, so we would not recommend that patients with residual CFTR function be managed differently from an infection control standpoint or that clinics should be segregated by CFTR function.
How should our results be interpreted when counseling families? Continuing to recommend the annual influenza vaccine is warranted. Our results do not reduce the controversy over the role of palivizumab in infants with CF. The negative effects of cigarette smoke exposure on lung function have been clearly documented [39], and socializing with others with CF is known to increase the risk of acquiring transmissible epidemic Pa strains [40] so these exposures should be avoided. Given that we were unable to detect evidence of earlier Pa acquisition among those exposed to swimming pools, hot tubs or wood burning stoves, limited contact with these exposures may be reasonable. Relaxing restrictions on these exposures may reduce anxiety and improve quality of life for some CF patients and their families.
Supplementary Material
Acknowledgments
The authors wish to thank all the site investigators and research coordinators, listed below, as well as all the participants in the EPIC Observational Study and their families.
Sources of support
Cystic Fibrosis Foundation grants OBSERV04K0 and EPIC0K0 to M. Rosenfeld, Seattle Children’s Hospital, Seattle, WA, Nemours Children’s Clinic Research Program, Jacksonville, FL. The study sponsors had no role in the study design; the collection, analysis and interpretation of data; the writing of the manuscript; or in the decision to submit the manuscript for publication.
Appendix A. Supplementary data
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.jcf.2012.04.003.
Footnotes
These results were presented in abstract form at the North American Cystic Fibrosis Conference, New Orleans, Louisiana, USA, May, 2009 [1].
Clinical trials registration
References
- 1.Rosenfeld M, Emerson J, Joubran K, Morgan W, Gibson R, for the EPIC Study Group Risk factors for early pseudomonas acquisition in a large cohort of young US CF patients. Pediatr Pulmonol. 2009;S32:373. [Google Scholar]
- 2.Cystic Fibrosis Foundation. National Patient Registry 2010 Annual Data Report. Bethesda, Maryland: 2011. [Google Scholar]
- 3.Kosorok MR, Zeng L, West SE, Rock MJ, Splaingard ML, Laxova A, et al. Acceleration of lung disease in children with cystic fibrosis after Pseudomonas aeruginosa acquisition. Pediatr Pulmonol. 2001;32(4):277–87. doi: 10.1002/ppul.2009.abs. [DOI] [PubMed] [Google Scholar]
- 4.Emerson J, Rosenfeld M, McNamara S, Ramsey B, Gibson RL. Pseudomonas aeruginosa and other predictors of mortality and morbidity in young children with cystic fibrosis. Pediatr Pulmonol. 2002;34(2):91–100. doi: 10.1002/ppul.10127. [DOI] [PubMed] [Google Scholar]
- 5.Konstan MW, Morgan WJ, Butler SM, Pasta DJ, Craib ML, Silva SJ, et al. Risk factors for rate of decline in forced expiratory volume in one second in children and adolescents with cystic fibrosis. J Pediatr. 2007;151(2):134–9 [9 e1]. doi: 10.1016/j.jpeds.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 6.Li Z, Kosorok MR, Farrell PM, Laxova A, West SE, Green CG, et al. Longitudinal development of mucoid Pseudomonas aeruginosa infection and lung disease progression in children with cystic fibrosis. JAMA. 2005;293(5):581–8. doi: 10.1001/jama.293.5.581. [DOI] [PubMed] [Google Scholar]
- 7.Pressler T, Frederiksen B, Skov M, Garred P, Koch C, Hoiby N. Early rise of anti-pseudomonas antibodies and a mucoid phenotype of Pseudomonas aeruginosa are risk factors for development of chronic lung infection—a case control study. J Cyst Fibros. 2006;5(1):9–15. doi: 10.1016/j.jcf.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 8.Doring G, Conway SP, Heijerman HG, Hodson ME, Hoiby N, Smyth A, et al. Antibiotic therapy against Pseudomonas aeruginosa in cystic fibrosis: a European consensus. Eur Respir J. 2000;16(4):749–67. doi: 10.1034/j.1399-3003.2000.16d30.x. [DOI] [PubMed] [Google Scholar]
- 9.Doring G, Hoiby N. Early intervention and prevention of lung disease in cystic fibrosis: a European consensus. J Cyst Fibros. 2004;3(2):67–91. doi: 10.1016/j.jcf.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 10.Hoiby N, Frederiksen B, Pressler T. Eradication of early Pseudomonas aeruginosa infection. J Cyst Fibros. 2005;4(Suppl. 2):49–54. doi: 10.1016/j.jcf.2005.05.018. [DOI] [PubMed] [Google Scholar]
- 11.Hansen CR, Pressler T, Hoiby N. Early aggressive eradication therapy for intermittent Pseudomonas aeruginosa airway colonization in cystic fibrosis patients: 15 years experience. J Cyst Fibros. 2008;7(6):523–30. doi: 10.1016/j.jcf.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 12.Borowitz D, Robinson KA, Rosenfeld M, Davis SD, Sabadosa KA, Spear SL, et al. Cystic Fibrosis Foundation evidence-based guidelines for management of infants with cystic fibrosis. J Pediatr. 2009;155(6 Suppl):S73–93. doi: 10.1016/j.jpeds.2009.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kerem E, Corey M, Stein R, Gold R, Levison H. Risk factors for Pseudomonas aeruginosa colonization in cystic fibrosis patients. Pediatr Infect Dis J. 1990;9(7):494–8. doi: 10.1097/00006454-199007000-00008. [DOI] [PubMed] [Google Scholar]
- 14.Maselli JH, Sontag MK, Norris JM, MacKenzie T, Wagener JS, Accurso FJ. Risk factors for initial acquisition of Pseudomonas aeruginosa in children with cystic fibrosis identified by newborn screening. Pediatr Pulmonol. 2003;35(4):257–62. doi: 10.1002/ppul.10230. [DOI] [PubMed] [Google Scholar]
- 15.Kosorok MR, Jalaluddin M, Farrell PM, Shen G, Colby CE, Laxova A, et al. Comprehensive analysis of risk factors for acquisition of Pseudomonas aeruginosa in young children with cystic fibrosis. Pediatr Pulmonol. 1998;26(2):81–8. doi: 10.1002/(sici)1099-0496(199808)26:2<81::aid-ppul2>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 16.Kubesch P, Dork T, Wulbrand U, Kalin N, Neumann T, Wulf B, et al. Genetic determinants of airways’ colonisation with Pseudomonas aeruginosa in cystic fibrosis. Lancet. 1993;341(8839):189–93. doi: 10.1016/0140-6736(93)90062-l. [DOI] [PubMed] [Google Scholar]
- 17.De Braekeleer M, Allard C, Leblanc JP, Aubin G, Simard F. Genetic determinants of Pseudomonas aeruginosa colonization in cystic fibrosis patients in Canada. Eur J Clin Microbiol Infect Dis. 1998;17(4):269–71. doi: 10.1007/BF01699984. [DOI] [PubMed] [Google Scholar]
- 18.Goubau C, Wilschanski M, Skalicka V, Lebecque P, Southern KW, Sermet I, et al. Phenotypic characterisation of patients with intermediate sweat chloride values: towards validation of the European diagnostic algorithm for cystic fibrosis. Thorax. 2009;64(8):683–91. doi: 10.1136/thx.2008.104752. [DOI] [PubMed] [Google Scholar]
- 19.Green DM, McDougal KE, Blackman SM, Sosnay PR, Henderson LB, Naughton KM, et al. Mutations that permit residual CFTR function delay acquisition of multiple respiratory pathogens in CF patients. Respir Res. 2010;11:140. doi: 10.1186/1465-9921-11-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang SS, FitzSimmons SC, O’Leary LA, Rock MJ, Gwinn ML, Khoury MJ. Early diagnosis of cystic fibrosis in the newborn period and risk of Pseudomonas aeruginosa acquisition in the first 10 years of life: a registry-based longitudinal study. Pediatrics. 2001;107(2):274–9. doi: 10.1542/peds.107.2.274. [DOI] [PubMed] [Google Scholar]
- 21.Sims EJ, McCormick J, Mehta G, Mehta A. Neonatal screening for cystic fibrosis is beneficial even in the context of modern treatment. J Pediatr. 2005;147(3 Suppl):S42–6. doi: 10.1016/j.jpeds.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 22.Baussano I, Tardivo I, Bellezza-Fontana R, Forneris MP, Lezo A, Anfossi L, et al. Neonatal screening for cystic fibrosis does not affect time to first infection with Pseudomonas aeruginosa. Pediatrics. 2006;118(3):888–95. doi: 10.1542/peds.2004-2599. [DOI] [PubMed] [Google Scholar]
- 23.Treggiari MM, Rosenfeld M, Mayer-Hamblett N, Retsch-Bogart G, Gibson RL, Williams J, et al. Early anti-pseudomonal acquisition in young patients with cystic fibrosis: rationale and design of the EPIC clinical trial and observational study’. Contemp Clin Trials. 2009;30(3):256–68. doi: 10.1016/j.cct.2009.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rosenfeld M, Emerson J, McNamara S, Joubran K, Retsch-Bogart G, Graff GR, et al. Baseline characteristics and factors associated with nutritional and pulmonary status at enrollment in the cystic fibrosis EPIC observational cohort. Pediatr Pulmonol. 2010;45(9):934–44. doi: 10.1002/ppul.21279. [DOI] [PubMed] [Google Scholar]
- 25.Farrell PM, Rosenstein BJ, White TB, Accurso FJ, Castellani C, Cutting GR, et al. Guidelines for diagnosis of cystic fibrosis in newborns through older adults: Cystic Fibrosis Foundation consensus report. J Pediatr. 2008;153(2):S4–S14. doi: 10.1016/j.jpeds.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McKone EF, Emerson SS, Edwards KL, Aitken ML. Effect of genotype on phenotype and mortality in cystic fibrosis: a retrospective cohort study. Lancet. 2003;361(9370):1671–6. doi: 10.1016/S0140-6736(03)13368-5. [DOI] [PubMed] [Google Scholar]
- 27.Rowntree RK, Harris A. The phenotypic consequences of CFTR mutations. Ann Hum Genet. 2003;67(Pt. 5):471–85. doi: 10.1046/j.1469-1809.2003.00028.x. [DOI] [PubMed] [Google Scholar]
- 28.Collaco JM, McGready J, Green DM, Naughton KM, Watson CP, Shields T, et al. Effect of temperature on cystic fibrosis lung disease and infections: a replicated cohort study. PLoS One. 2011;6(11):e27784. doi: 10.1371/journal.pone.0027784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Matsui H, Wagner VE, Hill DB, Schwab UE, Rogers TD, Button B, et al. A physical linkage between cystic fibrosis airway surface dehydration and Pseudomonas aeruginosa biofilms. Proc Natl Acad Sci U S A. 2006;103(48):18131–6. doi: 10.1073/pnas.0606428103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bajmoczi M, Gadjeva M, Alper SL, Pier GB, Golan DE. Cystic fibrosis transmembrane conductance regulator and caveolin-1 regulate epithelial cell internalization of Pseudomonas aeruginosa. Am J Physiol Cell Physiol. 2009;297(2):C263–77. doi: 10.1152/ajpcell.00527.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stoltz DA, Meyerholz DK, Pezzulo AA, Ramachandran S, Rogan MP, Davis GJ, et al. Cystic fibrosis pigs develop lung disease and exhibit defective bacterial eradication at birth. Sci Transl Med. 2010;2(29):29ra31. doi: 10.1126/scitranslmed.3000928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Levy H, Kalish LA, Cannon CL, Garcia KC, Gerard C, Goldmann D, et al. Predictors of mucoid Pseudomonas colonization in cystic fibrosis patients. Pediatr Pulmonol. 2008;43(5):463–71. doi: 10.1002/ppul.20794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ranganathan SC, Parsons F, Gangell C, Brennan S, Stick SM, Sly PD. Evolution of pulmonary inflammation and nutritional status in infants and young children with cystic fibrosis. Thorax. 2011;66(5):408–13. doi: 10.1136/thx.2010.139493. [DOI] [PubMed] [Google Scholar]
- 34.Ortiz JR, Neuzil KM, Victor JC, Wald A, Aitken ML, Goss CH. Influenza-associated cystic fibrosis pulmonary exacerbations. Chest. 2010;137(4):852–60. doi: 10.1378/chest.09-1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rosenfeld M, Emerson J, Accurso F, Armstrong D, Castile R, Grimwood K, et al. Diagnostic accuracy of oropharyngeal cultures in infants and young children with cystic fibrosis. Pediatr Pulmonol. 1999;28(5):321–8. doi: 10.1002/(sici)1099-0496(199911)28:5<321::aid-ppul3>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 36.Treggiari MM, Retsch-Bogart G, Mayer-Hamblett N, Khan U, Kulich M, Kronmal R, et al. Comparative efficacy and safety of 4 randomized regimens to treat early Pseudomonas aeruginosa infection in children with cystic fibrosis. Arch Pediatr Adolesc Med. 2011;165(9):847–56. doi: 10.1001/archpediatrics.2011.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Griffiths AL, Jamsen K, Carlin JB, Grimwood K, Carzino R, Robinson PJ, et al. Effects of segregation on an epidemic Pseudomonas aeruginosa strain in a cystic fibrosis clinic. Am J Respir Crit Care Med. 2005;171(9):1020–5. doi: 10.1164/rccm.200409-1194OC. [DOI] [PubMed] [Google Scholar]
- 38.Hayes D, Jr, West SE, Rock MJ, Li Z, Splaingard ML, Farrell PM. Pseudomonas aeruginosa in children with cystic fibrosis diagnosed through newborn screening: assessment of clinic exposures and microbial genotypes. Pediatr Pulmonol. 2010;45(7):708–16. doi: 10.1002/ppul.21263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Collaco JM, Vanscoy L, Bremer L, McDougal K, Blackman SM, Bowers A, et al. Interactions between secondhand smoke and genes that affect cystic fibrosis lung disease. JAMA. 2008;299(4):417–24. doi: 10.1001/jama.299.4.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Saiman L, Siegel J. Infection control recommendations for patients with cystic fibrosis: microbiology, important pathogens, and infection control practices to prevent patient-to-patient transmission. Am J Infect Control. 2003;31(3 Suppl):S1–S62. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


