Abstract
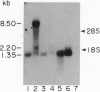
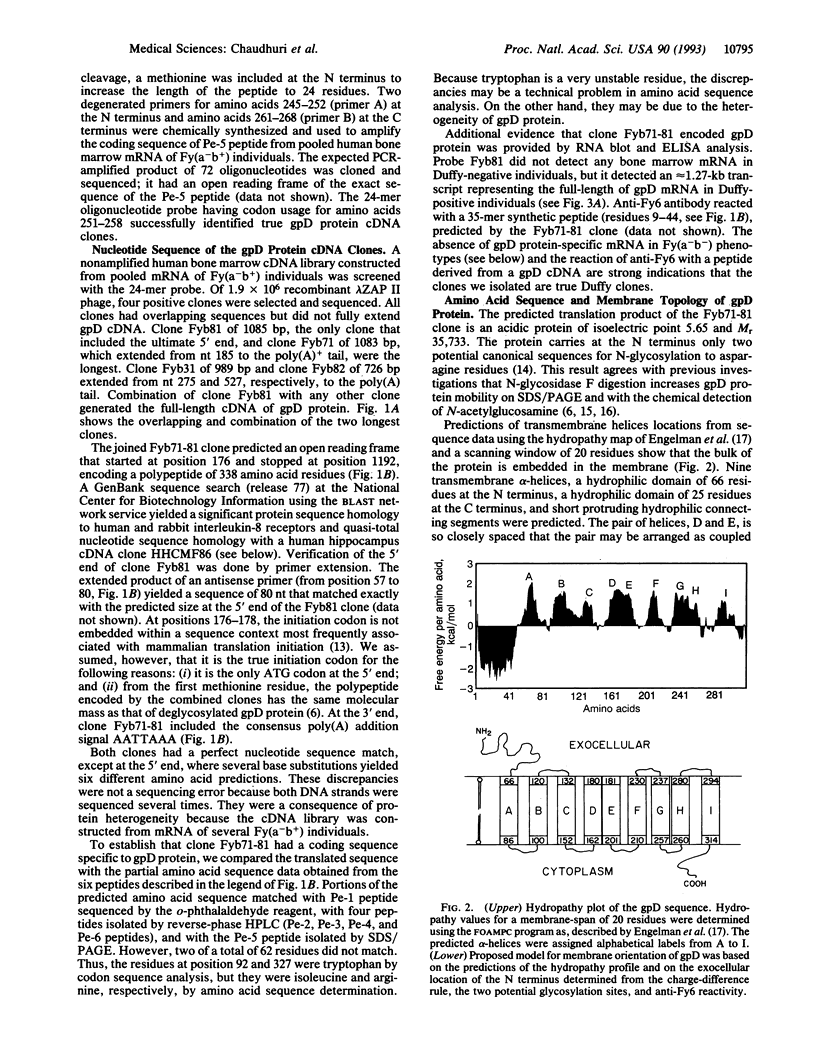
cDNA clones encoding the major subunit of the Duffy blood group were isolated from a human bone marrow cDNA library using a PCR-amplified DNA fragment encoding an internal peptide sequence of glycoprotein D (gpD) protein. The open reading frame of the 1267-bp cDNA clone indicated that gpD protein was composed of 338 amino acids, predicting a M(r) of 35,733, which was the same as a deglycosylated gpD protein. Portions of the predicted amino acid sequence, matched with six CNBr/pepsin peptides obtained from affinity-purified gpD protein. In ELISA analysis, an anti-Duffy murine monoclonal antibody reacted with a synthetic peptide deduced from the cDNA clone. Hydropathy analysis suggested the presence of 9 membrane-spanning alpha-helices. In bone marrow RNA blot analysis, the gpD cDNA detected a 1.27-kb mRNA in Duffy-positive but not in Duffy-negative individuals. It also identified the same size mRNA in adult kidney, adult spleen, and fetal liver; in brain, it detected a prominent 8.5-kb and a minor 2.2-kb mRNA. In Southern blot analysis, gpD cDNA identified a single gene in Duffy-positive and -negative individuals. Duffy-negative individuals, therefore, have the gpD gene, but it is not expressed in bone marrow. The same or a similar gene is active in adult kidney, adult spleen, and fetal liver of Duffy-positive individuals. Whether this is true in Duffy-negative individuals remains to be demonstrated. A GenBank sequence search yielded a significant protein sequence homology to human and rabbit interleukin-8 receptors.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams M. D., Dubnick M., Kerlavage A. R., Moreno R., Kelley J. M., Utterback T. R., Nagle J. W., Fields C., Venter J. C. Sequence identification of 2,375 human brain genes. Nature. 1992 Feb 13;355(6361):632–634. doi: 10.1038/355632a0. [DOI] [PubMed] [Google Scholar]
- Avent N. D., Ridgwell K., Tanner M. J., Anstee D. J. cDNA cloning of a 30 kDa erythrocyte membrane protein associated with Rh (Rhesus)-blood-group-antigen expression. Biochem J. 1990 Nov 1;271(3):821–825. doi: 10.1042/bj2710821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blobel G. Intracellular protein topogenesis. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1496–1500. doi: 10.1073/pnas.77.3.1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brauer A. W., Oman C. L., Margolies M. N. Use of o-phthalaldehyde to reduce background during automated Edman degradation. Anal Biochem. 1984 Feb;137(1):134–142. doi: 10.1016/0003-2697(84)90359-2. [DOI] [PubMed] [Google Scholar]
- Chaudhuri A., Zbrzezna V., Johnson C., Nichols M., Rubinstein P., Marsh W. L., Pogo A. O. Purification and characterization of an erythrocyte membrane protein complex carrying Duffy blood group antigenicity. Possible receptor for Plasmodium vivax and Plasmodium knowlesi malaria parasite. J Biol Chem. 1989 Aug 15;264(23):13770–13774. [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Chérif-Zahar B., Bloy C., Le Van Kim C., Blanchard D., Bailly P., Hermand P., Salmon C., Cartron J. P., Colin Y. Molecular cloning and protein structure of a human blood group Rh polypeptide. Proc Natl Acad Sci U S A. 1990 Aug;87(16):6243–6247. doi: 10.1073/pnas.87.16.6243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelman D. M., Steitz T. A., Goldman A. Identifying nonpolar transbilayer helices in amino acid sequences of membrane proteins. Annu Rev Biophys Biophys Chem. 1986;15:321–353. doi: 10.1146/annurev.bb.15.060186.001541. [DOI] [PubMed] [Google Scholar]
- Hartmann E., Rapoport T. A., Lodish H. F. Predicting the orientation of eukaryotic membrane-spanning proteins. Proc Natl Acad Sci U S A. 1989 Aug;86(15):5786–5790. doi: 10.1073/pnas.86.15.5786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes W. E., Lee J., Kuang W. J., Rice G. C., Wood W. I. Structure and functional expression of a human interleukin-8 receptor. Science. 1991 Sep 13;253(5025):1278–1280. doi: 10.1126/science.1840701. [DOI] [PubMed] [Google Scholar]
- Horuk R., Chitnis C. E., Darbonne W. C., Colby T. J., Rybicki A., Hadley T. J., Miller L. H. A receptor for the malarial parasite Plasmodium vivax: the erythrocyte chemokine receptor. Science. 1993 Aug 27;261(5125):1182–1184. doi: 10.1126/science.7689250. [DOI] [PubMed] [Google Scholar]
- Jay D., Cantley L. Structural aspects of the red cell anion exchange protein. Annu Rev Biochem. 1986;55:511–538. doi: 10.1146/annurev.bi.55.070186.002455. [DOI] [PubMed] [Google Scholar]
- Kozak M. An analysis of 5'-noncoding sequences from 699 vertebrate messenger RNAs. Nucleic Acids Res. 1987 Oct 26;15(20):8125–8148. doi: 10.1093/nar/15.20.8125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L., Suzuki T., Mori N., Greengard P. Identification of a functional silencer element involved in neuron-specific expression of the synapsin I gene. Proc Natl Acad Sci U S A. 1993 Feb 15;90(4):1460–1464. doi: 10.1073/pnas.90.4.1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh W. L. Present status of the Duffy blood group system. CRC Crit Rev Clin Lab Sci. 1975 Mar;5(4):387–412. doi: 10.3109/10408367509107049. [DOI] [PubMed] [Google Scholar]
- Marshall R. D. Glycoproteins. Annu Rev Biochem. 1972;41:673–702. doi: 10.1146/annurev.bi.41.070172.003325. [DOI] [PubMed] [Google Scholar]
- Miller L. H., Mason S. J., Clyde D. F., McGinniss M. H. The resistance factor to Plasmodium vivax in blacks. The Duffy-blood-group genotype, FyFy. N Engl J Med. 1976 Aug 5;295(6):302–304. doi: 10.1056/NEJM197608052950602. [DOI] [PubMed] [Google Scholar]
- Miller L. H., Mason S. J., Dvorak J. A., McGinniss M. H., Rothman I. K. Erythrocyte receptors for (Plasmodium knowlesi) malaria: Duffy blood group determinants. Science. 1975 Aug 15;189(4202):561–563. doi: 10.1126/science.1145213. [DOI] [PubMed] [Google Scholar]
- Murphy P. M., Tiffany H. L. Cloning of complementary DNA encoding a functional human interleukin-8 receptor. Science. 1991 Sep 13;253(5025):1280–1283. doi: 10.1126/science.1891716. [DOI] [PubMed] [Google Scholar]
- Nichols M. E., Rubinstein P., Barnwell J., Rodriguez de Cordoba S., Rosenfield R. E. A new human Duffy blood group specificity defined by a murine monoclonal antibody. Immunogenetics and association with susceptibility to Plasmodium vivax. J Exp Med. 1987 Sep 1;166(3):776–785. doi: 10.1084/jem.166.3.776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul C., Rosenbusch J. P. Folding patterns of porin and bacteriorhodopsin. EMBO J. 1985 Jun;4(6):1593–1597. doi: 10.1002/j.1460-2075.1985.tb03822.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schägger H., von Jagow G. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem. 1987 Nov 1;166(2):368–379. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- Stoffel W., Hillen H., Schröder W., Deutzmann R. The primary structure of bovine brain myelin lipophilin (proteolipid apoprotein). Hoppe Seylers Z Physiol Chem. 1983 Oct;364(10):1455–1466. doi: 10.1515/bchm2.1983.364.2.1455. [DOI] [PubMed] [Google Scholar]
- Tanner M. J., Anstee D. J., Mallinson G., Ridgwell K., Martin P. G., Avent N. D., Parsons S. F. Effect of endoglycosidase F-peptidyl N-glycosidase F preparations on the surface components of the human erythrocyte. Carbohydr Res. 1988 Jul 15;178:203–212. doi: 10.1016/0008-6215(88)80112-5. [DOI] [PubMed] [Google Scholar]
- Wasniowska K., Eichenberger P., Kugele F., Hadley T. J. Purification of a 28 kD non-aggregating tryptic peptide of the Duffy blood group protein. Biochem Biophys Res Commun. 1993 Apr 30;192(2):366–372. doi: 10.1006/bbrc.1993.1424. [DOI] [PubMed] [Google Scholar]
- Wessels H. P., Spiess M. Insertion of a multispanning membrane protein occurs sequentially and requires only one signal sequence. Cell. 1988 Oct 7;55(1):61–70. doi: 10.1016/0092-8674(88)90009-8. [DOI] [PubMed] [Google Scholar]
- Yamamoto F., Clausen H., White T., Marken J., Hakomori S. Molecular genetic basis of the histo-blood group ABO system. Nature. 1990 May 17;345(6272):229–233. doi: 10.1038/345229a0. [DOI] [PubMed] [Google Scholar]