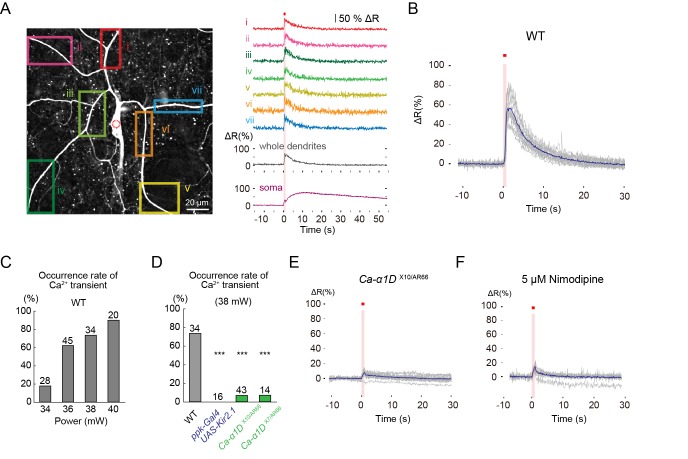
Figure 2. L-type VGCC-dependent global Ca2+ transients occur in Class IV neurons upon noxious thermal stimulation.
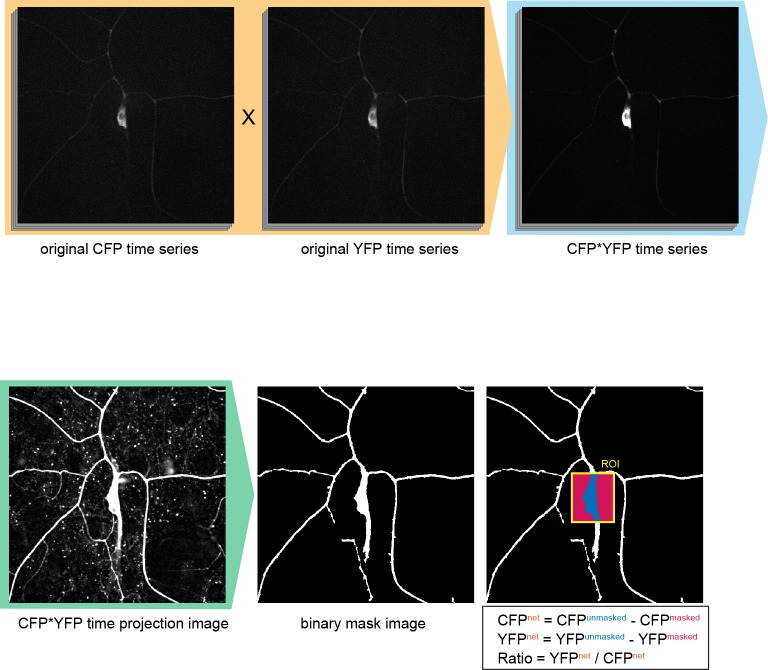
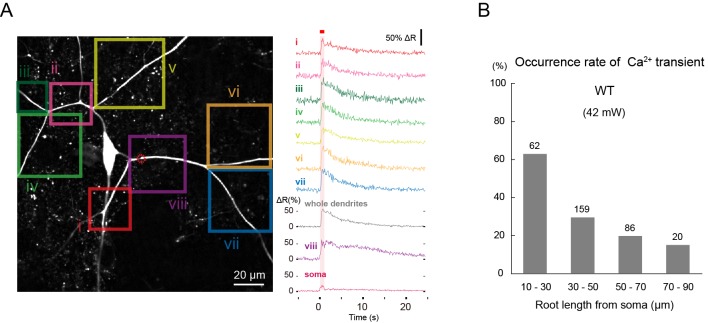
Ca2+ responses of Class IV neuron ddaC expressing TN-XXL in whole-mount larvae, except for F (fillet preparations), upon IR irradiation. Throughout this figure, somata were targeted (red dotted circle in A). The output power of the laser was 38 mW except for C. Red squares above traces and magenta shadings in A, B, E, and F indicate the 1 s irradiations. (A) (Left) Time-projected image that is constructed by multiplying CFP and YFP images at every time point. Rectangles i–vii indicate the regions of interest (ROIs). (Right) Global Ca2+ transients were detected in individual ROIs (i–vii). The transient was also detected when we drew a ROI around the entire dendritic arbor excluding the soma (whole dendrite). Ca2+ transient measured in somata displayed slower fluctuation compared to the dendritic ones (soma). (B) Time courses of Ca2+ transients in whole dendrites. Gray lines indicate dendritic Ca2+ transients of 14 cells, and the blue line represents the averaged amplitude. The data from cells that did not generate Ca2+ transients were excluded. (C) Occurrence rate of dendritic Ca2+ transients when stimulated with different IR-laser output powers. We performed one trial per cell and the number of cells examined is displayed at the top of each bar. (D) Occurrence rate of dendritic Ca2+ transients was dramatically reduced in Class IV neurons overexpressing Kir2.1 (ppk-Gal4 UAS-Kir2.1) or in neurons of larvae with mutations of the L-type VGCC gene (Ca-α1DX10/AR66 and Ca-α1DX7/AR66). *** p < 0.001 versus wild type by Fisher’s exact test. (E) Ca2+ fluctuations in dendrites of Ca-α1D mutant neurons (Ca-α1DX10/AR66). Gray lines indicate Ca2+ responses of 17 cells, and the blue line represents the averaged amplitude. (F) Ca2+ fluctuations in dendrites of fillet preparations when treated with 5 μM Nimodipine. Gray lines indicate Ca2+ responses of 11 cells, and the blue line represents the averaged amplitude. In E and F, we excluded data where ratiometric signals could not be continuously recorded due to movements of mounted larvae.
DOI: http://dx.doi.org/10.7554/eLife.12959.007