Abstract
Purpose of the review
Nonalcoholic steatohepatitis (NASH) is projected to become the most common indication for liver transplantation in the near future. NASH recipients have concurrent obesity, metabolic and cardiovascular risks, which directly impact patient selection, post-transplant morbidity and potentially long term outcomes. The purpose of this review is to highlight strategies to optimize pre-transplant selection, outcomes, and post-transplant risk modification to optimize patient and graft survival.
Recent findings
NASH recipients are at risk for pre-transplant cardiovascular disease, diabetes mellitus, and related reno-vascular complications. Stringent selection criteria identify those patients most likely to benefit from liver transplantation without adverse cardiovascular events, yet, the incidence of these events remains high in NASH recipients. High body mass index imparts post-operative morbidity due to infections, wound complications, and longer lengths of hospital stay. Aggressive management of modifiable risk factors such as obesity, hyperlipidemia, diabetes mellitus and hypertension is recommended.
Summary
Though patient and graft survival in NASH recipients is excellent, long term reduction in health care utilization and outcomes in these patients would benefit from risk factor modification. Periodic reassessment of coronary artery disease and early consideration of bariatric surgery is recommended in this population.
Keywords: Metabolic syndrome, liver transplantation, cardiovascular disease
INTRODUCTION
Non-alcoholic steatohepatitis (NASH) is an increasing indication for liver transplantation (LT) (1) and is projected to become the most frequent indication in the coming decades (2). NASH is associated with metabolic syndrome and other comorbidities that may impact liver transplant candidacy and post-transplant outcomes. At this time based on large database reviews, patients with NASH can anticipate similar graft and patient survival as other transplant recipients (1, 3). This review will address pre-LT issues specific to NASH as well as review the risk for recurrent disease and comorbidities on the long term outcome of the NASH recipient.
PRE-TRANSPLANT CONSIDERATIONS in NASH RECIPIENTS
The selection process for LT candidates is based on two important criteria: i) ability to withstand the complex surgery without major subsequent morbidity and ii) benefit from LT. Age, severity of liver disease, coronary artery disease (CAD), diabetes mellitus (DM), obesity and renal failure are individual risk predictors of poor postoperative and late outcomes. Among liver transplant candidates, patients with NASH represent a particularly challenging group because they are most likely to have these risk factors, which may contribute in both an independent and additive manner to patient selection and outcomes after LT.
Cardiovascular risk assessment
Multiple studies have demonstrated that nonalcoholic fatty liver disease (NAFLD) is associated with increased risk for cardiovascular events (4–8). NAFLD has been associated with increased intima-media thickness and plaque (4), arterial stiffness (5), coronary artery calcifications (6), CAD (9), subclinical myocardial remodeling, ventricular dysfunction (10), risk of atrial fibrillation (7) and heart valve calcifications (8). Whether the increased cardiovascular (CV) risk is directly attributed to NAFLD traits (such as simple steatosis, hepatic inflammation, or increased oxidative stress) independent of the coexisting dysmetabolic traits of obesity (such as insulin resistance, visceral adiposity and atherogenic dyslipidemia) remains to be elucidated. What remains clear is that, compared to other cirrhotics, NASH cirrhosis confers a higher risk of CV events after LT, more commonly in the early postoperative period (11). The most common CV events include acute pulmonary edema (18%), new onset atrial fibrillation (10%) and sudden cardiac arrest (8%) (11). The pro-atherogenic environment of NAFLD and obesity comes in addition to the predisposition to cirrhotic cardiomyopathy, which is present in up to 50% of cirrhotics, independent of liver disease etiology (12).
These findings highlight the need for careful selection of the NASH LT candidate; the general approach followed at our institution is depicted in Figure 1. However, there are currently no specific guidelines for preoperative assessment in this population. The sensitivity and specificity of non-invasive testing in diagnosing CAD in the general population is summarized in Table 1 (13). Dobutamine stress echocardiography (DSE) has been advocated as the assessment tool of choice in NASH LT candidates. The high negative predictive value of DSE, when target heart rates are achieved, allows it to be used to identify a low risk group. However, several studies have shown that DSE has a poor performance to predict the risk of myocardial injury during liver transplantation in patients with cirrhosis (14). Other emerging techniques for coronary artery and myocardial functional assessment, such as CT coronary artery calcification scoring and cardiac MRI may be useful to improve the identification of CAD in advanced cirrhotic patients on the waiting list, but further studies are needed to validate their use in this population. Invasive coronary angiography is often necessary. However, percutaneous revascularization pre-transplantation has demonstrated little evidence of benefit and surgical revascularization carries an increased risk of postoperative morbidity (25–58%) and mortality (17–30%) in these patients (15). Furthermore, in the presence of traditional risk factors such as DM, HTN, or hyperlipidemia, medical optimization with statins and beta-blockers (16) in the perioperative period is protective and likely underutilized. There is a great need for prospective studies to identify a standardized comprehensive approach to the CV risk of liver transplant candidates with NASH cirrhosis, who may have a different risk profile than the general population.
Figure 1.
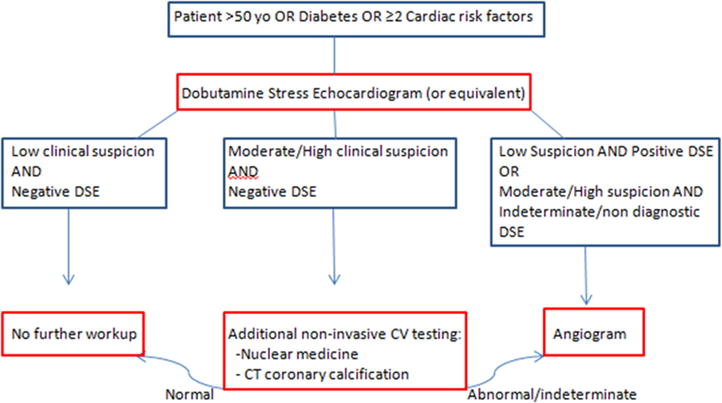
Cardiac workup algorithm. DSE= dobutamine stress echocardiogram, clinical suspicion= compilation of cardiac risk factors (smoking, previous cardiac disease, family history of cardiac disease, diabetes, hyperlipidemia, hypertension, obesity)
Table 1.
Accuracy of noninvasive methods for assessment of coronary artery disease in the general population
| Imaging Modality | Sensitivity (%) | Specificity (%) |
|---|---|---|
| Exercise electrocardiogram | 45–50 | 85–90 |
| Exercise stress echocardiogram | 80–85 | 80–88 |
| Exercise SPECT | 73–92 | 63–87 |
| Dobutamine stress echocardiogram | 79–83 | 82–86 |
| Dobutamine stress cardiac MRI | 79–88 | 81–91 |
| Adenosine SPECT | 90–91 | 75–84 |
| CT coronary angiography | 95–99 | 64–83 |
| Adenosine PET | 81–97 | 74–91 |
Based on 2013 ESC guidelines on the management of stable coronary artery disease (12). SPECT, single-photon emission computed tomography; MRI, magnetic resonance imaging; CT, computed tomography; PET, positron emission tomography.
Weight and body mass index (BMI) as selection criteria
The low number of studies with LT recipients with class III obesity (BMI >40 kg/m2) would suggest that BMI may play a role in patient selection. Most studies seem to agree that obesity alone does not negatively impact long term graft and patient survival, though it does impart an increase in early post-transplant morbidity (17, 18), while some have shown that BMI is an independent predictor of death if above 40 kg/m2 (19),(20). Additionally, intra-abdominal adiposity poses challenges to the surgical technique, but there are no data on its impact on postoperative complications(21). Except at the extremes of BMI, obesity alone should not represent a significant contraindication to LT and should not be considered in isolation as a factor precluding successful transplantation. In fact, these patients may be considered for concurrent bariatric surgery, an approach taken by some transplant centers in recent years. Heimbach et al. reported that sleeve gastrectomy at the time of liver transplant in a carefully selected population of obese liver transplant candidates who failed an aggressive noninvasive weight loss program results in effective and sustained weight loss and no post-transplant metabolic complications (allograft steatosis, diabetes mellitus) (22).
Pre-transplant diabetes mellitus
Pre-transplant DM in combination with obesity is a strong predictor of early postoperative complications (infections, CV events) and length of hospital stay compared to obesity alone or with other CV risk factor (23). Additionally, pre-transplant DM is associated with lower long term patient and graft survival, with deaths mainly attributed to CV complications, infections, and renal failure (24). In a Unites Network for Organ Sharing (UNOS) database study, patients with preexisting CAD and DM documented at the time of registration on the waiting list were 60% more likely to die after LT when compared to patients with either disease alone (24). Therefore, in patients with DM, especially in the presence of DM-related organ damage consistent with micro- or macro-vascular complications, in particular, renal and CV function should be carefully assessed, and periodically re-assessed if faced with long wait times to organ transplantation.
Other issues
Additional risk factors associated with obesity and NASH, such as chronic kidney disease (25) and obstructive sleep apnea and its cardiopulmonary complications (pulmonary hypertension and right heart failure) should be sought early and optimized. The greatest challenge in the selection of LT candidates with NASH is how to integrate the multiple risk factors into one accurate risk stratification tool. Until then, sound clinical judgment of the overall metabolic risk profile of a candidate, instead of independent predictors should be used (Table 2).
Table 2.
Metabolic risks factors and methods of assessment in liver transplant candidates
| Risk factor | Assessment methods |
|---|---|
| Age | >50 years |
| Cardiovascular disease | Medical history, family history, NT-pro brain natriuretic peptide, troponins, electrocardiogram, transthoracic echocardiogram, dobutamine stress test, CT coronary angiography, cardiac MRI, percutaneous coronary angiography, carotid ultrasound |
| Diabetes mellitus | Medical history, fasting blood glucose, hemoglobin A1c, assessment of organ-related complications |
| Hypertension | Medical history, blood pressure measurement |
| Hyperlipidemia | Medical history, lipid profile |
| Smoking | History - Active, previous history |
| Obesity | Weight, body mass index |
| Renal failure | Serum creatinine, measured glomerular filtration rate |
| Sleep related disorders | Medical history, overnight oximetry or sleep study in individuals at risk |
CT: computed tomography; MRI: magnetic resonance imaging
POST LIVER TRANSPLANTATION NAFLD
Recurrent and de novo NAFLD are common post LT; whereas, progressive NASH is less common. The incidence, outcomes, CV risks, and possible therapies are discussed in this section.
Incidence
Several studies have shown a high incidence of post-LT NAFLD in patients transplanted for NASH or cryptogenic cirrhosis (26–32) (Table 3). This is significantly greater than the prevalence of NAFLD in patients transplanted for indications other than NASH or cryptogenic cirrhosis, 25% in patients followed 5 years post LT (26, 30); Whereas, in another cohort, de novo steatosis was reported to occur in 40% of recipients and NASH in 13% of recipients transplanted for chronic hepatitis B or C (31). The use of corticosteroids for immunosuppression, pre-transplant BMI, post-LT BMI, tacrolimus-based immunosuppressive regimen, DM, hyperlipidemia, and arterial hypertension are risk factors for post-LT steatosis, (26, 32), whereas the use of angiotensin converting enzyme (ACE) inhibitors was associated with a lower steatosis risk (30). The incidence of NASH reported in various series, which includes both living donor and deceased donor LT, with patients followed for varying periods of time (28 – 64 months), ranges from 4% (median interval between transplant and first biopsy was 40 months, range 6–189 months, (32)) to 14% (median follow-up 64 months, (29)). However, the risk factors for progressive NASH are less well defined in those transplanted for NASH or cryptogenic cirrhosis, apart from the presence of co-existing metabolic syndrome with its inherent risk factors, at the time of transplant.
Table 3.
Incidence of recurrent or de novo allograft NAFLD and NASH
| First Author (Reference#) | Steatosis in NASH or Cryptogenic Recipients | Steatosis in Non-NASH Recipients | Progression to NASH in NASH or Cryptogenic Recipients | Median Duration of follow up (months) |
|---|---|---|---|---|
| Contos et al (25) | 100% | 25% | 10% | 42 ± 32 |
| Maor-Kendler et al (26) | 18% | 11.6%@ | 12& | |
| 38% | 10.3%@ | 0% | 45 ± 17 | |
| Tanaka et al (28)# | 14% | 64 | ||
| Seo et al (29) | 18% | 9% | 23 | |
| Lim et al (30) | 40% | 13% | 44 ± 4 | |
| Dumortier et al (31)$ | 31.1% | 10% | 40 (6–189) |
average for cholestatic, alcoholic, and HCV recipients
7 NASH recipients that received living donor liver transplants
only included non-NASH recipients without recurrent disease
one year protocol biopsy
Patient and graft survival
The reported 1 year survival for NASH recipients ranges from 85–90% and 5 year survival ranges from 70–80% (Figure 2) (1, 3, 33). A recent meta-analysis of 9 studies concluded non-inferior patient survival for NASH recipients (34). When subgroup and sensitivity analyses were performed, patient age and presence of hepatocellular carcinoma impacted patient survival at 1 year and 5 years, respectively. Moreover, patients transplanted for NASH were at greater risk for post-transplant CV events and sepsis. Two large data base analyses also showed that NASH recipients were older than others, consistent with the meta-analysis (1, 3). The SRTR database study performed by Charlton et al included 35,781 adults that received a transplant between 2001 and 2009. Of these, 1959 received a primary liver transplant for NASH as a primary or secondary indication. This study showed non-inferior 1 year (84%) and 3 year (78%) patient survival (1). The other large, UNOS database analysis by Afzali et al included 69,962 patients from 1997 to 2010. Of these NASH was a primary LT indication in 1810 recipients, and cryptogenic cirrhosis was a primary indication in 3843 recipients. This study showed decreased risk of death and graft failure when adjustments were made for both donor and recipient characteristics (3), with 87.6% 1 year, 82.2% 3 year, and 75.75% 5 year patient survival among NASH recipients. The study by Afzali et al excluded from the NASH category those recipients that also had a diagnosis of hepatocellular carcinoma. This might account for the better patient survival reported by them. Though both studies overall showed excellent patient and graft survival in those transplanted for NASH as a primary indication, the study by Afzali et al showed Post liver transplant survival among those transplanted for NASH was higher than some patient groups (hepatocellular carcinoma, hepatitis C, alcoholic liver disease, acute hepatic necrosis, hemochromatosis, cryptogenic cirrhosis), though inferior to others (primary biliary cirrhosis, primary sclerosing cholangitis, autoimmune hepatitis, hepatitis B). Thus, in the current era of LT, NASH imparts equivalent if not superior post LT patient and graft survival.
Figure 2.
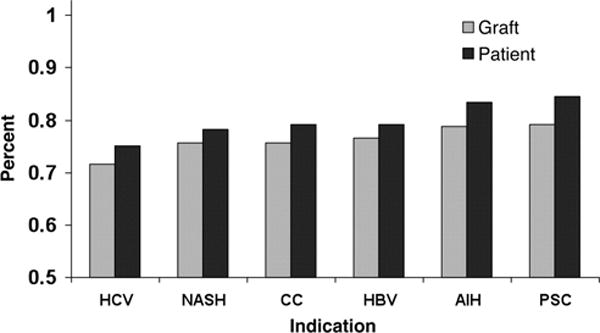
Three year post-liver transplantation graft and patient survival among adult recipients in the United States, by etiology of liver disease. AIH, autoimmune hepatitis; CC, cryptogenic cirrhosis; HBV, hepatitis B virus; HCV, hepatitis C virus; NASH, nonalcoholic steatohepatitis; PSC, primary sclerosing cholangitis. Reproduced with permission from the publisher from Charlton et al, Gastroenterology 2011,141(4):1249–1253.
Cardiovascular disease in NASH recipients
CVD imparts the most significant mortality risk in the NASH patient population, and this is likely true in the post-transplant NASH recipients as well (35, 36). Potential transplant recipients with cardiac risk factors are stringently screened for the presence of atherosclerotic coronary artery disease. Furthermore, many risk factors, such as hyperlipidemia, are masked secondary to impaired hepatic synthesis in cirrhosis. Therefore, the increased incidence of CV disease-related morbidity in NASH LT recipients (11, 37)compared with both those transplanted for other indications, and population matched controls is a significant problem. Moreover, this may reflect an accelerated disease course in these at-risk patients, who were screened for the absence of significant CVD pre-transplantation (36, 37). The presence of pre-transplant CVD, post-operative sepsis, DM, and elevated pre-LT serum troponins were predictors of post-LT CV disease in liver transplant recipients (16, 38, 39), though these studies did not perform sub-group analyses for NASH recipients. Extrapolating from the pre-transplant and general population based studies, male gender and age also impart significant risk, though remain unmodifiable. Among the modifiable cardio-metabolic risk factors are smoking, diabetes mellitus, hypertension (preexisting and de novo), hyperlipidemia, post-LT renal insufficiency, and the use of mycophenolate mofetil (MMF) (36, 37). In the study by Albeldawi which looked at cardiovascular events among all LT recipients (NASH and others), MMF use imparted a hazard ratio of 2.3 for the occurrence of a cardiovascular event post-LT (37), but confirmatory data for its role in cardiovascular disease is needed. All of these risk factors should be aggressively modified and treated. In the study by Fussner et al., tacrolimus-based immunosuppression was associated with a lower risk of CVD. This finding will need to be verified in unique cohorts (36). Patients with pre-LT elevated random serum troponins, left ventricular hypertrophy on ECHO, lower ejection fraction, and prior CVD identifies patients who should receive close follow up and aggressive risk factor modification therapy.
Pharmacotherapy for NASH and metabolic syndrome
There are no specific randomized controlled trials for pharmacotherapy for NASH in the LT population. Though Vitamin E, pioglitazone and obeticholic acid have shown promise in the treatment of NASH in the non-transplant population (40, 41), none of these agents has received regulatory agency approval for NASH therapy. Similarly, though several agents are available for the pharmacotherapy of obesity, such as orlistat, lorcaserin, liraglutide, buprepione-naltrexone, these agents have not been studied in the organ transplantation population. Metformin, statins, and ursodeoxycholic acid have been examined for use in NASH in the non-transplant population with no clear benefit (42–44). Thus, at present effective pharmacotherapy for NASH amelioration, both in the non-transplant and post-transplant population is lacking. However, recognizing the significant risk imparted by CVD in NASH patients, approved therapies for aggressive management of hyperlipidemia, DM, hypertension, and smoking cessation should be pursued in the NASH recipient.
Bariatric surgery in NASH recipients
Laparoscopic sleeve gastrectomy after solid organ transplantation including LT recipients or open sleeve gastrectomy at the time of LT are gaining traction as safe and effective bariatric procedures in liver transplant recipients (45, 46). Due to concerns regarding malabsorption of medications and the inability to access the biliary tree following roux-en-Y procedures, restrictive bariatric procedures have remained more popular in solid organ transplant recipients, though malabsorptive procedures have been reported. A recent review summarized two bariatric surgery case series each, before LT and during LT, and seven post-LT case series (47). Sleeve gastrectomy (SG) was the most frequent bariatric procedure, followed by roux-en-Y gastric bypass. Of the included 54 patients, 26 underwent laparoscopic SG (LSG) before transplant and 11 after transplant, open sleeve gastrectomy was performed during-LT in 7 patients. Percent excess weight loss ranged from 26% at 3 months in one study to 64.7% in another, though these numbers should be interpreted with caution due to the small numbers of patients included. Patients had comparable degrees of decrease in body mass index, or excess body weight loss regardless of the timing of bariatric surgery. Though increase in morbidity (1 leak from the gastric staple line and 1 excess weight loss) in patients with simultaneous transplantation and bariatric surgery has been reported, the post-transplant reduction in onset of diabetes, weight gain, and steatosis has endured to last follow up (mean follow up 33 months) in the study by Heimbach et al (22). Delayed sleeve gastrectomy, similarly, is associated with increased early post-operative morbidity, such as mesh dehiscence, bile leak and post-operative dysphagia, each occurring in one of 9 patients in this case series, however, acceptable long term outcomes (46).
Immunosuppression considerations in NASH recipients
Calcineurin inhibitor (CNI) therapy and mammalian target of rapamycin (mTOR) inhibitors impart CV risks; therefore every attempt should be made to maintain patients on the least effective immunosuppressive therapy. Dumortier et al. reported tacrolimus-based immunosuppression as a risk factor for the development of de novo steatosis in liver transplant recipients (32). Hypertension and hyperlipidemia are higher with cyclosporine based immunosuppression than tacrolimus, though the overall incidence of metabolic syndrome and post-transplant diabetes is unaffected by choice of calcineurin inhibitor(48). Use of the mTOR inhibitors (sirolimus and everolimus) leads to hyperlipidemia, which can be managed with pharmacotherapy. This hyperlipidemia does not increase the risk for CV or cerebrovascular events, as recently demonstrated in a large case series by Weick et al(49). A randomized controlled trial of CNI versus everolimus, though primarily done to look at renal function, also showed similar patient and graft outcomes, though the rate of discontinuation of therapy was higher for everolimus(50). Though ongoing trials are geared towards defining the lowest effective immunosuppressive agents for liver transplant recipients, in our practice, tacrolimus monotherapy is the most-widely used, and efforts should be made to reduce dosage to the minimal effective dose. Alternatively, mTOR inhibitors could be considered in patients with metabolic syndrome and multiple CV risk factors, to minimize the many metabolic side effects associated with CNI use (32, 39), but careful followup of lipid and glucose is still warranted. Prospective data is needed to show true benefit for mTOR inhibitor use over the CNI.
CONCLUSIONS
With NASH on trajectory to become the most frequent primary indication for LT in the near future; one provocative question is how might we improve patient survival further? Though, historically, 5 year patient survival has been an accepted metric for post-transplant outcomes, given the slow onset and progression of NASH, we do we need longer-term follow up and outcomes data. Furthermore, aggressive risk factor modification with life style modification and pharmacotherapy for metabolic syndrome and cardiovascular disease or bariatric surgery for obesity is recommended in NASH recipients.
KEY POINTS.
Recurrent and de novo steatosis are common after liver transplantation, though progressive NASH is less frequent. Moreover, NASH recipients have excellent 5 year patient and graft survival following liver transplantation.
NAFLD traits such as hepatic steatosis, obesity, and the coexisting dysmetabolic traits, such as insulin resistance, visceral adiposity and atherogenic dyslipidemia impart increased cardiovascular risk to these patients.
Pharmacotherapy for risk factor reduction is recommended in the NASH liver transplantation recipient, specifically targeting cardiovascular risk factors.
Bariatric surgery should be considered early in the NASH recipient, and may even be considered at the time of liver transplantation.
Acknowledgments
The authors are grateful for superb secretarial assistance provided by Ms. Courtney Hoover.
Financial support: This work is partially supported by National Institutes of Health grant DK97178 to HM.
Abbreviations
- CAD
coronary artery disease
- CV
cardiovascular
- DM
diabetes mellitus
- DSE
Dobutamine stress ECHO
- LT
liver transplantation
- NAFLD
nonalcoholic fatty liver disease
- NASH
nonalcoholic steatohepatitis
Footnotes
CONFLICTS OF INTEREST
None
References
- 1.Charlton MR, Burns JM, Pedersen RA, et al. Frequency and outcomes of liver transplantation for nonalcoholic steatohepatitis in the United States. Gastroenterology. 2011 Oct;141(4):1249–53. doi: 10.1053/j.gastro.2011.06.061. [DOI] [PubMed] [Google Scholar]
- 2•.Wong RJ, Cheung R, Ahmed A. Nonalcoholic steatohepatitis is the most rapidly growing indication for liver transplantation in patients with hepatocellular carcinoma in the U.S. Hepatology. 2014 Jun;59(6):2188–95. doi: 10.1002/hep.26986. Highlights that NASH cirrhosis is the most rapidly growing etiology of cirrhosis for patients transplanted for hepatocellular carcinoma. [DOI] [PubMed] [Google Scholar]
- 3.Afzali A, Berry K, Ioannou GN. Excellent posttransplant survival for patients with nonalcoholic steatohepatitis in the United States. Liver Transpl. 2012 Jan;18(1):29–37. doi: 10.1002/lt.22435. [DOI] [PubMed] [Google Scholar]
- 4.Sookoian S, Pirola CJ. Non-alcoholic fatty liver disease is strongly associated with carotid atherosclerosis: a systematic review. J Hepatol. 2008 Oct;49(4):600–7. doi: 10.1016/j.jhep.2008.06.012. [DOI] [PubMed] [Google Scholar]
- 5.Oni ET, Agatston AS, Blaha MJ, et al. A systematic review: burden and severity of subclinical cardiovascular disease among those with nonalcoholic fatty liver; should we care? Atherosclerosis. 2013 Oct;230(2):258–67. doi: 10.1016/j.atherosclerosis.2013.07.052. [DOI] [PubMed] [Google Scholar]
- 6.Kim D, Choi SY, Park EH, et al. Nonalcoholic fatty liver disease is associated with coronary artery calcification. Hepatology. 2012 Aug;56(2):605–13. doi: 10.1002/hep.25593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Targher G, Valbusa F, Bonapace S, et al. Non-alcoholic fatty liver disease is associated with an increased incidence of atrial fibrillation in patients with type 2 diabetes. PLoS One. 2013;8(2):e57183. doi: 10.1371/journal.pone.0057183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mantovani A, Pernigo M, Bergamini C, et al. Heart valve calcification in patients with type 2 diabetes and nonalcoholic fatty liver disease. Metabolism. 2015 Aug;64(8):879–87. doi: 10.1016/j.metabol.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 9.Targher G, Day CP, Bonora E. Risk of cardiovascular disease in patients with nonalcoholic fatty liver disease. N Engl J Med. 2010 Sep 30;363(14):1341–50. doi: 10.1056/NEJMra0912063. [DOI] [PubMed] [Google Scholar]
- 10.Petta S, Argano C, Colomba D, et al. Epicardial fat, cardiac geometry and cardiac function in patients with non-alcoholic fatty liver disease: association with the severity of liver disease. J Hepatol. 2015 Apr;62(4):928–33. doi: 10.1016/j.jhep.2014.11.030. [DOI] [PubMed] [Google Scholar]
- 11.Vanwagner LB, Bhave M, Te HS, et al. Patients transplanted for nonalcoholic steatohepatitis are at increased risk for postoperative cardiovascular events. Hepatology. 2012 Nov;56(5):1741–50. doi: 10.1002/hep.25855. [DOI] [PubMed] [Google Scholar]
- 12.Moller S, Henriksen JH. Cirrhotic cardiomyopathy. J Hepatol. 2010 Jul;53(1):179–90. doi: 10.1016/j.jhep.2010.02.023. [DOI] [PubMed] [Google Scholar]
- 13.Montalescot G, Sechtem U, Achenbach S, et al. 2013 ESC guidelines on the management of stable coronary artery disease: the Task Force on the management of stable coronary artery disease of the European Society of Cardiology. Eur Heart J. 2013 Oct;34(38):2949–3003. doi: 10.1093/eurheartj/eht296. [DOI] [PubMed] [Google Scholar]
- 14.Findlay JY, Keegan MT, Pellikka PP, et al. Preoperative dobutamine stress echocardiography, intraoperative events, and intraoperative myocardial injury in liver transplantation. Transplant Proc. 2005 Jun;37(5):2209–13. doi: 10.1016/j.transproceed.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 15.Ehtisham J, Altieri M, Salame E, et al. Coronary artery disease in orthotopic liver transplantation: pretransplant assessment and management. Liver Transpl. 2010 May;16(5):550–7. doi: 10.1002/lt.22035. [DOI] [PubMed] [Google Scholar]
- 16.Safadi A, Homsi M, Maskoun W, et al. Perioperative risk predictors of cardiac outcomes in patients undergoing liver transplantation surgery. Circulation. 2009 Sep 29;120(13):1189–94. doi: 10.1161/CIRCULATIONAHA.108.847178. [DOI] [PubMed] [Google Scholar]
- 17.Leonard J, Heimbach JK, Malinchoc M, et al. The impact of obesity on long-term outcomes in liver transplant recipients-results of the NIDDK liver transplant database. Am J Transplant. 2008 Mar;8(3):667–72. doi: 10.1111/j.1600-6143.2007.02100.x. [DOI] [PubMed] [Google Scholar]
- 18.Hakeem AR, Cockbain AJ, Raza SS, et al. Increased morbidity in overweight and obese liver transplant recipients: a single-center experience of 1325 patients from the United Kingdom. Liver Transpl. 2013 May;19(5):551–62. doi: 10.1002/lt.23618. [DOI] [PubMed] [Google Scholar]
- 19.Rustgi VK, Marino G, Rustgi S, et al. Impact of body mass index on graft failure and overall survival following liver transplant. Clin Transplant. 2004 Dec;18(6):634–7. doi: 10.1111/j.1399-0012.2004.00141.x. [DOI] [PubMed] [Google Scholar]
- 20.Dick AA, Spitzer AL, Seifert CF, et al. Liver transplantation at the extremes of the body mass index. Liver Transpl. 2009 Aug;15(8):968–77. doi: 10.1002/lt.21785. [DOI] [PubMed] [Google Scholar]
- 21.Watt KD. Reducing the load: the evolution and management of obesity and nonalcoholic steatohepatitis before liver transplantation. Liver Transpl. 2012 Nov;18(Suppl 2):S52–8. doi: 10.1002/lt.23515. [DOI] [PubMed] [Google Scholar]
- 22.Heimbach JK, Watt KD, Poterucha JJ, et al. Combined liver transplantation and gastric sleeve resection for patients with medically complicated obesity and end-stage liver disease. Am J Transplant. 2013 Feb;13(2):363–8. doi: 10.1111/j.1600-6143.2012.04318.x. [DOI] [PubMed] [Google Scholar]
- 23.Trail KC, Stratta RJ, Larsen JL, et al. Results of liver transplantation in diabetic recipients. Surgery. 1993 Oct;114(4):650–6. discussion 6–8. [PubMed] [Google Scholar]
- 24.Yoo HY, Thuluvath PJ. The effect of insulin-dependent diabetes mellitus on outcome of liver transplantation. Transplantation. 2002 Oct 15;74(7):1007–12. doi: 10.1097/00007890-200210150-00019. [DOI] [PubMed] [Google Scholar]
- 25.Musso G, Cassader M, Cohney S, et al. Emerging Liver-Kidney Interactions in Nonalcoholic Fatty Liver Disease. Trends Mol Med. 2015 Oct;21(10):645–62. doi: 10.1016/j.molmed.2015.08.005. [DOI] [PubMed] [Google Scholar]
- 26.Contos MJ, Cales W, Sterling RK, et al. Development of nonalcoholic fatty liver disease after orthotopic liver transplantation for cryptogenic cirrhosis. Liver Transpl. 2001 Apr;7(4):363–73. doi: 10.1053/jlts.2001.23011. [DOI] [PubMed] [Google Scholar]
- 27.Maor-Kendler Y, Batts KP, Burgart LJ, et al. Comparative allograft histology after liver transplantation for cryptogenic cirrhosis, alcohol, hepatitis C, and cholestatic liver diseases. Transplantation. 2000 Jul 27;70(2):292–7. doi: 10.1097/00007890-200007270-00009. [DOI] [PubMed] [Google Scholar]
- 28.Garcia RF, Morales E, Garcia CE, et al. Recurrent and de novo non-alcoholic steatohepatitis following orthotopic liver transplantation. Arq Gastroenterol. 2001 Oct-Dec;38(4):247–53. doi: 10.1590/s0004-28032001000400007. [DOI] [PubMed] [Google Scholar]
- 29.Tanaka T, Sugawara Y, Tamura S, et al. Living donor liver transplantation for non-alcoholic steatohepatitis: A single center experience. Hepatol Res. 2014 Oct;44(10):E3–E10. doi: 10.1111/hepr.12200. [DOI] [PubMed] [Google Scholar]
- 30.Seo S, Maganti K, Khehra M, et al. De novo nonalcoholic fatty liver disease after liver transplantation. Liver Transpl. 2007 Jun;13(6):844–7. doi: 10.1002/lt.20932. [DOI] [PubMed] [Google Scholar]
- 31.Lim LG, Cheng CL, Wee A, et al. Prevalence and clinical associations of posttransplant fatty liver disease. Liver Int. 2007 Feb;27(1):76–80. doi: 10.1111/j.1478-3231.2006.01396.x. [DOI] [PubMed] [Google Scholar]
- 32.Dumortier J, Giostra E, Belbouab S, et al. Non-alcoholic fatty liver disease in liver transplant recipients: another story of “seed and soil”. Am J Gastroenterol. 2010 Mar;105(3):613–20. doi: 10.1038/ajg.2009.717. [DOI] [PubMed] [Google Scholar]
- 33.Malik SM, deVera ME, Fontes P, et al. Outcome after liver transplantation for NASH cirrhosis. Am J Transplant. 2009 Apr;9(4):782–93. doi: 10.1111/j.1600-6143.2009.02590.x. [DOI] [PubMed] [Google Scholar]
- 34•.Wang X, Li J, Riaz DR, et al. Outcomes of liver transplantation for nonalcoholic steatohepatitis: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2014 Mar;12(3):394–402 e1. doi: 10.1016/j.cgh.2013.09.023. A systematic meta-analysis of outcomes of patients transplated for NASH cirrhosis. [DOI] [PubMed] [Google Scholar]
- 35.Rinella ME. Nonalcoholic Fatty Liver Disease: A Systematic Review. Jama. 2015 Jun 9;313(22):2263–73. doi: 10.1001/jama.2015.5370. [DOI] [PubMed] [Google Scholar]
- 36•.Fussner LA, Heimbach JK, Fan C, et al. Cardiovascular disease after liver transplantation: When, What, and Who Is at Risk. Liver Transpl. 2015 Jul;21(7):889–96. doi: 10.1002/lt.24137. The high incidence of post-liver transplant obesity and cardiovascular disease is demonstrated in this retrospective review. The authors also idnetify risk factors for cardiovascular disease by uni-variate and multi-variate analysis. [DOI] [PubMed] [Google Scholar]
- 37.Albeldawi M, Aggarwal A, Madhwal S, et al. Cumulative risk of cardiovascular events after orthotopic liver transplantation. Liver Transpl. 2012 Mar;18(3):370–5. doi: 10.1002/lt.22468. [DOI] [PubMed] [Google Scholar]
- 38.Fouad TR, Abdel-Razek WM, Burak KW, et al. Prediction of cardiac complications after liver transplantation. Transplantation. 2009 Mar 15;87(5):763–70. doi: 10.1097/TP.0b013e318198d734. [DOI] [PubMed] [Google Scholar]
- 39.Coss E, Watt KD, Pedersen R, et al. Predictors of cardiovascular events after liver transplantation: a role for pretransplant serum troponin levels. Liver Transpl. 2011 Jan;17(1):23–31. doi: 10.1002/lt.22140. [DOI] [PubMed] [Google Scholar]
- 40.Neuschwander-Tetri BA, Loomba R, Sanyal AJ, et al. Farnesoid X nuclear receptor ligand obeticholic acid for non-cirrhotic, non-alcoholic steatohepatitis (FLINT): a multicentre, randomised, placebo-controlled trial. Lancet. 2015 Mar 14;385(9972):956–65. doi: 10.1016/S0140-6736(14)61933-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sanyal AJ, Chalasani N, Kowdley KV, et al. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med. 2010 May 6;362(18):1675–85. doi: 10.1056/NEJMoa0907929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Musso G, Gambino R, Cassader M, et al. A meta-analysis of randomized trials for the treatment of nonalcoholic fatty liver disease. Hepatology. 2010 Jul;52(1):79–104. doi: 10.1002/hep.23623. [DOI] [PubMed] [Google Scholar]
- 43.Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of non-alcoholic fatty liver disease: practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology. 2012 Jun;55(6):2005–23. doi: 10.1002/hep.25762. [DOI] [PubMed] [Google Scholar]
- 44.Lindor KD, Kowdley KV, Heathcote EJ, et al. Ursodeoxycholic acid for treatment of nonalcoholic steatohepatitis: results of a randomized trial. Hepatology. 2004 Mar;39(3):770–8. doi: 10.1002/hep.20092. [DOI] [PubMed] [Google Scholar]
- 45.Butte JM, Devaud N, Jarufe NP, et al. Sleeve gastrectomy as treatment for severe obesity after orthotopic liver transplantation. Obes Surg. 2007 Nov;17(11):1517–9. doi: 10.1007/s11695-008-9432-z. [DOI] [PubMed] [Google Scholar]
- 46.Lin MY, Tavakol MM, Sarin A, et al. Safety and feasibility of sleeve gastrectomy in morbidly obese patients following liver transplantation. Surg Endosc. 2013 Jan;27(1):81–5. doi: 10.1007/s00464-012-2410-5. [DOI] [PubMed] [Google Scholar]
- 47.Lazzati A, Iannelli A, Schneck AS, et al. Bariatric surgery and liver transplantation: a systematic review a new frontier for bariatric surgery. Obes Surg. 2015 Jan;25(1):134–42. doi: 10.1007/s11695-014-1430-8. [DOI] [PubMed] [Google Scholar]
- 48.Bianchi G, Marchesini G, Marzocchi R, et al. Metabolic syndrome in liver transplantation: relation to etiology and immunosuppression. Liver Transpl. 2008 Nov;14(11):1648–54. doi: 10.1002/lt.21588. [DOI] [PubMed] [Google Scholar]
- 49.Weick A, Chacra W, Kuchipudi A, et al. Incidence of cardiovascular and cerebrovascular events associated with sirolimus use after liver transplantation. Transplant Proc. 2015 Mar;47(2):460–4. doi: 10.1016/j.transproceed.2014.11.036. [DOI] [PubMed] [Google Scholar]
- 50.Fischer L, Klempnauer J, Beckebaum S, et al. A randomized, controlled study to assess the conversion from calcineurin-inhibitors to everolimus after liver transplantation--PROTECT. Am J Transplant. 2012 Jul;12(7):1855–65. doi: 10.1111/j.1600-6143.2012.04049.x. [DOI] [PubMed] [Google Scholar]


