Abstract
Background
There is a paucity of evidence-based interventions for mild traumatic brain injury (mTBI).
Objective
To evaluate the feasibility and potential benefits of an interactive, web-based intervention for mTBI.
Setting
Emergency department (ED) and outpatient settings.
Participants
Of the 21 adolescents ages 11–18 years with mTBI recruited from November 2013 to June 2014 within 96 hours of injury, 13 completed the program.
Design
Prospective, open pilot.
Intervention
The web-based Self-Management Activity-restriction and Relaxation Training (SMART) program incorporates anticipatory guidance and psychoeducation, self-management and pacing of cognitive and physical activities, and cognitive behavioral principles for early management of mTBI in adolescents.
Main Measures
Primary: Daily post-concussion symptom score (PCSS). Secondary: Daily self-reported ratings of activities and satisfaction survey
Results
Average time from injury to baseline testing was 14.0 (SD:16.7) hours. Baseline PCSS was 23.6 (range:0–46), and daily activity was 1.8 hours (range:0–5.75). Repeated-measures, generalized linear mixed-effects model analysis demonstrated a significant decrease of PCSS at a rate of 2.0 points/day that stabilized after about two weeks. Daily activities, screen time and physical activity increased by 0.06 (SE=0.04, p=0.09), 0.04 (SE=0.02, p=0.15) and 0.03 (SE=0.02, p=0.05) hours/day, respectively, over the 4-week follow-up. Satisfaction was rated highly by parents and youth.
Conclusions
SMART is feasible and reported to be helpful and enjoyable by participants. Future research will need to determine the comparative benefits of SMART and ideal target population.
Keywords: Adolescents, Concussion, Intervention, Mild traumatic brain injury, Web-based
Background
Pediatric traumatic brain injury (TBI) is among the most common causes of acquired morbidity and mortality in children, resulting in over 6,000 deaths, 60,000 hospitalizations, and 630,000 emergency department (ED) visits annually in the U.S. Approximately, 75–85% of these injuries are mild TBIs (mTBI).1 Although most individuals recover within 2–4 weeks, up to one-third of individuals remain symptomatic at 1–3 months after injury.2–5 Persistent symptoms are associated with significant lost time from school and potential social isolation.6 Development of interventions to reduce or avoid persistent symptoms would be a huge step towards reducing the public health burden of mTBI.
There is a paucity of evidence-based interventions for management of mTBI.7–13 Prior research has shown that anticipatory guidance and psychoeducation provided soon after injury may reduce long-term behavioral problems.13–15 Consensus statements recommend cognitive and physical rest as a cornerstone of treatment of mTBI;8–11 however, the specific intensity, timing, and duration of rest are unclear.16 Recent research indicates that a limited rest period may promote more rapid recovery than a longer rest period,17,18 and moderate activity may actually be beneficial; specifically, sub-symptom exacerbation aerobic exercise and pacing return to school and other activities may reduce symptom duration.17,19–24 Cognitive and behavioral therapy and relaxation training may also benefit individuals with persistent symptoms after mTBI.13,21,25–27
In moderate to severe pediatric TBI, web-based delivery of online-problem solving therapy can assist patients and families in managing common behavioral sequelae.28–33 To our knowledge, similar web-based systems have not been utilized in mTBI. The goal of this study was to build on prior research and evaluate the feasibility and potential benefits of a web-based Self-Monitoring Activity-restriction and Relaxation Training (SMART) program that incorporates anticipatory guidance and psychoeducation about mTBI, self-management and pacing of cognitive and physical activities, and cognitive behavioral principles for early management of mTBI in adolescents. Prior usability testing of the SMART program was positive and resulted in patient-driven modifications in content.34 The goal of this open-pilot study was to evaluate the feasibility of delivering SMART soon after mTBI in adolescents and assess its potential benefits.
Methods
This was a prospective, open-label, non-comparative, pilot evaluation of SMART. Participants were eligible if they were between 11 and 18 years of age; had a mTBI within 96 hours of emergency department (ED) presentation; English was their primary language; internet access was available in their home; and they resided with a parent or legal guardian. mTBI was defined using the following criteria: 1) a blow to the head or body, 2) the injury was witnessed or there was physical evidence of a head injury and 3) loss of consciousness, amnesia or a change in mental status occurred.35 Loss consciousness was limited to 30 minutes or less, amnesia was limited to 24 hours or less, and changes in mental status included feeling dazed, disoriented, or confused at the time of the injury.35 Determination of whether or not a participant met the criteria for mTBI was based on review of medical records and discussion with the treating ED physician and/or family. Participants were excluded if they had a history of two or more extra-cranial injuries or an extra-cranial injury with an Abbreviated Injury Severity Scale score36 above four; attention deficit hyperactivity disorder requiring more than one controlling medication; or other pre-existing conditions that may impair baseline cognition such as neurologic (e.g., stroke, CSF shunt, brain tumor, seizure disorder), cognitive (e.g., intellectual disability), psychological (e.g., depression, schizophrenia, or bipolar disorder), or developmental delays.
Study procedures
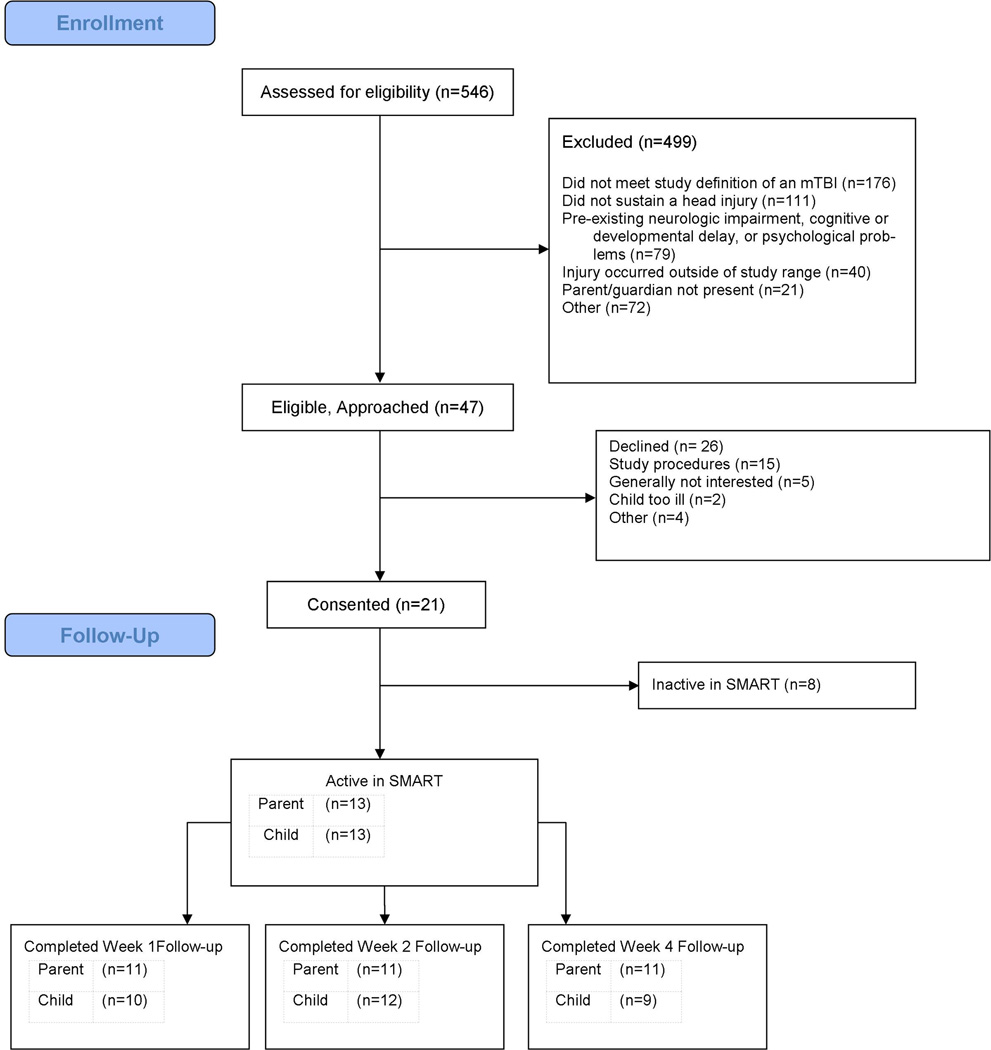
Participants were screened for eligibility by trained clinical research coordinators in our institution’s pediatric ED. Once patient eligibility was confirmed with an ED physician, research coordinators approached patients and families about the study. Forty-seven potential participants were approached and 21 adolescents were recruited prospectively by research coordinators (see Figure 1. CONSORT flow chart). The main reasons that participants declined to participate were study procedures were too burdensome (n=15), generally not interested (n=5), child being too ill when approached (n=2), and other reasons (n=4).
Figure 1.
Consort Flow diagram
After obtaining consent and assent, baseline data were collected using Research Electronic Data Capture (REDCap).37 REDCap is a free, web-based application for collecting structured, secure data for research studies. Research coordinators collected demographic and injury information. The child and parent were asked to complete a battery of baseline assessment tools, after which the research coordinator provided an overview of SMART using screenshots of the program and gave participants an instructional pamphlet and information on what to expect for study follow-up.
SMART Program
Within 24 hours of enrollment, participants were sent an email with login information for SMART. The participant was asked to log in daily throughout the 4-week program and complete the symptoms and activities questions. Reminder emails, text messages and phone calls were made by the lead research coordinator if the participant was inactive in SMART for greater than two days. Participants were also given small monetary incentives to encourage completion of study measures at baseline in the ED and after completion of electronic follow-up measures at 1, 2 and 4 weeks post-injury. Reminders were sent until the participant logged into the system or was inactive for two weeks.
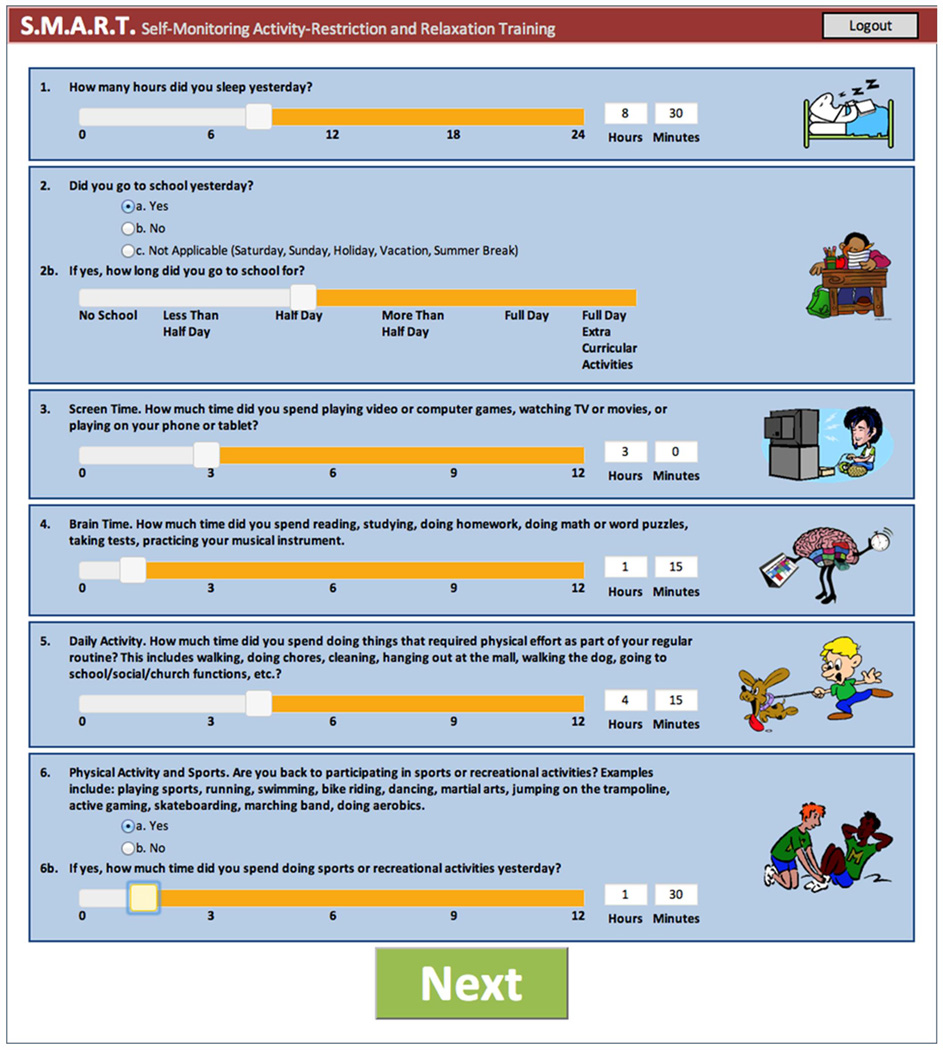
SMART consisted of two components: a symptom and activity monitoring interface and eight psychoeducational modules about topics related to mTBI (Table 1). As part of the self-monitoring interface, participants were asked to rate their activity level in various domains of daily activities (mental, screen-time, and physical activities; see Figure 2) and complete the 22-item post-concussion symptom survey (PCSS)38 each day. After completion of the PCSS, the participants received feedback about whether their symptoms were improving, remaining stable, or worsening, as well as a graph of the trajectory of the participant’s symptom score. The logic for presentation of the graphic feedback was built into the SMART program based upon total PCSS trajectory. Participants were then asked to identify potential contributing factors responsible for their change in symptom burden using a prepopulated graded list (sleep, school, brain activity, screen time, daily activities, physical activities) and an open text box. They were then asked what they planned to do the next day in regards to their activities (i.e., increase or decrease these activities). This self-monitoring interface was designed to promote the concept of self-management, i.e. that the patient is responsible for the day-to-day management of their symptoms by engaging in healthy behaviors.
Table 1.
Overview of the SMART Program
| SELF MANAGEMENT | |
| Symptom Monitoring | |
| 22- item Post Concussion Symptom Score (PCSS) | |
| Total initial PCSS in the ED used to determine injury severity | |
| Ongoing total PCSS assessed at each log-on used to gauge recovery | |
| Activity Tracking | |
| Self-reported prior day’s hours spent | |
| Activities: sleep, school, screen, brain, activities of daily living, physical activities | |
| Feedback | |
| Based on progression of daily total symptoms: better or worse | |
| Self-insight as to what level of participation in each activity may have contributed to current symptom burden | |
| Self-Regulation | |
| Self-management of level of participation in next day's activities to facilitate recovery | |
| EDUCATIONAL MODULES | |
| Module | Skills Addressed |
| Introduction and Self-monitoring | Introduction to the SMART program and information about common symptoms experienced after an mTBI |
| Symptom Management | Information about common symptoms, timelines for recovery, and strategies for coping |
| Returning to Activities | Provides guidelines and strategies for working with the school and other non-athletic activities to ensure a successful re-entry without symptom exacerbation |
| Taking Care of You | Provides strategies for maintaining optimal self-care and informs about the impact of fatigue on recovery |
| Staying Positive | Teaches cognitive reframing strategies to address worries and negative cognitions about symptoms and missed activities |
| Staying Focused | Teaches strategies for improving attention and memory |
| Stop, Think, Problem Solve | Provides training in 5-step problem solving heuristic (Aim, Brainstorm, Choose, Do It, Evaluate) to address concerns regarding PCS-related issues |
| Managing Stress | Provides instruction in relaxation and imagery to more effectively handle stress and manage headaches and other pain |
Figure 2.
Example of daily activity ratings in the SMART program
The SMART modules were adapted from similar programs used for managing sequelae of moderate and severe TBI28–33 and were designed to promote further self-management using cognitive-behavioral and psychoeducational principles. Cognitive-behavioral therapy is a form of psychotherapy that requires the individual to examine their belief structures that may be contributing to their maladaptive behaviors and hampering their recovery.39 Psychoeducation is education that empowers individuals to deal with their condition in an optimal way.40 The eight SMART models were Introduction and Self-Monitoring; Symptom Management; Staying Positive; Managing Stress; Stop, Think, Problem Solve; Returning to School/Activities; Taking Care of You, and Staying Focused (Table 1). Within each module, information pertaining to that topic was presented in a combination of graphic and written materials, along with voice overwrite, and participants were instructed on pertinent cognitive-behavioral exercises and relaxation techniques. The introduction module was available to all users 24 hours after injury. The additional modules were released based on the injury date and the reported severity score from the daily questions. Based on review of normative data for the PCSS that indicated the mean (SD) for boys is 4.8 (7.9) and girls is 7.7 (13.7)41 and consensus between two physicians with clinical expertise in mTBI management (B.G.K and L.B.), cut-offs for release of modules were developed. Participants were considered to have no or very mild symptoms burden with a PCSS score <= 8, to have mild symptom burden with scores 9–14, moderate symptom burden with a score of 15–30, severe symptom burden with a score of 31–49, and very severe symptom burden with a score of 50–132. Participants with normal and mild PCSS scores had all modules open to them by day four. Participants with moderate, severe, and very severe scores had an extra day of rest suggested by the system before any modules were open. The module order of completion varied slightly (Table 2) depending on the severity of the injury and current symptom burden determined by consensus opinion of two physicians with expertise in mTBI management (B.G.K. and L.B.). By one-week post-injury, all modules were available for completion for all participants. Guidelines for return to physical and/or sports activities were described in the program; however, the program referred participants to their clinical provider for formal clearance for these types of activities as required by state law.
Table 2.
Timing of module display based on symptom burden
| Symptom burden | Day 0 | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|---|
| Normal | Rest | 1,2 | 3,4 | 5,6 | 7,8 | |
| Mild | Rest | 1,2 | 3,4 | 5,6 | 7,8 | |
| Moderate | Rest | Rest | 1,2 | 3,4 | 5,6 | 7,8 |
| Severe | Rest | Rest | 1,2 | 3,4 | 5,8 | 6,7 |
| Very Severe | Rest | Rest | 1,2 | 4,5 | 8,6 | 3,7 |
1 = Introduction and Self-Monitoring, 2 = Symptom Management, 3 = Staying Positive, 4 = Managing Stress, 5 = Stop, Think, Problem Solve, 6 = Returning to School/Activities, 7 = Taking Care of You, 8 = Staying Focused
The primary outcome for this analysis was daily symptom score ratings on the PCSS. Secondary outcomes consisted of daily self-reports of the amount of time spent in daily activities, including physical, mental, and screen time. We hypothesized that symptom scores would decrease and activities proportionally increase over time with module completion. To assess daily activities, slider bar scales were developed to allow participants to indicate time they spent in each of respective activity (Figure 2). In addition, to assess acceptability and helpfulness of the program, participating youth and parents rated their agreement (strongly disagree, disagree, agree, or strongly agree) with a series of statements after completion of the 4-week program. The teen statements were “I have reached the goals that I had when I began the program”, “My questions about my injury have been answered”, “I have a plan for handling future problems with my concussion”, “I understand my injury better”, “I would do the program over again”, “I would recommend the program to other teens”, “The program was too long”, and “The program was too short”. The parent statements were “I have reached the goals that I had when I began the program”, “My questions about my child’s injury have been answered”, “I have a plan for handling problems related to my child’s concussion”, “I understand my child’s injury better”, “I would do the program over again”, “I would recommend the program to other parents”, “The program was too long”, and “The program was too short”. Both teens and parents were asked to rate the helpfulness and enjoyment of the program on a ten point Likert scale ranging from 0 (not all) to 10 (completely/extremely).
Analysis
Descriptive statistics were used for the demographics of participants. Independent t-tests and Chi Square statistics were used to compare demographics between participants and non-participants. Repeated-measures analyses using generalized linear mixed models were used to evaluate the trajectory of symptom recovery and participation in daily activities. All analyses were performed using SAS 9.3 (SAS Institute, Cary NC). Significance was defined as p < 0.05.
Results
Participants
Twenty-one individuals were originally enrolled for participation. Gender, race, age, and time since injury were similar between completers (n=13) and non-completers (n=8) (Table 3). The 8 non-completers were unable to be contacted following enrollment and thus the reasons for failure to complete the modules are unknown. Of the 13 participants that completed the study, the mean age was 14.3 years and time from injury to baseline testing was 14.0 hours; 9 participants were males and all were Caucasian. The mean baseline PCSS was 23.6.
Table 3.
Comparison of completers and non-completers (n=21)
| Completers (n=13) |
Non-completers (n=8) |
P-value* | |
|---|---|---|---|
| Mean Age at baseline, years (SD) | 14.3 (2.0) | 15.3 (2.5) | .33 |
| Mean Time since injury at baseline, hours (SD) | 14.0 (16.7) | 7.8 (17.1) | .42 |
| Gender (% Female [n]) | 31 [4] | 39 [5] | .75 |
| Race/Ethnicity (% [n]) White Non-White |
100[13] 0 [0] |
75 [6] 25 [2] |
.06 |
| Mean GCS score, initial (SD) | 14.9 (.3) | 15 (0) | .45 |
| Mean GCS score, 2 hours after presentation (SD) | 15 (0) | 15 (0) | |
| Loss of consciousness | |||
| absent | 46% [6] | 13% [1] | .08 |
| present | 38% [5] | 88% [7] | |
| unknown | 15% [2] | 0% [0] |
Comparison of completers and non-completers by either Chi-square or T test
SD = Standard Deviation; GCS = Glasgow Coma Scale score
SMART Use
The intervention has eight distinct modules. Over the four weeks of the program, participants interacted with the program a median of six times (range 2–11). The median total time spent on the modules was 35.45 minutes (range 1.07 – 107.57 minutes). Participants spent the most time on the “Stop, Think, Problem Solve” module (median = 9.8 minutes, range 0.07 to 19.2 minutes), and the least time on the “Taking Care of You” module (median 0.64 minute, with range from 0.03 to 8.00 minutes).
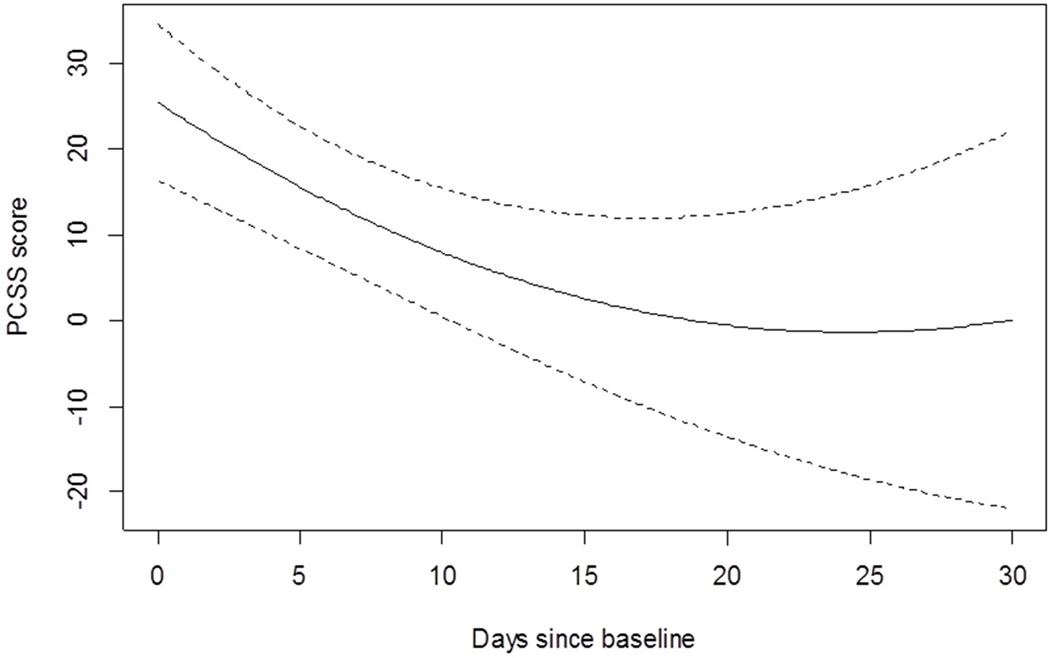
Recovery rate
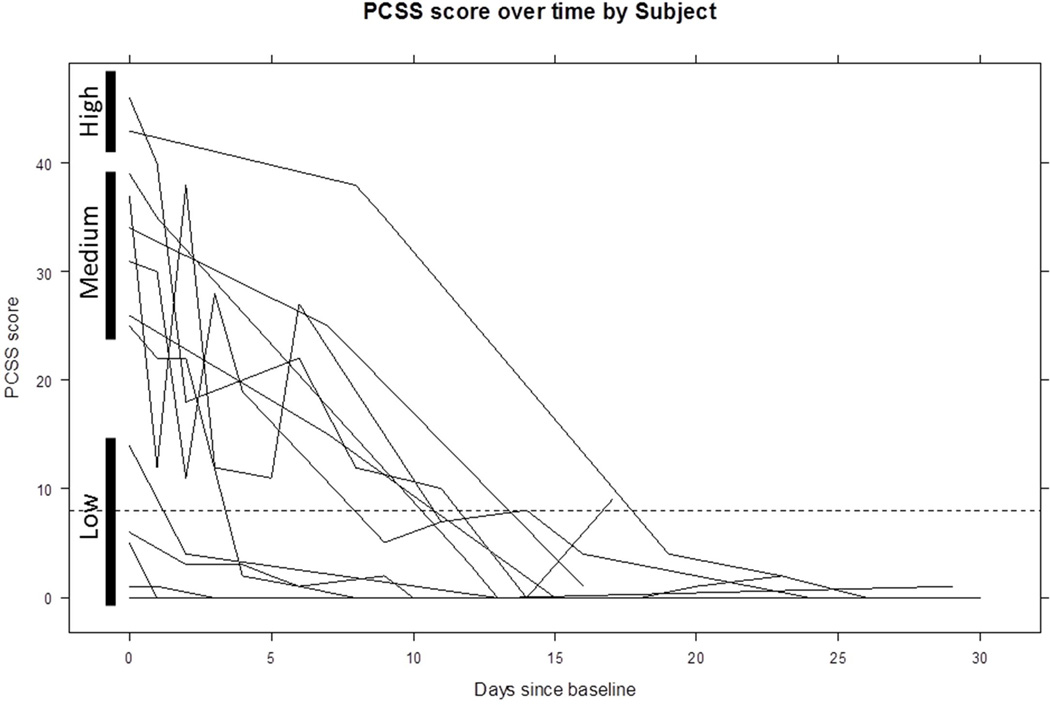
The rate of recovery of PCSS was 2.0 points/day soon after the intervention (p< 0.01), stabilizing after about two weeks (Figure 3). Individual trajectories of recovery indicate within-subject variability over time, with most participants having resolution of symptoms (PCSS <= 8) by two weeks post-injury (Figure 4). Qualitative review of trajectories shows three symptom burden groups: low, medium, and high. There were five participants with initial PCSS scores between 0 – 15, six with initial scores between 20 – 40, and two with initial scores above 40 (Figure 4). The low symptom burden group achieved full recovery the most quickly. The medium group took slightly longer to recover than the low initial symptom burden group. One of the two participants in the high initial symptom burden group took the longest to recover.
Figure 3.
Rate of improvement of post-concussion symptoms over time
PCSS = Post Concussion Symptom Scale
Dotted lines represent 95% confidence intervals
Figure 4.
Individual trajectories of post-concussion symptom recovery
Solid bars delineate groups that had low, medium, and high initial symptom burden
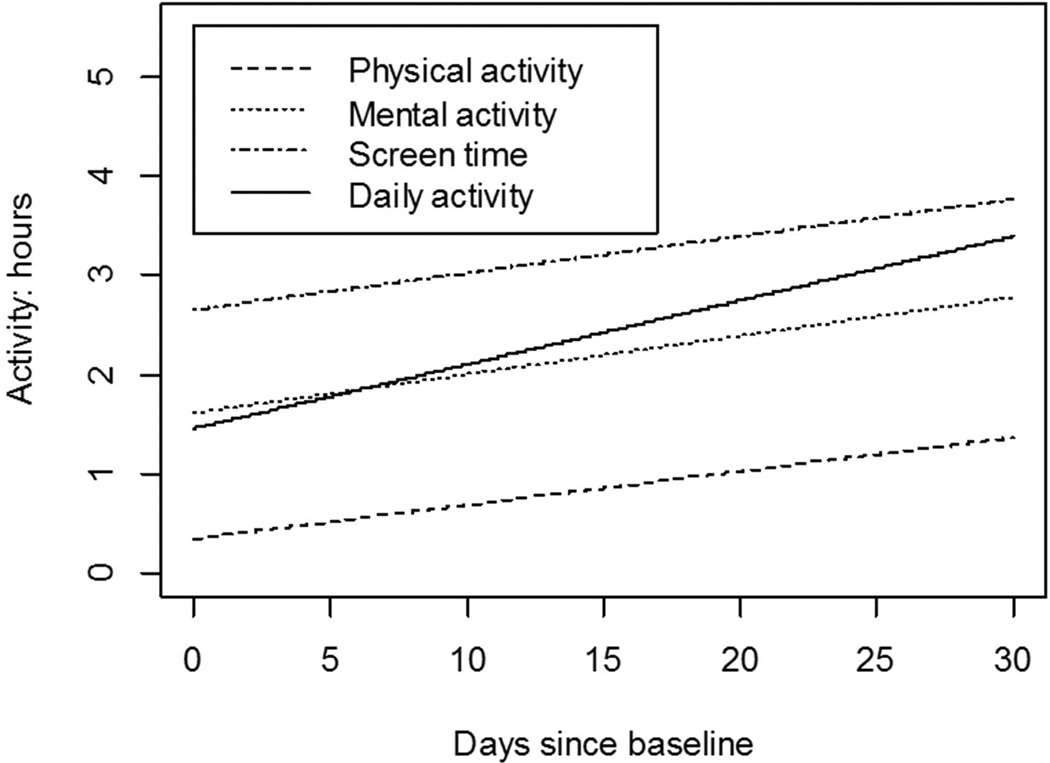
The rates of increased physical, mental, screen time, and overall daily activities were 0.03, 0.04, 0.04, and 0.06 hours/day (p=0.05, 0.30, 0.15, 0.09), respectively (Figure 5). Qualitative review of individual trajectories of increased physical, mental, screen time, and overall daily activities revealed large variability in trajectories among participants..
Figure 5.
Rate of improvement in physical, mental, screen time, and daily activity
Results of the survey completed at the end of the 4-week program showed that all eleven parents and nine youth who completed the survey agreed or strongly agreed that the program met their goals, their questions had been answered, they have a plan for handling future problems with mTBI, and they understood the injury better. All parents and youth agreed or strongly agreed that they would do the program over and recommend it to others. No parents or youth felt that the program was too short or too long. Likert ratings on a 10-point scale indicated high levels of parental and youth satisfaction with helpfulness and enjoyment rated highly by both (Helpfulness: parent = 8.36/10; SD = 2.11; youth = 7.33/10; SD = 1.50; enjoyment: parent = 8.00/10; SD = 2.41; youth = 7.00/10; SD = 1.50).
Discussion
Our findings indicate that a web-based program for mTBI that provides anticipatory guidance, psychoeducation, and incorporates recommendations for self-guided return to activities is feasible to initiate in the ED acutely after a mTBI and is reported to be helpful by parents and adolescents who used the program.. The majority of participant’s symptoms resolved within two weeks after injury, with all participants becoming asymptomatic within four weeks. Future comparative studies are needed to determine the efficacy of the SMART intervention compared to standard care or other interventions.
In this small pilot study, all participants’ symptoms resolved by four weeks post-injury, with most resolving within the initial two weeks. This time trajectory is similar to the expected recovery trajectory and may represent natural recovery.2,42 Similar to prior research showing that early education about mTBI may reduce development of persisting problems14, early introduction of the SMART program may reduce the risk of longer-term problems post-injury. However, larger, comparative studies are needed to determine efficacy. SMART recommends rest for only 1–2 days before initiation of modules, in agreement with recent work that demonstrated potential benefits associated with both a shorter rest period and moderate activity during recovery.18,20,43,44 Furthermore, in agreement with prior work45, qualitative review of individual trajectories in this study indicated that lower initial symptom burden was associated with quicker recovery. Further work is needed to determine optimal guidelines for introduction of physical and cognitive activity after mTBI.
The SMART program allows individuals to self-guide return to daily activities, which is in agreement with consensus statements that recommend individualized management with return to activities.8,10,11 Due to state law requiring clearance by a health care provider before returning to full sports or recreational activities, specific recommendations on return to these activities was not provided in SMART. However, general information on typical return to play guidelines was provided in SMART that would allow participants to discuss specific return to activity questions with their health care provider. Future research needs to better determine how information provided in this application intersects with medical care and opens up potential conduits to facilitate information-sharing with pertinent health care providers. Participants using the SMART program rate symptoms and activities daily and are asked throughout the program to think about types of activities that may have contributed to improvement or worsening of symptoms. This self-management of pacing return to activities encourages the participant to actively take control of their recovery, thus encouraging a more active coping style, which has been linked to improved recovery after mTBI in adolescents.46 Furthermore, it encourages participants to titrate activities based on symptoms. This idea of titrating activities is in agreement with other research that has demonstrated moderate and sub-symptom exacerbation activities may help recovery after mTBI.20,21,43,44
The few pediatric intervention trials performed thus far during the acute timeframe have focused on anticipatory guidance and psychoeducation.13–15,47 Education about the common issues children face following mTBI was the cornerstone of our modules. To date, cognitive-behavioral interventions have shown the greatest utility for management of symptoms after pediatric brain injury, especially when there are persistent symptoms7,27,48,49 However, the efficacy of the introduction of cognitive-behavioral therapy soon after injury has not been well-studied. The problem-solving and relaxation component of SMART incorporates these cognitive-behavioral principles into the management of mTBI symptoms acutely after injury and allows the adolescent to guide implementation. Introduction of cognitive-behavioral components earlier after injury may promote symptom resolution and, in part, reduce the development of persistent symptoms.
There was variability in the utilization of the SMART modules. The most time was spent on the “Stop, Think, and Problem Solve” module while less time was spent on other modules. These findings suggest that some modules may be more beneficial or appealing than others. Also, certain modules may be more beneficial depending on time since injury and burden of symptoms. Larger studies are needed to determine the relative benefits of the modules and characteristics of individuals most likely to benefit from each module. Because SMART allows self-pacing and access to modules to be guided by the user, individualization is determined by the user. The web-based, self-guided approach used in SMART provides individuals with daily access to information and recommendations regarding management of mTBI symptoms. This frequency and level of access is typically more than can be provided via standard clinical visits and may support more rapid return to activities in conjunction with improved symptoms. Integration of interaction between health care providers and/or school personnel and SMART users into the program may be consider in the future as a way to further enhance the program and incorporate it into standard care.
Limitations
Since this is a prospective open pilot, we were unable to draw conclusions on the efficacy of SMART compared to standard care or other treatments. Although participants rated the helpfulness of SMART highly, we are also unable to comment on whether participation in SMART led to specific behavior changes. Almost half of the participants originally enrolled did not complete the study. There was a high rate of initial refusal, suggesting a need to determine the optimal timing for interventions like SMART, as well as to identify the population at highest risk of persistent symptoms that would benefit the most from such an intervention. Higher drop-out was anticipated, as most children recover within a few weeks from mTBI and are unlikely to remain in an intervention study once symptoms have resolved. However, without data from the dropouts, we are unable to determine whether they chose not to participate because they had recovered or for other reasons (e.g., too symptomatic to participate). Also, specific recommendations for pattern of utilization and incentives for completing modules may have improved adherence. Although not statistically significant, there was a trend for non-completers to be less likely to have loss of consciousness associated with the injury and for non-white participants to be less likely to complete the study.
Conclusions
SMART, a novel web-based application of anticipatory guidance, psychoeducation and cognitive-behavioral therapy, is feasible to initiate soon after mTBI in adolescents and is reported to be helpful and enjoyable by parents and adolescents who used the program.. A web-based program potentially provides more convenient access to patients and families to a treatment for mTBI than standard care. Future research will need to determine the comparative benefits of SMART to standard care and the ideal target population. Adaptation of the program for adolescents presenting with persistent symptoms should also be considered.
Acknowledgements
Funding for this study was supported in part by the Ohio Department of Public Safety, Cincinnati Children’s Research Foundation Place Outcomes Grant, National Institute for Child Health and Human Development K23HD074683-01A1, and Grant 8 UL1 TR000077 from the National Center for Advancing Translational Sciences (NCATS) of the National Institutes of Health (NIH). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or other supporting agencies.
References
- 1.Faul M, Xu L, Wald M, Coronado V. Atlanta (GA): Centers for Disease Control and Prevention, National Center for Injury Prevention and Control; 2010. [Accessed September 9, 2013]. Traumatic brain injury in the United States: emergency department visits, hospitalizations and deaths 2002–2006. http://www.cdc.gov/traumaticbraininjury/pdf/blue_book.pdf. [Google Scholar]
- 2.Barlow KM, Crawford S, Stevenson A, Sandhu SS, Belanger F, Dewey D. Epidemiology of postconcussion syndrome in pediatric mild traumatic brain injury. Pediatrics. 2010;126(2):e374–e381. doi: 10.1542/peds.2009-0925. [DOI] [PubMed] [Google Scholar]
- 3.Sroufe NS, Fuller DS, West BT, Singal BM, Warschausky SA, Maio RF. Postconcussive symptoms and neurocognitive function after mild traumatic brain injury in children. Pediatrics. 2010;125(6):e1331–e1339. doi: 10.1542/peds.2008-2364. [DOI] [PubMed] [Google Scholar]
- 4.Ponsford J, Willmott C, Rothwell A, et al. Cognitive and behavioral outcome following mild traumatic head injury in children. Journal of Head Trauma Rehabilitation. 1999;14(4):360–372. doi: 10.1097/00001199-199908000-00005. [DOI] [PubMed] [Google Scholar]
- 5.Meehan WP, 3rd, Mannix RC, Stracciolini A, Elbin RJ, Collins MW. Symptom severity predicts prolonged recovery after sport-related concussion, but age and amnesia do not. The Journal of pediatrics. 2013;163(3):721–725. doi: 10.1016/j.jpeds.2013.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Babcock L, Byczkowski T, Wade SL, Ho M, Mookerjee S, Bazarian JJ. Predicting postconcussion syndrome after mild traumatic brain injury in children and adolescents who present to the emergency department. JAMA pediatrics. 2013;167(2):156–161. doi: 10.1001/jamapediatrics.2013.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sports-Related Concussions in Youth: Improving the Science, Changing the Culture. Washington, DC: The National Academies Press; 2014. [PubMed] [Google Scholar]
- 8.Giza CC, Kutcher JS, Ashwal S, et al. Summary of evidence-based guideline update: evaluation and management of concussion in sports: report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology. 2013;80(24):2250–2257. doi: 10.1212/WNL.0b013e31828d57dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McCrory P. Traumatic brain injury: revisiting the AAN guidelines on sport-related concussion. Nat Rev Neurol. 2013;9(7):361–362. doi: 10.1038/nrneurol.2013.88. [DOI] [PubMed] [Google Scholar]
- 10.McCrory P, Meeuwisse WH, Aubry M, et al. Consensus statement on concussion in sport--the 4th International Conference on Concussion in Sport held in Zurich, November 2012. PM & R : the journal of injury, function, and rehabilitation. 2013;5(4):255–279. doi: 10.1016/j.pmrj.2013.02.012. [DOI] [PubMed] [Google Scholar]
- 11.Harmon KG, Drezner JA, Gammons M, et al. American Medical Society for Sports Medicine position statement: concussion in sport. British journal of sports medicine. 2013;47(1):15–26. doi: 10.1136/bjsports-2012-091941. [DOI] [PubMed] [Google Scholar]
- 12.Borg J, Holm L, Peloso PM, et al. Non-surgical intervention and cost for mild traumatic brain injury: results of the WHO Collaborating Centre Task Force on Mild Traumatic Brain Injury. Journal of rehabilitation medicine. 2004;(43 Suppl):76–83. doi: 10.1080/16501960410023840. [DOI] [PubMed] [Google Scholar]
- 13.Gravel J, D'Angelo A, Carriere B, et al. Interventions provided in the acute phase for mild traumatic brain injury: a systematic review. Systematic reviews. 2013;2:63. doi: 10.1186/2046-4053-2-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ponsford J, Willmott C, Rothwell A, et al. Impact of early intervention on outcome after mild traumatic brain injury in children. Pediatrics. 2001;108(6):1297–1303. doi: 10.1542/peds.108.6.1297. [DOI] [PubMed] [Google Scholar]
- 15.Bell KR, Hoffman JM, Temkin NR, et al. The effect of telephone counselling on reducing post-traumatic symptoms after mild traumatic brain injury: a randomised trial. Journal of neurology, neurosurgery, and psychiatry. 2008;79(11):1275–1281. doi: 10.1136/jnnp.2007.141762. [DOI] [PubMed] [Google Scholar]
- 16.Moser RS, Glatts C, Schatz P. Efficacy of immediate and delayed cognitive and physical rest for treatment of sports-related concussion. The Journal of pediatrics. 2012;161(5):922–926. doi: 10.1016/j.jpeds.2012.04.012. [DOI] [PubMed] [Google Scholar]
- 17.Schneider KJ, Iverson GL, Emery CA, McCrory P, Herring SA, Meeuwisse WH. The effects of rest and treatment following sport-related concussion: a systematic review of the literature. British journal of sports medicine. 2013;47(5):304–307. doi: 10.1136/bjsports-2013-092190. [DOI] [PubMed] [Google Scholar]
- 18.Thomas DG, Apps JN, Hoffmann RG, McCrea M, Hammeke T. Benefits of Strict Rest After Acute Concussion: A Randomized Controlled Trial. Pediatrics. 2015 doi: 10.1542/peds.2014-0966. [DOI] [PubMed] [Google Scholar]
- 19.Tan CO, Meehan WP, 3rd, Iverson GL, Taylor JA. Cerebrovascular regulation, exercise, and mild traumatic brain injury. Neurology. 2014;83(18):1665–1672. doi: 10.1212/WNL.0000000000000944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Majerske CW, Mihalik JP, Ren D, et al. Concussion in sports: postconcussive activity levels, symptoms, and neurocognitive performance. J Athl Train. 2008;43(3):265–274. doi: 10.4085/1062-6050-43.3.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leddy JJ, Sandhu H, Sodhi V, Baker JG, Willer B. Rehabilitation of Concussion and Post-concussion Syndrome. Sports health. 2012;4(2):147–154. doi: 10.1177/1941738111433673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Master CL, Gioia GA, Leddy JJ, Grady MF. Importance of 'return-to-learn' in pediatric and adolescent concussion. Pediatric annals. 2012;41(9):1–6. doi: 10.3928/00904481-20120827-09. [DOI] [PubMed] [Google Scholar]
- 23.Vidal PG, Goodman AM, Colin A, Leddy JJ, Grady MF. Rehabilitation strategies for prolonged recovery in pediatric and adolescent concussion. Pediatric annals. 2012;41(9):1–7. doi: 10.3928/00904481-20120827-10. [DOI] [PubMed] [Google Scholar]
- 24.Halstead ME, McAvoy K, Devore CD, Carl R, Lee M, Logan K. Returning to learning following a concussion. Pediatrics. 2013;132(5):948–957. doi: 10.1542/peds.2013-2867. [DOI] [PubMed] [Google Scholar]
- 25.Azulay J, Smart CM, Mott T, Cicerone KD. A pilot study examining the effect of mindfulness-based stress reduction on symptoms of chronic mild traumatic brain injury/postconcussive syndrome. J Head Trauma Rehabil. 2013;28(4):323–331. doi: 10.1097/HTR.0b013e318250ebda. [DOI] [PubMed] [Google Scholar]
- 26.Potter S, Brown RG. Cognitive behavioural therapy and persistent post-concussional symptoms: integrating conceptual issues and practical aspects in treatment. Neuropsychological rehabilitation. 2012;22(1):1–25. doi: 10.1080/09602011.2011.630883. [DOI] [PubMed] [Google Scholar]
- 27.Ross KA, Dorris L, McMillan T. A systematic review of psychological interventions to alleviate cognitive and psychosocial problems in children with acquired brain injury. Dev Med Child Neurol. 2011;53(8):692–701. doi: 10.1111/j.1469-8749.2011.03976.x. [DOI] [PubMed] [Google Scholar]
- 28.Kurowski BG, Wade SL, Kirkwood MW, Brown TM, Stancin T, Taylor HG. Online problem-solving therapy for executive dysfunction after child traumatic brain injury. Pediatrics. 2013;132(1):e158–e166. doi: 10.1542/peds.2012-4040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kurowski BG, Wade SL, Kirkwood MW, Brown TM, Stancin T, Taylor HG. Long-term benefits of an early online problem-solving intervention for executive dysfunction after traumatic brain injury in children: a randomized clinical trial. JAMA pediatrics. 2014;168(6):523–531. doi: 10.1001/jamapediatrics.2013.5070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wade SL, Carey J, Wolfe CR. The Efficacy of an Online Cognitive-Behavioral, Family Intervention in Improving Child Behavior and Social Competence Following Pediatric Brain Injury. Rehabilitation Psychology. 2006;51(3):179–189. [Google Scholar]
- 31.Wade SL, Walz NC, Carey J, McMullen KM. A randomized trial of teen online problem solving: Efficacy in improving caregiver outcomes after brain injury. Health Psychology. 2012;31(6):767. doi: 10.1037/a0028440. [DOI] [PubMed] [Google Scholar]
- 32.Wade SL, Walz NC, Carey J, et al. A randomized trial of teen online problem solving for improving executive function deficits following pediatric traumatic brain injury. J Head Trauma Rehabil. 2010;25(6):409–415. doi: 10.1097/HTR.0b013e3181fb900d. [DOI] [PubMed] [Google Scholar]
- 33.Wade SL, Kurowski BG, Kirkwood MW, et al. Online Problem-Solving Therapy After Traumatic Brain Injury: A Randomized Controlled Trial. Pediatrics. 2015 doi: 10.1542/peds.2014-1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dexheimer JW, Kurowski B, Anders SH, Wade SL, Babcock L. Usability evaluation of SMART application for youth with mTBI; Pediatric Academic Society Annual Conference; Vancouver, CA. 2014. [Google Scholar]
- 35.Kay T, Harrington DE, Adams R, et al. Definition of mild traumatic brain injury. J Head Trauma Rehabil. 1993;8(3):86–87. [Google Scholar]
- 36.Civil ID, Schwab CW. The Abbreviated Injury Scale, 1985 revision: a condensed chart for clinical use. The Journal of trauma. 1988;28(1):87–90. doi: 10.1097/00005373-198801000-00012. [DOI] [PubMed] [Google Scholar]
- 37.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. Journal of biomedical informatics. 2009;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schatz P, Pardini JE, Lovell MR, Collins MW, Podell K. Sensitivity and specificity of the ImPACT Test Battery for concussion in athletes. Arch Clin Neuropsychol. 2006;21(1):91–99. doi: 10.1016/j.acn.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 39.Hofmann SG, Asnaani A, Vonk IJ, Sawyer AT, Fang A. The Efficacy of Cognitive Behavioral Therapy: A Review of Meta-analyses. Cognitive therapy and research. 2012;36(5):427–440. doi: 10.1007/s10608-012-9476-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lukens E, McFarlane WR. Psychoeducation as evidence-based practice: considerations for practice, research, and policy. Brief Treatment and Crisis Intervention. 2004;(4):205–225. [Google Scholar]
- 41.Immediate Post-Concussion Assessment Testing (ImPACT) Test: Technical Manual Online ImPaCT 2007–2012. ImPACT Applications, Inc.; 2011. [Google Scholar]
- 42.Eisenberg MA, Andrea J, Meehan W, Mannix R. Time interval between concussions and symptom duration. Pediatrics. 2013;132(1):8–17. doi: 10.1542/peds.2013-0432. [DOI] [PubMed] [Google Scholar]
- 43.Moor HM, Eisenhauer RC, Killian KD, et al. The relationship between adherence behaviors and recovery time in adolescents after a sports-related concussion: an observational study. International journal of sports physical therapy. 2015;10(2):225–233. [PMC free article] [PubMed] [Google Scholar]
- 44.Silverberg ND, Iverson GL. Is rest after concussion "the best medicine?": recommendations for activity resumption following concussion in athletes, civilians, and military service members. J Head Trauma Rehabil. 2013;28(4):250–259. doi: 10.1097/HTR.0b013e31825ad658. [DOI] [PubMed] [Google Scholar]
- 45.Meehan WP, 3rd, Mannix R, Monuteaux MC, Stein CJ, Bachur RG. Early symptom burden predicts recovery after sport-related concussion. Neurology. 2014;83(24):2204–2210. doi: 10.1212/WNL.0000000000001073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Woodrome SE, Yeates KO, Taylor HG, et al. Coping strategies as a predictor of post-concussive symptoms in children with mild traumatic brain injury versus mild orthopedic injury. Journal of the International Neuropsychological Society : JINS. 2011;17(2):317–326. doi: 10.1017/S1355617710001700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Casey R, Ludwig S, McCormick MC. Minor head trauma in children: an intervention to decrease functional morbidity. Pediatrics. 1987;80(2):159–164. [PubMed] [Google Scholar]
- 48.Ylvisaker MLCKMMMJ. Behavioural interventions for children and adults with behaviour disorders after TBI: A systematic review of the evidence. Brain Injury. 2007;21(8):769–805. doi: 10.1080/02699050701482470. [DOI] [PubMed] [Google Scholar]
- 49.Burke MJ, Fralick M, Nejatbakhsh N, Tartaglia MC, Tator CH. In search of evidence-based treatment for concussion: characteristics of current clinical trials. Brain injury : [BI] 2014:1–6. doi: 10.3109/02699052.2014.974673. [DOI] [PMC free article] [PubMed] [Google Scholar]