Abstract
Transient receptor potential melastatin 7 (TRPM7) is a bi-functional protein comprising a magnesium (Mg2+)/cation channel and a kinase domain. We previously demonstrated that vasoactive agents regulate vascular TRPM7. Whether TRPM7 plays a role in the pathophysiology of hypertension and associated cardiovascular dysfunction is unknown. We studied TRPM7kinase-deficient mice (TRPM7Δkinase) (heterozygous for TRPM7kinase) and wildtype (WT) mice infused with Ang II (400 ng/kg/min, 4 weeks). TRPM7kinase expression was lower in heart and aorta from TRPM7Δkinase versus WT mice, effects that were further reduced by Ang II infusion. Plasma Mg2+ was lower in TRPM7Δkinase versus WT mice in basal and stimulated conditions. Ang II increased blood pressure in both strains with exaggerated responses in TRPM7Δkinase versus WT groups (p<0.05). Acetylcholine-induced vasorelaxation was reduced in Ang II-infused TRPM7Δkinase mice, an effect associated with Akt and eNOS downregulation. Vascular VCAM-1 expression was increased in Ang II-infused TRPM7kinase-deficient mice. TRPM7 kinase targets, calpain and annexin-1, were activated by Ang II in WT but not in TRPM7Δkinase mice. Echocardiographic and histopathological analysis demonstrated cardiac hypertrophy and LV dysfunction in Ang II-treated groups. In TRPM7kinase-deficient mice, Ang II-induced cardiac functional and structural effects were amplified compared with WT counterparts. Our data demonstrate that in TRPM7Δkinase mice, Ang II-induced hypertension is exaggerated, cardiac remodelling and LV dysfunction are amplified and endothelial function is impaired. These processes are associated with hypomagnesemia, blunted TRPM7kinase expression/signaling, eNOS downregulation and pro-inflammatory vascular responses. Our findings identify TRPM7kinase as a novel player in Ang II-induced hypertension and associated vascular and target organ damage.
Keywords: Blood pressure, endothelial dysfunction, TRPM, magnesium, signal transduction
Introduction
The transient receptor potential (TRP) melastatin 7 (TRPM7) cation channel is a member of the TRP ion channel superfamily which, together with its homolog TRPM6, has the unique property of possessing a cation channel domain coupled to a cytosolic α-type serine/threonine kinase domain that comprises the carboxy-terminal region of the protein (hence also termed “chanzyme”) (1, 2). While the channel domain regulates transmembrane magnesium (Mg2+) influx, the kinase domain influences downstream signaling targets such as annexin-1, calpain, myosin-IIA heavy chain, Ef2-k and PLCγ2 (3–6). The kinase domain can also influence TRPM7 channel function (7). TRPM7 regulates many cellular processes including Mg2+ homeostasis, cell growth/apoptosis, motility, actin-myosin interaction, differentiation, and mechanosensitivity (8–10). Studies in TRPM7-deficient mice indicate that TRPM7 plays an essential role in embryonic development, kidney morphogenesis, cardiac function and systemic Mg2+ homeostasis (11–13). TRPM7 dysfunction has been implicated in numerous pathologies including neurodegenerative disorders, cancer and cardiovascular disease (14–18). Although the physiological and pathological significance of TRPM7 is becoming increasingly apparent, the biology of TRPM7, particularly in the cardiovascular system, still remains elusive.
We previously demonstrated that TRPM7 is expressed in the vasculature and that it is regulated by vasoactive agents, such as bradykinin, aldosterone, endothelin-1, and angiotensin II (Ang II) (19–23). Physical factors such as shear stress and flow stimulate TRPM7 activity implicating TRPM7 as a mechanotransducer in vascular cells (24, 25). TRPM7 plays an important role in modulating vascular smooth muscle cell (VSMC) Mg2+ homeostasis (1, 19–22, 26), a major determinant of VSMC function and vascular tone. Experimental evidence indicates that Mg2+ deficiency causes endothelial dysfunction, arterial remodeling and hypertension and that normalization of Mg2+ improves vascular function and reduces blood pressure (BP) (27).
Mechanisms regulating vascular Mg2+ in health and disease remain unclear but TRPM7 could be important. Aberrant TRPM7 expression/activity may contribute to impaired intracellular free Mg2+ concentration ([Mg2+]i) and VSMC contraction, proliferation, inflammation, and fibrosis, important determinants of vascular dysfunction and remodelling in hypertension (28). In support of this 1) pro-hypertensive factors, such as Ang II and oxidative stress, regulate TRPM7 expression/activity (22, 29), 2) vascular TRPM7 is dysfunctional in spontaneously hypertensive rats (30), 3) renal TRPM7 is downregulated in aldosterone-infused mice (31), 4) pulsatile atheroprone shear stress stimulates endothelial TRPM7 expression (32), 5) TRPM7 is upregulated after renal ischemia (33), and 6) TRPM7 is implicated in vascular remodeling and inflammation induced by pressure overload (34). Moreover clinical studies showed decreased expression of TRP channels in cerebral vascular tissue from patients after hypertensive intracerebral hemorrhage (35).
The critical importance of TRPM7 is emerging from studies in which TRPM7 gene has been deleted. In cells from TRPM7-knockout mice and in cells in which siRNA is targeted to TRPM7, growth is abnormal, proliferation is arrested and cells undergo apoptosis and death (11, 12). In embryonic cardiomyocytes in which TRPM7 is disrupted, spontaneous Ca2+ transient firing is reduced with associated downregulation of voltage-dependent Ca2+ channel (36). At the whole animal level, homozygous TRPM7 kinase (Δ kinase) knockout is embryonic lethal, indicating the critical role of TRPM7 kinase for normal development (11,12,37). Heterozygous TRPM7kinase mice are viable, but exhibit signs of hypomagnesaemia.
Considering the importance of TRPM7 in the cardiovascular system, we hypothesised that TRPM7 disruption plays a role in the pathophysiology of hypertension. To address this we induced hypertension by Ang II infusion in mice deficient in TRPM7 kinase (TRPM7Δkinase) and investigated BP responses and effects on cardiac, renal, and vascular function. In addition, we explored putative molecular mechanisms underlying TRPM7kinase-dependent cardiovascular effects.
Methods
Please see supplemental text for detailed methods
Animals
Male mice, 3 months of age, mixed background (C57B6 and SV129), were studied including: wildtype (TRPM7+/+) mice and mice heterozygous for the deletion of the TRPM7kinase domain (TRPM7Δkinase) (12). Homozygous mice were not studied because of embryonic lethality (12). Mice were infused with Ang II (osmotic minipumps, 4 weeks). Systolic BP was measured by plethysmography.
Echocardiography
Transthoracic echocardiography was performed at the end of the study (Vevo 2100 imaging system with 40-MHz transducer).
Vascular function
Vascular function of small mesenteric resistance arteries was studied by wire myography (38,39). Vessel contractility was assessed by concentration-response curves to norepinephrine (NE). Endothelium-dependent and -independent relaxation was assessed by measuring dilatory responses to acetylcholine (ACh) and diethylamine NONOate (DEA-NO) respectively in pre-contracted vessels.
Vascular structure and mechanics
Vascular structure and mechanics were studied by pressure myography (38).
Western Blotting
Total protein and membrane-enriched fractions were analyzed by immunoblotting.
Reactive Oxygen Species (ROS) Assessment
NADPHoxidase-derived O2•− was assessed by lucigenin-enhanced chemiluminescence, hydrogen peroxide (H2O2) by Amplex Red, and nitric oxide (NO) by the Nitrate/Nitrite Colorimetric Assay Kit.
Statistics
Data are presented as means±SEM. Groups were compared using one-way ANOVA with Tukey’s correction to compensate for multiple testing. p<0.05 was significant.
Results
Reduced cardiac and vascular TRPM7kinase expression in TRPM7Δkinase and Ang II-infused mice
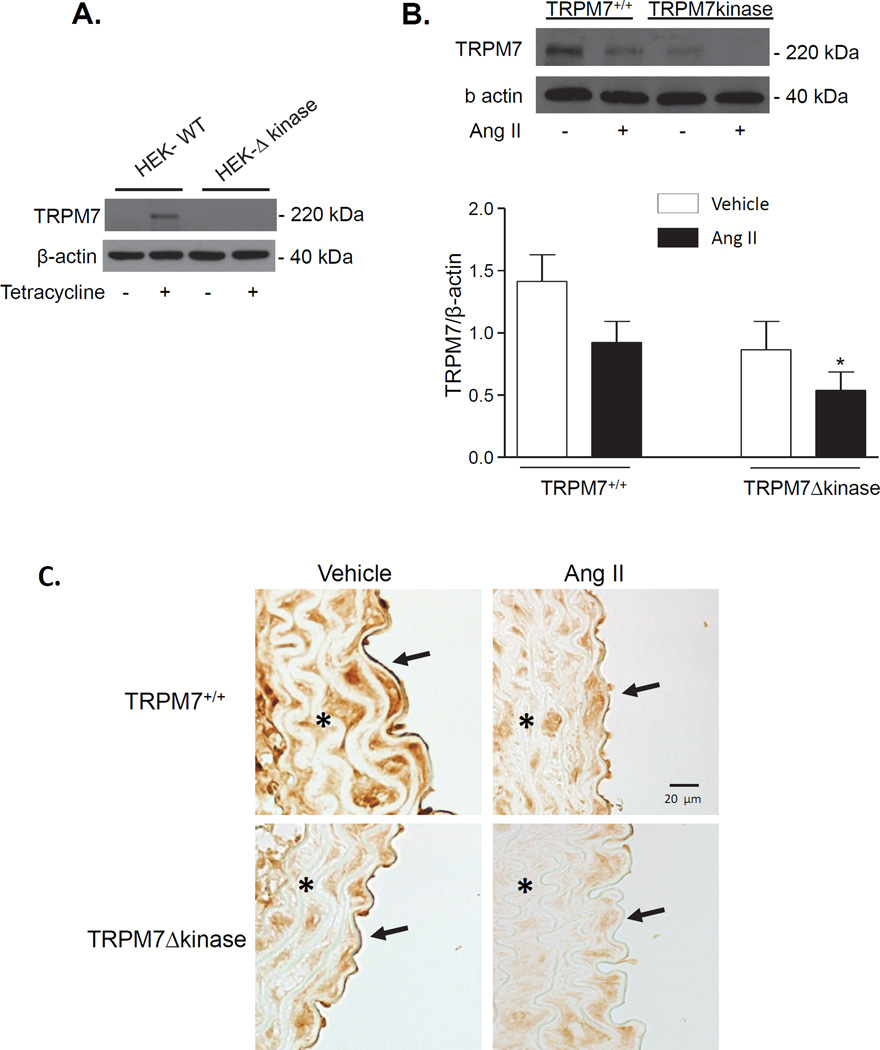
To analyze TRPM7kinase protein expression in mice, we first sought to investigate the specificity of the TRPM7kinase antibody. HEK cells overexpressing full length TRPM7 under the control of tetracycline (tet) and HEK cells deficient in the kinase domain (Δkinase) were used as we described, (23). Tet-on HEK cells with full length TRPM7 showed significant TRPM7 expression, while in Tet-on Δkinase cells, protein was undetectable (Fig. 1A). These findings confirmed the specificity of the antibody for TRPM7kinase. Using this antibody, we probed for TRPM7kinase in cardiac and vascular tissue in our experimental groups. TRPM7kinase expression was reduced ≈ 50% in cardiac and vascular tissue in TRPM7Δkinase mice compared to TRPM7+/+ mice, which reflects the heterozygosity of the TRPM7Δkinase mice (Figs 1B, 1C). Ang II reduced TRPM7kinase expression in heart and aorta in both strains.
Figure 1. TRPM7kinase expression in heart and aorta of TRPM7+/+ and TRPM7Δkinase mice.
A. Validation of TRPM7kinase antibody (Epitomics; # 3828-1). TRPM7 kinase expression in HEK cells expressing TRPM7 full length protein (WT) and TRPM7 lacking the kinase domain (Δ kinase) under the control of tetracycline. Cells deficient in TRPM7kinase failed to express the protein. B. Hearts were isolated from TRPM7+/+ and TRPM7Δkinase mice infused with vehicle or Ang II. Protein expression of TRPM7kinase was analyzed as a ratio to β-actin. Upper panels, representative immunoblots of TRPM7 and β-actin. Bar graphs represent mean ±SEM of 9–11 mice per group. *p<0.05 vs vehicle-infused TRPM7+/+. C. TRPM7kinase expression was analyzed in aortas from vehicle and Ang II-infused mice. Panels are representative photomicrographs of paraffin-embedded aortic sections processed for immunohistochemistry of TRPM7kinase, with original magnification of ×100. Data were obtained from 5 mice/group. Stars (*) and arrows (↑) indicate the presence of TRPM7kinase in vascular media and endothelium respectively.
Immunohistochemical analysis demonstrated that in aorta, TRPM7kinase is expressed in both the vascular media (vascular smooth muscle cells (VSMC)) and in the endothelium (endothelial cells (EC)) (Fig. 1C). In Ang II-infused mice, TRPM7kinase expression in VSMC and ECs was reduced in WT and TRPM7kinase-deficient mice.
Morphological characteristics and biochemical analysis
Body weight was similar between groups (Table 1). Cardiac mass was increased in Ang II-infused TRPM7+/+ and TRPM7Δkinase mice versus controls. Ang II-induced cardiac hypertrophy was significantly greater in TRPM7Δkinase mice versus Ang II-infused TRPM7+/+. Renal mass was similar between vehicle-infused groups, and no kidney hypertrophy was observed in Ang II-infused group.
Table 1.
Body weight, kidney and heart mass of vehicle and Ang II-infused TRPM7+/+ and TRPM7Δkinase mice.
| TRPM7+/+ vehicle |
TRPM7+/+ Ang II |
TRPM7Δkinase vehicle |
TRPM7Δkinase Ang II |
|
|---|---|---|---|---|
| Morphological Parameter |
||||
| BW (g) | 24.8 ± 0.7 | 25.5 ± 1.0 | 23.4 ± 0.7 | 24.2 ± 1.0 |
| HM (mg)/BW(g) | 4.4 ± 0.1 | 5.4 ± 0.1* | 4.5 ± 0.1 | 5.8 ± 0.1*† |
| KM (mg)/BW(g) | 6.1 ± 0.2 | 5.8 ± 0.1 | 6.2 ± 0.1 | 6.2 ± 0.1 |
TRPM7+/+ and TRPM7Δkinase mice were infused with vehicle or Ang II (400 ng/Kg/min, 4 weeks). Results are mean ± SEM. n=6–16 mice per group. Body weight (BW), heart mass (HM), kidney mass (KM).
p<0.05 versus vehicle counterparts;
p<0.05 versus Ang II-infused TRPM7+/+.
Biochemical analysis is summarized in Table 2. In vehicle-infused mice, plasma Mg2+ levels were lower in TRPM7Δkinase than WT mice. Ang II increased plasma Mg2+ in TRPM+/+, but not in TRPM7Δkinase mice. Plasma urea levels and urinary albumin-creatinine ratio were increased in Ang II-infused mice, reflecting renal dysfunction. Ang II reduced plasma albumin, calcium and chloride levels only in TRPM7Δkinase mice. Urine nitrate/nitrite, marker of systemic NO production, was reduced in Ang II-infused groups versus control counterparts.
Table 2.
Plasma and urine profile in vehicle- and Ang II-infused TRPM7+/+ and TRPM7Δkinase mice.
| TRPM7+/+ vehicle |
TRPM7+/+ Ang II |
TRPM7Δkinase vehicle |
TRPM7Δkinase Ang II |
|
|---|---|---|---|---|
| Plasma | ||||
| Total protein | 44.1 ± 0.7 | 41.4 ± 0.9 * | 43.2 ± 0.9 | 39.8 ±1.2 * |
| Albumin | 6.0 ± 0.4 | 5.2 ± 0.5 | 6.2 ± 0.7 | 4.4 ± 0.3 * |
| Cholesterol | 2.3 ± 0.07 | 2.4 ± 0.09 | 2.2 ± 0.06 | 2.2 ± 0.07 |
| Triglycerides | 1.5 ± 0.1 | 1.2 ± 0.1 | 1.3 ± 0.1 | 1.9 ± 0.4 |
| Glucose | 11.2 ± 0.5 | 11.2 ± 0.4 | 10.5 ± 0.5 | 10.5 ± 0.6 |
| Urea | 7.9 ± 0.2 | 11.2 ± 0.7 * | 7.9 ± 0.4 | 10.6 ± 0.5 * |
| Creatinine | 14.9 ± 0.9 | 14.0 ± 0.8 | 13.0 ± 1.3 | 12.3 ± 1.0 |
| Calcium | 1.51 ± 0.04 | 1.55 ± 0.06 | 1.46 ± 0.06 | 1.28 ± 0.08 * |
| Chloride | 96.8 ± 1.2 | 92.8 ± 1.9 | 95.0 ± 1.2 | 88.7 ± 2.4 * |
| Phosphorus | 1.2 ± 0.04 | 1.2 ± 0.07 | 1.1 ± 0.07 | 1.2 ± 0.06 |
| Bicarbonate | 19.1 ± 0.4 | 26.5 ± 0.5 | 20.0 ± 1.1 | 20.9 ± 1.7 |
| Magnesium | 0.65 ± 0.02 | 0.74 ± 0.04* | 0.60 ± 0.01‡ | 0.64 ± 0.02† |
| Urine | ||||
|
Albumin:creatinine ratio (µg/mg) |
25 ± 6 | 394 ± 99* | 47 ± 11 | 449 ± 118* |
| Nitric oxide (µM) | 3.7 ± 0.2 | 3.0 ± 0.2* | 3.9 ± 0.2 | 3.2 ± 0.1* |
TRPM7+/+ and TRPM7Δkinase mice were infused with vehicle or Ang II (400 ng/Kg/min, 4 weeks). Results are mean ± SEM. n=6–16 mice per group.
p<0.05 versus vehicle-treated counterparts.
p<0.05 versus Ang II-infused TRPM7+/+.
p<0.05 versus vehicle-treated TRPM7+/+
Echocardiography
Echocardiography was performed to determine left ventricle (LV) dimensions and function (Table 3). No differences in echocardiographic measurements were observed between vehicle infused-groups. LV mass and intraventricular septum (IVS) thickness in diastole (d) were increased in Ang II-infused TRPM7+/+ mice versus vehicle-infused counterparts. In TRPM7Δkinase mice EF and FS were significantly decreased upon Ang II infusion compared to vehicle-infused TRPM7+/+. LV mass, LVIDd, LVID in systole (s) and LVPWd were increased in TRPM7Δkinase mice versus vehicle-infused TRPM7+/+. In Ang II-infused TRPM7Δkinase mice there was a more pronounced decrease in EF, increase in LV mass and LVIDd than in Ang II-infused controls.
Table 3.
Echocardiographic measurements from M-mode images of vehicle- and Ang II-infused TRPM7+/+ and TRPM7Δkinase mice.
| TRPM7+/+ vehicle |
TRPM7+/+ Ang II |
TRPM7Δkinase vehicle |
TRPM7Δkinase Ang II |
|
|---|---|---|---|---|
| Echocardiographic Parameter |
||||
| EF (%) | 49.9 ± 5.5 | 43.6 ± 7.2 | 42.8 ± 3.1 | 22.9 ± 3.7 *† |
| FS (%) | 25.1 ± 3.3 | 19.8 ± 4.3 | 21.6 ± 1.7 | 10.5 ± 1.9 * |
| LV mass (mg) | 89.9 ± 3.4 | 127.1 ± 10.2 * | 105.7 ± 5.9 | 160.9 ± 8.3 *† |
| IVS; d (mm) | 0.77 ± 0.06 | 1.03 ± 0.03 * | 0.89 ± 0.04 | 0.94 ± 0.04 |
| LVID; d (mm) | 3.6 ± 0.07 | 3.6 ± 0.18 | 3.6 ± 0.10 | 4.13 ± 0.15 *† |
| LVID; s (mm) | 2.7 ± 0.1 | 3.1 ± 0.2 | 2.9 ± 0.1 | 3.7 ± 0.2 * |
| LVPW; d (mm) | 0.76 ± 0.04 | 0.9 ± 0.03 | 0.79 ± 0.05 | 0.96 ± 0.05 * |
|
Heart rate (bpm) |
501 ± 20 | 472 ± 15 | 455 ± 13 | 474 ± 23 |
TRPM7+/+ and TRPM7Δkinase mice were infused with vehicle or Ang II (400 ng/Kg/min., osmotic minipumps, 4 weeks). EF = Ejection fraction, FS = Fractional shortening, LV= Left ventricle, IVS = Interventricular septum, LVID = Left ventricle internal diameter, LVPW= Left ventricle posterior wall, d= in diastole, s= in systole, bpm= beats per minute. Results are mean ± SEM. n=5–10 mice per group.
p<0.05 vs vehicle-treated counterparts;
p<0.05 vs Ang II-infused TRPM7+/+.
Cardiac histopathology
Cardiac hypertrophy was further analyzed histologically using hematoxylin and eosin (Fig. S1A). Hematoxylin-eosin staining revealed that vehicle-infused TRPM7+/+ and TRPM7Δkinase mice had similar cardiac morphology with no gross abnormalities. Ang II promoted an increase in heart size in both groups, with a greater effect in Ang II-infused TRPM7Δkinase mice, especially in the left ventricle (Fig. S1B).
Cardiac collagen accumulation was assessed by Sirius Red staining (Fig. S2A and table 4) and interstitial fibrosis was assessed by Masson’s trichrome staining (Fig. S2B). Hearts from vehicle-infused TRPM7+/+ and TRPM7Δkinase mice did not exhibit collagen deposition nor fibrosis. In hearts from Ang II-infused mice there were foci of collagen deposition and interstitial fibrosis (Table 4). Perivascular collagen content and fibrosis were increased in Ang II-infused mice versus vehicle-infused controls (Fig. S2A, S2B).
Table 4.
Scores for collagen accumulation and fibrosis of vehicle- and Ang II-infused TRPM7+/+ and TRPM7Δkinase mice.
| Sirius Red |
Masson’s Trichrome |
|||||||
|---|---|---|---|---|---|---|---|---|
| − | + | ++ | +++ | − | + | ++ | +++ | |
| Experimental Group | ||||||||
| TRPM7+/+ Vehicle | 8 | 0 | 0 | 0 | 8 | 0 | 0 | 0 |
| TRPM7+/+ Ang II | 0 | 2 | 4 | 1 | 0 | 1 | 4 | 2 |
| TRPM7Δkinase Vehicle | 9 | 0 | 0 | 0 | 9 | 0 | 0 | 0 |
| TRPM7Δkinase Ang II | 0 | 2 | 3 | 2 | 0 | 1 | 5 | 2 |
Hearts were isolated from TRPM7+/+ and TRPM7Δkinase mice infused with vehicle or Ang II (400 ng/Kg/min., osmotic minipumps, 4 weeks). Paraffin-embedded of heart sections were processed for Sirius Red (collagen) and Masson’s trichrome (fibrosis). Staining for both collagen content and fibrosis were scored according to the following: – indicates intact; +, mild, ++, moderate, +++, severe. Values are numbers of mice displaying collagen deposition or fibrosis. n= 8–9 mice per group.
Aortic histopathology
Photomicrographs of Masson’s trichrome-stained cross-sections of thoracic aorta showed that vehicle-infused TRPM7+/+ and TRPM7Δkinase mice have similar media thickness. Ang II infusion led to a significant increase in the media thickness in both groups (Fig. S3A, S3B). The thickening of the aortic media is evidenced by the distance between each concentric elastic lamina intermingled with the smooth muscle cell layers. A pronounced thickening of the perivascular adventitial layer was also observed in Ang II-infused groups.
BP effects of Ang II in TRPM7Δkinase mice
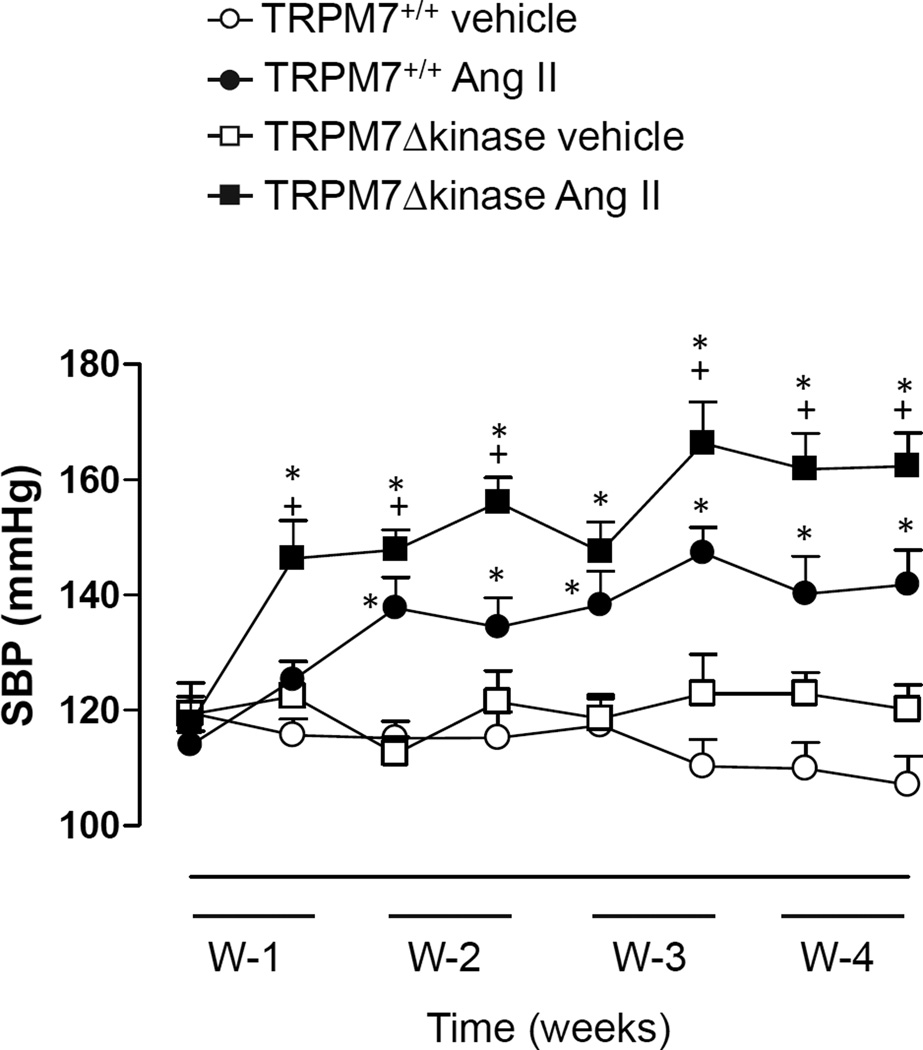
Figure 2 shows BP in TRPM7+/+ and TRPM7Δkinase mice infused with vehicle or Ang II for 4 weeks. No differences in basal BP were observed between vehicle-infused groups. In Ang II-infused TRPM7+/+ mice, BP increased gradually over 10 days at which time BP became significantly higher than vehicle-treated controls. BP remained elevated until the end of the experiment. In Ang II-infused TRPM7Δkinase mice, the onset of hypertension was rapid, occurring within 6 days of Ang II infusion. The magnitude of BP elevation by Ang II was significantly greater in TRPM7Δkinase mice versus vehicle-infused counterparts.
Figure 2. Systolic blood pressure (BP) in TRPM7+/+ and TRPM7+/− mice infused with Ang II.
TRPM7+/+ and TRPM7+/− mice were infused with vehicle or Ang II (400 ng/Kg/min., osmotic minipumps; 4 weeks). BP was measured weekly for 4 weeks (W) by tail-cuff plethysmography. Line graphs represent mean ± SEM of 10–19 mice per group. *p<0.05 vs vehicle-infused counterparts: +p<0.05 vs Ang II-infused TRPM7+/+.
Effect of Ang II on vascular function in TRPM7Δkinase mice
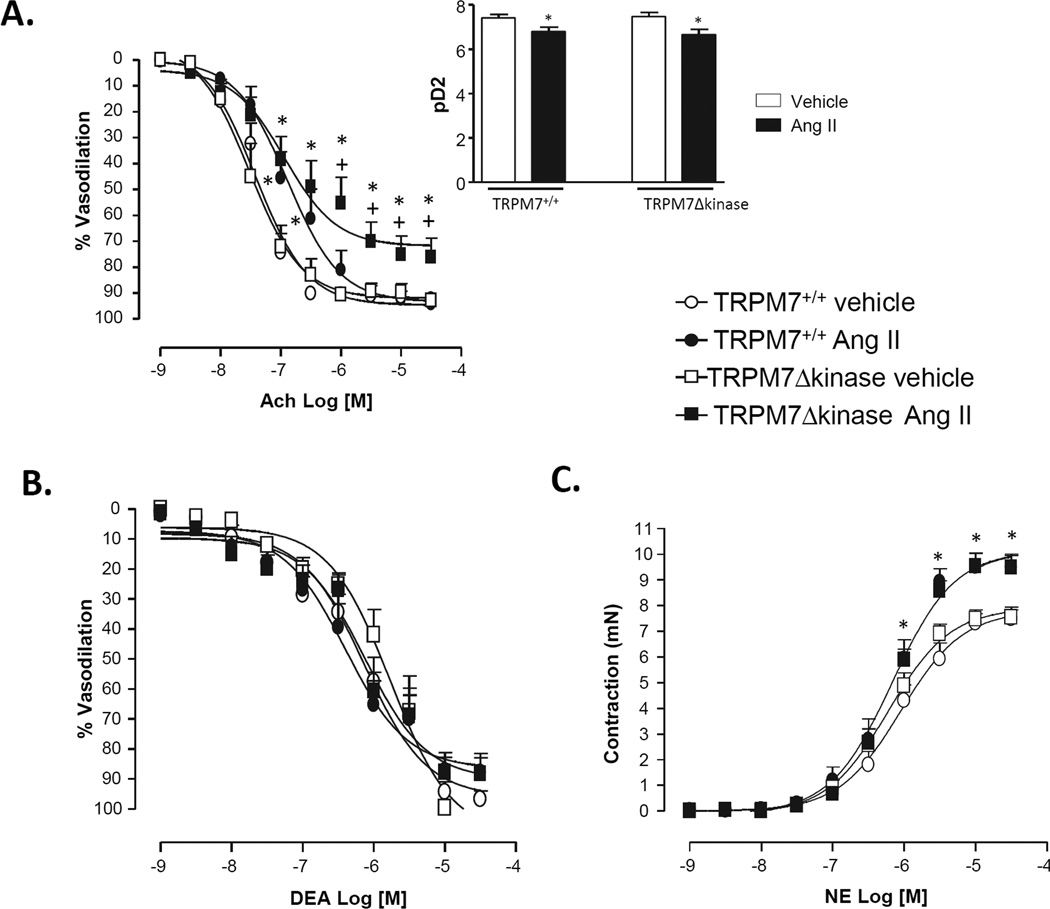
As shown in figure 3A, maximal Ach-induced relaxation was significantly reduced in mesenteric resistance arteries from Ang II-infused TRPM7Δkinase versus vehicle-infused TRPM7+/+ mice. Decreased sensitivity to Ach (pD2) was observed in arteries from both Ang II-infused groups (Inset Fig. 3A). This impairment was specific for endothelium-dependent relaxation since arteries from Ang II-infused TRPM7+/+ and TRPM7Δkinase mice showed similar relaxation to DEA-NO (NO donor) (Fig. 3B).
Figure 3. Functional responses in mesenteric arteries from Ang II-infused TRPM7+/+ and TRPM7+/− mice.
Mesenteric arteries were isolated from TRPM7+/+ and TRPM7+/− mice infused with vehicle or Ang II. Concentration-response curves to cumulative concentrations of acetylcholine (Ach, 10−9- 3 × 10−5 M) (A), diethylamine NONOate (DEA-NO, 10−9- 3 × 10−5 M) (B), and norepinephrine (NE, 10−9- 3 × 10−5 M) (C) were analyzed in vessels mounted on wire myographs. Line graphs represent mean ± SEM of 7- 17 mice per group. Inset (A) shows the sensitivity to Ach expressed by pD2 values. *p<0.05 vs vehicle-infused counterparts: +p<0.05 vs Ang II-infused TRPM7+/+.
Contractile responses to NE were similar in vehicle-infused TRPM7+/+ and TRPM7Δkinase mice (Fig. 3C). Ang II-infused mice displayed increased contraction to NE compared to vehicle-infused counterparts.
Ang II effects on vascular structure
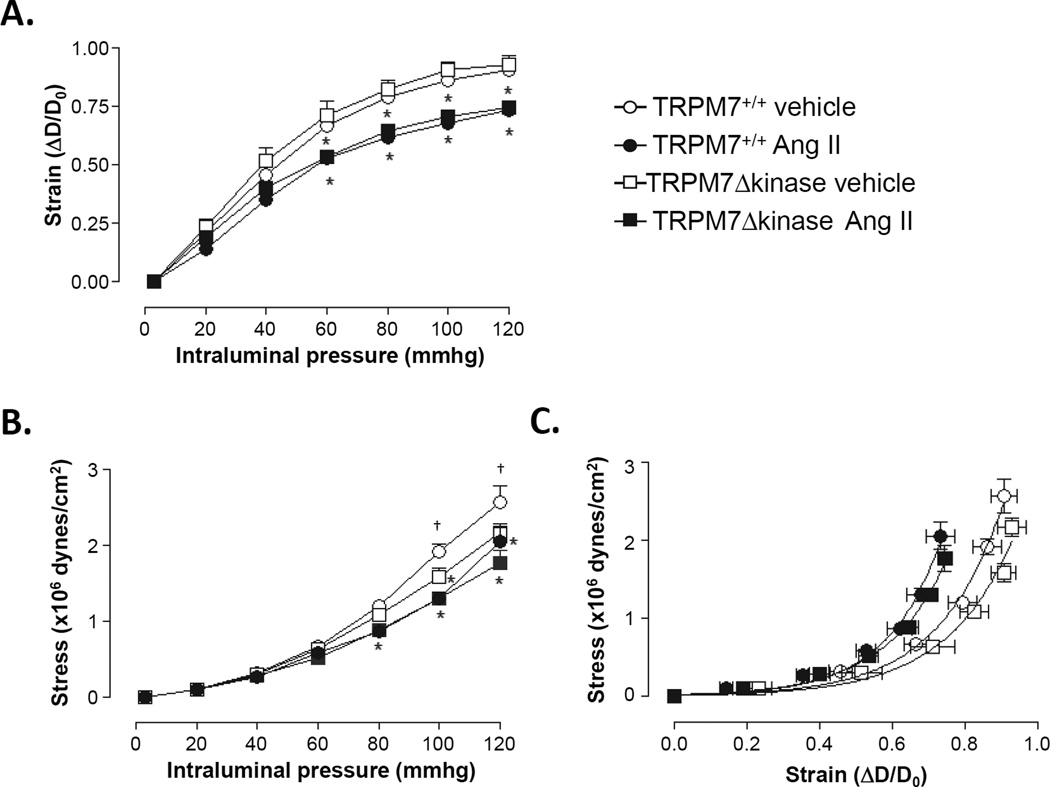
Pressurized arteries had comparable lumen diameter at 3 mmHg of intraluminal pressure in all four groups (Fig. S4A). With a progressive increase of intraluminal pressure, the lumen diameter increased to a lesser degree in Ang II-infused versus vehicle-infused TRPM7+/+ mice (Fig. 4A). Media cross-sectional area (Fig. S4B) and media to lumen ratio (Fig. S4C) were similar in all groups at intraluminal pressure of 3 mmHg. In response to stepwise increments of intraluminal pressure these parameters were not affected by Ang II.
Figure 4. Mechanical properties in mesenteric resistance arteries from Ang II-infused mice.
Mesenteric resistance arteries were isolated from TRPM7+/+ and TRPM7+/− mice infused with vehicle or Ang II. Strain (A), Stress (B), and Stress-strain relationship curves (C) were analyzed by pressure myography. Mesenteric arteries were incubated in 0 Ca2+−physiological salt solution containing 10 mmol/L EGTA and were subjected to increasing levels of intraluminal pressure. Line graphs represent mean ± SEM of 5 mice per group. *p<0.05 vs vehicle-infused counterparts; †p<0.05 vehicle-infused TRPM7+/−
Vascular mechanics
Mesenteric resistance arteries from all groups had comparable strain (Fig. 4A) at an intraluminal pressure of 3 mmHg. In arteries from vehicle-infused groups, TRPM7Δkinase mice showed decreased stress-pressure (Fig. 4B) in response to stepwise increments of intraluminal pressure compared to TRPM7+/+. In both Ang II treated groups, artery deformation and stress-pressure in response to stepwise increments of intraluminal pressure, were lower than in vehicle-infused TRPM7+/+ mice. Ang II evoked a shift to the left in the stress-strain curve to an equal extent in both groups, indicating that vessels from both of the Ang II-infused groups are stiffer than vehicle-infused counterparts (Fig. 4C).
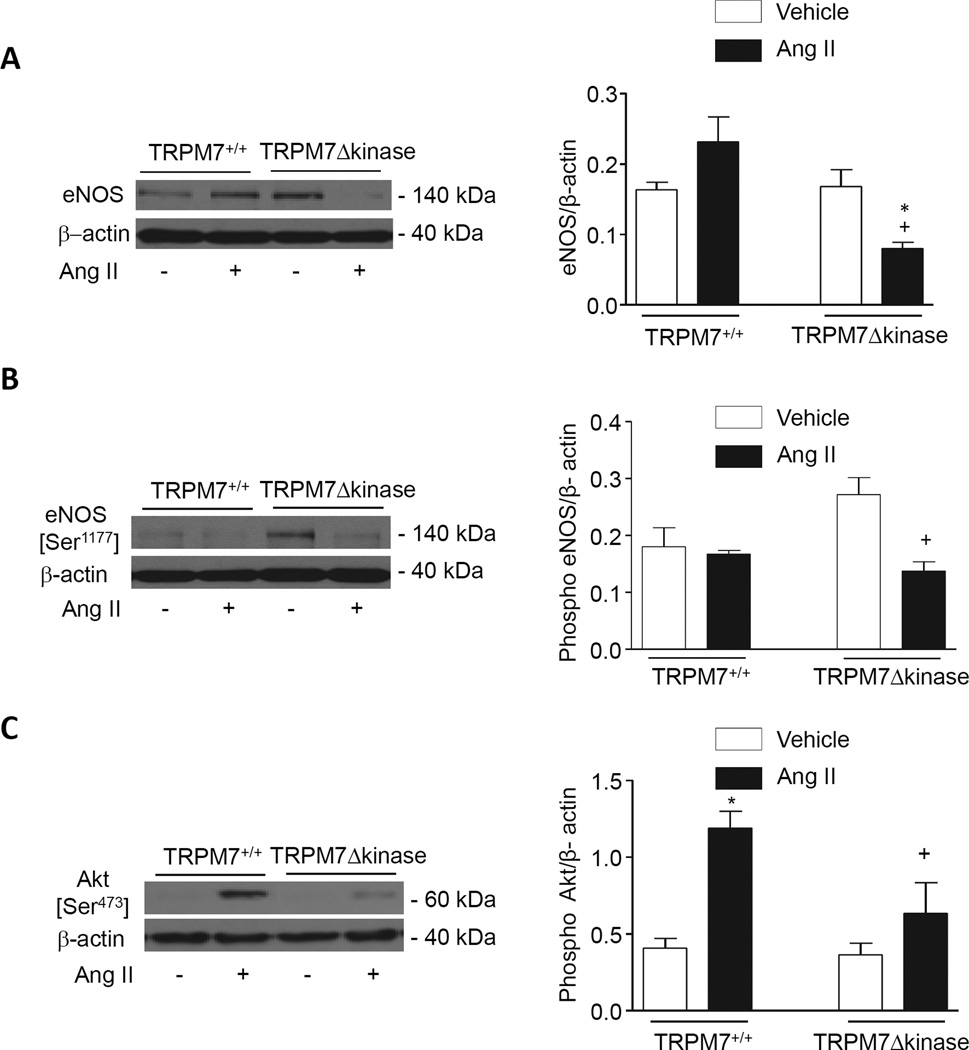
Activation of vascular eNOS, Akt and caveolin-1
To investigate putative molecular mechanism underlying endothelial dysfunction in Ang II-infused TRPM7Δkinase mice, we examined vascular expression and phosphorylation of eNOS and its upstream regulator Akt in Ang II-infused TRPM7+/+ and TRPM7Δkinase mice. As demonstrated in figure 5A basal eNOS was similar in TRPM7+/+ and TRPM7Δkinase mice. However, in Ang II-infused TRPM7kinase-deficient mice, eNOS levels were significantly reduced compared with vehicle- and Ang II-infused TRPM7+/+ mice. Phosphorylation of eNOS (serine 1177, activation site) was significantly decreased in Ang II-infused TRPM7Δkinase mice versus vehicle-treated mice (Fig. 5B). Akt was phosphorylated in Ang II-infused TRPM7+/+ but not in Ang II-infused TRPM7Δkinase mice (Fig. 5C). In membrane-enriched fractions, expression of eNOS was lower and that of caveolin-1 was higher in Ang II-infused TRPM7Δkinase mice versus control counterparts (Figs. S5A, S5B).
Figure 5. Ang II effects on expression and phosphorylation of eNOS and Akt in aorta from TRPM7+/+ and TRPM7+/− mice.
Representative immunoblots and bar graphs of eNOS content (A), eNOS (B) and Akt (C) phosphorylation in vessels from vehicle- and Ang II-infused TRPM7+/+ and TRPM7+/− mice. Levels of total eNOS, phospho eNOS and phospho Akt are expressed relative to expression of β-actin. Results are presented as mean ±SEM of 8–9 mice per group. *p<0.05 vs vehicle-treated groups; + p<0.05 vs Ang II-infused TRPM7+/+ mice.
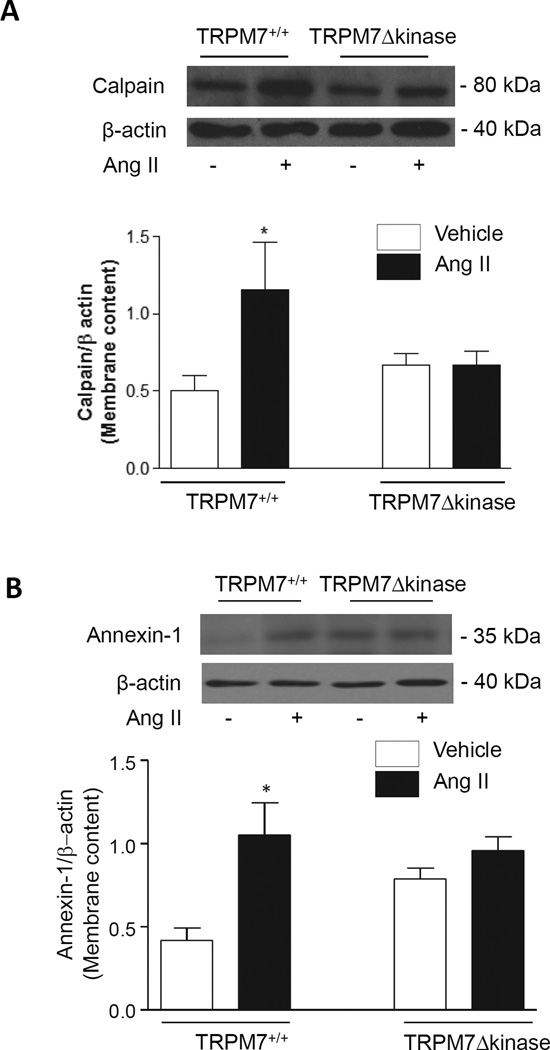
Ang II effects on TRPM7 targets, mitogen-activated protein (MAP) kinases and pro-inflammatory mediators in arteries from TRPM7+/+ and TRPM7Δkinase mice
Upon activation, calpain and annexin-1, TRPM7 kinase downstream targets, translocate to the plasma membrane. The content of calpain and annexin in membrane-enriched fractions was evaluated as a functional index of TRPM7. As shown in figure 6 Ang II increased calpain and annexin-1 membrane content in arteries from TRPM7+/+ mice, but failed to elicit any response in TRPM7Δkinase mice.
Figure 6. Expression of TRPM7 kinase targets calpain and annexin-1 in membrane-enriched fractions of mesenteric arteries from Ang II-infused TRPM7+/+ and TRPM7Δkinase mice.
Mesenteric arteries were isolated from TRPM7+/+ and TRPM7Δkinase mice infused with vehicle or Ang II. Protein content of calpain (A) and annexin-1 (B) was measured in membrane-enriched fractions. Data are presented as the expression of calpain or annexin-1 relative to the expression of β-actin. Upper panels, representative immunoblots of calpain, annexin-1 and α actin. Results are presented as mean ±SEM of 6–9 mice per group. *p<0.05 vs vehicle-infused TRPM7+/+.
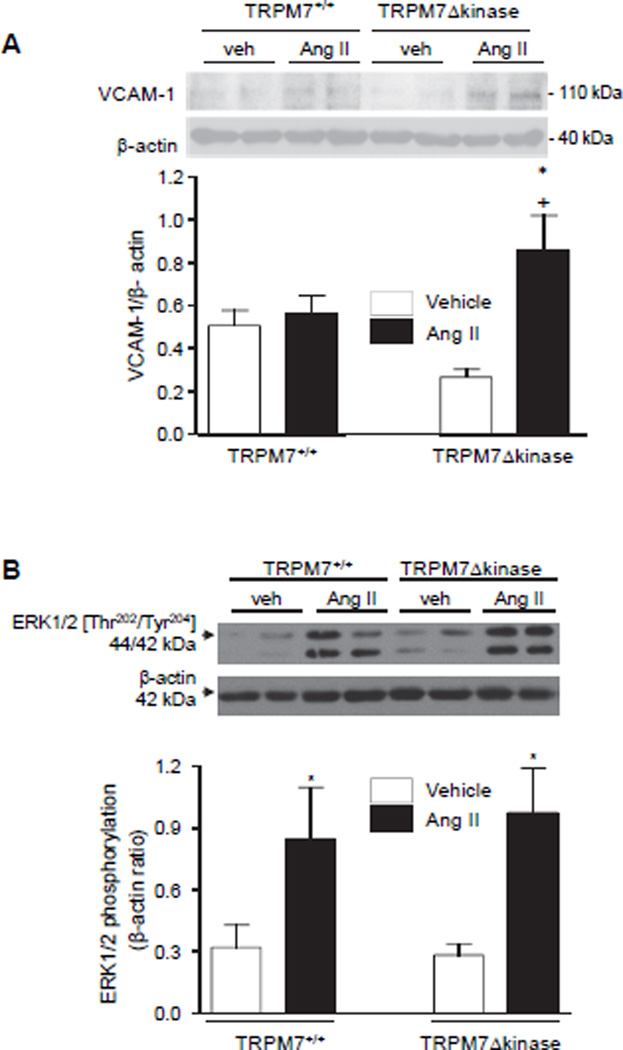
Expression of the VCAM-1, an adhesion molecule induced by inflammatory mediators, was significantly increased in aorta from TRPM7+/− mice compared to other groups (Fig. 7A).
Figure 7. Expression of VCAM-1 and ERK1/2 in vessels from Ang II-infused TRPM7+/+ and TRPM7Δkinase mice.
Aortas were isolated from TRPM7+/+ and TRPM7Δkinase mice infused with vehicle or Ang II. (A) Upper panels, representative immunoblots of VCAM-1 and β-actin. Data are presented as the expression of VCAM-1 relative to the expression of β-actin. (B) Phosphorylation levels of ERK1/2 are presented relative to expression of β-actin. Upper panels, representative immunoblots of phospho-ERK1/2 and β-actin. Results are presented as mean ±SEM of 5–9 mice per group. *p<0.05 vs vehicle infused counterparts; +p<0.05 vs Ang II-infused TRPM7+/+.
At the molecular level, MAP kinases play a key role in vascular regulation by Ang II. We evaluated activation of vascular ERK1/2 and JNK in Ang II-infused TRPM7+/+ and TRPM7+/− mice. As shown in figure 7B and figure S6 Ang II significantly increased phosphorylation of ERK1/2 and JNK in arteries from TRPM7+/+ mice. Similar responses were observed in TRPM7Δkinase suggesting that TRPM7 kinase is not critically involved in MAP kinase activation by Ang II.
Ang II effects on systemic and vascular ROS in TRPM7+/+ and TRPM7Δkinase mice
The role of ROS generation by Ang II was analyzed in TRPM7+/+ and TRPM7Δkinase mice. Plasma H2O2 concentration was similar between vehicle-infused TRPM7+/+ and TRPM7Δkinase mice. Ang II had no significant effect on plasma H2O2 in either group (Figs. S7A, S7B). NADPH-dependent superoxide anion generation was measured in aortic homogenates by lucigenin-enhanced chemiluminescence and showed similar responses in TRPM7+/+ and TRPM7Δkinase mice (Fig. S7C). Translocation of the Nox cytosolic subunit p47phox from the cytosol to membrane was evaluated as an index of oxidase activation. Expression of p47phox in membrane-enriched fractions was similar between groups (Fig. S7D).
Discussion
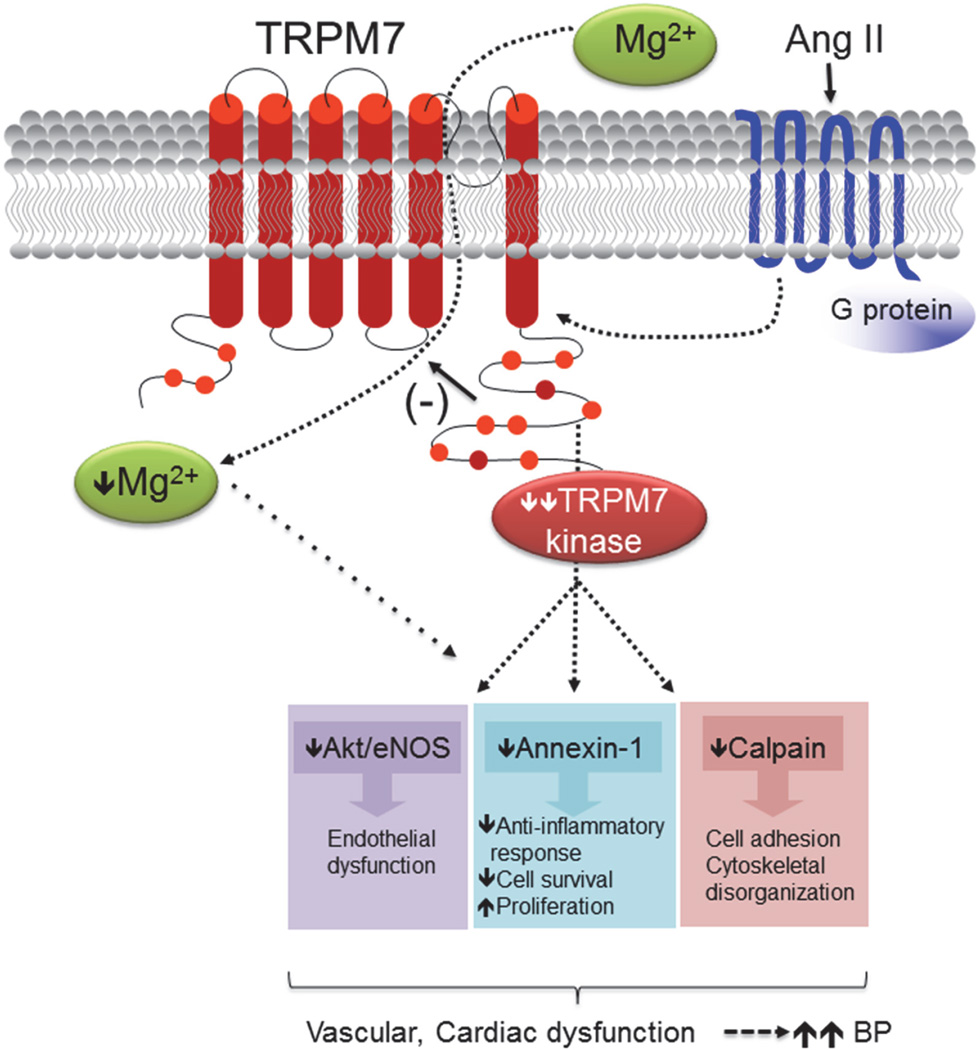
This study demonstrates a distinct cardiovascular phenotype in Ang II-infused TRPM7Δkinase mice characterised by severe hypertension, exaggerated cardiac hypertrophy, worsening of left ventricular dysfunction and persistent hypomagnesemia compared with wildtype counterparts. Associated with these changes is impaired endothelial function, downregulation of eNOS and blunted signaling through TRPM7kinase, without effect on MAP kinases or redox status. Our findings identify TRPM7kinase as a novel player in the regulation of BP and cardiovascular function and indicate that TRPM7kinase perturbations may be important in the pathophysiology of Ang II-induced hypertension and associated vascular and target organ damage (Fig. 8). Cardiac and vascular expression of TRPM7kinase was significantly downregulated by Ang II in WT and TRPM7Δkinase mice indicating an important negative interaction between these systems.
Figure 8. Possible molecular mechanisms whereby TRPM7 kinase deficiency amplifies Ang II cardiovascular and blood pressure (BP) effects.
Blunted TRPM7 kinase signaling by Ang II through its G protein-coupled receptor is associated with reduced activation of TRPM7 kinase downstream targets, including Akt, calpain and annexin-1, leading to vascular and cardiac dysfunction and amplified increase in BP. These processes may also be influenced by reduced intracellular Mg2+, possibly due to decreased transmembrane Mg2+ transport as a consequence of TRPM7 kinase downregulation (as we previously demonstrated (1)). (−) denotes inhibitory effect. Dashed lines are possible pathways.
Phenotypically TRPM7Δkinase mice in basal conditions were similar to wildtype controls, except for significant hypomagnesemia, likely due to reduced intestinal Mg2+ absorption and increased renal Mg2+ wasting, since TRPM7 and its homologue TRPM6 are major Mg2+ transporters in epithelial cells in the gastrointestinal tract and kidneys as we reported (1–3, 12, 13). Similar to other knockout models used to interrogate the pathophysiology of hypertension, a pathological phenotype was only unmasked when TRPM7Δkinase mice were challenged with Ang II. In mice deficient in TRPM7kinase, Ang II-induced hypertension was exaggerated and target organ damage was amplified. These phenomena were associated with pronounced endothelial dysfunction and blunted TRPM7kinase signaling through Akt, calpain and annexin-1. The relationship between reduced TRPM7 expression/activity, hypomagnesemia and BP has previously been suggested in experimental models of hypertension (16, 27, 40–42). Here we advance this concept and suggest that TRPM7kinase fine-tunes Ang II actions, because when it is downregulated, Ang II effects were augmented. This is supported by our previous findings in another mouse model with hereditary hypomagnesemia and associated downregulation of TRPM7 (42). While exact molecular mechanisms underlying the regulatory role of TRPM7 are still unclear, dampened TRPM7 signaling, as demonstrated here, may be important. However, we cannot exclude the possibility that some of the observed effects in our study may also relate to altered Mg2+ homeostasis and hypomagnesemia, especially since our previous studies demonstrated that alterations in TRPM7Δkinase mice were ameliorated by Mg2+ supplementation (12).
TRPM7 plays an important role in the regulation and function of endothelial cells as demonstrated in human umbilical vein endothelial cells, where TRPM7 deficiency leads to altered growth and migration (43). In our in vivo study here, endothelial function was not significantly altered by Ang II in wildtype mice but was markedly impaired in Ang II-infused TRPM7Δkinase mice as evidenced by reduced endothelium-dependent vasodilation. Reasons why Ang II did not influence endothelial function, or ROS production, in wildtype mice, as expected, are unclear but may relate, in part, to the fact that the mice studied here were of mixed genetic background, where responses to vasoactive agents are variable.
Decreased acetylcholine-induced vasorelaxation in Ang II-infused TRPM7Δkinase mice was associated with decreased eNOS phosphorylation and Akt activation and increased membrane-associated caveolin-1. Since eNOS is negatively modulated by caveolin-1, upregulation of caveolin-1 may reduce eNOS bioactivity, as previously reported (44,45). In addition blunted effects of Ang II on Akt, an upstream regulator of eNOS, may play a role in reduced eNOS activation in TRPM7Δkinase mice. These molecular processes likely contribute to dampened eNOS phosphorylation and NO generation with consequent endothelial dysfunction and reduced vasorelaxation, important determinants of BP elevation. It is also possible that hypomagnesemia in TRPM7Δkinase mice may influence endothelial function, since decreased [Mg2+]i enhances agonist-stimulated vasoconstriction and impairs vasodilation, while increased [Mg2+]i is associated with vasorelaxation. These effects may occur through Mg2+ interactions with Ca2+, or through eNOS modulation by Mg2+ (46).
One of the earliest indicators of endothelial inflammation is expression of adhesion molecules, such as VCAM-1, which promotes leukocyte adhesion to the endothelium and migration of macrophages through the vascular media. Increased VCAM expression has been demonstrated in atherosclerosis and hypertension and is closely associated with vascular inflammation through processes that involve activation of MAP kinases and oxidative stress. In TRPM7kinase-deficient mice, Ang II induced a significant increase in VCAM-1 expression. Molecular mechanisms underlying the inflammatory response are unclear, but may relate to decreased activation of TRPM7kinase targets, annexin-1 and calpain, which have anti-inflammatory and pro-migratory functions respectively (3, 47). Reduced annexin-1 expression and increased inflammation have been demonstrated in aortas from hypomagnesemic mice (40). We recently demonstrated that the TRPM7kinase domain, but not the TRPM7 channel domain, regulates annexin-1 and calpain signalling (23). The inflammatory and endothelial responses observed in our study appear to be independent of changes in oxidative stress or redox-sensitive MAP kinase signaling, because TRPM7kinase-deficiency had little impact on Ang II-induced phosphorylation of ERK1/2 and JNK.
The hypertensive vascular phenotype is characterised not only by endothelial dysfunction, but also by vascular remodeling (28). Previous studies demonstrated a role for TRPM7 and Mg2+ in vascular hypertrophy in hypertension (28,41). Here we show that in Ang II-treated mice, lumen diameter was reduced and the stress-strain relationship had a leftward shift compared with controls. Effects were similar in TRPM7+/+ and TRPM7Δkinase mice indicating that remodeling and altered mechanics (increased stiffness) induced by Ang II are independent of TRPM7 kinase. These findings are in contrast to those for endothelial dysfunction, which had a significant TRPM7 component, suggesting that TRPM7 kinase may be more important in endothelial than vascular smooth muscle cell regulation, at least in 3 month-old mice. Whether vascular structural changes become more apparent with aging in TRPM7Δkinase mice awaits confirmation.
TRPM7 has also been implicated in cardiac regulation and growth, since disturbed TRPM7 activity leads to abnormal cardiogenesis and impaired ventricular function (36, 37). In rat cardiomyocytes increased TRPM7 activity was involved in Ang II-induced cardiac fibrosis (48). In mouse heart and aorta, upregulation of TRPM7 channels by Ang II was associated with a proliferative phenotype of ascending aortic smooth muscle cells, an effect that was mediated through TRPM7 suppression of Pyk2-ERK1/2-Elk-1 signaling (49). Moreover cardiac arrhythmias have been linked to both up- and down-regulation of TRPM7 (35, 36). In our study, decreased TRPM7kinase activity was associated with exaggerated cardiac hypertrophy and pronounced left ventricular dysfunction in Ang II-infused mice. While this might be an adaptive response secondary to severe hypertension, it is also possible that decreased myocardial TRPM7kinase activity itself may contribute to structural and functional cardiac changes.
In conclusion, we show that in heterozygous TRPM7Δkinase mice Ang II induces an exaggerated hypertensive response, pronounced cardiac hypertrophy, and worsening of left ventricular function. Associated with these changes is endothelial dysfunction and inflammation, processes linked to blunted TRPM7kinase signaling and eNOS downregulation. Vascular remodeling and vascular MAPK activation, typically associated with Ang II-induced hypertension, were not influenced by TRPM7kinase deficiency. These observations indicate that TRPM7kinase differentially regulates vascular function in hypertension, with a primary target being the endothelium. Our findings identify TRPM7kinase as a modulator of BP regulation, which when downregulated promotes severe hypertension and worsening of cardiovascular function. Moreover, we show that Ang II is a negative regulator of TRPM7. Taken together this study defines a novel TRPM7kinase-sensitive mechanism involved in Ang II-induced hypertension and target organ damage.
Perspectives
Alterations in electrolyte status and ion transporters have been implicated in the pathophysiology of hypertension. While there is extensive data on calcium, sodium and potassium in hypertension, there is a paucity of information about magnesium and its transporters. Here we identify TRPM7, a magnesium/cation channel with kinase activity, as a regulator of magnesium homeostasis and modulator of BP regulation. Altered activity of TRPM7, particularly the kinase domain, is associated with hypomagnesemia, endothelial dysfunction, vascular inflammation, cardiac dysfunction and exaggerated pressor responses to Ang II. Our findings identify TRPM7kinase and its downstream signaling pathways as a novel paradigm in Ang II-induced hypertension. Perturbed activation of TRPM7kinase may be an important mechanism linking hypomagnesemia and cardiovascular injury in hypertension,
Supplementary Material
Novelty and Significance.
What Is New?
TRPM7, a unique Mg2+ transporter with kinase activity, influences vascular function and blood pressure in Ang II-induced hypertension.
TRPM7 kinase deficiency leads to exaggerated hypertension with associated eNOS downregulation and amplified endothelial dysfunction.
TRPM7 kinase/eNOS is a novel system through which Ang II signals.
What Is Relevant?
Clinical hypertension is associated with perturbed Mg2+ homeostasis and vascular dysfunction, possibly through TRPM7 dysfunction.
Targeting TRPM7 kinase may have potential as a novel therapeutic target in hypertension.
Summary
TRPM7 kinase regulates vascular function in hypertension, with a primary target being the endothelium. TRPM7 kinase may have vasoprotective functions, which when dysregulated contributes to endothelial dysfunction, vascular inflammation and hypertension. We identify a novel TRPM7 kinase-sensitive mechanism in Ang II-induced hypertension.
Acknowledgments
Source of Funding
This study was funded by a grant from the Canadian Institute of Health Research (FRN57786). This work was supported by grants from PO1 GM078195 from the National Institutes of Health (AR). RMT was supported through a Canada Research Chair/Canadian Foundation for Innovation award and a British Heart Foundation Chair (CH/12/4/29762). Dr Alex Gutsol is thanked for expert histological assistance.
Footnotes
Disclosures: There are no disclosures to declare.
References
- 1.Bates-Withers C, Sah R, Clapham DE. TRPM7, the Mg(2+) inhibited channel and kinase. Adv Exp Med Biol. 2011;704:173–183. doi: 10.1007/978-94-007-0265-3_9. [DOI] [PubMed] [Google Scholar]
- 2.Zhang Z, Yu H, Huang J, Faouzi M, Schmitz C, Penner R, Fleig A. The TRPM6 kinase domain determines the Mg·ATP sensitivity of TRPM7/M6 heteromeric ion channels. J Biol Chem. 2014;289:5217–5227. doi: 10.1074/jbc.M113.512285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dorovkov MV, Kostyukova AS, Ryazanov AG. Phosphorylation of annexin A1 by TRPM7 kinase: a switch regulating the induction of an α-helix. Biochemistry. 2011;50:2187–2193. doi: 10.1021/bi101963h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Perraud AL, Zhao X, Ryazanov AG, Schmitz C. The channel-kinase TRPM7 regulates phosphorylation of the translational factor eEF2 via eEF2-k. Cell Signal. 2011;23:586–593. doi: 10.1016/j.cellsig.2010.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Su LT, Agapito MA, Li M, Simonson WT, Huttenlocher A, Habas R, Yue L, Runnels LW. TRPM7 regulates cell adhesion by controlling the calcium-dependent protease calpain. J Biol Chem. 2006;281:11260–11270. doi: 10.1074/jbc.M512885200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clark K, Middelbeek J, Lasonder E, Dulyaninova NG, Morrice NA, Ryazanov AG, Bresnick AR, Figdor CG, van Leeuwen FN. TRPM7 regulates myosin IIA filament stability and protein localization by heavy chain phosphorylation. J Mol Biol. 2008;378:790–803. doi: 10.1016/j.jmb.2008.02.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Desai BN, Krapivinsky G, Navarro B, Krapivinsky L, Carter BC, Febvay S, Delling M, Penumaka A, Ramsey IS, Manasian Y, Clapham DE. Cleavage of TRPM7 releases the kinase domain from the ion channel and regulates its participation in Fas-induced apoptosis. Dev Cell. 2012;22:1149–1162. doi: 10.1016/j.devcel.2012.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Numata T, Shimizu T, Okada Y. TRPM7 is a stretch- and swelling-activated cation channel involved in volume regulation in human epithelial cells. Am J Physiol Cell Physiol. 2007;292:C460–C467. doi: 10.1152/ajpcell.00367.2006. [DOI] [PubMed] [Google Scholar]
- 9.Chen HC, Xie J, Zhang Z, Su LT, Yue L, Runnels LW. Blockade of TRPM7 channel activity and cell death by inhibitors of 5-lipoxygenase. PLoS One. 2010;5:e11161. doi: 10.1371/journal.pone.0011161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schlingmann KP, Waldegger S, Konrad M, Chubanov V, Gudermann T. TRPM6 and TRPM7--Gatekeepers of human magnesium metabolism. Biochim Biophys Acta. 2007;1772:813–821. doi: 10.1016/j.bbadis.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 11.Li X, Wang X, Wang Y, Li X, Huang C, Li J. Inhibition of transient receptor potential melastatin 7 (TRPM7) channel induces RA FLSs apoptosis through endoplasmic reticulum (ER) stress. Clin Rheumatol. 2014;33:1565–1574. doi: 10.1007/s10067-014-2599-x. [DOI] [PubMed] [Google Scholar]
- 12.Ryazanova LV, Rondon LJ, Zierler S, Hu Z, Galli J, Yamaguchi TP, Mazur A, Fleig A, Ryazanov AG. TRPM7 is essential for Mg(2+) homeostasis in mammals. Nat Commun. 2010;1:109. doi: 10.1038/ncomms1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jin J, Wu LJ, Jun J, Cheng X, Xu H, Andrews NC, Clapham DE. The channel kinase, TRPM7, is required for early embryonic development. Proc Natl Acad Sci U S A. 2012;109:E225–E233. doi: 10.1073/pnas.1120033109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jin J, Desai BN, Navarro B, Donovan A, Andrews NC, Clapham DE. Deletion of Trpm7 disrupts embryonic development and thymopoiesis without altering Mg2+ homeostasis. Science. 2008;322:756–766. doi: 10.1126/science.1163493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fleig A, Chubanov V. Trpm7. Handb Exp Pharmacol. 2014;222:521–546. doi: 10.1007/978-3-642-54215-2_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yogi A, Callera GE, O'Connor SE, He Y, Correa JW, Tostes RC, Mazur A, Touyz RM. Dysregulation of renal transient receptor potential melastatin 6/7 but not paracellin-1 in aldosterone-induced hypertension and kidney damage in a model of hereditary hypomagnesemia. J Hypertens. 2011;29:1400–1410. doi: 10.1097/HJH.0b013e32834786d6. [DOI] [PubMed] [Google Scholar]
- 17.Menè P, Punzo G, Pirozzi N. TRP channels as therapeutic targets in kidney disease and hypertension. Curr Top Med Chem. 2013;13:386–397. doi: 10.2174/1568026611313030013. [DOI] [PubMed] [Google Scholar]
- 18.Park HS, Hong C, Kim BJ, So I. The Pathophysiologic Roles of TRPM7 Channel. Korean J Physiol Pharmacol. 2014;18:15–23. doi: 10.4196/kjpp.2014.18.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Callera GE, He Y, Yogi A, Montezano AC, Paravicini T, Yao G, Touyz RM. Regulation of the novel Mg2+ transporter transient receptor potential melastatin 7 (TRPM7) cation channel by bradykinin in vascular smooth muscle cells. J Hypertens. 2009;27:155–166. doi: 10.1097/hjh.0b013e3283190582. [DOI] [PubMed] [Google Scholar]
- 20.Yogi A, Callera GE, Tostes R, Touyz RM. Bradykinin regulates calpain and proinflammatory signaling through TRPM7-sensitive pathways in vascular smooth muscle cells. Am J Physiol Regul Integr Comp Physiol. 2009;296:R201–R207. doi: 10.1152/ajpregu.90602.2008. [DOI] [PubMed] [Google Scholar]
- 21.Touyz RM, He Y, Montezano AC, Yao G, Chubanov V, Gudermann T, Callera GE. Differential regulation of transient receptor potential melastatin 6 and 7 cation channels by ANG II in vascular smooth muscle cells from spontaneously hypertensive rats. Am J Physiol Regul Integr Comp Physiol. 2006;290:R73–R78. doi: 10.1152/ajpregu.00515.2005. [DOI] [PubMed] [Google Scholar]
- 22.He Y, Yao G, Savoia C, Touyz RM. Transient receptor potential melastatin 7 ion channels regulate magnesium homeostasis in vascular smooth muscle cells: role of angiotensin II. Circ Res. 2005;96:207–215. doi: 10.1161/01.RES.0000152967.88472.3e. [DOI] [PubMed] [Google Scholar]
- 23.Yogi A, Callera GE, O'Connor S, Antunes TT, Valinsky W, Miquel P, Montezano AC, Perraud AL, Schmitz C, Shrier A, Touyz RM. Aldosterone signaling through transient receptor potential melastatin 7 cation channel (TRPM7) and its α-kinase domain. Cell Signal. 2013;25:2163–2175. doi: 10.1016/j.cellsig.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oancea E, Wolfe JT, Clapham DE. Functional TRPM7 channels accumulate at the plasma membrane in response to fluid flow. Circ Res. 2006;98:245–253. doi: 10.1161/01.RES.0000200179.29375.cc. [DOI] [PubMed] [Google Scholar]
- 25.Numata T, Shimizu T, Okada Y. Direct mechano-stress sensitivity of TRPM7 channel. Cell Physiol Biochem. 2007;19:1–8. doi: 10.1159/000099187. [DOI] [PubMed] [Google Scholar]
- 26.Zholos A, Johnson C, Burdyga T, Melanaphy D. TRPM channels in the vasculature. Adv Exp Med Biol. 2011;704:707–729. doi: 10.1007/978-94-007-0265-3_37. [DOI] [PubMed] [Google Scholar]
- 27.Yogi A, Callera GE, Antunes TT, Tostes RC, Touyz RM. Transient receptor potential melastatin 7 (TRPM7) cation channels, magnesium and the vascular system in hypertension. Circ J. 2011;75:237–245. doi: 10.1253/circj.cj-10-1021. [DOI] [PubMed] [Google Scholar]
- 28.Savoia C, Burger D, Nishigaki N, Montezano A, Touyz RM. Angiotensin II and the vascular phenotype in hypertension. Expert Rev Mol Med. 2011;13:e11. doi: 10.1017/S1462399411001815. [DOI] [PubMed] [Google Scholar]
- 29.Inoue H, Murayama T, Tashiro M, Sakurai T, Konishi M. Mg2+ and ATP-dependent inhibition of TRPM7 by oxidative stress. Free Radic Biol Med. 2014;72:257–266. doi: 10.1016/j.freeradbiomed.2014.04.015. [DOI] [PubMed] [Google Scholar]
- 30.Yogi A, Callera GE, Antunes TT, Tostes RC, Touyz RM. Vascular biology of magnesium and its transporters in hypertension. Magnes Res. 2010;23:S207–S215. doi: 10.1684/mrh.2010.0222. [DOI] [PubMed] [Google Scholar]
- 31.Sontia B, Montezano AC, Paravicini T, Tabet F, Touyz RM. Downregulation of renal TRPM7 increased inflammation fibrosis in aldosterone-infused mice: effects ofmagnesium. Hypertension. 2008;51:915–921. doi: 10.1161/HYPERTENSIONAHA.107.100339. [DOI] [PubMed] [Google Scholar]
- 32.Thilo F, Vorderwülbecke BJ, Marki A, Krueger K, Liu Y, Baumunk D, Zakrzewicz A, Tepel M. Pulsatile atheroprone shear stress affects the expression of transient receptor potential channels in human endothelial cells. Hypertension. 2012;59:1232–1240. doi: 10.1161/HYPERTENSIONAHA.111.183608. [DOI] [PubMed] [Google Scholar]
- 33.Meng Z, Wang X, Yang Z, Xiang F. Expression of transient receptor potential melastatin 7 up-regulated in the early stage of renal ischemia-reperfusion. Transplant Proc. 2012;44:1206–1210. doi: 10.1016/j.transproceed.2011.11.068. [DOI] [PubMed] [Google Scholar]
- 34.Li Y, Jiang H, Ruan C, Zhong J, Gao P, Zhu D, Niu W, Guo S. The interaction of transient receptor potential melastatin 7 with macrophages promotes vascular adventitial remodeling in transverse aortic constriction rats. Hypertens Res. 2014;37:35–42. doi: 10.1038/hr.2013.110. [DOI] [PubMed] [Google Scholar]
- 35.Thilo F, Suess O, Liu Y, Tepel M. Decreased expression of transient receptor potential channels in cerebral vascular tissue from patients after hypertensive intracerebral hemorrhage. Clin Exp Hypertens. 2011;33:533–537. doi: 10.3109/10641963.2011.561903. [DOI] [PubMed] [Google Scholar]
- 36.Sah R, Mesirca P, Van den Boogert M, Rosen J, Mably J, Mangoni ME, Clapham DE. Ion channel-kinase TRPM7 is required for maintaining cardiac automaticity. Proc Natl Acad Sci U S A. 2013;110:E3037. doi: 10.1073/pnas.1311865110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sah R, Mesirca P, Mason X, Gibson W, Bates-Withers C, Van den Boogert M, Chaudhuri D, Pu WT, Mangoni ME, Clapham DE. Timing of myocardial trpm7 deletion during cardiogenesis variably disrupts adult ventricular function, conduction, and repolarization. Circulation. 2013;128:101–114. doi: 10.1161/CIRCULATIONAHA.112.000768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Briones AM, Nguyen Dinh Cat A, Callera GE, Yogi A, Burger D, He Y, Corrêa JW, Gagnon AM, Gomez-Sanchez CE, Gomez-Sanchez EP, Sorisky A, Ooi TC, Ruzicka M, Burns KD, Touyz RM. Adipocytes produce aldosterone through calcineurin-dependent signaling pathways: implications in diabetes mellitus-associated obesity and vascular dysfunction. Hypertension. 2012;59:1069–1078. doi: 10.1161/HYPERTENSIONAHA.111.190223. [DOI] [PubMed] [Google Scholar]
- 39.Endemann D, Touyz RM, Li JS, Deng LY, Schiffrin EL. Altered angiotensin II-induced small artery contraction during the development of hypertension in spontaneously hypertensive rats. Am J Hypertens. 1999;12:716–723. doi: 10.1016/s0895-7061(99)00036-9. [DOI] [PubMed] [Google Scholar]
- 40.Paravicini TM, Yogi A, Mazur A, Touyz RM. Dysregulation of vascular TRPM7 and annexin-1 is associated with endothelial dysfunction in inherited hypomagnesemia. Hypertension. 2009;53:423–429. doi: 10.1161/HYPERTENSIONAHA.108.124651. [DOI] [PubMed] [Google Scholar]
- 41.Touyz RM. Transient receptor potential melastatin 6 and 7 channels, magnesium transport, and vascular biology: implications in hypertension. Am J Physiol Heart Circ Physiol. 2008;294:H1103–H1118. doi: 10.1152/ajpheart.00903.2007. [DOI] [PubMed] [Google Scholar]
- 42.Baldoli E, Maier JA. Silencing TRPM7 mimics the effects of magnesium deficiency in human microvascular endothelial cells. Angiogenesis. 2012;15:47–57. doi: 10.1007/s10456-011-9242-0. [DOI] [PubMed] [Google Scholar]
- 43.Baldoli E, Castiglioni S, Maier JA. Regulation function of TRPM7 in human endothelial cells: TRPM7 as a potential novel regulator of endothelial function. PLoS One. 2013;8(3):e5989. doi: 10.1371/journal.pone.0059891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Howard AB, Alexander RW, Taylor WR. Effects of magnesium on nitric oxide synthase activity in endothelial cells. Am J Physiol. 1995;269:C612–C618. doi: 10.1152/ajpcell.1995.269.3.C612. [DOI] [PubMed] [Google Scholar]
- 45.Goligorsky MS, Li H, Brodsky S, Chen J. Relationships between caveolae eNOS: everything in proximity the proximity of everything. Am J Physiol Renal Physiol. 2002;283:F1–F10. doi: 10.1152/ajprenal.00377.2001. [DOI] [PubMed] [Google Scholar]
- 46.Basralı F, Koçer G, Ülker Karadamar P, Nasırcılar Ülker S, Satı L, Özen N, Özyurt D, Şentürk ÜK. Effect of magnesium supplementation on blood pressure and vascular reactivity in nitric oxide synthase inhibition-induced hypertension model. Clin Exp Hypertens. 2015;37:1–10. doi: 10.3109/10641963.2015.1036063. [DOI] [PubMed] [Google Scholar]
- 47.Su LT, Chen HC, González-Pagán O, Overton JD, Xie J, Yue L, Runnels LW. TRPM7 activates m-calpain by stress-dependent stimulation of p38 MAPK and c-Jun N-terminal kinase. J Mol Biol. 2010;396:858–869. doi: 10.1016/j.jmb.2010.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yu Y, Chen S, Xiao C, Jia Y, Guo J, Jiang J, Liu P. TRPM7 is involved in angiotensin II induced cardiac fibrosis development by mediating calcium and magnesium influx. Cell Calcium. 2014;55:252–260. doi: 10.1016/j.ceca.2014.02.019. [DOI] [PubMed] [Google Scholar]
- 49.Zhang Z, Wang M, Fan XH, Chen JH, Guan YY, Tang YB. Upregulation of TRPM7 channels by angiotensin II triggers phenotypic switching of vascular smooth muscle cells of ascending aorta. Circ Res. 2012;111:1137–1146. doi: 10.1161/CIRCRESAHA.112.273755. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.