Abstract
Objective
(1) Examine relationships between persistent hypogonadotropic hypogonadism (PHH) and long-term outcomes after severe traumatic brain injury (TBI); (2) determine if sub-acute testosterone levels can predict PHH.
Setting
Level 1 trauma center at a university hospital.
Participants
Consecutive sample of men with severe TBI between 2004 and 2009.
Design
Prospective cohort study.
Main Measures
Post-TBI blood samples were collected during week 1, every 2 weeks until 26 weeks, and at 52 weeks. Serum hormone levels were measured, and individuals were designated as having PHH if ≥50% of samples met criteria for hypogonadotropic hypogonadism. At 6 and 12 months post-injury, we assessed global outcome, disability, functional cognition, depression, and quality-of-life.
Results
We recruited 78 men; median (IQR) age was 28.5 (22–42) years. 34 patients (44%) had PHH during the first year post-injury. Multivariable regression, controlling for age, demonstrated PHH status predicted worse global outcome scores, more disability, and reduced functional cognition at 6 and 12 months post-TBI. Two-step testosterone screening for PHH at 12–16 weeks post-injury yielded a sensitivity of 79% and specificity of 100%.
Conclusion
PHH status in men predicts poor outcome after severe TBI, and PHH can accurately be predicted at 12–16 weeks.
Keywords: TBI, brain injury, post-traumatic hypogonadotrophic hypogonadism, hypopituitarism, outcome, biomarker screening assay, sensitivity and specificity, Rehabilomics
Introduction
Traumatic brain injury (TBI) results in 2.5 million hospital visits annually in the United States (US).1 Men are at an increased risk to sustain a TBI in the US, with an annual incidence of 932/100,000, compared to 720/100,000 in women.2 Further, ~1.1% of Americans live with TBI-related disabilities.3 Individuals with TBI are at high risk for developing hypothalamic-pituitary deficiencies, including hypogonadotropic hypogonadism (HH).4 Hypogonadism shares many symptoms with TBI (e.g. lack of energy, poor concentration, and mood disturbances), making it difficult for clinicians to assess and diagnose this problem in individuals recovering from TBI. Prevalence estimates for chronic hypogonadism after TBI vary widely, from 8–41%, likely due to differences with time of screening, injury severity, and study design.5–7 Most prior studies use single laboratory measurements to screen for HH, even though clinical guidelines generally recommend repeat testosterone testing for diagnosis if an initial level is found to be low, especially when symptoms are non-specific.8 Thus, multiple hormone measurements may more accurately determine hypogonadism status. Yet longitudinal data characterizing hormone levels across acute, sub-acute, and chronic phases of TBI recovery are limited.
Emerging evidence suggests that individuals with hypogonadism post-TBI may have poorer long-term outcomes than those without this complication. Retrospective studies involving men with hypogonadism upon presentation to inpatient rehabilitation after TBI have yielded both positive and negative results on testosterone associations with functional outcomes.9,10 A prospective study of 72 patients entering inpatient TBI rehabilitation (mean 250d post-TBI) reported that those with hypogonadism exhibited worse functional dependence, disability, and cognitive function at discharge compared to individuals without hypogonadism.11 Testosterone and gonadotropin serum levels were also positively correlated with visuoconstructional abilities in a cross-sectional study one year post-TBI.12 In a previously reported small cohort (N=38) of men with severe TBI,6 individuals with persistent hypogonadotropic hypogonadism (PHH) over the first year after TBI had worse disability, cognitive function, and neurological outcomes at 6 and 12 months post-injury. Although these data support that hypogonadism contributes to poor recovery post-TBI, clinical predictors about which patients will develop hypogonadism have not been identified, and relevant screening windows post-TBI have not been established.
The present study builds upon previous work by studying a larger prospective cohort of men with severe TBI to: 1) determine the time course of serum sex hormone levels over the first year post-TBI; 2) confirm prior findings regarding relationships between hypogonadism and multidimensional outcomes at 6 and 12 months post-TBI; and 3) develop a novel two-step screening algorithm using sub-acute testosterone levels to predict PHH.
Materials and Methods
Study Design and Population
The local Institutional Review Board approved this research. We conducted a prospective cohort study that consecutively recruited patients presenting to a level 1 trauma center (defined by Glasgow Coma Scale [GCS] ≤8 at presentation and confirmed computed tomography findings). The GCS is the standard scale used to categorize TBI severity.13 This analysis included men aged 16–70 years in whom we were able to collect at least two subacute (>1 week) blood samples. Individuals were excluded if they had a history of hypothalamic or pituitary tumors, orchiectomy, luteinizing hormone (LH) therapy, or untreated thyroid disease prior to injury. Informed consent was provided by next-of-kin. Subjects were admitted to a neurotrauma intensive care unit and treated according to the Brain Trauma Foundation’s Guidelines for the Management of Severe Head Injury.14 Demographic data (age, body mass index [BMI], race, and education) were recorded. Clinical data obtained included GCS (best score in 24 hours), Injury Severity Score (ISS), length of hospital stay, mechanism of injury, and acute stay neuroradiology reports. Fourteen healthy male volunteers (median age, 21.5 y; range, 19–58 y), without history of head injury, neurological disorder, or endocrine disorder, had serum samples drawn for hormone analysis to serve as controls.
Hormone Analysis and Hypogonadism Definition
Blood samples were collected daily when possible for the first week after TBI. Blood was collected up to every two weeks for six months, and again at 52 weeks post-injury. Since previous data show acute hypogonadism takes ≥2 days to manifest post-TBI,15 we averaged individuals’ hormone levels measured days 3–7 and assigned this value as the week 1 value. Upon collection, samples were centrifuged, aliquoted in polypropylene cryovials, and stored at −80 °C until analysis.
Serum testosterone (25μl samples) was measured in duplicate using radioimmunoassay with the Coat-A-Count® In-vitro Diagnostic Test Kit (Siemens Healthcare Diagnostics). Kits included a solid-phase 125I radioimmunoassay designed for direct, quantitative measurements of each hormone. Serum LH levels were measured in duplicate using a highly sensitive fluoroimmunometric assay (Delfia, Perkin-Elmer-Wallac). Inter-assay and intra-assay coefficients of variation were <10%. Samples with out of range (low) levels were assigned the detection limit of the respective assay.
We prospectively defined PHH based on criteria defined in previous work and used serial hormone measurements to determine PHH status in this study.6 Individuals were designated as having PHH if ≥50% of their samples had testosterone and LH values meeting criteria for hypogonadotropic hypogonadism (testosterone <10 nmol/L [minimum normal level] with LH <5.6 IU/L [maximum normal level]). These cut-offs are the medical center’s pathology lab reference values.
Outcome Measures
At 6 and 12 months, trained assessors obtained information on functional outcome, depression, quality of life (QOL), and administered a comprehensive cognitive battery.
Functional outcome measures included the Glasgow Outcome Scale (GOS),16 Disability Rating Scale (DRS),17 and Functional Independence Measure (FIM).18,19 We dichotomized individuals’ GOS scores into favorable outcome (scores 4–5) or unfavorable outcome (scores 2–3) for analysis. All individuals were alive at 12 months. The five cognition questions of FIM were analyzed separately (FIM-Cog), assessing comprehension, expression, social interaction, problem solving, and memory, with total FIM-Cog scores ranging from 5–30.
Depression was assessed using the PHQ-9, a self-administered depression symptom screening tool that derives its testing items directly from the American Psychiatric Association’s Diagnostic and Statistical Manual of Disorders, Fourth Edition diagnostic criteria for major depressive disorder.20 We categorized individuals as depressed if they endorsed ≥5 symptoms on the PHQ-9, including one of the cardinal symptoms (little interest or pleasure in doing things; or feeling down, depressed, or hopeless). The PHQ-9 is a validated measure of depressive symptoms post-TBI.21 In addition, we used responses to question 4 on the PHQ-9 (“feeling tired or having little energy”) as a measure representing a physical symptom of hypogonadism.
In order to assess the impact of PHH status on QOL, we used two outcome measures: Perceived Quality of Life (PQOL) and Percent Back to Normal. The PQOL is an 18-item questionnaire that quantitatively measures QOL by asking individuals to assess different life domains on a scale of 0–100, with higher scores indicating better QOL.22 Individuals’ scores for these questions were averaged for analysis. The Percent Back to Normal measure, previously used in TBI,23 is a single question asking individuals how close they feel to being back to normal, on a scale of 0–100.
We conducted a comprehensive cognitive testing battery, described previously,24 consisting of eight tests from a larger neuropsychological battery examining attention, language fluency, memory, and executive function. Tests of attention included the Trail Making Test A and the Digit Span sub-test of the Wechsler Adult Intelligence Scale-R (WAIS-R).25,26 Language fluency tests included the Controlled Oral Word Association test27 and the Delis-Kaplan Executive Function Systems Verbal Fluency test.28 To test memory, we used the Rey-Osterreith Complex Figure Task and the California Verbal Learning Test II.29,30 Executive function was measured by the Trail Making Test B and the Stroop Color and Word Test.25,31 Each test was scored in a standardized manner, compared to normative data, and reported as a T-score. T-scores for individual tests were then averaged to produce one aggregate Cognitive Composite score for each individual.
Statistical Analysis
All variables were assessed for normality using the Shapiro-Wilk test. Normally distributed data were expressed as mean ± standard deviation (SD). Descriptive statistics for skewed data were reported as median (interquartile range[IQR]), unless otherwise stated. Mann-Whitney U tests were used to conduct group comparisons for: DRS, FIM, FIM-Cog, and lack of energy (question 4 of PHQ-9) scores. Independent two-tailed t-tests were used to compare the Cognitive Composite scores and hormone levels. Chi-square tests were used for GOS outcome and depression status. Outlier hormone levels were removed if they were greater than 5x the IQR above the 75th percentile at each time point. Demographic/clinical characteristics associated (p<0.2) with outcome measures and PHH status in bivariate analysis were analyzed using linear or logistic multivariable regression. Odds ratios (OR) and 95% confidence intervals (CI) are reported for logistic regression results, and regression coefficients (β) with standard error (SE) are reported for linear regression results.
We developed a two-step testing process (screening test followed by a confirmatory test) for predicting PHH status using receiver operating characteristic (ROC) curve analysis with testosterone serum levels drawn 12–16 weeks post-injury. This time window is consistent with 2005 consensus guidelines on post-traumatic hypopituitarism screening, which recommend initial screening for hormone deficiencies at 3 months post-TBI.32 For this analysis, we included individuals with at least two testosterone values during the four week period. While LH and testosterone were concurrently used in defining PHH, only testosterone was used in ROC curve analysis since only a single continuous measure can be used to determine cutpoints for sensitivity and specificity assessments. We chose testosterone given that this marker was most often below clinical reference ranges with our PHH categorization strategy. This is still expected to be representative of PHH considering primary hypogonadism is rare in younger populations,33 and all group mean LH values were within normal clinical range. A screening ROC curve was generated using individuals’ first testosterone value in the time period to find a cut-off testosterone value with 100% sensitivity for determining PHH status. From there, individuals with testosterone levels below this screening cut-off were used to generate a confirmatory ROC curve analysis with a second, subsequent serum testosterone value in the 12–16 week time period being evaluated to predict PHH status. A confirmatory test cut-off was determined with 100% specificity. This cut-off was chosen to create a clinical testing protocol to identify, without false positives, individuals with PHH who may benefit from treatment. Post-hoc cross-sectional analyses were conducted to further analyze individuals in the PHH group that had hormone measurements at 6 and 12 months. These analyses compared frequency and multi-dimensional outcomes among those that met hypogonadotropic hypogonadism (HH) criteria specifically at 6 or 12 months post-injury vs. those that did not.
Bivariate analyses and ROC curve analysis were conducted using SPSS Version 22.0 (Armonk, NY), and multivariable analyses were conducted with SAS Version 9.4 (Cary, NC). P-values <0.05 were considered statistically significant.
Results
Demographics and characteristics of study sample
Demographic and clinical information is reported in Table 1 for the 78 men recruited. The PHH group (median, 35 years) was 11 years older than the non-PHH group (median, 24 years, p=0.04). Groups had similar BMI, education level, and race. Injury and clinical characteristics, including GCS, ISS, length of hospital stay, mechanism of injury, and radiographic injury types, were also similar, with the exception of the PHH group having only 2 individuals (6%) with diffuse axonal injury compared to 16 (36%) in the non-PHH group (p=0.002). Control subjects did not differ from study participants in age (p=0.16).
Table 1.
Demographics and clinical characteristics
| All | Non-PHH | PHH | p-value | |
|---|---|---|---|---|
| n | 78 | 44 | 34 | - |
| Age, median (IQR), y | 29 (22–42) | 24 (21–40) | 35 (22–42) | 0.044 |
| BMI, median (IQR), kg/m2 | 26 (24–29) | 26 (24–29) | 27 (24–30) | 0.343 |
| Education, No. (%) | ||||
| < HS | 14 (18) | 15 (34) | 13 (41) | 0.242 |
| HS | 34 (45) | 23 (52) | 11 (34) | |
| > HS | 28 (37) | 6 (14) | 8 (25) | |
| Race, No. (%) | ||||
| Caucasian | 72 (92) | 40 (91) | 32 (94) | 0.815 |
| African American | 4 (5) | 3 (7) | 1 (3) | |
| Other | 2 (3) | 1 (2) | 1 (3) | |
| GCS (best in 24 h), median (IQR) | 7 (6–9) | 7 (6–9) | 7 (6–9) | 0.782 |
| Injury severity score, median (IQR) | 30 (26–38) | 30 (26–38) | 30 (25–39) | 0.541 |
| Length of hospital stay, median (IQR) | 20 (16–27) | 19 (15–26) | 23 (18–35) | 0.076 |
| Mechanism of injury, No. (%) | ||||
| Motor vehicle accident | 39 (51) | 26 (61) | 13 (39) | 0.173 |
| Motorcycle accident | 22 (29) | 11 (26) | 11 (33) | |
| Fall/jump | 12 (16) | 4 (9) | 8 (24) | |
| Bicycle accident | 3 (4) | 2 (5) | 1 (3) | |
| Radiographic Injury Type, No. (%) | ||||
| Subdural hematoma | 47 (62) | 26 (59) | 21 (66) | 0.563 |
| Subarachnoid hemorrhage | 49 (65) | 28 (64) | 21 (66) | 0.858 |
| Diffuse axonal injury | 18 (24) | 16 (36) | 2 (6) | 0.002 |
| Epidural hemorrhage | 10 (13) | 6 (14) | 4 (13) | 1.000 |
| Contusion | 26 (24) | 14 (32) | 12 (38) | 0.606 |
| Intraventricular hemorrhage | 15 (20) | 9 (21) | 6 (19) | 0.854 |
| Intracerebral hemorrhage | 26 (34) | 13 (30) | 13 (41) | 0.315 |
| Other | 4 (5) | 2 (5) | 2 (6) | 1.000 |
Neuroimaging reports for computed tomography (CT) and/or magnetic resonance imaging (MRI) during acute hospital stay were available for 74 men. One had subacute hemorrhage in the left thalamus and hypothalamus on MRI nine days post-injury. The remaining 73 individuals had no radiographic evidence of pituitary or hypothalamic injury.
Medication data was available at 6 months (n=72) and 12 months (n=57). None of these individuals were on exogenous testosterone.
Serum hormone levels
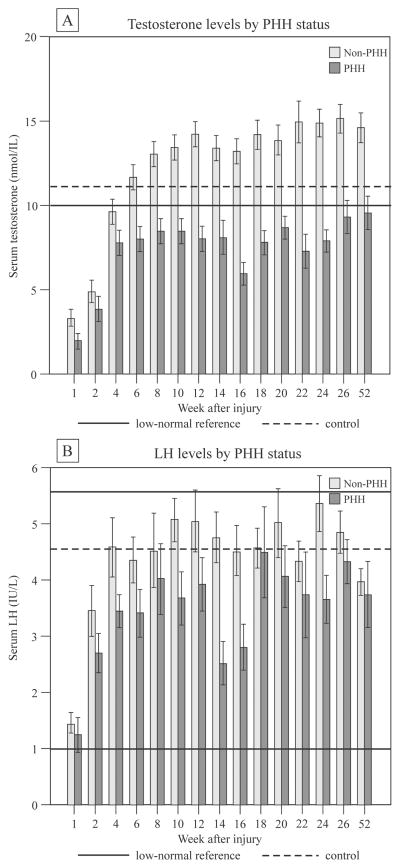
Thirty-four individuals (44%) had PHH. A total of 618 samples were collected between 2–52 weeks post-TBI. An additional 152 samples were collected on days 3–7 post-injury and averaged to provide a week 1 value for each individual. Two testosterone values and one LH value were removed as outliers. The time-course by PHH status for serum testosterone and LH is presented in Figure-1. The control group mean±SD testosterone level (n=14) was 11.057±7.557 nmol/L, and LH level (n=7) was 4.559±2.473 IU/L. PHH group testosterone levels were significantly lower than those of the non-PHH group at week 1 (p<0.05) and from weeks 6–52 (p<0.001), but were similar at weeks 2–4. While starting in the low-normal range, LH levels were within the normal reference range at all weeks for both PHH and non-PHH groups. LH levels were lower in the PHH group at weeks 10–16 and 24 (p<0.05).
Figure 1.
Serum levels of testosterone (upper) and LH (lower) by persistent hypogonadotropic hypogonadism (PHH) status after severe TBI in men. Data represent mean ± SEM. Testosterone levels were significantly lower in the PHH group at week 1 (p<0.05) and weeks 6–52 (p<0.001). LH levels were significantly lower in the PHH group at weeks 10, 12 (p<0.05), 14 (p<0.01), 16, and 24 (p<0.05).
6 and 12 month cross-sectional HH assessment
Among those in the PHH group with cross-sectional hormone levels at 6 and 12 months, 13 individuals (65%) met and 7 individuals (35%) did not meet HH criteria at 6 months. 12 individuals (71%) met and 5 individuals (29%) did not meet HH criteria. Individuals who met HH criteria at 6 and 12 months did not significantly vary (p>0.05 for all comparisons) from those who did not on any of the multidimensional outcomes measured in this study (GOS, DRS, FIM, FIM-Cog, and lack of energy).
PHH association with outcomes
Bivariate outcome associations with PHH status are shown in Table-2. Compared to the non-PHH group, the PHH group exhibited worse FIM scores 6 and 12 months, worse FIM-COG scores at 6 and 12 months, worse DRS scores at 12 months, and more commonly endorsed a lack of energy on the PHQ-9 at 12 months (p<0.05, all comparisons). Groups did not differ by depression status, PHQ-9 scores, PQOL, Percent Back to Normal, or cognitive composite scores, including all cognitive subtest scores. Trends were noted for GOS at 6 and 12 months and DRS at 6 months.
Table 2.
PHH status and functional outcomes at 6 and 12 months post-TBI
|
6 months GOS |
n 74 |
Non-PHH | PHH | p value |
|---|---|---|---|---|
| Favorable (4 or 5), no. (%) | 29 (67) | 14 (45) | 0.055 | |
| Unfavorable (2 or 3), no. (%) | 14 (33) | 17 (55) | ||
| DRS, median (IQR) | 73 | 3 (1–5) | 5 (1–9) | 0.084 |
| FIM-Total, median (IQR) | 71 | 123 (117–124) | 117 (80–122) | 0.011 |
| FIM-Cog, median (IQR) | 71 | 32 (29–34) | 29 (13–32) | 0.007 |
| Cognitive composite, mean ± SD | 53 | 40.4 ± 6.0 | 40.7 ± 7.8 | 0.867 |
| Depression | 51 | |||
| Depressed, no. (%) | 9 (28) | 7 (41) | 0.354 | |
| Not depressed, no. (%) | 23 (72) | 10 (59) | ||
| Lack of energy (PHQ-9 Q4), mean ± SD | 51 | .62 ± .92 | .83 ± .99 | 0.409 |
| Percent back to normal, median (IQR) | 43 | 77 (50–89) | 60 (40–75) | 0.132 |
| Perceived quality-of-life, median (IQR) | 45 | 78 (49–88) | 58 (46–82) | 0.173 |
|
12 months GOS |
67 |
|||
| Favorable (4 or 5), no. (%) | 32 (84) | 19 (66) | 0.075 | |
| Unfavorable (2 or 3), no. (%) | 6 (16) | 10 (34) | ||
| DRS, median (IQR) | 66 | 1 (0–4) | 3 (1–7) | 0.010 |
| FIM-Total, median (IQR) | 63 | 124 (121–125) | 117 (105–122) | 0.002 |
| FIM-Cog, median (IQR) | 59 | 33 (31–34) | 28 (22–32) | 0.001 |
| Cognitive Composite, mean ± SD | 47 | 43.2 ± 5.9 | 40.5 ± 7.3 | 0.163 |
| Depression | 51 | |||
| Depressed, no. (%) | 8 (24) | 6 (33) | 0.525 | |
| Not depressed, no. (%) | 25 (76) | 12 (67) | ||
| Lack of energy (PHQ-9 Q4), mean ± SD | 51 | .55 ± .75 | 1.2 ± 1.0 | 0.028 |
| Percent back to normal, median (IQR) | 44 | 80 (50–95) | 80 (50–90) | 0.871 |
| Perceived quality-of-life, median (IQR) | 46 | 81 (61–91) | 88 (58–94) | 0.981 |
Age was the only demographic variable that correlated (p<0.2) with both PHH status and any outcome (data not shown). Multivariable analyses were thus adjusted for age (Table-3). Those with PHH had greater than a 3-fold increased risk of having an unfavorable GOS outcome at 6 and 12 months compared to those in the non-PHH group (p<0.05, both comparisons). Further, PHH was a strong predictor of worse DRS scores, where adjusted mean scores were 3.2 points higher among those with PHH compared to non-PHH (p<0.05, both comparisons). PHH also significantly predicted worse FIM scores at 6 and 12 months, where, after age adjustment, those with PHH scored at least 18 points lower than those with non-PHH (p<0.05, both comparisons). Similarly, adjusted mean FIM-Cog scores were at least 6.7 points lower for the PHH group than the non-PHH group at 6 and 12 months (p<0.01, all comparisons). Finally, the PHH group reported having a lack of energy (PHQ-9 question 4) more often at 12 months, such that there adjusted mean scores were 0.6 points higher (range 0–3) than the non-PHH group (p=0.03).
Table 3.
Multivariate analysis of outcome associations with PHH status
| GOS | DRS | FIM | FIM-Cog | Lack of energy | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | p value | Beta (SE) | p value | Beta (SE) | p value | Beta (SE) | p value | Beta (SE) | p value | |
|
6 m Age |
0.972 (0.937, 1.009) | 0.135 | −0.050 (0.049) | 0.317 | .424 (0.278) | 0.132 | 0.082 (0.075) | 0.282 | 0.028 (0.009) | 0.004 |
| PHH | 3.100 (1.120, 8.579) | 0.029 | 3.243 (1.384) | 0.022 | −23.762 (7.819) | 0.003 | −7.093 (2.126) | 0.001 | −0.027 (0.269) | 0.919 |
|
12 m Age |
0.965 (0.919, 1.013) | 0.148 | −0.071 (0.056) | 0.208 | 0.320 (0.268) | 0.238 | 0.072 (0.070) | 0.307 | 0.003 (0.009) | 0.774 |
| PHH | 3.612 (1.046, 12.466) | 0.042 | 3.189 (1.573) | 0.047 | −18.107 (7.714) | 0.022 | −6.709 (2.036 | 0.002 | 0.604 (0.268) | 0.029 |
PHH prediction and ROC curve analysis
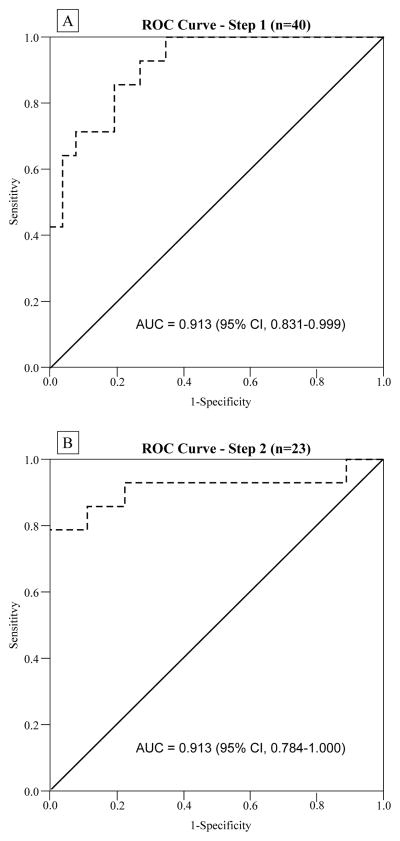
Forty individuals (51%) had ≥2 blood samples during the proposed PHH screening period (12–16 weeks). Of these individuals, 14 (35%) had PHH. Using the first testosterone value obtained in the screening period for these 40 individuals, the ROC curve analysis area under the curve (AUC) was 0.915 (95% CI, 0.831–0.999) (Figure-2A), with a 100% sensitivity at a cutoff value of 13.47 nmol/L. Seventeen individuals were above this cutoff and categorized as not having PHH, while 23 individuals were then included in the second step confirmatory test. For this second testosterone measurement in the screening period for these 23 men, the AUC was 0.913 (95% CI, 0.784–1.000) as shown in Figure-2B. A corresponding cutoff of 9.05 nmol/L had 100% specificity and 78.6% sensitivity. The positive predictive value was 100%, and the negative predictive value was 90%.
Figure 2.
A: Receiver operating characteristic (ROC) curve analysis for initial screening test of testosterone serum values to predict PHH status. The 100% sensitivity cutoff point was selected (13.47 nmol/L). Subjects testing positive (below the cutoff) in the screening test were analyzed in a confirmatory test. B: ROC curve analysis for confirmatory test of testosterone serum values to predict PHH status. The 100% specificity cutoff point was selected (9.05 nmol/L). Two-step testing of serum testosterone yielded an overall sensitivity of 78.6% and specificity of 100% for prediction of PHH.
Discussion
This prospective cohort study undertakes the most extensive longitudinal assessment of post-traumatic hypogonadism currently available in the literature. We report that PHH in men after severe TBI is associated with worse functional outcomes compared to men who do not develop PHH. Further, two-step testing of testosterone levels 12–16 weeks post-injury predicts PHH status, providing a novel, accurate testing method for long-term hypogonadism after TBI.
PHH prevalence after severe TBI in this cohort was 44%, larger than prior estimates.5 While others have used single serum measurements to define hypogonadism,11,34,35 our PHH categorization captures a longitudinal definition of hypogonadism, establishing the persistence of hypogonadism for some individuals after TBI. Our multi-measure algorthism may more precisely and accurately reflect men’s hypogonadism status over the first year post-injury. Our cohort consisted of men with severe TBI, and more severe TBI may confer higher hypogonadism risk.36 Serial hormone measurements up to 1yr post-TBI confirmed prior data6 showing uniformly low testosterone levels at weeks 1, 2, and 4, followed by testosterone recovery in the non-PHH group and sustained hypotestosteronemia in the PHH group by week 6. Some literature suggests withholding screening/diagnosis of post-traumatic hypogonadism until 1yr post-TBI, since self-recovery may occur during this time.32,34 However, our data suggest that recovery of testosterone levels begins ~6 weeks post-TBI. Normal range LH levels at all time-points suggest that hypothalamic-pituitary suppression is uniform during the first 4 weeks post-TBI which then resolves in some, but not all individuals. Traditionally, hypopituitarism after TBI has been attributed to injury and/or ischemia of the pituitary gland or infundibulum.37 Pituitary abnormalities on acute MRI have been noted in 30% of individuals post-TBI,38 and a small study (N=22) found post-traumatic hypopituitarism to be associated with pituitary abnormalities on CT conducted long after TBI (mean 17.4y).39 Radiographic pituitary abnormalities were not detected in our cohort, which may reflect less stringent study of the pituitary in clinical practice than in careful research protocol examination. Post-traumatic autoimmunity to CNS proteins is an emerging area of research.40,41 Recent data suggest that autoimmunity to pituitary antigens develops after TBI42; more data are needed to determine how autoimmunity development after TBI might contribute to PHH.
Our findings corroborate a growing literature that post-traumatic hypogonadism adversely impacts disability, functional independence, cognition, and global neurological outcome.6,9–11 Hypogonadism manifests with physical symptoms and functional limitations in men across a wide age-range.43 Therefore, it is not surprising that post-traumatic hypogonadism impedes recovery post-TBI. Preclinical experiments report mixed effects of testosterone on brain injury recovery.44 Testosterone is a precursor of estradiol, a well-established neuro-protective agent.44 Better neurological outcomes in the non-PHH group potentially may be explained by either direct or downstream effects of testosterone on brain function. It was reported previously that PHH was associated with worse cognitive test scores6; however, the study did not correct for normal variance in the general population. The present study used norm-referenced cognitive data and yielded no difference in cognitive test scores. However, despite no cognitive differences based neuropsychological testing, participants with PHH reported a clinically meaningful difference in functional cognition compared to those without hypogonadism.45 These findings may, in part, be due to increased fatigue (lack of energy endorsement) reported in the PHH group. Individuals with PHH may have the cognitive ability to perform within similar limits on cognitive testing as those without PHH, however decreased effort in applying cognitive skills to everyday tasks may impede functional cognition. Further study with a larger sample may allow for more detailed analysis of individual cognitive testing domains where finer differences may be observed. No between group differences were detected with depression (PHQ-9 scores ranged from ~4–7 points) or PQOL (scores range from ~57%-74%), findings which may be due to already poor baseline scores post-TBI for which PHH does not further discriminate. Future work should evaluate if/how fatigue influences functional cognition and other multidimensional outcomes using a Rehabilomics approach, which applies grounded methodologies for assessing biomarker relationships with complications/conditions arising from injury, and relating how these relationships affect multimodal outcome.46,47
Age is important to consider when studying hypogonadism. Testosterone levels naturally decline in men starting around age 30 at a rate of ~1% per year.48 The PHH group’s median age was 11 years older than the non-PHH group, however, PHH showed stronger prognostic ability to discriminate multimodal outcomes after controlling for age than in bivariate analysis. These findings suggest that outcome differences are primarily due to hypogonadism, independent of age. Higher DAI rates in the non-PHH group is consistent with observations that younger age is associated with DAI after TBI.49,50 Also, multivariable analyses showed that lack of energy endorsement was age-driven at 6 months but PHH-driven at 12 months. This may suggest that age has a particularly heavy influence on energy levels earlier during the recovery process. This finding could also reflect that low testosterone levels take a longer time-frame to manifest with noticeable fatigue symptoms.
A published consensus guideline recommends withholding hypogonadism therapy until 1 year post-TBI since deficits may self-correct between 3–12 months.32 However, new deficits may also arise in this timeframe.51,52 Our data show that low testosterone levels persist over time and measurable outcome differences already exist at 6 and 12 months post-injury. Thus, hypogonadism is a clinically important problem in this timeframe that may warrant treatment. In our post-hoc cross-sectional analysis, some individuals with PHH had normal hormone levels based on the HH definition at 6 and 12 months. However, at 6 and 12 months, those meeting HH criteria were not significantly different than those with testosterone in the normal range on any outcome measure assessed, suggesting that persistence of HH over time is what contributes to health outcomes and recovery at these later time points.
Thus, we developed a prediction model of PHH, hypothesizing that earlier intervention may improve health outcomes and recovery during the first year post-injury. A clinical testing protocol to predict post-traumatic hypogonadism should be conservative considering there is no published clinical trial of hormone replacement therapy for this population. Clinical characteristics do not sufficiently predict PHH, so testosterone measurements were utilized, yielding 79% sensitivity and 100% specificity. The 79% sensitivity is sufficient considering post-traumatic hypogonadism is largely either not recognized by clinicians or undertreated, as evidenced by our data showing that no men were being treated with testosterone during follow-up. Two-step testing enabled elimination of false-positives, and identified men with PHH that would qualify for treatment without the risk of treating an individual needlessly. This work represents a novel prediction algorithm to screen for post-traumatic hypogonadism, although we cannot yet make any definitive treatment recommendations. These results must be validated in an independent study that also evaluates for evidence of other concurrent neuroendocrinopathy. If validated, however, the current data showing association between PHH and poor outcome, combined with this PHH prediction method, could provide a rationale for a clinical trial of hormone replacement therapy in men with hypogonadism after severe TBI.
Other endocrinopathies can occur after TBI in isolation or in the setting of hypogonadism7,36,53. Thus, those in either the PHH group or the no-PHH group could be at risk for other neuroendocrinopathies after TBI. In 322 men with TBI screened for hypopituitarism upon presentation to a neurorehabilitation facility (median 7wks post-TBI), 131 (40%) were hypotestosteronemic, and 28 of these men (21%) had disturbances in at least one other pituitary axis.7 Therefore one must consider this study’s results within the clinical likelihood that multiple axes are affected. Notably, many hormones can directly influence testosterone levels. Experimentally elevated serum cortisol rapidly decreases testosterone levels in men,54 which in vitro experiments suggest is due to direct glucocorticoid inhibition of testicular testosterone synthesis.55 In our population there was no association between endogenous serum cortisol levels and PHH status (data not shown), however, more work with stimulated cortisol responses would be needed to draw a definitive conclusion about the HPA axis’ role in HPG suppression. Primary hypothyroidism in men is associated with HH that is reversible with thyroxine replacement.56 Lastly, hyperprolactinemia in male rats decreases gonadal testosterone synthesis and pituitary LH release.57,58 Notably, Kopczak reported that 30% of men with post-traumatic hypotestosteronemia also had hyperprolactinemia.7 A complete, systematic temporal characterization of these hormone levels after TBI is lacking, but is an important topic for ongoing research.
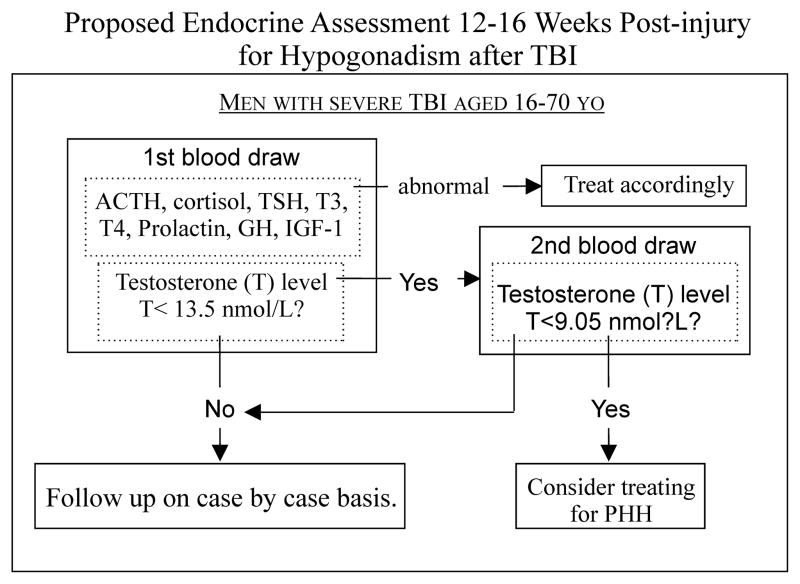
We have devised a preliminary assessment protocol, based on our work and the literature, regarding possible involvement of other hypothalamic-pituitary axes to identify individuals with PHH who may benefit from hormone replacement therapy, should clinical trials demonstrate such a benefit (Figure-3).
Figure 3.
Diagnosis of post-traumatic hypogonadotropic hypogonadism should be incorporated into a full endocrinological workup for hypopituitarism in order to determine an appropriate course of therapy. Adrenal insufficiency, hypothyroidism, and hyperprolactinemia should all be treated prior to hypogonadism since these hormones may affect testosterone levels. In the absence of these endocrinopathies, two-step testosterone testing is recommended to detect persistent hypogonadotropic hypogonadism (PHH). This should be conducted between 12–16 weeks after TBI. If a testosterone screening test level is below 13.5 nmol/L, then a second confirmatory test should be conducted. If the confirmatory test level is below 9.05 nmol/L, then this individual is at risk for poor outcome, and treatment for PHH may be beneficial.
There are limitations associated with this study. PHH status was assigned retrospectively and not clinically diagnosed. We did not determine if PHH was due to hypothalamic or pituitary dysfunction, however, hypothalamic vs. hypopituitary hormone dysfunction would not likely impact clinical decision making for treatment. Serum samples and outcome measures were unable to be collected at all time-points for individuals, representing a challenge of conducting long-term prospective studies. We also did not measure free testosterone or sex hormone binding globulin, which may further inform hypogonadism in men.8 However, our findings show a strong, clinically relevant relationship between injury, serum testosterone levels over time, and patient outcomes.
In conclusion, men with PHH exhibit poorer outcomes at 6 and 12 months after TBI. Accurate clinical testing for PHH can be performed at 12–16 weeks post-TBI, suggesting men may benefit from screening. However before clinical treatment recommendations can be made, this algorithm requires: 1) validation in an independent population, and 2) rigorous testing in a placebo-controlled randomized control trial. Future work should also examine other pituitary hormone axes, and determine whether these conclusions about PHH generalize to mild/moderate TBI populations. Importantly, the impact of post-traumatic hypogonadism on outcome in women remains unknown and requires study.
Acknowledgments
Sources of Funding: This study was funded by CDC grant #R49-CCR-323155-03, NIDRR grant #H133A120087, and the Rehabilitation Research Experience for Medical Students (RREMS) Program.
This work was supported by CDC grant number R49 CCR 323155-03, NIDRR grant number H133A120087, the Rehabilitation Research Experience for Medical Students (RREMS) Program, and the UPMC Rehabilitation Institute.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Centers for Disease Control and Prevention, National Center for Injury Prevention and Control Website. Traumatic Brain Injury in the United States: Fact Sheet. [Google Scholar]
- 2.Centers for Disease Control and Prevention, National Center for Injury Prevention and Control. Rates of TBI-related Emergency Department Visits, Hospitalizations, and Deaths by Sex — United States, 2001–2010. [Google Scholar]
- 3.Zaloshnja E, Miller T, Langlois JA, Selassie AW. Prevalence of long-term disability from traumatic brain injury in the civilian population of the United States, 2005. J Head Trauma Rehabil. 2008;23(6):394–400. doi: 10.1097/01.HTR.0000341435.52004.ac. [DOI] [PubMed] [Google Scholar]
- 4.Fernandez-Rodriguez E, Bernabeu I, Castro AI, Kelestimur F, Casanueva FF. Hypopituitarism following traumatic brain injury: determining factors for diagnosis. Front Endocrinol. 2011;2:25. doi: 10.3389/fendo.2011.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hohl A, Ronsoni MF, van de Sande-Lee S, et al. Androgens, Male Hypogonadism and Traumatic Brain Injury. Open J Endocr Metab Dis. 2014;04(01):13–23. [Google Scholar]
- 6.Wagner AK, Brett CA, McCullough EH, et al. Persistent hypogonadism influences estradiol synthesis, cognition and outcome in males after severe TBI. Brain Inj. 2012;26(10):1226–1242. doi: 10.3109/02699052.2012.667594. [DOI] [PubMed] [Google Scholar]
- 7.Kopczak A, Kilimann I, von Rosen F, et al. Screening for Hypopituitarism in 509 Patients with Traumatic Brain Injury or Subarachnoid Hemorrhage. J Neurotrauma. 2014;31(1):99–107. doi: 10.1089/neu.2013.3002. [DOI] [PubMed] [Google Scholar]
- 8.Petak SM, Nankin HR, Spark RF, Swerdloff RS, Rodriguez-Rigau LJ American Association of Clinical Endocrinologists. American Association of Clinical Endocrinologists Medical Guidelines for clinical practice for the evaluation and treatment of hypogonadism in adult male patients--2002 update. Endocr Pract. 2002;8(6):440–456. [PubMed] [Google Scholar]
- 9.Young TP, Hoaglin HM, Burke DT. The role of serum testosterone and TBI in the in-patient rehabilitation setting. Brain Inj. 2007;21(6):645–649. doi: 10.1080/02699050701210426. [DOI] [PubMed] [Google Scholar]
- 10.Carlson NE, Brenner LA, Wierman ME, et al. Hypogonadism on admission to acute rehabilitation is correlated with lower functional status at admission and discharge. Brain Inj. 2009;23(4):336–344. doi: 10.1080/02699050902788535. [DOI] [PubMed] [Google Scholar]
- 11.Bondanelli M, Ambrosio MR, Cavazzini L, et al. Anterior pituitary function may predict functional and cognitive outcome in patients with traumatic brain injury undergoing rehabilitation. J Neurotrauma. 2007;24(11):1687–1697. doi: 10.1089/neu.2007.0343. [DOI] [PubMed] [Google Scholar]
- 12.Popovic V, Pekic S, Pavlovic D, et al. Hypopituitarism as a consequence of traumatic brain injury (TBI) and its possible relation with cognitive disabilities and mental distress. J Endocrinol Invest. 2004;27(11):1048–1054. doi: 10.1007/BF03345308. [DOI] [PubMed] [Google Scholar]
- 13.Teasdale G, Jennett B. Assessment of coma and impaired consciousness. A practical scale. Lancet. 1974;2(7872):81–84. doi: 10.1016/s0140-6736(74)91639-0. [DOI] [PubMed] [Google Scholar]
- 14.Brain Trauma Foundation, American Association of Neurological Surgeons, Congress of Neurological Surgeons. . Guidelines for the management of severe traumatic brain injury. J Neurotrauma. 2007;24 (Suppl 1):S1–106. doi: 10.1089/neu.2007.9999. [DOI] [PubMed] [Google Scholar]
- 15.Wagner AK, McCullough EH, Niyonkuru C, et al. Acute serum hormone levels: characterization and prognosis after severe traumatic brain injury. J Neurotrauma. 2011;28(6):871–888. doi: 10.1089/neu.2010.1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jennett B, Bond M. Assessment of outcome after severe brain damage. Lancet. 1975;1(7905):480–484. doi: 10.1016/s0140-6736(75)92830-5. [DOI] [PubMed] [Google Scholar]
- 17.Rappaport M, Hall KM, Hopkins K, Belleza T, Cope DN. Disability rating scale for severe head trauma: coma to community. Arch Phys Med Rehabil. 1982;63(3):118–123. [PubMed] [Google Scholar]
- 18.Hamilton BB, Laughlin JA, Fiedler RC, Granger CV. Interrater reliability of the 7-level functional independence measure (FIM) Scand J Rehabil Med. 1994;26(3):115–119. [PubMed] [Google Scholar]
- 19.Corrigan JD, Smith-Knapp K, Granger CV. Validity of the functional independence measure for persons with traumatic brain injury. Arch Phys Med Rehabil. 1997;78(8):828–834. doi: 10.1016/s0003-9993(97)90195-7. [DOI] [PubMed] [Google Scholar]
- 20.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- 21.Fann JR, Bombardier CH, Dikmen S, et al. Validity of the Patient Health Questionnaire-9 in assessing depression following traumatic brain injury. J Head Trauma Rehabil. 2005;20(6):501–511. doi: 10.1097/00001199-200511000-00003. [DOI] [PubMed] [Google Scholar]
- 22.Dikmen SS, Machamer JE, Powell JM, Temkin NR. Outcome 3 to 5 years after moderate to severe traumatic brain injury1. Arch Phys Med Rehabil. 2003;84(10):1449–1457. doi: 10.1016/s0003-9993(03)00287-9. [DOI] [PubMed] [Google Scholar]
- 23.Powell JM, Machamer JE, Temkin NR, Dikmen SS. Self-report of extent of recovery and barriers to recovery after traumatic brain injury: a longitudinal study. Arch Phys Med Rehabil. 2001;82(8):1025–1030. doi: 10.1053/apmr.2001.25082. [DOI] [PubMed] [Google Scholar]
- 24.Failla MD, Myrga JM, Ricker JH, Dixon CE, Conley YP, Wagner AK. Posttraumatic Brain Injury Cognitive Performance Is Moderated by Variation Within ANKK1 and DRD2 Genes. J Head Trauma Rehabil. 2015 doi: 10.1097/HTR.0000000000000118. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reitan R, Wolfson D. The Halstead-Reitan Neuropsychological Test Battery: Theory and Clinical Interpretation. Tucson, AZ: Neuropsychology Press; 1985. [Google Scholar]
- 26.Larrabee GJ, Curtiss G. Construct validity of various verbal and visual memory tests. J Clin Exp Neuropsychol. 1995;17(4):536–547. doi: 10.1080/01688639508405144. [DOI] [PubMed] [Google Scholar]
- 27.Borkowski J, Benton A, Spreen O. Word fluency and brain damage. Neuropsychologia. 1967;5(2):135–140. [Google Scholar]
- 28.Delis D, Kaplan E, Kramer J. Delis-Kaplan Executive Function System. San Antonio, TX: Psychological Corporation; 2001. [Google Scholar]
- 29.Shin M-S, Park S-Y, Park S-R, Seol S-H, Kwon JS. Clinical and empirical applications of the Rey-Osterrieth Complex Figure Test. Nat Protoc. 2006;1(2):892–899. doi: 10.1038/nprot.2006.115. [DOI] [PubMed] [Google Scholar]
- 30.Delis D, Kramer J, Kaplan E, Ober B. California Verbal Learning Test – Second Edition. Adult Version. Manual. San Antonio, TX: Psychological Corporation; 2000. [Google Scholar]
- 31.Stroop J. Studies of interference in serial verbal reactions. J Exp Psychol. 1935;18(6):643–662. [Google Scholar]
- 32.Ghigo E, Masel B, Aimaretti G, et al. Consensus guidelines on screening for hypopituitarism following traumatic brain injury. Brain Inj. 2005;19(9):711–724. doi: 10.1080/02699050400025315. [DOI] [PubMed] [Google Scholar]
- 33.Harman SM, Metter EJ, Tobin JD, Pearson J, Blackman MR Baltimore Longitudinal Study of Aging. Longitudinal effects of aging on serum total and free testosterone levels in healthy men. Baltimore Longitudinal Study of Aging. J Clin Endocrinol Metab. 2001;86(2):724–731. doi: 10.1210/jcem.86.2.7219. [DOI] [PubMed] [Google Scholar]
- 34.Kleindienst A, Brabant G, Bock C, Maser-Gluth C, Buchfelder M. Neuroendocrine function following traumatic brain injury and subsequent intensive care treatment: a prospective longitudinal evaluation. J Neurotrauma. 2009;26(9):1435–1446. doi: 10.1089/neu.2008.0601. [DOI] [PubMed] [Google Scholar]
- 35.Kozlowski Moreau O, Yollin E, Merlen E, Daveluy W, Rousseaux M. Lasting pituitary hormone deficiency after traumatic brain injury. J Neurotrauma. 2012;29(1):81–89. doi: 10.1089/neu.2011.2048. [DOI] [PubMed] [Google Scholar]
- 36.Schneider H, Kreitschmann-Andermahr I, Ghigo E, Stalla G, Agha A. Hypothalamopituitary dysfunction following traumatic brain injury and aneurysmal subarachnoid hemorrhage: A systematic review. JAMA. 2007;298(12):1429–1438. doi: 10.1001/jama.298.12.1429. [DOI] [PubMed] [Google Scholar]
- 37.Dusick JR, Wang C, Cohan P, Swerdloff R, Kelly DF. Pathophysiology of hypopituitarism in the setting of brain injury. Pituitary. 2012;15(1):2–9. doi: 10.1007/s11102-008-0130-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maiya B, Newcombe V, Nortje J, et al. Magnetic resonance imaging changes in the pituitary gland following acute traumatic brain injury. Intensive Care Med. 2008;34(3):468–475. doi: 10.1007/s00134-007-0902-x. [DOI] [PubMed] [Google Scholar]
- 39.Schneider HJ, Sämann PG, Schneider M, et al. Pituitary imaging abnormalities in patients with and without hypopituitarism after traumatic brain injury. J Endocrinol Invest. 2007;30(4):RC9–RC12. doi: 10.1007/BF03346291. [DOI] [PubMed] [Google Scholar]
- 40.Zhang Z, Zoltewicz JS, Mondello S, et al. Human traumatic brain injury induces autoantibody response against glial fibrillary acidic protein and its breakdown products. PloS One. 2014;9(3):e92698. doi: 10.1371/journal.pone.0092698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tanriverdi F, De Bellis A, Bizzarro A, et al. Antipituitary antibodies after traumatic brain injury: is head trauma-induced pituitary dysfunction associated with autoimmunity? Eur J Endocrinol Eur Fed Endocr Soc. 2008;159(1):7–13. doi: 10.1530/EJE-08-0050. [DOI] [PubMed] [Google Scholar]
- 42.Tanriverdi F, De Bellis A, Ulutabanca H, et al. A five year prospective investigation of anterior pituitary function after traumatic brain injury: is hypopituitarism long-term after head trauma associated with autoimmunity? J Neurotrauma. 2013;30(16):1426–1433. doi: 10.1089/neu.2012.2752. [DOI] [PubMed] [Google Scholar]
- 43.Tajar A, Forti G, O’Neill TW, et al. Characteristics of Secondary, Primary, and Compensated Hypogonadism in Aging Men: Evidence from the European Male Ageing Study. J Clin Endocrinol Metab. 2010;95(4):1810–1818. doi: 10.1210/jc.2009-1796. [DOI] [PubMed] [Google Scholar]
- 44.Liu M, Kelley MH, Herson PS, Hurn PD. Neuroprotection of Sex Steroids. Minerva Endocrinol. 2010;35(2):127–143. [PMC free article] [PubMed] [Google Scholar]
- 45.Beninato M, Gill-Body KM, Salles S, Stark PC, Black-Schaffer RM, Stein J. Determination of the minimal clinically important difference in the FIM instrument in patients with stroke. Arch Phys Med Rehabil. 2006;87(1):32–39. doi: 10.1016/j.apmr.2005.08.130. [DOI] [PubMed] [Google Scholar]
- 46.Wagner AK, Sowa G. Rehabilomics research: a model for translational rehabilitation and comparative effectiveness rehabilitation research. Am J Phys Med Rehabil. 2014;93(10):913–916. doi: 10.1097/PHM.0000000000000114. [DOI] [PubMed] [Google Scholar]
- 47.Wagner AK, Zitelli KT. A Rehabilomics focused perspective on molecular mechanisms underlying neurological injury, complications, and recovery after severe TBI. Pathophysiology. 2013;20(1):39–48. doi: 10.1016/j.pathophys.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 48.Huhtaniemi I, Forti G. Male late-onset hypogonadism: pathogenesis, diagnosis and treatment. Nat Rev Urol. 2011;8(6):335–344. doi: 10.1038/nrurol.2011.47. [DOI] [PubMed] [Google Scholar]
- 49.Failla MD, Kumar RG, Peitzman AB, Conley YP, Ferrell RE, Wagner AK. Variation in the BDNF Gene Interacts With Age to Predict Mortality in a Prospective, Longitudinal Cohort with Severe TBI. Neurorehabil Neural Repair. Jul; doi: 10.1177/1545968314542617. 1545968314542617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Skandsen T, Kvistad KA, Solheim O, Strand IH, Folvik M, Vik A. Prevalence and impact of diffuse axonal injury in patients with moderate and severe head injury: a cohort study of early magnetic resonance imaging findings and 1-year outcome. J Neurosurg. 2010;113(3):556–563. doi: 10.3171/2009.9.JNS09626. [DOI] [PubMed] [Google Scholar]
- 51.Giordano G, Aimaretti G, Ghigo E. Variations of pituitary function over time after brain injuries: the lesson from a prospective study. Pituitary. 2005;8(3–4):227–231. doi: 10.1007/s11102-006-6045-1. [DOI] [PubMed] [Google Scholar]
- 52.Bavisetty S, Bavisetty S, McArthur DL, et al. Chronic hypopituitarism after traumatic brain injury: risk assessment and relationship to outcome. Neurosurgery. 2008;62(5):1080–1093. doi: 10.1227/01.neu.0000325870.60129.6a. discussion 1093–1094. [DOI] [PubMed] [Google Scholar]
- 53.Schneider HJ, Schneider M, Kreitschmann-Andermahr I, et al. Structured assessment of hypopituitarism after traumatic brain injury and aneurysmal subarachnoid hemorrhage in 1242 patients: the German interdisciplinary database. J Neurotrauma. 2011;28(9):1693–1698. doi: 10.1089/neu.2011.1887. [DOI] [PubMed] [Google Scholar]
- 54.Cumming DC, Quigley ME, Yen SS. Acute suppression of circulating testosterone levels by cortisol in men. J Clin Endocrinol Metab. 1983;57(3):671–673. doi: 10.1210/jcem-57-3-671. [DOI] [PubMed] [Google Scholar]
- 55.Welsh TH, Bambino TH, Hsueh AJ. Mechanism of glucocorticoid-induced suppression of testicular androgen biosynthesis in vitro. Biol Reprod. 1982;27(5):1138–1146. doi: 10.1095/biolreprod27.5.1138. [DOI] [PubMed] [Google Scholar]
- 56.Donnelly P, White C. Testicular dysfunction in men with primary hypothyroidism; reversal of hypogonadotrophic hypogonadism with replacement thyroxine. Clin Endocrinol (Oxf) 2000;52(2):197–201. doi: 10.1046/j.1365-2265.2000.00918.x. [DOI] [PubMed] [Google Scholar]
- 57.Huang WJ, Yeh JY, Kan SF, Chang LS, Wang PS. Effects of hyperprolactinemia on testosterone production in rat Leydig cells. J Cell Biochem. 2001;80(3):313–320. doi: 10.1002/1097-4644(20010301)80:3<313::aid-jcb30>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 58.Smith MS, Bartke A. Effects of hyperprolactinemia on the control of luteinizing hormone and follicle-stimulating hormone secretion in the male rat. Biol Reprod. 1987;36(1):138–147. doi: 10.1095/biolreprod36.1.138. [DOI] [PubMed] [Google Scholar]
- 59.De Rosa M, Ciccarelli A, Zarrilli S, et al. The treatment with cabergoline for 24 month normalizes the quality of seminal fluid in hyperprolactinaemic males. Clin Endocrinol (Oxf) 2006;64(3):307–313. doi: 10.1111/j.1365-2265.2006.02461.x. [DOI] [PubMed] [Google Scholar]
- 60.Kelly DF, McArthur DL, Levin H, et al. Neurobehavioral and quality of life changes associated with growth hormone insufficiency after complicated mild, moderate, or severe traumatic brain injury. J Neurotrauma. 2006;23(6):928–942. doi: 10.1089/neu.2006.23.928. [DOI] [PubMed] [Google Scholar]
- 61.Moreau OK, Cortet-Rudelli C, Yollin E, Merlen E, Daveluy W, Rousseaux M. Growth hormone replacement therapy in patients with traumatic brain injury. J Neurotrauma. 2013;30(11):998–1006. doi: 10.1089/neu.2012.2705. [DOI] [PubMed] [Google Scholar]
- 62.Gardner CJ, Mattsson AF, Daousi C, Korbonits M, Koltowska-Haggstrom M, Cuthbertson DJ. Growth Hormone Deficiency after Traumatic Brain Injury: improvement in quality of life with GH therapy - analysis of the KIMS database. Eur J Endocrinol Eur Fed Endocr Soc. 2015 Jan; doi: 10.1530/EJE-14-0654. [DOI] [PubMed] [Google Scholar]
- 63.Reimunde P, Quintana A, Castañón B, et al. Effects of growth hormone (GH) replacement and cognitive rehabilitation in patients with cognitive disorders after traumatic brain injury. Brain Inj. 2011;25(1):65–73. doi: 10.3109/02699052.2010.536196. [DOI] [PubMed] [Google Scholar]
- 64.High WM, Briones-Galang M, Clark JA, et al. Effect of growth hormone replacement therapy on cognition after traumatic brain injury. J Neurotrauma. 2010;27(9):1565–1575. doi: 10.1089/neu.2009.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]