Abstract
Objective
Failure on performance validity tests (PVTs) is common in Veterans with histories of mild traumatic brain injury (mTBI), leading to questionable validity of clinical presentations.
Participants
Using diffusion tensor imaging (DTI), we investigated white matter (WM) integrity and cognition in 79 Veterans with history of mTBI who passed PVTs (n = 43; TBI-Passed), history of mTBI who failed at least one PVT (n = 13; TBI-Failed), and military controls (n = 23; MCs) with no history of TBI.
Results
The TBI-Failed group demonstrated significantly lower cognitive scores relative to MCs and the TBI-Passed group; however, no such differences were observed between MCs and the TBI-Passed group. On a global measure of WM integrity (i.e., white matter burden), the TBI-Failed group showed more overall WM abnormalities than the other groups. However, no differences were observed between the MCs and TBI-Passed group on white matter burden. Interestingly, regional WM analyses revealed abnormalities in the anterior internal capsule and cingulum of both TBI subgroups relative to MCs. Moreover, compared to the TBI-Passed group, the TBI-Failed group demonstrated significantly decreased WM integrity in the corpus callosum.
Conclusions
Findings revealed that, within our sample, WM abnormalities are evident in those who fail PVTs. This study adds to the burgeoning PVT literature by suggesting that poor PVT performance does not negate the possibility of underlying WM abnormalities in military personnel with history of mTBI.
Keywords: Performance Validity, mild traumatic brain injury (mTBI), diffusion tensor imaging (DTI), white matter, effort testing
INTRODUCTION
Invalid presentation of cognitive functioning during neuropsychological assessment limits clinical inferences and complicates diagnostic decision-making. Performance validity tests (PVTs) have been utilized in exams of Veterans with mild traumatic brain injury (mTBI) to provide objective measurement of whether observed cognitive test performances are valid and reliable. Failure on PVT measures has been associated with decreased cognitive test performance, evaluation context, and increased endorsement on post-concussion symptom checklists.1–9 The utility of PVTs in clinical populations has primarily been demonstrated by the failure to establish an association between poor PVT performance and bona fide clinical syndromes.10–12 The ease of the PVT task is such that even individuals with significant neurological deficits typically pass PVTs, 13,14 indicating that poor PVT performance is generally not a consequence of cognitive impairment. This insensitivity to cognitive impairment is further shown within TBI as those with more severe injuries demonstrate lower rates of poor PVT performance when compared to those with milder injuries.15–17 As such, PVT failure has largely been interpreted as a non-neurological factor that obscures the investigation of residual symptoms and cognition in Veteran mTBI samples.
The vast majority of research examining the role of PVTs in the context of a clinical evaluation has occurred in forensic settings where external incentives are salient.18, 19 In such settings, external financial or legal gain has been implicated as the source for PVT failure. However, in non-forensic settings, where there is less obvious motivation for secondary gain, why poor PVT failure occurs is less clear. Proposed explanations outside of direct monetary incentives include attempts to gain access to clinical care, overly focus on or exaggerate genuine deficits, adoption of social psychological factors (e.g., illness perception, diagnosis threat), and iatrogenic consequences.20–24 However, such explanations are largely speculative, and despite the increasing study of PVT performance in Veterans, 1–3,5–9 the clinical presentations of those who fail PVTs remain poorly understood.
While poor PVT performance alerts examiners to potentially invalid cognitive test performance and patterns of exaggerated symptom reporting, invalid test results do not negate the possibility of brain damage or genuine cognitive impairment25–28; thus non-performance based biomarkers of neurotrauma (e.g., imaging findings) may therefore prove useful in determining the presence of damage that may be related to the reported symptoms and clinical outcome. For example, a recent study examined magnetic resonance spectroscopic (MRS) metabolites in a sample of Veterans with self-reported memory impairment and history of blast-related TBI in order to investigate correlates of brain alterations.28 Results of this study showed that those who failed PVTs demonstrated MRS metabolite values roughly 1.5–2 standard deviations below the mean of control participants in the hippocampus, suggesting that a biological correlate of neural impairment was present, even in those who failed PVTs.
Clarifying whether underlying brain abnormalities are present among those failing PVTs is an important clinical issue, especially in Veterans’ health settings where TBI is regarded as the signature injury of the wars in Iraq and Afghanistan.29 Therefore, using diffusion tensor imaging (DTI), we investigated cerebral white matter (WM) microstructure of Veterans with reported histories of head injury and compared them to a military control (MC) sample without history of TBI. Our mTBI sample was subdivided into two groups on the basis of performances above or below recommended cut-points on PVTs. We hypothesized that, although cognitive test performance would be significantly reduced in the TBI-Failed group relative to the other two groups, the TBI-Failed group would consist of a mixed sample of those with and without WM microstructural damage; thus their level of WM damage was expected to fall between MC and PVT-passed groups.
METHODS
Participants
Study participants were 79 (TBI: n = 56, MCs: n = 23) Operation Enduring Freedom, Operation Iraqi Freedom, and Operation New Dawn (OEF/OIF/OND) service members who were recruited from the VA San Diego Healthcare System (VASDHS) and University of California, San Diego (UCSD) via word-of-mouth, posted recruitment fliers, and referrals from the VASDHS TBI Clinic. Only participants with mild TBI were included in the study, and of the TBI participants n = 43 passed PVT measures (TBI-Passed), while n = 13 failed (TBI-Failed). Study participants received comprehensive neuropsychological testing and MRI scanning, as well as administration of self-report measures of psychiatric symptomatology. All participants provided written and informed consent in compliance with the institutional review boards of the VASDHS and UCSD.
The following exclusionary criteria were applied to the study sample: (1) failure to complete PVT testing; (2) moderate or severe TBI; (3) current or past history of a significant neurological condition (e.g., seizures, multiple sclerosis); (4) current or past serious medical illness (e.g., cerebrovascular accident, myocardial infarction, etc.); (5) hearing or vision impairment that interfered with neuropsychological performance; (6) lack of English proficiency; (7) current psychiatric illnesses that are likely to impact brain morphometry and/or neuroendocrine functioning (e.g., schizophrenia, bipolar disorder); (8) current substance/alcohol abuse or dependence as indicated by concordance with the Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition) criteria; (9) any contraindications to magnetic resonance scanning (MRI) (e.g., shrapnel, ferromagnetic implants); and (10) involvement in current or pending litigation.
TBI Diagnostic Procedure
Diagnosis of mild TBI was based on Department of Defense (DOD) and VA TBI Task Force guidelines for mTBI30: (1) Glasgow Coma Scale (when available) score of 13–15; (2) presence and duration of loss of conciousness (LOC) ≤ 30 minutes; (3) presence and duration of alteration of conciousness (AOC) ≤ 24 hours; and/or (4) presence and duration of post-traumatic amnesia (PTA) of ≤ 24 hours. All participants were assessed for non-military (prior to or after discharge from the military) and military-related head injuries. Military-related injuries were assessed separately for blast and blunt mechanisms of injury. With respect to blasts, participants are also asked to estimate total number of blast exposures, distance, and the direction from which the blast was initiated (i.e., front, back, left, right).
TBI relevant information was obtained via open-ended questionning and prompts via a lab-based questionnaire modeled on the VA’s semistructured clinical interview for TBI identification.31 This measure was developed specifically to address the aims of the broader TBI study, and at present lacks the rigorous psychometric evaluation of more established clinical interviews31–33 and screening measures34–36 for TBI. However, this measure, is similar to other available instruments in that it addresses several key aspects of traumatic events including the number of head injuries sustained, important diagnostic data for each (e.g., duration of LOC, AOC, and PTA), and the mode of injury (i.e., blast or blunt force). Specifically, participants are asked to recall in detail any falls, fights, blast expsoures, sporting events, or any other experiences in which they may have hit, or suffered a blow to their head. A trained interviewer probed for details about diagnostic criteria (i.e., LOC, AOC, and PTA) and collected information with respect to whether injuries were occurred in combat, medical attention was received, and about the presence and persistence of neurological symptoms (i.e., nausea, headaches, fatigue, blurry vision) after each reported injury.
Neuropsychological Assessment
Participants completed a neuropsychological test battery that included: (1) Trail Making and Verbal Fluency tests of the Delis Kaplan Executive Function System (D-KEFS) 37; (2) California Verbal Learning Test-2nd Edition (CVLT-II)38; (3) Wisconsin Card Sorting Task- 64 Card Version (WCST-64)39 and (4) Reading subtest of the Wide Range Achievement Test- 4th edition (WRAT-4) 40. Participants also completed self-report measures including the Neurobehavioral Symptom Inventory (NSI) 41; PTSD Checklist (PCL-M) 42; and Beck-Depression Inventory-II (BDI-II). 43 Due to its later inclusion in the larger study, NSI data were available for only 45 TBI participants (TBI-Passed: n = 36, TBI-Passed: n = 9).
Assessments of Performance Validity
The Test of Memory Malingering (TOMM) is a stand-alone PVT designed to detect inadequate test motivation,14 and is especially sensitive and specific to detecting inadequate test engagement in head-trauma samples.44,45 Similarly, the CVLT-II Forced Choice Recognition (CVLT-FCR) is a brief, embedded measure of performance validity that has been validated in TBI samples.45 Invalid test performance was determined by a TOMM Trial 2 score of less than 45 or CVLT-FCR less than 15.14,45
Neuroimaging Protocols, Processing, and Analysis
All participants underwent structural MRI and diffusion tensor imaging (DTI) at the UCSD Functional Magnetic Resonance Imaging Center. Scans were acquired on a 3T General Electric MRI scanner using the MR750 platform. A radiologic technician with expertise in neuroimaging processing and analysis reviewed all structural scans for lesions.
Structural Scanning
A sagitally acquired high-resolution 3D T1-weighted anatomical MRI was collected with the following parameters: FOV = 24 cm, 256 × 256 × 192 matrix, .94 × .94 × 1 mm voxels, 176 slices, TR = 20 ms, TE 4.8 ms., flip angle 12°, over approximately 7 minutes.
Diffusion Tensor Imaging
All DTI images were collected via dual spin echo EPI acquisition.46 The b=0 was used for anatomical reference. DTI scan parameters were: FOV = 240 mm, slick thickness = 3mm, matrix size 128 × 128, in-plane resolution = 1.875 × 1.875, TR 8000ms, TE 93 ms. Thirty-four slices were acquired with 61 diffusion directions distributed on the surface of a sphere in conjunction with the electrostatic repulsion model47 and a b value of 1500 s/mm2, in addition to one T2 weighted image with no diffusion (β = 0). Distortions due to magnetic field heterogeneity were corrected with two field maps with identical spatial parameters as those of collected DTI scans. Total scan time for DTI acquisition and field mapping was approximately 12 to 16 minutes.
DTI was used for in vivo quantification of the direction and magnitude of water molecules within WM.48 Fractional anisotropy (FA) is a directional measure of diffusion ranging from 0 (isotropic diffusion) and 1 (perfectly anisotropic diffusion) that is reflective of fiber integrity.49–52 Subsequent DTI parameters are obtained through diagonalization and resulting eigenvalues provide further information about WM microstructures.48, 52 Specifically, axial diffusivity (AD) defined by the principal eigenvalue (i.e., AD = λ1), reflects the degree of diffusion parallel to axon fibers.51,53 Decreased AD has been shown to be reflective of axonal injury in ischemic WM lesions.54 Radial diffusivity (RD) was defined as the average of the second and third eigenvalues (i.e., RD = (λ2 + λ3)/2), and is a measure of diffusivity perpendicular to axonal fibers.49,53 Increased RD has been linked to demyelination after traumatic injury.53 DTI Processing. DTI image processing was performed utilizing the Oxford Centre for Functional Magnetic Resonance Imaging of the Brain (FMRIB) Software Library (FSL). 55 Two field maps were utilized to unwarp EPI acquisitions and all images were correction for motion artifact using the eddy correct FSL command. Visual inspection of all images was performed for quality assurance and the FSL bet command was utilized to remove non-brain voxels from analyses. The FSL program ditfit framed a diffusion tensor model to each voxel in order to generate the DTI index of FA and corresponding eigenvalues on a voxel-by-voxel basis.
Tractography
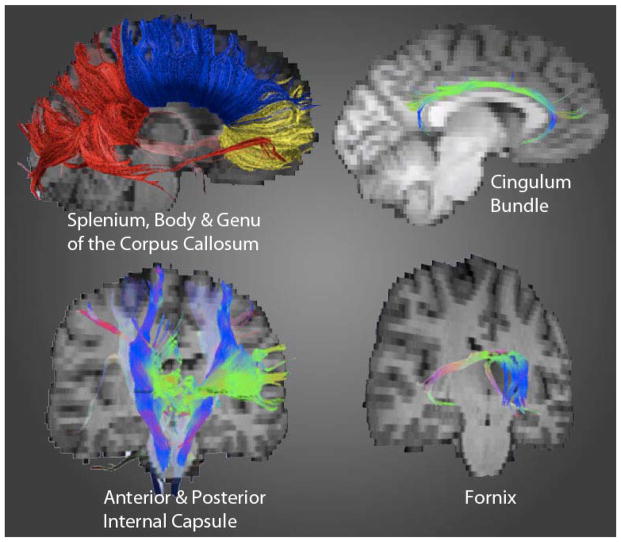
Using the Fiber Assignment by Continuous Tracking (FACT) method,56 fiber tracts were generated in TrackVis (MGH) according to “seed points” placed in regions of interest (ROIs). This occurred via bilateral seed point placement within the following WM tracts: anterior and posterior limbs of the internal capsule (IC); genu, body and splenium of the corpus callosum (CC); the fornix; and cingulum bundle. A blind rater placed seed ROIs in every subject’s color-map image in TrackVis. A color-coded scheme, seen by loading the principle eigenvector image in FSL, was generated to display each voxel’s main orientation of diffusion. This information, in conjunction with a non-diffusion weighted map, allowed the rater to delineate seed point ROIs for fiber tracking. A mean FA, AD, and RD value was extracted from the length of each track and utilized for composition of our main outcomes measures of white matter burden. The reduction of partial voluming effects due to encroaching gray matter was achieved by the inclusion of voxels with FA values greater than .2057, and irregular tracking was restricted by the implementation of an angle threshold of 41.4 degrees.58
Region of Interest Seeding
Internal capsule
DTI segmentation of the IC followed Wakana and colleagues’ procedures.59 ROI placement for the anterior IC occurred within green-colored voxels (see Figure 1). ROI seeds were placed in the axial plane between the putamen and caudate. ROI placement for the posterior IC occurred within blue-colored voxels. ROI seeds were placed medial to the lenticular nucleus (pallidum and putamen) and lateral to the thalamus.
Figure 1.
Fiber tracks of interest
Corpus Callosum
The entire CC was tracked by ROI seed placement within red-colored voxels in a mid-saggital slice of known CC anatomy utilizing Wakana and colleagues procedures.59 Subdivisions of the CC (e.g., genu, body, splenium) were identified using an adapted fiber tracking method.60 Cingulum: Placement occurred within green-colored voxels inferior to the cingulum gyrus and superior to the corpus callosum in the coronal plane. Distinct ROIs were placed for the anterior, middle, and posterior aspects of the cingulum following Concha et al. methodology.61 Fornix: Per Concha and colleagues, 61 ROI placements occurred in the body, crus and column.
Total WM Burden
Overall white matter burden (WMB), an index of global WM integrity, was calculated to capture the number of ROIs with compromised WM integrity (see 62). This measure reduces individual variability due to the heterogeneous nature of TBI, has been demonstrated to distinguish TBI participants from controls, and is a more tightly associated with reduced cognition relative to DTI-derived ROI indices.62 First, z-scores were calculated for the 10 ROIs of interest across each DTI index using the MCs’ means and standard deviations. Next, WMB was calculated by summing the total number of ROIs greater than 1 standard deviation below the control mean for FA and AD, separately. As increased RD may be indicative of membrane permeability or demyelination, 53 WMB for RD was calculated by summing the total number of ROIs greater than 1 standard deviation above the control mean. In all, 3 WMB variables were generated for each DTI index and total WMB loads ranged from 0 to 10. Higher WMB (e.g., 10) is indicative of a greater number of ROIs that displayed values below (for FA and AD) or above (RD) the control means and is representative of worse overall WM integrity.
Statistical Analyses
Formal group comparisons for all categorical data utilized chi-squared analyses. For continuous data, Shapiro Wilk’s test was conducted to determine whether the assumption of normality was met, while Levene’s test was utilized to determine whether there were homogeneous variances between the groups. For quantitative data, outlier analyses were conducted using Hoaglin and Igelwicz63 recommendations, which are more sensitive and appropriate for non-normal distributions and small-to-moderate sample sizes.63,64 Group comparisons for continuous data were conducted using the one-way analysis of variance (ANOVA) tests, followed by planned contrast testing (t- tests). For WMB and neuropsychological variables, multiple comparison corrections were conducted using Tukey’s Honestly Significant Differences (HSD). Dunnett’s T3 was used for multiple comparisons when there was heterogeneous variances between the groups and no multiple comparisons corrections were not performed for group comparisons of WM ROIs due to the exploratory nature of these analyses. The Kruskall-Wallis test was conducted to verify any findings in which the assumption of normality was violated. All statistical analyses were conducted using the Statistical Package for the Social Sciences (SPSS) version 21 (SPSS IBM, New York, USA). 65
RESULTS
PVT Performance
No MCs performed below PVT cut-off scores. Of those with TBI, 43 passed both PVTs (TBI-Passed) and 13 failed at least one PVT (TBI-Failed) reflected as obtaining a score below published cutoffs on either test. Within the TBI-Failed subgroup, nine individuals (69.2%) scored below threshold on the CVLT-FCR, three scored below threshold on only the TOMM (23.1%), and one individual (7.7%) scored below threshold on both measures. There were no individuals who scored below chance on the CVLT-FCR or the TOMM. This overall rate of poor PVT performance within the sample (16%) is commensurate with previously reported PVT failure rates in Veteran mTBI samples within a research context.7
Participant Demographic and Clinical Characteristics
Participant demographics, injury characteristics, and self-reported symptom rating scales are presented in Table 1. The groups did not significantly differ with respect to age or gender (all p-values > .10); however, ANOVA revealed significant differences with respect to years of education (p = .02). Both the TBI-Passed and TBI-Failed subgroups demonstrated significantly fewer years of education than the MC group (p = .03, p = .01, respectively), yet there were no differences in years of education between the TBI-Passed and TBI-Failed subgroups. There were no group differences with respect to estimated premorbid verbal ability (WRAT Reading, p > .10). However, chi-squared analysis revealed the groups significantly differed with respect to ethnicity (p = .001), with the TBI-Passed subgroup comprised of more Hispanic individuals compared to the MCs and the TBI-Failed group.
Table 1.
Participant Characteristics, Mean (SD)
| Controls | TBI-Passed | TBI-Failed | p-value | |
|---|---|---|---|---|
|
| ||||
| N | 23 | 43 | 13 | |
|
| ||||
| Age | 32.9 (7.9) | 32.9 (8.2) | 31.5 (8.5) | p = .86 |
|
| ||||
| * Gender (men: women) | 16:7 | 38:5 | 12:1 | p = .11 |
|
| ||||
| * Ethnicity | ||||
| Caucasian | 19 | 17 | 9 | |
| African American | 1 | 5 | 0 | p < .001 |
| Hispanic | 0 | 16 | 1 | |
| Asian | 0 | 2 | 3 | |
| Other | 3 | 3 | 0 | |
|
| ||||
| Years of Education | 15.1 (2.0) | 14.1 (1.6) | 13.5 (1.9) | p = .02 |
|
| ||||
| WRAT Reading Standard Score | 101.3 (16.9) | 99.4 (14.4) | 98.9 (8.0) | p = .85 |
|
| ||||
| Number of mTBIs | - | 2.4 (1.3) | 3.2 (2.0) | p = .11 |
|
| ||||
| *% Reporting Blast exposure | - | 69.8% | 69.2% | p = .97 |
|
| ||||
| # Self reported Blast exposures | - | 4.1 (12.92) | 4.8 (7.0) | p = .85 |
|
| ||||
| *% Reporting any LOC | - | 53.5% | 76.9% | p = .12 |
|
| ||||
| “Worst” TBI LOC duration in minutes | - | 6.08 (9.3) | 7.25 (7.0) | p = .75 |
|
| ||||
| Months Since Last TBI | - | 64.37 (43.8) | 34.00 (20.3) | p = .02 |
|
| ||||
| NSI Total Score | - | 32.7 (17.4) | 48.8 (10.8) | p = .01 |
|
| ||||
| BDI-II Total Score | 3.0 (4.3) | 19.1 (11.1) | 28.3 (13.3) | p < .001 |
|
| ||||
| PCL-M Total Score | 19.9 (4.1) | 43.6 (17.0) | 63.8 (10.4) | p < .001 |
Likelihood ratio utilized, WRAT = Wide Range Achievement Test-4; NSI = Neurobehavioral Symptom Inventory; BDI-II = Beck Depression Inventory-II; PCL-M = Posttraumatic Stress Disorder Symptom Checklist-Military
ANOVAs revealed the TBI-Passed and TBI-Failed subgroups did not differ in percentage of individuals with blast exposure, number of self-reported blast events, or most TBI characteristics (all p-values > .10). The last TBI event for the TBI-Failed subgroup was closer in time to the date of assessment than the TBI-Passed group (p = .02). The groups significantly differed in terms of self-reported psychiatric and neurological symptoms (all p-values < .001). Both the TBI-Passed and TBI-Failed subgroups showed significantly greater BDI, and PCL scores (all p’s < .001) when compared to MCs. The TBI-Failed subgroup endorsed significantly greater psychiatric and neurobehavioral symptoms when compared to the TBI-Passed subgroup (all p-values < .05).
Exploration of WMB Indices
One-way ANOVAs were used to examine whether there were group differences on WMB. The independent variable utilized in each analysis represented the three groups: MCs, TBI-Passed and TBI-Failed. The dependent variables were WMB indices of FA, RD and AD. See Table 2 for means, standard deviations and significance levels. With respect to FA-WMB, outlier analyses revealed there were no extreme values across any of the groups. The Shapiro-Wilk test demonstrated the fundamental assumption of normality was violated across both the MCs and TBI-Pass groups (all p’s < .001), but not the TBI-Failed group (p = .328). Visual inspection of the distributions for each group showed positively skewed distributions for the MCs and TBI-Pass group, while the distributions for the TBI-Failed group appeared relatively uniform in shape. Additionally, Levene’s test of homogeneity of variances was not significant, (F (2, 76) = 2.319, p = .105), indicating this underlying assumption was met and outlier analyses revealed no extreme values.
Table 2.
White Matter Burden (WMB) by Group
| DTI Index of WMB | Military Controls | TBI-Passed | TBI-Failed | MCs vs. TBI- Passed | MCs vs. TBI-Failed | TBI-Passed vs. TBI- Failed | |||
|---|---|---|---|---|---|---|---|---|---|
| Mean (SD) | Mean (SD) | Mean (SD) | p-value | ||||||
| FA WMB | 1.30 | (1.77) | 1.77 | (2.12) | 3.69 | (2.92) | .69 | .01 | .02 |
| RD WMB | 1.56 | (2.59) | 2.28 | (2.36) | 2.46 | (2.50) | .16 | .24 | .97 |
| AD WMB | .45 | (0.85) | .73 | (1.31) | 1.69 | (2.02) | .65 | .14 | .33 |
FA = Fractional anisotropy; RD = Radial diffusivity; AD = Axial diffusivity
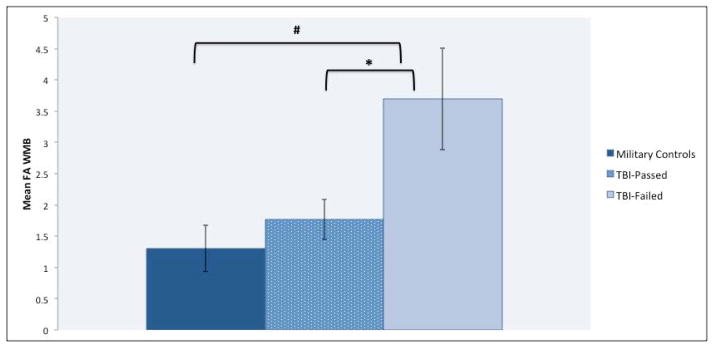
ANOVA showed a statistically significant main effect of group, (F (2,76) = 5.310, p = .007, η2p = .12), for FA-WMB. Follow-up contrasts demonstrated that the TBI-Failed subgroup had significantly greater FA-WMB (more ROIs differed from that of controls) than both the MC (Cohen’s d = 1.08, p = .006) and TBI-Passed (Cohen’s d = .83, p = .018) groups (see Figure 2). However, no significant differences were observed between MCs and the TBI-Passed groups (p > .50). Although the one-way ANOVA is generally robust against violations of non-normality66, non-parametric statistics were used to verify findings. The Kruskal-Wallis test revealed there was a statistically significant difference between the groups on FA-WMB (χ2 (2)= 7.647, p = .022) with a mean rank of 34.22 for the MCs, 38.58 for the TBI-Passed group, and 54.92 for the TBI-Failed group. Post-hoc analyses of the Kruskal-Wallis test revealed similar differences between the groups; that is, mean ranks for TBI-Failed group were statistically significant from both the TBI-Passed (p = .020) and MC (p = .007) groups, but there were no significant differences (p = .446) between the MCs and TBI-Passed groups.
Figure 2.
Fractional Anisotropic Index of White Matter Burden by Group
# p = .01, * p = .02
With respect to RD-WMB, Shapiro-Wilk tests revealed the distributions of all groups were not normally distributed (p’s < .05). Visual inspection of the distributions for each group showed positively skewed distributions for the MCs and TBI-Pass group, while the distribution for the TBI-Failed group appeared relatively uniform in shape. Outlier analyses revealed there was an outlier (MCs group; value of 10), which was removed prior to conducting subsequent analyses. Levene’s test demonstrated the fundamental assumption of for homogeneous variances was met (F (2, 75) = 1.241, p = .295). Inspection of the group means for the RD-WMB showed a WMB pattern similar to that of FA-WMB (MCs < TBI-Passed < TBI-Failed). However, ANOVA revealed there was no main effect of group (F (2,75) = 2.040, p = .137), nor did follow-up contrasts reveal any significant differences between the groups (all p’s > .05). The Kruskal-Wallis test revealed there were no significant differences between the groups on RD-WMB (χ2 (2)= 5.014, p = .082) as well.
With respect to AD-WMB, Shapiro-Wilk tests revealed the fundamental assumption of normality was violated across all groups (all p’s < .005). Visual inspection of the distributions for each group showed positively skewed distributions for the MCs and TBI-Pass group, while the distributions for the TBI-Failed group appeared relatively uniform in shape. Outlier analyses revealed two outliers (MCs group, value of 10; TBI-Pass group, value of 7), which were removed prior to conducting the group analyses. Levene’s test revealed the groups had heterogeneous variances was not met, (F (2, 74) = 5.941, p = .004) therefore, group differences were examined via the more appropriate Welch ANOVA.67 The Welch ANOVA is especially robust to both violations of normality and heterogeneous variances.67–69 Inspection of the group means for the AD-WMB showed a pattern similar to that of the other WMB indices (MCs < TBI-Passed < TBI-Failed). However, results revealed no main effect of group (F (2, 28.546) = 2.348, p = .114), nor follow-up contrasts revealed there were any significant differences between the groups (all p’s > .05). The Kruskal-Wallis test was not conducted for AD-WMB, as this test is not robust to heterogeneous variances.70
Exploration of WM ROIs
ANOVAs were conducted to examine differences across WM ROIs (see Table 3). There were some violations of normality and heterogeneity across the groups; however, when Kruskal-Wallis tests were conducted for variables with non-normal distributions, the results did not differ from ANOVA results. Outlier analyses revealed no extreme values.
Table 3.
Group Comparisons of ROIs across DTI Indices
| Track Name | Control | TBI, Pass | TBI, Fail | Control vs. TBI-Pass | Control vs. TBI-Failed | TBI-Passed vs. TBI- Failed | |
|---|---|---|---|---|---|---|---|
| FA | AIC, Left | .413 (.017) | .410 (.020) | .408 (.030) | .55 | .49 | .78 |
| AIC, Right | .422 (.020) | .419 (.018) | .407 (.023) | .54 | .04 | .07 | |
| CC Body | .493 (.020) | .498 (.016) | .487 (.014) | .24 | .33 | .04 | |
| Cing, Left | .451 (.018) | .445 (.020) | .432 (.019) | .27 | .01 | .04 | |
| Cing, Right | .430 (.018) | .428 (.019) | .417 (.021) | .71 | .06 | .08 | |
| Fornix | .381 (.032) | .386 (.019) | .381 (.017) | .40 | .91 | .58 | |
| CC Genu | .475 (.030) | .475 (.026) | .461 (.023) | .99 | .14 | .11 | |
| PIC, Left | .490 (.013) | .484 (.015) | .475 (.013) | .10 | .00 | .06 | |
| PIC, Right | .485 (.018) | .479 (.017) | .475 (.015) | .18 | .09 | .39 | |
| CC Splenium | .515 (.017) | .517 (.017) | .505 (.018) | .69 | .10 | .03 | |
|
| |||||||
| RDx10−4 | AIC, Left | 5.17 (.23) | 5.36 (.22) | 5.31 (.27) | .00 | .10 | .47 |
| AIC, Right | 5.13 (.24) | 5.26 (.19) | 5.29 (.20) | .02 | .03 | .73 | |
| CC Body | 5.18 (.33) | 5.15 (.26) | 5.08 (.30) | .67 | .32 | .46 | |
| Cing, Left | 4.97 (.23) | 5.08 (.19) | 5.14 (.19) | .04 | .02 | .36 | |
| Cing, Right | 5.08 (.22) | 5.15 (.19) | 5.24 (.24) | .19 | .03 | .16 | |
| Fornix | 8.90 (.96) | 9.06 (1.04) | 9.03 (.84) | .54 | .69 | .94 | |
| CC Genu | 5.33 (.35) | 5.38 (.26) | 5.41 (.27) | .46 | .40 | .75 | |
| PIC, Left | 4.58 (.20) | 4.66 (.16) | 4.69 (.18) | .09 | .08 | .59 | |
| PIC, Right | 4.60 (.26) | 4.70 (.18) | 4.70 (.19) | .08 | .18 | .97 | |
| CC Splenium | 5.29 (.33) | 5.30 (.28) | 5.28 (.31) | .92 | .88 | .80 | |
|
| |||||||
| ADx10−3 | AIC, Left | 1.00 (.05) | 1.04 (.05) | 1.02 (.05) | .02 | .30 | .40 |
| AIC, Right | 1.02 (.05) | 1.04 (.05) | 1.02 (.05) | .08 | .81 | .24 | |
| CC Body | 1.19 (.06) | 1.21 (.06) | 1.16 (.05) | .29 | .13 | .01 | |
| Cing, Left | 1.05 (.04) | 1.07 (.05) | 1.05 (.04) | .43 | .69 | .28 | |
| Cing, Right | 1.03 (.04) | 1.04 (.05) | 1.03 (.04) | .43 | .71 | .81 | |
| Fornix | 1.63 (.14) | 1.67 (.17) | 1.66 (.13) | .22 | .52 | .77 | |
| CC Genu | 1.18 (.06) | 1.20 (.06) | 1.17 (.04) | .31 | .43 | .10 | |
| PIC, Left | 1.05 (.04) | 1.05 (.04) | 1.04 (.03) | .39 | .51 | .15 | |
| PIC, Right | 1.04 (.04) | 1.06 (.04) | 1.04 (.04) | .20 | .96 | .32 | |
| CC Splenium | 1.28 (.07) | 1.29 (.07) | 1.25 (.04) | .40 | .23 | .05 | |
FA = Fractional anisotropy; RD = Radial diffusivity; AD = Axial diffusivity AIC= Anterior Internal Capsule; CC = Corpus Callosum; Cing = Cingulum Bundle; PIC = Posterior Internal Capsule
In regards to FA, there was a main effect of group for the left cingulum (η2p = .09, p = .02) and left posterior IC (η2p = .10, p = .01). Post-hoc analyses revealed no significant differences across all ROIs between MCs and the TBI-Passed subgroup. However, there were significant FA reductions in the TBI-Failed subgroup for the right anterior IC (Cohen’s d = .69, p = .04), left cingulum (Cohen’s d = 1.04, p = .01), and left posterior IC (Cohen’s d = 1.14, p < .01) when compared to MCs. Moreover, the TBI-Failed subgroup demonstrated significantly lower FA for the left cingulum (Cohen’s d = .67, p = .04), and in the body (Cohen’s d = .71, p = .04) and splenium (Cohen’s d = .69, p = .03) of the CC in comparison to the TBI-Passed subgroup. All other FA ROIs did not reach significance (all p’s > .05).
In regards to RD, there was a significant main effect of group for the left (η2p = .09, p = .01) and right (η2p = .08, p = .03) anterior IC, as well as for the left cingulum (η2p = .09, p = .04). Post hoc analyses revealed increased RD in the TBI-Passed subgroup for the left (Cohen’s d = .86, p < .01) and right (Cohen’s d = .63, p = .02) anterior IC, in addition to the left cingulum (Cohen’s d = .53, p = .04) when compared to MCs. Similarly, increased RD in the TBI-Failed subgroup was observed for the right anterior IC (Cohen’s d = .71, p = .03), in addition to the left (Cohen’s d = .79, p = .02) and right (Cohen’s d = .71, p = .03) cingulum relative to MCs. All other RD ROIs did not reach significance, nor were there any significant differences between the TBI-Passed and TBI-Failed subgroups detected (all p’s > .05).
ANOVA revealed a main effect of group for AD of the CC body (η2p = .08, p = .04). Post analyses revealed that compared to MCs, the TBI-Passed subgroup showed decreased AD for the left anterior IC (Cohen’s d = .24, p = .02), yet there were no significant differences between MCs and the TBI-Failed subgroup across any ROI. However, the TBI-Failed subgroup showed significantly reduced AD in the body (Cohen’s d = .82, p = .01) and splenium (Cohen’s d = .68, p = .05) of the CC, relative to the TBI-Passed subgroup. All other AD ROIs did not reach significance (all p’s > .05).
Neuropsychological Test Performances
There were some violations of normality, yet all variances were homogenous across the groups for each neuropsychological variable. Outliers were removed prior to group analyses and Kruskal-Wallis tests revealed the results did not differ from ANOVA results. ANOVA results are presented in Table 4. One-way ANOVAs revealed significant group differences across CVLT-II and D-KEFS variables (all p-values < .05), and post hoc comparisons revealed that the TBI-Failed subgroup performed significantly worse than both MCs and the TBI-Passed subgroup across multiple tests (all p’s < .05). When the TBI-Passed subgroup was directly compared to MCs, results revealed that the TBI-Passed subgroup generally demonstrated significantly poorer performance across multiple cognitive measures (all p’s < .05).
Table 4.
Cognitive Test Performance by Group
| Cognitive Test | Control | TBI-Passed | TBI-Failed | Control vs. TBI-Passed | Control vs. TBI- Failed | TBI- Passed vs. TBI- Failed | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean (SD) | Mean (SD) | Mean (SD) | p | p | ||||||
| CVLT-II 1-5 Total T-Score | 54.57 | (10.06) | 49.25 | (9.79) | 38.31 | (7.80) | p < .001 | .09 | .00 | .01 |
| CVLT-II Long Delay Free Recall Z-score | .15 | (1.10) | −.23 | (1.10) | −1.77 | (1.09) | p < .001 | .39 | .00 | .00 |
| CVLT-II Total Recognition Discriminability | .08 | (1.05) | −.22 | (1.01) | −1.59 | (1.13) | p < .001 | .50 | .00 | .00 |
| D-KEFS Letter Fluency Scaled Score | 11.61 | (2.86) | 9.95 | (3.33) | 9.00 | (2.58) | p < .05 | .10 | .05 | .62 |
| D-KEFS Category Fluency Scaled Score | 12.35 | (2.77) | 11.17 | (3.26) | 9.00 | (2.86) | p < .05 | .31 | .01 | .09 |
| D-KEFS Category- Switching Total Correct Scaled Score | 12.65 | (3.02) | 10.65 | (3.45) | 8.25 | (3.25) | p < .001 | .06 | .00 | .07 |
| D-KEFS Trails Number Sequencing Scaled Score | 11.52 | (2.19) | 10.73 | (2.49) | 9.33 | (2.71) | p = .05 | .43 | .04 | .20 |
| D-KEFS Trails Letter Sequencing Scaled Score | 11.39 | (2.06) | 10.51 | (2.63) | 9.42 | (2.35) | p = .05 | .49 | .04 | .18 |
| D-KEFS Trails Number-Letter Sequencing Scaled Score | 10.91 | (1.59) | 9.37 | (2.89) | 7.17 | (3.51) | p < .005 | .07 | .00 | .04 |
| WCST-64 Preservative Responses T- Score | 47.41 | (5.80) | 46.95 | (7.18) | 48.08 | (11.64) | p = .90 | .97 | .97 | .90 |
| WCST-64 Total Errors T-Score | 50.95 | (5.73) | 50.59 | (7.19) | 49.00 | (13.69) | p = .79 | .98 | .79 | .83 |
CVLT-II = California Verbal Learning Test-II Edition; D-KEFS = Delis-Kaplan Executive Function System; WCST-64 = Wisconsin Card Sorting Test- 64 Card Version
DISCUSSION
Using DTI, we explored neuroimaging biomarkers of WM microstructure in Veterans with history of mTBI who failed performance validity tests (PVTs), those with mTBI who passed PVTs, and military controls with no neurotrauma history. The groups significantly differed in FA-WMB, an aggregate score reflecting the number of ROIs in which there is a significant deviation from the control mean. Specifically, the TBI-Failed subgroup showed greater WMB when compared to either MCs or TBI-Passed subgroups; and no significant differences in WMB were observed between MCs and the TBI-Passed subgroup. Exploratory analyses of WM tracts revealed decreased WM integrity across both TBI subgroups when compared to MCs. Furthermore, in line with our WMB findings, there were significant WM alterations across several ROIs in the TBI-Failed subgroup relative to the TBI-Passed subgroup. Finally, significantly lower cognitive scores were observed in the TBI-Failed group relative to both the MCs and TBI-Passed group.
While some studies show notable and robust abnormalities of WM microstructure in both civilians and Veterans with mTBI when compared to control groups, 71–74 other studies have failed to find such differences.75–77 Reasons for variability across studies remain unclear, but could be related to heterogeneity of diagnostic criteria used,78,79 differences in clinic-pathologic characteristics of mTBI, 80 and variation in imaging acquisition or analysis techniques.81, 82 Further complicating matters, many military controls are exposed to blast during their service, and there is debate regarding whether blast exposure causes measurable brain structural alterations.83,84 General exposure to blast may obscure group differences between controls and mTBI participants.
Contrary to expectations, comparisons between the TBI subgroups revealed a greater degree of overall WM damage in the TBI-Failed subgroup when compared to the TBI-Passed subgroup. Examination of WM ROIs showed a similar pattern and revealed an increased level of WM microstructural abnormalities across commissural tracks in the TBI-Failed subgroup relative to the TBI-Passed subgroup. Those with poor PVT performance in our study generally displayed worse WM integrity and reported elevated psychiatric symptoms. Thus, those who fail PVTs may subjectively experience greater cognitive or psychiatric difficulties than those who pass PVT measures because of greater underlying brain abnormalities. Consistent with this possibility, a study by Lippa and colleagues85 showed that poor PVT performance, above and beyond various demographic and injury factors, predicted less community integration and participation in Veterans with mTBI. Findings of their study are of particular importance as the veracity of impairment may be called into question due to poor PVT performance. Results of the present study align with those of Lippa and colleagues85 and underscore the possibility that individuals who fail PVTs may in fact experience considerable difficulties and be in need of clinical resources, as the increased symptom endorsement, albeit potentially exaggerated, may be associated with genuine structural impairments.
Although we failed to detect differences between MCs and the TBI-Passed group on our global measure of WM integrity (WMB), ROI analyses revealed WM alterations across both TBI subgroups relative to MCs. More specifically, both TBI subgroups demonstrated consistent WM alterations in the anterior IC and cingulum. Importantly, these fronto-limbic and fronto-striatal tracts connect regions critical for emotional and higher-order cognitive functioning, and may be especially vulnerable to shear and tensile strain that occurs during neurotrauma. 86,87 Additionally, ROI analyses between the TBI-Passed and TBI-Failed subgroups revealed significantly decreased FA and AD in the body and splenium of the CC in the TBI-Failed subgroup. As detailed in a meta-analysis by Aoki et al.88, the unique organization of the CC may contribute to its increased vulnerability to neurotrauma when compared to less organized tracts. Furthermore, the falx cerebri may come into contact with the anterior and posterior aspects of the CC, acting as a fulcrum in the mechanical distortion of the CC during trauma. As such, disrupted inter-hemispheric communication, or integration of the CC with frontal and temporal lobes, may play a pivotal role in the clinical presentation of the TBI-Failed subgroup. Mechanisms behind lower FA-WMB in the TBI-Failed subgroup are unclear. The TBI-Failed subgroups reported slightly greater LOC durations and were closer in proximity to incurred head injuries; thus, it is possible the increased WM damage within the TBI-Failed subgroup may be the product of more severe75 and less remote injuries.89 It must be acknowledged that a number of studies have demonstrated associations between DTI-measured WM differences and psychiatric symptoms.90–92 In other words, it is possible that WM damage contributes to increased psychiatric symptomatology within the TBI-Failed subgroup and better explains observed WM differences between the groups.
Clinical decision-making in the context of PVT failure is complex and several theories have been proffered to explain possible factors behind poor PVT performance. Bigler26,27 has noted that at least some PVT failures may be related to neurological deficits,93–95, or disruption of brain regions actively engaged during PVT performance (see Bigler26 for review). For example, performance during the Word Memory Test, a commonly used PVT, initiates activation of fronto-executive neural systems96,97 which have been linked to structural impairments in both civilians and Veterans with mTBI.73,74 Additionally, the practice of dichotomizing PVT performance may be limiting, as useful information may be gleaned from the evaluation of PVT performance on a continuum.26,27,98 Clearly, additional research examining these proposed hypotheses—especially in the context of neuroimaging findings—is needed.
To the best of our knowledge, this represents the first study to compare the structural integrity of cerebral WM tracts between those who pass and fail PVTs in the context of military TBI. However, there are some weaknesses of our study that are important to note. For example, diagnosis of TBI within this study relies largely on restropective self-report and may be subject to recall bias; although this is a common limitation in TBI assessment, and it must be acknowledged that these time differences may have caused differential effects between the groups99. Importantly, the reliability and validity of the TBI measure utilized has not been established, though such efforts are currently underway. In addition, poor PVT was defined by reduced performance on at least one of two measures, and the application of stricter criteria,100 may have yielded different findings. The majority of those with poor PVT performance in our study failed the CVLT-FCR (n = 9), as opposed to the TOMM (n = 3), which may be a reflection of varying degrees of sensitivity and specificity of each test. Moreover, recently, there has been a distinction made between PVTs and symptom validity tests (SVTs)101, as SVTs may better speak to self-reported symptomatic complaints. Furthermore, although our sample is likely representative of OEF/OIF/OND Veterans with history of mTBI, our current sample was relatively small, therefore sampling bias must be considered and replication of these findings is needed. Finally, due to the exploratory nature, ROI analyses were not corrected for multiple comparisons, thus there is an increased likelihood of Type I error.
Taken together, our results demonstrate that WM microstructural alterations were evident in a sample of Veterans with mTBI who failed PVTs, and the extent of WM damage in this subgroup was unexpectedly greater than those with mTBI who passed PVTs. Results of the current study highlight that, despite the understandable cautious interpretation of cognitive scores in the context of poor PVT performance, such individuals may still have genuine deficits owing to the presence of WM abnormalities. As our understanding of the relationship between measures of WM microstructure and cognitive functions improves, DTI may represent an invaluable marker and objective measure of WM damage and poor clinical outcome.
Acknowledgments
Sources of Funding: This work was supported by grants awarded by the Veterans Affairs (Career Development Awards [CDA]: L.D.-W., D.S.; Merit Award; L.D.-W.) as well as the Department of Defense (Investigator-Initiated Research Grant [IIRG]: L.D.-W.). This material is further supported with resources of the Veterans Affairs Center of Excellence for Stress and Mental Health (CESAMH: L.D.-W.).
The authors would like to thank all OEF/OIF/OND Veterans for their service and are extremely appreciative of those who volunteered to participate in this study. In addition, a special thanks is owed to the Veterans Affairs CESAMH at the Veterans Affairs San Diego Healthcare System for their organizational assistance and to the wonderful research assistants (RK, EL, NL) who dedicate their time to our research lab.
Footnotes
Conflicts of Interest: The authors report no conflicts of interests.
Author Contributions: AC, SS, MB, LDW contributed to manuscript conception. AC performed the data analysis and SS aided with interpretation. AC drafted the paper with contributions from SS. LDW, EB, MB, DS, AJ, & MJ provided critical revisions of paper drafts. NL, EL, and RK collected the data.
References
- 1.Armistead-Jehle P, Hansen CL. Comparison of the Repeatable Battery for the Assessment of Neuropsychological Status Effort Index and stand-alone symptom validity tests in a military sample. Arch Clin Neuropsychol. 2011;26(7):592–601. doi: 10.1093/arclin/acr049. [DOI] [PubMed] [Google Scholar]
- 2.Armistead-Jehle P. Symptom validity test performance in U.S. veterans referred for evaluation of mild TBI. Appl Neuropsychol. 2010;17(1):52–59. doi: 10.1080/09084280903526182. [DOI] [PubMed] [Google Scholar]
- 3.Clark AL, Amick MM, Fortier C, Milberg WP, McGlinchey RE. Poor performance validity predicts clinical characteristics and cognitive test performance of OEF/OIF/OND Veterans in a research setting. Clin Neuropsychol. 2014;28(5):802–825. doi: 10.1080/13854046.2014.904928. [DOI] [PubMed] [Google Scholar]
- 4.Gfeller JD, Roskos PT. A comparison of insufficient effort rates, neuropsychological functioning, and neuropsychiatric symptom reporting in military veterans and civilians with chronic traumatic brain injury. Behav Sci Law. 2013;31(6):833–849. doi: 10.1002/bsl.2084. [DOI] [PubMed] [Google Scholar]
- 5.Lange RT, Iverson GL, Brooks BL, Rennison VL. Influence of poor effort on self-reported symptoms and neurocognitive test performance following mild traumatic brain injury. J Clin Exp Neuropsychol. 2010;32(9):961–972. doi: 10.1080/13803391003645657. [DOI] [PubMed] [Google Scholar]
- 6.Lange RT, Brickell T, French LM, et al. Risk factors for postconcussion symptom reporting after traumatic brain injury in U.S. military service members. J Neurotrauma. 2013;30(4):237–246. doi: 10.1089/neu.2012.2685. [DOI] [PubMed] [Google Scholar]
- 7.McCormick CL, Yoash-Gantz RE, McDonald SD, Campbell TC, Tupler LA. Performance on the Green Word Memory Test following Operation Enduring Freedom/Operation Iraqi Freedom-era military service: Test failure is related to evaluation context. Arch Clin Neuropsychol. 2013;28(8):808–823. doi: 10.1093/arclin/act050. [DOI] [PubMed] [Google Scholar]
- 8.Nelson NW, Hoelzle JB, McGuire KA, Ferrier-Auerbach AG, Charlesworth MJ, Sponheim SR. Evaluation context impacts neuropsychological performance of OEF/OIF veterans with reported combat-related concussion. Arch Clin Neuropsychol. 2010;25(8):713–723. doi: 10.1093/arclin/acq075. [DOI] [PubMed] [Google Scholar]
- 9.Whitney KA, Shepard PH, Williams AL, Davis JJ, Adams KM. The Medical Symptom Validity Test in the evaluation of Operation Iraqi Freedom/Operation Enduring Freedom soldiers: a preliminary study. Arch Clin Neuropsychol. 2009;24(2):145–152. doi: 10.1093/arclin/acp020. [DOI] [PubMed] [Google Scholar]
- 10.Drane DL, Williamson DJ, Stroup ES, et al. Cognitive impairment is not equal in patients with epileptic and psychogenic nonepileptic seizures. Epilepsia. 2006;47(11):1879–1886. doi: 10.1111/j.1528-1167.2006.00611.x. [DOI] [PubMed] [Google Scholar]
- 11.Gervais RO, Russell AS, Green P, Allen LM, 3rd, Ferrari R, Pieschl SD. Effort testing in patients with fibromyalgia and disability incentives. J Rheumatol. 2001;28(8):1892–1899. [PubMed] [Google Scholar]
- 12.Wisdom NM, Brown WL, Chen DK, Collins RL. The use of all three Test of Memory Malingering trials in establishing the level of effort. Arch Clin Neuropsychol. 2012;27(2):208–212. doi: 10.1093/arclin/acr107. [DOI] [PubMed] [Google Scholar]
- 13.Hou R, Moss-Morris R, Peveler R, Mogg K, Bradley BP, Belli A. When a minor head injury results in enduring symptoms: a prospective investigation of risk factors for postconcussional syndrome after mild traumatic brain injury. J Neurol Neurosurg Psychiatry. 2012;83(2):217–223. doi: 10.1136/jnnp-2011-300767. [DOI] [PubMed] [Google Scholar]
- 14.Tombaugh TN. Test of memory malingering. Toronto, Ontario, Canada: Multi-Health Systems, Inc; 1996. [Google Scholar]
- 15.Carone DA. Children with moderate/severe brain damage/dysfunction outperform adults with mild-to-no brain damage on the Medical Symptom Validity Test. Brain Inj. 2008;22(12):960–971. doi: 10.1080/02699050802491297. [DOI] [PubMed] [Google Scholar]
- 16.Lange RT, Pancholi S, Bhagwat A, Anderson-Barnes V, French LM. Influence of poor effort on neuropsychological test performance in U.S. military personnel following mild traumatic brain injury. J Clin Exp Neuropsychol. 2012;34(5):453–466. doi: 10.1080/13803395.2011.648175. [DOI] [PubMed] [Google Scholar]
- 17.Tsanadis J, Montoya E, Hanks RA, Millis SR, Fichtenberg NL, Axelrod BN. Brain injury severity, litigation status, and self-report of postconcussive symptoms. Clin Neuropsychol. 2008;22(6):1080–1092. doi: 10.1080/13854040701796928. [DOI] [PubMed] [Google Scholar]
- 18.Flaro L, Green P, Robertson E. Word Memory Test failure 23 times higher in mild brain injury than in parents seeking custody: the power of external incentives. Brain Inj. 2007;21(4):373–383. doi: 10.1080/02699050701311133. [DOI] [PubMed] [Google Scholar]
- 19.Wolfe PL, Millis SR, Hanks R, Fichtenberg N, Larrabee GJ, Sweet JJ. Effort indicators within the California Verbal Learning Test-II (CVLT-II) Clin Neuropsychol. 2010;24(1):153–168. doi: 10.1080/13854040903107791. [DOI] [PubMed] [Google Scholar]
- 20.Zabecki DT. Stolen Valor: How the Vietnam Generation Was Robbed of Its Heroes and Its History. Mil Rev. 2000;80(2):106. [Google Scholar]
- 21.Elhai JD, Sweet JJ, Guidotti-Breting LM, Kaloupek D. Assessment in contexts that threaten response validity. PTSD and mild traumatic brain injury [Google Scholar]
- 22.Frueh BC, Elhai JD, Grubaugh AL, et al. Documented combat exposure of US veterans seeking treatment for combat-related post-traumatic stress disorder. Br J Psychiatry. 2005;186:467–472. doi: 10.1192/bjp.186.6.467. discussion 473–465. [DOI] [PubMed] [Google Scholar]
- 23.Robert J, Spencer RSR. Iatrogenic Risk in the Management of Mild Traumatic Brain Injury among Combat Veterans: A Case Illustration and Commentary. Intl Jour of Phys Med & Rehab. 2013;01(01) [Google Scholar]
- 24.Silver JM. Effort, exaggeration and malingering after concussion. J Neurol Neurosurg Psychiatr. 2012;83(8):836–841. doi: 10.1136/jnnp-2011-302078. [DOI] [PubMed] [Google Scholar]
- 25.Merten T, Bossink L, Schmand B. On the limits of effort testing: Symptom validity tests and severity of neurocognitive symptoms in nonlitigant patients. J Clin Exp Neuropsychol. 2007;29(3):308–318. doi: 10.1080/13803390600693607. [DOI] [PubMed] [Google Scholar]
- 26.Bigler ED. Effort, symptom validity testing, performance validity testing and traumatic brain injury. Brain Inj. 2014;28(13–14):1623–1638. doi: 10.3109/02699052.2014.947627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bigler ED. Symptom validity testing, effort, and neuropsychological assessment. J Int Neuropsychol Soc. 2012;18(4):632–640. doi: 10.1017/s1355617712000252. [DOI] [PubMed] [Google Scholar]
- 28.Hetherington HP, Hamid H, Kulas J, et al. MRSI of the medial temporal lobe at 7 T in explosive blast mild traumatic brain injury. Magn Reson Med. 2014;71(4):1358–1367. doi: 10.1002/mrm.24814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hoge CW, McGurk D, Thomas JL, Cox AL, Engel CC, Castro CA. Mild traumatic brain injury in U.S. soldiers returning from iraq. N Engl J Med. 2008;358(5):453–463. doi: 10.1056/NEJMoa072972. [DOI] [PubMed] [Google Scholar]
- 30.Traumatic Brain Injury Task Force. [Accessed June 11, 2015];Report to the surgeon general: TBI task force. https://www.hsdl.org/?view&did=482727. Published January, 2008.
- 31.Vanderploeg RD, Groer S, Belanger HG. Initial developmental process of a VA semistructured clinical interview for TBI identification. J Rehabil Res Dev. 2012;49(4):545–556. doi: 10.1682/jrrd.2011.04.0069. [DOI] [PubMed] [Google Scholar]
- 32.Corrigan JD, Bogner J. Initial reliability and validity of the Ohio State University TBI Identification Method. J Head Trauma Rehabil. 2007;22(6):318–329. doi: 10.1097/01.HTR.0000300227.67748.77. [DOI] [PubMed] [Google Scholar]
- 33.Fortier CB, Amick MM, Kenna A, Milberg WP, McGlinchey RE. Correspondence of the Boston Assessment of Traumatic Brain Injury-Lifetime (BAT-L) clinical interview and the VA TBI screen. J Head Trauma Rehabil. 2015;30(1):E1–7. doi: 10.1097/HTR.0000000000000008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schwab KA, Ivins B, Cramer G, et al. Screening for traumatic brain injury in troops returning from deployment in Afghanistan and Iraq: initial investigation of the usefulness of a short screening tool for traumatic brain injury. J Head Trauma Rehabil. 2007;22(6):377–389. doi: 10.1097/01.HTR.0000300233.98242.87. [DOI] [PubMed] [Google Scholar]
- 35.Terrio H, Brenner LA, Ivins BJ, et al. Traumatic brain injury screening: preliminary findings in a US Army Brigade Combat Team. J Head Trauma Rehabil. 2009;24(1):14–23. doi: 10.1097/HTR.0b013e31819581d8. [DOI] [PubMed] [Google Scholar]
- 36.Donnelly KT, Donnelly JP, Dunnam M, et al. Reliability, sensitivity, and specificity of the VA traumatic brain injury screening tool. J Head Trauma Rehabil. 2011;26(6):439–453. doi: 10.1097/HTR.0b013e3182005de3. [DOI] [PubMed] [Google Scholar]
- 37.Delis D, Kaplan E, Kramer GL. D-KEFS examiner’s and technical manual. San Antonio, TX: Pearson Education; 2001. [Google Scholar]
- 38.Delis DC, Kramer J, Kaplan E, Ober B. California Verbal Learning Test-second edition. San Antonio, TX: The Psychological Corporation; 2000. [Google Scholar]
- 39.Kongs SK, Thompson LL, Iverson GL, Heaton RK. Wisconsin Card Sorting Test-64 Card Version: Professional Manual. Odessa, FL: Psychological Assessment Resources, Inc; 2000. [Google Scholar]
- 40.Wilkinson GS, Robertson GJ. Wide Range Achievement Test—Fourth Edition. Lutz, FL: Psychological Assessment Resources; 2006. [Google Scholar]
- 41.Cicerone KD, Kalmar K. Persistent postconcussion syndrome: the structure of subjective complaints after mTBI. J Head Trauma Rehabil. 1995;10:1–17. [Google Scholar]
- 42.Asmundson GJ, Frombach I, McQuaid J, Pedrelli P, Lenox R, Stein MB. Dimensionality of posttraumatic stress symptoms: a confirmatory factor analysis of DSM-IV symptom clusters and other symptom models. Behav Res Ther. 2000;38(2):203–214. doi: 10.1016/s0005-7967(99)00061-3. [DOI] [PubMed] [Google Scholar]
- 43.Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory-II. San Antonio, TX: Psychological Corporation; 1996. [Google Scholar]
- 44.Haber AH, Fichtenberg NL. Replication of the test of memory malingering (TOMM) in a traumatic brain injury and head trauma sample. Clin Neuropsychol. 2006;20(3):524–532. doi: 10.1080/13854040590967595. [DOI] [PubMed] [Google Scholar]
- 45.Moore BA, Donders J. Predictors of invalid neuropsychological test performance after traumatic brain injury. Brain Inj. 2004;18(10):975–984. doi: 10.1080/02699050410001672350. [DOI] [PubMed] [Google Scholar]
- 46.Reese TG, Heid O, Weisskoff RM, Wedeen VJ. Reduction of eddy-current-induced distortion in diffusion MRI using a twice-refocused spin echo. Magn Reson Med. 2003;49(1):177–182. doi: 10.1002/mrm.10308. [DOI] [PubMed] [Google Scholar]
- 47.Jones DK, Horsfield MA, Simmons A. Optimal strategies for measuring diffusion in anisotropic systems by magnetic resonance imaging. Magn Reson Med. 1999;42(3):515–525. [PubMed] [Google Scholar]
- 48.Pierpaoli C, Basser PJ. Toward a quantitative assessment of diffusion anisotropy. Magn Reson Med. 1996;36(6):893–906. doi: 10.1002/mrm.1910360612. [DOI] [PubMed] [Google Scholar]
- 49.Basser PJ. Inferring microstructural features and the physiological state of tissues from diffusion-weighted images. NMR Biomed. 1995;8(7–8):333–344. doi: 10.1002/nbm.1940080707. [DOI] [PubMed] [Google Scholar]
- 50.Beaulieu C. The basis of anisotropic water diffusion in the nervous system - a technical review. NMR Biomed. 2002;15(7–8):435–455. doi: 10.1002/nbm.782. [DOI] [PubMed] [Google Scholar]
- 51.Burzynska AZ, Preuschhof C, Bäckman L, et al. Age-related differences in white matter microstructure: Region-specific patterns of diffusivity. Neuroimage. 2010;49(3):2104–2112. doi: 10.1016/j.neuroimage.2009.09.041. [DOI] [PubMed] [Google Scholar]
- 52.Basser PJ, Pierpaoli C. Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. J Magn Reson B. 1996;111(3):209–219. doi: 10.1006/jmrb.1996.0086. [DOI] [PubMed] [Google Scholar]
- 53.Song S, Sun S, Ramsbottom MJ, Chang C, Russell J, Cross AH. Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. Neuroimage. 2002;17(3):1429–1436. doi: 10.1006/nimg.2002.1267. [DOI] [PubMed] [Google Scholar]
- 54.Song S, Yoshino J, Le TQ, et al. Demyelination increases radial diffusivity in corpus callosum of mouse brain. Neuroimage. 2005;26(1):132–140. doi: 10.1016/j.neuroimage.2005.01.028. [DOI] [PubMed] [Google Scholar]
- 55.Smith SM, Jenkinson M, Woolrich MW, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23 (Suppl 1):S208–219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- 56.Mori S, Crain BJ, Chacko VP, van Zijl PC. Three-dimensional tracking of axonal projections in the brain by magnetic resonance imaging. Ann Neurol. 1999;45(2):265–269. doi: 10.1002/1531-8249(199902)45:2<265::aid-ana21>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 57.Vassar RL, Barnea-Goraly N, Rose J. Identification of neonatal white matter on DTI: influence of more inclusive thresholds for atlas segmentation. PLoS One. 2014;9(12):e115426. doi: 10.1371/journal.pone.0115426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mori S, van Zijl PCM. Fiber tracking: Principles and strategies - a technical review. NMR Biomed. 2002;15(7–8):468–480. doi: 10.1002/nbm.781. [DOI] [PubMed] [Google Scholar]
- 59.Wakana S, Jiang H, Nagae-Poetscher LM, van Zijl PC, Mori S. Fiber tract-based atlas of human white matter anatomy. Radiology. 2004;230(1):77–87. doi: 10.1148/radiol.2301021640. [DOI] [PubMed] [Google Scholar]
- 60.Hofer S, Frahm J. Topography of the human corpus callosum revisited--comprehensive fiber tractography using diffusion tensor magnetic resonance imaging. Neuroimage. 2006;32(3):989–994. doi: 10.1016/j.neuroimage.2006.05.044. [DOI] [PubMed] [Google Scholar]
- 61.Concha L, Gross DW, Beaulieu C. Diffusion tensor tractography of the limbic system. AJNR Am J Neuroradiol. 2005;26(9):2267–2274. [PMC free article] [PubMed] [Google Scholar]
- 62.Kraus MF, Susmaras T, Caughlin BP, et al. White matter integrity and cognition in chronic traumatic brain injury: a diffusion tensor imaging study. Brain. 2007;130(Pt 10):2508–2519. doi: 10.1093/brain/awm216. [DOI] [PubMed] [Google Scholar]
- 63.Hoaglin DC, Iglewicz B. Fine-tuning some resistant rules for outlier labeling. Jour Amer Stat Assoc. 1987;82(400):1147–1149. [Google Scholar]
- 64.Iglewicz B, Hoaglin DC. How to detect and handle outliers. Milwaukee, WI: ASQC Quality Press; 1993. [Google Scholar]
- 65.IBM Corp. IBM SPSS Statistics for Mac [computer program]. Version 22.0. Armonk, NY: [Google Scholar]
- 66.Khan A, Rayner GD. Robustness to non-normality of common tests for the many sample location problem. Jour Appl Math & Dec Sci. 2003;7(4):187–206. [Google Scholar]
- 67.De Beuckelaer A. A closer examination on some parametric alternatives to the ANOVA F-test. Statistical Papers. 1996;37(4):291–305. [Google Scholar]
- 68.Levy KJ. An empirical comparison of the ANOVA F-test with alternatives which are more robust against heterogeneity of variance. Jour of stat compu simul. 1978;8(1):49–57. [Google Scholar]
- 69.Schneider PJ, Penfield DA. Alexander and Govern’s approximation: Providing an alternative to ANOVA under variance heterogeneity. Journ of experi educ. 1997;65(3):271–286. [Google Scholar]
- 70.Zimmerman DW. Statistical significance levels of nonparametric tests biased by heterogeneous variances of treatment groups. Journal of gen psych. 2000;127(4):354–364. doi: 10.1080/00221300009598589. [DOI] [PubMed] [Google Scholar]
- 71.Petrie EC, Cross DJ, Yarnykh VL, et al. Neuroimaging, behavioral, and psychological sequelae of repetitive combined blast/impact mild traumatic brain injury in Iraq and Afghanistan war veterans. J Neurotrauma. 2014;31(5):425–436. doi: 10.1089/neu.2013.2952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mac Donald CL, Johnson AM, Cooper D, et al. Detection of blast-related traumatic brain injury in U.S. military personnel. N Engl J Med. 2011;364(22):2091–2100. doi: 10.1056/NEJMoa1008069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wäljas M, Lange RT, Hakulinen U, et al. Biopsychosocial outcome after uncomplicated mild traumatic brain injury. J Neurotrauma. 2014;31(1):108–124. doi: 10.1089/neu.2013.2941. [DOI] [PubMed] [Google Scholar]
- 74.Jorge RE, Acion L, White T, et al. White matter abnormalities in veterans with mild traumatic brain injury. Am J Psychiatry. 2012;169(12):1284–1291. doi: 10.1176/appi.ajp.2012.12050600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sorg SF, Delano-Wood L, Luc N, et al. White matter integrity in veterans with mild traumatic brain injury: associations with executive function and loss of consciousness. J Head Trauma Rehabil. 2014;29(1):21–32. doi: 10.1097/HTR.0b013e31828a1aa4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lange RT, Panenka WJ, Shewchuk JR, et al. Diffusion tensor imaging findings and postconcussion symptom reporting six weeks following mild traumatic brain injury. Arch Clin Neuropsychol. 2015;30(1):7–25. doi: 10.1093/arclin/acu060. [DOI] [PubMed] [Google Scholar]
- 77.Davenport ND, Lim KO, Sponheim SR. White matter abnormalities associated with military PTSD in the context of blast TBI. Hum Brain Mapp. 2015;36(3):1053–1064. doi: 10.1002/hbm.22685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ruff RM, Jurica P. In search of a unified definition for mild traumatic brain injury. Brain Inj. 1999;13(12):943–952. doi: 10.1080/026990599120963. [DOI] [PubMed] [Google Scholar]
- 79.Tellier A, Marshall SC, Wilson KG, et al. The heterogeneity of mild traumatic brain injury: Where do we stand? Brain Inj. 2009;23(11):879–887. doi: 10.1080/02699050903200555. [DOI] [PubMed] [Google Scholar]
- 80.Culotta VP, Sementilli ME, Gerold K, Watts CC. Clinicopathological heterogeneity in the classification of mild head injury. Neurosurgery. 1996;38(2):245–250. doi: 10.1097/00006123-199602000-00002. [DOI] [PubMed] [Google Scholar]
- 81.Hulkower MB, Poliak DB, Rosenbaum SB, Zimmerman ME, Lipton ML. A decade of DTI in traumatic brain injury: 10 years and 100 articles later. AJNR Am J Neuroradiol. 2013;34(11):2064–2074. doi: 10.3174/ajnr.A3395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lange RT, Shewchuk JR, Heran MK, et al. To exclude or not to exclude: further examination of the influence of white matter hyperintensities in diffusion tensor imaging research. J Neurotrauma. 2014;31(2):198–205. doi: 10.1089/neu.2013.2866. [DOI] [PubMed] [Google Scholar]
- 83.Taber KH, Hurley RA, Haswell CC, et al. White matter compromise in veterans exposed to primary blast forces. J Head Trauma Rehabil. 2015;30(1):E15–25. doi: 10.1097/HTR.0000000000000030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Levin HS, Wilde E, Troyanskaya M, et al. Diffusion tensor imaging of mild to moderate blast-related traumatic brain injury and its sequelae. J Neurotrauma. 2010;27(4):683–694. doi: 10.1089/neu.2009.1073. [DOI] [PubMed] [Google Scholar]
- 85.Lippa SM, Pastorek NJ, Romesser J, et al. Ecological validity of performance validity testing. Arch Clin Neuropsychol. 2014;29(3):236–244. doi: 10.1093/arclin/acu002. [DOI] [PubMed] [Google Scholar]
- 86.McAllister TW, Ford JC, Ji S, et al. Maximum principal strain and strain rate associated with concussion diagnosis correlates with changes in corpus callosum white matter indices. Ann Biomed Eng. 2012;40(1):127–140. doi: 10.1007/s10439-011-0402-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yeh PH, Wang B, Oakes TR, et al. Postconcussional disorder and PTSD symptoms of military-related traumatic brain injury associated with compromised neurocircuitry. Hum Brain Mapp. 2014;35(6):2652–2673. doi: 10.1002/hbm.22358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Aoki Y, Inokuchi R, Gunshin M, Yahagi N, Suwa H. Diffusion tensor imaging studies of mild traumatic brain injury: A meta-analysis. J Neurol Neurosurg Psychiatry. 2012;83(9):870–876. doi: 10.1136/jnnp-2012-302742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Toth A, Kovacs N, Perlaki G, et al. Multi-modal magnetic resonance imaging in the acute and sub-acute phase of mild traumatic brain injury: Can we see the difference? J Neurotrauma. 2013;30(1):2–10. doi: 10.1089/neu.2012.2486. [DOI] [PubMed] [Google Scholar]
- 90.Dalby RB, Frandsen J, Chakravarty MM, et al. Depression severity is correlated to the integrity of white matter fiber tracts in late-onset major depression. Psychiatry Res. 2010;184(1):38–48. doi: 10.1016/j.pscychresns.2010.06.008. [DOI] [PubMed] [Google Scholar]
- 91.Matthews SC, Strigo IA, Simmons AN, O’Connell RM, Reinhardt LE, Moseley SA. A multimodal imaging study in U.S. veterans of Operations Iraqi and Enduring Freedom with and without major depression after blast-related concussion. Neuroimage. 2011;54 (Suppl 1):S69–75. doi: 10.1016/j.neuroimage.2010.04.269. [DOI] [PubMed] [Google Scholar]
- 92.Sanjuan PM, Thoma R, Claus ED, Mays N, Caprihan A. Reduced white matter integrity in the cingulum and anterior corona radiata in posttraumatic stress disorder in male combat veterans: a diffusion tensor imaging study. Psychiatry Res. 2013;214(3):260–268. doi: 10.1016/j.pscychresns.2013.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Merten T, Bossink L, Schmand B. On the limits of effort testing: Symptom validity tests and severity of neurocognitive symptoms in nonlitigant patients. J Clin Exp Neuropsychol. 2007;29(3):308–318. doi: 10.1080/13803390600693607. [DOI] [PubMed] [Google Scholar]
- 94.Hall VL, Worthington A, Venables K. A UK pilot study: The specificity of the word memory test effort sub-tests in acute minimal to mild head injury. J Neuropsychol. 2014;8(2):216–230. doi: 10.1111/jnp.12021. [DOI] [PubMed] [Google Scholar]
- 95.Willis PF, Farrer TJ, Bigler ED. Are effort measures sensitive to cognitive impairment? Mil Med. 2011;176(12):1426–1431. doi: 10.7205/milmed-d-11-00168. [DOI] [PubMed] [Google Scholar]
- 96.Allen MD, Bigler ED, Larsen J, Goodrich-Hunsaker NJ, Hopkins RO. Functional neuroimaging evidence for high cognitive effort on the word memory test in the absence of external incentives. Brain Inj. 2007;21(13–14):1425–1428. doi: 10.1080/02699050701769819. [DOI] [PubMed] [Google Scholar]
- 97.Larsen JD, Allen MD, Bigler ED, Goodrich-Hunsaker NJ, Hopkins RO. Different patterns of cerebral activation in genuine and malingered cognitive effort during performance on the word memory test. Brain Inj. 2010;24(2):89–99. doi: 10.3109/02699050903508218. [DOI] [PubMed] [Google Scholar]
- 98.Lippa SM, Agbayani KA, Hawes S, Jokic E, Caroselli JS. Effort in acute traumatic brain injury: Considering more than pass/fail. Rehabil Psychol. 2014;59(3):306–312. doi: 10.1037/a0037217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Vagnozzi R, Signoretti S, Cristofori L, et al. Assessment of metabolic brain damage and recovery following mild traumatic brain injury: a multicentre, proton magnetic resonance spectroscopic study in concussed patients. Brain. 2010;133(11):3232–3242. doi: 10.1093/brain/awq200. [DOI] [PubMed] [Google Scholar]
- 100.Slick DJ, Sherman EM, Iverson GL. Diagnostic criteria for malingered neurocognitive dysfunction: proposed standards for clinical practice and research. Clin Neuropsychol. 1999;13(4):545–561. doi: 10.1076/1385-4046(199911)13:04;1-Y;FT545. [DOI] [PubMed] [Google Scholar]
- 101.Larrabee GJ. Performance validity and symptom validity in neuropsychological assessment. J Int Neuropsychol Soc. 2012;18(4):625–630. doi: 10.1017/s1355617712000240. [DOI] [PubMed] [Google Scholar]