Abstract
Novel molecular, cellular and pharmacological therapies to stimulate repair of sensorimotor circuits after stroke are entering clinical trials. Compared to acute neuroprotection and thrombolysis studies, clinical trials for repair in subacute and chronic hemiplegic participants have a different time course for delivery of an intervention, different mechanisms of action within the milieu of the injury, distinct relationships to the amount of physical activity and skills practice of participants, and need to include more refined outcome measures. This review examines the biological interaction of targeted rehabilitation with neural repair strategies to optimize outcomes. We suggest practical guidelines for the incorporation of inexpensive skills training and exercise at home. In addition, we describe some novel outcome measurement tools, including wearable sensors, to obtain the more detailed outcomes that may identify at least some minimal level of success from cellular and regeneration interventions. Thus, proceeding in the shadow of acute stroke trial designs may unnecessarily limit the mechanisms of action of new repair strategies, reduce their impact on participants, and risk missing important behavioral outcomes.
Keywords: stroke rehabilitation, mHealth, brain tissue regeneration, neuronal plasticity, axonal sprouting, physical therapy, clinical trials, stem cell
Introduction
Therapies that stimulate CNS repair for recovery after stroke are reaching the stage of phase II and III clinical trials,1,2 as have several cellular interventions for spinal cord injury.3,4 Basic science studies of stroke have provided a growing number of potential molecular targets and types of cells that may serve as recovery-promoting agents. Compared to acute endovascular or neuroprotective interventions for ischemic stroke, new biological therapies will be directed at later time points after onset of physical and cognitive impairments and disability, will have to alter different cellular and molecular events in the brain, and may depend on neurorehabilitation strategies carried out in parallel. In addition, the outcome measures of preclinical and clinical acute stroke trials, such as infarct size or typical neurological impairment, disability, and quality of life scales, may not be as relevant for neural repair. Yet, many of the proposed clinical repair trial designs aimed primarily at improving motor control appear to include the same major elements as neuroprotection trials. The safe familiarity of proceeding as in the past may unnecessarily limit the mechanisms of action of new repair strategies, reduce their impact on participants in trials, and risk missing important behavioral outcomes.
This review focuses on the mechanisms and time course of tissue repair after stroke with hemiplegia and the necessity for incorporation of rehabilitation strategies to augment the goals of novel molecular, cellular and pharmacological interventions. We also pose practical considerations to improve the design and outcome measurements of restorative trials. The particular requirements of controlled trials for neural repair are essential to optimize the opportunity for moderate to severely impaired patients to achieve better motor outcomes, as well as to combat the hype of stem cell spas that claim to treat stroke and other neurological diseases.5
Phases of Recovery after Stroke
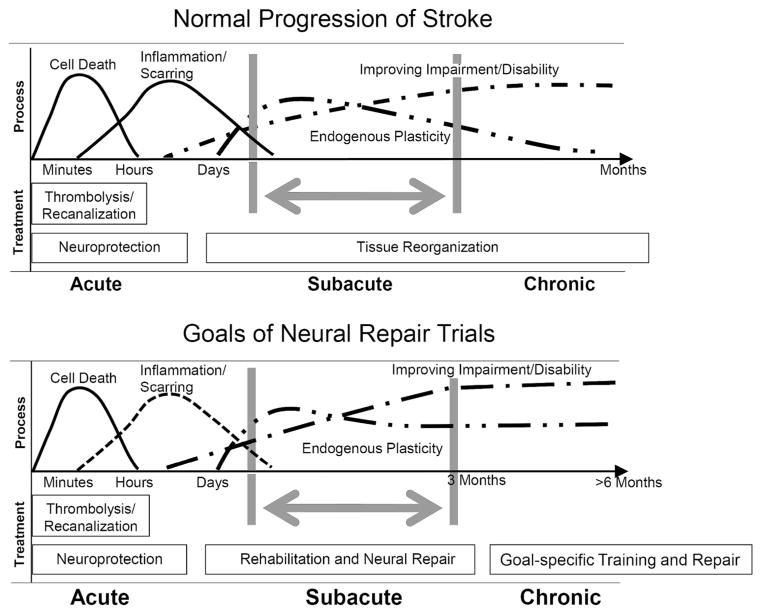
The global incidence of stroke is increasing while at the same time the incidence of death from stroke is declining.6 This means that stroke is transitioning even more into a disease of chronically disabled survivors. Patients improve most rapidly in their physical and sensorimotor functioning within the first three months and then reach a relative asymptote on standardized scales of impairment, self-care and mobility (Figure).7,8 In this first 3–4 months, recovery improves from one week to the next, i.e., time itself explains significant changes.9 Neurological recovery, then, may mostly evolve spontaneously early after onset, regardless of lesion size and location, perhaps mostly based on severity of sensorimotor deficits.10–12. Spontaneous gains are related to intrinsic neural mechanisms such as resolution of the effects of edema, blood, and diaschisis, restoration of neuronal excitability and network responsiveness ipsilateral to the stroke, and tissue repair mechanisms. Patients that engage in rehabilitation, such as in task-related, repetitive practice have greater improvement in those skills compared to no practice,13 via activity-induced residual neural ensembles that represent an action, as well as behavioral and functional adaptations that enable compensation.14,15 The overall increase in functional recovery in the first 2–3 months, as animal models confirm, is especially a period that utilizes endogenous mechanisms of brain plasticity for repair. This period is the subacute phase of stroke recovery (Figure).
Figure.
Progression of stroke damage, tissue reorganization and behavioral gains in the normal condition and after a neural repair therapy.
The top panel (A) shows the progression from initial cell death through secondary damage and then the limited repair and recovery after stroke. The bottom panel (B) shows the goals of a neural repair therapeutic, in which endogenous neuroplasticity is enhanced and extended in time, leading to a higher level and more prolonged recovery of neurological impairments.
Beyond 2–3 months, the chronic period (Figure), improvements in focused goals can still be induced by rehabilitative therapies. Gains were evident in this period in large stroke rehabilitation trials such as the VA robotic assist trial,16 EXCITE for the affected arm,17 and LEAPS for walking.16 These trials included at least 30 hours of practice. However, using standardized impairment measures such as the Fugl-Meyer scale, improvement beyond 3–12 months after stroke may be as little as one-tenth of that seen within the subacute period.16,19 This duality is also found in the animal literature, but with different temporal milestones. In many rat and mouse stroke models, a phase of rapid neurological recovery and plasticity with rehabilitation occurs within the first week to one month after onset, followed by a slower recovery phase.20–22 This animal literature indicates that a subacute phase within one month after stroke and a chronic phase beyond one month may model the 3-month (subacute) and greater than three-month (chronic) human conditions.
We anticipate that repair approaches after stroke will be tested in moderate to severely impaired participants, perhaps those who have no functional use of an upper extremity or cannot walk without human assistance. This criteria is suggested because initial trials may be invasive, have uncertain side effects, and such participants have greater functional needs compared patients with minimal to moderate impairment. Participant characteristics may include a highly impaired arm and hand with minimal selective movement against gravity at the wrist and fingers. On the Fugl-Meyer Motor Assessment Scale for the upper extremity, they might score less than 28/66 in the severe range and 29–40 in the moderate-severe range. Goals for repair may include functional reaching, gripping, pinching, and using the upper extremity for tasks within one’s peri-personal space to eat, groom, and assist other valued tasks. For the affected lower extremity, hip flexion and knee extension will likely be less than full movement against gravity at entry. Goals may include walking without human assistance in the home and community at greater than 0.4m/s. These need not be the only motor-related goals of a trial, but they are sought by most persons after disabling stroke.
Mechanisms of Action of Neural Repair Therapies
Pre-clinical studies of neural repair therapies have established that at least five mechanisms of action promote recovery after stroke.23–25 As discussed below, they are uniquely tied into conditions of behavioral/physical activity, which should be key features in the design of clinical trials of biological interventions. These five mechanisms include manipulation of excitatory neuronal signaling; induction of neuronal or neurovascular growth programs (axonal sprouting, dendritic spine morphogenesis, angiogenesis); reduction of the blockade of glial growth inhibitory molecules; promotion of endogenous progenitor responses (glial and neuronal progenitors); and cellular therapies. Examples include blockade of tonic GABA signaling26,27, facilitation of glutamate signaling,26 blockade of Nogo receptor 1 (NgR1),27,28 ephrin-A5 signaling,29 and Rho kinase pathways,30 as well as small molecule activation of growth factor signaling.31 Cellular therapies may do much more than possibly replace damaged neurons and glia, including local modulation of excitability and trophic actions, alteration in neuronal connections, angiogenesis, and immune and glial modulation.32,33 With few exceptions,34 a definitive mechanism of action for cellular therapies in stroke has not been established.
A main point of clinical significance is that the mechanisms for treatment-induced neural repair in animal models are similar to those seen in the mechanisms of successful physical activity after stroke, so repair may be impacted by neurorehabilitation and overall activity levels during stroke recovery. Repetitive limb use, for example, induces the formation of new connections24,35 and physical activity induces angiogenesis in motor cortex.36 Learning and memory activities during motor training utilize NgR1, Ephrin and Rho kinase pathways, as well as many of the molecular systems involved in a neuronal growth program that is induced by stroke, such as GAP43, stathmin family members and SPRR1.24 Motor learning induces dendritic spine formation37 through mechanisms that utilize some of the same molecular systems as stroke recovery.38 In regard to cellular therapies, behavioral activity levels directly impact transplant survival and engraftment in pre-clinical models of stroke and other neurological diseases.38,40 These data indicate that the CNS substrate for neural repair and that of repetitive rehabilitation practice substantially overlap.
Neural Repair Trials: Interaction with Patient Activity Patterns
If the mechanism of action of a candidate repair drug or cell is similar to the mechanism of action of rehabilitation and behavioral activity after stroke, then it is quite possible that a patient’s behavioral activity will directly impact the effect of a neural repair strategy. The type and amount of neurorehabilitation or physical activity may augment, interfere with or otherwise alter the efficacy of the candidate therapy. Thus, variations in physical activity may confound the outcomes of a clinical trial. Indeed, this activity-repair interaction has been demonstrated in pre-clinical stroke models. For example, rehabilitative practice augments repair and recovery in NgR1 antagonist studies when begun days after the drug was given to rodents. However, the temporal sequence was critical. If the rehabilitation activity was provided at the same time as the injection of an NgR1 antagonist, then motor function was actually worse than that seen after stroke.28 In clinical neurorehabilitation, progressive repetitive use of a moderately paretic upper extremity after stroke enhances its function.17 In support of this approach in pre-clinical studies, overuse of the affected limb induces local axonal sprouting in peri-infarct cortex. However, when forced overuse of a limb is paired with a glial blocker (anti-Ephrin A5), the resultant axonal sprouting is so extensive that it covers much of the ipsilateral brain.29 It is not clear that such exuberant growth of new connections is beneficial. In cellular therapy, behavioral activity patterns may enhance or hinder transplant engraftment. This finding led to the catchy phrase of “training the transplant” in pre-clinical studies in the Parkinson’s field.39 Thus, candidate neural repair treatments may be exquisitely sensitive to the behavioral activity patterns of the recipients.
There are two important clinical implications from this interaction of training and exercise with a neural repair therapy. First, a clinical trial for stroke recovery must control for overall levels of behavioral activity. Real-world measurement of such activity has been technically difficult, so estimates have been made utilizing self-report scales, step counters, and diaries. New wearable sensor technologies (see below) provide ways to measure behavioral activity at home over extended periods and provide linear outcomes that also more closely approximate the activity measures in some pre-clinical development protocols. Without quantitative activity monitoring, consider the confounder to trial validity if a cohort of patients receives a cellular therapy for stroke and continues to be sedentary, but a cohort in the control group is energized by participation in the trial and increases its activity, exercise, and skills practice. As in stroke models, the former cohort may not drive functional biological repair and the latter may improve modestly. In a similar vein, how would the results of a clinical trial be interpreted if a subset of patients engaged in self-directed activity patterns that promoted use of the unaffected side - a pattern that can limit plasticity and recovery in stroke-affected circuits.39 A subacute or chronic stroke repair trial that has no measure of the types and quantity of activity of participants misses one of the most important dose-response variables in the processes of recovery. This error of omission would be similar to a neuroprotection trial not controlling for age or initial severity of impairments of its participants.
A second interaction of training or exercise for a neural repair trial arises in chronic stroke, in which endogenous recovery has largely plateaued. It is possible that a neural repair therapy applied in a chronically degraded neural milieu may induce a degree of plasticity that can only be realized if it is coupled to the motor learning induced by rehabilitation. Such an outcome has been reported in animal studies, where combined motor learning plus a candidate neural repair therapy (chondroitinase ABC) in a late time point after stroke improved recovery and increased markers of local connections, but administration of the neural repair therapy alone did not boost these local connections when a behavioral activity program was not in place.41 Both processes induce CNS repair, but may be independently incapable of rising to the point of noticeable recovery in the chronic period of diminished molecular and cellular plasticity.
In terms of trial design, the energy and focus of resources toward recovery that comes with participation in a new trial when a patient is in a chronic disability state will themselves lead to some degree of improved function. This is reflected in the improvements seen in motor function in the activity-control arms of recent stroke recovery trials, such as the VA robot trial and the LEAPS trial16,18. This placebo effect reinforces the importance for control arms that are not just standard of care for stroke recovery, but that match as closely as possible not just the activity patterns of a new intervention, but any change in therapy, patient procedures (such as skull burr holes in a cell transplant study) and outcomes evaluation.
Practical Strategies For Trials
Stroke recovery trials will be initiated at different time points during the period of brain recovery, may activate distinct biological events during this time course, and will interface with patients at different stages of the rehabilitation process. These present challenges for the effective design of a repair trial. A key first consideration is time after stroke onset: subacute versus chronic. A trial in subacute stroke can piggyback on the substantial endogenous plasticity that occurs in this phase. It will be initiated during a period in which most moderate to severely disabled patients are still undergoing some rehabilitation. A potential problem is that this rehabilitation is not standardized, may occur during inpatient or outpatient care, and takes place in clinics, community centers, and the home. A trial in the chronic phase will occur in a period of reduced spontaneous recovery and a plateau in the biological mechanisms of repair (Figure). Patients at this chronic stage often have fallen into reduced physical activity patterns and may even have experienced a decline in function.9,42 Thus, the term chronic stroke does not imply a stable level of impairment and disability; fluctuation or recent decline that is not related to the stroke may make for an unreliable baseline assessment in a trial. For both stages, the neural repair strategy may benefit from a parallel program of continuous rehabilitation designed to engage and activate residual and eventually new components of the neuronal networks involved in the trial’s targeted motor tasks.43
Grant review panels, biopharma and the U.S. Food and Drug Administration (FDA) may express concern that the combination of a novel repair intervention plus rehabilitation would test two different therapies simultaneously, so culling out adverse responses and positive outcomes may be challenging. The funder of a combinational therapy trial may balk at the added cost. More likely, however, targeted rehabilitation, through mechanisms of activity-dependent plasticity, will maximize the potential efficacy of successful biological therapies (Table).28
Table.
Enrichment strategy to augment a repair trial with rehabilitation for a motor deficit.
|
Neurorehabilitation Augmentation
The contribution of rehabilitation in a repair trial is to complement and augment the repair strategy, but not put burdens on the participants and on the budget for these expensive phase II and III studies. Directed, home-based therapy, primarily conducted by the subject with some family support, may meet these requirements. Practice that can be accomplished without a visiting therapist at home, but with intermittent supervision and feedback that encourages progression of voluntary movement would be ideal for disabled persons who live hours away from participating sites. Home-based feedback about compliance and performance also helps maintain routine contact with participants, which may retain them in the trial and improve monitoring for adverse events. Indeed,
Important motor goals for a biological intervention in moderate-severe hemiparetic participants will be to lessen the upper extremity impairments that prevent functional reaching within peri-personal space for grasp or pinch of commonly used items. A trial in chronic stroke is likely to encounter participants with disuse of their affected limb and a subsequent decline in its function. Because of this, increases in function of the affected limb may simply arise because patients are stimulated to begin utilizing it more. This modest boost in function is seen in the active control group in rehabilitation trials, so it may confound a trial by producing the equivalent of the sought-after effect of a neural repair therapy. For this reason, we recommend a 2–3 week lead-in of neurorehabilitation therapy prior to a chronic stroke trial, so that all patients have experienced structured activities of their affected limbs and attain a truly stable plateau in function. This phase-in could be accomplished with 3–4 hours a week of formal physical/occupational therapy, but a less burdensome strategy might use only home-based practice of repetitive reaching and grasping. In the home, the experimental and control groups would be shown how to practice a limited set of movements, get retested for stability of function after 2–3 weeks, then continue the same skills practice throughout the trial (Table).
Upper Extremity Interventions
Cost-free practice at home with the upper extremity can start with the paretic forearm resting on a table at mid-chest height with the elbow at the mid-axillary line. The arm should be clothed to reduce friction. With as little truncal forward flexion as possible, the subject slides the forearm forward as far as feasible, tries to pre-shape the hand (wrist and finger extension) to the size of a 5 cm or so cone set at that distance, then grasps it with the palm or the thumb and several fingers. She can use the other hand to steady the item as needed. If grasped, the subject then attempts to lift the cone to the opposite shoulder or lips, then places it back on the table near the original position. The sequences might be repeated 10–30 times or more per session, twice a day (perhaps a total of 20 minutes daily). As the hand opens more or grasp and lift improve with more accurate performance, movement speed or the size of the object can be increased. The subject can also increase the difficulty by placing the object further away from the body to reach and grasp by more fully extending the elbow. Further progression could include reaching with the forearm not supported on the tabletop and using small food items such as grapes or dry slices of fruit or vegetables to bring to the mouth and eat. The subject can also use the unaffected hand to practice each component of the movement and then use mental imagery to prepare for using the affected one.42 This practice is not simply exercise. It aims to recruit a wide array of neural structures that can contribute to reach, pre-shaping the hand, prehension and pinch – all of the pathways within the corticospinal tract and their cortical and subcortical inputs, as well as regions for planning, sensorimotor reward modulation, visuomotor integration, and explicit and implicit learning through reinforcement. The trainees are, in a sense, trying to find successful movements. This simple but broad attempt especially aims to engage repairing tissue to modulate its growth, secretory influences, connectivity, and synaptic strength on targets.
Lower Extremity Interventions
For the lower extremities, the most frequent goal will be to improve the gait pattern with better hip flexion, knee extension for swing and stance, and balance to enable more functional walking. Home-based practice might include sit-to-stand-to-sit repetitions in front of a table for support and with hands-on assistance as necessary. The subject can also lift the affected leg’s heel 6 inches above the bed while supine, holding it for 3–5 seconds. If able to do that, participants can try to lift the unaffected leg with the affected one crossed under at the ankle and hold. While seated, knee extension of the affected leg can be added in sets as is feasible, followed by crossing the better leg’s ankle over the affected one and lifting and extending the knee against this resistance if feasible. These light resistance, skillful movements can be repeated twice a day for 10 or more attempted lifts per session. The lifts with the ankles crossed should also be performed with the affected ankle crossed on top of the better leg’s ankle so the better one can get some exercise and perhaps better pattern the muscle coordination needed by the affected leg. The subject should also aim to walk a feasible distance within her capacity. This may require hands-on assistance. Four scheduled walks daily for 3–5 minutes each or preferably longer, as is feasible, should be attempted in these highly impaired subjects. The aim will be to walk faster and farther in that time, as well as to increase the duration of these walks weekly.
In these sample scenarios, at least 30 minutes of daily home practice is encouraged. As noted earlier, gains in arm function and walking across stroke rehabilitation trials were found when total practice time reached up to 30 hours, regardless of the practice intervention, so a higher dose over the duration of a trial ought to be encouraged. The patient maintains or even gains a modest level of fitness and strengthening to support repair-related motor and functional gains. The experimental group should show better final outcomes over the control group if the biological intervention has been engaged by practice and is capable of augmenting recovery. Keep in mind that the highly impaired participants in a repair trial, once they have been shown to have reached a plateau with pre-trial therapy, would not be expected to improve their motor control very much by this modest focused therapy in the chronic stage of recovery, so this process will not produce functional gains that would reduce overall gains by the neural repair arm of the trial. In the subacute stage, the experimental and control groups might improve with this design, but equally only if there is no additional biological effect.
Monitoring Home-Based Strategies
Ideally, practice should be monitored and timely feedback provided that rewards compliance. Mobile health (mHealth) applications use wearable wireless inertial, physiological and biological sensors that send these signals to a smartphone for real time analysis or for transmission to a server that performs analytics.45 Inexpensive systems can remotely capture data about patients in the home and community. One simple solution for repair trials is to wear wireless triaxial accelerometers on the affected arm and leg. Most commercial devices merely perform activity counts, however, which are accelerations/decelerations that tend to correlate with the inertial forces elicited by arm swing or forward body movements during routine reaching, walking and running. These devices do not measure speed, smoothness of movement or accurately, if at all, recognize walking at speeds under 0.6m/s, which is the most likely speed for participants in a repair trial. Smartphone applications with GPS can also provide step counts and estimate speed and distance, but do not usually function indoors. Bilateral triaxial accelerometers placed over the ankles or laces of shoes can use machine-learning algorithms that determine the type, quantity and quality of locomotor movements. For walking, for example, the signals fused from bilateral ankle sensors can capture each bout of walking, cycling or leg exercise performed at any speed. The system then measures walking speed or repetitions per minute, distance, duration of activity, and asymmetries in stance and swing times, as well as inertial smoothness of repetitive movements.46 Summary data throughout a trial can then reveal whether patients are trying to be active and practicing skills as instructed. Continuous monitoring may also show gradual gains in daily activity in the setting of the home and community to serve as a measure of real-world participation, e.g., by revealing more bouts of walking for longer durations indoors and outdoors, faster walking speeds, and less sedentary time. This data can augment the standard clinic-based 6-min walking distance and 10-m walking speed outcomes, and give insight into standard self-reports of activity.7,48.
Although continuous sensing data could be of great interest, to keep costs down, sensing of activity probably only needs to be sampled for 1–2 weeks at a time at routinely scheduled trial follow-up visits. That way, each sensor kit can be used by many subjects. Over a 1-year trial, for example, key activity data might be acquired at pre-entry, baseline, and 1, 3, 6, 9, and 12 months after the start. At each follow-up, the investigator can see how the subject is practicing and make suggestions about how to try to make further progress. Feedback about levels of activity that are falling below what the home rehabilitation program requires can be provided by a phone call, so that education, goal setting and further instruction can be given.
A modest tele-rehabilitation program could also be instituted.49 In this design, every 2–4 weeks a centrally located therapist might watch all subjects at practice at home via a smartphone or tablet camera, review summarized activity-recognition data from wearable sensors about the quantity and quality of standard practice and activity during the week, and make suggestions about how to continue. Splints and orthotics may be useful to better position a joint so that newly acquired movements can be practiced and made more functional. With these innovations, the trial can capture the practice actually carried out, improve the reliability of these supportive rehabilitation procedures, develop dose-response data over time for practice and motor changes, obtain multiple serial measures related to outcomes, maximize the interaction between the repair intervention and rehabilitation, increase the interest potential subjects may have in participating and then staying in the trial, and generally increase the reliability of the trial.
Many other rehabilitation strategies might be considered, but they would require additional oversight and expertise, expensive equipment or frequent clinic visits. Most important, their efficacy has not been demonstrated in moderate-severely impaired patients after stroke.47,50 However, if an additional rehabilitation intervention were shown to amplify, for example, an axonal sprouting therapy, such as transcranial magnetic stimulation of the intact hemisphere’s motor cortex to induce corticospinal fibers to re-cross into the denervated motor pools of the spinal cord,51 then more complex multi-dimensional therapies might be combined.
Outcome Assessments
The STEPS participants recommended that cellular therapy trials should include at least two pre-treatment baseline examinations to assure a stable baseline in a homogeneous group of subjects.52 We would go a step further. For trials that start in a late subacute or chronic period after injury onset, spontaneous degradation of function may have intervened or latent function may not be brought out by the neurological examination. As described above, we suggest a short phase-in of rehabilitation aimed at the motor gains that are the primary target of the trial’s outcomes. The subjects are then tested to assure the stability of their baseline findings.
The STEPS participants also suggested employing modality-specific outcome measures, tested in a Phase II design and possibly serving as the primary outcome in a Phase III cellular trial.52 This approach could lead to a modality-specific FDA label for the approach, but that is not a deterrent for a study of motor recovery.53 Again, we would go a step further, drawing upon the most specific measurement tools available, rather than relying on the NIH Toolbox or other off-the-shelf tools that may not be precise enough to capture a clinical signal of repair. Commonly used ordinal scale tools for acute stroke trials include the NIH Stroke Scale, modified Rankin Scale, and Fugl-Meyer Motor Assessment. The NIHSS looks only at gross sensorimotor impairment. The Rankin emphasizes walking ability with a mix of impairment and disability categories, but does not provide any standard way to assess the details of motor functions and motor- or cognitive-related disability. The Fugl-Meyer assesses limb movements in and out of upper motor neuron synergies. The scale cannot assess more subtle single joint motor changes, so it may not serve as a targeted outcome measure. Also, tools are needed that do not have floor and ceiling effects if they are to detect small incremental changes.
Motor assessments for repair trials can consider returning to an old friend, the British Medical Council Scale, by testing 3–4 muscle groups from each spinal root level for the arm and leg, where voluntary movement most often will be ≤3/5 before the intervention. By adding a few additional muscles to the standard upper extremity motor score after cervical spinal cord injury, greater sensitivity for outcome prediction was added.54 Surface electromyography and wireless sensors such as accelerometers, gyroscopes, and goniometers may be applicable as monitoring tools for newly organized and larger movements across joints for both subacute and chronic stroke rehabilitation trials. Indeed, highly accurate wearable sensing data (e.g., total amount of daily walking at the subject’s average speed) could also serve as a stratification or entry criteria tool, e.g., stratify those who walk <15 minutes daily vs more at walking speeds <1m/s or eliminate those who already walk >1 m/s for >30 minutes daily. Scales such as the Fugl-Meyer for selective multi-joint movements would supplement the targeted decrease in motor impairment, as would timed hand tasks (e.g., Nine Hole Peg Test, the Wolf Motor Function Test) and functional scales that were relevant to the goals of the rehabilitation plus biological approach.
Investigators may initially be satisfied with any signal that is consistent with motor network repair. For example, structural, functional, and connectivity magnetic resonance imaging of the brain may provide a means to detect anatomic and physiologic responses to rehabilitation plus repair. Imaging could even serve as a stratification tool, such as revealing upper and lower limits in the volume of spared corticospinal and bulbospinal fibers at the level of the internal capsule. One might anticipate better outcomes in those with more residual fibers. Transcranial magnetic stimulation to test for improvement in cortically elicited motor evoked potentials could also physiologically suggest new axonal sprouting and supraspinal input to the spinal motor pools. So might sensory evoked potentials. Given the present state of knowledge, even compelling findings from these tests can serve only as biomarkers of change in phase II and III trials, not as clinically meaningful outcome measures.
Although the clinical science of repair will benefit from any demonstration of restoration, whether an MRI structural biomarker or a modest additional movement of the hand, clinicians will look for outcomes such as extension of the wrist and fingers against gravity when none had existed at baseline. The participants, however, may not benefit in their daily activities if they do not regain reach, grasp and release to hold and manipulate objects or achieve the ability to walk longer distances in more difficult environments. An intervention that carries risk, such as invasive procedures to implant cells, must ultimately enable useful new function, not only send a signal of repair.
Conclusion
Neural repair therapies interact with higher levels of physical activity and may be potentiated or even hindered by particular rehabilitative treatments. Thus, neural repair trials should at least track physical activity and would be well served by incorporating specific elements of exercise and skills practice leading into the trial and during the trial. Wearable sensors and phone or video feedback about activity can inexpensively enable this. Sensor data can also serve as an outcome measurement. Rehabilitation strategies that make use of the neurobiology underlying skills learning within a flexible, distributed motor system may help drive neural repair processes, as well as help incorporate reconnected nodes into the motor controllers that lessen the impairment and disability of patients.
Acknowledgments
Supported by the Dr. Miriam and Sheldon G. Adelson Medical Research Foundation, Richard Merkin Foundation for Neural Repair at UCLA, the American Stroke Association/Bugher Foundation, and the National Institues of Health NS085019, NS081055, NS077521, NS071481, HD071809.
References
- 1.Mack G. ReNeuron and StemCells get green light for neural stem cell trials. Nat Biotechnol. 2011;29:95–7. doi: 10.1038/nbt0211-95. [DOI] [PubMed] [Google Scholar]
- 2. [Accessed at 4-25-15];2015 http://www.firstwordpharma.com/node/1188336-axzz3YWUZHFBZ.
- 3.Lima C, Escada P, Pratas-Vital J, et al. Olfactory mucosal autografts and rehabilitation for chronic traumatic spinal cord injury. Neurorehab Neural Repair. 2010;24:10–22. doi: 10.1177/1545968309347685. [DOI] [PubMed] [Google Scholar]
- 4.Tabakow P, Jarmundowicz W, Czapiga B, et al. Transplantation of autologous olfactory ensheathing cells in complete human spinal cord injury. Cell Transplant. 2013;22:1591–612. doi: 10.3727/096368912X663532. [DOI] [PubMed] [Google Scholar]
- 5.Enserink M. Selling the stem cell dream. Science. 2006;313:160–3. doi: 10.1126/science.313.5784.160. [DOI] [PubMed] [Google Scholar]
- 6.Krishnamurthi R, Feigin V, Forouzanfar M, et al. Global and regional burden of first-ever ischaemic and haemorrhagic stroke during 1990–2010: findings from the Global Burden of Disease Study 2010. Lancet Glob Health. 2013;1:e259–81. doi: 10.1016/S2214-109X(13)70089-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jorgensen HS, Nakayama H, Raaschou HO, Vive-Larsen J, Stoier M, Olsen TS. Outcome and time course of recovery in stroke. Part I: Outcome. The Copenhagen Stroke Study. Arch Phys Med Rehabil. 1995;76:399–405. doi: 10.1016/s0003-9993(95)80567-2. [DOI] [PubMed] [Google Scholar]
- 8.Jorgensen HS, Nakayama H, Raaschou HO, Vive-Larsen J, Stoier M, Olsen TS. Outcome and time course of recovery in stroke. Part II: Time course of recovery. The Copenhagen Stroke Study. Arch Phys Med Rehabil. 1995;76:406–12. doi: 10.1016/s0003-9993(95)80568-0. [DOI] [PubMed] [Google Scholar]
- 9.de Port IG, Kwakkel G, van Wijk I, Lindeman E. Susceptibility to deterioration of mobility long-term after stroke: a prospective cohort study. Stroke. 2006;37:167–71. doi: 10.1161/01.STR.0000195180.69904.f2. [DOI] [PubMed] [Google Scholar]
- 10.Prabhakaran S, Zarahn E, Riley C, et al. Inter-individual variability in the capacity for motor recovery after ischemic stroke. Neurorehabil Neural Repair. 2008;22:64–71. doi: 10.1177/1545968307305302. [DOI] [PubMed] [Google Scholar]
- 11.Winters C, van Wegen EE, Daffertshofer A, Kwakkel G. Generalizability of the proportional recovery model for the upper extremity after an ischemic stroke. Neurorehabil Neural Repair. 2015;29:614–22. doi: 10.1177/1545968314562115. [DOI] [PubMed] [Google Scholar]
- 12.Byblow WD, Stinear CM, Barber PA, Petoe MA, Ackerley SJ. Proportional recovery after stroke depends on corticomotor integrity. Ann Neurol. 2015 Jul 7; doi: 10.1002/ana.24472. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 13.Pollock A, Baer G, Campbell P, et al. Physical rehabilitation approaches for the recovery of function and mobility after stroke: Major update. Cochrane Database Syst Rev. 2014;4:CD001920. doi: 10.1002/14651858.CD001920.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Levin M, Kleim J, Wolf S. What do motor “recovery” and “compensation” mean in patients following stroke? Neurorehabil Neural Repair. 2009;23:313–9. doi: 10.1177/1545968308328727. [DOI] [PubMed] [Google Scholar]
- 15.Alaverdashvili M, Whishaw I. A behavioral method for identifying recovery and compensation: Hand use in a preclinical stroke model using the single pellet reaching task. Neurosci Biobehav Rev. 2013;37:950–67. doi: 10.1016/j.neubiorev.2013.03.026. [DOI] [PubMed] [Google Scholar]
- 16.Lo A, Guarino P, Richards L, et al. Robot-assisted therapy for long-term upper-limb impairment after stroke. N Engl J Med. 2010;362:1772–83. doi: 10.1056/NEJMoa0911341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wolf SL, Winstein CJ, Miller JP, et al. Effect of constraint-induced movement therapy on upper extremity function 3 to 9 months after stroke: the EXCITE randomized clinical trial. JAMA. 2006;296:2095–104. doi: 10.1001/jama.296.17.2095. [DOI] [PubMed] [Google Scholar]
- 18.Duncan P, Sullivan K, Behrman A, et al. Body-weight-supported treadmill rehabilitation program after stroke. N Engl J Med. 2011;364:2026–36. doi: 10.1056/NEJMoa1010790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chollet F, Tardy J, Albucher J, et al. Fluoxetine for motor recovery after acute ischemic stroke (FLAME): a randomised placebo-controlled trial. Lancet Neurol. 2011;10:123–30. doi: 10.1016/S1474-4422(10)70314-8. [DOI] [PubMed] [Google Scholar]
- 20.Biernaskie J, Chernenko G, Corbett D. Efficacy of rehabiliitative experience declines with time after focal ischemic brain injury. J Neurosci. 2004;24:1245–54. doi: 10.1523/JNEUROSCI.3834-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Clarkson A, Overman J, Zhong S, Mueller R, Lynch G, Carmichael S. AMPA receptor-induced local brain-derived neurotrophic factor signaling mediates motor recovery after stroke. J Neurosci. 2011;31:3766–75. doi: 10.1523/JNEUROSCI.5780-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Krakauer J, Carmichael S, Corbett D, Wittenberg G. Getting neurorehabilitation right: what can be learned from animal models? Neurorehabil Neural Repair. 2012;26:923–31. doi: 10.1177/1545968312440745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hermann D, Chopp M. Promoting brain remodelling and plasticity for stroke recovery: therapeutic promise and potential pitfalls of clinical translation. Lancet Neurol. 2012;11:369–80. doi: 10.1016/S1474-4422(12)70039-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Overman J, Carmichael S. Plasticity in the injured brain: more than molecules matter. Neuroscientist. 2014;20:15–28. doi: 10.1177/1073858413491146. [DOI] [PubMed] [Google Scholar]
- 25.Starkey M, Schwab M. How plastic is the brain after a stroke? Neuroscientist. 2014;20:359–71. doi: 10.1177/1073858413514636. [DOI] [PubMed] [Google Scholar]
- 26.Clarkson A, Huang B, Macisaac S, Mody I, Carmichael S. Reducing excessive GABA-mediated tonic inhibition promotes functional recovery after stroke. Nature. 2010;468:305–9. doi: 10.1038/nature09511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fang P, Barbay S, Plautz E, Hoover E, Strittmatter S, Nudo R. Combination of NEP 1-40 treatment and motor training enhances behavioral recovery after a focal cortical infarct in rats. Stroke. 2010;41:544–49. doi: 10.1161/STROKEAHA.109.572073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wahl A-S, Schwab M. Finding an optimal rehabilitation paradigm after stroke: enhancing fiber growth and training of the brain at the right moment. Front Hum Neurosci. 2014;8:1–13. doi: 10.3389/fnhum.2014.00381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Overman J, Clarkson A, Wanner I, et al. A role for ephrin-A5 in axonal sprouting, recovery, and activity-dependent plasticity after stroke. Proc Natl Acad Sci U S A. 2012;109:E2230–9. doi: 10.1073/pnas.1204386109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lemmens R, Jaspers T, Robberecht W, Thijs V. Modifying expression of EphA4 and its downstream targets improves functional recovery after stroke. Hum Mol Genet. 2013;22:2214–20. doi: 10.1093/hmg/ddt073. [DOI] [PubMed] [Google Scholar]
- 31.Han J, Pollak J, Yang T, et al. Delayed administration of a small molecule tropomyosin-related kinase B ligand promotes recovery after hypoxic-ischemic stroke. Stroke. 2012;43:1918–24. doi: 10.1161/STROKEAHA.111.641878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tornero D, Wattananit S, Madsen M, et al. Human induced pluripotent stem cell-derived cortical neurons integrate in stroke-injured cortex and improve functional recovery. Brain. 2013;136:3561–77. doi: 10.1093/brain/awt278. [DOI] [PubMed] [Google Scholar]
- 33.Kokaia Z, Lindvall O. Stem cell repair of striatal ischemia. Prog Brain Res. 2012;201:35–53. doi: 10.1016/B978-0-444-59544-7.00003-2. [DOI] [PubMed] [Google Scholar]
- 34.Horie N, Pereira M, Niizuma K, et al. Transplanted stem cell-secreted vascular endothelial growth factor effects poststroke recovery, inflammation, and vascular repair. Stem Cells. 2011;29:274–85. doi: 10.1002/stem.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Adkins DL, Boychuk J, Remple MS, Kleim JA. Motor training induces experience-specific patterns of plasticity across motor cortex and spinal cord. J Appl Physiol. 2006;101:1776–82. doi: 10.1152/japplphysiol.00515.2006. [DOI] [PubMed] [Google Scholar]
- 36.Kleim J, Cooper N, VandenBerg P. Exercise induces angiogenesis but does not alter movement representations within rat motor cortex. Brain Res. 2002;201:1–6. doi: 10.1016/s0006-8993(02)02239-4. [DOI] [PubMed] [Google Scholar]
- 37.Fu M, Yu X, Lu J, Zuo Y. Repetitive motor learning induces coordinated formation of clustered dendritic spines in vivo. Nature. 2012;483:92–5. doi: 10.1038/nature10844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zemmar A, Weinmann O, Kellner Y, et al. Neutralization of Nogo-A enhances synaptic plasticity in the rodent motor cortex and improves motor learning in vivo. J Neurosci. 2014;26:8685–98. doi: 10.1523/JNEUROSCI.3817-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dunnett S. Neural tissue transplantation, repair, and rehabilitation. Handb Clin Neurol. 2013;110:43–59. doi: 10.1016/B978-0-444-52901-5.00004-6. [DOI] [PubMed] [Google Scholar]
- 40.Hicks A, Hewlett K, Windle V, et al. Enriched environment enhances transplanted SVZ stem cell survival, migration and functional recovery after stroke. Neurosci. 2007;136:31–40. doi: 10.1016/j.neuroscience.2007.01.020. [DOI] [PubMed] [Google Scholar]
- 41.Gherardini L, Gennaro M, Pizzorusso T. Perilesional treatment with chondroitinase ABC and motor training promote functional recovery after stroke in rats. Cereb Cortex. 2015;25:202–12. doi: 10.1093/cercor/bht217. [DOI] [PubMed] [Google Scholar]
- 42.Meyer S, Verheyden G, Brinkmann N, et al. Functional and motor outcome 5 years after stroke is equivalent to outcome at 2 months. Stroke. 2015;46 doi: 10.1161/STROKEAHA.115.009421. Epub. [DOI] [PubMed] [Google Scholar]
- 43.Dong Y, Winstein CJ, Dobkin B, Singh M. Changes in cortical motor activity with rehabilitative therapy in stroke: a longitudinal fMRI study. Soc for Neurosci; San Diego: 2004. p. 181.11. [Google Scholar]
- 44.Ietswaart M, Johnston M, Dijkerman H, et al. Mental practice with motor imagery in stroke recovery. Brain. 2011;134:1373–86. doi: 10.1093/brain/awr077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dobkin B. Wearable motion sensors to continuously measure real-world activities. Curr Opin Neurol. 2013;26:602–8. doi: 10.1097/WCO.0000000000000026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dorsch A, Thomas S, Xu X, Kaiser W, Dobkin B. SIRRACT: An international randomized clinical trial of activity feedback during inpatient stroke rehabilitation enabled by wireless sensing. Neurorehabil Neural Repair. 2014 Sep 26; doi: 10.1177/1545968314550369. epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dobkin B, Nadeau S, Behrman A, et al. Prediction of responders for outcome measures of locomotor Experience Applied Post Stroke trial. J Rehabil Res Dev. 2014;51:39–50. doi: 10.1682/JRRD.2013.04.0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dobkin B, Dorsch A. The promise of mHealth: Daily activity monitoring and outcome assessments by wearable sensors. Neurorehabil Neural Repair. 2011;25:788–98. doi: 10.1177/1545968311425908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Benvenuti F, Stuart M, Cappena V, et al. Community-based exercise for upper limb paresis: A controlled trial with telerehabilitation. Neurorehabil Neural Repair. 2014;28:611–20. doi: 10.1177/1545968314521003. [DOI] [PubMed] [Google Scholar]
- 50.Dobkin B, Dorsch A. New evidence for therapies in stroke rehabilitation. Curr Atheroscler Rep. 2013;15:331–40. doi: 10.1007/s11883-013-0331-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Carmel J, Kimura H, Martin J. Electrical stimulation of motor cortex in the uninjured hemisphere after chronic unilateral injury promotes recovery of skilled locomotion through ipsilateral control. J Neurosci. 2014;34:462–66. doi: 10.1523/JNEUROSCI.3315-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wechsler L, Steindler D, Borlongan C The STEPS Participants a. Stem cell therapies as an emerging paradigm in stroke. Stroke. 2009;40:510–5. doi: 10.1161/STROKEAHA.108.526863. [DOI] [PubMed] [Google Scholar]
- 53.Cramer S, Koroshetz W, Finklestein S. The case for modality-specific outcome measures in clinical trials of stroke recovery-promoting agents. Stroke. 2007;38:1393–95. doi: 10.1161/01.STR.0000260087.67462.80. [DOI] [PubMed] [Google Scholar]
- 54.Velstra I, Curt A, Frotzler A, et al. Changes in strength, sensation, and prehension in acute cervical spinal cord injury: GRASSP. Neurorehabil Neural Repair. 2015 doi: 10.1177/1545968314565466. epub Jan 7. [DOI] [PubMed] [Google Scholar]