Abstract
BACKGROUND:
This prospective, randomized trial was undertaken to evaluate the utility of adding end-tidal capnometry (ETC) to pulse oximetry (PO) in patients undergoing procedural sedation and analgesia (PSA) in the emergency department (ED).
METHODS:
The patients were randomized to monitoring with or without ETC in addition to the current standard of care. Primary endpoints included respiratory adverse events, with secondary endpoints of level of sedation, hypotension, other PSA-related adverse events and patient satisfaction.
RESULTS:
Of 986 patients, 501 were randomized to usual care and 485 to additional ETC monitoring. In this series, 48% of the patients were female, with a mean age of 46 years. Orthopedic manipulations (71%), cardioversion (12%) and abscess incision and drainage (12%) were the most common procedures, and propofol and fentanyl were the sedative/analgesic combination used for most patients. There was no difference in patients experiencing de-saturation (SaO2<90%) between the two groups; however, patients in the ETC group were more likely to require airway repositioning (12.9% vs. 9.3%, P=0.003). Hypotension (SBP<100 mmHg or <85 mmHg if baseline <100 mmHg) was observed in 16 (3.3%) patients in the ETC group and 7 (1.4%) in the control group (P=0.048).
CONCLUSIONS:
The addition of ETC does not appear to change any clinically significant outcomes. We found an increased incidence of the use of airway repositioning maneuvers and hypotension in cases where ETC was used. We do not believe that ETC should be recommended as a standard of care for the monitoring of patients undergoing PSA.
KEY WORDS: Procedural sedation and analgesia, Capnography, Adverse events, Emergency medicine
INTRODUCTION
Procedural sedation and analgesia (PSA) in the emergency department (ED) has allowed patients to safely undergo painful procedures that in earlier times would have entailed a trip to the operating room, or performance in the ED under inadequate analgesia for fear of cardio-respiratory depression or airway compromise. These complications can, however, still occur during PSA in the ED. Using a liberal definition of respiratory depression, Miner et al[1] described the rate of up to 44.6% during ED PSA. Non-invasive pulse oximetry (PO) has become a standard of care to diagnose oxygen desaturation during PSA.[1–3] End-tidal capnometry (ETC) has been shown to identify respiratory depression (RD), airway obstruction, apnea and laryngospasm earlier than PO, and theoretically offers a greater safety margin when monitoring patients.[1,4–8] Other investigators, using different methods and definitions, found PO to reveal respiratory depression before ETC.[9] The use of combined PO and ETC has been strongly recommended.[10] The clinical use of ETC has not yet to be determined, as the level of RD at which adverse events are more likely has not yet to be determined.[9] Furthermore, variability in ETC readings at baseline has been noted. Sivilotti et al[9] found fluctuations in ETC readings even before drug administration and also considerable variation between participating physicians in the way they intervened in ETC changes.
Improving patient safety is of critical importance in patients undergoing PSA, and if ETC does this, then it should be used. A recommendation for use of ETC in EDs, however, raises questions about the burden of false positive triggers, the cost of purchasing machines, training practitioners adequately and quality control. The potential for unexpected harm has not been established. This study aimed to investigate the practical use of ETC monitoring during PSA at a busy academic ED.
METHODS
Study design
This was a prospective, randomized, non-blinded study of ETC monitoring of PSA in the ED. Patients undergoing PSA were, after informed consent, randomized to have PSA conducted with “standard of care” monitoring plus continuous ETC, versus standard of care monitoring alone. The study was approved by the institutional ethics review board.
Screening and selection of participants
The study was conducted at the Charles V. Keating Emergency and Trauma Centre, a 600-bed teaching hospital ED with an annual ED census of 65 000. All patients (age> 16 years) undergoing PSA were eligible to participate. Patients were excluded if they were unable to give consent or if they were critically ill, and/or required immediate endotracheal intubation.
In our institution, PSA was performed by senior paramedic acute care practioners (ACPs) with additional training in PSA.[11] Current standard care at our institution included pre-procedure intravenous analgesia when indicated, informed patient consent, continuous oxygen therapy, cardiac and PO monitoring, and blood pressure measurement every 5 minutes during the procedure. The paramedic monitored the patient until full recovery from the PSA, according to a standardized recovery assessment tool. All clinical details and drug dosages were recorded on a specific PSA patient care record. Prior to the study, all ACPs involved in the study received training on the interpretation of ETC readings.
Intervention
Patients were randomized using a computer generated table to have PSA conducted with a standard care monitoring plus continuous ETC, versus standard care monitoring alone. Allocation was concealed using opaque white envelopes. Treatment was identical except for the use of ETC monitoring (Medtronic physio-control Lifecap Capnograph) and recording of end-tidal carbon dioxide (ETCO2) every 5 minutes, or the absence of a ETCO2 waveform at any time during the procedure. ACPs were instructed to use their clinical judgment to intervene clinically in response to ETC or PO changes as they felt appropriate. Paramedics were permitted to select medications for PSA as well as doses and methods of titration. Intravenous fluid administration was left to the discretion of the paramedic.
Outcomes
The primary outcomes focusing on respiratory adverse events included: need for airway intervention which included: (i) airway repositioning maneuver; (ii) positive pressure (bag mask valve) ventilation (PPV); (iii) oral/nasal airway placement; (iv) endotracheal intubation; and (v) oxygen desaturation SaO2 of < 90% for 30 seconds. Secondary outcomes included: (i) level of sedation greater than intended (overshoot); (ii) hypotension; (iii) sedation time (time from the first dose of drug administration to commencement of procedure; (iv) recovery time (time from the end of procedure to cessation of monitoring); (v) patient satisfaction; and (vi) procedure success.
Analysis
Summary statistics to describe patient characteristics included mean and standard deviations for the continuous variables and percentages for categorical variables. Differences in the study variables were assessed using Student’s t test (continuous) and the Chi-square (categorical) test. The analysis consisted of the standard descriptive statistics and univariate statistics (dichotomized by the intervention). Statistical significance was set at 0.05 for all analyses. All analyses were completed using Stata 11.1 (College Station, Texas, USA).
The sample size calculation was based on the hypothesis that RD, defined by the need for airway intervention during PSA or an oxygen desaturation ≤ 90% for 30 seconds, would occur in 25% of the standard care group. A minimum of 429 patients would have to be enrolled in each arm of the study to detect a 32% risk reduction at a power of 80% with a 2-tailed α–value of 0.05.
RESULTS
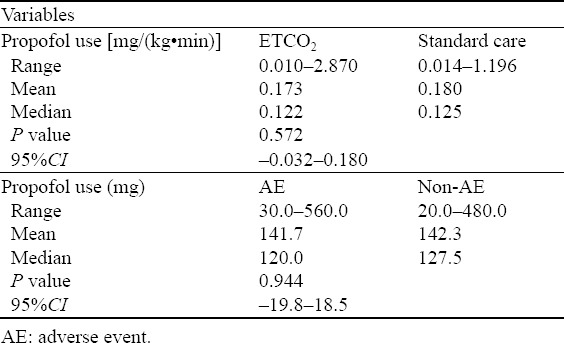
Between April 4, 2006 and April 12, 2010, 986 patients were randomized, 501 to usual care and 485 to additional ETC monitoring, of which 48% were female with a mean age of 46±18 years. Orthopedic manipulations (71%), cardioversion (12%) and abscess incision and drainage (12%) were the most common procedures, and propofol and fentanyl were the sedative/analgesic combination used for most patients. ASA scores, height, weight and age were similar between the two groups (Table 1), as were the sedative drugs chosen by the ACP (Table 2). There was no significant difference in the dose of propofol, the most frequently used sedative medication, between the two groups (Table 3). In this series, 973 (98.7%) patients received oxygen, 958 received oxygen via a non-re-breather face mask and 15 by nasal prongs.
Table 1.
Demograhpic data for both cohorts
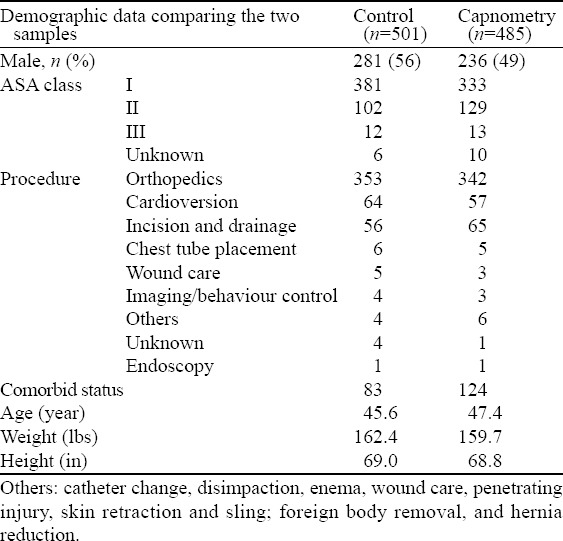
Table 2.
Sedative medications prescribed in each cohort, n (%)

Table 3.
Propofol dose administered in each group
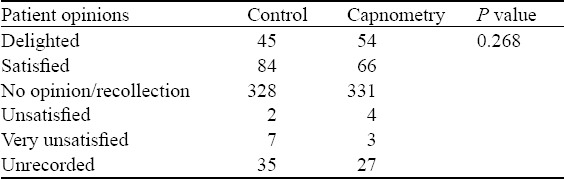
With regard to outcomes (Tables 4 and 5), there was no difference between the two groups in patients experiencing desaturation (SaO2<90%), although patients in the ETC group were more likely than those in the control group to require airway repositioning (26.2%, 95%CI 22.5–30.3 vs. 18.4%, 95%CI 15.2–22.0, P=0.003). There was no difference in the need for more aggressive airway intervention (2 patients in the control group received PPV and no patients in either group required endotracheal intubation). Time taken for the procedure, vomiting, arrhythmia, degree of “overshoot”, and patient satisfaction (Table 6) were not different between the groups. Hypotension (SBP<100 mmHg or <85 mmHg if baseline <100 mmHg) was observed in 16 (3.3%, 95%CI 2.0–5.3) patients in the ETC group, and 7 (1.4%, 95%CI 0.7–2.9, P=0.048) in the control group.
Table 4.
Incidence rates of interventions

Table 5.
Incidence rates of adverse events
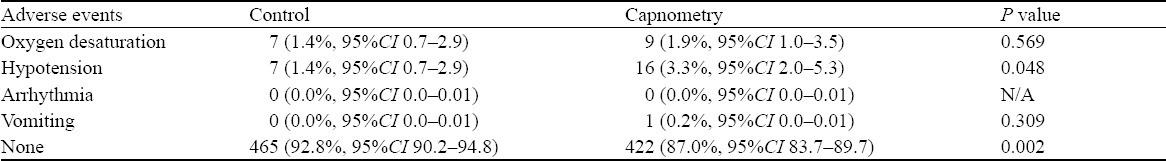
Table 6.
Patient opinions post PSA
DISCUSSION
The question of whether ETC provides benefit to patients undergoing PSA is an important one. Intuitively one would predict that a machine that warns practitioners earlier that their patient’s respiration is being suppressed would be an improvement. On the other hand, a machine that adds complexity to a procedure without improving patient outcome might distract the seditionist from more important clinical monitoring and would add expense to an already strapped system. Our findings suggest that ETC, although not associated with a decrease in oxygen de-saturation, is in fact associated with an increase in hypotension.
One likely explanation for this increase in hypotension in the group managed with ETC is that the monitoring modality gave seditionists a sense of security that led them to administer sedatives more aggressively than if they were more concerned about missing respiratory depression. Medications used for PSA have both respiratory and circulatory system depressive effects, and the feeling of safety about the former may have allowed a lower level of vigilance against the latter, with a subsequent increase in the incidence of hypotension.
Although not being actually “adverse events”, the increase in airway repositioning interventions in the ETC group does suggest an earlier response to hypoventilation, possibly because of earlier detection as a result of more sensitive monitoring. The clinical significance of transient hypoventilation, however, is questionable in that it denotes patients at risk of hypoxia rather than clinically important RD.[9] Although it did not occur in our study, premature diagnosis of RD may, in fact, be deleterious to patients might be unnecessarily subjected to bag valve mask ventilation, insufflating their stomachs with air, and increasing their chances of aspiration.[10] This might be especially true in the case of ultra short acting agents, where momentary apnea (safe in the presence of adequate pre-oxygenation) is likely to correct itself spontaneously as the effect of the drug wears off.[10] Hypercapnea itself is an important respiratory stimulant,[12] and suppression thereof by hasty positive pressure ventilation may in theory complicate the smooth recovery of sedated patients.
An alternative explanation for the finding of increased airway repositioning in the ETC group, however, is that it supports the above contention that the seditionists may have been more aggressive with drug use, resulting in a need for airway repositioning that would have been less likely with more cautious drug administration.
Although oxygen applied during PSA has been shown to be associated with decreased hypoxic events,[4,13–15] injudicious use of high flow oxygen prior to or during PSA may contribute to hypoventilation that may not be detected by PO[3,10,16] and that events of hypoventilation would be detected by ETC.[14] Again, the clinical significance of a brief period of hypoventilation without hypoxia is questionable, especially when considering the tolerance of hypercapnea is considered in ventilated patients.[17]
Although the jury is still out regarding whether ETC improves patient safety during PSA, there is less of a doubt that apnea is common during the process and that ETC does detect this earlier than PO alone. Soto et al[5] showed that apnea of greater than 20 seconds occurred in 26% (10 of 39) cases of monitored anesthesia care/sedation, all of whom were diagnosed by capnography, while none was detected by the provider monitoring the patient. Miner et al[1] found that in 74 patients undergoing PSA, 33 were diagnosed with RD, all of whom met the ETC criteria for RD, yet only 11 (33%) were detected by PO. Burton et al[7] stopped their study early after 20 of 60 PSA patients suffered from RD. Of these, abnormal ETC findings were documented in 14 patients before changes in PO or clinically observed hypoventilation.
Pediatric studies have reached similar conclusions.[18,19] In a recent meta-analysis, Waugh et al[8] concluded that respiratory events were 17.6 (95%CI 2.5–122.1) times more likely to be detected during PSA if ETC was used than if not used; however, significant heterogeneity was identified calling into the question the decision to pool the data. Other investigators, however, have failed to show a benefit to patients when comparing ETC with PO for detecting RD.[9,20]
With regard to ETC preventing hypoxia, Deitch et al[13–15] found hypoxia in 25% of patients monitored by ETC, compared with 42% of those where the sedationist was blinded to the ETC reading [difference of 17% (95%CI 1.3–33)]. They also noted that all cases of hypoxia were identified by ETC before onset (100% sensitivity) although 32/132 ETC monitored patients exhibited respiratory depression without going on to hypoxia [64% specificity (95%CI 53%–73%)].[4] Again, the clinical significance of this transient hypoxia is unclear, especially considering that a quarter of patients with ETC monitoring experienced hypoxia, with no apparent long-term effects. Furthermore, the risk of unnecessary intervention in respiratory depression that does not lead to hypoxia has also not yet to be quantified.
The American Society of Anesthesiologists standards for anesthetic monitoring mandate capnography for general anesthesia. The standards are less categorical about the use of ETC in the ED because of the lack of evidence supporting its use.[3,5,6]
Limitations
The principal strengthness of this study is that it was conducted in a real world setting. Patients were only excluded if they were unable to give consent, and seditionists were free to decide what medications or doses to use according to the clinical situation. Because the PSA administration regimen was not standardized, we were able to detect an outcome that resulted from a change in clinical behavior related in some way to the modality in question. Furthermore, unlike studies that recorded ETC in both groups, blinding the results in the PO one,[4] outcomes used were clinical and not surrogate markers defined partially by the monitoring modality being tested.
In conclusion, ETC did not appear to clinically benefit patients undergoing PSA in our study. It was, however, associated with an increase in both the use of airway repositioning maneuvers and hypotension. We do not believe that ETC should be recommended as a standard care for the monitoring of patients undergoing PSA in the ED.
ACKNOWLEDGEMENT
The authors would like to thank Saleema Karim for her statistical expertise in this study.
Footnotes
Funding: The study was supported by a grant from the Capital Health Research Fund, Halifax, Nova Scotia, Canada.
Ethical approval: The study was approved by the institutional ethics review board.
Conflicts of interest: No authors declare any actual or potential conflicts of interest.
Contributors: SC KM and GK conceived the study, and designed the trial. SC, RM and PF obtained research funding and conducted ethics submission. PF, RM, GE AL and DW supervised the conduct of the trial and data collection and undertook recruitment of patients. MB managed the data, including quality control. PZ provided statistical advice on study design and analyzed the data, SC and PZ drafted the manuscript, and all authors contributed substantially to its revision. SC takes responsibility for the paper as a whole.
REFERENCES
- 1.Miner JR, Heegaard W, Plummer D. End-tidal carbon dioxide monitoring during procedural sedation. Acad Emerg Med. 2002;9:275–280. doi: 10.1111/j.1553-2712.2002.tb01318.x. [DOI] [PubMed] [Google Scholar]
- 2.Wright SW. Conscious sedation in the emergency department: the value of capnography and pulse oximetry. Ann Emerg Med. 1992;21:551–555. doi: 10.1016/s0196-0644(05)82523-5. [DOI] [PubMed] [Google Scholar]
- 3.American Society of Anesthesiologists Task Force on Sedation and Analgesia by Non-Anesthesiologists. Practice guidelines for sedation and analgesia by non-anesthesiologists. Anesthesiology. 2002;96:1004–1017. doi: 10.1097/00000542-200204000-00031. [DOI] [PubMed] [Google Scholar]
- 4.Deitch K, Miner J, Chudnofsky CR, Dominici P, Latta D. Does end tidal CO2 monitoring during emergency department procedural sedation and analgesia with propofol decrease the incidence of hypoxic events? A randomized, controlled trial. Ann Emerg Med. 2010;55:258–264. doi: 10.1016/j.annemergmed.2009.07.030. [DOI] [PubMed] [Google Scholar]
- 5.Soto RG, Fu ES, Vila H, Jr, Miguel RV. Capnography accurately detects apnea during monitored anesthesia care. Anesth Analg. 2004;99:379–382. doi: 10.1213/01.ANE.0000131964.67524.E7. [DOI] [PubMed] [Google Scholar]
- 6.Green SM, Pershad J. Should capnographic monitoring be standard practice during emergency department procedural sedation and analgesia? Pro and con. Ann Emerg Med. 2010;55:265–267. doi: 10.1016/j.annemergmed.2009.08.019. [DOI] [PubMed] [Google Scholar]
- 7.Burton JH, Harrah JD, Germann CA, Dillon DC. Does end-tidal carbon dioxide monitoring detect respiratory events prior to current sedation monitoring practices? Acad Emerg Med. 2006;13:500–504. doi: 10.1197/j.aem.2005.12.017. [DOI] [PubMed] [Google Scholar]
- 8.Waugh JB, Epps CA, Khodneva YA. Capnography enhances surveillance of respiratory events during procedural sedation: a meta-analysis. J Clin Anesth. 2011;23:189–196. doi: 10.1016/j.jclinane.2010.08.012. [DOI] [PubMed] [Google Scholar]
- 9.Sivilotti ML, Messenger DW, van Vlymen J, Dungey PE, Murray HE. A comparative evaluation of capnometry versus pulse oximetry during procedural sedation and analgesia on room air. CJEM. 2010;12:397–404. doi: 10.1017/s1481803500012549. [DOI] [PubMed] [Google Scholar]
- 10.Green SM, Krauss B. Propofol in emergency medicine: pushing the sedation frontier. Ann Emerg Med. 2003;42:792–797. doi: 10.1016/s0196-0644(03)00746-7. [DOI] [PubMed] [Google Scholar]
- 11.Campbell SG, Petrie DA, MacKinley Froese P, Etsell G, Warren DA, et al. Procedural sedation and analgesia facilitator – expanded scope role for paramedics in the Emergency Department. Journal of Emergency Primary Health Care. 2008;6 [Article 990294]. Available from: http://www.jephc.com/full_article.cfm?content_id=486 . [Google Scholar]
- 12.Guyenet PG, Stornetta RL, Bayliss DA. Central respiratory chemoreception. J Comp Neurol. 2010;518:3883–3906. doi: 10.1002/cne.22435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deitch K, Chudnofsky CR, Dominici P. The utility of supplemental oxygen during emergency department procedural sedation with propofol: a randomized, controlled trial. Ann Emerg Med. 2008;52:1–8. doi: 10.1016/j.annemergmed.2007.11.040. [DOI] [PubMed] [Google Scholar]
- 14.Deitch K, Chudnofsky CR, Dominici P. The utility of supplemental oxygen during emergency department procedural sedation and analgesia with midazolam and fentanyl: a randomized, controlled trial. Ann Emerg Med. 2007;49:1–8. doi: 10.1016/j.annemergmed.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 15.Deitch K, Chudnofsky CR, Dominici P, Latta D, Salamanca Y. The utility of high-flow oxygen during emergency department procedural sedation and analgesia with propofol: a randomized, controlled trial. Ann Emerg Med. 2011;58:360–364. doi: 10.1016/j.annemergmed.2011.05.018. [DOI] [PubMed] [Google Scholar]
- 16.Green SM. Research advances in procedural sedation and analgesia. Ann Emerg Med. 2007;49:31–36. doi: 10.1016/j.annemergmed.2006.09.020. [DOI] [PubMed] [Google Scholar]
- 17.Rogovik A, Goldman R. Permissive hypercapnia. Emerg Med Clin North Am. 2008;26:941–952. doi: 10.1016/j.emc.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 18.Yldzdas D, Yapcoglu H, Ylmaz HL. The value of capnography during sedation or sedation/analgesia in pediatric minor procedures. Pediatr Emerg Care. 2004;20:162–165. doi: 10.1097/01.pec.0000117922.65522.26. [DOI] [PubMed] [Google Scholar]
- 19.Langhan ML, Chen L, Marshall C, Santucci KA. Detection of hypoventilation by capnography and its association with hypoxia in children undergoing sedation with ketamine. Pediatr Emerg Care. 2011;27:394–397. doi: 10.1097/PEC.0b013e318217b538. [DOI] [PubMed] [Google Scholar]
- 20.Koniaris LG, Wilson S, Drugas G, Simmons W. Capnographic monitoring of ventilatory status during moderate (conscious) sedation. Surg Endosc. 2003;17:1261–1265. doi: 10.1007/s00464-002-8789-7. [DOI] [PubMed] [Google Scholar]


