Abstract
BACKGROUND:
Pain in the emergency department (ED) is common but undertreated. The objective of this study was to examine the efficacy and safety of intranasal (IN) ketamine used as an analgesic for patients with acute injury with moderate to severe pain.
METHODS:
This study was a cross sectional, observational study of patients more than 8 years old experiencing moderate to severe pain [visual analog score (VAS) >50 mm]. The initial dose of IN ketamine was 0.7 mg/kg with an additional dose of 0.3 mg/kg if VAS was more than 50 mm after 15 minutes. Pain scores and vital signs were recorded at 0, 15, 30 and 60 minutes. Side-effects, sedation level and patient’s satisfaction were also recorded. The primary outcome was the number of patients achieving ≥ 20 mm reductions in VAS at 15 minutes. Other secondary outcome measures were median reduction in VAS at 15, 30 and 60 minutes, changes of vital signs, adverse events, satisfaction of patients, and need for additional ketamine.
RESULTS:
Thirty-four patients with a median age of 29.5 years (IQR 17.5–38) were enrolled, and they had an initial median VAS of 80 mm (IQR 67–90). The VAS decreased more than 20 mm at 15 minutes in 27 (80%) patients. The reduction of VAS from baseline to 40 mm (IQR 20–40), 20 mm (IQR 14–20) and 20 mm (IQR 10–20) respectively at 15, 30 and 60 minutes (P<0.001). No critical changes of vital signs were noted and adverse effects were mild and transient.
CONCLUSION:
This study showed that IN ketamine is an analgesic choice for patients with acute injury in moderate to severe pain in an overcrowded and resource limited ED.
KEY WORDS: Analgesia, Intranasal, Ketamine, Pain, Visual analog score
INTRODUCTION
Pain is one of the most common presenting symptoms in emergency department (ED), yet the provision of timely and appropriate analgesia and early assessment of its effectiveness is often a challenge.[1] The use of the intranasal (IN) route for ketamine provides an efficient, relatively painless noninvasive and well-tolerated mode of analgesia delivery.[2]
The provision of analgesia is a fundamental necessity of the ED and in our setting is usually accomplished using intramuscular NSAIDS (80%) which is usually inadequate or intravenous (IV) opioid.[3] However, significant barriers like scarcity of available emergency doctors/nurses, bed, and monitoring exist to the provision of timely analgesia by the IV route especially in overcrowded and resource limited ED like ours. Ketamine, labeled as a general anesthetic agent, is also an analgesic agent and demonstrates analgesic properties at doses 10–15 times less than that required for anesthesia.[4] Because of this analgesic effect at a low dose, patients often remain completely awake and alert and no deleterious or clinically significant hemodynamic or respiratory effects were noted previously, therefore close physiological monitoring of the patients can be avoided in contrast to opioids.[2,4–7] IN ketamine in the ED has been explored in recent years internationally;[5–7] however, the results are controversial. Recent studies[5,6] have demonstrated adequate analgesia with IN ketamine, whereas one study[7] concluded that IN ketamine had a relatively low response rate. Moreover, there is a paucity of information about this topic in our context using drops rather than mucosal atomizer device for the delivery of IN ketamine.
The primary objective of this study was to examine the analgesic effect of IN ketamine (0.7 mg/kg) in treating moderate-to-severe pain due to injury in ED setting defined as a pain reduction of 20 mm or more on a 100-mm visual analog score (VAS). The secondary objectives of the study were to determine safety of the agent by describing the level of sedation and adverse events.
METHODS
This was a cross sectional observational study conducted at a busy 35-bed general ED (average annual 30 000 patients) of one of the largest teaching hospitals in Patan Academy of Health Sciences, Nepal from July 15, 2014 to December 15, 2014. The doctors in the ED were trained by the investigator to perform patient selection, treatment and data collection. The study was approved by the Institutional Review Board of the hospital.
Study procedure
Informed written consent (with verbal explanation to minimize delays in treatment) was obtained from the patients or their caregivers. The patients self-reported baseline pain scores which were recorded using a standard 100-mm VAS with “no pain” marked at the left-hand end and “worst pain ever” at the right-hand end. The 100-mm VAS was validated in previous studies and it was proposed that 13-18 mm change in pain severity is the minimum clinically significant.[8] Severity of the pain was recorded from the left-hand end of the line.
Patients with acute injuries with moderate or severe pain (VAS≥50 mm) aged more than 8 years were included in this study. The history of allergy or intolerance to ketamine, structural or functional nasal occlusion, inability to understand VAS, Glasgow coma scale <15, systolic blood pressure >180 mmHg, a history of schizophrenia, pregnancy, prior administration of opioid analgesia within 4 hours and clinical necessity for immediate intravenous access as judged by the treating physician were set as exclusion criteria.
The initial dose of IN ketamine was 0.7 mg/kg as reported in the previous studies.[5–7] The patients received a single dose of 0.3 mg/kg or an additional dose of IN ketamine if the VAS remained 50 mm or higher at 15 minutes after the initial administration of the agent. Dosing was calculated previously for 0.7 mg/kg and 0.3 mg/kg and doses were rounded in the table in five kg weight bands for ease of dose and volume calculation so as to reduce risk of dosing error. The maximum total volume was 1 mL as the average weight of an adult in our series was 50–70 kg. An equal amount of the agent was administered in each nostril by drops using a 1-mL syringe in lateral head low position when a mucosal atomiser device was not available. The procedure was shown to the doctors before the study. The initial VAS, weight and baseline vital parameters (O2sat, HR, RR, and BP) were recorded and then IN ketamine was administered according to the precalculated weight based the dosing table. Subsequent VAS and vital signs were recorded at 15, 30 and 60 minutes respectively.
Adverse effects were recorded based on the Side Effects Rating Scale for Dissociative Anesthetics (SERSDA)[9] at 30 and 60 minutes. The effects included fatigue, dizziness, nausea, headache, feeling of unreality, changes in hearing, mood change, general discomfort, and hallucinations. The severity of adverse effects were graded on a five-point scale, with “0” representing the absence of any adverse effects and “4” representing an effect severity the patient rated as bothersome.
Sedation was graded by using the University of Michigan Sedation Scale (UMSS)[10] at 30 and 60 minutes: 0=awake and alert, 1=mild sedation, 2=moderate sedation, 3=deep sedation, 4=unarousable.
After 60 minutes patients were asked to rate the severity of nasal irritation on a 1 to 10 ordinal scale anchored with “1=none” and “10=very severe”, as well as their overall satisfaction with the pain relief provided anchored with “1= not satisfied” and “10=very satisfied”.
Outcome measures
Primary outcome measures were defined as the number of patients achieving a 20 mm or more reduction in pain as measured on VAS within 15 minutes. Secondary outcome measures were considered a median maximum reduction in VAS pain scores achieved within 15, 30 and 60 minutes, the number of patients requiring additional IN ketamine at 15 minutes, vital signs changes at 15, 30 and 60 minutes, adverse effects at 30 and 60 minutes, levels of sedation at 30 and 60 minutes, nasal irritation at 60 minutes, and overall patients/caregivers satisfaction at 60 minutes.
Data analysis
Types of papers from the clinical records were recorded by the treating doctor in the ED. Data were recorded in Excel and then analyzed with SPSS 13.0. Baseline variables like age, gender, injury types and other data were described as number, percentage or median with interquartile range (IQR) as appropriate. VAS was reported as median with IQR and compared using Wilcoxon’s signed-rank test. A reduction in VAS was compared with other variables using the Chi-square test. A P value less than 0.05 was considered statistically significant.
RESULTS
Patient demographics
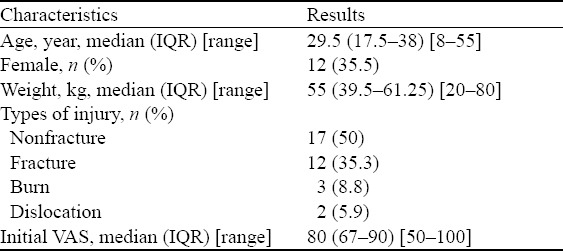
A total of 39 patients with various acute injuries and VAS pain score >50 mm were enrolled in the study (the study period of 5 months). Five patients were excluded as they did not have a VAS score and vital signs recorded after 30 minutes for various reasons. The characteristics of the remaining 34 patients are shown in Table 1. The initial median VAS score was 80 mm (IQR 67–90). There was no statistically significant relationship among the baseline VAS score, age, gender, and types of injury.
Table 1.
Characteristics of patients (n=34) receiving IN ketamine
Efficacy
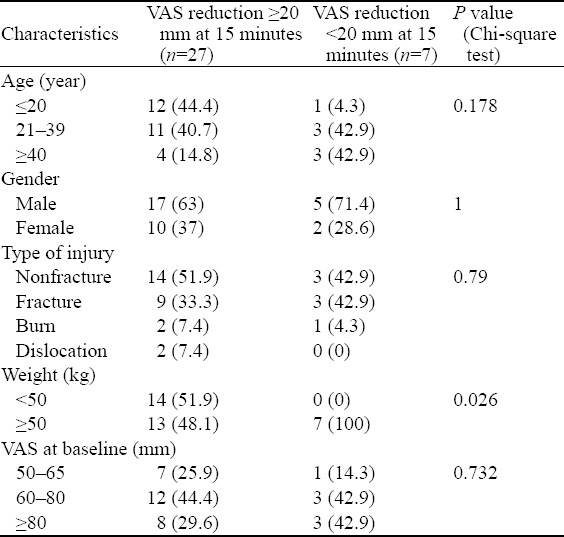
The median VAS pain score at enrollment and 15, 30 and 60 minutes after IN ketamine was represented in Box and whisker plot (Figure 1). The reduction from enrollment to each time point was statistically significant (P<0.001, Wilcoxon’s signed-rank test). The median reductions in VAS scoring at 15, 30 and 60 minutes were 40 (IQR 24–50), 50 (IQR 40–70) and 58 (IQR 45–70) respectively. Seventy-nine percent (n=27) of the patients achieved a ≥20 mm reduction in VAS at 15 minutes which increased to 100% at 30 and 60 minutes. The characteristics of patients with a VAS reduction of ≥20 mm were compared with those with a VAS reduction <20 mm (Table 2). Additional dose of ketamine was administered to six (17.6%) patients at 15 minutes.
Figure 1.

Box and Whisker plot of VAS pain scoring at baseline, 15, 30 and 60 minutes. Wilcoxon’s signed-rank test (P<0.001).
Table 2.
Comparison of characteristics between patients with a VAS reduction ≥20 mm and VAS reduction <20 mm, n (%)
Safety and adverse effect
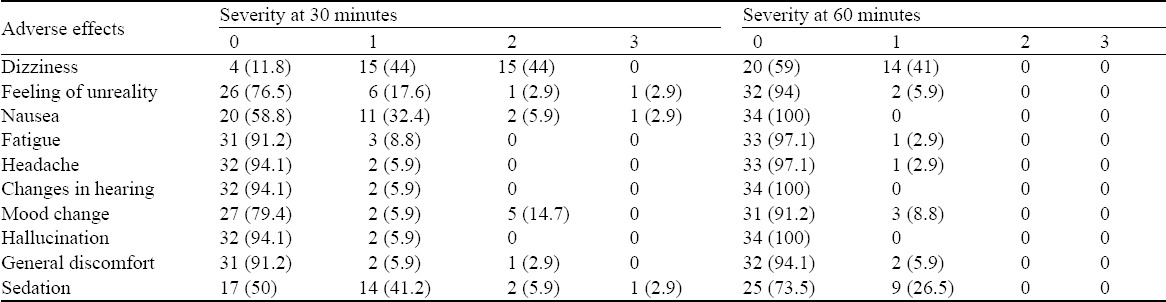
Vital signs at enrollment, 15, 30 and 60 minutes are shown in Table 3. Adverse events were generally mild and transient in nature, resolving mostly within 60 minutes after administration of ketamine (Table 4). After 60 minutes when patients were asked to rate the severity of nasal irritation on a 1 to 10 ordinal scale anchored with “1=none” and “10=very severe”, 25 (73.5%) patients did not report any nasal irritation, 6 (17.6%) and 3 (8.8%) reported grade “2” and “3” nasal irritation respectively. The median satisfaction rating with the pain relief provided anchored with “1=not satisfied” and “10=very satisfied was 8 (IQR 7–9) [range 2–9].
Table 3.
VAS score and vital signs at baseline, 15, 30 and 60 minutes, median (IQR) [range]

Table 4.
Adverse effects and their severity at 30 and 60 minutes, n (%)
DISCUSSION
In this study we found that administration of IN ketamine at a dose of 0.7 mg/kg in patients with acute injury showed a significant pain relief (≥20 mm in VAS) in 27 (79%) of the patients at 15 minutes, which increased to 100% at 30 and 60 minutes. The relief was not dependent on age, gender, type of injury, and the initial VAS score. Adverse events were mild and transient and no significant changes in physiological parameters were recorded.
Pain management in the ED is crucial and challenging, but it is often ignored and undermanaged for various reasons like limited doctors and nurses, limited time, limited beds, and limited monitoring devices. Bhandari et al[3] did an observational retrospective study in the ED of a tertiary hospital in Nepal focusing on the practice of analgesia. The study revealed that pain was poorly managed and recorded and was limited primarily to intramuscular diclofenac (NSAID) injection (80%). Intranasal delivery of pain medication is an attractive, convenient, efficient, cheap and rapid form of pain control in the ED where time, manpower, space and proper monitoring to all patients with pain are not feasible, especially in the setting where IV initiation or opioid administration exists. Researchers[11,12] have reported that the nose-brain pathway leads to a rapid delivery of some nasal medications across the olfactory mucosa to the cerebral spinal fluid and the brain, by-passing the first pass metabolism and the blood brain barrier.
Current evidence suggests that IN ketamine is an effective safe and well tolerated method of analgesia in burn dressing[13] and pediatric laceration repair,[14] in control of post-operative pain[2] and neuropathic pain,[12] and in prehospital setting[15] and the ED.[5–7]
In 1996 Kulbe et al[13] published a case report confirming that intranasal ketamine spray can be effective for acute pain control during burn dressing changes, suggesting this was an area that deserved further investigation. Carr et al[16] found that doses of up to 50 mg of IN ketamine were effective in many patients with uncontrollable pain (mean pain score reductions of about 23 mm on a 100 mm VAS), and detectable blood level was noted after 2 minutes, which peaked at 30 minutes. Another study[12] investigated the pharmacokinetics of low doses of intranasal ketamine as well as its impact on pain control and the resulting side effects which demonstrated a measurable impact on pain control within 15 minutes and peaked at 60 minutes, lasting several hours. Christensen et al[2] investigated intranasal ketamine for treating post-operative pain following wisdom tooth removal and found that meaningful pain relief was achieved by 14 minutes in 70% of patients receiving a 50 mg dose of IN ketamine. Peak analgesic effect (44 mm drop on VAS) appeared at 30 minutes in their study. Our study has better results with a 80% reduction in VAS (>20 mm) at 15 minutes, which reached 100% at 30 minutes. The better result was probably because this study included only injured patients and the effect of supplementary treatments like splinting, reduction of fracture and dislocation was not taken into account but these treatments could have alleviated the pain. Our results are consistent with those reported in children with isolated limb injury, where a total dose of approximately 1.0 mg/kg resulted in a median VAS reduction of 43 mm at 30 minutes, with 82% reporting a reduction of ≥20 mm.[6] By contrast, another study conducted by the authors in adult patients with pain from a variety of causes in ED had a median reduction in VAS rating of 24 mm at 30 minutes and only 56% reported a VAS reduction ≥20 mm with an IN ketamine dose around 1 mg/kg.[7] They also noted lack of increasing analgesic effect at the higher ketamine dose. Andolfatto et al[5] prospectively evaluated the effectiveness of IN ketamine (0.5 to 0.75 mg/kg) as an analgesic in patients with primarily orthopedic injuries and found to have a clinically significant impact on pain reduction in 88% of patients which was higher than in our study.
Intranasal ketamine has been compared with other intranasal opioids. A RCT comparing IN ketamine (1.5 mg/kg) vs. IN fentanyl (1.5 mcg/kg) for controlling postoperative pain following sinus surgery showed equivalent pain control (much better than placebo) with a slight increase in dissociative side effects in the Ketamine group.[17]
No critical changes in vital signs were noted in this study requiring intervention or withdrawal from the study. In general, the vital signs remained stable at baseline levels. Oxygen saturation remained normal over a 60-minute observation indicating that ketamine did not suppress ventilation. None of the previous studies reported delirious changes in vital signs requiring intervention.[2,5–7] This finding suggests that physiologic monitoring is not required which is essential when opioid is administered for the same purpose. This would be a great complement for most EDs in Nepal which are overcrowded and lack adequate monitoring equipments.
No serious side effects requiring intervention occurred. Some patients experienced more than one adverse effect; however none reported any bothersome symptoms of scale 4. Dizziness of scale one and two was the most reported unpleasant effect. Half of the subjects (n=17) were not sedated at 30 minutes and the rest had mild degree of sedation (grade 1 and 2) at that time. Very mild adverse effects that did not need treatment and were transient (dizziness being the most common reported side effect) are similar to the previous studies.[5–7]
VAS reduction was significantly lower in patients who weighed >50 kg. The median weight was 55 kg (IQR 39.5–61.25). The ideal volumes of intranasal drug are about 0.3 mL per nostril to reduce runoff but allow maximal mucosal coverage.[18] As the weight increased the patients required a large amount of ketamine and though the maximum volume was 1 mL (50 mg) capped at 70 kg, the excess volumes could have lost into the pharynx or out the nostril leading to its less effectiveness.
Previous studies[4–6] used mucosal atomizer device to administer IN ketamine. IN ketamine had less aversive reaction, rapid onset and recovery of sedation when administered with the mucosal atomization device as compared with the drop of the same agent; however it had similar effect using both methods in a study.[19] Our study demonstrated comparable efficacy of IN ketamine by drops and suggested that ketamine can be delivered with drops where mucosal atomization device is unavailable.
Limitations of study
There were several limitations to this study. The sample size was small. Patients were enrolled as a convenience sample for the investigators and drug administration and data collection were done by the same investigator and was unblinded. Thus there is a chance of bias in the results. Whether the patients were alcohol dependent or opioid naïve is not investigated in the study which could have affected the efficacy of ketamine. Moreover, the study was not compared randomly with placebo or other drugs.
In conclusion, IN ketamine at the initial dose of 0.7 mg/kg reduced VAS pain scores to a clinically significant degree in 80% of patients and was well tolerated without any deleterious changes of vital signs and adverse effects. This study suggests a potential use for IN ketamine in the setting of acute severe pain associated with acute injury that is cheap, fast, needle-free, safe and relatively effective with minimal side effects, in contrast to intramuscular NSAIDs and/or IV opioid which is inconvenient, invasive and requires proper monitoring of the patients for a prolonged duration.
Doses of initial 0.7 mg/kg of IN ketamine via drops is safe and effective but should be administered in aliquots if >0.3 mL of ketamine needs to be administered in a nostril to avoid runoff. Further larger randomized study, investigating the role of IN ketamine as an analgesic in the ED, needs to be conducted in future.
ACKNOWLEDGEMENT
The authors gratefully acknowledge Prof Dr Bharat Kumar Yadav, Chair, Department of General Practice and Emergency Medicine, Coordinator, Fellowship in Emergency Medicine, Patan academy of health sciences (PAHS) and Dr Darren Nichols, Associate Professor, international coordinator for fellowship in Emergency Medicine, PAHS for their guidance and inspiration. We would also like to express our gratitude to Dr Ian Walker for his constructive comments in the manuscript.
Footnotes
Funding: None.
Ethical approval: The study was approved by the Institutional Review Board of the hospital.
Conflicts of interest: The authors declare there is no competing interest related to the study, authors, other individuals or organizations.
Contributors: Roshana S proposed the study and wrote the first draft. All authors read and approved the final version of the paper.
REFERENCES
- 1.Todd KH, Ducharme J, Choiniere M, Crandall CS, Fosnocht DE, Homel P, et al. Pain in the emergency department: results of the Pain and Emergency Medicine Initiative (PEMI) Multicenter Study. J Pain. 2007;8:460–466. doi: 10.1016/j.jpain.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 2.Christensen K, Rogers E, Greenb GA, Hamiltonb DA, Mermelsteinb F, Liaob E, et al. Safety and efficacy of intranasal ketamine for acute postoperative pain. Acute Pain. 2007;9:183–192. [Google Scholar]
- 3.Bhandari R, Malla G, Mahato IP, Gupta R. Use of analgesia in an emergency department. J Nepal Med Assoc. 2013;52:224–228. [PubMed] [Google Scholar]
- 4.Kronenberg RH. Ketamine as an analgesic: parental, oral, rectal, subcutaneous, transdermal and intranasal administration. J Pain Palliat Care Pharmacother. 2002;16:27–235. doi: 10.1080/j354v16n03_03. [DOI] [PubMed] [Google Scholar]
- 5.Andolfatto G, Willman E, Joo D, Miller P, Wong WB, Koehn M, et al. Intranasal Ketamine for Analgesia in the Emergency Department: A Prospective Observational Series. Acad Emerg Med. 2013;20:1050–1054. doi: 10.1111/acem.12229. [DOI] [PubMed] [Google Scholar]
- 6.Yeaman F, Oakley E, Meek R, Graudins A. Sub-dissociative dose intranasal ketamine for limb injury pain in children in the emergency department: A pilot study. Emerg Med Australas. 2013;25:161–167. doi: 10.1111/1742-6723.12059. [DOI] [PubMed] [Google Scholar]
- 7.Yeaman F, Meek R, Egerton-Warburton D, Rosengarten P, Graudins A. Sub-dissociative dose intranasal ketamine for moderate to severe pain in adult emergency department patients. Emerg Med Australas. 2014;26:237–242. doi: 10.1111/1742-6723.12173. [DOI] [PubMed] [Google Scholar]
- 8.Todd K, Funk KG, Funk JP, Bonacci R. Clinical significance of reported changes in pain severity. Ann Emerg Med. 1996;27:485–489. doi: 10.1016/s0196-0644(96)70238-x. [DOI] [PubMed] [Google Scholar]
- 9.Eide P, Jorum E, Stubhaug A, Bremnes J, Breivik H. Relief of post-herpetic neuralgia with the N-methyl-D-aspartic acid receptor antagonist ketamine: a double-blind, cross-over comparison with morphine and placebo. Pain. 1994;58:347–354. doi: 10.1016/0304-3959(94)90129-5. [DOI] [PubMed] [Google Scholar]
- 10.Malviya S, Voepel-Lewis T, Tait AR, Merkel S, Tremper K. Depth of sedation in children undergoing computed tomography: validity and reliability of the University of Michigan Sedation Scale (UMSS) Br J Anaesth. 2002;88:241–245. doi: 10.1093/bja/88.2.241. [DOI] [PubMed] [Google Scholar]
- 11.Westin EA. direct nose-to-brain transfer of morphine after administration to rats. Pharm Res. 2006;23:565–572. doi: 10.1007/s11095-006-9534-z. [DOI] [PubMed] [Google Scholar]
- 12.Huge V, Lauchart M, Magerl W, Schelling G, Beyer A, Thieme D, et al. Effects of low-dose intranasal (S)-ketamine in patients with neuropathic pain. Eur J Pain. 2010;14:387–394. doi: 10.1016/j.ejpain.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 13.Kulbe J. The use of ketamine nasal spray for short-term analgesia. Home Health Nurse. 1998;16:367–370. [PubMed] [Google Scholar]
- 14.Tsze D, Steele W, Akhlaghi F, Linakis JG. Intranasal ketamine for procedural sedation in pediatric laceration repair. Pediatr Emer Care. 2012;28:767–770. doi: 10.1097/PEC.0b013e3182624935. [DOI] [PubMed] [Google Scholar]
- 15.Johansson J, Sjöberg J, Nordgren M, Sandström E, Sjöberg F, Zetterström H. Prehospital analgesia using nasal administration of S-ketamine--a case series. Scand J Trauma Resusc Emerg Med. 2013;21:38. doi: 10.1186/1757-7241-21-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carr DB, Goudas LC, Denman WT, Brookoff D, Staats PS, Brennen L, et al. Safety and efficacy of intranasal ketamine for the treatment of breakthrough pain in patients with chronic pain: a randomized, double-blind, placebo-controlled, crossover study. Pain. 2004;108:17–27. doi: 10.1016/j.pain.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 17.Abdel-Ghaffar HS, Salem MAM. Safety and analgesic efficacy of pre-emptive intranasal ketamine versus fentanyl in patients undergoing endoscopic nasal surgery. Journal of Americal Science. 2012;8:430–436. [Google Scholar]
- 18.Dale O, Hjortkjaer R, Kharasch ED. Nasal administration of opioids for pain management in adults. Acta Anaesthesiol Scand. 2002;46:759–770. doi: 10.1034/j.1399-6576.2002.460702.x. [DOI] [PubMed] [Google Scholar]
- 19.Pandey RK, Bahetwar SK, Saksena AK, Chandra G. A comparative evaluation of drops versus atomized administration of intranasal ketamine for the procedural sedation of young uncooperative pediatric dental patients: a prospective crossover trial. J Clin Pediatr Dent. 2011 Fall;36:79–84. doi: 10.17796/jcpd.36.1.1774746504g28656. [DOI] [PubMed] [Google Scholar]


