Abstract
BACKGROUND:
Emergency physicians (EPs) often care for patients with acute small bowel obstruction. While some patients require exploratory laparotomy, others are managed successfully with supportive care. We aimed to determine features that predict the need for operative management in emergency department (ED) patients with small bowel obstruction (SBO).
METHODS:
We performed a retrospective chart review of 370 consecutive patients admitted to a large urban academic teaching hospital with a diagnosis of SBO over a two-year period. We evaluated demographic characters (prior SBO, prior abdominal surgery, active malignancy) and clinical findings (leukocytosis and lactic acid) to determine features associated with the need for urgent operative intervention.
RESULTS:
Patients with a prior SBO were less likely to undergo operative intervention [20.3% (42/207)] compared to those without a prior SBO [35.2% (57/162)]. Abnormal bloodwork was not associated with need for operative intervention. 68% of patients with CT scan findings of both an SBO and a hernia, however, were operatively managed.
CONCLUSIONS:
Patients with a history of SBO were less likely to require operative intervention at any point during their hospitalization. Abnormal bloodwork was not associated with operative intervention. The CT finding of a hernia, however, predicted the need for operative intervention, while other findings (ascites, duodenal thickening) did not. Further research would be helpful to construct a prediction rule, which could help community EPs determine which patients may benefit from expedited transfer for operative management, and which patients could be safely managed conservatively as an initial treatment strategy.
KEY WORDS: Small bowel obstruction, Computed tomography, Hernia
INTRODUCTION
Approximately 300 000 patients are hospitalized annually in the United States with acute small bowel obstruction (SBO).[1] The majority of these patients are admitted to the hospital from the emergency department (ED) after imaging studies and initial stabilization. While a history of prior abdominal surgery, abnormal bowel sounds, abdominal distention, and constipation raise the likelihood of an intestinal obstruction, it would be useful if the history, physical exam, or diagnostic test results could identify patients who are more likely to require urgent operative intervention.[2] In a retrospective review of 192 patients admitted with SBO and operatively managed, Jancelwicz et al[3] found a modest pre-operative correlation between peritoneal signs, leukocytosis, abdominal CT findings of reduced wall enhancement, and an operative diagnosis of bowel strangulation. Zielinksi et al,[4] who examined 100 admitted patients with SBO, determined that vomiting, and three different abdominal CT findings (ascites, mesenteric edema, absence of the ’small bowel feces sign’) were independent predictors of the need for operative intervention. At this time there is no compelling evidence to help the emergency physician (EP) identify which patients will require operative management and which patients will likely experience symptom resolution with conservative treatment alone (nasogastric tube placement, bowel rest, and intravenous hydration).
METHODS
Setting and study population
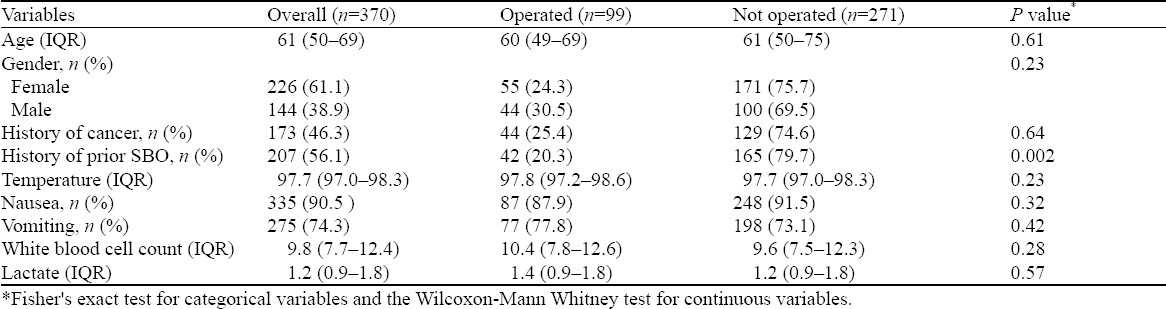
This is a retrospective, single-center cohort study in a large tertiary care academic center. We reviewed 370 consecutive admissions for small bowel obstructions from January 2012 to January 2014. This study was approved by the hospital’s Institutional Review Board (IRB) with a waiver of informed consent. We recorded each patient’s age, gender, date of visit, history of abdominal surgeries, history of prior SBO, and whether there was a history of active malignancy (Table 1). We documented each patients’ oral temperature, WBC count, lactic acid level, and noted whether neutrophilia was present. We logged what type of imaging was performed (plain films, bedside ultrasound, and/or abdominal CT scan) in the ED. Finally, we recorded each patient’s treatment (conservative, operative). If an operative intervention was performed during the patient’s hospitalization, we recorded when this occurred relative to the patient’s ED visit.
Table 1.
Baseline characteristics of patients stratified by treatment (operative versus conservative)
Analysis
The primary outcome of interest was whether a patient with a bowel obstruction required operative intervention. Secondary outcome was time to operation. We collected variables by structured chart abstraction. The initial chart review was conducted by author AH and 10% of those charts were reviewed by SF to check internal validity on history of cancer, history of bowel obstruction, and CT reading. There was 100% agreement between the two reviewers. Initial post-hoc review of the data suggested that patients with hernia appeared to require operative intervention as compared with those without hernia. For descriptive statistics, we compared proportions with fisher’s exact test and continuous variables with the Wilcoxon-Mann Whitney test. We used logistic regression to model the association between clinical variables and the need for operative intervention. For our multivariable model, we included apriori clinical and demographic variables (age, gender, history of cancer, history of prior small bowel obstruction, fever, elevated WBC, and elevated lactate) and other variables with P value of less than 0.20, which only included those with a CT finding of a hernia. We estimated odds ratio and from the logistic model we estimated predicted probabilities for requiring an operation. For the multivariable models, we evaluated the predicted probabilities evaluated at the mean value of other covariates in the model. To model time to operation, we used Poisson regression with robust variances with the same variables as our logistic model above. We considered a two-sided P value of less than 0.05 to be statistically significant and conducted our analysis using STATA 12.0 and R v 3.02.
RESULTS
There were 370 patients with small bowel obstruction in our cohort. The baseline characteristics of patients who underwent an operation and those who did not are noted in Table 1. Overall, 27% (99/370) of patients required an operation. Table 2 depicts the univariate association of our baseline factors (history of malignancy, history of recurrent SBO, vital signs, bloodwork, etc) with the probability of requiring operative intervention when diagnosed with a SBO along with the results of a multivariable model. We found that patients with a history of SBO were less likely to undergo operative intervention [32.0% (24.2%–39.9%) vs. 19.2% (13.5%–25.0%), P=0.010], whereas patients with hernia on abdominal CT imaging were much more likely to receive surgery compared with those without hernia [68.6 (95% CI=vs 19.3%)]. Prior history of malignancy, leukocytosis and elevated lactic acid levels were not associated with increased likelihood of operative intervention.
Table 2.
Association of baseline factors with the probability of requiring operative intervention for small bowel obstruction
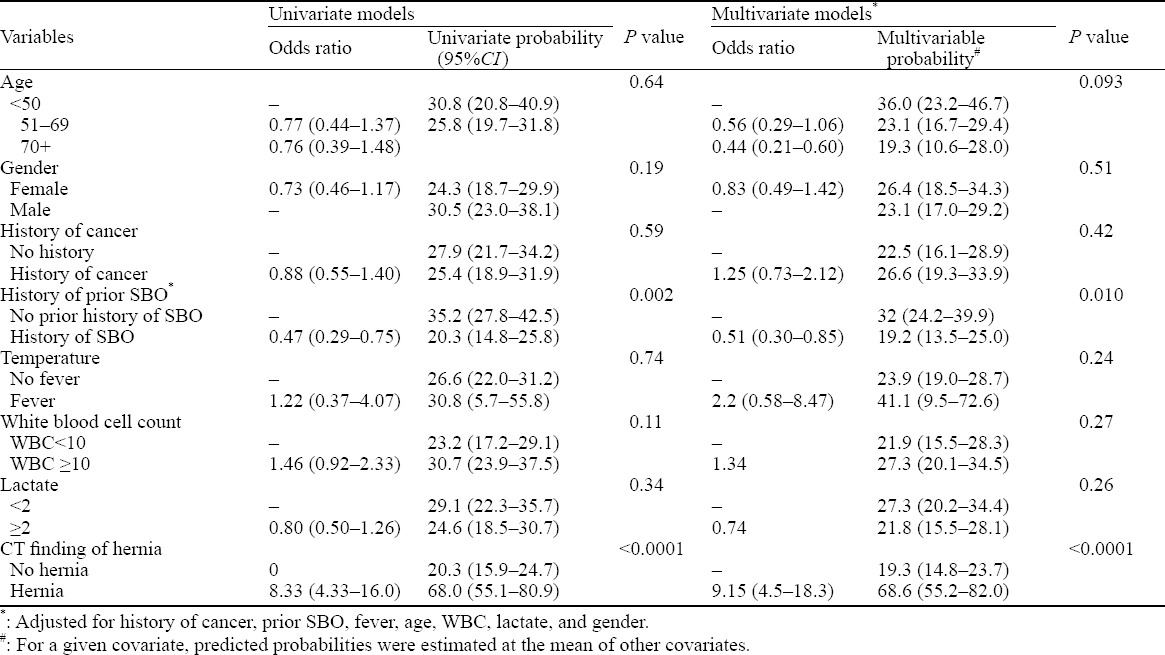
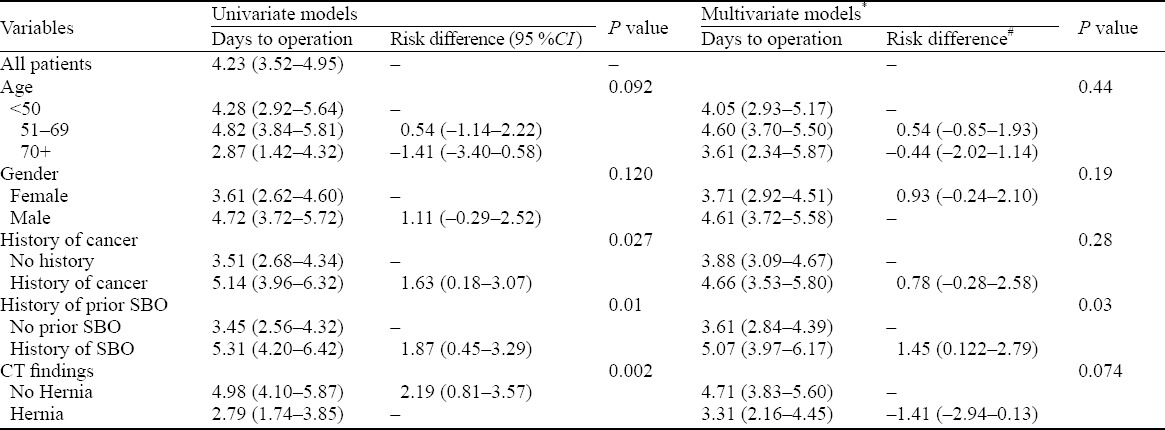
Table 3 describes all patients who went to the OR. Patients with a history of SBO went to the OR 1.45 days later [95%CI 1.22–2.79, P=0.030]) than those without SBO. Patients with a history of malignancy also went to the OR later than those without active cancer. But these findings could be explained by chance alone (P=0.28). Finally, in our study there was no difference between timing of patients with hernia and those without (P=0.074).
Table 3.
Time to operation
DISCUSSION
Our ED is affiliated to a large urban academic hospital and has an annual patient volume of approximately 70 000. In addition, the ED is affiliated with one of the country’s leading cancer institiutes, which has approximately 230 000 patient visits per year. Consequently, a significant number of our patients not only have an active oncologic history, but routinely present to the ED with a variety of complications specific to their disease, including febrile neutropenia, anaphylaxis to chemotherapeutic agents, pleural and pericardial effusions, malignant ascites, and recurrent SBOs. We wished to determine whether specific patient characteristics, such as a history of SBO, abdominal surgery, or active malignancy, correlated with the type of treatment (operative vs conservative). We theorized that patients with active cancer and a history of SBO would more likely undergo conservative treatment, whereas those who had abdominal surgeries but no history of active cancer were more likely to undergo operative management. We also examined whether specific markers of inflammation (WBC, neutrophilia, lactic acid) were higher in patients who ultimately underwent operative management during their hospitalization. Finally, we examined whether specific additional abdominal CT findings (ascites, hernia) were associated with the need for operative management.
Although we hypothesized that patients with active malignancy would be more likely conservatively managed, they were just as likely as those without malignancy to undergo surgery. Treatment of obstructions in patients with active malignancy is challenging and surgery may be delayed in order to attempt conservative management.[5] These patients often have poor baseline health or a poor overall prognosis that may affect treatment decisions and when malignant disease causes an obstruction, it is often associated with very poor survival.[6,7] Surgical management in oncology patients is also associated with higher post-operative morbidity.[6] Furthermore, operative interventions are valuable in oncology patients only when they resolve a benign cause of the obstruction.[8] In a retrospective review, Ellis et al[9] noted that 30% of patients with colorectal malignancy had a benign and, thus, potentially curable cause for SBO. In such cases operative treatment can improve survival and quality of life.[6,8,10]
Similarly to patients without active malignancy, those with SBOs secondary to cancer often respond well to non-operative treatment.[9] Non-operative resolution of both malignant and non-malignant obstructions generally occurs within 72 hours.[11,12] Malignant obstructions also have lower strangulation rates than obstructions secondary to adhesions or hernias, which may prompt a longer trial of non-operative management.[11,13]
We also found that patients with a history of SBO were less likely to require operative intervention at any point during their hospitalization. Adhesions are the most common cause of obstruction, with malignancy being the second most common cause.[4,14] Thus, patients with a first-time obstruction but without a history of surgery are more likely to undergo surgery to determine the cause of the obstruction and attempt to rule out a malignant cause. Obstructions due to adhesions also have a lower rate of ischemia, which may account for the finding that patients with recurrent SBOs were less likely to be operatively managed.[4,15]
It can be challenging to differentiate between partial and complete small bowel obstructions. Patients with a partial obstruction are very likely to have spontaneous resolution with conservative management.[15] If the obstruction has not resolved within 48 hours, however, surgery is often indicated.[5] Perhaps recurrent obstructions are more often conservatively managed because patients are likely to present to the ED earlier in their course (once they recognize the symptoms of SBO) and surgeons are less likely to operate on patients who have undergone successful conservative management for prior SBOs.
Neither elevated lactate levels nor leukocytosis on presentation are associated with an increased likelihood of operative intervention. Likewise, an oral temperature >100.4 °F is not associated with an increased likelihood of operative intervention. Thus, similar to prior studies, laboratory values do not predict the need for surgical management.[16–20] It appears as though most patients with an obstruction undergo the proper treatment (whether conservative or surgical) before the development of late complications, such as bowel necrosis, perforation, or peritonitis, regardless of their laboratory values.[5,21] To date no specific marker has been discovered that can identify early mucosal damage or differentiate between early and full-thickness intestinal infarction, likely due to the effect of first pass hepatic metabolism.[20]
The CT finding of a hernia predictes the need for operative intervention, while other findings (ascites, duodenal thickening) do not. Although it is difficult to estimate the incidence of abdominal hernias because many remain asymptomatic, nearly 10%–15% of abdominal surgeries result in an incisional hernia.[22] We found that any hernia visualized on CT scan predicted the need for operative management. Perhaps the identification of this particular defect encourages surgical intervention as it is easily corrected, whereas adhesions have a high frequency of recurrence despite repeated lysis in the operating room.[21]
Limitations
There are several limitations to our study. Our data were obtained through a retrospective chart review, and we must contend with missing data. Not every patient had a lactic acid drawn or an oral temperature recorded. A few patients had no imaging performed, making it impossible to confirm the diagnosis of SBO on admission. There were several reasons to explain the lack of imaging studies. In a few instances patients wished to defer imaging given their history of multiple prior conservatively managed SBOs and a strong desire to avoid further radiation. A few very ill patients with a presumptive diagnosis of SBO who were deemed non-operative candidates per surgical consultation in the ED also elected to forego CT imaging instead they were diagnosed with a SBO based on a bedside ultrasound or plain radiograph. Some patients were transferred to our institution from smaller community EDs once the diagnosis of SBO was made. We recorded bloodwork from their initial ED visit when it was accessible. Unfortunately, however, these results were not always available to us. Thus, we logged repeat bloodwork that was obtained in our ED once the patient had arrived. Transfer patients had uniformly been given intravenous fluids and antibiotics prior to their arrival at our institution; thus, WBC counts and lactic acid levels may have been ’improved’ leading to falsely normal results at our institution. Finally, we did not examine if patients were more or less likely to go to the OR depending on which attending surgeon was on call for the ED.
In conclusion, most patients with a diagnosis of SBO are conservatively managed. Further research would be helpful to construct a prediction rule, which could help community EPs determine which patients may benefit from expedited transfer for operative management, and which patients could be safely managed conservatively as an initial treatment strategy, hereby avoiding costly transfers.
Footnotes
Funding: None.
Ethical approval: This study was approved by the hospital’s Institutional Review Board (IRB) with a waiver of informed consent.
Conflicts of interest: The authors declare that there are no conflicts of interest related to the publication of this paper.
Contributors: Frasure SE proposed the study, analyzed the data and wrote the first draft. All authors contributed to the design and interpretation of the study and to further drafts.
REFERENCES
- 1.Irvine TT. Abdominal pain: a surgical audit of 1190 emergency admissions. Br J Surg. 1989;76:1121–1125. doi: 10.1002/bjs.1800761105. [DOI] [PubMed] [Google Scholar]
- 2.Taylor MR, Lalani N. Adult small bowel obstruction. Acad Emerg Med. 2013;20:528–544. doi: 10.1111/acem.12150. [DOI] [PubMed] [Google Scholar]
- 3.Jancelwicz T, Vu LT, Shawo AE. Predicting strangulated small bowel obstruction: an old problem revisited. J Gastrointest Surg. 2009;13:93–99. doi: 10.1007/s11605-008-0610-z. [DOI] [PubMed] [Google Scholar]
- 4.Zielinski MD, Eiken PW, Bannon MP, Heller SF, Lohse CM, Huebner M, et al. Small bowel obstruction – who needs an operation? A multivariate prediction model. World J Surg. 2010;34:910–919. doi: 10.1007/s00268-010-0479-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cappell MS, Batke M. Mechanical obstruction of the small bowel and colon. Med Clin North Am. 2008;98:575–597. doi: 10.1016/j.mcna.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 6.Miller G, Boman J, Shrier I, Gordon PH. Small bowel obstruction secondary to malignant disease: an 11-year audit. Can J Surg. 2000;43:353–358. [PMC free article] [PubMed] [Google Scholar]
- 7.Parker MC, Baines MJ. Intestinal obstruction in patients with advanced malignant disease. Br J Surg. 1996;83:1–2. doi: 10.1002/bjs.1800830102. [DOI] [PubMed] [Google Scholar]
- 8.Woolfson RG, Jennings K, Whalen GF. Management of bowel obstruction in patients with abdominal cancer. Arch Surg. 1997;132:1093–1097. doi: 10.1001/archsurg.1997.01430340047006. [DOI] [PubMed] [Google Scholar]
- 9.Ellis CN, Boggs HW, Jr, Slagle GW, Cole PA. Small bowel obstruction after colon resection for benign and malignant diseases. Dis Colon Rectum. 1991;34:367–371. doi: 10.1007/BF02053685. [DOI] [PubMed] [Google Scholar]
- 10.Lau PW, Lorentz TG. Results of surgery for malignant bowel obstruction in advanced, unresectable, recurrent colorectal cancer. Dis Colon Rectu. 1993;36:61–64. doi: 10.1007/BF02050303. [DOI] [PubMed] [Google Scholar]
- 11.Bizer LS, Liebling RW, Delany HM, Gliedman ML. Small bowel obstruction: the role of nonoperative treatment in simple intestinal obstruction and predictive criteria for strangulation obstruction. Surgery. 1981;89:401–413. [PubMed] [Google Scholar]
- 12.Osteen RT, Guyton S, Steele G, Jr, Wilson RE. Malignant intestinal obstruction. Surgery. 1980;87:611–615. [PubMed] [Google Scholar]
- 13.Glass RL, LeDuc RJ. Small intestinal obstruction from peritoneal carcinomatosis. Am J Surg. 1973;125:316–317. doi: 10.1016/0002-9610(73)90049-4. [DOI] [PubMed] [Google Scholar]
- 14.Menzies D, Ellis H. Intestinal obstruction from adhesions – how big is the problem? Ann R Coll Surg Eng. 1990;72:60–63. [PMC free article] [PubMed] [Google Scholar]
- 15.Millet I, Ruyer A, Alili C, Curros Doyon F, Molinari N, Pages E, et al. Adhesive small-bowel obstruction: value of CT in identifying findings associated with the effectiveness of nonsurgical treatment. Radiology. 2014;273:425–432. doi: 10.1148/radiol.14132872. [DOI] [PubMed] [Google Scholar]
- 16.Brolin RE, Krasna MJ, Marst BA. Use of tubes and radiographs in the management of small bowel obstruction. Ann Surg. 1987;206:126–133. doi: 10.1097/00000658-198708000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Silen W, Hein MF, Goldman L. Strangulation obstruction of the small intestine. Arch Surg. 1962;85:121–129. doi: 10.1001/archsurg.1962.01310010125017. [DOI] [PubMed] [Google Scholar]
- 18.Sarr MG, Bulkley GB, Zuidema GD. Preoperative recognition of intestinal strangulation obstruction: prospective evaluation of diagnostic capability. Am J Surg. 1983;145:176–182. doi: 10.1016/0002-9610(83)90186-1. [DOI] [PubMed] [Google Scholar]
- 19.Block T, Nilsson TK, Björck M, Acosta S. Diagnostic accuracy of plasma biomarkers in intestinal ischemia. Scand J Clin Lab Invest. 2008;68:242–248. doi: 10.1080/00365510701646264. [DOI] [PubMed] [Google Scholar]
- 20.Evennett NJ, Petrov MS, Mittal A, Windsor JA. Systematic review and pooled estimates for the diagnostic accuracy of serological markers for intestinal ischemia. World J Surg. 2009;33:1374–1383. doi: 10.1007/s00268-009-0074-7. [DOI] [PubMed] [Google Scholar]
- 21.Hayden GE, Sprouse KL. Bowel obstruction and hernia. Emerg Med Clin N Amer. 2011;29:319–345. doi: 10.1016/j.emc.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 22.Kingsnorth A, LeBlanc K. Hernias: inguinal and incisional. Lancet. 2003;362:1561–1571. doi: 10.1016/S0140-6736(03)14746-0. [DOI] [PubMed] [Google Scholar]


