Abstract
BACKGROUND:
Periplaneta americana extract is recognized to have a positive effect on gastrointestinal mucosa. This study aimed to investigate the effects of periplaneta americana extract on immune function, nutrition status and gastrointestinal complications of early enteral nutrition patients with systemic inflammatory response syndrome (SIRS).
METHODS:
Patients with SIRS were randomly divided into two groups: treatment and control groups. All patients in the two groups received conventional therapy including enteral nutrition, but periplaneta americana extract, an additional Chinese medicine, was given to the patients in the treatment group. At the beginning of treatment (0 day) and 1, 3, and 7 days after treatment, the levels of immunoglobulin (IgA), total lymphocyte count (TLC), total protein (TP) and prealbumin (PA) were respectively tested in patients’ venous blood. The incidences of bloating, diarrhea, aspiration pneumonia and high blood sugar at 7 days after treatment were recorded. The mortality of the patients in 28 days was recorded.
RESULTS:
At 3 and 7 days after treatment, the levels of IgA and TLC in the treatment group were higher than those in the control group (P<0.05). At 7 days after treatment, the levels of TP and PA in the treatment group were higher than those in the control group (P<0.05). The incidences of bloating and diarrhea in the treatment group were lower than those in the control group, the differences were significant (P<0.05). The mortality of treatment group was lower than that of the control group (P>0.05).
CONCLUSION:
Periplaneta americana extract could reduce gastrointestinal complications and improve immune function and nutritional status in patients with systemic inflammatory response syndrome.
KEY WORDS: Periplaneta americana extract, Systemic inflammatory response syndrome, Gastrointestinal function, immune function, Nutritional status, Enteral nutrition
INTRODUCTION
Systemic inflammatory response syndrome (SIRS) is an inflammatory state affecting the whole body, which is a response to an infectious or noninfectious insult.[1] If SIRS is due to an infection, it is considered sepsis. Noninfectious causes of SIRS include burns, trauma, ischemia, hemorrhage, etc.[2] If patients with SIRS are not treated effectively, multiple organ dysfunction syndrome appears as a major leading cause of death in the intensive care unit.[3] As patients with SIRS are in a high metabolic state, nutritional support is often necessary. Early enteral nutrition can help to reduce metabolic complications, protect the integrity of gastrointestinal mucosal structure and function, reduce intestinal permeability, and prevent bacterial translocation and enterogenous infection. However, early enteral nutrition support often leads to a variety of adverse reactions. Effective medicine is expected to protect gastrointestinal mucosal barrier and reduce gastrointestinal complications.
Studies[4,5] have shown that periplaneta americana extract has a positive effect on the gastrointestinal mucosa. Since periplaneta americana extract has been used in early enteral nutrition patients with SIRS in our hospitals, the present study was to determine whether it is effective in improving immune function, nutrition status and gastrointestinal complications.
METHODS
General data
From January 2008 to December 2010, a total of 72 patients with SIRS aged from 18 to 79 years were treated at the ICU of our hospital. All patients met early enteral nutrition standards, and their APACHE II scores were ≥11 points. They fulfilled SIRS criteria recommended by the American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference.[6,7] SIRS is characterized by serious trauma, cardiopulmonary resuscitation, poisoning, and injuries of the brain nervous system, respiratory system, cardio-cerebral vascular system. The patients were divided into two groups, treatment and control groups (36 patients in each group). The study was approved by the ethics committee of our hospital and was conducted in accordance with the Declaration of Helsinki. All patients signed an informed consent form before their enrollment in this study.
Exclusion criteria
Excluded were patients who used gastrointestinal motility or anticholinergic agents for a week; those with gastrointestinal resection or gastrointestinal primary injury; those with suspected intestinal obstruction; those with malignant tumor, HIV infection, systemic lupus erythematosus and serious cardiovascular disorders including arrhythmia; and those who were intolerable to enteral nutrition.
Nutrition
After the patients were admitted to the ICU, they were given enteral nutrition during 24 to 48 hours according to the Harris-Benedict formula. Nutrison (Nutricia Pharmaceutical Wuxi Co., Ltd.) was used. Enteral nutrition was input through a nasogastric or nose intestinal tube. Nutrison was given by enteral nutrition pumps for 16 to 20 hours and enteral nutrition pump controlled input temperature at 37–40 °C. According to patient’s conditions and clinical manifestations, the quantity of Nutrison gradually increased from half to fully amount. There was no significant difference in the treatment with enteral nutrition between the two groups (P>0.05).
Interventions
All patients were subjected to a comprehensive treatment, which was in line with the international guidelines,[8] including the treatment of primary diseases, blood and fluid transfusion to maintain the stability of the circulatory system, protective mechanical ventilation to maintain oxygenation, analgesic, sedative, anti-inflammatory, and nutritional support, maintain water and electrolyte balance, and other conventional therapy. But periplaneta americana extract (kangfuxin solution, Hunan the Nanke Lun Pharmaceutical Co., Ltd. China), an additional Chinese medicine, was given to the patients of the treatment group. The diagnosis of SIRS was confirmed within 24 hours, periplaneta americana extract was taken with a gastric tube, 10 mL/time, tid, 7 days for a course of treatment.
Outcome measures
At 0, 1, 3, and 7 days after the treatment, the levels of immunoglobulin (IgA), total lymphocyte count (TLC), total protein (TP) and prealbumin (PA) were respectively detected in venous blood of the patients. The incidences of bloating, diarrhea, aspiration pneumonia and high blood sugar at 7 days were recorded. The mortality of the patients at 28 days was also recorded. The levels of TP and PA were tested by an automatic biochemical analyzer. TLC was detected by a blood cell analyzer.
Outcome measures were performed by an independent researcher who was not informed of the treatment sequence.
Statistical analysis
Statistical analysis was conducted using statistical software SPSS17.0. The data were expressed in mean ± standard deviation and analyzed by Student’s t test. The numeration data were analyzed by the Chi-square test (P<0.05).
RESULTS
The general data of the two groups
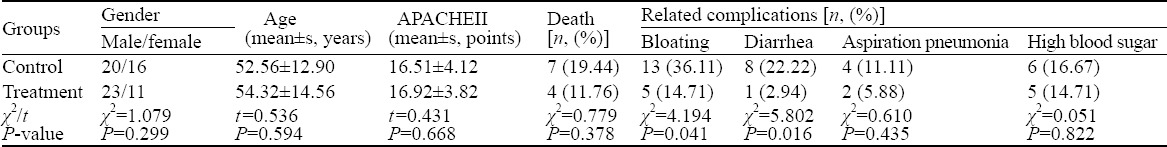
A total of 72 patients were enrolled in this study. None of the patients was dropped out in the control group. Two patients were excluded from the treatment group because they had poor compliance and refused outcome measures. Finally, 70 patients were included in the study: 34 patients in the treatment group and 36 in the control group. There was no significant difference in gender, age, and APACHE II score between the two groups (P>0.05). Four and seven patients in the treatment group and control group died, respectively. The mortality of the treatment group at 28 days was lower than that of the control group (11.76% vs. 19.44%) (P>0.05). The incidences of bloating and diarrhea in the treatment group at 7 days were significantly lower than those in the control group (P<0.05) (Table 1).
Table 1.
The general data of the two groups
Comparison of immunity function
Before treatment, the levels of IgA and TLC in the two groups were not significantly different (P>0.05). At 3 and 7 days after treatment, the levels of IgA and TLC in the treatment group were higher than those in the control group (P<0.05) (Figures 1–2).
Figure 1.
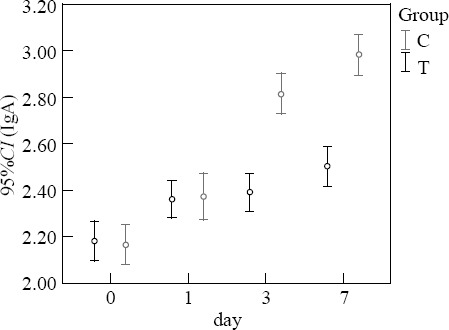
Comparison of IgA (g/L).
Figure 2.
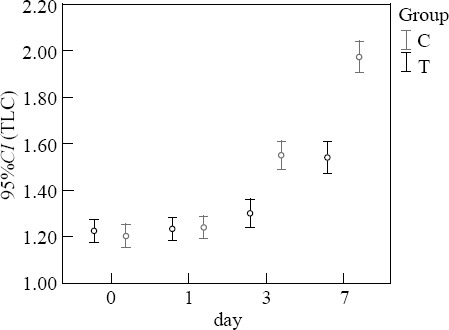
Comparison of TLC (×109/L).
Comparison of nutritional status
Before treatment, the levels of TP and PA in the two groups were not significantly different (P>0.05). At 7 days after treatment, the levels of TP and PA in the treatment group were higher than those in the control group (P<0.05) (Figures 3–4).
Figure 3.
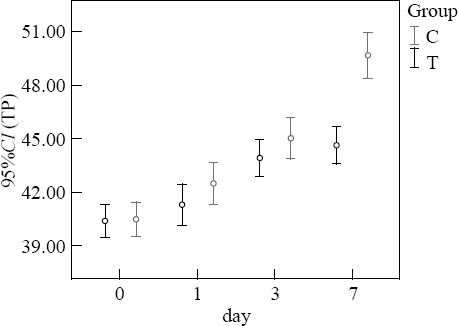
Comparison of TP (g/L).
Figure 4.
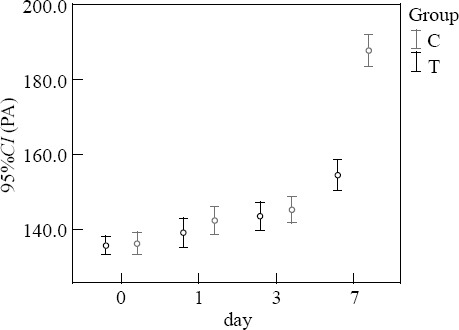
Comparison of PA (mg/L).
DISCUSSION
SIRS is considered as a self-defense mechanism. Inflammation is body’s response to nonspecific insults that arise from chemical, traumatic or infectious spur. The inflammatory mechanism is a complex process involving humeral and cellular responses, complement, and cytokine cascades. The pathophysiological changes of SIRS have been clarified in recent years.[9–11] Patients with SIRS are usually in a pathological state such as gastrointestinal mucosal ischemia and hypoxia, which lead to the appearance of mucosal lesions or mucosal barrier damage. At the same time, patient’s body is in a high catabolic state and energy consumption is significantly increased; therefore nutritional support is particularly important in the treatment. Nutritional support can provide patients with energy and protein, while improving nitrogen balance and promoting injured tissue healing and functional recovery. The gastrointestinal tract is an active participant of pathophysiological process in critically ill patients. Early enteral nutrition is superior to parenteral nutrition in preventing infection, metabolic complications, and bacterial translocation. Therefore, the protection of the gastrointestinal mucosal barrier and nutritional support play an important role in the treatment of patients with SIRS.
Periplaneta americana extract is derived from American periplaneta dried worm by ethanol, containing peptides, polyols, epidermal growth factor, sticky sugar acid, amino acids, and other active substances. The present study showed that Periplaneta americana extract had preventive and therapeutic effect on peptic ulcer.[12] Periplaneta americana extract can increase the levels of gastric mucosal hexosamine and prostaglandin E2 (PGE2). Hexosamine has a protective effect on the gastric mucosa, and PGE2 can inhibit acid secretion and increase mucosal blood flow, both of which contribute to the repair of gastrointestinal mucosa.[13] Moreover, they also inhibit the release of inflammatory mediators in the gastric mucusa, and inhibit neutrophils, monocytes and macrophages at inflammatory sites.[14] The protective effect of Periplaneta americana extract on the function of the gastrointestinal mucosal barrier could be induced through the above-mentioned mechanism. A recent study[4] found that Periplaneta americana extract could reduce the level of endotoxin, thus protecting the function of the gastrointestinal mucosal barrier.
The gastrointestinal tract plays an important role in the immune system. The epithelial surface of the gastrointestinal mucosa is covered with a mucus layer, which is essential to the function of the gastrointestinal barrier.[15] IgA secretes on the gastrointestinal mucosal surface.[16] As the gastrointestinal immune system is activated, lymphoid tissue of the gastrointestinal mucosa is stimulated while increasing the secretion of IgA and enhancing the immune function of the gastrointestinal tract.[17] In our study, the levels of IgA and TLC in the treatment group were higher than those in the control group at 3 and 7 days after treatment (P<0.05). Bloating and diarrhea were reduced in the treatment group (P<0.05). These results revealed that Periplaneta americana extract could improve the immune barrier of the gastrointestinal mucosa. Seven days after treatment, nutrition indicators (TP and PA) in the treatment group were better than those in the control group (P<0.05), indicating that Periplaneta americana extract has a protective effect on the gastrointestinal mucosa.
In summary, Periplaneta americana extract can improve the immune barrier of the gastrointestinal mucosa in patients with SIRS. Thus it could reduce adverse reactions of the gastrointestinal tract, increase the tolerability of enteral nutrition, promote nutrition digestion and absorption in the gastrointestinal tract, and ultimately improve the general nutritional status, conditions, and prognosis of the patients.
Footnotes
Funding: The study was supported by Tangshan City Science and Technology Development Project (No.15130219a).
Ethical approval: The study was approved by Ethics Committee of Affiliated Orthopedic Hospital of North China University of Science and Technology, Tangshan, China.
Conflicts of interest: The authors declare that there are no conflicts of interest related to the publication of this paper.
Contributors: Zhang HW proposed the study, analyzed the data and wrote the first draft. All authors contributed to the design and interpretation of the study and to further drafts.
REFERENCES
- 1.Robertson CM, Coopersmith CM. The systemic inflammatory response syndrome. Microbes Infect. 2006;8:1382–1389. doi: 10.1016/j.micinf.2005.12.016. [DOI] [PubMed] [Google Scholar]
- 2.Balk RA. Systemic inflammatory response syndrome (SIRS): where did it come from and is it still relevant today? Virulence. 2014;5:20–26. doi: 10.4161/viru.27135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang HW, Wei LY. Effects of xuebijing injection on septic patients’ high-density lipoprotein cholesterol. Chin J Crit Care Med (in Chinese) 2010;30:171–173. [Google Scholar]
- 4.Zhang H, Wei L, Zhang Z, Liu S, Zhao G, Zhang J, et al. Protective effect of periplaneta americana extract on intestinal mucosal barrier function in patients with sepsis. J Tradit Chin Med. 2013;33:70–73. doi: 10.1016/s0254-6272(13)60103-x. [DOI] [PubMed] [Google Scholar]
- 5.Zhang HW, Zhang ZY, Zhao G, Wei LY, Liu SZ, Zhang J, et al. Effect of periplaneta americana extract on gastrointestinal mucosal barrier function in severe brain injury patients. Chin J Crit Care Med (in Chinese) 2013;33:447–449. doi: 10.1016/s0254-6272(13)60103-x. [DOI] [PubMed] [Google Scholar]
- 6.Bone RC, Balk RA, Cerra FB, Dellinger RP, Fein AM, Knaus WA, et al. Definitions for sepsis an organ failure and guidelines for the use of innovative the rapiesi sepsis. Chest. 1992;101:1644. doi: 10.1378/chest.101.6.1644. [DOI] [PubMed] [Google Scholar]
- 7.Meeran H, Messent M. The systemic infammatory response syndrome. Trauma. 2001;3:89–100. [Google Scholar]
- 8.Dellinger RP, Levy MM, Carlet JM, Bion J, Parker MM, Jaeschke R, et al. Surviving Sepsis Campaign: international guidelines for management of severe sepsis andseptic shock: 2008. Crit Care Med. 2008;36:1394–1396. doi: 10.1097/01.CCM.0000298158.12101.41. [DOI] [PubMed] [Google Scholar]
- 9.Kalita J, Bastia J, Bhoi SK, Misra UK. Systemic inflammatory response syndrome predicts severity of stroke and outcome. J Stroke Cerebrovasc Dis. 2015;24:1640–1648. doi: 10.1016/j.jstrokecerebrovasdis.2015.03.057. [DOI] [PubMed] [Google Scholar]
- 10.Kaukonen KM, Bailey M, Pilcher D, Cooper DJ, Bellomo R. Systemic inflammatory response syndrome criteria in defining severe sepsis. N Engl J Med. 2015;372:1629–1638. doi: 10.1056/NEJMoa1415236. [DOI] [PubMed] [Google Scholar]
- 11.Nogueira C, Borges F, Lameu E, Franca C, Rosa CL, Ramalho A. Retinol, β-carotene and oxidative stress in systemic inflammatory response syndrome. Rev Assoc Med Bras. 2015;61:116–120. doi: 10.1590/1806-9282.61.02.116. [DOI] [PubMed] [Google Scholar]
- 12.Zhang HW, Wei LY, Zhang ZY, Liu SZ, Zhang J. Preventive effect of periplaneta americana extract on stress ulcer bleeding in patients with acute lung injury or acute respiratory distress syndrome. Chin Gen Pract (in Chinese) 2012;15:3878–3879. [Google Scholar]
- 13.Li Y, Wu HW, Dong XJ. Mechanism of kangfuxin solution on gastric ulcer. Chinese Remedies & Clinics (in Chinese) 2008;8:495–496. [Google Scholar]
- 14.Xiao XQ, Wang SP, Luo C, Xu SR, Wu SJ, Zhong F. Preliminary study of periplaneta americana extract on gastric ulcer. Journal of Tropical Medicine (in Chinese) 2006;6:1274–1276. [Google Scholar]
- 15.Lichtenberger LM. The hydrcphobic barrier properties of gastrointestinal mucus. Ann Rev Physiol. 1995;57:565–583. doi: 10.1146/annurev.ph.57.030195.003025. [DOI] [PubMed] [Google Scholar]
- 16.Cerutti A, Rescigno M. The biology of intestinal immunoglobulin A responses. Immunity. 2008;28:740–750. doi: 10.1016/j.immuni.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lundin A, Bok CM, Aronsson L, Björkholm B, Gustafsson JA, Pott S, et al. Toll-like receptors and nuclear receptors: a tripartite communication that tunes innate immunity in large intestine. Cell Microbiol. 2008;10:1093–1103. doi: 10.1111/j.1462-5822.2007.01108.x. [DOI] [PubMed] [Google Scholar]


