Abstract
Pulmonary fibrosis (PF) can severely disrupt lung function, leading to fatal consequences. Salidroside is a principal active ingredient of Rhodiola rosea and has recently been reported to protect against lung injures. The present study was aimed at exploring its therapeutic effects on PF. Lung fibrotic injuries were induced in SD rats by a single intratracheal instillation of 5 mg/kg bleomycin (BLM). Then, these rats were administrated with 50, 100, or 200 mg/kg salidroside for 28 days. BLM-triggered structure distortion, collagen overproduction, excessive inflammatory infiltration, and pro-inflammatory cytokine release, and oxidative stress damages in lung tissues were attenuated by salidroside in a dose-dependent manner. Furthermore, salidroside was noted to inhibit IκBα phosphorylation and nuclear factor kappa B (NF-κB) p65 nuclear accumulation while activating Nrf2-antioxidant signaling in BLM-treated lungs. Downregulation of E-cadherin and upregulation of vimentin, fibronectin, and α-smooth muscle actin (α-SMA) indicated an epithelial-mesenchymal transition (EMT)-like shift in BLM-treated lungs. These changes were suppressed by salidroside. The expression of TGF-β1 and the phosphorylation of its downstream targets, Smad-2/-3, were enhanced by BLM, but weakened by salidroside. Additionally, salidroside was capable of reversing the recombinant TGF-β1-induced EMT-like changes in alveolar epithelial cells in vitro. Our study reveals that salidroside’s protective effects against fibrotic lung injuries are correlated to its anti-inflammatory, antioxidative, and antifibrotic properties.
Keywords: Salidroside, Pulmonary fibrosis, EMT, NF-κB p65 signaling pathway, Nrf2 signaling pathway, TGF-β1/Smad2/3 signaling pathway
Introduction
Pulmonary fibrosis (PF) is the end result of various insults arising from known or unknown etiologies, ultimately leading to death within 5 years once diagnosed (Olson et al. 2007; White et al. 2003). Idiopathic pulmonary fibrosis (IPF) is a progressive, usually lethal, form of fibrotic lung disease, with failure of alveolar reepithelialization and proliferation of fibroblasts/myofibroblasts as well as overproduction of extracellular matrix (ECM) as its major features (Noble et al. 2012; White et al. 2003). Numerous pharmacological therapies have been evaluated for IPF, but a great number of the formerly tested agents failed in clinical trials, including N-acetylcysteine, warfarin, and macitentan (Martinez et al. 2014; Noth et al. 2012; Raghu et al. 2013). Encouragingly, pirfenidone (an antifibrotic and anti-inflammatory agent) and nintedanib (a tyrosine kinase inhibitor) have recently been demonstrated to reduce the decline in forced vital capacity (King et al. 2014; Richeldi et al. 2014). However, so far, these two drugs have only been able to slow the progression of PF. Identification and development of novel therapeutic components or agents for IPF are still needed.
Rhodiola rosea grows at high altitude and in cold regions and has long been used as a medicinal herb to prevent high altitude sickness in China due to its stress-protective and antioxidative activities (Qu et al. 2009, 2012). Salidroside isolated from R. rosea is a phenolic glycoside and possesses various pharmacological properties (Bai et al. 2014; Li and Chen 2001). Zheng and coworkers demonstrated that salidroside could attenuate ischemic heart damage (Zheng et al. 2014), while Qu et al. found that salidroside could improve the streptozotocin-impaired neurogenesis in rat hippocampus (Qu et al. 2012). Besides these previously reported cardioprotective and neuroprotective effects, salidroside has recently been noted to be beneficial in ameliorating lung injuries. It has been reported that pretreatment of salidroside can mitigate acute lung injuries in lipopolysaccharide- or paraquat-challenged animals (Guan et al. 2012; Zhang et al. 2014). A study from Huang et al. also revealed a protective role of salidroside in the lung by showing that salidroside can mitigate chronic hypoxia-induced pulmonary arterial hypertension in mice (Huang et al. 2015). These earlier studies are mainly focused on the anti-inflammatory and antiapoptopic/pro-apoptotic effects of salidroside in lung tissues and/or cells, but not on its antifibrotic potential. Interestingly, administration of salidroside was observed to synergistically strengthen the therapeutical effects of rat mesenchymal stem cell transplantation on hepatic fibrotic lesions in rats (Ouyang et al. 2010). This previous study reveals a novel antifibrotic effect of salidroside in liver, promoting us to investigate whether it is also effective in alleviating fibrotic lung injury. Moreover, excessive fibroblast accumulation is the hallmark for PF pathogenesis, where epithelial-mesenchymal transition (EMT) is considered as a key mechanism (Kim et al. 2006; Lamouille et al. 2014). Although Wang and coworkers found a suppressive effect of salidroside on transforming growth factor-β (TGF-β)-1 (a potent EMT inducer)-induced EMT in A549 lung epithelial cells (Wang et al. 2014), the underlying mechanisms are still not clear and await further investigations.
The aims of the present study were to explore the effects of salidroside on fibrotic injuries in the lung. In vivo and in vitro experiments were performed in bleomycin (BLM)-challenged rats and recombinant TGF-β1-stimulated primarily cultured rat alveolar epithelial cells (AECs) and human A549 cells, respectively.
Materials and methods
Animals and treatments
All work in this study was approved by the Institutional Animal Care and Use Committee of Dalian Medical University. Eight-week-old Sprague–Dawley (SD) rats (weighing approximately 250 g) were kept on a 12-h light/dark cycle at a controlled temperature with free access to food and tap water.
Fibrotic injuries were established in rats by BLM (Melonepharma, Dalian, China). In short, rats were anesthetized with injection of 10 % chloride hydrate (3.5 mL/kg; Sinopharm, Shanghai, China) and given an intratracheal instillation of 5 mg/kg BLM (BLM group). The rats were rotated immediately to ensure the thorough drug distribution in the lungs. Control rats undergoing the same surgical procedures were given an equal volume of saline (control group). Then, the BLM-treated rats were given a daily intraperitoneal injection of 50, 100, or 200 mg/kg salidroside (purity ≥ 98 %; Melonepharma). Control rats were administrated with an equal volume of salidroside vehicle. Twenty-eight days later, after collection of bronchoalveolar lavage fluids (BALFs), all animals were sacrificed. The lung tissues were removed then fixed in 4 % paraformaldehyde (Sinopharm) or stored in −80 °C before assay.
Cell culture and treatment
Human A549 cells were obtained from the Cell Bank of the Chinese Academy of Sciences (Shanghai, China) and cultured in Dulbecco’s modified Eagle’s medium (DMEM; Gibco, Grand Island, NY, USA) supplemented with 10 % fetal bovine serum (FBS; HyClone, Logan, UT, USA) under a 95 % air/5 % CO2 (37 °C) atmosphere. Type II primary AECs were isolated from lung tissues of neonatal rats according to protocols reported by Abraham et al. (1999). Recombinant TGF-β1 (5 or 10 ng/mL; Sino Biological Inc., Beijing, China) and salidroside (50 μM) were used to co-treat the primarily cultured rat AECs or human A549 cells for 48 h.
BAL
The right main bronchus of each animal was ligated, and BAL was performed in the left lung through a tracheal cannula with 1.5 mL sterile saline for three times. The BALFs were immediately centrifuged to obtain the cell pellet. Then, the cell pellet was resuspended in cold PBS, smeared on a glass slide, and stained with Giemsa (JianCheng Bioengineering Institute, Nanjing, China) to distinguish different cell types under a light microscope. The total cell number was determined with a hemocytometer. Furthermore, the production of pro-inflammatory cytokines such as tumor necrosis factor alpha (TNF-α) and interleukin 6 (IL-6) was assessed in cell-free BAL supernatant by using corresponding ELISA Kits (USCN Life Science, Wuhan, China) according to the manufacturer’s protocols. The obtained data were presented as picograms per milliliter of BALF.
Assessment of indices for oxidative stress and collagen deposition
Activities of superoxide dismutase (SOD) and glutathione peroxidase (GSH-Px) and contents of malondialdehyde (MDA), glutathione (GSH), and hydroxyproline in rat lung tissues were assessed with commercially available kits (JianCheng Bioengineering Institute) according to the manufacturer’s standard instructions.
Histological examination
The fixed lung tissue samples were dehydrated in graded ethanol and embedded in paraffin. Each paraffin block was cut into 5-μm-thick slices. Then, these slices were dewaxed in xylene, rehydrated in gradient alcohols, and rinsed with distilled water. For hematoxylin and eosin (H&E) staining, these sections were incubated with hematoxylin for 5 min and then with eosin for 3 min. For evaluation of collagen deposition, these slices were stained with Masson’s trichrome, and the collagen deposition areas were assessed with Image-Pro Plus 6.0 software and expressed as a percentage to the total analyzed areas.
Immunofluorescence
Cells growing on the glass slides were first fixed with 4 % paraformaldehyde and then incubated with 0.1 % Triton X-100 (Amresco, Solon, OH, USA) at room temperature (RT) for 30 min. The 5-μm-thick lung slices were dewaxed, rehydrated, and retrieved antigens. Both cell and tissue samples were blocked with goat serum and then incubated with rabbit polyclonal antibodies for E-cadherin (1:100 diluted for tissues and 1:200 diluted for cells; Boster, Wuhan, China) and fibronectin (1:200 diluted for cells; Boster) overnight (4 °C). Thereafter, Cyanine-3-CTP (Cy3)- or fluorescein isothiocyanate (FITC)-labeled goat anti-rabbit secondary antibodies (1:200 diluted; Beyotime, Shanghai, China) were utilized to treat these samples for 60 min (RT) in the dark. Cell nuclei were counterstained with 4′,6-diamidino-2-phenylindole (DAPI; Biosharp, Hefei, China).
Western blot analysis
Rabbit polyclonal antibodies against phosphorylated nuclear factor kappa B (NF-κB) inhibitor α (p-IκBα), small mother against decapentaplegic (Smad)-2, p-Smad2, Smad3, p-Smad3, vimentin, NF-E2-related factor 2 (Nrf2), and NADPH/quinone oxidoreductase (NQO1) were all purchased from Bioss (Beijing, China). The dilution for each antibody was 1:500. Rabbit polyclonal antibodies against E-cadherin, fibronectin, and NF-κB subunit p65 and mouse monoclonal antibody against α-smooth muscle actin (α-SMA) were obtained from Boster, and the dilution was 1:400. Rabbit polyclonal antibodies against TGF-β1 and hemoxygenase 1 (HO-1) were obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA), and the dilution was 1:200.
Lung tissues and cells were lysed in RIPA lysis buffer (Beyotime) to isolate the total proteins. Then, equal amount of each protein sample was separated on SDS-PAGE, transferred onto PVDF membranes, and then blocked in 5 % skimmed milk. After blocking, the membranes were incubated overnight (4 °C) with the above-mentioned antibodies, followed by goat anti-rabbit or mouse secondary antibodies (1:5000 diluted; Beyotime) for 45 min (RT). In order to detect the nuclear expression levels of NF-κB p65 and Nrf-2 in rat lung tissues, nuclear extracts were first prepared with a nuclear and cytoplasmic protein extraction kit (Beyotime) and then subjected to Western blot analysis. Finally, the proteins bands were visualized with an enhanced peroxidase/luminol chemiluminescence reaction kit (Seven-sea Pharmtech Co., Ltd, Shanghai, China). The density of each protein blot was compared with that of β-actin or Histone H3 and was shown as a ratio to the endogenous control.
Real-time quantitative RT-PCR
Two pairs of primers of E-cadherin (NM_031334.1) and TGF-β1 (NM_021578.2) for real-time quantitative RT-PCR were as follows: E-cadherin F, 5′ aaagcaggaagaaaacaccactc 3′; E-cadherin R, 5′ aaagggcacgctatcaacattag 3′; TGF-β1 F, 5′ caacaattcctggcgttacct 3′; and TGF-β1 R, 5′ agccctgtattccgtctcctt 3′. Total RNAs were isolated from rat lung tissues with a fast RNApure kit (BioTeke, Beijing, China) and processed into cDNAs with a Super M-MLV Reverse Transcriptase Kit (BioTeke). Real-time PCR was performed with SYBR Green (Solarbio, Beijing, China). Ct values of the indicated genes in each sample were calculated, and the transcript levels were calculated by 2-ΔΔCt method. Endogenous Ct values of β-actin were used as control.
Data analysis
The data were presented as mean ± standard deviation (SD) of n determinations, where n represents the number of samples studied. One-way analysis of variance (ANOVA) was performed to analyze the significance of differences in the measured values between groups, and Bonferroni correction was used as a post hoc test. A P value less than 0.05 was considered to be statistically significant.
Results
Salidroside mitigates BLM-induced lung injuries in rats
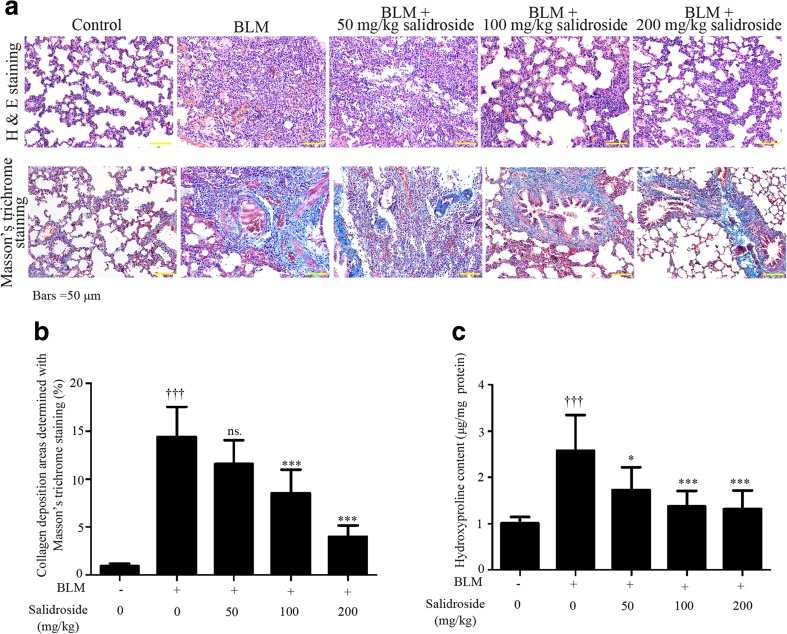
H&E staining and Masson’s trichrome staining were applied to evaluate the establishment of fibrotic injuries in rats. Histopathological results indicated that BLM treatment induced obvious structure distortion and excessive collagen deposition (Fig. 1a, b). In addition, significant upregulation of hydroxyproline was also presented in fibrotic lung tissues (Fig. 1c). These pathological changes were alleviated by salidroside in a dose-dependent manner. Treatment of 200 mg/kg salidroside had the most efficacious effect (Fig. 1). These data indicated that salidroside prevented BLM-induced fibrotic lesions in rats.
Fig. 1.
Salidroside mitigates BLM-induced lung injuries in rats. Following a single administration of 5 mg/kg BLM at day 0, the SD rats received daily injection of salidroside (50, 100, or 200 mg/kg) or vehicle for 28 days and then sacrificed, and lung tissue samples were collected. a Photomicrograph of rat lung tissues stained with H&E and Masson, respectively (×200). b Lung fibrosis levels were calculated as percentages to the total analyzed areas (n = 6). c Hydroxyproline contents were analyzed with a commercially available kit (n = 8). The data were presented as mean ± standard deviation (SD). ††† P < 0.001 was compared to the control group; ns P > 0.05, *P < 0.05, and ***P < 0.001 were compared to the BLM group
Salidroside exerts anti-inflammatory and antioxidative effects in BLM-treated rat lungs
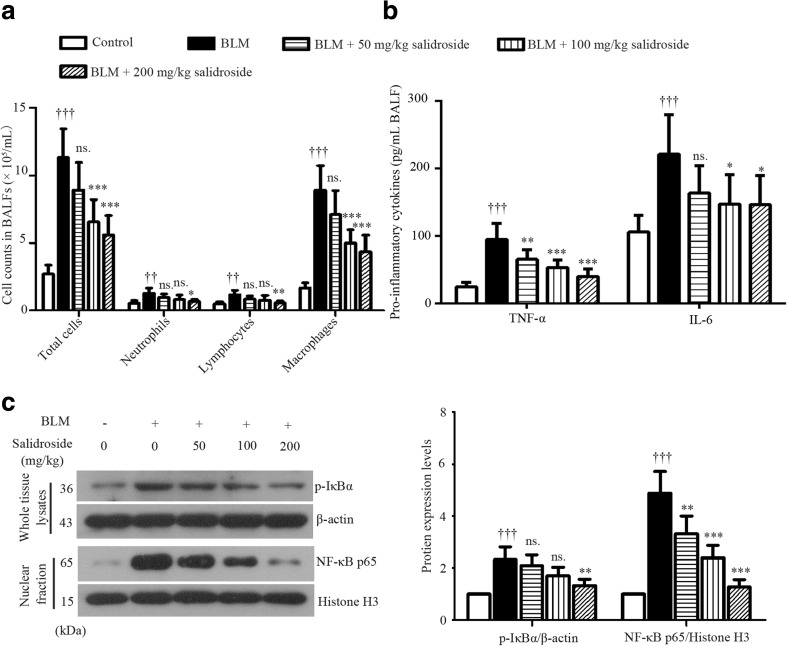
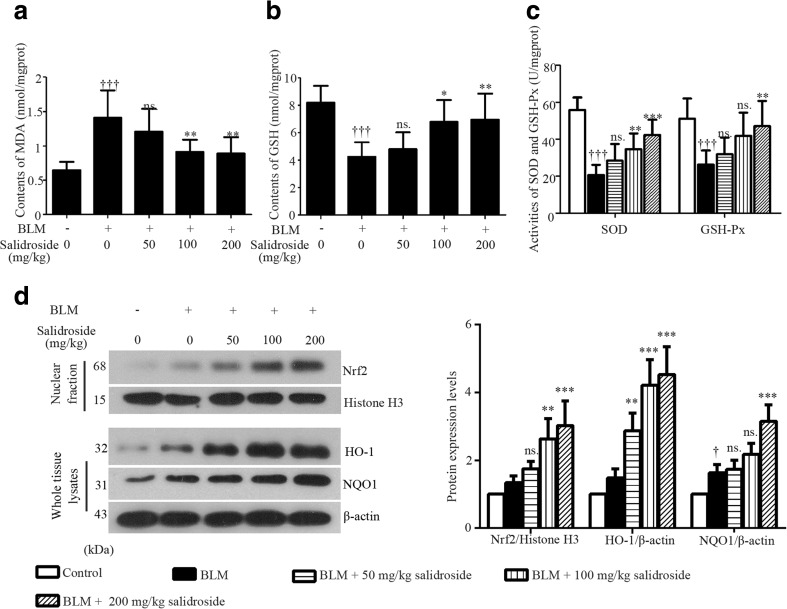
To evaluate BLM-induced inflammatory infiltration, the total cell number in 1 mL BALF was counted, and the different cell types were assessed by Giemsa staining. As illustrated in Fig. 2a, a great number of inflammatory cells were infiltrated into the lung in response to BLM challenge, with macrophages being the dominating cell types. This detrimental inflammatory infiltration was suppressed by salidroside. Higher dose of salidroside was more effective (Fig. 2a). Also, the overproduction of pro-inflammatory TNF-α and IL-1β induced by BLM was inhibited by salidroside (Fig. 2b). Additionally, the phosphorylation of IκBα and the nuclear accumulation of p65 were enhanced by BLM, but weakened by salidroside (Fig. 2c). Moreover, treatment with BLM resulted in a 2.2-fold increase in MDA production (byproducts of lipid peroxidation) in rat lungs (Fig. 3a). In contrast, antioxidants such as SOD, GSH, and GSH-Px were significantly decreased in response to BLM stimulation (SOD, 55.84 ± 6.73 U/mg protein in the control group vs. 20.69 ± 5.47 U/mg protein in the BLM group; GSH, 8.20 ± 1.23 vs. 4.25 ± 1.05 nmol/mg protein; GSH-Px, 51.07 ± 11.04 vs. 26.23 ± 7.72 U/mg protein) as indicated in Fig. 3b, c. Furthermore, Nrf2 accumulation in cell nuclei was slightly provoked by BLM and further enhanced by salidroside (Fig. 3d). Additionally, the expression levels of Nrf2-inducible antioxidant HO-1 and NQO1 were also upregulated by salidroside (Fig. 3d). Taken together, these data illustrated that salidroside had anti-inflammatory and antioxidative properties in BLM-induced lung lesions, wherein NFκB and Nrf-2 signaling pathways played critical roles.
Fig. 2.
Salidroside inhibits inflammatory infiltration and blocks NF-κB signaling transduction in BLM-treated lung tissues. a The total cell number in 1 mL BALF was counted, and the different cell types were assessed with Giemsa staining (n = 6). b TNF-α and IL-6 levels in cell-free BALFs were determined with ELISA kits. c Protein expression levels of p-IκBα and nuclear translocation of NFκB p65 were assessed with Western blot analysis. The endogenous β-actin was used as a control for p-IκBα, while Histone H3 was used as a control for p65. The data were presented as mean ± standard deviation (SD). ††† P < 0.001 was compared to the control group; ns P > 0.05, *P < 0.05, **P < 0.01, and ***P < 0.001 were compared to the BLM group. BALF bronchoalveolar lavage fluid
Fig. 3.
Salidroside exerts antioxidative effects and activates Nrf-2 signaling in BLM-treated lung tissues. Contents of MDA (a) and GSH (b) and activities of SOD and GSH-Px (c) were detected with commercially available kits. d Protein expression levels of nuclear Nrf-2, HO-1, and NQO1 were analyzed with Western blot assay. Histone H3 was used as a control for Nrf-2; β-actin for HO-1 and NQO1. The data were presented as mean ± standard deviation (SD). † P < 0.05 and ††† P < 0.001 were compared to the control group; ns. P > 0.05 *P < 0.05, **P < 0.01 and ***P < 0.001 were compared to the BLM group
Salidroside blocks TGF-β1/Smad-2/-3 signaling transduction in lung tissues of BLM-treated rats
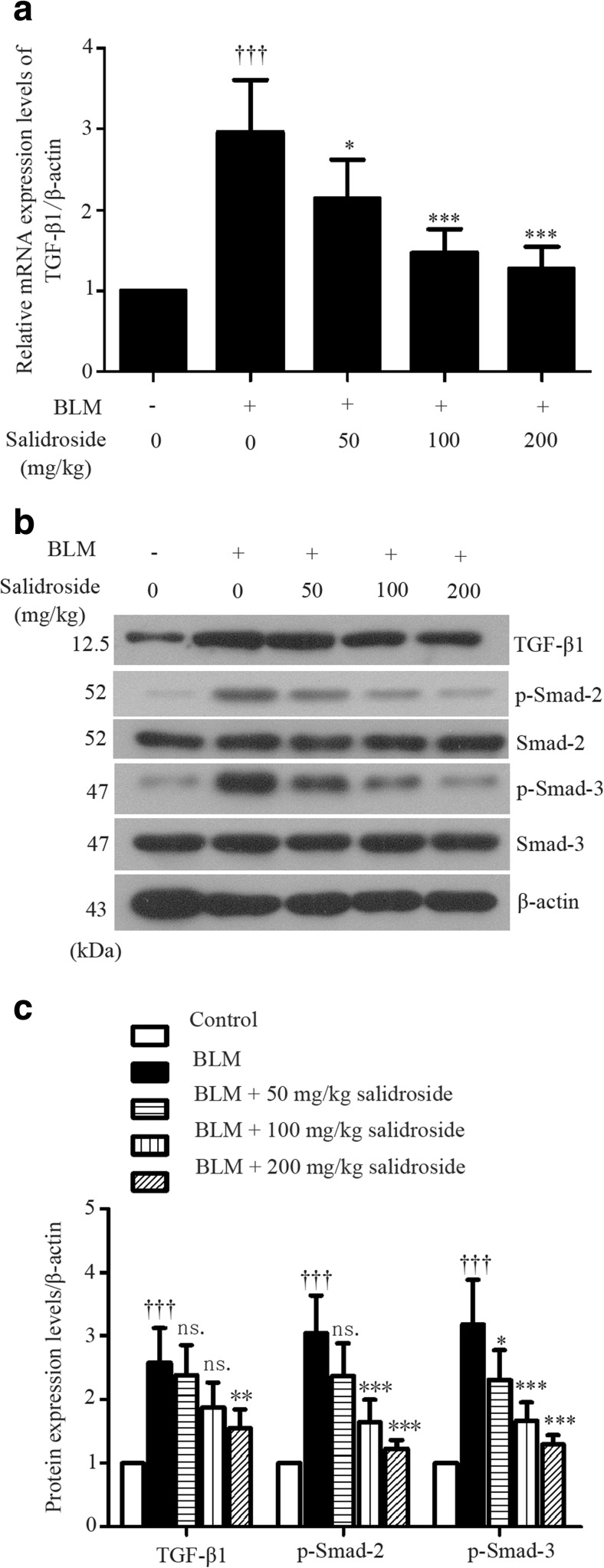
Since the activation of TGF-β/Smad pathway is involved in BLM-stimulated fibrotic injury (Della Latta et al. 2015), we next examined whether salidroside affected TGF-β signaling activation. Results of quantitative RT-PCR showed that the mRNA expression level of TGF-β1 was significantly elevated in BLM-treated lungs, but decreased after salidroside treatment (Fig. 4a). Such inhibitory effects of salidroside on BLM-induced TGF-β1 expression was also confirmed by Western blot analysis at protein level (Fig. 4b, c). Furthermore, BLM-induced phosphorylation of Smad-2 and Smad-3 were attenuated by salidroside, while no detectable expression alterations of their total proteins were observed between different groups (Fig. 4b, c). Our data illustrated that salidroside was able to block BLM-induced TGF-β/Smad signaling transduction in lung tissues.
Fig. 4.
Salidroside blocks TGF-β1/Smad-2/-3 signaling transduction in BLM-treated lung tissues. a The mRNA expression levels of TGF-β1 were determined with real-time quantitative RT-PCR. β-actin was used as the endogenous control. b Protein expression levels of TGF-β1, smad2, p-smad2, smad3, and p-smad3 were analyzed with Western blot assay and c calculated as ratios to that of β-actin in the same sample. The data were presented as mean ± standard deviation (SD). ††† P < 0.001 was compared to the control group; ns P > 0.05, *P < 0.05, **P < 0.01, and ***P < 0.001 were compared to the BLM group
Salidroside inhibits BLM-induced EMT in rat lung tissues
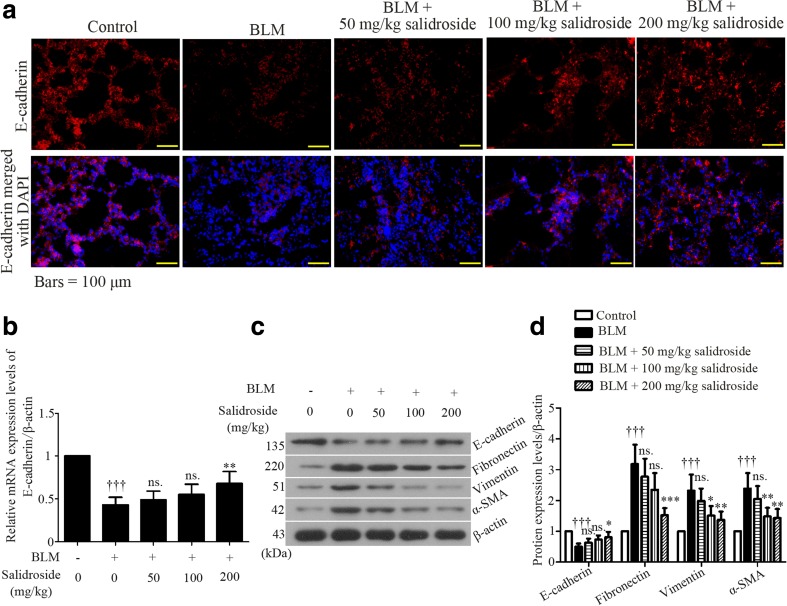
Loss of E-cadherin is considered as a hallmark for EMT-like cellular responses (Gonzalez and Medici 2014). To determine whether BLM treatment could drive EMT in rat lung, immunofluorescent assay was performed to detect E-cadherin protein expression in rat lung tissues at the end of the experiments. Results showed that E-cadherin was mainly detected in cell junctions and cell membranes, and its expression was markedly decreased in BLM-treated lung samples than in normal ones (Fig. 5a). However, such reduction was partly rescued by salidroside, and the most efficacious dose was 200 mg/kg (Fig. 5a). Also, data from quantitative real-time PCR (Fig. 5b) and Western blot (Fig. 5c, d) analyses confirmed the results from immunofluorescent assay. On BLM stimulation, the expression of vimentin, in contrast to E-cadherin, was increased (Fig. 5c, d). Such increase was suppressed by salidroside. Additionally, the protein expression of another two EMT makers, fibronectin and α-SMA, was upregulated by BLM but downregulated by salidroside (Fig. 5c, d). These results indicate that salidroside has an inhibitory effect on BLM-induced mesenchymal shift in rat lung tissues.
Fig. 5.
Salidroside inhibits BLM-induced EMT in rat lung tissues. a Immunofluorescent staining was performed in rat lung tissue slices by using a specific antibody against E-cadherin (red). DAPI was used to visualize the cell nuclei (blue). Bars = 50 μM. b The mRNA expression levels of E-cadherin were determined with real-time quantitative RT-PCR. β-actin was used as the endogenous control. c Protein expression levels of E-cadherin, fibronectin, vimentin, and α-SMA were analyzed with Western blot assay and d calculated as ratios to that of β-actin in the same sample. The data were presented as mean ± standard deviation (SD). ††† P < 0.001 was compared to the control group; ns P > 0.05, *P < 0.05, **P < 0.01, and ***P < 0.001 were compared to the BLM group
Salidroside suppresses recombinant TGF-β1-induced EMT-like shift in AECs in vitro
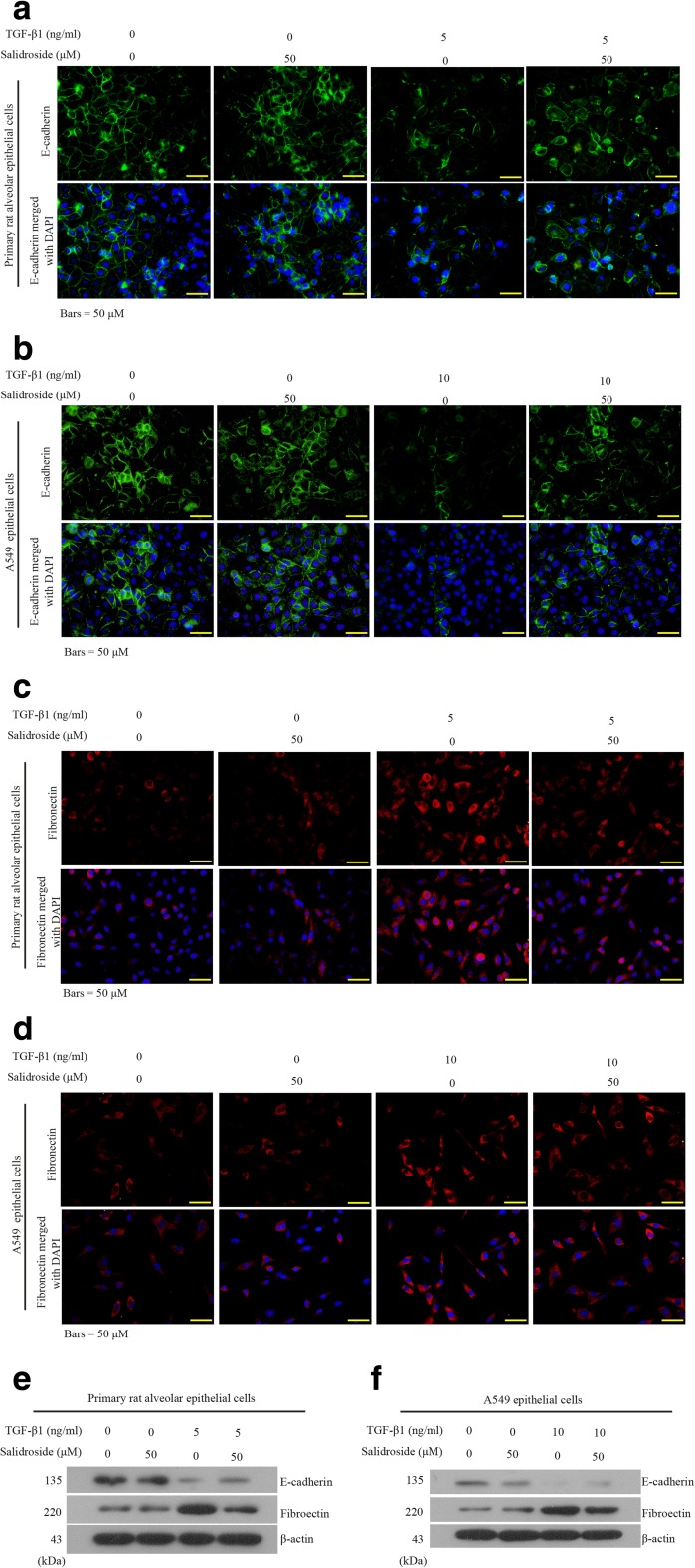
The above in vivo results revealed novel inhibitory effects of salidroside on EMT and TGF-β/Smad signaling activation in fibrotic lung tissues. Since TGF-β is a potent inducer of EMT (Wang et al. 2014), we next explored whether and how salidroside inhibited TGF-β-dependent EMT in human A549 cells and primarily cultured rat AECs. Control primary AECs expressed abundant E-cadherin (Fig. 6a), but weak fibronectin, as indicated in immunofluorescent assessments (Fig. 6c). Salidroside alone did not affect E-cadherin expression (Fig. 6a, c). Moreover, TGF-β1 stimulation induced a significant decrease in E-cadherin and an increase in fibronectin in primary rat AECs, and these alterations were partly reversed by salidroside (Fig. 6a, c). Similar expression patterns of these two EMT-related proteins were observed in A549 cells (Fig. 6a, d). Data from Western blot analysis echoed the immunofluorescent results (Fig. 6e, f). These data provide evidence that salidroside is able to inhibit TGF-β1-triggered EMT-like shift in AECs in vitro.
Fig. 6.
Salidroside blocks recombinant TGF-β1-induced EMT-like shift in AECs in vitro. Recombinant TGF-β1 (5 or 10 ng/mL) and salidroside (50 μM) were utilized to co-treat the primarily cultured rat AECs or human A549 cells for 48 h. a–d Then, these cells were subjected to immunohistochemical analysis using antibodies against E-cadherin (green) and fibronectin (red). Cell nuclei were stained with DAPI (blue). e, f Protein expression levels of E-cadherin and fibronectin were analyzed with Western blot assay. β-Actin was used as the endogenous control
Discussion
The onset of fibrotic changes after lung injuries plays a causal role in causing death in PF patients. Hence, identification of novel agents possessing antifibrotic effects is of great importance for PF therapy. Both anti-inflammatory and antioxidative effects of salidroside have been reported in accumulating studies, but its antifibrotic potential remains largely unknown. We here explored the therapeutic effects of salidroside on PF by conducting in vivo and in vitro experiments.
BLM is a common drug applied in chemotherapy for a variety of neoplasms and now is widely used in fundamental researches by inducing lung fibrosis (Della Latta et al. 2015). In this study, we first evaluated the effects of salidroside on BLM-induced lung injuries by examining the histological changes in rats. Our data in keeping with previous studies (Arizmendi et al. 2014; Della Latta et al. 2015) showed a significant structure distortion of lung tissues in rats challenged with BLM. Abnormal infiltration of inflammatory cells and overproduction of TNFα and IL-6 as well as collagens were presented in BLM-treated lungs. However, these pathological alterations were attenuated by salidroside in a dose-dependent way within the indicated doses. Extensive evidence demonstrates that NF-κB signaling can be activated by BLM stimulation and plays a critical role in regulating inflammation during pulmonary fibrogenesis (Alvira 2014). Interestingly, endotoxin-induced NF-κB signaling transduction in mouse lung tissues is noted to be inhibited by salidroside (Guan et al. 2012). Our results corresponded with this previous study revealing that salidroside could block NF-κB signaling delivery by inhibiting IκBα phosphorylation and p65 nuclear translocation.
Oxidative stress contributes to various pathological conditions and diseases (Birben et al. 2012). BLM treatment has been proven to promote the initiation of oxidative stress damage and the generation of reactive oxygen species (ROS) (Teixeira et al. 2008). ROS often react with multiple fatty acids to produce lipid peroxidation products (Bocchino et al. 2010). We used MDA, an index of lipid peroxidation, to evaluate oxidative tissue damage in this study. We found that BLM application to the rats resulted in marked increase in MDA in lung tissues, yet this elevation was suppressed by salidroside. Aside from the aberrant upregulation of MDA, BLM-induced oxidative injury can also be shown by measuring the antioxidants such as SOD, GSH, and GSH-Px. Therefore, the contents or activities of the above-mentioned antioxidants were also examined in rat lung tissues. Herein, SOD, GSH, and GSH-Px were decreased in BLM-treated lung tissues, whereas they increased after salidroside administration. Activation of Nrf2 signaling-mediated antioxidant defense represents a novel strategy to abrogate the progression of fibrotic injury (Kikuchi et al. 2010). Salidroside is noted to promote the transcription of Nrf-2 downstream targets, such as heme oxygenase-1 and quinone oxidoreductase 1, in cardiomyocytes (Zheng et al. 2014). However, its direct effects on Nrf-2 signaling activation in fibrotic lung injury have not been defined yet. Upon pathological challenges, Nrf-2 is released from Kelch-like ECH-associated protein 1 (Keap1)/Nrf2 complex and enters and accumulates in cell nuclei, thus inducing antioxidant defense gene expression such as HO-1 and NQO1 through its binding to specific antioxidant response elements (AECs) (Shelton and Jaiswal 2013; Walters et al. 2008). We here demonstrated for the first time that salidroside could promote the nuclear accumulation of Nrf-2 and increase the expression of Nrf2-inducible antioxidants HO-1 and NQO1 in injured lungs. Further experiments using pharmaceutical agents or genetic approaches to inhibit Nrf-2 activation are needed to confirm the regulatory patterns of salidroside.
Our understanding of the pathogenesis of PF includes a growing consensus that pathological EMT provoked by severe initial injury to epithelium is implicated in PF development and progress (Friedman et al. 2013). TGF-β is a pro-sclerotic cytokine that can promote EMT during fibrotic diseases (Jiang et al. 2015; Wolters et al. 2014). Interference with TGF-β signaling can attenuate fibroblast accumulation and subsequently reverse the established fibrosis (Hashimoto et al. 2010). Interestingly, salidroside was found to suppress paraquat-induced TGF-β1 expression in rat lung (Zhang et al. 2014), but the precise regulatory mechanisms have not been fully studied. Therefore, we investigated the effects of salidroside on TGF-β-related EMT in fibrotic lung.
In this study, BLM-triggered epithelial alterations in rat lung tissues were evaluated by assessing the expression of EMT markers. We found that BLM could induce expression decrease in the key epithelial-specific marker E-cadherin and increases in mesenchymal markers vimentin, fibronectin, and α-SMA in rat lung. These alterations were partly inhibited by administrating salidroside, revealing an inhibitory effect of salidroside on injury-triggered EMT-like shift in the lung. Smad-2 and Smad-3 serve principally as substrates for TGF-β receptors, and their phosphorylation induced by the activated TGF-β receptor complex is considered as a pivotal event in the initiation of TGF-β signaling transduction (Massague et al. 2005). Despite a study showing inhibitory effects of salidroside on Smad-1/5/8 activation (Zhao et al. 2014), its effects on other Smads are unknown. Intriguingly, we here found that the BLM-induced activation of TGF-β/Smad-2/3 signaling in rat lung was partly blocked by salidroside.
Further in vitro experiments were performed in recombinant TGF-β1-stimulated AECs to elucidate whether salidroside could affect TGF-β-induced EMT changes. Our data showed that the exogenous TGF-β1 significantly reduced E-cadherin expression in AECs. Like in vivo results, the loss of E-cadherin in AECs was partly rescued by adding salidroside. Reportedly, overactive TGF-β/Smad-2 signaling cascades cause epithelial gene-specific silencing during EMT by promoting methyltransferase 1 (DNMT1) binding to epithelial gene promoters (Papageorgis et al. 2010). Currently, little is known about salidroside on DNA methylation. Whether salidroside exerts its inhibitory effects on TGF-β-induced EMT by affecting TGF-β-induced hypermethylation of E-cadherin requires further investigation. As demonstrated by Tanjore and coworkers, a portion of fibroblasts in pulmonary fibroblastic foci are derived from epithelial cells through EMT (Tanjore et al. 2009). When tissues become more fibrotic, fibroblasts can differentiate into ECM-producing myofibroblast phenotype, further exacerbating tissue stiffness and injury (Balestrini et al. 2012; Hinz 2009). However, lung epithelial cells undergoing EMT are not a principal resource to lung myofibroblasts (Tanjore et al. 2009). Although we demonstrated an inhibitory effect of salidroside on TGF-β-induced EMT-like shift in lung epithelial cells, whether and how this agent regulates the proliferation, differentiation, recruitment, and activation of myofibroblasts require further elucidation.
In summary, we demonstrate that treatment with salidroside can attenuate BLM-induced fibrotic lung injuries in rats. Such protective effects of salidroside are correlated to its anti-inflammatory, antioxidative, and antifibrotic potentials. Our study suggests the implication of salidroside for clinical use in PF therapy in the future.
Acknowledgments
This study was supported by grants from the National Nature Science Foundation of China (No. 81273924) and the Nature Science Foundation of Liaoning Province (No. 2013023028).
Footnotes
Haiying Tang and Lili Gao contributed equally to this work.
Contributor Information
Hongli Lin, Email: linhongli@vip.163.com.
Taihua Wu, Phone: +86-411-83635963, Email: wutaihua@sina.com.
References
- Abraham V, Chou ML, DeBolt KM, Koval M. Phenotypic control of gap junctional communication by cultured alveolar epithelial cells. Am J Physiol. 1999;276:L825–L834. doi: 10.1152/ajplung.1999.276.5.L825. [DOI] [PubMed] [Google Scholar]
- Alvira CM. Nuclear factor-kappa-B signaling in lung development and disease: one pathway, numerous functions. Birth Defects Res A Clin Mol Teratol. 2014;100:202–216. doi: 10.1002/bdra.23233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arizmendi N, et al. Rac2 is involved in bleomycin-induced lung inflammation leading to pulmonary fibrosis. Respir Res. 2014;15:71. doi: 10.1186/1465-9921-15-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai Y, et al. Production of salidroside in metabolically engineered Escherichia coli. Sci Rep. 2014;4:6640. doi: 10.1038/srep06640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balestrini JL, Chaudhry S, Sarrazy V, Koehler A, Hinz B. The mechanical memory of lung myofibroblasts. Integr Biol (Camb) 2012;4:410–421. doi: 10.1039/c2ib00149g. [DOI] [PubMed] [Google Scholar]
- Birben E, Sahiner UM, Sackesen C, Erzurum S, Kalayci O. Oxidative stress and antioxidant defense. World Allergy Organ J. 2012;5:9–19. doi: 10.1097/WOX.0b013e3182439613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bocchino M, et al. Reactive oxygen species are required for maintenance and differentiation of primary lung fibroblasts in idiopathic pulmonary fibrosis. PLoS One. 2010;5:e14003. doi: 10.1371/journal.pone.0014003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Della Latta V, Cecchettini A, Del Ry S, Morales MA. Bleomycin in the setting of lung fibrosis induction: from biological mechanisms to counteractions. Pharmacol Res. 2015;97:122–130. doi: 10.1016/j.phrs.2015.04.012. [DOI] [PubMed] [Google Scholar]
- Friedman SL, Sheppard D, Duffield JS, Violette S. Therapy for fibrotic diseases: nearing the starting line. Sci Transl Med. 2013;5:167sr161. doi: 10.1126/scitranslmed.3004700. [DOI] [PubMed] [Google Scholar]
- Gonzalez DM, Medici D. Signaling mechanisms of the epithelial-mesenchymal transition. Sci Signal. 2014;7:re8. doi: 10.1126/scisignal.2005189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan S, et al. Protective effects of salidroside from Rhodiola rosea on LPS-induced acute lung injury in mice. Immunopharmacol Immunotoxicol. 2012;34:667–672. doi: 10.3109/08923973.2011.650175. [DOI] [PubMed] [Google Scholar]
- Hashimoto N, et al. Endothelial-mesenchymal transition in bleomycin-induced pulmonary fibrosis. Am J Respir Cell Mol Biol. 2010;43:161–172. doi: 10.1165/rcmb.2009-0031OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinz B. Tissue stiffness, latent TGF-beta1 activation, and mechanical signal transduction: implications for the pathogenesis and treatment of fibrosis. Curr Rheumatol Rep. 2009;11:120–126. doi: 10.1007/s11926-009-0017-1. [DOI] [PubMed] [Google Scholar]
- Huang X, et al. Salidroside attenuates chronic hypoxia-induced pulmonary hypertension via adenosine A2a receptor related mitochondria-dependent apoptosis pathway. J Mol Cell Cardiol. 2015;82:153–166. doi: 10.1016/j.yjmcc.2015.03.005. [DOI] [PubMed] [Google Scholar]
- Jiang L, et al. Metabolic reprogramming during TGFbeta1-induced epithelial-to-mesenchymal transition. Oncogene. 2015;34:3908–3916. doi: 10.1038/onc.2014.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi N, et al. Nrf2 protects against pulmonary fibrosis by regulating the lung oxidant level and Th1/Th2 balance. Respir Res. 2010;11:31. doi: 10.1186/1465-9921-11-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KK, et al. Alveolar epithelial cell mesenchymal transition develops in vivo during pulmonary fibrosis and is regulated by the extracellular matrix. Proc Natl Acad Sci U S A. 2006;103:13180–13185. doi: 10.1073/pnas.0605669103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King TE, Jr, et al. A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N Engl J Med. 2014;370:2083–2092. doi: 10.1056/NEJMoa1402582. [DOI] [PubMed] [Google Scholar]
- Lamouille S, Xu J, Derynck R. Molecular mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell Biol. 2014;15:178–196. doi: 10.1038/nrm3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li HB, Chen F. Preparative isolation and purification of salidroside from the Chinese medicinal plant Rhodiola sachalinensis by high-speed counter-current chromatography. J Chromatogr A. 2001;932:91–95. doi: 10.1016/S0021-9673(01)01232-8. [DOI] [PubMed] [Google Scholar]
- Martinez FJ, de Andrade JA, Anstrom KJ, King TE, Jr, Raghu G. Randomized trial of acetylcysteine in idiopathic pulmonary fibrosis. N Engl J Med. 2014;370:2093–2101. doi: 10.1056/NEJMoa1401739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massague J, Seoane J, Wotton D. Smad transcription factors. Genes Dev. 2005;19:2783–2810. doi: 10.1101/gad.1350705. [DOI] [PubMed] [Google Scholar]
- Noble PW, Barkauskas CE, Jiang D. Pulmonary fibrosis: patterns and perpetrators. J Clin Invest. 2012;122:2756–2762. doi: 10.1172/JCI60323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noth I, et al. A placebo-controlled randomized trial of warfarin in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2012;186:88–95. doi: 10.1164/rccm.201202-0314OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson AL, Swigris JJ, Lezotte DC, Norris JM, Wilson CG, Brown KK. Mortality from pulmonary fibrosis increased in the United States from 1992 to 2003. Am J Respir Crit Care Med. 2007;176:277–284. doi: 10.1164/rccm.200701-044OC. [DOI] [PubMed] [Google Scholar]
- Ouyang J, Gao Z, Ren Z, Hong D, Qiao H, Chen Y. Synergistic effects of rMSCs and salidroside on the experimental hepatic fibrosis. Pharmazie. 2010;65:607–613. [PubMed] [Google Scholar]
- Papageorgis P, et al. Smad signaling is required to maintain epigenetic silencing during breast cancer progression. Cancer Res. 2010;70:968–978. doi: 10.1158/0008-5472.CAN-09-1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu ZQ, Zhou Y, Zeng YS, Li Y, Chung P. Pretreatment with Rhodiola rosea extract reduces cognitive impairment induced by intracerebroventricular streptozotocin in rats: implication of anti-oxidative and neuroprotective effects. Biomed Environ Sci. 2009;22:318–326. doi: 10.1016/S0895-3988(09)60062-3. [DOI] [PubMed] [Google Scholar]
- Qu ZQ, Zhou Y, Zeng YS, Lin YK, Li Y, Zhong ZQ, Chan WY. Protective effects of a Rhodiola crenulata extract and salidroside on hippocampal neurogenesis against streptozotocin-induced neural injury in the rat. PLoS One. 2012;7:e29641. doi: 10.1371/journal.pone.0029641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghu G, Million-Rousseau R, Morganti A, Perchenet L, Behr J. Macitentan for the treatment of idiopathic pulmonary fibrosis: the randomised controlled MUSIC trial. Eur Respir J. 2013;42:1622–1632. doi: 10.1183/09031936.00104612. [DOI] [PubMed] [Google Scholar]
- Richeldi L, et al. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N Engl J Med. 2014;370:2071–2082. doi: 10.1056/NEJMoa1402584. [DOI] [PubMed] [Google Scholar]
- Shelton P, Jaiswal AK. The transcription factor NF-E2-related factor 2 (Nrf2): a protooncogene? FASEB J. 2013;27:414–423. doi: 10.1096/fj.12-217257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanjore H, et al. Contribution of epithelial-derived fibroblasts to bleomycin-induced lung fibrosis. Am J Respir Crit Care Med. 2009;180:657–665. doi: 10.1164/rccm.200903-0322OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teixeira KC, et al. Attenuation of bleomycin-induced lung injury and oxidative stress by N-acetylcysteine plus deferoxamine. Pulm Pharmacol Ther. 2008;21:309–316. doi: 10.1016/j.pupt.2007.07.006. [DOI] [PubMed] [Google Scholar]
- Walters DM, Cho HY, Kleeberger SR. Oxidative stress and antioxidants in the pathogenesis of pulmonary fibrosis: a potential role for Nrf2. Antioxid Redox Signal. 2008;10:321–332. doi: 10.1089/ars.2007.1901. [DOI] [PubMed] [Google Scholar]
- Wang J, Li JZ, Lu AX, Zhang KF, Li BJ. Anticancer effect of salidroside on A549 lung cancer cells through inhibition of oxidative stress and phospho-p38 expression. Oncol Lett. 2014;7:1159–1164. doi: 10.3892/ol.2014.1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White ES, Lazar MH, Thannickal VJ. Pathogenetic mechanisms in usual interstitial pneumonia/idiopathic pulmonary fibrosis. J Pathol. 2003;201:343–354. doi: 10.1002/path.1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolters PJ, Collard HR, Jones KD. Pathogenesis of idiopathic pulmonary fibrosis. Annu Rev Pathol. 2014;9:157–179. doi: 10.1146/annurev-pathol-012513-104706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Ding L, Wu L, Xu L, Zheng L, Huang X. Salidroside alleviates paraquat-induced rat acute lung injury by repressing TGF-beta1 expression. Int J Clin Exp Pathol. 2014;7:8841–8847. [PMC free article] [PubMed] [Google Scholar]
- Zhao HB, et al. Salidroside induces neuronal differentiation of mouse mesenchymal stem cells through Notch and BMP signaling pathways. Food Chem Toxicol. 2014;71:60–67. doi: 10.1016/j.fct.2014.05.031. [DOI] [PubMed] [Google Scholar]
- Zheng K, Sheng Z, Li Y, Lu H. Salidroside inhibits oxygen glucose deprivation (OGD)/re-oxygenation-induced H9c2 cell necrosis through activating of Akt-Nrf2 signaling. Biochem Biophys Res Commun. 2014;451:79–85. doi: 10.1016/j.bbrc.2014.07.072. [DOI] [PubMed] [Google Scholar]