Abstract
Reactive oxygen species (ROS) are responsible for lung damage during inhalation of cold air. However, the mechanism of the ROS production induced by cold stress in the lung is still unclear. In this work, we measured the changes of ROS and the cytosolic Ca2+ concentration ([Ca2+]c) in A549 cell. We observed that cold stress (from 20 to 5 °C) exposure of A549 cell resulted in an increase of ROS and [Ca2+]c, which was completely attenuated by removing Ca2+ from medium. Further experiments showed that cold-sensing transient receptor potential subfamily member 1 (TRPA1) agonist (allyl isothiocyanate, AITC) increased the production of ROS and the level of [Ca2+]c in A549 cell. Moreover, HC-030031, a TRPA1 selective antagonist, significantly inhibited the enhanced ROS and [Ca2+]c induced by AITC or cold stimulation, respectively. Taken together, these data demonstrated that TRPA1 activation played an important role in the enhanced production of ROS induced by cold stress in A549 cell.
Keywords: Cold stress, Reactive oxygen species, TRPA1, [Ca2+]c
Introduction
Reactive oxygen species (ROS) is a universal and pleiotropic signaling molecule in the pathogenesis of disease states (Ray et al. 2012). In physiological states, ROS are normally kept at low levels, which makes very important for normal cellular function (Stowe and Camara 2009). However, the overproduction of ROS can cause serious damage to a variety of biomolecules (Belousov et al. 2006; Oliveira-Marques et al. 2009). In disease states, the dysregulated ROS signaling may contribute to be exclusively toxic to cells and tissues such as the lung (Finkel 2011). The production of ROS is provoked by a wide range of factors in the lung. These endogenous or exogenous mediators include cytokines and chemical irritants in polluted air (Thannickal and Fanburg 2000; Valavanidis et al. 2013).
As the interface with the outside air environment, the airway epithelial cell is critical to lung defense in response to exogenous stimulants. Cold temperature exposure can cause respiratory responses such as cough, bronchoconstriction, and mucosal secretion (Giesbrecht 1995; Koskela 2007). In addition, it has been reported cold-induced injury to lung epithelial cells is associated to ROS formation (Pizanis et al. 2011). A recent study showed that cold-sensing transient receptor potential subfamily member 1 (TRPA1) was expressed in the alveolar epithelial cells which increased release of IL-8 chemokine in response to activation of the TRPA1 (Mukhopadhyay et al. 2011). We have reported that cold stress enhances the production of nitric oxide through the activation of TRPA1 ion channel in A549 cells (Sun et al. 2014). However, the effect of TRPA1 activation on the production of ROS induced by cold stress in lung epithelial cells has not been studied in detail. In the present study, we investigated the role of TRPA1 in the production of ROS induced by cold stress using ROS and Ca2+ signaling fluorescence imaging.
Materials and methods
Reagents
Fluo-2 acetoxymethyl ester (Fluo-2AM) was from Biotium Inc. (Hayward, CA, USA). Ethylene glycol-bis (β-amiROSethyl ether)-N,N,N,N-tetraacetic acid (EGTA), ROS probe dihydroethidium (DHE), TRPA1 antagonist HC-030031 and Calcium Ionophore A23187 were obtained from Sigma-Aldrich (St. Louis, MO, USA). Allyl isothiocyanate (AITC) was obtained from Dewei chem (Chuzhou, China). The other agents were analysis grade.
Cell culture
Human lung carcinoma A549 cells, a human alveolar epithelial cell line, were grown in Dulbecco’s modified Eagle’s medium supplemented with 10 % fetal bovine serum, 2 mM glutamine, 56 U/ml of penicillin-G, and 56 μg/ml of streptomycin sulfate. The cells were seeded on cover glass (24 × 24 × 0.17 mm) in six-well dishes (Costar, NY, USA) and were regulated at an initial density of 250,000 cells suspension in each well. Cells were located in a close chamber with a thermosensor and two pipes. The cold medium was rapidly infused into the chamber through one pipe and effused by another pipe. The change of solution temperature was recorded.
Solutions
Cells were bathed in an isotonic medium (ISO). The medium contained 140 mM NaCl, 5.4 mM KCl, 0.5 mM MgCl2, 0.4 mM MgSO4, 3.3 mM NaHCO3, 2.0 mM CaCl2, 10 mM HEPES, and 5.5 mM glucose (pH 7.4, adjusted with NaOH, having an osmolarity of 300 mOsm/l). For studies in the absence of extracellular calcium ([Ca2+]o), the medium was made with CaCl2 substituted by the same concentration of MgCl2, with 1 mM EGTA to chelate trace Ca2+.
Measurement intracellular ROS by DHE fluorescence
A549 cells were washed with the above-mentioned isotonic medium buffer, incubated with 5 μM DHE in ISO buffer for 30 min at room temperature, and washed again with the buffer. Fluorescence (exCitation wavelength, 535 nm; emission wavelength, 610 nm) was measured using a fluorescence microscope (Olympus IX53, Tokyo. Japan). The cells’ images were monitored through cool CCD (Q-imaging; Retiga EXI FAST 1394, Mono, 12-bit, cooled; Canada). Fluorescence images were collected at 60 min after stimulation. The ROS image experiments were repeated at least three times, and ten wells were used in each experiment. For quantization, the area of the cell was selected, and image quantization was performed using the Image J software (National Institutes of Health, USA). The background fluorescence was subtracted and the mean fluorescence intensity of the images was determined.
Measurement of [Ca2+]c
Fura 2-AM was dissolved in DMSO, with isotonic medium added to make a final concentration of 5 μmol/L. Cells were incubated for 1 h at room temperature in the dark and then were washed on the slip three times. Changes in the fluorescence intensity of Fura-2 at excitation wavelengths of 340 and 380 nm and an emission wavelength of 510 nm were monitored. The exposure time was 200 ms. Fluorescence intensity was measured through cool CCD and analyzed with Meta Fluor software (Sunnyvale, USA). F340/F380 was directly represented to [Ca2+]c. At least three experiments were carried out for each condition, and for each condition, 30 individual cells were selected. The representative calcium trace has been selected among more than 10 similar traces.
Immunohistochemistry
We performed immunocytochemistry analyses in A549 cells. Cells were fixed with PFA for 30 min at room temperature. After three washing steps with PBS, cells were subjected to 0.1 % Triton-X 100 for cell membrane perforation. After treatment with protein block for 30 min, cells were stained with rabbit polyclonal TRPA1 antibody (1:250 Sigma, Saint Louis, USA) overnight at 4 °C. After three washings with TBS, FITC-conjugated goat anti-rabbit antibody (EarthOXford, San Francisco, CA, USA) were added and incubated for 90 min at room temperature. After removing excess of fluorescence-conjugated secondary antibody, cells were examined using a fluorescence microscope.
Statistical analysis
All data were expressed as the means ± standard deviation. Statistical analysis was assessed by Student’s t test. In all cases, p < 0.05 was considered significant; p < 0.01 was considered statistically significant.
Results and discussion
The respiratory epithelial cells constantly interact with the external air environment. Cold air induces respiratory inflammation in high-altitude region or in winter (Koskela 2007). especially in patients with respiratory disorders (Seys et al. 2013; Hyrkäs et al. 2014). At addition, reactive oxygen species are involved in physiological and pathophysiological processes in the lung (Al Ghouleh et al. 2011). Lung epithelial cells have been identified in ROS production induced by various stimulants (Faux et al. 2009; Rosanna and Salvatore 2012). It has been reported that lung epithelial cells might increase ROS production after the cells were kept at 4 °C for varying periods in medium and then rewarmed (for 3 h) (Pizanis et al. 2011). However, the mechanism of the ROS production in pulmonary epithelial cells induced by rapid cold stress is still unclear.
Production of reactive oxygen species is dependent on [Ca2+]c elevation induced by cold stimulation
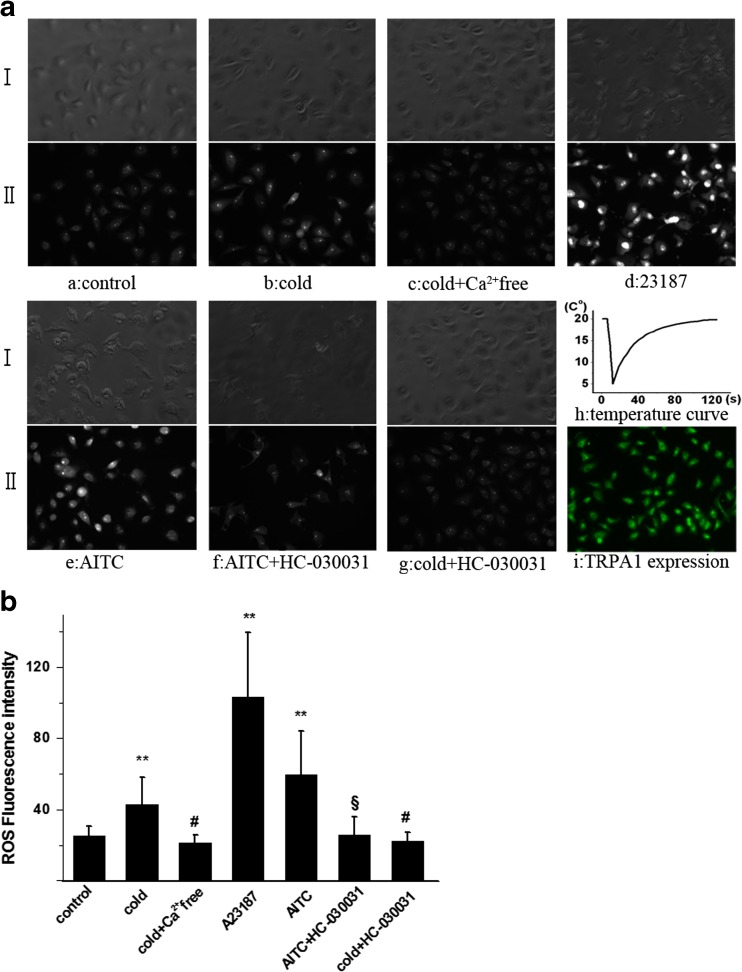
We first examined the effect of cold stress on the ROS formation in A549 cells. The intensity of ROS in single cell was detected with ROS-specific fluorescence probe DHE. The change curve of medium temperature was shown in Fig. 1a (h). The ROS fluorescence images showed cold stress (from 20 to 5 °C) induced a substantial increase in A549 cells, which was significantly higher than control (Fig. 1a (a, b)). Statistical analysis of multicellular data confirmed that the enhanced production of ROS was induced by cold stimulation (Fig. 1b; P < 0.01). In addition, we have observed the cellular viability with trypan blue. The results showed one-time cold stimulation did not bring damage to cell. These results showed that cold stress could increase the ROS formation in A549 cells.
Fig. 1.
The effect of stimuli with or without inhibitor on the ROS generation in A549 cells. a The fluorescence images of intracellular ROS produced in A549 cells. (I) A549 cells were imaged by interference contrast microscopy. (II) Fluorescence images are shown with the fluorescence intensity representing the ROS concentration. (a) Control. (b) Cold with 2 mM Ca2+ in the medium. (c) Cold and Ca2+ free in the medium. (d) 2 μM A32187. (e) 100 μM AITC. (f) 100 μM AITC and HC-030031 (50 μM pre-treatment for 20 min). (g) Cold and HC-030031 (50 μM pre-treatment for 20 min). (h) The change curve of temperature. (i) The protein expression of TRPA1 in A549. The immunocytochemistry revealed TRPA1 protein expression in A549. Cells were stained with rabbit TRPA1 antibody and FITC-conjugated goat anti-rabbit antibody. Immunocytochemistry experiments were carried out without primary antibody as control. No signals in cells were detected (control pictures no shown). b Statistical analysis of fluorescence intensity of ROS in A549 cells. Values are expressed as an averaged response (mean ± S.E.M.) of at least 30 individual cells from three independent experiments. **P < 0.01, Cold, A23187 and AITC increased the production of ROS compared with control. § P < 0.01; HC-030031 inhibited the production of ROS stimulated by AITC. # P < 0.01; Ca free and HC-030031 inhibited the production of ROS stimulated by cold stress, respectively
The Ca2+ is an important signaling messenger that involves in the regulation of various cellular functions (Clapham 2007). It has been reported that ROS generated from mitochondrial electron transport chain which is modulated by mitochondrial Ca2+ uptake (Aldakkak et al. 2013). Furthermore, the mitochondria can accumulate a large amount of Ca2+ as a consequence of cytosolic Ca2+ increase (Rizzuto et al. 1999; Carafoli 2003). In addition, low temperature might increase ROS production in isolated mitochondria from murine brain (Ali et al. 2010). For the above reasons, the importance of [Ca2+]c in the enhanced production of ROS induced by cold stress was further assessed in the following experiments. At first, the change of [Ca2+]c has been examined during cold stimulation. The results showed cold stress (from 20 to 5 °C) induced a significant increase in [Ca2+]c (Fig. 2a (b), Fig. 2b; P < 0.01). In view of evidence that cold stress increases both [Ca2+]c and ROS levels, the extracellular Ca2+ ions have been removed in the next experiment. The results showed Ca2+-free medium almost completely blocked cold-induced [Ca2+]c elevation (Fig. 2a (c)). At the same time, the increased production of ROS was also inhibited by cold stress without Ca2+ in the medium (Fig. 1a (c)). Statistical results confirmed the inhibitory effects of Ca2+-free medium on the increase in [Ca2+]c and ROS induced by cold stress in A549 cells (Figs. 1b and 2b; P < 0.01, respectively). Next, the role of Ca2+ influx in the formation of ROS was studied with an alternative mechanism to increase [Ca2+]c. A23187, an ionophore for Ca2+, has been used in the following experiments. The result showed A23187 induced robust elevation of both [Ca2+]c and ROS in A549 (Figs. 1a (d) and 2a (d), Figs. 1b and 2b; P < 0.01). These results indicate that [Ca2+]c elevation from extracellular Ca2+ entry induced by cold stress plays an important role in the enhanced production of ROS in A549.
Fig. 2.
The change of [Ca2+]c in A549 induced by stimuli with or without inhibitor. a Representative tracings of intracellular Ca2+ fluorescence intensity in A549 cells. (a) Control: without change of [Ca2+]c. (b) Cold with 2 mM Ca2+ in the medium. (c) Cold with Ca2+-free medium. (d) 2 μM A23187. (e) 100 μM AITC. (f) 100 μM AITC and HC-030031 (50 μM pre-treatment for 20 min). (g) Cold and HC-030031 (50 μM pre-treatment for 20 min). Arrows point to the beginning of cold stress or agonist treatment. b Statistical analysis of the peak of [Ca2+]c in multiple experiments. Values are expressed as an averaged response (mean ± S.E.M.) of at least 10 individual cells from three independent experiments. **P < 0.01; cold, A23187 and AITC increased the peak of [Ca2+]c elevation compared with control, respectively. # P < 0.01; Ca free and HC-030031 inhibited the peak of [Ca2+]c elevation stimulated by cold stress, respectively. § P < 0.01; HC-030031 blocked the peak of [Ca2+]c elevation induced by AITC
Production of reactive oxygen species is through the activation of TRPA1 during cold stimulation
In recent years, it has been demonstrated that transient receptor potential channel of the ankyrin-binding repeat subfamily, TRPA1, is the sole member of TRPA subgroup and a Ca2+-permeable non-selective cation channel. TRPA1 could cause Ca2+ influx following activation by cold stress (<17 °C) or agonists (Nilius et al. 2012). Environmental chemicals have been identified to be TRPA1 activators such as allyl isothiocyanate (AITC) (Qian et al. 2013). The antagonist of TRPA1 is HC-030031 (Eid et al. 2008). It has been reported that cold temperature significantly increased mitochondrial ATP levels in cells which were transfected with cold-sensing transient receptor potential TRPM8 or TRPA1 (Park et al. 2013). However, the effect of TRPA1 channel on the production of ROS has not been reported. We hypothesized that [Ca2+]c elevation following TRPA1 activated by the noxious cold is involved in the ROS production.
TRPA1 protein has been found in the airway epithelial cells toward the air in the human lung (Büch et al. 2013). The previous research has reported TRPA1 expression at mRNA and protein levels in A549 cell line (Mukhopadhyay et al. 2011). We also confirmed the conclusion of TRPA1 mRNA expression in A549 cell using RT-PCR in the previous research (Sun et al. 2014). Here, the expression of TRPA1 protein has been confirmed by immunocytochemistry in A549 cells (Fig. 1a (i)). In order to further investigate the possible role of TRPA1 channel in the production of ROS, A549 cells were treated with the TRPA1-specific agonist, allyl isothiocyanate (AITC), in our expirations. The results showed that AITC dramatically increased both [Ca2+]c level (Fig. 2a (e), Fig. 2b, P < 0.01) and ROS formation in A549 cells at room temperature (20 °C) (Fig. 1a (e), Fig. 1b; P < 0.01). Next, HC-030031, a TRPA1-specific channel blocker, inhibited [Ca2+]c elevation induced by AITC significantly (Fig. 2a (f), Fig. 2b; P < 0.01). At the same time, HC-030031 also blocked the AITC-induced enhanced ROS production in A549 cells (Fig. 1a (g), Fig. 1b; P < 0.01). Those results indicate that TRPA1 activation is involved in ROS formation in cells. In the following experiments, the results showed HC-030031 also largely prevented the cold-induced ROS and [Ca2+]c elevation in A549 cells (Figs. 1a (j) and 2a (g)), respectively. Statistical results in Figs. 1b and 2b confirmed the suppressive effect of TRPA1 antagonist on the increase in ROS and [Ca2+]c induced by cold stimulation. Taken together, those results suggest that [Ca2+]c elevation via TRPA1 activation induced by cold stress is a possible mechanism underlying the enhanced production of ROS. Accumulating evidence has implicated that transient receptor potential canonical 3 is responsible for the increase in Ca2+/calmodulin-dependent kinase II (CaMKII) activity and ROS production (Kitajima et al. 2011). Further study showed that mitochondrial ROS generation was mediated by Ca2+/CaM/CaMKII signaling pathway (Toledo et al. 2014). We speculate that the increased ROS formation might be via Ca2+/CaM/CaMKII signaling pathway which is elicited by TRPA1 activation induced by cold stimulation.
Reactive oxygen species (ROS) are well known to play a major role in the pathogenesis of a variety of lung disorders such as asthma, chronic obstructive lung disease, acute lung injury, pulmonary fibrosis, and cancer (Henricks and Nijkamp 2011; MacNee 2001). It has been reported that cold stress increased the production of ROS in animal model and cellular and mitochondrial experiments, respectively (Ali et al. 2010; García-Díaz et al. 2015; Awad et al. 2013). Our results suggest that TRPA1 is mainly involved in the production of ROS via [Ca2+]c elevation pathway induced by cold stress in lung epithelial cell. The pulmonary expression of TRPA1 has been found in sensory nerve endings and pulmonary epithelial cells, which is involved in the acceleration of inflammatory responses in the lung (Büch et al. 2013). On the other hand, ROS are important components of signaling cascades that respond to extracellular stimuli, including tumor necrosis factor, interleukin-1, adenosine-5′-triphosphate, cigarette smoke extract, lipoteichoic acid, or lipopolysaccharide, which are associated with many of the known inflammatory target proteins, such as metalloproteinase-9, intercellular adhesion molecule-1, vascular cell adhesion molecule-1, cyclooxygenase-2, and cytosolic phospholipase A2. Noxious stimuli-induced ROS result in the modulation of inflammatory gene expression associated with pulmonary diseases (Lee and Yang 2013). In addition, it is worth noting that TRPA1 channel exhibits a cooperative effect during cold and other chemical stimulation simultaneously (del Camino et al. 2011). Therefore, we speculate the ROS elevation which is dependent on TRPA1 activation aggravates the pulmonary disease during cold environment. Perhaps this work might improve the understanding of the pathological process of the pulmonary disease.
Acknowledgments
This work was supported by the 12th 5 years project of medicine (No. BWS12J007), The Natural Sciences Foundation of Liaoning Province (No. 2011225008), and the National S&T project (No. 2012ZX09303-016).
References
- Al Ghouleh I, Khoo NK, Knaus UG, Griendling KK, Touyz RM, Thannickal VJ, Barchowsky A, Nauseef WM, Kelley EE, Bauer PM, Darley-Usmar V, Shiva S, Cifuentes-Pagano E, Freeman BA, Gladwin MT, Pagano PJ. Oxidases and peroxidases in cardiovascular and lung disease: new concepts in reactive oxygen species signaling. Free Radic Biol Med. 2011;51:1271–1288. doi: 10.1016/j.freeradbiomed.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldakkak M, Stowe DF, Dash RK, Camara AK. Mitochondrial handling of excess Ca2+ is substrate-dependent with implications for reactive oxygen species generation. Free Radic Biol Med. 2013;56:193–203. doi: 10.1016/j.freeradbiomed.2012.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali SS, Marcondes MC, Bajova H, Dugan LL, Conti B. Metabolic depression and increased reactive oxygen species production by isolated mitochondria at moderately lower temperatures. J Biol Chem. 2010;285:32522–32528. doi: 10.1074/jbc.M110.155432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awad EM, Khan SY, Sokolikova B, Brunner PM, Olcaydu D, Wojta J, Breuss JM, Uhrin P. Cold induces reactive oxygen species production and activation of the NF-kappa B response in endothelial cells and inflammation in vivo. J Thromb Haemost. 2013;11:1716–1726. doi: 10.1111/jth.12357. [DOI] [PubMed] [Google Scholar]
- Belousov VV, Fradkov AF, Lukyanov KA, Staroverov DB, Shakhbazov KS, Terskikh AV, Lukyanov S. Genetically encoded fluorescent indicator for intracellular hydrogen peroxide. Nat Methods. 2006;3:281–286. doi: 10.1038/nmeth866. [DOI] [PubMed] [Google Scholar]
- Büch TR, Schäfer EA, Demmel MT, Boekhoff I, Thiermann H, Gudermann T, Steinritz D, Schmidt A. Functional expression of the transient receptor potential channel TRPA1, a sensor for toxic lung inhalants, in pulmonary epithelial cells. Chem Biol Interact. 2013;206:462–471. doi: 10.1016/j.cbi.2013.08.012. [DOI] [PubMed] [Google Scholar]
- Carafoli E. Historical review: mitochondria and calcium: ups and downs of an unusual relationship. Trends Biochem Sci. 2003;28:175–181. doi: 10.1016/S0968-0004(03)00053-7. [DOI] [PubMed] [Google Scholar]
- Clapham DE. Calcium signaling. Cell. 2007;131:1047–1058. doi: 10.1016/j.cell.2007.11.028. [DOI] [PubMed] [Google Scholar]
- del Camino D, Murphy S, Heiry M, Barrett LB, Earley TJ, Cook CA, Petrus MJ, Zhao M, D’Amours M, Deering N, Brenner GJ, Costigan M, Hayward NJ, Chong JA, Fanger CM, Woolf CJ, Patapoutian A, Moran MM. TRPA1 contributes to cold hypersensitivity. J Neurosci. 2011;30:15165–15174. doi: 10.1523/JNEUROSCI.2580-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eid SR, Crown ED, Moore EL, Liang HA, Choong KC, Dima S, Henze DA, Kane SA, Urban MO. HC-030031, a TRPA1 selective antagonist, attenuates inflammatory- and neuropathy-induced mechanical hypersensitivity. Mol Pain. 2008;27:48. doi: 10.1186/1744-8069-4-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faux SP, Tai T, Thorne D, Xu Y, Breheny D, Gaca M. The role of oxidative stress in the biological responses of lung epithelial cells to cigarette smoke. Biomarkers. 2009;14(Suppl 1):90–96. doi: 10.1080/13547500902965047. [DOI] [PubMed] [Google Scholar]
- Finkel T. Signal transduction by reactive oxygen species. J Cell Biol. 2011;194:7–15. doi: 10.1083/jcb.201102095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Díaz EC, Gómez-Quiroz LE, Arenas-Ríos E, Aragón-Martínez A, Ibarra-Arias JA, Retana-Márquez MD. Oxidative status in testis and epididymal sperm parameters after acute and chronic stress by cold-water immersion in the adult rat. Syst Biol Reprod Med. 2015;61:150–160. doi: 10.3109/19396368.2015.1008071. [DOI] [PubMed] [Google Scholar]
- Giesbrecht GG. The respiratory system in a cold environment. Aviat Space Environ Med. 1995;66:890–902. [PubMed] [Google Scholar]
- Henricks PA, Nijkamp FP. Reactive oxygen species as mediators in asthma. Pulm Pharmacol Ther. 2011;14:409–420. doi: 10.1006/pupt.2001.0319. [DOI] [PubMed] [Google Scholar]
- Hyrkäs H, Jaakkola MS, Ikäheimo TM, Hugg TT, Jaakkola JJ. Asthma and allergic rhinitis increase respiratory symptoms in cold weather among young adults. Respir Med. 2014;108:63–70. doi: 10.1016/j.rmed.2013.10.019. [DOI] [PubMed] [Google Scholar]
- Kitajima N, Watanabe K, Morimoto S, Sato Y, Kiyonaka S, Hoshijima M, Ikeda Y, Nakaya M, Ide T, Mori Y, Kurose H, Nishida M. TRPC3-mediated Ca2+ influx contributes to Rac1-mediated production of reactive oxygen species in MLP-deficient mouse hearts. Biochem Biophys Res Commun. 2011;409:108–113. doi: 10.1016/j.bbrc.2011.04.124. [DOI] [PubMed] [Google Scholar]
- Koskela HO. Cold air-provoked respiratory symptoms: the mechanisms and management. Int J Circumpolar Health. 2007;66:91–100. doi: 10.3402/ijch.v66i2.18237. [DOI] [PubMed] [Google Scholar]
- Lee IT, Yang CM. Inflammatory signalings involved in airway and pulmonary diseases. Mediat Inflamm. 2013;2013:791231. doi: 10.1155/2013/791231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacNee W. Oxidative stress and lung inflammation in airways disease. Eur J Pharmacol. 2001;1429:195–207. doi: 10.1016/S0014-2999(01)01320-6. [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay I, Gomes P, Aranake S, Shetty M, Karnik P, Damle M, Kuruganti S, Thorat S, Khairatkar-Joshi N. Expression of functional TRPA1 receptor on human lung fibroblast and epithelial cells. J Recept Signal Transduct Res. 2011;31:350–358. doi: 10.3109/10799893.2011.602413. [DOI] [PubMed] [Google Scholar]
- Nilius B, Appendino G, Owsianik G. The transient receptor potential channel TRPA1: from gene to pathophysiology. Pflugers Arch. 2012;464:425–458. doi: 10.1007/s00424-012-1158-z. [DOI] [PubMed] [Google Scholar]
- Oliveira-Marques V, Marinho HS, Cyrne L, Antunes F. Role of hydrogen peroxide in NF-kappaB activation: from inducer to modulator. Antioxid Redox Signal. 2009;11:2223–2243. doi: 10.1089/ars.2009.2601. [DOI] [PubMed] [Google Scholar]
- Park S, Chun S, Kim D. Cold exposure lowers energy expenditure at the cellular level. Cell Biol Int. 2013;37:638–642. doi: 10.1002/cbin.10086. [DOI] [PubMed] [Google Scholar]
- Pizanis N, Gillner S, Kamler M, de Groot H, Jakob H, Rauen U. Cold-induced injury to lung epithelial cells can be inhibited by iron chelators—implications for lung preservation. Eur J Cardiothorac Surg. 2011;40:948–955. doi: 10.1016/j.ejcts.2011.01.052. [DOI] [PubMed] [Google Scholar]
- Qian X, Francis M, Solodushko V, Earley S, Taylor MS. Recruitment of dynamic endothelial Ca2+ signals by the TRPA1 channel activator AITC in rat cerebral arteries. Microcirculation. 2013;20:138–148. doi: 10.1111/micc.12004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray PD, Huang BW, Tsuji Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell Signal. 2012;24:981–990. doi: 10.1016/j.cellsig.2012.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzuto R, Pinton P, Brini M, Chiesa A, Filippin L, Pozzan T. Mitochondria as biosensors of calcium microdomains. Cell Calcium. 1999;26:193–199. doi: 10.1054/ceca.1999.0076. [DOI] [PubMed] [Google Scholar]
- Rosanna DP, Salvatore C. Reactive oxygen species, inflammation, and lung diseases. Curr Pharm Des. 2012;18:3889–3900. doi: 10.2174/138161212802083716. [DOI] [PubMed] [Google Scholar]
- Seys SF, Daenen M, Dilissen E, Van Thienen R, Bullens DM, Hespel P, Dupont LJ. Effects of high altitude and cold air exposure on airway inflammation in patients with asthma. Thorax. 2013;68:906–913. doi: 10.1136/thoraxjnl-2013-203280. [DOI] [PubMed] [Google Scholar]
- Stowe DF, Camara AK. Mitochondrial reactive oxygen species production in excitable cells: modulators of mitochondrial and cell function. Antioxid Redox Signal. 2009;11:1373–1414. doi: 10.1089/ars.2008.2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun W, Wang Z, Cao J, Wang X, Han Y, Ma Z. Enhanced production of nitric oxide in A549 cells through activation of TRPA1 ion channel by cold stress. Nitric Oxide. 2014;40:31–35. doi: 10.1016/j.niox.2014.04.009. [DOI] [PubMed] [Google Scholar]
- Thannickal VJ, Fanburg BL. Reactive oxygen species in cell signaling. Am J Physiol Lung Cell Mol Physiol. 2000;279:L1005–L1028. doi: 10.1152/ajplung.2000.279.6.L1005. [DOI] [PubMed] [Google Scholar]
- Toledo FD, Pérez LM, Basiglio CL, Ochoa JE, Sanchez Pozzi EJ, Roma MG. The Ca2+-calmodulin-Ca2+/calmodulin-dependent protein kinase II signaling pathway is involved in oxidative stress-induced mitochondrial permeability transition and apoptosis in isolated rat hepatocytes. Arch Toxicol. 2014;88(9):1695–1709. doi: 10.1007/s00204-014-1219-5. [DOI] [PubMed] [Google Scholar]
- Valavanidis A, Vlachogianni T, Fiotakis K, Loridas S. Pulmonary oxidative stress, inflammation and cancer: respirable particulate matter, fibrous dusts and ozone as major causes of lung carcinogenesis through reactive oxygen species mechanisms. Int J Environ Res Public Health. 2013;27:3886–3907. doi: 10.3390/ijerph10093886. [DOI] [PMC free article] [PubMed] [Google Scholar]