Abstract
As a survival strategy to environmental water deficits, desiccation-tolerant organisms are commonly known for their ability to recruit stress-protective biomolecules such as trehalose. We have previously reported the pivotal role of trehalose in larval desiccation tolerance in Drosophila melanogaster. Trehalose has emerged as a versatile molecule, serving mainly as energy source in insects and also being a stress protectant. While several recent reports have revealed the unconventional role of trehalose in scavenging reactive oxygen species in yeast and plants, this aspect has not received much attention in animals. We examined the status of desiccation-induced generation of reactive oxygen species in D. melanogaster larvae and the possible involvement of trehalose in ameliorating the harmful consequences thereof. Insect trehalose synthesis is governed by the enzyme trehalose 6-phosphate synthase 1 (TPS1). Using the ubiquitous da-GAL4-driven expression of the dTps1-RNAi transgene, we generated dTps1-downregulated Drosophila larvae possessing depleted levels of dTps1 transcripts. This resulted in the inability of the larvae for trehalose synthesis, thereby allowing us to elucidate the significance of trehalose in the regulation of desiccation-responsive redox homeostasis. Furthermore, the results from molecular genetics studies, biochemical assays, electron spin resonance analyses and a simple, non-invasive method of whole larval live imaging suggested that trehalose in collaboration with superoxide dismutase (SOD) is involved in the maintenance of redox state in D. melanogaster.
Electronic supplementary material
The online version of this article (doi:10.1007/s12192-015-0658-0) contains supplementary material, which is available to authorized users.
Keywords: Drosophila, Desiccation stress, Trehalose, ROS, SOD, dTps1-RNAi transgene
Introduction
Imbalances in abiotic factors such as temperature and humidity can lead to desiccating conditions in nature. Body water loss in response to desiccation stress can pose severe challenges for survival under physiological water deficits (Chown and Nicolson 2004). To escape hostile dehydration bouts in nature, desiccation-tolerant organisms possess the ability to sustain the dehydrated state followed by revival and resumption of active metabolism upon rehydration (Keilin 1959). One of the common mechanisms of desiccation tolerance is the ability of organisms to synthesise compatible solutes like proline, glycine betaine, umbelliferose, trehalose, mannitol, sorbitol, glycerol and LEA proteins (Tunnacliffe and Lapinski 2003). Among these biomolecules, trehalose is well known as a stress protectant (Reyes-DelaTorre et al. 2012) and is also drawing attention for its novel roles in nutrition-associated behaviour in insects (Liua et al. 2013). Trehalose is extensively found in bacteria, fungi, plants and invertebrate animals but is absent in vertebrates (Shukla et al. 2015). In recent times, the unconventional role of trehalose to scavenge superoxide radicals (O2·−) has been widely appreciated in plants and yeast (França et al. 2007). To our knowledge, in barring a few studies (Echigo et al. 2012), this aspect has hardly been studies in animals.
In the purview of dehydration, loss of body water causes osmotic perturbations resulting in ionic fluctuations and the generation of reactive oxygen species (ROS) (Rizzo et al. 2010). ROS are highly reactive entities and comprise a whole gamut of species like singlet oxygen, superoxide and hydroxyl ions, hydrogen peroxide, nitric oxide and peroxynitrite ions (Gutteridge and Halliwell 2001). In living systems, endogenous sources of ROS generation include a variety of cellular activities such as respiration, photosynthesis, metal-catalysed reactions, etc. (Mittler 2002). Under normal physiological conditions, there exists equilibrium in the amount of ROS and the antioxidants. However, this equilibrium is perturbed when the rate of ROS generation exceeds that of the antioxidant defence machinery leading to the onset of oxidative stress. Thus, the level of antioxidants often reflect the ROS pools in organisms and have been used as indicators in studies concerning the oxidative stress response inflicted by stressors like hypoxia, radiation, desiccation and chill (Benoit and Lopez-Martinez 2012).
Previous work from our laboratory has reported that trehalose confers desiccation tolerance ability in the larvae of Drosophila melanogaster (Thorat et al. 2012). In this study, we extended our investigation to find out the status of desiccation-induced ROS generation in the larvae and the possible involvement of trehalose in ameliorating the harmful consequences involved therein. Members of the family Drosophilidae make excellent model systems for molecular genetics-based inquiries because they are highly amenable to genetic manipulations. We took advantage of genetic tools to generate dTps1-downregulated larvae in which trehalose synthesis was severely reduced. Trehalose 6-phosphate synthase (TPS1; EC 2.4.1.15) is the first enzyme involved in insect trehalose synthesis (Wyatt 1967), and thus, dTps1-downregulated progeny provided proof of the concept that trehalose indeed plays a pivotal role in larval desiccation tolerance. Furthermore, we elucidated the involvement of trehalose in the regulation of desiccation-responsive redox homeostasis in the larvae. To execute our approach, we categorised the larvae into two groups, viz. trehalose fed and unfed. Using molecular genetics tools, biochemical assays, electron spin resonance analyses and a simple, non-invasive method of whole larval real-time imaging, we demonstrate that trehalose in collaboration with superoxide dismutase (SOD) is involved in the maintenance of redox state in the larvae of D. melanogaster.
Materials and methods
Flies
Wild-type flies of D. melanogaster (Oregon K+ strain) were used in this study. dTps1-RNAi and GAL4 fly lines were received as a kind gift from Prof. Alexander Brehm (Philipps Universität Marburg, Germany). Males of dTps1-RNAi transgenic line of flies (carrying UAS-RNAi construct against dTps1) were mated with virgin females of ubiquitous daughterless Gal4 flies (P{da-Gal4} expressing Gal4 in da pattern) to obtain progeny depleted with dTps1 (henceforth referred to as the progeny expressing the dTps1-RNAi transgene or the dTps1-downregulated progeny). These downregulated individuals were incapable of producing trehalose due to the block in trehalose biosynthesis. Early-third instar ORK and dTps1-downregulated larvae were used for all subsequent experiments. All fly lines were reared on a standard cornmeal-agar food medium and maintained in a biological oxygen demand (BOD) chamber (CI-6S Remi, India) at 23 ± 1 °C under conditions of 14:10-h L/D.
Trehalose feeding
Wild-type and dTps1-downregulated larvae were incubated in glass vials containing either 5 ml of 25 mM trehalose (Sigma, USA) solution or water for 1 h prior to desiccation. The former batch formed the trehalose-fed group, while the later was referred to as the trehalose-unfed group. After 1 h of feeding, groups of ten larvae were picked up and blotted on a filter paper sheet followed by desiccation treatment.
Desiccation stress
Desiccation treatment was carried out using the method published before (Thorat et al. 2012). In brief, unfed and fed wild-type and dTps1-RNAi transgene-expressing larvae were exposed to acute desiccation stress at <5 % relative humidity for defined hours (h) followed by rehydration with water. Larvae were judged for survival based on abdominal contractions using a stereo zoom microscope (Magnus MS 24; Olympus Pvt. Ltd., India). Undesiccated wild-type and dTps1-downregulated larvae were used as the respective controls.
Detection of trehalose accumulation
Trehalose quantification was carried out by HPLC analysis as described before (Thorat et al. 2012). Briefly, groups of 30 pre-weighed larvae were picked up at appropriate time points of desiccation and homogenised in 300 μl of 90 % ethanol with 0.1 mg sorbitol as an internal standard. The samples were centrifuged at 1500g for 10 min, and the supernatants were analysed on an HPLC system (Waters) as per the manufacturer’s instructions. The assembly comprised of a guard column attached to the analytical column (Sugar-Pak, 6.5 mm × 300 mm) equipped with a reflective index detector (RID-2410). The oven was heated to 70 °C, and Ca2+/EDTA was used as the mobile phase with a constant flow rate of 0.4 ml/min. Standard solution of trehalose was analysed, and content of trehalose in the larvae (μg/larva) was estimated.
RNA isolation and reverse transcription quantitative PCR analysis
Larvae were picked up at specific time points and frozen down in liquid nitrogen followed by total RNA extraction using the peqGOLD Total RNA Kit (Germany) according to the manufacturer’s instructions as described previously (Thorat and Nath 2015). RNA was quantified and was used for cDNA synthesis using M-MLV Reverse Transcriptase Kit (Invitrogen, USA). Reverse transcription quantitative PCR (RT-qPCR) analysis was carried out on Step One System (Applied Biosystems, USA) using appropriate primer sequences for quantifying dTps1 transcripts in the larvae relative to rp49 transcripts (dTps1: For-5′-TTCTTCCTGCACATCCCATT and Rev-5′-CAGGTCACAACCCAACATACC; rp49: For-5′- GATCGTGAAGAAGCGCACCAAG and Rev-5′- CCGGATTCAAGAAGTTCCTGGTG). Undesiccated wild-type control values were set to 1 against which all other values were compared. The results were represented as fold induction relative to rp49.
Determination of enzyme activity
Biochemical assay for determination of SOD activity was carried out according to published procedures (Datkhile et al. 2009). The larvae were picked up at specified time points and homogenised in extraction buffer, and the supernatants obtained on centrifugation were used as crude extracts for spectrophotometric measurements of enzyme activity on a UV-Vis spectrophotometer (V-630; JASCO International, Japan). Protein estimations were carried out by Lowry et al. (1951).
For determination of SOD activity, absorbance was monitored at 560 nm. One unit of enzyme activity was defined as the amount of enzyme required to cause 50 % inhibition in the rate of NBT reduction under standard assay conditions.
Measurement of superoxide radicals
After desiccation treatment, the larvae were incubated in the dark in 2′,7′-dicholorodihydrofluorescein diacetate (DCF-DA; Cayman, USA) solution for 20 min for the detection of O2·−. Post incubation, the larvae were homogenised in 100 mM phosphate-buffered saline (PBS) and centrifuged at 5000g at 4 °C for 10 min. The supernatants obtained were measured by fluorimetry (Varian Cary Eclipse UV-Vis Fluorescence Spectrophotometer, Australia) at dye specific excitation and emission wavelength. DCF-DA dye solution was run as standard and data were normalised according to the weight of the larvae.
Whole organismal live imaging
As described above, after desiccation, the larvae were incubated in DCF-DA solution in the dark for 20 min. At the end of the incubation period, the larvae were given a quick rinse in water and the whole larvae were mounted in PBS on a glass slide for live imaging. Fluorescence images were taken using an Olympus fluorescence microscope with DP71 camera attachment (Olympus 1X71, Japan).
Electron spin resonance (ESR) analysis
ESR analysis for validation of the presence of O2·− was adopted from a published procedure (Kumarov 2005). 5-Diethoxyphosphoryl-5-methyl-1-pyrroline-N-oxide (DEPMPO; Enzo Life Sciences, USA) was used as the spin-trapping agent that forms DEPMPO-OOH spin adducts upon detection of O2·−. Larval hemolymph was extracted in 100 mM PBS (pH 7.4) and centrifuged at 5000g at 4 °C for 10 min. The reaction mixture comprising 50 μl of supernatant and 30 mM DEPMPO was placed into a 200-ml quartz flat cell, and spectra were acquired on an ESR system (JEOL, Japan) with a modulation amplitude of 2 G and a microwave power of 20 mW.
Statistics
All experiments were replicated thrice (n = 30 larvae per replica) under standard laboratory conditions. Mean ± SD values obtained were subjected to Student’s t test using SPSS, version 12.0 (SPSS Inc., Chicago, IL, USA).
Results and discussion
Desiccation tolerance threshold
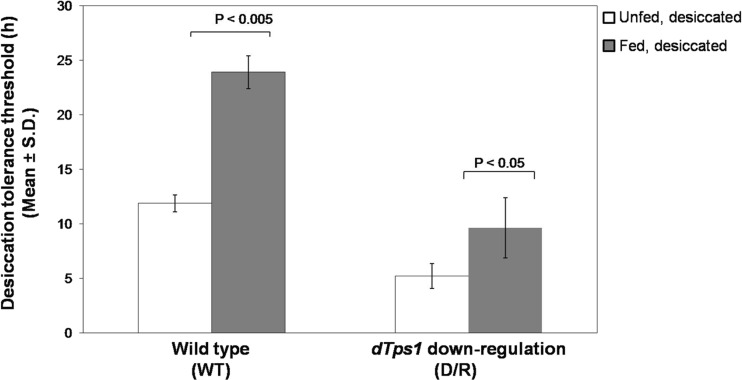
Threshold tolerance (in hours) of the unfed wild-type (WT) larvae to desiccation was found to be 11.87 ± 0.78 h (WTunfed, desiccated) (Fig. 1), whereas trehalose feeding caused a statistically significant increase to 23.91 ± 1.14 h in the tolerance threshold in the fed larvae (WTfed, desiccated) (P < 0.005). As expected, dTps1-downregulated (D/R) larvae showed a low tolerance threshold of 5.22 ± 1.5 h (D/Runfed, desiccated). Interestingly, exogenous trehalose feeding enhanced the ability of dTps1-downregulated larvae to withstand prolonged desiccation exposure up to 9.62 ± 2.75 h (D/Rfed, desiccated). Upon rehydration with water, the wild-type larvae revived and resumed metabolism followed by normal life activities. In contrast, although dTps1-downregulated larvae showed revival, they did not survive beyond 5 h of rehydration and thus could not reach the pupal stage (data not shown). Based on these data, all further results described in this study represent measurements/analyses carried out at 10-h desiccation and 12-h rehydration for the wild-type larvae and at 5-h desiccation and 3-h rehydration for the dTps1-downregulated larvae.
Fig. 1.
Comparative threshold desiccation tolerance (in hours) in the unfed and trehalose-fed wild-type and dTps1-downregulated larvae after desiccation
Trehalose is a very crucial component of insect physiology (Reyes-DelaTorre et al. 2012). In our study, exogenous feeding of trehalose dramatically increased the in vivo trehalose pools and also enhanced larval desiccation tolerance. These observations are in agreement with previous findings in the Antarctic midge larva, Belgica antarctica, wherein exogenously injected trehalose resulted in augmented tolerance towards desiccation (Benoit et al. 2009). Earlier, Chen et al. (2002) also demonstrated a role of trehalose towards anoxia tolerance in D. melanogaster. Using dTps1 over-expressing fly lines and dTps1 mutants, they showed that over-expression of dTps1 in D. melanogaster led to doubling of the fly trehalose content and also increased anoxia tolerance whereas the mutants suffered death at early developmental stages. They further concluded that apart from stress protection, dTps1/trehalose play crucial roles in the development and key physiological functions in fly. Very recently, dTps1 mutants of D. melanogaster have been reported to exhibit diet-dependent phenotypes and development and growth-related defects (Matsuda et al. 2015). A trehalose transporter in insects is responsible for the bidirectional transport of trehalose synthesised in the fat body across other tissues. Thus, the trehalose transporter is abundantly expressed in the fat body while low expression profiles are also seen in the insect gut and the nervous tissues (Kikawada et al. 2007; Liua et al. 2013). We therefore believe that the trehalose taken up by the larvae upon feeding was transported to the hemolymph via the trehalose transporter from the gut. Analogous studies in plants have also confirmed that exogenous supplementation of trehalose in several plant species improves desiccation tolerance (Shafiq et al. 2015).
Effects of trehalose feeding
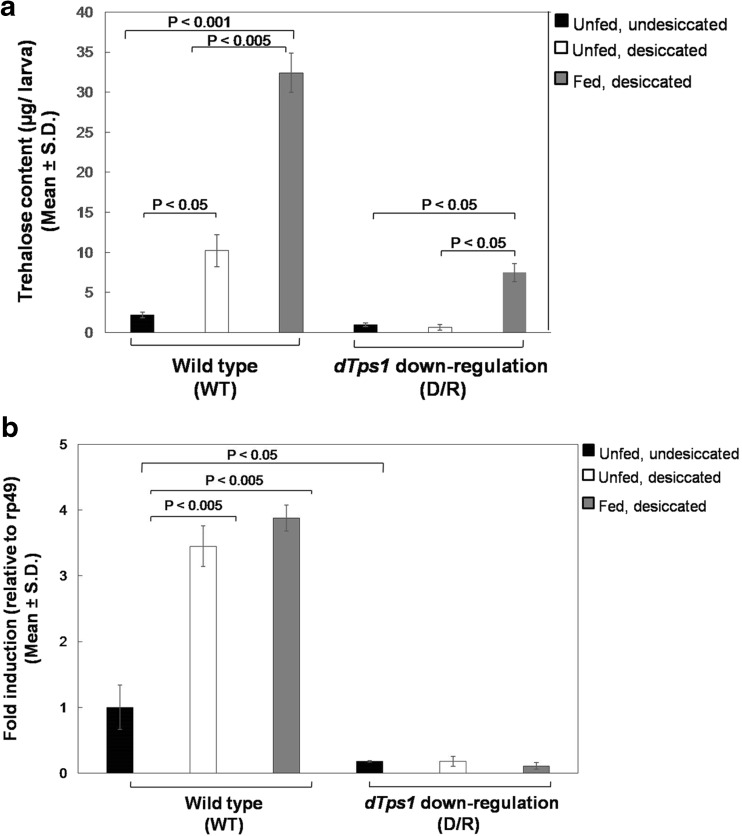
At the end of desiccation, the wild-type undesiccated larvae showed a basal trehalose level of 2.2 ± 0.34 μg/larva (WTunfed, undesiccated control) while the level in desiccated larvae was as high as 10.22 ± 1.97 μg/larva (WTunfed, desiccated) (P < 0.005) (Fig. 2a). Interestingly, trehalose feeding remarkably elevated the total trehalose content of the fed larvae to 32.41 ± 2.44 μg/larva (WTfed, desiccated) which was almost a 15-fold increase over the wild-type undesiccated control level (P < 0.001). In contrast, the incapability for trehalose synthesis of dTps1-RNAi-expressing larvae resulted in negligible accumulation of trehalose that averaged to 0.69 ± 0.22 μg/larva (D/Runfed, undesiccated control) and 0.52 ± 0.34 μg/larva (D/Runfed, desiccated) (Fig. 2a). In the fed larvae, trehalose levels rose to 7.5 ± 1.12 μg/larva (D/Rfed, desiccated), indicating an approximately 11-fold increase in comparison to their unfed, undesiccated controls (P < 0.05). As evident, dTps1 downregulation led to marked reduction in desiccation tolerance threshold which was rescued to a considerable extent upon exogenous trehalose feeding.
Fig. 2.
Estimation of trehalose and dTps1 transcripts at the end of desiccation. a Total trehalose content (μg/larva) in the control, unfed and trehalose-fed wild-type and dTps1-downregulated larvae after desiccation. b Fold induction of dTps1 transcripts after desiccation in the wild-type and dTps1-downregulated larvae. Undesiccated wild-type control values were set to 1 against which all other values were compared. The results were represented as fold induction relative to rp49
RT-qPCR confirmed that irrespective of exogenous trehalose feeding, the larvae expressing the dTps1-RNAi transgene possessed negligible levels of dTps1 transcripts and thus were incapable of producing trehalose (Fig. 2b). Thus, the relatively higher trehalose accumulation observed in the fed dTps1-downregulated larvae (Fig. 2a) could be attributed mainly to the exogenous trehalose. In contrast, unfed and fed wild-type larvae showed nearly a 3.5-fold increment in dTps1 levels, leading to a concomitant increase in trehalose in response to desiccation. These results confirm a significant role of trehalose in D. melanogaster larval desiccation tolerance.
Desiccation-responsive oxidative stress and involvement of trehalose in the regulation of redox state
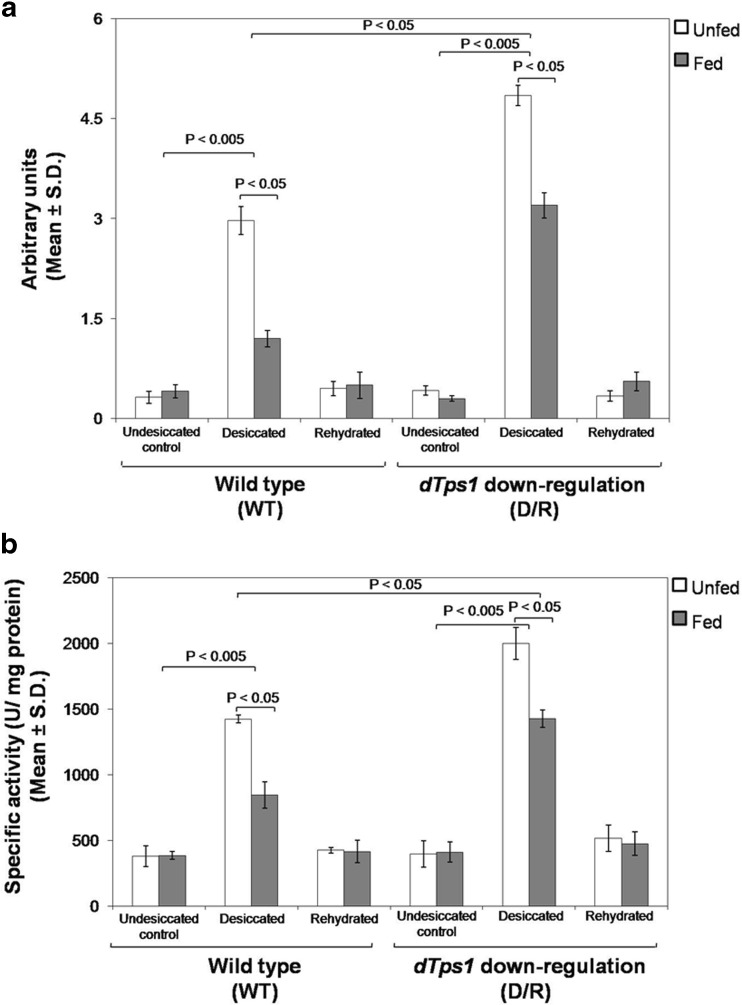
We next examined the status of desiccation-induced oxidative stress and whether trehalose plays any role in mitigating the harmful consequences of ROS accumulation. Spectrofluorimetric measurements using DCF-DA dye revealed the generation of O2·− upon exposure to desiccation stress in the wild-type as well as the dTps1-downregulated larvae (Fig. 3a). In both the larval groups, trehalose feeding notably reduced the amount of O2·−. In the case of the wild type, the undesiccated controls exhibited significantly lower levels of O2·− as compared to those of the desiccated larvae. Furthermore, among the desiccated groups, the unfed larvae possessed substantially higher O2·− content than those fed with trehalose (P < 0.05). dTps1-downregulated larvae also showed maximum O2·− levels in the unfed desiccated group as compared to the levels recorded from the undesiccated control and the trehalose-fed larvae (P < 0.05). The overall accumulation of O2·− was considerably high in the dTps1-RNAi transgene-expressing larvae in comparison to their wild-type counterparts. Upon rehydration, levels of O2·− started to decline and eventually matched the control values at the end of the recovery period in both the larval groups. Overall, these data confirmed desiccation-induced oxidative stress in both the wild-type larvae and those expressing the dTps1-RNAi transgene.
Fig. 3.
ROS levels and SOD activity in the desiccated and rehydrated control, unfed and trehalose-fed wild-type and dTps1-downregulated larvae. a Detection of O2 ·− using DCF-DA. b Determination of specific activity of SOD (U/mg protein)
Having confirmed the presence of O2·− in the larvae, we next elucidated the involvement of superoxide dismutase (SOD), the universal O2·− scavenger in most plants and animals (Mittler 2002). We found that in the unfed wild-type desiccated larvae, the SOD activity averaging to 1423.3 ± 30.67 (WTunfed, desiccated) was substantially higher than the basal SOD activity of 380.24 ± 79.35 U/mg protein (WTunfed, undesiccated control) in undesiccated controls (P < 0.005) (Fig. 3b). Remarkably, trehalose-fed wild-type larvae showed lower SOD levels up to 845.61 ± 101.26 U/mg protein (WTfed, desiccated). Similarly, the unfed dTps1-downregulated larvae showed a significantly higher SOD activity of 1998.7 ± 176.31 (D/Runfed, desiccated) as compared to the undesiccated control value of 415.9 ± 86.12 U/mg protein (D/Runfed, undesiccated control) (P < 0.005) and that of the trehalose-fed larvae with a value of 1425.5 ± 66.32 U/mg protein (D/Rfed, desiccated) (P < 0.05). Following rehydration, the activity of SOD matched the controls in both the larval groups.
It thus appeared that upon desiccation, the wild-type and the dTps1-downregulated larvae showed an increase in SOD levels to scavenge the O2·− generated during desiccation exposure. However, it is important to note that the trehalose-fed larvae (endogenous + exogenous trehalose) harboured lower levels of SOD in comparison to their unfed (endogenous trehalose only) counterparts. In other words, SOD activity was markedly reduced in the presence of trehalose, suggesting that the degree of SOD activity was largely influenced by the available trehalose pools in the larvae. Thus, in the fed larvae, O2·− scavenging could be achieved majorly by trehalose and, to some extent, by SOD which is the reason why levels of SOD remained significantly low. On the other hand, upon rehydration, trehalose itself is known to undergo hydrolysis (Thorat et al. 2012) and hence one can expect that the declining trehalose concentration (data not shown) signalled the elevation in SOD activity. These observations pointed out a negative correlation between trehalose levels and SOD levels, suggesting the trehalose-dependent recruitment of SOD in the larvae. This interplay between trehalose and SOD levels could be attributed to the cell’s economising strategy to avoid the diversion of energy and resources for unwarranted cellular activities, especially given the hypometabolic state during desiccation. The fact that trehalose and SOD show intermolecular interactions (http://www.ncbi.nlm.nih.gov/Structure/mmdb/mmdbsrv.cgi?uid=4L2D) also lends weight to our findings of their collaborative ability to ameliorate the effects of ROS generation as a result of dehydration.
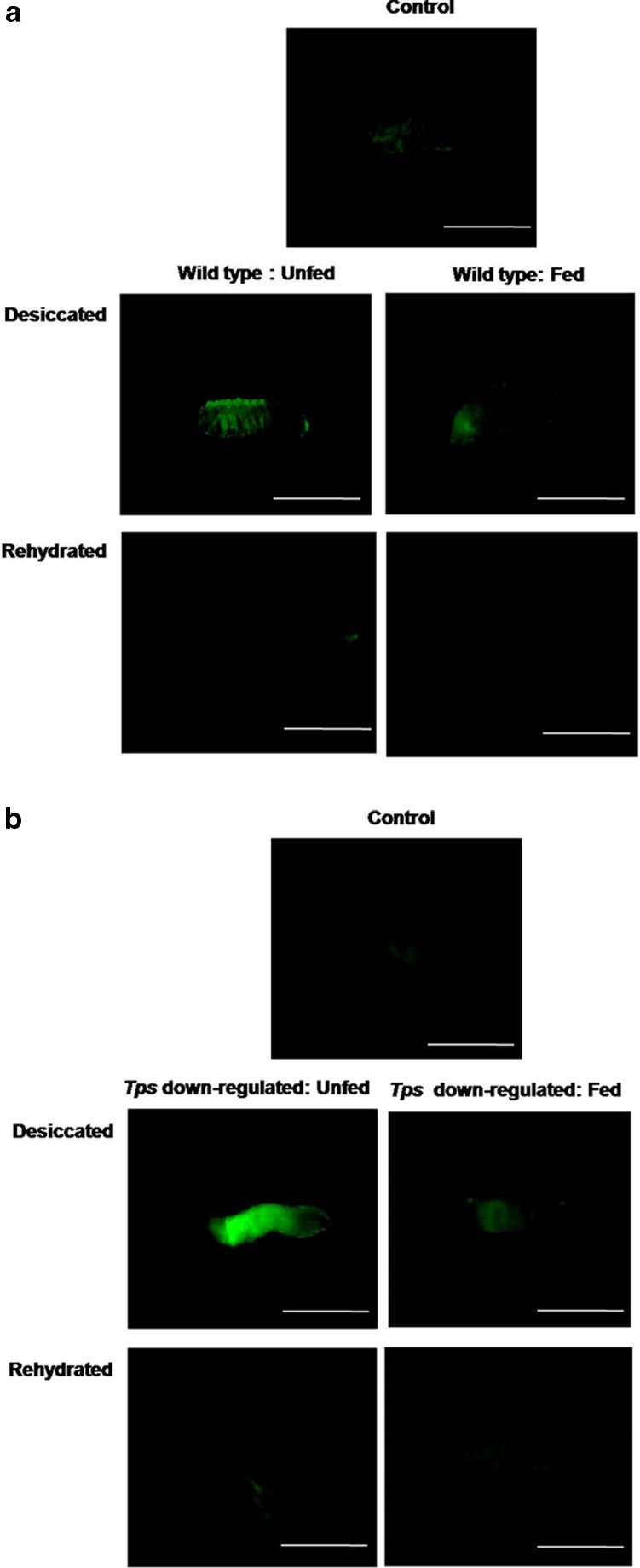
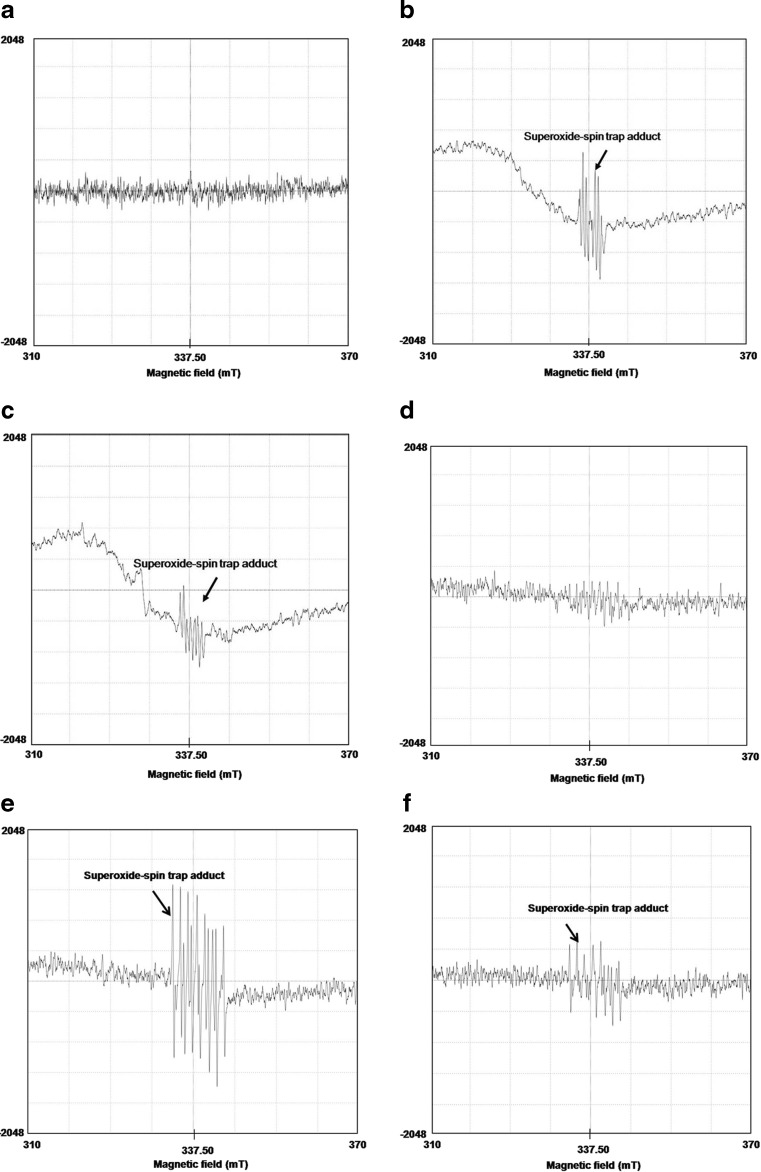
To confirm a role of trehalose as an O2·− scavenger, we investigated the effects of trehalose on scavenging of free radicals by whole larval live imaging. Being short-lived ions, temporal detection of ROS is often a difficult task. Here, we adopted a straightforward and non-invasive approach of whole larval real-time imaging to visualise the presence of ROS. Live imaging of whole larvae confirmed the above results on the basis of fluorescence signals of DCF-DA dye as shown in the video clippings (Online Resource 1–6) wherein higher fluorescence indicated the presence of higher O2·− content. Substantially high fluorescence signal was seen in the unfed wild type (Fig. 4a) at the end of desiccation when compared with undesiccated control and the trehalose-fed larvae. A similar trend was seen in the dTps1-downregulated group wherein the trehalose-fed larvae showed strikingly reduced fluorescence intensity in comparison to the unfed larvae. Rehydration led to the decrease in signal intensities, indicating the decline in O2·− in both the larval groups. To validate these observations, we performed ESR analysis which facilitated the detection of short-lived radicals using the spin trap (DEPMPO) to form long-lived radical spin trap adduct (DEPMPO-OOH) depending on the presence of O2·− in the larvae. The undesiccated wild-type control (Fig. 5a) showed the near absence of the characteristic radical spin trap adduct (337.5 mT). On the other hand, desiccation-responsive O2·− accumulation was reflected from the high peak intensity of the adduct in the unfed larvae (Fig. 5b) whereas the trehalose-fed larvae showed a decline in O2·− levels as evident from the low peak intensity (Fig. 5c). In perfect corroboration with our hypothesis, the unfed dTps1-downregulated larvae displayed a very high radical spin trap adduct peak intensity indicating substantially high O2·− content (Fig. 5e) in comparison to the undesiccated control (Fig. 5d). Again, trehalose feeding resulted in a noticeable decline in the peak intensity of the ESR spectrum (Fig. 5f).
Fig. 4.
Representative images of whole larval live imaging under a fluorescence microscope. a Visualisation of O2 ·− using DCF-DA dye in the control, unfed and trehalose-fed wild-type larvae. b Visualisation of O2 ·− using DCF-DA dye in the control, unfed and trehalose-fed dTps1-downregulated larvae. Bar = 1 mm
Fig. 5.
Electron spin resonance analysis showing a peak corresponding to that of radical spin trap adduct (337.5 mT). a Unfed, undesiccated wild-type control. b Unfed, desiccated wild type. c Fed, desiccated wild type. d Unfed, undesiccated dTps1-downregulated control. e Unfed, desiccated dTps1 downregulated. f Fed, desiccated dTps1-downregulated larvae
Our hypothesis was that larvae expressing the dTps1-RNAi transgene would be enormously vulnerable to ROS accumulation during desiccation and that dietary supplement of trehalose would be able to reduce the ROS content as a redox regulator. Our data on in vivo fluorescence imaging and ESR analysis confirmed the ameliorative effects of exogenous trehalose feeding in the larvae. Notably, the highest fluorescence intensity signal and the highest peak intensity of radical spin trap adduct were seen in the unfed dTps1-downregulated larvae which were indicative of higher O2·− content in the larvae. Thus, dTps1 downregulation led to increased sensitivity to oxidative stress which was also evident from their low desiccation tolerance threshold in contrast to their wild-type counterparts.
Our findings are in agreement with earlier reports which demonstrated the ROS scavenging ability of trehalose under desiccation-inflicted oxidative stress in SOD-deficient yeast cells and plants (França et al. 2007). In fact, in the food industry, trehalose is known to confer protection against oxidative damage by virtue of its SOD-like activity, thereby reducing a number of issues associated with drying and storage of foodstuffs without compromising on their visual appeal and quality (Ohtake and Wang 2011). Under heat stress in wheat, trehalose is known to play a direct role in eliminating O2·− and H2O2 in a concentration-dependent manner (Luo et al. 2008). In vitro studies have provided a mechanistic basis of the antioxidant property of trehalose since the α,α-1,1 linkage of trehalose allows it to take a characteristic gauche conformation which significantly decreases the oxidation of biomolecules (Oku et al. 2003). In animals, the role of enzymatic and non-enzymatic antioxidants has been correlated with ROS generation during desiccation stress. For instance, in the Antarctic midge, B. antarctica, antioxidant enzymes mainly SOD have been implicated in desiccation-responsive antioxidant defence (Benoit and Lopez-Martinez 2012). Observations on desiccation-induced upregulation of specific antioxidants like thioredoxin in the African midge larva, Polypedilum vanderplanki (Cornette et al. 2010); glutathione and glutathione peroxidase in the tardigrade, Paramacrobiotus richtersi (Rizzo et al. 2010); and glutaredoxin in the nematode, Aphelenchus avenae (Browne et al. 2004) have provided evidence for desiccation-responsive oxidative damage in animals. However, none of these studies have provided a direct evidence for the involvement of trehalose in ROS scavenging. The present study thus adds trehalose to the growing list of non-enzymatic antioxidants that ameliorate the harmful consequences of ROS in desiccation-tolerant animals. We conclude that trehalose along with SOD establishes desiccation-responsive redox homeostasis in the larvae of D. melanogaster.
Electronic supplementary material
Live imaging of unfed, undesiccated control wild type larva. (AVI 4032 kb)
Live imaging of unfed, desiccated wild type larva. (AVI 11540 kb)
Live imaging of trehalose fed, desiccated wild type larva. (AVI 2214 kb)
Live imaging of unfed, undesiccated control dTps1 down-regulated larva. (AVI 6982 kb)
Live imaging of unfed, desiccated dTps1 down-regulated larva. (AVI 951 kb)
Live imaging of trehalose fed, desiccated dTps1 down-regulated larva. (AVI 2630 kb)
Acknowledgments
We thank Dr. Takashi Okuda (NIAS, Japan) for his critical advice and suggestions on insect desiccation tolerance. We are extremely grateful to Prof. Alexander Brehm for the kind gift of dTps1-RNAi and GAL4 fly lines. Thanks are also due to the ESR analysis facility provided by SAIF, IIT Bombay, and to Vrindha Vishwanathan for her technical advice. This work was partially supported by UGC-CAS and DST-PURSE grants to the Department of Zoology, SPPU, and UoP-BCUD grants to BBN. LJT acknowledges the financial support received from the Council of Scientific and Industrial Research-Senior Research Fellowship (CSIR-SRF), New Delhi, India. The authors thank the two anonymous reviewers for their valuable comments on our manuscript.
Authors’ contributions
B. Nath, L. Thorat and S. Chatterjee designed the experiments. L. Thorat, K. Mani and P. Thangaraj performed the experiments. L. Thorat, B. Nath and S. Chatterjee analysed the data. L. Thorat and B. Nath wrote the paper with contributions from S. Chatterjee.
References
- Benoit JB, Lopez-Martinez G, Elnitsky MA, Lee RE, Jr, Denlinger DL. Dehydration-induced cross tolerance of Belgica antarctica larvae to cold and heat is facilitated by trehalose accumulation. Comp Biochem Physiol Part A. 2009;152:518–523. doi: 10.1016/j.cbpa.2008.12.009. [DOI] [PubMed] [Google Scholar]
- Benoit JB, Lopez-Martinez G. Role of conventional and unconventional stress proteins during the response of insects to traumatic environmental conditions. In: Tufail M, Takeda M, editors. Hemolymph proteins and functional peptides: recent advances in insects and other arthropods. Oak Park: Bentham Science; 2012. pp. 128–160. [Google Scholar]
- Browne JA, Dolan KM, Tyson T, Goyal K, Tunnacliffe A, Burnell AM. Dehydration-specific induction of hydrophilic protein genes in the anhydrobiotic nematode Aphelenchus avenae. Eukaryot Cell. 2004;3:966–975. doi: 10.1128/EC.3.4.966-975.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, Ma E, Behar KL, Xu T, Haddad GG. Role of trehalose phosphate synthase in anoxia tolerance and development in Drosophila melanogaster. J Biol Chem. 2002;277:3274–3279. doi: 10.1074/jbc.M109479200. [DOI] [PubMed] [Google Scholar]
- Chown SL, Nicolson SW. Insect physiological entomology, mechanisms and patterns. Oxford: University; 2004. [Google Scholar]
- Cornette R, Kanamori Y, Watanabe M, Nakahara Y, Gusev O, Mitsumasu K, Kadono-Okuda K, Shimomura M, Mita K, Kikawada T, Okuda T. Identification of anhydrobiosis-related genes from an expressed sequence tag database in the cryptobiotic midge Polypedilum vanderplanki (Diptera; Chironomidae) J Biol Chem. 2010;285:35889–35899. doi: 10.1074/jbc.M110.150623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datkhile KD, Mukhopadhyaya RM, Dongre TK, Nath BB. Increased level of superoxide dismutase (SOD) activity in larvae of Chironomus ramosus (Diptera: Chironomidae) subjected to ionizing radiation. Comp Biochem Physiol Part C. 2009;149:500–506. doi: 10.1016/j.cbpc.2008.11.003. [DOI] [PubMed] [Google Scholar]
- Echigo R, Shimohata N, Karatsu K, Yano F, Kayasuga-Kariya Y, Fujisawa A, Ohto T, Kita Y, Nakamura M, Suzuki S, Mochizuki M, Shimizu T, Chung U, Sasaki N. Trehalose treatment suppresses inflammation, oxidative stress, and vasospasm induced by experimental subarachnoid haemorrhage. J. Transl. Med. 2012;10:80–93. doi: 10.1186/1479-5876-10-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- França MB, Panek AD, Eleutherio ECA. Oxidative stress and its effects during dehydration. Comp Biochem Physiol Part A. 2007;146:621–631. doi: 10.1016/j.cbpa.2006.02.030. [DOI] [PubMed] [Google Scholar]
- Gutteridge JMC, Halliwell B. Antioxidants: molecules, medicines, and myths. Biochem Biophys Res Commun. 2001;393:561–564. doi: 10.1016/j.bbrc.2010.02.071. [DOI] [PubMed] [Google Scholar]
- Keilin D. The problem of anabiosis or latent life: history and current concepts. Proc R Soc Lond B. 1959;150:149–191. doi: 10.1098/rspb.1959.0013. [DOI] [PubMed] [Google Scholar]
- Kikawada T, Saito A, Kanamori Y, Nakahara Y, Iwata K, Tanaka D, Watanabe M, Okuda T. Trehalose transporter 1, a facilitated and high-capacity trehalose transporter, allows exogenous trehalose uptake into cells. Proc Natl Acad Sci U S A. 2007;104:11585–11590. doi: 10.1073/pnas.0702538104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumarov ES. Detection of free radicals formation in haemolymph of insects by EPR spectroscopy. Appl Magn Reson. 2005;28:411–419. doi: 10.1007/BF03166772. [DOI] [Google Scholar]
- Liua K, Donga Y, Huangb Y, Rasgonc JL, Agrea P. Impact of trehalose transporter knockdown on Anopheles gambiae stress adaptation and susceptibility to Plasmodium falciparum infection. Proc Natl Acad Sci U S A. 2013;110:17504–17509. doi: 10.1073/pnas.1316709110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Luo Y, Li W, Wang W. Trehalose: protector of antioxidant enzymes or reactive oxygen species scavenger under heat stress? Environ. Exp. Bot. 2008;63:378–384. doi: 10.1016/j.envexpbot.2007.11.016. [DOI] [Google Scholar]
- Matsuda H, Yamada T, Yoshida M, Nishimura T. Flies without trehalose. J Biol Chem. 2015;290:1244–1255. doi: 10.1074/jbc.M114.619411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittler R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002;7:405–410. doi: 10.1016/S1360-1385(02)02312-9. [DOI] [PubMed] [Google Scholar]
- Ohtake S, Wang YJ. Trehalose: current use and future applications. J Pharm Sci. 2011;100:2020–2053. doi: 10.1002/jps.22458. [DOI] [PubMed] [Google Scholar]
- Oku K, Watanabe H, Kubota M, Fakuda S, Kurimoto M, Tsujisaka Y, Komori M, Inoue Y, Sakurai M. NMR and quantum chemical study on the OH…pi and CH…O interactions between trehalose and unsaturated fatty acids: implication for the mechanism of antioxidant function of trehalose. J Am Chem Soc. 2003;125:12739–12748. doi: 10.1021/ja034777e. [DOI] [PubMed] [Google Scholar]
- Reyes-DelaTorre A, Peña-Rangel MT, Riesgo-Escovar JR. Carbohydrate metabolism in Drosophila: reliance on the disaccharide trehalose. In: Chang C, editor. Carbohydrates-comprehensive studies on glycobiology and glycotechnology. Winchester: InTech; 2012. pp. 317–338. [Google Scholar]
- Rizzo M, Negroni M, Altiero T, Montorfano G, Corsetto P, Berselli P, Berra B, Guidetti R, Rebecchi L. Antioxidant defences in hydrated and desiccated states of the tardigrade Paramacrobiotus richtersi. Comp Biochem Physiol Part B. 2010;156:115–121. doi: 10.1016/j.cbpb.2010.02.009. [DOI] [PubMed] [Google Scholar]
- Shafiq S, Akram NA, Ashraf M. Does exogenously-applied trehalose alter oxidative defense system in the edible part of radish (Raphanus sativus L.) under water-deficit conditions? Sci. Hortic. 2015;185:68–75. doi: 10.1016/j.scienta.2015.01.010. [DOI] [Google Scholar]
- Shukla E, Thorat LJ, Nath BB, Gaikwad SM. Insect trehalase: physiological significance and potential applications. Glycobiology. 2015;25:357–367. doi: 10.1093/glycob/cwu125. [DOI] [PubMed] [Google Scholar]
- Thorat LJ, Gaikwad SM, Nath BB. Trehalose as an indicator of desiccation stress in Drosophila melanogaster larvae: a potential marker of anhydrobiosis. Biochem Biophys Res Commun. 2012;419:638–642. doi: 10.1016/j.bbrc.2012.02.065. [DOI] [PubMed] [Google Scholar]
- Thorat L, Nath BB. Tolerance to desiccation stress in Chironomus ramosus through an efficient homeostatic control. Eur J Environ Sci. 2015;5:86–91. doi: 10.14712/23361964.2015.81. [DOI] [Google Scholar]
- Tunnacliffe A, Lapinski J. Resurrecting Van Leeuwenhoek’s rotifers: a reappraisal of the role of disaccharides in anhydrobiosis. Phil Trans R Soc Lond B. 2003;358:1755–1771. doi: 10.1098/rstb.2002.1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyatt GR. The biochemistry of sugars and polysaccharides in insects. Adv Insect Physiol. 1967;4:287–360. doi: 10.1016/S0065-2806(08)60210-6. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Live imaging of unfed, undesiccated control wild type larva. (AVI 4032 kb)
Live imaging of unfed, desiccated wild type larva. (AVI 11540 kb)
Live imaging of trehalose fed, desiccated wild type larva. (AVI 2214 kb)
Live imaging of unfed, undesiccated control dTps1 down-regulated larva. (AVI 6982 kb)
Live imaging of unfed, desiccated dTps1 down-regulated larva. (AVI 951 kb)
Live imaging of trehalose fed, desiccated dTps1 down-regulated larva. (AVI 2630 kb)