Abstract
Changes in the levels of three structurally and functionally different important thermoprotectant molecules, namely small heat shock proteins (sHsps), trehalose, and lipids, have been investigated upon heat shock in Schizosaccharomyces pombe. Both α-crystallin-type sHsps (Hsp15.8 and Hsp16) were induced after prolonged high-temperature treatment but with different kinetic profiles. The shsp null mutants display a weak, but significant, heat sensitivity indicating their importance in the thermal stress management. The heat induction of sHsps is different in wild type and in highly heat-sensitive trehalose-deficient (tps1Δ) cells; however, trehalose level did not show significant alteration in shsp mutants. The altered timing of trehalose accumulation and induction of sHsps suggest that the disaccharide might provide protection at the early stage of the heat stress while elevated amount of sHsps are required at the later phase. The cellular lipid compositions of two different temperature-adapted wild-type S. pombe cells are also altered according to the rule of homeoviscous adaptation, indicating their crucial role in adapting to the environmental temperature changes. Both Hsp15.8 and Hsp16 are able to bind to different lipids isolated from S. pombe, whose interaction might provide a powerful protection against heat-induced damages of the membranes. Our data suggest that all the three investigated thermoprotectant macromolecules play a pivotal role during the thermal stress management in the fission yeast.
Electronic supplementary material
The online version of this article (doi:10.1007/s12192-015-0662-4) contains supplementary material, which is available to authorized users.
Keywords: S. pombe, Fission yeast, Heat stress, Small Hsps, Membrane, Lipids, Trehalose accumulation
Introduction
The small heat shock proteins (sHsps) belong to a family of 10–40-kDa proteins that can form large multimeric structures and display a wide range of cellular functions, including the endowment of cells with thermotolerance in vivo and the ability to act as molecular chaperones in vitro. Like other chaperones, sHsps bind partially unfolded polypeptides so that they may retain the capacity to refold. As “holding” chaperones, they protect cells from protein losses or toxicity caused by aggregation, but they must necessarily release their still aggregation-prone clients to other downstream chaperones that facilitate folding. The sHsps are nature’s molecular sponges: their direct temperature modulation is at the heart of the response to heat stress: increased temperature exposes hydrophobic surfaces in such a way that the species populated at high temperatures bind client proteins better (Eyles and Gierasch 2010).
Besides their basic chaperone function, the critical role of sHsps in controlling the physical state, bilayer stability, and integrity of membranes via specific lipid interactions has basically been established (reviewed in (Horváth et al. 2008)). There are numerous indications of the important physiological roles of membrane-associated sHsps (Horváth et al. 2008). They can protect against stress conditions (heat, light, and oxidative stress) in prokaryotes (Lee et al. 1992; Kitagawa et al. 2000, 2002; Nitta et al. 2005; Balogi et al. 2008). unicellular eukaryotes (de Miguel et al. 2005). or higher eukaryotes (Adamska and Kloppstech 1991; Eisenberg-Domovich et al. 1994; Heckathorn et al. 1998; Bausero et al. 2005; de Miguel et al. 2008; Fujita et al. 2011). Whether different Hsps interact only with membrane proteins, only with membrane lipids, or with both remains to be explored. However, it has been proved for some sHsps that their interaction with membranes is lipid dependent and influences the physical properties of the membrane. It has been suggested that a subset of sHsps function in the cellular stress management by acting as membrane-stabilizing factors (Delmas et al. 2001; Zhang et al. 2005; Chowdary et al. 2007; Horváth et al. 2008; Weidmann et al. 2010; Welker et al. 2010). For example, a human small Hsp, Hsp16.2, inhibits stress-induced cell death via stabilization of the mitochondrial membrane system and lipid rafts (Bellyei et al. 2007a, b). Hsp16.2 displays very distinct cholesterol-dependent binding to cholesterol-sphingomyelin Langmuir monolayers (Török et al. 2012). The alternative, membrane-associated regulation of stress protein genes, the membrane sensor model, also highlights the importance of the Hsps in the stabilization of the membrane structure (Vigh et al. 2005, 2007a, b). In this concept, mild stress, or “membrane defects” caused by different stress or pathophysiological conditions, is sensed by changes in the fluidity and/or microdomain structure of the membranes, without inducing a protein-unfolding signal (Vigh et al. 1998, 2007a; Balogh et al. 2005; Nagy et al. 2007; Brameshuber et al. 2010; Gombos et al. 2011).
Schizosaccharomyces pombe contains only two genes of sHsp family, hsp15.8 (also known as hsp20; PomBase ID SPCC338.06c) and hsp16 (SPBC3E7.02c (Wood et al. 2012)). Both sHsps were induced within 15 min, and their messenger RNA (mRNA) levels remained high up to 1 h when cells were subjected to heat stress (Chen et al. 2003; Lackner et al. 2012) Moreover, the hsp16 transcript displayed a strong induction even after a 4-h heat shock (Taricani et al. 2001). “ORFeome” experiments revealed Hsp16 is localized in the cytosol and in the nucleus, while Hsp15.8 is accumulated in the mitochondria when YFP-tagged versions are overexpressed under normal growth conditions (Matsuyama et al. 2006). It was previously shown that both sHsps of S. pombe exert their chaperone function in vitro (Hirose et al. 2005; Sugino et al. 2009).
In addition to the stress proteins, the non-reducing disaccharide, trehalose functions as a thermoprotectant in yeasts and many other organisms (Elbein et al. 2003; Gancedo and Flores 2004). Trehalose can protect either proteins (Singer and Lindquist 1998) or membranes (Crowe 2007) under different stress conditions. In Saccharomyces cerevisiae, the trehalose level is regulated in a complex manner (Eleutherio et al. 2015). but the deletion of the tps1 gene (trehalose-6-phosphate synthase subunit) leads to heat sensitivity both in exponential (Argüelles 1994) and in stationer growth phase (Elliott et al. 1996). It should be noted that trehalose-deficient mutants failed to grow on glucose, indicating metabolomic changes as well (Hohmann et al. 1993). Similar to the budding yeast, trehalose is also accumulated and provided protection against thermal stress in S. pombe (De Virgilio et al. 1990). Deletion of the tps1 gene caused heat sensitivity, while its overexpression provided extra protection against heat stress (Soto et al. 1999). In contrast to S. cerevisiae, fission yeast tps1Δ mutants were able to grow on glucose (Blazquez et al. 1994).
Since both sHsps and trehalose are suggested to protect proteins and cellular membranes, we examined their kinetics of accumulation upon heat stress conditions. We addressed the question whether the deletion of shsp genes affect the trehalose level and vice versa. Finally, we provided experimental data indicating that the cellular lipid composition of the fission yeast changes with the growth temperature, and both sHsps are able to bind to S. pombe lipids and might have membrane protecting effect.
Methods
Strains and growth conditions
S. pombe strains used in this study are listed in Table 1. BRC1 (Shiozaki et al. 1997) and BRC24 (Soto et al. 1999) were used to generate the different mutant strains. Cells were grown at 30 °C in EMM medium supplemented with leucine and uracil according to (Forsburg 2003). Cells in exponential phase (3–5 × 106 cells/ml) were used in all experiments.
Table 1.
S. pombe strains used in this study
| Name | Genotype | Source/Reference |
|---|---|---|
| BRC1 | h−; leu1-32; ura4-D18 | K. Shiozaki (Shiozaki et al. 1997) |
| BRC24 | h+; ade6M216; leu1-32; ura4-D18; tps1:: ura4+; (pREP3X-tps1) | T. Soto (Soto et al. 1999) |
| BRC28 | h−; leu1-32; ura4-D18; hsp15.8::ura4+ | This study |
| BRC29 | h−; leu1-32; ura4-D18; hsp16::ura4+ | This study |
| BRC37 | h−; leu1-32; ura4-D18; hsp15.8::KmR; hsp16::ura4+ | This study |
| BRC39 | h−; leu1-32; ura4-D18; hsp15.8:GFP(KmR) | This study |
| BRC40 | h−; leu1-32; ura4-D18; hsp16:GFP(KmR) | This study |
| BRC43 | h−; leu1-32; ura4-D18; tps1:: ura4+; hsp15.8:GFP(KmR) | BRC24 × BRC39 |
| BRC44 | h−; leu1-32; ura4-D18; tps1:: ura4+; hsp16:GFP(KmR) | BRC24 × BRC40 |
| BRC45 | h−; leu1-32; ura4-D18; tps1:: ura4+ hsp15.8::KmR; hsp16::ura4+ | BRC24 × BRC37 |
Construction of the sHsp mutant strains
Mutants were constructed according to the PCR method described by Krawchuk and Walls (Krawchuk and Wahls 1999). For constructing the null mutants, pBSK(−) plasmid (Stratagene) containing either the 1.8-kb ura4+ fragment of pREP41 (kindly provided by S. Forsburg) or the 1-kb KmR fragment of pKT 127 (Sheff and Thorn 2004) was used as template. Plasmid pKT127 (Sheff and Thorn 2004) served for generating the GFP-tagged mutants. The tps1Δ/shsp:GFP double mutants were obtained by genetic crossing. The primers used are listed in Supplementary Table 1. Transformations were carried out according to the standard protocol (Forsburg and Rhind 2006). The mutants were checked by PCR and genomic Southern analysis.
GFP measurements
Cultures of GFP-tagged strains (BRC39, BRC40) were grown at 30 °C, divided into 35-ml aliquots, and incubated at the indicated temperatures. GFP fluorescence measurements were carried out by Accuri C6 flow cytometer (50,000 events) at every 20 min (200 μl, three parallels per sample). The measured fluorescence intensity (mean FL1-H) values were corrected by subtracting the autofluorescence value of a non-tagged strain (BRC1).
Determination of thermosensitivity
BRC1, BRC28, BRC29, and cells were grown as described above and then subjected to 48 °C for 15–60 min. Samples then were serially diluted (10×) and 10-μl aliquots were spotted onto YES plates and incubated at 30 °C for 4 days.
Measurement of the intracellular trehalose level
An 35-ml aliquot of heat-shocked cells were filtered, washed once with 35 ml ice-cold PBS (137 mM NaCl, 2.7 mM KCl, 4.3 mM Na2HPO4·7H2O, 1.4 mM KH2PO4 pH 7.3) resuspended in 100 μl of PBS, and disrupted in Bullet Blender (Next Advance Inc.) with 0.5-mm zirconium oxide beads (3 min, speed 8, 4 °C). Beads were washed 2× with 200 μl PBS, and 100 μl of the lysates were boiled for 10 min and centrifuged at 10,000×g for 5 min. Trehalase digestion of 25 μl lysates were carried out in 100 μl 135 mM citrate buffer (pH 5.6) with 1.15 mU trehalase (Sigma-Aldrich) at 37 °C, overnight. Glucose determinations were carried out by adding 200 μl of Assay Reagent (GO Assay kit, Sigma-Aldrich), and samples were incubated at 37 °C for 30 min. Reactions were stopped by the addition of 200 μl 12 N sulfuric acid, and the absorbance (560 nm) of 200 μl of the mixtures was determined by Multiskan EX (Thermo Scientific) plate reader. Trehalose and glucose solutions (25–100 μg/ml) were used as standards. Protein levels of the samples were measured according to (Lowry et al. 1951).
Overexpression and purification of Hsp15.8 and Hsp16 proteins
Both shsp genes of S. pombe were overexpressed in Escherichia coli and purified with a modified protocol of Chowdary et al. (Chowdary et al. 2007). The hsp16 and hsp15.8 genes were amplified with genomic DNA as a template using the following primers containing NdeI and BamHI sites (underlined):
hsp16.0_cl_fw: ATTTTAACATATGTCTTTGCAACC
hsp16.0_cl_rev: GGAAGAGAGGATCCTTACTTAATAGC
hsp15.8_cl_fw: CATTTCATATGTTGTTTGATGC
hsp15.8_cl_rev: GTATTGGATCCTACTGAATTTCAAC
The PCR reaction was carried out with High-Fidelity PCR Enzyme Mix (Fermentas) according to the manufacturer’s instructions. The amplified fragments were cloned into the NdeI and BamHI sites of the pET11a(+) vector (Novagen), and the resulted clones were verified by sequencing. E. coli BL21 Codon + RIL were transformed with the pET11a(+) vectors containing hsp16 and hsp15.8. The transformed cells were cultured in a 200-ml LB medium (containing 35 μg/ml chloramphenicol and 100 μg/ml ampicillin) at 37 °C. Protein expression was induced at OD600 = 0.6 with 0.5 mM isopropyl β-d-1-thiogalactopyranoside (Sigma-Aldrich). After 4 h of induction, cells were harvested by centrifugation at 4000×g for 10 min at 4 °C and were frozen at −20 °C. Frozen cells (~0.5 g bacteria) were resuspended in 15 ml TNE buffer (100 mM Tris/HCl pH 8.0, 100 mM NaCl, 2 mM EDTA) containing 1:1000 (v/v) Protease Inhibitor Cocktail (Sigma-Aldrich) and were disrupted on ice by sonication (TYPE UM-1, techpan) until the lysates become clear. The suspension of disrupted cells was centrifuged at 18,000×g for 15 min at 4 °C. The supernatant was precipitated by adding ammonium sulfate (slowly, under continuous stirring at 4 °C) to obtain concentrations of 67 % for Hsp16 and 40 % for Hsp15.8. After stirring for 30 min, the suspension was centrifuged again at 17,000×g for 15 min at 4 °C, and the resulting supernatant was precipitated again with final ammonium sulfate concentrations of 100 % for Hsp16 and 67 % for Hsp15.8. The suspension was centrifuged at 17,000×g for 15 min at 4 °C. The pellet was redissolved in TNE buffer and dialyzed o/n against Tris/HCl pH 7.6 buffer. After dialysis, the Hsp16 was sufficiently pure for monolayer experiments. Protein concentration was measured with micro BCA Protein Assay Kit (Thermo Scientific). In the case of Hsp15.8, the dialyzed solution was applied on a Resource Q anion-exchange column (Amersham Pharmacia) and eluted with a linear gradient from 20 mM Tris/HCl pH 7.6 to 20 mM Tris/HCl pH 7.6, 1 M NaCl at a flow rate of 1 ml/min. The fraction containing Hsp15.8 (around 0.6 M NaCl) was dialyzed o/n against Tris/HCl pH 7.6 buffer. From 100 ml of culture, we obtained 37 mg of Hsp16 and 22 mg of Hsp15.8. Purified protein was analyzed by SDS- and non-denaturing PAGE (on 15 % and 6 % gels, respectively) and visualized by staining with Coomassie Brilliant Blue.
Extraction, separation, and fatty acid analysis of S. pombe lipids
For monolayer experiments and for fatty acid (FA) analysis, lipids were extracted from 500 ml exponential S. pombe culture grown at 30 °C or at 36 °C. Cells were washed two times with 250 ml PBS, resuspended in 10 ml PBS, and disrupted with a French press (20,000 psi, two times, AMINCO, Silver Spring, MD, USA).
Extraction of lipids from the cell lysate and the separation of total polar lipid (TPL) fraction was done according to Balogh et al. (Balogh et al. 2010). Polar phospholipid classes (phosphatidylcholine (PC), phosphatidylethanolamine (PE), phosphatidylinositol (PI), phosphatidylserine (PS), phosphatidylglycerol (PG), and cardiolipin (CL)) were separated from lipid extract (corresponding to 5 ml cell lysate) on Kieselgel 60 silica gel TLC plates in two steps. First, plates were developed to two thirds with chloroform/methanol/glacial acetic acid/water/n-hexane (60:40:2:2:3, vol:vol:vol:vol) and, after drying, with n-hexane/diethylether/acetic acid = 85:25:2 (vol:vol:vol) to the top. To localize the lipid classes, a thin stripe on the edges of the plates was visualized with 8-anilinonaphthalene-1-sulfonate (0.05 % in methanol/water = 1:1, vol:vol) under UV and identified with the use of authentic standards. For FA analysis, aliquots of TPL and each lipid classes were methylated and analyzed by GC-MS as in Balogh et al. (2010).
Monolayer experiments
Monolayer experiments were carried out with a KSV3000 Langmuir-Blodget instrument (KSV, Finland) in a mini Teflon trough (V = 6.5 ml) at room temperature (Török et al. 1997). Surface pressure was measured by the Wilhelmy method, using a platinum plate. Monolayers of different lipids, extracted from S. pombe cells grown at 30 or 36 °C, were spread from chloroform solution at a different initial surface pressure on a PBS subphase (pH 7.2) which was continuously stirred with a magnetic bar. After the monolayer had reached an equilibrium pressure (5–10 min), 1 μM protein was injected underneath the monolayer and the surface pressure was monitored.
Steady-state fluorescence anisotropy
Large unilamellar vesicles (LUVs) were prepared in PBS by the extrusion technique using a Liposofast extruder (Avestin, Ottawa, Canada), with two stacked polycarbonate filters with a pore size of 200 nm, as previously described (Török et al. 1997). LUVs were labeled by adding diphenyl-hexatriene (DPH) probe directly to the lipids in organic solvent before the lipid film was dried. The lipid-to-probe molar ratio in the liposome solution was 500:1. Fluorescence anisotropy was measured on a T-format fluorescence spectrometer (Quanta Master QM-1, Photon Technology International, Princeton, NJ). Excitation and emission wavelengths were 360 and 430 nm, respectively (5-nm slits). Temperature was controlled by a circulating water bath. sHsps were added to the LUV suspension (50 μM lipid) with continuous stirring.
Results
Hsp16 and Hsp15.8 are induced by heat shock
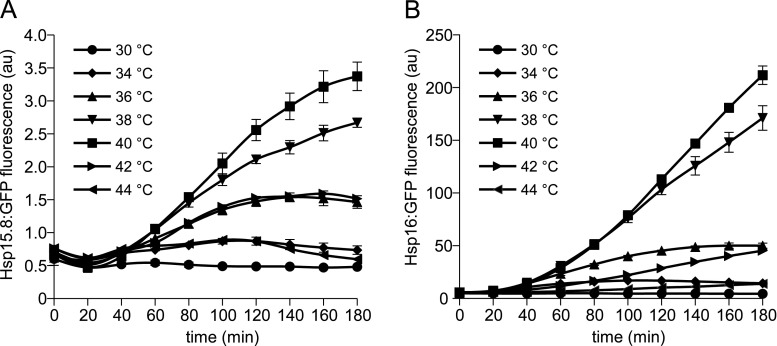
In order to study the inducibility of the two sHsps during heat stress, chromosomal hsp16:GFP and hsp15.8:GFP fusion constructs were made. Cells in the exponential growth phase were subjected to different temperatures, and the kinetics of Hsp16:GFP and Hsp15.8:GFP synthesis was followed by measuring fluorescence intensity similarly as described in Taricani et al. (2001). Mild heat shock (34 and 36 °C) caused a very slight induction of both sHsps compared to more severe heat stress (38 and 40 °C, Fig. 1). The level of both sHsps reached a plateau within 2 h under mild heat stress conditions. Incubation at 38 °C and 40 °C resulted in continuously increasing sHsp:GFP protein levels throughout the heat treatment (Fig. 1). Despite the similarities of the heat induction kinetics of the two sHsps, it should be noted that the cellular level of Hsp16 is much higher at all temperatures and its maximum amount (at 40 °C, 180 min) is approximately 60-fold higher than that observed in the case of Hsp15.8 (Fig. 1a, b). Interestingly, at the highest tested temperatures (42 and 44 °C), the fluorescence intensities drop down to the levels similar to that observed upon mild heat stress conditions. Since viability was not affected after 180-min incubation at 44 °C (Supplementary Fig. 1), we assume that this phenomenon might be coupled to the impairment of the de novo protein synthesis (Ribeiro et al. 1997).
Fig. 1.
Induction of the small Hsps during heat shock. a hsp15.8:GFP and the b hsp16:GFP S. pombe cells were grown at 30 °C until exponential phase, aliquoted, and subjected to the indicated temperatures for 3 h. Samples were taken every 20 min for flow cytometry analysis
Effects of sHsps on the thermotolerance of S. pombe
To investigate the roles of sHsps in the thermotolerance of S. pombe, mutants were generated that lacked hsp15.8, hsp16, or both. Since the wild-type and the null mutant cells displayed only a slight difference in thermosensitivity when incubated at 46 °C for 1 h (100 % vs. 75 ± 15 % survival; data not shown), their viability were compared after exposing cells to 48 °C heat shock for 15–60 min. The spot tests indicated that the mutants showed higher heat sensitivity than the wild-type cells especially after 30–45-min heat stress (Fig. 2a). Since the induction of the sHsps tends to decrease drastically above 40 °C, change in either the function and/or the localization of the pre-existing proteins should be involved in the acute thermal stress tolerance. When cells were heat shocked at 44 °C, Hsp15.8:GFP virtually did not change its localization. However, Hsp16:GFP displayed significant intracellular redistribution after 5-min incubation at 44 °C (Fig. 2b, lower panel). The “dot-like” arrangement of the Hsp16:GFP closely resembles structures observed in the case of Golgi-associated proteins (Matsuyama et al. 2006).
Fig. 2.
Thermosensitivity of the sHspΔ mutants and intracellular localization of Hsp15.8 and Hsp16. a Cells were grown at 30 °C and subjected to 48 °C for the indicated times, then tenfold serial dilutions were spotted onto YES plates. b Intracellular localization of sHsps during heat stress. Cells were incubated at 44 °C for the indicated time and images were taken using Leica TCS SP8 confocal microscope. Scale bars represent 10 μm
Heat induction of the sHsps are altered in trehalose-deficient S. pombe cells, but the trehalose levels are not affected in the shsp mutants
It is well documented that trehalose6-P-synthase (tps1Δ) mutants are unable to accumulate trehalose during heat shock and are sensitive to heat stress (De Virgilio et al. 1990; Ribeiro et al. 1997). Therefore, we examined whether the heat-induced expression of the sHsps is affected in the tps1Δ mutants. Alterations of the induction profiles are demonstrated on samples incubated for 120 min at temperatures indicated (Fig. 3a; for more time points of tps1Δ mutant cells, see Supplementary Fig. 2a). The most obvious common feature of both sHsps is that their maximum induction temperature is lower in tps1Δ background (approx. 38 vs. 40 °C). The kinetic profiles up to 38 °C were very similar to those observed in the wild-type cells. Interestingly, the intracellular level of Hsp16 and Hsp15.8 tends to be lower in the tps1Δ cells at high temperatures (e.g., by 30 and 40 % at 30 °C). Whether it is the direct consequence of trehalose deficiency or due to other effect(s) of tps1 mutation needs to be investigated. Since more than 90 % of the mutants survived the heat treatment (Supplementary Fig. 2b), the decreased induction rate above 38 °C is probably the consequence of the reduced de novo protein synthesis. Next we examined whether the heat-induced accumulation of trehalose at 40 °C is influenced by the loss of sHsps. Surprisingly, the levels of the disaccharide in the shsp mutant cells were similar to that found in wt cells at all time points examined (Fig. 3c).
Fig. 3.
Accumulation of thermoprotectants in different S. pombe mutants during heat shock. Induction of a hsp15.8:GFP and b hsp16:GFP in wt and tps1Δ mutant cells are sHsp mutant S. pombe strains were determined following an incubation at 40 °C for the indicated times(two-tailed t test, n = 3, *p < 0.05, **p < 0.01, ***p < 0.001). c Accumulation of trehalose in wt and sHsp mutants following a 1-h heat shock at 40 °C
Membrane lipid composition of S. pombe cells grown at different temperatures
According to the principle of homeoviscous adaptation, living organisms are able to cope with heat by adjusting the physical properties of their membranes when propagated at higher, but still tolerable, growth temperatures. These changes resulted in the reduction in heat-induced membrane hyperfluidization through decreased FA unsaturation, or stabilization of the bilayer phase through decreasing the amount of the non-bilayer forming lipid classes such as PE (Hazel and Williams 1990) or by interaction with chaperone proteins (Török et al. 1997; Tsvetkova et al. 2002). In order to test whether S. pombe is able to use these tools, we first analyzed the lipid and FA composition of cells grown at normal (30 °C) and at a higher (36 °C) temperature (Table 2). The overall membrane unsaturation level can be characterized by the double bond index/saturated FAs (DBI/sat). DBI/sat proved to be significantly lower at higher growth temperature, as indicated by the values calculated for the total polar lipids (TPL) (3.8 and 2.9 for 30 and 36 °C, respectively; Table 2), demonstrating that the membranes are enriched in saturated FAs at higher temperatures. Apart from the overall decrease in DBI/sat, the individual polar lipid classes displayed quite different contributions. PC and PE were the most unsaturated (i.e., the highest DBI/sat values) phospholipids, thereby possessing a stronger ability to adjust their FA chain composition to adapt to higher temperatures. Indeed, with the ca. 30 % decrease in the DBI/sat values, the unsaturation level of these lipid classes was affected to a great extent at elevated growth temperature. Furthermore, S. pombe not only used the FA chain saturation as a tool to increase the rigidity of its membranes at higher temperature, but the ratio of PE, a non-bilayer forming lipid, was also reduced from 20 to 16 %. These alterations could reflect the striving of the organism to adapt the dynamics of the membrane-associated processes to the altered environmental conditions.
Table 2.
Fatty acid composition of total polar lipids and different phospholipids and phospholipid compositions of S. pombe grown at either 30 or 36 °C
| Growth temp. (°C) | TPL | PC | PE | PI | PS | PG + CL | |
|---|---|---|---|---|---|---|---|
| 16:0 | 30 | 14.8 ± 0.4 | 7.4 ± 0.4 | 11.2 ± 0.3 | 28.5 ± 1.0 | 24.4 ± 0.2 | 13.9 ± 0.8 |
| 36 | 18.4 ± 1.0 | 11.6 ± 1.6 | 16.3 ± 0.1 | 30.2 ± 0.8 | 27.2 ± 0.1 | 17.5 ± 1.0 | |
| 16:1 | 30 | 1.2 ± 0.1 | 0.5 ± 0.8 | 2.6 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 1.0 ± 1.4 |
| 36 | 1.3 ± 0.0 | 0.6 ± 0.8 | 3.1 ± 0.8 | 0.3 ± 0.5 | 0.0 ± 0.0 | 1.1 ± 1.6 | |
| 18:0 | 30 | 5.6 ± 0.5 | 4.6 ± 0.7 | 2.2 ± 0.4 | 7.9 ± 1.0 | 7.0 ± 0.7 | 12.3 ± 0.6 |
| 36 | 7.3 ± 0.4 | 6.3 ± 1.5 | 3.6 ± 1.1 | 8.9 ± 0.1 | 7.8 ± 0.3 | 14.0 ± 0.4 | |
| 18:1 | 30 | 77.9 ± 0.1 | 87.4 ± 0.3 | 84.0 ± 0.7 | 63.6 ± 2.0 | 68.6 ± 0.5 | 58.1 ± 3.6 |
| 36 | 72.4 ± 0.8 | 81.5 ± 2.3 | 77.0 ± 0.3 | 60.5 ± 1.1 | 64.9 ± 0.2 | 49.6 ± 2.7 | |
| 30:0 | 30 | 0.5 ± 0.1 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 14.7 ± 5.2 |
| 36 | 0.6 ± 0.3 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 17.8 ± 4.9 | |
| DBI/sat | 30 | 3.8 ± 0.0 | 7.3 ± 0.7 | 6.5 ± 0.4 | 1.7 ± 0.2 | 2.2 ± 0.0 | 1.5 ± 0.3 |
| 36 | 2.9 ± 0.1* | 4.6 ± 0.5* | 4.0 ± 0.3* | 1.6 ± 0.0 | 1.9 ± 0.0* | 1.0 ± 0.2 | |
| Lipid class (%) # | 30 | n/a | 38.7 ± 2.7 | 20.4 ± 1.8 | 25.6 ± 3.1 | 7.4 ± 1.3 | 4.4 ± 1.0 |
| 36 | n/a | 40.9 ± 5.2 | 16.4 ± 1.6 | 27.0 ± 4.2 | 7.0 ± 0.6 | 4.9 ± 0.8 |
S. pombe lipids were separated on TLC and analyzed by GC-MS (see “Methods”). FA data are expressed as weight % of total FAs present in each lipid classes and presented as means ± SD (n = 3). DBI/sat, double bond index/saturated FAs, was calculated as the ratio of the sum of the weight % of unsaturated FAs multiplied by the number of double bonds for each FA and the sum of the weight % of saturated fatty acids. The lipid composition was determined by GC-MS of the FA of each PL, using an internal standard and is given as a % of the total PL
n/a not applicable
*Values significantly different from the corresponding data at 30 °C (p < 0.05); #The difference between the sum of polar lipid class percentages and 100 % represents the sum of minor components (3–4 %) not given in the table
Interaction of the S. pombe sHsps with phospholipid monolayers
Besides the well-established adaptive remodeling of membranes involving changes in the extent of lipid unsaturation and ratio between lipid classes, it was shown that association between sHsps and membranes may constitute a general mechanism that preserves membrane integrity during thermal fluctuations (Tsvetkova et al. 2002). To explore the possible interaction of sHsps with lipids, the proteins were expressed in E. coli and purified to homogeneity (Hsp16) or to 95 % purity (Hsp15.8) (Fig. 4a). For hsp15.8, we detected a single ~100 kDa species on non-denaturing gel, suggesting a hexamer complex of this protein and a major band at around 200 kDa (possibly 12-mer) and a faint band at 400 kDa (24-mer) for Hsp16 (Fig. 4b). The interaction of the purified Hsps with lipid membranes was studied with Langmuir lipid monolayers. The proteins were injected into the aqueous phase beneath a phospholipid monolayer made of S. pombe lipids, the surface area of which was kept constant, and the surface pressure was monitored. To minimize the possibility of incomplete mixing and the formation of patches of pure protein in the monolayer, lipids were spread to a surface pressure greater than the equilibrium spreading pressure of the protein at an air-water interface, 22 mN/m. Thus, an increase in surface pressure indicates that the protein specifically interacts with and/or inserts into the lipid monolayer. Both sHsps interacted in a concentration-dependent manner with monolayers made of TPL extracts of S. pombe grown at 30 °C (Fig. 4c); however, Hsp15.8 exhibited a much higher affinity toward this monolayer.
Fig. 4.
Interaction of purified S. pombe sHsps with lipids. a SDS-PAGE and b non-denaturing PAGE analysis of sHsps. c Surface pressure increase of TPL30 (total phospholipid of S. pombe grown at 30 °C) monolayers upon interaction with increasing concentration of Hsp15.8 and Hsp16 at an initial surface pressure of 22 mN/m
The ability of a surface-active molecule to penetrate a lipid monolayer depends on the initial surface pressure (Verger and Pattus 1982). The critical pressure for insertion (CPI) provides information on the membrane binding ability of proteins. This value corresponds to the surface pressure up to which the protein can insert into the monolayer. CPI is obtained by measuring the pressure increase at different initial surface pressures and extrapolating to a pressure increase of zero. The CPI value of Hsp15.8 and Hsp16 with TPL extracts of S. pombe grown at 30 °C (TPL30) monolayers was calculated to be 29.6 and 27.9 mN/m, respectively (Fig. 5a), meaning again that Hsp15.8 has a higher affinity to this monolayer. The interactions of Hsp15.8 were almost identical for all of the major lipid classes (PI < PS ≈ PC ≈ PE ≈ PG + CL), whereas for Hsp16, the CPI values were highly lipid specific showing an affinity order of PS < PI < PE < PG + CL < PC. Interestingly, both proteins induced smaller surface pressure increases with significantly lower CPI values for total polar lipid extracts than for any individual lipid class (Fig. 5b). This phenomenon may indicate that the packing density in the lipid mixture is higher than that in the pure lipid monolayer.
Fig. 5.
Lipid-specific interaction of sHsps with monolayers. a Surface pressure increase after injection of Hsp15.8 and Hsp16 underneath TPL30 monolayers at different initial pressures. The protein concentration in the subphase was 1 μM. b Critical insertion pressures for Hsp15.8 and Hsp16 above which the proteins are no longer able to insert into monolayers of polar phospholipids (isolated from cells grown at 30 °C): phosphatidylcholine (PC), phosphatidylglycerol + cardiolipin (PG + CL), phosphatidylinositol (PI), phosphatidylserine (PS), phosphatidylethanolamine (PE), and total polar lipids extracted either from 30 °C (TPL30) or from 36 °C (TPL36) grown cells. Critical pressures for insertion were determined by extrapolating the experimental data through linear regression analysis to the initial surface pressure resulting in zero pressure increase (as shown in a)
sHsps elevate the rigidity (thermostability) of bilayers made of S. pombe polar lipids
To estimate the contribution of sHsps to the overall fluidity and thereby the stability of lipid bilayers, fluorescence anisotropy measurements were carried out at 40 °C corresponding to heat shock. Increasing amounts of proteins were added to large unilamellar vesicles (LUVs) made of TPL of S. pombe grown at 30 °C (TPL30) or at 36 °C (TPL36) and labeled with the hydrophobic probe (DPH; Fig. 6). The fluorescence anisotropy of LUVs made of TPL36 was higher than that of LUVS made of TPL30 (Fig. 6, 0 μM), indicating that the lipid changes described above resulted in a lower membrane fluidity for cells grown at higher temperatures. The addition of both Hsp15.8 and Hsp16 made the membranes less fluid in a protein concentration-dependent manner, and the extent of protein-induced fluidity change was dependent on the lipid composition of the vesicles (Fig. 6 and Table 2). Interestingly, in the case of Hsp15.8, the rigidifying effect of the protein above 2 μM was higher on the more fluid LUV, which is in accordance to the result gained by monolayer studies, while Hsp16 did not differentiate between the more or less fluid LUVs. This difference between the two sHsps was especially evident in the case of TPL30 LUVs in which the lipids are more fluid (Fig. 6a).
Fig. 6.
Membrane stabilization by S. pombe sHsps. Fluorescence anisotropy of DPH was measured at 40 °C as a function of sHsp concentration. a Hsp15.8 and b Hsp16 were added to LUVs made of total polar lipid extracts from S. pombe cells grown at 30 °C (TPL30) or at 36 °C (TPL36)
Discussion
In the current study, S. pombe was applied as a model to address the question whether sHsps, trehalose, and cellular lipids play a defensive role during heat stress and the sHsps could have evolved to serve as alternative membrane-stabilizing tools. Vegetatively growing cells contain approx. 2–40 times more copy of Hsp16 than Hsp15.8 (Marguerat et al. 2012; Carpy et al. 2014). Based on fluorescence intensity measurements, we found circa tenfold difference which is in a good agreement with the above cited data (Fig. 1). Protein levels started to increase after 20 min of heat stress reaching a moderate expression level at 60 min (Hsp15.8: 1.2-fold, Hsp16: 5.1-fold increase at 40 °C), similar to Hsp70 and Hsp104 induction in S. cerevisiae (Lee and Goldberg 1998) (Fig. 1). A common feature of Hsp15.8 and Hsp16 induction is that they are both very moderately induced upon mild heat stress (34 and 36 °C) that are unlikely to cause any protein denaturation in the cell (Park et al. 2005; Balogh et al. 2013) reaching a plateau after incubation for 100 min at these temperatures (Fig. 1). Incubation at higher temperatures (38 and 40 °C) led to continuous increases in the protein levels of both sHsps, even in the third hour of heat treatment. There is a significant difference in the rate of induction of the two sHsps: the maximum induction of Hsp15.8 is about 2.5-fold (40 °C, 3 h), while that of Hsp16 under the same conditions is more than 30-fold, suggesting different physiological functions. Hsp16 has been shown to be involved in the protection of nuclear mRNA transport under heat stress (Yoshida and Tani 2005). while Hsp15.8 has been shown to be located in the mitochondria under conditions tested. It might be speculated that Hsp15.8 is involved in the protection of the mitochondria under stress conditions (mitochondrial membranes?). This question is currently under examination in our laboratory. Irrespective of their functions, neither of these sHsps is essential, since the removal of their genes did not cause obvious phenotype under normal conditions (30 °C); however, these mutations caused a difference in the heat sensitivity: both mutants proved to be more heat sensitive than the wild-type strain (Fig. 2a). It should also be noted that the heat sensitivity of the two shsp mutants is much less (at least five times (De Virgilio et al. 1990). Glatz A, unpublished) than that observed in the trehalose synthase mutant S. pombe (tps1Δ), indicating the important role of metabolites during thermal stress as recently has been described for baker’s yeast (Gibney et al. 2013). Their different localization and heat induction rates might indicate that the two α-crystallin-type chaperones of S. pombe have distinct physiological functions. A correlation between intracellular trehalose content and thermotolerance has been confirmed in S. pombe (De Virgilio et al. 1990; Ribeiro et al. 1997; Soto et al. 1999). Due to its protective role under different stress conditions, trehalose was considered as a “chemical chaperone” (Crowe 2007). therefore, one should suppose that sHsps and trehalose might have a common target to protect. However, according to our results, the importance of trehalose is superior to that of sHsps at least during the first hour of the heat stress. The heat-induced accumulation of trehalose was independent of the presence of any of the two sHsps (Fig. 3c). It has to be mentioned that the time course of the trehalose and sHsp accumulation is different: the disaccharide content of the cells reached its maximum at 40 °C in 40–60 min when the inductions of the sHsps are still barely detected. Our data support the previous finding that trehalose protection is important at the early stage of the thermal stress, while elevated sHsp levels are required in a later phase (Ribeiro et al. 1997). On the other hand, the altered timing might also suggest the presence of two different (an “early” and a “late”) sensors and/or pathways in fission yeast. However, these observations might not rule out the existence of a common heat-sensitive target (protein?, membrane?). For an evaluation of the potential contributions of these thermoprotective factors, it is important to study their effects on and the interactions with the cellular targets of heat-induced damage. With a view to dissecting the roles of membranes and sHsps in the thermal stability of S. pombe, a detailed analysis of the membrane lipids was carried out for cells adapted to different temperatures. S. pombe not only made the use of the FA saturation as a tool to increase the rigidity and thereby the stability of its membranes at higher temperature but also reduced the ratio of PE, a non-bilayer forming lipid from 20 to 16 % (Table 2). These alterations could promote the adaptation of the membrane-associated processes to the altered environmental conditions. With increasing growth temperature, the enhanced saturation in the main lipid classes, together with the decreased level of the non-bilayer forming PE, could counterbalance the harmful membrane-disordering effect of heat.
Small Hsps can regulate membrane physical properties in vitro, thereby preventing the formation of “hyperfluid” states (Tsvetkova et al. 2002). Thus, we propose that sHsps associate reversibly with membrane lipids in vivo and, by regulating membrane fluidity, preserve membrane structure and integrity during the initial stages of stress conditions. Further, we suggest that this stabilization precedes the thermal adaptation that occurs by adjustment of the lipid composition. We propose that membrane domains may act as sensors where stress-induced membrane perturbations are transduced as a signal leading to activation of heat-shock genes. The association of sHsps with membranes causing increased molecular order may lead to downregulation of the gene expression. Such “crosstalk” between the primary stress sensor in the membranes and sHsps suggests a feedback mechanism in the regulation of heat-shock genes and provides a model for sHsp function. We tested the interaction of purified S. pombe sHsps with lipids with Langmuir monolayers. For these studies, the proteins were overexpressed in E. coli and purified to homogeneity. On “non-denaturing” gels, they formed hexamers (Hsp15.8) and dodecamers (Hsp16) (Fig. 4a). The molecular mass of the Hsp15.8 oligomer was earlier found by using size exclusion chromatography–multiangle light scattering (SEC–MALS) to be about 200 kDa corresponding to a 12-mer (Sugino et al. 2009). It is difficult to examine the precise oligomeric size of Hsp15.8 by chromatography due to the strong adhesive nature of Hsp15.8 (Sugino et al. 2009). The molecular weight of Hsp16 oligomers measured with SEC–MALS was 250 kDa (Hirose et al. 2005). The different molecular masses of 12-mers and 16-mers measured earlier by SEC-MALS (Hirose et al. 2005; Sugino et al. 2009) could be a result of the differences in methodology, though the molecular mass observed for Hsp16 in our study was strengthened by the appearance of higher order oligomers with double apparent size on non-denaturing gels (Fig. 4a).
Both sHsps interacted with monolayers made of total polar lipid extracts or the individual lipid classes of S. pombe, but Hsp15.8 exhibited much higher affinity toward lipids (Fig. 4a). The interaction of Hsp15.8 was not specific for the lipid composition, but in the case of Hsp16, the CPI values were highly lipid specific, with the affinity sequence of PS < PI < PG + CL < PC. Interestingly, both proteins induced smaller surface pressure increase with significantly lower CPI values for total polar lipid extracts than for any individual lipids (Fig. 5b).
CPI values similar to that of Hsp15.8 have been reported for membrane-associated proteins such as colicin A, rat apolipoprotein AI, LamB signal peptide of E. coli (Briggs et al. 1985). E. coli GroEL (Török et al. 1997). mitochondrial presequences (Török et al. 1994), and mitochondrial creatine kinase (Rojo et al. 1991). To test whether the lipid interacting sHsps could stabilize the bilayer, membrane fluidity measurements were carried out in LUVs made of S. pombe lipids. This organism adapted to temperature, as expected from the different levels of FA unsaturation and the principle of homeoviscous adaptation: the fluidity of LUVs made of TPL36 was significantly lower than in the case of TPL30 indicating that the cells strive for lower membrane fluidity at higher temperatures (Fig. 6). Both sHsps decrease membrane fluidity; however, Hsp15.8 interacted much more strongly with the more fluid lipid bilayer, indicating that this protein may “intelligently” sense the fluidity of the bilayer and stabilize it at high temperature (Fig. 6a). Taken together, the sHsps of S. pombe are interacting with lipids in a lipid- and fluidity-dependent manner. While Hsp15.8 revealed a much stronger affinity, Hsp16 interacted with lipids with higher specificity. The experiments summarized above highlight that both Hsp16.0 and Hsp15.8 in S. pombe could have an important moonlighting function during heat stress. Their interaction with membranes could help in their classical chaperoning function to rescue membrane-associated heat-denatured proteins, but they could also stabilize the structure of the bilayer during heat stress. We observed that the interactions of Hsp15.8 with lipid monolayers and bilayers were driven by the pre-existing fluidity of the lipids, thereby making this chaperone an excellent candidate for the control and stabilization of stress-induced perturbation in the membrane.
Electronic supplementary material
Primers used to generate the different S. pombe mutants. (DOCX 13 kb)
Survival of GFP-tagged shsp mutants. Spot tests of both shsp-mutants were carried out before and after incubation at 44 °C for 180 min as described in Methods. (GIF 41 kb)
Heat induction of sHsps in tps1Δ cells (a) and survival of the shsp:GFP/tps1Δ mutants after the heat treatments (b). Experiments were performed as described in Methods. (GIF 95 kb)
Acknowledgments
This work was supported by the Hungarian Basic Research Fund (OTKA NN111006, NK100857, ANN112372), by the “Social Renewal Operational Programme” of the Hungarian National Development Agency (TÁMOP-4.1.1.C-13/1/KONV-2014-0001), and by the European Union and the State of Hungary and co-financed by the European Social Fund in the framework of TÁMOP-4.2.4.A/ 2-11/1-2012-0001 “National Excellence Program” and from the New Szechenyi Plan, GOP (GOP-1.1.1-11-2012-0452).
References
- Adamska I, Kloppstech K. Evidence for the localization of the nuclear-coded 22-kDa heat-shock protein in a subfraction of thylakoid membranes. Eur J Biochem. 1991;198:375–381. doi: 10.1111/j.1432-1033.1991.tb16025.x. [DOI] [PubMed] [Google Scholar]
- Argüelles JC. Heat-shock response in a yeast tps1 mutant deficient in trehalose synthesis. FEBS Lett. 1994;350:266–270. doi: 10.1016/0014-5793(94)00786-1. [DOI] [PubMed] [Google Scholar]
- Balogh G, Horváth I, Nagy E, et al. The hyperfluidization of mammalian cell membranes acts as a signal to initiate the heat shock protein response. FEBS J. 2005;272:6077–6086. doi: 10.1111/j.1742-4658.2005.04999.x. [DOI] [PubMed] [Google Scholar]
- Balogh G, Péter M, Liebisch G, et al. Lipidomics reveals membrane lipid remodelling and release of potential lipid mediators during early stress responses in a murine melanoma cell line. Biochim Biophys Acta. 2010;1801:1036–1047. doi: 10.1016/j.bbalip.2010.04.011. [DOI] [PubMed] [Google Scholar]
- Balogh G, Péter M, Glatz A, et al. Key role of lipids in heat stress management. FEBS Lett. 2013;587:1970–1980. doi: 10.1016/j.febslet.2013.05.016. [DOI] [PubMed] [Google Scholar]
- Balogi Z, Cheregi O, Giese KC, et al. A mutant small heat shock protein with increased thylakoid association provides an elevated resistance against UV-B damage in synechocystis 6803. J Biol Chem. 2008;283:22983–22991. doi: 10.1074/jbc.M710400200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bausero M a, Bharti A, Page DT, et al. Silencing the hsp25 gene eliminates migration capability of the highly metastatic murine 4T1 breast adenocarcinoma cell. Tumour Biol. 2005;27:17–26. doi: 10.1159/000090152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellyei S, Szigeti A, Boronkai A, et al. Inhibition of cell death by a novel 16.2 kD heat shock protein predominantly via Hsp90 mediated lipid rafts stabilization and Akt activation pathway. Apoptosis. 2007;12:97–112. doi: 10.1007/s10495-006-0486-x. [DOI] [PubMed] [Google Scholar]
- Bellyei S, Szigeti A, Pozsgai E, et al. Preventing apoptotic cell death by a novel small heat shock protein. Eur J Cell Biol. 2007;86:161–171. doi: 10.1016/j.ejcb.2006.12.004. [DOI] [PubMed] [Google Scholar]
- Blazquez M a, Stucka R, Feldmann H, Gancedo C. Trehalose-6-P synthase is dispensable for growth on glucose but not for spore germination in Schizosaccharomyces pombe. J Bacteriol. 1994;176:3895–3902. doi: 10.1128/jb.176.13.3895-3902.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brameshuber M, Weghuber J, Ruprecht V, et al. Imaging of mobile long-lived nanoplatforms in the live cell plasma membrane. J Biol Chem. 2010;285:41765–41771. doi: 10.1074/jbc.M110.182121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs MS, Gierasch LM, Zlotnick a, et al. In vivo function and membrane binding properties are correlated for Escherichia coli lamB signal peptides. Science. 1985;228:1096–1099. doi: 10.1126/science.3158076. [DOI] [PubMed] [Google Scholar]
- Carpy A, Krug K, Graf S, et al. Absolute proteome and phosphoproteome dynamics during the cell cycle of Schizosaccharomyces pombe (fission yeast) Mol Cell Proteomics. 2014;13:1925–1936. doi: 10.1074/mcp.M113.035824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D, Toone WM, Mata J, et al. Global transcriptional responses of fission yeast to environmental stress. Mol Biol Cell. 2003;14:214–229. doi: 10.1091/mbc.E02-08-0499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdary TK, Raman B, Ramakrishna T, Rao CM. Interaction of mammalian Hsp22 with lipid membranes. Biochem J. 2007;401:437–445. doi: 10.1042/BJ20061046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowe JH. Trehalose as a “chemical chaperone”: fact and fantasy. In: Csermely P, Vígh L, editors. Molecular aspects of the stress response: chaperones, membranes and networks. New York: Springer; 2007. pp. 143–158. [Google Scholar]
- de Miguel N, Echeverria PC, Angel SO. Differential subcellular localization of members of the Toxoplasma gondii small heat shock protein family. Eukaryot Cell. 2005;4:1990–1997. doi: 10.1128/EC.4.12.1990-1997.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Miguel N, Lebrun M, Heaslip A, et al. Toxoplasma gondii Hsp20 is a stripe-arranged chaperone-like protein associated with the outer leaflet of the inner membrane complex. Biol Cell. 2008;100:479–489. doi: 10.1042/BC20080004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Virgilio C, Simmen U, Hottiger T, et al. Heat shock induces enzymes of trehalose metabolism, trehalose accumulation, and thermotolerance in Schizosaccharomyces pombe, even in the presence of cycloheximide. FEBS Lett. 1990;273:107–110. doi: 10.1016/0014-5793(90)81062-S. [DOI] [PubMed] [Google Scholar]
- Delmas F, Pierre F, Coucheney F, et al. Biochemical and physiological studies of the small heat shock protein Lo18 from the lactic acid bacterium Oenococcus oeni. J Mol Microbiol Biotechnol. 2001;3:601–610. [PubMed] [Google Scholar]
- Eisenberg-Domovich Y, Kloppstech K, Ohad I. Reversible membrane association of heat-shock protein 22 in Chlamydomonas reinhardtii during heat shock and recovery. Eur J Biochem. 1994;222:1041–1046. doi: 10.1111/j.1432-1033.1994.tb18956.x. [DOI] [PubMed] [Google Scholar]
- Elbein AD, Pan YT, Pastuszak I, Carroll D. New insights on trehalose: a multifunctional molecule. Glycobiology. 2003;13:17–27. doi: 10.1093/glycob/cwg047. [DOI] [PubMed] [Google Scholar]
- Eleutherio E, Panek A, De Mesquita JF, et al. Revisiting yeast trehalose metabolism. Curr Genet. 2015;61:263–274. doi: 10.1007/s00294-014-0450-1. [DOI] [PubMed] [Google Scholar]
- Elliott B, Haltiwanger RS, Futcher B. Synergy between trehalose and Hsp104 for thermotolerance in Saccharomyces cerevisiae. Genetics. 1996;144:923–933. doi: 10.1093/genetics/144.3.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyles SJ, Gierasch LM. Nature’s molecular sponges: small heat shock proteins grow into their chaperone roles. Proc Natl Acad Sci U S A. 2010;107:2727–2728. doi: 10.1073/pnas.0915160107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsburg SL. Growth and manipulation of S. pombe. Curr Protoc Mol Biol. 2003 doi: 10.1002/0471142727.mb1316s64. [DOI] [PubMed] [Google Scholar]
- Forsburg SL, Rhind N. Basic methods for fission yeast. Yeast. 2006;23:173–183. doi: 10.1002/yea.1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita R, Ounzain S, Wang ACY, et al. Hsp-27 induction requires POU4F2/Brn-3b TF in doxorubicin-treated breast cancer cells, whereas phosphorylation alters its cellular localization following drug treatment. Cell Stress Chaperones. 2011;16:427–439. doi: 10.1007/s12192-011-0256-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gancedo C, Flores CL. The importance of a functional trehalose biosynthetic pathway for the life of yeasts and fungi. FEMS Yeast Res. 2004;4:351–359. doi: 10.1016/S1567-1356(03)00222-8. [DOI] [PubMed] [Google Scholar]
- Gibney PA, Lu C, Caudy AA, et al. Yeast metabolic and signaling genes are required for heat-shock survival and have little overlap with the heat-induced genes. Proc Natl Acad Sci U S A. 2013;110:E4393–E4402. doi: 10.1073/pnas.1318100110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gombos I, Crul T, Piotto S, et al. Membrane-lipid therapy in operation: the HSP co-inducer BGP-15 activates stress signal transduction pathways by remodeling plasma membrane rafts. PLoS One. 2011;6:e28818. doi: 10.1371/journal.pone.0028818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazel JR, Williams EE. The role of alterations in membrane lipid composition in enabling physiological adaptation of organisms to their physical environment. Prog Lipid Res. 1990;29:167–227. doi: 10.1016/0163-7827(90)90002-3. [DOI] [PubMed] [Google Scholar]
- Heckathorn SA, Downs CA, Sharkey TD, Coleman JS. The small, methionine-rich chloroplast heat-shock protein protects photosystem II electron transport during heat stress. Plant Physiol. 1998;116:439–444. doi: 10.1104/pp.116.1.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirose M, Tohda H, Giga-Hama Y, et al. Interaction of a small heat shock protein of the fission yeast, Schizosaccharomyces pombe, with a denatured protein at elevated temperature. J Biol Chem. 2005;280:32586–32593. doi: 10.1074/jbc.M504121200. [DOI] [PubMed] [Google Scholar]
- Hohmann S, Neves MJ, de Koning W, et al. The growth and signalling defects of the ggs1 (fdp1/byp1) deletion mutant on glucose are suppressed by a deletion of the gene encoding hexokinase PII. Curr Genet. 1993;23:281–289. doi: 10.1007/BF00310888. [DOI] [PubMed] [Google Scholar]
- Horváth I, Multhoff G, Sonnleitner A, Vígh L. Membrane-associated stress proteins: more than simply chaperones. Biochim Biophys Acta - Biomembr. 2008;1778:1653–1664. doi: 10.1016/j.bbamem.2008.02.012. [DOI] [PubMed] [Google Scholar]
- Kitagawa M, Matsumura Y, Tsuchido T. Small heat shock proteins, IbpA and IbpB, are involved in resistances to heat and superoxide stresses in Escherichia coli. FEMS Microbiol Lett. 2000;184:165–171. doi: 10.1111/j.1574-6968.2000.tb09009.x. [DOI] [PubMed] [Google Scholar]
- Kitagawa M, Miyakawa M, Matsumura Y, Tsuchido T. Escherichia coli small heat shock proteins, IbpA and IbpB, protect enzymes from inactivation by heat and oxidants. Eur J Biochem. 2002;269:2907–2917. doi: 10.1046/j.1432-1033.2002.02958.x. [DOI] [PubMed] [Google Scholar]
- Krawchuk MD, Wahls WP. High-efficiency gene targeting in Schizosaccharomyces pombe using a modular, PCR-based approach with long tracts of flanking homology. Yeast. 1999;15:1419–1427. doi: 10.1002/(SICI)1097-0061(19990930)15:13<1419::AID-YEA466>3.0.CO;2-Q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lackner D, Schmidt M, Wu S, et al. Regulation of transcriptome, translation, and proteome in response to environmental stress in fission yeast. Genome Biol. 2012;13:R25. doi: 10.1186/gb-2012-13-4-r25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DH, Goldberg AL. Proteasome inhibitors cause induction of heat shock proteins and trehalose, which together confer thermotolerance in Saccharomyces cerevisiae. Mol Cell Biol. 1998;18:30–38. doi: 10.1128/MCB.18.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee BY, Hefta SA, Brennan PJ. Characterization of the major membrane protein of virulent Mycobacterium tuberculosis. Infect Immun. 1992;60:2066–2074. doi: 10.1128/iai.60.5.2066-2074.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Marguerat S, Schmidt A, Codlin S, et al. Quantitative analysis of fission yeast transcriptomes and proteomes in proliferating and quiescent cells. Cell. 2012;151:671–683. doi: 10.1016/j.cell.2012.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuyama A, Arai R, Yashiroda Y, et al. ORFeome cloning and global analysis of protein localization in the fission yeast Schizosaccharomyces pombe. Nat Biotechnol. 2006;24:841–847. doi: 10.1038/nbt1222. [DOI] [PubMed] [Google Scholar]
- Nagy E, Balogi Z, Gombos I, et al. Hyperfluidization-coupled membrane microdomain reorganization is linked to activation of the heat shock response in a murine melanoma cell line. Proc Natl Acad Sci U S A. 2007;104:7945–7950. doi: 10.1073/pnas.0702557104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitta K, Suzuki N, Honma D, et al. Ultrastructural stability under high temperature or intensive light stress conferred by a small heat shock protein in cyanobacteria. FEBS Lett. 2005;579:1235–1242. doi: 10.1016/j.febslet.2004.12.095. [DOI] [PubMed] [Google Scholar]
- Park HG, Han SI, Oh SY, Kang HS. Cellular responses to mild heat stress. Cell Mol Life Sci. 2005;62:10–23. doi: 10.1007/s00018-004-4208-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro MJS, Reinders A, Boller T, et al. Trehalose synthesis is important for the acquisition of thermotolerance in Schizosaccharomyces pombe. Mol Microbiol. 1997;25:571–581. doi: 10.1046/j.1365-2958.1997.4961856.x. [DOI] [PubMed] [Google Scholar]
- Rojo M, Hovius R, Demel R, et al. Interaction of mitochondrial creatine kinase with model membranes a monolayer study. FEBS Lett. 1991;281:123–129. doi: 10.1016/0014-5793(91)80374-C. [DOI] [PubMed] [Google Scholar]
- Sheff MA, Thorn KS. Optimized cassettes for fluorescent protein tagging in Saccharomyces cerevisiae. Yeast. 2004;21:661–670. doi: 10.1002/yea.1130. [DOI] [PubMed] [Google Scholar]
- Shiozaki K, Shiozaki M, Russell P (1997) Mcs4 mitotic catastrophe suppressor regulates the fission yeast cell cycle through the Wik1-Wis1-Spc1 kinase cascade. Mol Biol Cell 8:409–419 [DOI] [PMC free article] [PubMed]
- Singer M a, Lindquist S. Multiple effects of trehalose on protein folding in vitro and in vivo. Mol Cell. 1998;1:639–648. doi: 10.1016/S1097-2765(00)80064-7. [DOI] [PubMed] [Google Scholar]
- Soto T, Fernandez J, Vicente-Soler J, et al. Accumulation of trehalose by overexpression of tps1, coding for trehalose-6-phosphate synthase, causes increased resistance to multiple stresses in the fission yeast Schizosaccharomyces pombe. Appl Environ Microbiol. 1999;65:2020–2024. doi: 10.1128/aem.65.5.2020-2024.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugino C, Hirose M, Tohda H, et al. Characterization of a sHsp of Schizosaccharomyces pombe, SpHsp15.8, and the implication of its functional mechanism by comparison with another sHsp, SpHsp16.0. Proteins. 2009;74:6–17. doi: 10.1002/prot.22132. [DOI] [PubMed] [Google Scholar]
- Taricani L, Feilotter HE, Weaver C, Young PG. Expression of hsp16 in response to nucleotide depletion is regulated via the spc1 MAPK pathway in Schizosaccharomyces pombe. Nucleic Acids Res. 2001;29:3030–3040. doi: 10.1093/nar/29.14.3030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Török Z, Demel RA, Leenhouts JM, de Kruijff B. Presequence-mediated intermembrane contact formation and lipid flow. A model membrane study. Biochemistry. 1994;33:5589–5594. doi: 10.1021/bi00184a030. [DOI] [PubMed] [Google Scholar]
- Török Z, Horváth I, Goloubinoff P, et al. Evidence for a lipochaperonin: association of active protein-folding GroESL oligomers with lipids can stabilize membranes under heat shock conditions. Proc Natl Acad Sci U S A. 1997;94:2192–2197. doi: 10.1073/pnas.94.6.2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Török Z, Pilbat A-M, Gombos I, et al. Evidence on cholesterol-controlled lipid raft interaction of the small heat shock protein HSPB11. In: Henderson B, Pockley AG, et al., editors. Cellular trafficking of cell stress proteins in health and disease. Dordrecht: Springer Netherlands; 2012. pp. 75–85. [Google Scholar]
- Tsvetkova NM, Horváth I, Török Z, et al. Small heat-shock proteins regulate membrane lipid polymorphism. Proc Natl Acad Sci U S A. 2002;99:13504–13509. doi: 10.1073/pnas.192468399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verger R, Pattus F. Lipid-protein interactions in monolayers. Chem Phys Lipids. 1982;30:189–227. doi: 10.1016/0009-3084(82)90052-4. [DOI] [Google Scholar]
- Vigh L, Maresca B, Harwood JL. Does the membrane’s physical state control the expression of heat shock and other genes? Trends Biochem Sci. 1998;23:369–374. doi: 10.1016/S0968-0004(98)01279-1. [DOI] [PubMed] [Google Scholar]
- Vigh L, Escribá PV, Sonnleitner A, et al. The significance of lipid composition for membrane activity: new concepts and ways of assessing function. Prog Lipid Res. 2005;44:303–344. doi: 10.1016/j.plipres.2005.08.001. [DOI] [PubMed] [Google Scholar]
- Vigh L, Horváth I, Maresca B, Harwood JL. Can the stress protein response be controlled by “membrane-lipid therapy”? Trends Biochem Sci. 2007;32:357–363. doi: 10.1016/j.tibs.2007.06.009. [DOI] [PubMed] [Google Scholar]
- Vigh L, Nakamoto H, Landry J, et al. Membrane regulation of the stress response from prokaryotic models to mammalian cells. Ann N Y Acad Sci. 2007;1113:40–51. doi: 10.1196/annals.1391.027. [DOI] [PubMed] [Google Scholar]
- Weidmann S, Rieu A, Rega M, et al. Distinct amino acids of the Oenococcus oeni small heat shock protein Lo18 are essential for damaged protein protection and membrane stabilization. FEMS Microbiol Lett. 2010;309:8–15. doi: 10.1111/j.1574-6968.2010.01999.x. [DOI] [PubMed] [Google Scholar]
- Welker S, Rudolph B, Frenzel E, et al. Hsp12 is an intrinsically unstructured stress protein that folds upon membrane association and modulates membrane function. Mol Cell. 2010;39:507–520. doi: 10.1016/j.molcel.2010.08.001. [DOI] [PubMed] [Google Scholar]
- Wood V, Harris MA, McDowall MD, et al. PomBase: a comprehensive online resource for fission yeast. Nucleic Acids Res. 2012;40:D695–D699. doi: 10.1093/nar/gkr853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida J, Tani T. Hsp16p is required for thermotolerance in nuclear mRNA export in fission yeast Schizosaccharomyces pombe. Cell Struct Funct. 2005;29:125–138. doi: 10.1247/csf.29.125. [DOI] [PubMed] [Google Scholar]
- Zhang H, Fu X, Jiao W, et al. The association of small heat shock protein Hsp16.3 with the plasma membrane of Mycobacterium tuberculosis: dissociation of oligomers is a prerequisite. Biochem Biophys Res Commun. 2005;330:1055–1061. doi: 10.1016/j.bbrc.2005.03.092. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Primers used to generate the different S. pombe mutants. (DOCX 13 kb)
Survival of GFP-tagged shsp mutants. Spot tests of both shsp-mutants were carried out before and after incubation at 44 °C for 180 min as described in Methods. (GIF 41 kb)
Heat induction of sHsps in tps1Δ cells (a) and survival of the shsp:GFP/tps1Δ mutants after the heat treatments (b). Experiments were performed as described in Methods. (GIF 95 kb)