Abstract
Atherosclerosis is a chronic inflammatory disease of the artery wall, and both innate and adaptive immunity play important roles in the pathogenesis of this disease. In several experimental and human experiments of early atherosclerotic lesions, it has been shown that the first pathogenic event in atherogenesis is intimal infiltration of T cells at predilection sites. These T cells react to heat shock protein 60 (HSP60), which is a ubiquitous self-antigen expressed on the surface of endothelial cells (ECs) together with adhesion molecules in response to classical risk factors for atherosclerosis. When HSP60 is expressed on the EC surface, it can act as a “danger-signal” for both cellular and humoral immune reactions. Acquired by infection or vaccination, beneficial protective immunity to microbial HSP60 and bona fide autoimmunity to biochemically altered autologous HSP60 is present in all humans. Thus, the development of atherosclerosis during aging is paid by the price for lifelong protective preexisting anti-HSP60 immunity by harmful (auto)immune cross-reactive attack on arterial ECs maltreated by atherosclerosis risk factors. This is supported by experiments, which shows that bacterial HSP60 immunization can lead and accelerate experimental atherosclerosis. This review article presents accumulating proof that supports the idea that tolerization with antigenic HSP60 protein or its peptides may arrest or even prevent atherosclerosis by increased production of regulatory T cells and/or anti-inflammatory cytokines. Recent data indicates that HSP60, or more likely some of its derivative peptides, has immunoregulatory functions. Therefore, these peptides may have important potential for being used as diagnostic agents or therapeutic targets.
Keywords: Atherosclerosis, Heat shock protein 60, Tolerization, Peptides, Therapy
Introduction
Indicators of a cellular heat shock response were first discovered more than 50 years ago. Ritossa and coworkers first described the phenomenon of puffing in the large chromosomes of the salivary glands of Drosophila melanogaster after being exposed to heat (Ritossa 1962, 1964; Ashburner 1970). Later, the first gene and protein products of this morphological puffing pattern were identified and the term “heat shock proteins” (HSPs) has been created (Tissieres et al. 1974; McKenzie et al. 1975; Spradling et al. 1975; Moran et al. 1978). HSPs are grouped in families according to their molecular weight, and constitutive members of each family can be found in different cell compartments under non-stress conditions (Lindquist and Craig 1988). The genes coding for these proteins have been sequenced, their structure described, their chromosomal localization defined, and their mode of interaction with nuclear heat shock transcription actors characterized (Westwood et al. 1991). Both prokaryotic and eukaryotic cells are expressing HSPs under physiological conditions as well as all cells that are exposed to various forms of stress. They have a wide range of physiological functions. Their cellular involvement includes intracellular protein transport, protein folding, cellular signaling, protein degradation, and also certain chaperone functions. Between all mammalian and bacterial species, the members of the HSP60 (the 60-kDa HSP) family (mammalian HSP60 (hSP60), the Mycobacterium tuberculosis homologue HSP65 (mbHSP65), Chlamydia pneumoniae homologue (cHSP60), and the Escherichia coli homologue (GroEL) are highly conserved. That is the reason why extensive immunological cross-reactions between autologous and pathogenic HSP60 can occur (Young and Elliott 1989). During different stress conditions, the endogenous mitochondria-bound HSP60 protein can be translocated to the cytoplasm and the cell surface. The exact pathway, however, is still not completely understood.
In addition to the immunity against organism-specific epitopes, all humans develop protective beneficial adaptive immunity against the phylogenetically highly conserved microbial HSP60 antigen via infection or vaccination. Under physiological conditions, vascular endothelial cells (ECs) do not express HSP60. When stressed, however, HSP60 expression can be induced on the EC surface by classical atherosclerosis risk factors, such as mechanical stress, temperature, oxygen radicals, infections, toxins, heavy metals, cigarette smoke, and pro-inflammatory cytokines (Lamb et al. 2003; Xu and Wick 1996). Importantly, the same stressors can simultaneously induce the expression of both adhesion molecules (ICAM-1, ELAM-1, and VCAM-1) and HSP60 on the EC surface (Seitz et al. 1996; Amberger et al. 1997). This mechanism provides the prerequisite for potentially bacterial/human HSP60 cross-reactive antibodies and destruction of the EC by preexisting cellular and humoral immunity against HSP60, entailing intimal infiltration by mononuclear cells. Thus, HSP60 that is expressed on the cell surface can act as a “danger signal” both for cellular and humoral immune reactions. In other words, protective, preexisting anti-HSP60 immunity may cause harmful (auto)immune cross-reactive attack on arterial ECs maltreated by atherosclerosis risk factors. These early inflammatory stage of atherosclerosis is still reversible, but if atherosclerosis risk factors persist, the inflammatory stage proceeds to plaque formation with deleterious consequences. At later stages of atherogenesis, intralesional T cells, macrophages, dendritic cells (DCs), and smooth muscle cells (SMCs) can also express HSP60, and the anti-HSP60 cellular immune reaction could therefore be perpetuated in situ. These experimentally and clinically proven findings represent the basis for the “Autoimmune Concept of Atherosclerosis” (Wick et al. 2004, 2014; Grundtman et al. 2011; Grundtman and Wick 2011). This concept was first presented in 1992 and showed that normocholesterolemic rabbits immunized with mbHSP65 develop atherosclerotic plaques irrespective of their cholesterol levels (Wick et al. 1992; Xu et al. 1992). Moreover, during the last two decades, we and other laboratories have identified HSP60 as one of the most important antigens in early stages of atherosclerosis (Xu and Wick 1996; Wick et al. 1995, 2004). Proof of concept for the presence of antigenic mimicry has been thoroughly investigated in different animal models and humans (Wick et al. 2014).
Albeit already much can be accomplished only through certain lifestyle changes and several medicinal therapies for atherosclerosis exist, e.g., oxidized low-density lipoprotein (oxLDL)-lowering therapies, there are still a large number of adverse cardiovascular events indicating an obvious need for new specifically targeted therapeutic interventions. In this review, the focus will be put on the possible beneficial use of HSP60 and HSP60-derived peptides with the aim to avoid atherogenesis and specifically treat already ongoing atherosclerosis.
HSP60 in human atherosclerosis
As mentioned, all healthy humans display innate and adaptive anti-HSP60 immunity induced by infection, by vaccination, or as bona fide autoimmunity against biochemically altered autologous HSP60, probably derived from damaged or necrotic ECs. Soluble HSP60 (sHSP60) and/or anti-hHSP60 antibody concentrations may be used as prognostic biomarkers for the risk of develop cardiovascular disease (CVD) as several studies have demonstrated a correlation between high anti-hHSP60 antibody titers and/or elevated sHSP60 levels in individuals suffering from CVD (Willeit and Kiechl 1993; Xu et al. 1993a, b; Hoppichler et al. 2000; Pockley et al. 2000; Zhang et al. 2008; Almanzar et al. 2012). Also, common carotid artery intima media thickness (IMT; the combined thickness of both the tunica intima and tunica media) correlates with elevated sHSP60 levels in individuals with prevalent carotid atherosclerosis (Xu et al. 2000; Xiao et al. 2005). Anti-hHSP60 antibody titer has also not only been identified as a new early biomarker for morbidity but also for mortality from atherosclerosis (Xu et al. 1999). In addition, lifelong infectious load has also been discussed as correlated with antimicrobial HSP60 antibody titers and with atherosclerosis (Mayr et al. 2000; Burian et al. 2001; Ford et al. 2005). Cross-reactive antibodies between bacterial/human HSP60 can induce cytotoxic damage of stressed ECs (Mayr et al. 1999; Schett et al. 1995), indicating that humoral immune reactions to bacterial HSPs may play an important role in the process of vascular endothelial injury, which is believed to be a key event in the pathogenesis of atherosclerosis. As discussed below, it seems most likely that T cells initiate the disease while anti-hHSP60 antibodies has an accelerating and perpetuating effect (Knoflach et al. 2007).
Specific cellular immunity to HSP60 exists in the early stages of atherosclerosis (Knoflach et al. 2003, 2007, 2009). For example, it has been demonstrated in several studies that T cells are one of the first cells to invade the arterial intima, later followed by macrophages, DCs, and SMCs in predisposed sites (Xu et al. 1990; Kleindienst et al. 1993; Millonig et al. 2002). However, it has been shown that preexisting vascular-associated dendritic cells (VADCs) are presented in the tertiary lymphoid structures in the aortic adventitia at predisposed sites, even before the invasion of T cells (Bobryshev and Lord 1996, 1998; Waltner-Romen et al. 1998; Millonig et al. 2001a, b; Liu et al. 2008; Bobryshev 2010; Cybulsky and Jongstra-Bilen 2010). These DCs can function as antigen-presenting cells (APCs) and thereby capture potentially harmful exogenous or autoantigens and present these to T cells and macrophages. Lesion-derived T cells display an oligoclonally restricted repertoire in contrast to the polyclonal pattern of peripheral blood mononuclear cells (PBMCs), indicating that oligoclonal T cell expansion can take place in human atherosclerotic lesions (Rossmann et al. 2008). We have recently shown that these early autoreactive intralesional T cells, derived from early, clinically still inapparent human atherosclerotic lesions, can specifically react to certain hHPS60 epitopes (Almanzar et al. 2012). Similarly, also T cells derived from late complicated human atherosclerotic plaques harbored specific hHSP60 epitope reaction, which confirms earlier data where also cross-reactive epitopes were found between cHSP60 and hHSP60 (Almanzar et al. 2012; Benagiano et al. 2005). Interestingly, some potentially atherogenic hHSP60 epitopes were only found in early lesions vs. late plaques while others were shared (Almanzar et al. 2012). T cells from atherosclerotic lesions from rabbits do also give strong proliferative response to mbHSP65 (Xu et al. 1993a, b). Furthermore, oxLDL and LDLx (human group X-secreted phospholipase A2) but not native LDL can activate plaque T cells through DCs and HSP60 and 90 seem to play a role in this immune reactivity as T cell antigens (Liu et al. 2015). This congruence a strong indication that these hHSP60 epitopes recognized already by early lesional T cells plays a pathogenic role throughout atherogenesis and may represent interesting early candidates for investigation in diagnostic, preventive, and therapeutic approaches; however, this needs further investigations with larger cohorts of patients. Moreover, besides being specific T cell antigens per se, presented on APCs, HSP60 or peptides thereof could promote immune activation by other mechanisms, in a non-mutually exclusive way. HSPs being chaperone can form immune complexes with other antigens including tumor antigens, and these can be presented as antigens through classes I or II antigen presenting pathways (Murshid et al. 2012). Although HSPs, including HSP60, are potent activators of the innate immune system, very few data are available for their role in the context of atherosclerosis (Wallin et al. 2002). HSPs can be actively released through exosomes or passively as in cell necrosis. Such HSP could function as endogenous ligands in the extracellular space and activate the innate immune system, through toll-like receptors (TLRs) or by association with ligands as endotoxin (Tamura et al. 2012).
The importance of HSP60 B cell epitopes in atherosclerosis has also been investigated, however, to a much less extent. For example, atherosclerosis patients show common T and/or B cell epitope specificities with cross-reactivity between Porphyromonas gingivalis HSP60 and hHSP60 (Choi et al. 2004). Furthermore, antibodies to microbial HSP60/65 recognize specific epitopes on hHSP60. These cross-reactive epitopes were shown to serve as autoimmnune targets in incipient atherosclerosis (Perschinka et al. 2003).
HSP60 in experimental atherosclerosis
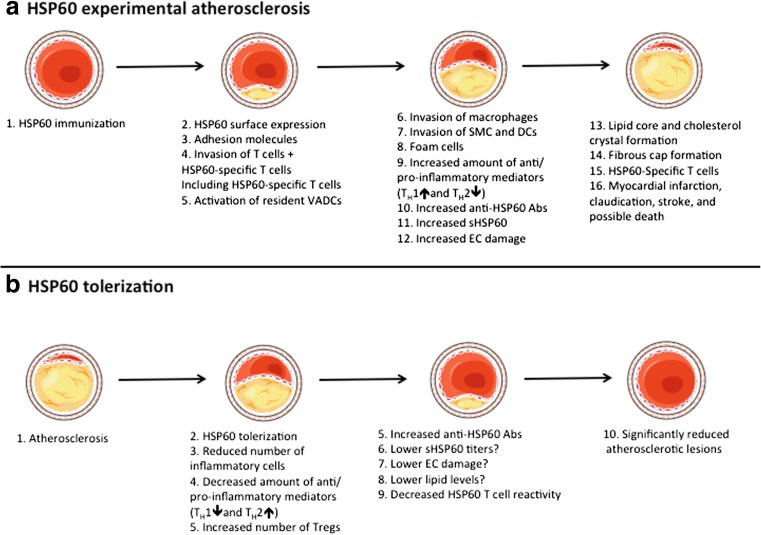
Early atherosclerotic lesions show a strong upregulation of hHSP60 and the stress-inducible form hHSP70 in ApoE−/− mice (Kanwar et al. 2001). The increased expression can already be found in 3-week-old mice before lesion formation is visually detectable. This is followed in 8 to 20-week-old mice by a strong and hererogeneous expression in lesional ECs of early to advanced fibrofatty plaques, macrophages, SMCs, and CD3+ T cells, with levels correlating to disease severity (Kanwar et al. 2001). However, in advanced collagenous acellular calcified plaques in 40- to 69-week-old mice, the expression is markedly downregulated. In 3- to 69-week-old normocholesterolemic ApoE+/+ mice, no expression could be found, indicating that HSPs might be a good marker for progression stages of atherosclerosis (Kanwar et al. 2001). A schematic overview of the experimental atherosclerosis development can be found in Fig. 1.
Fig. 1.
a Under physiological conditions, vascular endothelial cells (ECs) do not express heat shock protein (HSP)60 on the surface; however, after HSP60/65 immunizations (or other kind of stressors), HSP60 is transported and appears on the EC cell surface. The surfaces expression of HSP60 appears simultaneously with the expression of adhesion molecules. Activated T cells are the first invaders of the arterial intima in early atherosclerotic lesions. Early, still inapparent, atherosclerotic lesions show HSP60-specific T cells. Pre-existing resident vascular-associated dendritic cells (VADCs) might present the HSP60 antigen, either locally in the intima or after transport to draining lymph nodes. An increased number of macrophages, smooth muscle cells (SMCs), lipid deposition, foam cells formation, and release of pro-inflammatory mediators both locally and into the circulation are seen in the more developed plaque. Increased titers of anti-HSP60 autoantibodies and soluble HSP60 (sHSP60) are detected in the circulation. Stressed, but not unstressed ECs can be lysed by anti-HSP60 anti-HSP60 antibodies in a complement-mediated fashion or via antibody-dependent cellular cytotoxicity. Also, late complicated plaques show HSP60-specific T cells. Some of these epitopes are shared in early vs. late lesions; however, some only exist in each subset. If exposure of stress persists, the plaque becomes more complex and forms a core of necrotic and apoptotic cells, cell debris, and cholesterol crystals, along with a fibrous cap. Rupture of unstable plaques exposes the core and can lead to thrombus formation, myocardical infarction, claudication, stroke, and death. b After tolerization with full-length HSP60/65 or preferable with their peptide(s), a lower number of lesional T cells, macrophages, and SMCs are seen. A reduced level of TH1 and increased level of TH2 mediators can be found locally, in secondary lymphoid organs, and/or in the circulation. An increased number and suppressive capacity of regulatory T cells has also been found. Moreover, increased anti-HSP60 IgG1 (auto)antibodies are found in the circulation after HSP60 treatment, which may lead to a lower titer of sHSP60 and decreased EC damage. The lipid reduction that has been found in tolerized animals is probably a by-product of HSP60/65 immune interference and not a consequence of the tolerance. However, it is still not yet fully elucidated if lipid levels can be reduced after HSP60/65 tolerization. A decreased T cell reactivity in the secondary lymphoid organs against HSP60 antigens indicates an induction of tolerance to HSP60. Partly adapted from Servier Medical Art
Genetically normocholesterolemic rabbits immunized with mbHSP65 (in the present context, mbHSP65 is always used as a paradigmatic and potent representative of bacterial HSP60) develop atherosclerotic plaques irrespective of their diet with low or high-cholesterol levels, and T cells isolated from these lesions specifically respond to mbHSP65 in vitro (Xu et al. 1992, 1993a, b; Metzler et al. 1999), a finding similar to that in humans (Rossmann et al. 2008; Benagiano et al. 2005). Both C57BL/6J mice, fed high-cholesterol diet, and LDLr−/− mice, fed a normal chow diet, revealed enhanced early atherosclerotic lesions after immunization with mbHSP65 (Afek et al. 2000; George et al. 1999). When C56BL/6NJcl mice were immunized with hHSP60 and fed with high-cholesterol diet, an enhanced fatty streak formation resulted (Mori et al. 2000). In rats that were immunized with mbHSP65, a brisk and sustained humoral response together with increased neointimal growth could be observed (George et al. 2003). On the other hand, in the absence of traditional risk factors for atherosclerosis and T cell activation, early inflammatory stages of atherosclerotic lesions induced by mbHSP65 immunization can be regressed (Sun et al. 2010; Xu et al. 1996). After mbHSP65 immunization, enhanced progression of atherosclerosis and an increase in intralesional CD3+ T cells have been documented in C57BL/6J, LDLr−/−, and ApoE−/− mice (Shoenfeld et al. 2000). Transfer of these mbHSP65 reactive lymphocytes to syngenic mice led to an enhancement of fatty streak formation, supporting a selective immunomodulation of the atherosclerotic plaques. Similarly, in ApoE−/− mice, high-titer immunoglobulin treatment with human anti-HSP60 autoantibodies can accelerate atherosclerosis (Foteinos et al. 2005). In contrast, immunization with mbHSP65-alum protects ApoE−/− mice against progression of early atherosclerosis (Klingenberg et al. 2012). However, it has been shown that alum displays strong atheroprotective properties by itself by increasing Th2 responses, anti-MDA-LDL IgM titers, the number of CD4+CD25+Foxp3+Tregs, and downregulating T cell activation markers (Khallou-Laschet et al. 2006; Wigren et al. 2009). It seems likely that alum boosts immune reaction against self-antigens (mbHSP65) by facilitating the uptake of mbHSP65 by local APCs or by the recruitment of inflammatory APCs at the injection site that then migrate to the peripheral lymphatic tissues where they activate antigen-specific Tregs that protect against mbHSP65 autoimmunity.
Comparable results can be seen when animals are immunized with peptides of the corresponding HSP60. For example, immunizations with mbHSP65 peptide (91-105) leads to enhanced atherosclerosis in rabbits and aortic EC injury in mice (Zhang et al. 2012). Adopted transfer of mbHSP65 peptide (91-105)-specific splenic cells that secrete increased levels of interferon-γ (IFN-γ) can accelerate atherosclerosis (Zhang et al. 2012). When immunized with mbHSP65 peptide (153-171), different mice strains (with different H-2 haplotypes) induced a cross-reactive T cell proliferative response to homologous GroEL (Brett et al. 1989). Similarly, immunization of ApoE−/− mice with a specific monoclonal mouse antibody (II-13) that recognizes amino acid residues 288-266 of hHSP60, effectively induced atherosclerosis due to the recognition of specific epitopes expressed on arterial ECs (Foteinos et al. 2005). II-13 injection resulted in EC damage, followed by increased leukocyte attachment and accumulation of macrophages and SMC in lesions, whereas the monoclonal antibody ML-30, which binds to amino acids 315-318 of HSP60, lacked cytotoxic effects against cells in vitro (Foteinos et al. 2005).
Notably, HSPs are rather large proteins that give, when processed, rise to a multitude of potential epitopes. Only a few of these, however, are atherogenic. In atherosclerosis, different epitopes from the same HSP may therefore have very different functional effect on the immune response, some being pro-inflammatory and others tolerogenic. Therefore, our laboratory’s scientific goal during the last years has been to identify epitopes, rather than the full-lengths HSP60 protein, that can be found in a majority of patients and to characterize the appropriate immune response in order to identify the most pro-inflammatory epitope for the induction of tolerance.
HSP60 tolerization in atherosclerosis
Having investigated and proven the autoimmunological HSP60-modulated concept of atherogenesis, the idea about the development of a tolerization against atherogenic HSP60 epitopes has centered our laboratory’s and others research activities since it may be a plausible approach to preventing or even treating atherosclerosis. By treating hypercholesterolemic ApoE−/− and LDLr−/− mice either intranasally or orally with whole mbHSP65 preparations or immunize them with specific derived peptides thereof, the principle of tolerization has been successfully applied (Fig. 2). In one study, LDLr−/− mice were fed mbHSP65 in different concentrations every other day for 10 days, and after the last feeding, they were challenged with either (i) an immunization with M. tuberculosis (containing large amounts of bacterial HSP65) or (ii) recombinant mbHSP65 or (iii) by high-cholesterol diet (Harats et al. 2002). The results showed that oral tolerance with mbHSP65 significantly attenuated atherogenesis (Harats et al. 2002). Moreover, the reactivity of lymphocytes in mice that have been fed with mbHSP65 and immunized against mbHSP65 or M. tuberculosis was significantly reduced. Also the specific HSP65-reactivity in splenocytes was reduced in these mice. Cells extracted from the lymph nodes of these mice produced more interleukin (IL)-4 (TH2 cytokine) compared with cells of non-tolerized animals (Harats et al. 2002). However, no suppressive effect was seen on TH1 cytokine secretion, as evidenced by the unaltered IFN-γ production (Harats et al. 2002). The role of IL-4 in atherosclerosis has been proven previously (Huber et al. 2001). Feeding mbHSP65 orally suppressed high-cholesterol diet-induced atherosclerosis where spontaneous reactivity to mbHSP65 was not evident compared to the M. tuberculosis and mbHSP65-driven fatty-streak model (Huber et al. 2001). We have also successfully orally tolerized ApoE−/− and C57BL/6J mice with mbHSP65. We found that atherosclerotic lesions were significantly reduced together with a decrease in pro-inflammatory cytokines and increased in anti-inflammatory cytokines in the aorta. This was accompanied with increased numbers of CD4++CD25++Foxp3+regulatory T cells (Grundtman et al. 2015) (Fig. 2). Importantly, we could identify and functionally characterize novel atherogenic and atheroprotective mbHSP65 epitopes (Grundtman et al. 2015). To further analyze and understand the functionality of these peptides and to investigate if they could be used as anti-atherosclerosis vaccines without compromising protective immunity against other, non-atherosclerosis-associated domains of the HSP60 molecule not associated with atherosclerosis are needed. Another method of oral immunization with mbHSP65 used genetically modified recombinant Lactococcus lactis stains to deliver the protein to the mucosa and induce intracellular or extracellular production of the protein (Jing et al. 2011). Using this method, atherosclerosis was attenuated in LDLr−/− mice. This antigen-specific tolerance was probably mediated by a shift from a TH1 cell immune response to a TH2 cell response, because IL-10 concentrations increased and IFN-γ levels decreased in vitro (Jing et al. 2011). Maron et al. investigated the effect of nasal and oral administration of mbHSP65 using LDLr−/− mice that maintained on a high-cholesterol diet (Maron et al. 2002). A significant decrease in the size of atherosclerotic plaques, a reduction in macrophage-positive area in the aortic arch, decreased IFN-γ expression (TH1), increased IL-10 expression (TH2), a reduced number of CD4+ T cells, and decreased levels of anti-mbHSP65 antibodies were found in nasally treated mice (Maron et al. 2002). The antibodies showed a TH2-phenotype pattern with significantly increased amounts of IgG1 antibodies, which also is consistent with the cytokine profile found in these mice (Maron et al. 2002). Maron et al. also showed that mucosal treatment with mbHSP65 stimulates the development of adaptive immune cells that secrete anti-inflammatory cytokines (IL-10) and that these cells can then migrate from mucosal inductive sites to the aorta, where they are restimulated by HSP to secrete anti-inflammatory cytokines (Maron et al. 2002). The anti-inflammatory environment in the vascular wall then leads to a decrease in inflammatory IFN-γ secreting cells, which can result in an enhanced secretion of IL-10 by macrophages and SMCs (Fig. 1). Most of these studies have only used full-length HSP60/65 and not defined proatherogenic peptides, therefore, not taking into account the importance of bacterial-human cross-reactive epitopes. However, several studies have successfully investigated the possibility to treat experimental atherosclerosis with HSP60-specific peptides.
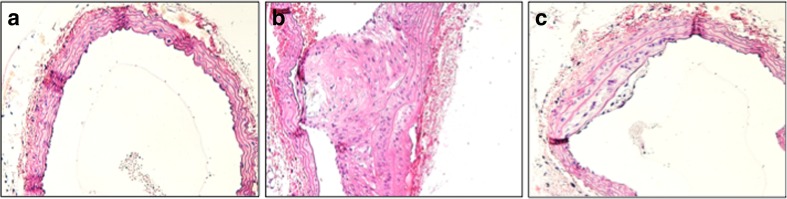
Fig. 2.
Mayer’s hematoxylin and eosin staining of the brachiocephalic artery of an ApoE–/– mouse fed with a conventional diet, in addition b immunized with mbHSP65, and c orally tolerized with full-length mbHSP65. A significant increase in lesion size is seen after mbHSP65 immunization. In contrast, an amelioration of the lesion size is seen after oral mbHSP65 treatment. Original magnification ×200
Recently, oral tolerance against mbHSP60, mbHSP60 peptide 253-268, and HSP70 peptide 111-125 (the sequence was based on a partially conserved human, rat, and mouse sequence of the HSP70 molecule) were scrutinized (van Puijvelde et al. 2007). In mbHSP60 and mbHSP60-peptide-treated LDLr−/− mice, the plaque size in carotid arteries have been reduced by 80 % and by 27 % in the aortic root (van Puijvelde et al. 2007). The plaque size reduction correlated with an increase in CD4+CD25+Foxp3+ regulatory T cells in several organs and an increased mRNA expression of Foxp3, CD25, and CTLA-4 in atherosclerotic lesions of treated mice (van Puijvelde et al. 2007). A 13- and 9-fold increased T cell proliferation confirmed that mbHSP60 but also the mbHSP60-peptide can induce a specific T cell response. However, after oral treatment, mice showed a significant reduction in proliferative responses to mbHSP60 (van Puijvelde et al. 2007). Moreover, tolerance induction lead to the production of IL-10 and transforming growth factor (TGF)-β by lymph nodes cells in response to mbHSP60 (van Puijvelde et al. 2007). Induced oxLDL-specific regulatory T cells are responsible for the reduction in atherosclerotic plaque formation (van Puijvelde et al. 2006). When a combination between human apolipoprotein B (ApoB) (688-707) and hHSP60 (153-163) peptides were used to immunize mice, an additive effect on atheroproduction was found (41.2 % reduction in early atherosclerotic lesions) compared to when the ApoB (14.7 %) or hHSP60 (21.2 %) peptides were applied alone by following atherosclerotic lesion development (Lu et al. 2010). In another study, orally induced tolerance to a combination of hApoB peptide 661-680 and hHSP60 peptide 153-163 prevented progression of atherosclerotic lesions and enable plaque stabilization, induction of CD4++CTLA-4+ regulatory T cells and CD4++CD25++Foxp3+ regulatory T cells secreting increased amounts of TGF-β (Mundkur et al. 2013a). Again, the same human ApoB (688-707) and hHSP60 peptide (153-163) were used with the aim to develop an in vitro assay to screen peptide molecules for their inflammatory properties. The results were similar to earlier studies using these peptides, with induced T cell proliferation and expansion of regulatory T cells with IL-10 and TGF-β secretion and reduction of early atherosclerotic lesion formation in mice by 32.1 and 33.5 %, respectively (Mundkur et al. 2013b). It has recently been shown that resident commensal bacterial GroEL, but not mouse-derived HSP60, could cause naïve T cells to differentiate into CD4++CD25++Foxp3+ T cells, indicating that the production of regulatory T cells depends on the type of HSP (Ohue et al. 2011). Furthermore, mice that were immunized with a construct containing multiple epitopes from ApoB100 (688-707), hHSP60 peptide (153-163), and Chlamydia pneumonia (67-74 and 283-291) showed significantly smaller early atherosclerotic lesions (Lu et al. 2012). The reduction in lesion size correlated with cellular infiltration and cytokine/chemokine secretion in the serum or by stimulated spleen cells as well as specific cellular immune responses when compared to controls (Lu et al. 2012) (Fig. 1).
Nasally induced tolerance to HSP60 in mice lead to suppression of atherosclerosis accompanied by a significant increase in CD4++LAP+ and CD4++CD25++Foxp3+ regulatory T cells and a simultaneously increased production of TGF-β (Li et al. 2012). Furthermore, the productive effect of mbHSP65 was neutralized by injection of an antibody to TGF-β (Li et al. 2012). Also in cholesterol-fed wild-type rabbits, nasal immunizations with mbHSP65 effectively attenuated atherosclerosis with a 15 % reduction in aortal lesion size (Xiong et al. 2009). Tolerance to mbHSP65 lead to a suppression of T cell proliferation, increase of IL-10 production, an absence of related antibodies, and a downregulation of serum lipid levels in this group (Xiong et al. 2009). Results from another group of rabbits nasally immunized with HSP65+CTB-P277, a conjugated protein (CTB; is the non-toxic B subunit of the cholera enterotoxin and is used as an adjuvant/fusion protein), used as a vaccine against autoimmune diabetes (Elias and Cohen 1996; Jin et al. 2008), showed a lipid reduction after immunization. However, no tolerance or reduction in lesion size was found (Xiong et al. 2009). Reduction of lipids is therefore not necessarily associated with immune tolerance to HSP65 but probably a by-product of HSP65 immune interference. It might also not be a consequence or a combined phenomenon of HSP65-specific tolerance.
HSP tolerization in other autoimmune diseases
HSP tolerization has been shown to ameliorate a number of autoimmune diseases; however, the mechanism of protection is still largely unclear. For example, a single mbHSP70 immunization can suppress inflammation and tissue damage, and enhance regulatory response as shown by the antigen-specific IL-10 production, in a pristane-induced arthritis (PGIA) model (Wieten et al. 2009). Furthermore, immunization with Mycobacterium vaccae (a mycobacterial strain expressing large amounts of HSP65) resulted in protection or exacerbation of PGIA (Thompson et al. 1991). HSP60-specific T cells response modulating atherogenic responses in adjuvant arthritis have also been shown after DNA vaccination with human HSP70 and HSP90 (Quintana et al. 2004). Pretreatment with a M. tuberculosis (TB-HSP70) peptide 234-252 could suppress the development of adjuvant-induced arthritis in Lewis rats, generating peptide-specific T cells, produced high levels of IL-10 and low levels of IFN-γ (Tanaka et al. 1999). Similarly, a different HSP70 peptide, peptide 111-125, could trigger self-HSP cross-reactive T cells to downregulate arthritis via IL-10 and when given intra-nasally it protect Lewis rats from the development of arthritis (Wendling et al. 2000). Interestingly, the same peptide has been used in another study with the aim to treat atherosclerosis; however, no effect could be found (van Puijvelde et al. 2007). Transfer of B29-induced CD4+CD25+Foxp3+ T cells (B29 is a conserved HSP70-epitope) can suppress established PGIA in mice (van Herwijnen et al. 2012). Recently, a clinical pilot phase II trial with the objection to induce immune deviation by mucosal dnaJP1 peptide-specific immunotherapy in active early rheumatoid arthritis (RA) patients was completed. Immunological analysis at initial, intermediate, and end treatment points showed a change from pro-inflammatory to regulatory T cell function (Prakken et al. 2004). Conclusively, a T cell-dependent, pro-inflammatory pathway can be specifically and safely modulated in patients with RA. Epitope-specific mucosal therapy does not seem to lead to an increased number of epitope-specific T cells, but rather to a functional readjustment of the responding antigen-specific T cells. This study and others (Prakken et al. 2004; Lee et al. 2000) show that committed TH1 cells can still undergo phenotypic change, which previously was considered to be impossible.
Moreover, HSP90 can inhibit spontaneous diabetes in NOD mice (model for spontaneous type I diabetes) (Elias et al. 1991; Birk et al. 1996). Preclinical studies of HSP peptides in NOD mice have gone onto develop DiaPep277 (residues 437-460 of the human HSP60 molecule), with the aim to treat developing diabetes mellitus in humans. This peptide may well be the first therapeutic vaccine with the capacity to reinstall the HSP-mediated immune regulation in this important clinical entity (Aldridge 2012). The results from the phase II and III clinical studies are very promising. DiaPep277 treatment preserved beta-cell functions and improved clinical outcomes over 2 years in newly diagnosed type I diabetes patients (Raz et al. 2001). Other experimental autoimmune diseases inhibited by immunization of HSPs are colitis (Tanaka et al. 2007), acute rejection of skin and tumor allografts (Borges et al. 2010), and experimental autoimmune encephalomyelitis (Billetta et al. 2012).
Conclusions
The role for both pro- and anti-atherogenic innate and adaptive immune responses in atherosclerosis has been proven in several studies. Moreover, common HSP60 autoantigens, against which an immune response with an activation of atheroprotective or atherogenic adaptive immune responses occurs, have been identified in animal and human models of atherosclerosis. Therefore, an induction of immune tolerance through the activation of cellular and humoral immune reactions to these antigens is hypothesized being atheroprotective. The success of using the recently identified specific immunoreactive antigenic HSP60 epitopes for tolerization further supports the idea that active vaccination may emerge as a novel immuno-modulating atheroprotective strategy. The intricate regulatory networks governing these tolerizations, however, are not yet fully understood. Moreover, there is still a lot to learn how certain HSP epitopes are atherogenic while others are atheroprotective. There are some characteristics of a peptide that is desirable to fulfill if it should be used as a tolerization peptide. Firstly, the peptide should be recognized by the human immune system and thus be able to bind to HLA molecules. Secondly, the peptide should mimic the naturally processed epitope, as altered peptides may behave unpredictably, and thirdly, a peptide needs to have high homology to self and still be immunogenic. Furthermore, the peptide should not cause excessive immune activation or inappropriate immune tolerance. There are several HSP60-specific peptide candidates for immunotherapy proven to be effective in different animal models of atherosclerosis. Even though mouse models of atherosclerosis have very much increased our understanding of atherosclerosis, it is still to note that there may be several problems with translating mouse data to humans. Thus, even if the tolerizing approach in mice may form the basis for the subsequent development of such a vaccine in humans, it is rather improbable that the same HSP60 peptide candidate will emerge as atherogenic in both species. However, promising results from clinical trials for treating rheumatoid arthritis and type I diabetes are currently ongoing (Prakken et al. 2004; Raz et al. 2001, 2007; Huurman et al. 2007, 2008; Lazar et al. 2007; Koffeman et al. 2009). A better understanding of these networks is highly warranted. Enhancement of peptide immunogenicity and combination of peptide therapy with immune-modulating agents would be of great importance.
References
- Afek A, George J, Gilburd B, Rauova L, Goldberg I, Kopolovic J, Harats D, Shoenfeld Y. Immunization of low-density lipoprotein receptor deficient (LDL-RD) mice with heat shock protein 65 (HSP-65) promotes early atherosclerosis. J Autoimmun. 2000;14:115–121. doi: 10.1006/jaut.1999.0351. [DOI] [PubMed] [Google Scholar]
- Aldridge S. Toll-like receptor blocker slows beta cell death in type 1 diabetes. Nat Biotechnol. 2012;30:124. doi: 10.1038/nbt0212-124c. [DOI] [PubMed] [Google Scholar]
- Almanzar G, Ollinger R, Leuenberger J, Onestingel E, Rantner B, Zehm S, Cardini B, van der Zee R, Grundtman C, Wick G. Autoreactive HSP60 epitope-specific T-cells in early human atherosclerotic lesions. J Autoimmun. 2012;39:441–450. doi: 10.1016/j.jaut.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amberger A, Maczek C, Jurgens G, Michaelis D, Schett G, Trieb K, Eberl T, Jindal S, Xu Q, Wick G (1997) Co-expression of ICAM-1, VCAM-1, ELAM-1 and Hsp60 in human arterial and venous endothelial cells in response to cytikones and oxidized low-density lipoproteins. Cell Stress Chaperones 2:94–103 [DOI] [PMC free article] [PubMed]
- Ashburner M. Patterns of puffing activity in the salivary gland chromosomes of Drosophila. V. Responses to environmental treatments. Chromosoma. 1970;31:356–376. doi: 10.1007/BF00321231. [DOI] [PubMed] [Google Scholar]
- Benagiano M, D'Elios MM, Amedei A, Azzurri A, van der Zee R, Ciervo A, Rombola G, Romagnani S, Cassone A, Del Prete G. Human 60-kDa heat shock protein is a target autoantigen of T cells derived from atherosclerotic plaques. J Immunol. 2005;174:6509–6517. doi: 10.4049/jimmunol.174.10.6509. [DOI] [PubMed] [Google Scholar]
- Billetta R, Ghahramani N, Morrow O, Prakken B, de Jong H, Meschter C, Lanza P, Albani S. Epitope-specific immune tolerization ameliorates experimental autoimmune encephalomyelitis. Clin Immunol. 2012;145:94–101. doi: 10.1016/j.clim.2012.08.004. [DOI] [PubMed] [Google Scholar]
- Birk OS, Douek DC, Elias D, Takacs K, Dewchand H, Gur SL, Walker MD, van der Zee R, Cohen IR, Altmann DM. A role of HSP60 in autoimmune diabetes: analysis in a transgenic model. Proc Natl Acad Sci U S A. 1996;93:1032–1037. doi: 10.1073/pnas.93.3.1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobryshev YV. Dendritic cells and their role in atherogenesis. Lab Invest. 2010;90:970–984. doi: 10.1038/labinvest.2010.94. [DOI] [PubMed] [Google Scholar]
- Bobryshev YV, Lord RS. Structural heterogeneity and contacting interactions of vascular dendritic cells in early atherosclerotic lesions of the human aorta. J Submicrosc Cytol Pathol. 1996;28:49–60. [PubMed] [Google Scholar]
- Bobryshev YV, Lord RS. Mapping of vascular dendritic cells in atherosclerotic arteries suggests their involvement in local immune-inflammatory reactions. Cardiovasc Res. 1998;37:799–810. doi: 10.1016/S0008-6363(97)00229-0. [DOI] [PubMed] [Google Scholar]
- Borges TJ, Porto BN, Teixeira CA, Rodrigues M, Machado FD, Ornaghi AP, de Souza AP, Maito F, Pavanelli WR, Silva JS, Bonorino C. Prolonged survival of allografts induced by mycobacterial Hsp70 is dependent on CD4+CD25+ regulatory T cells. PLoS One. 2010;5:e14264. doi: 10.1371/journal.pone.0014264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brett SJ, Lamb JR, Cox JH, Rothbard JB, Mehlert A, Ivanyi J. Differential pattern of T cell recognition of the 65-kDa mycobacterial antigen following immunization with the whole protein or peptides. Eur J Immunol. 1989;19:1303–1310. doi: 10.1002/eji.1830190723. [DOI] [PubMed] [Google Scholar]
- Burian K, Kis Z, Virok D, Endresz V, Prohaszka Z, Duba J, Berencsi K, Boda K, Horvath L, Romics L, Fust G, Gonczol E (2001) Independent and joint effects of antibodies to human heat-shock protein 60 and Chlamydia pneumoniae infection in the development of coronary atherosclerosis. Circulation 103:1503–1508 [DOI] [PubMed]
- Choi JI, Chung SW, Kang HS, Rhim BY, Park YM, Kim US, Kim SJ. Epitope mapping of Porphyromonas gingivalis heat-shock protein and human heat-shock protein in human atherosclerosis. J Dent Res. 2004;83:936–940. doi: 10.1177/154405910408301209. [DOI] [PubMed] [Google Scholar]
- Cybulsky MI, Jongstra-Bilen J. Resident intimal dendritic cells and the initiation of atherosclerosis. Curr Opin Lipidol. 2010;21:397–403. doi: 10.1097/MOL.0b013e32833ded96. [DOI] [PubMed] [Google Scholar]
- Elias D, Cohen IR. The HSP60 peptide p277 arrests the autoimmune diabetes induced by the toxin streptozotocin. Diabetes. 1996;45:1168–1172. doi: 10.2337/diab.45.9.1168. [DOI] [PubMed] [Google Scholar]
- Elias D, Reshef T, Birk OS, van der Zee R, Walker MD, Cohen IR. Vaccination against autoimmune mouse diabetes with a T-cell epitope of the human 65-kDa heat shock protein. Proc Natl Acad Sci U S A. 1991;88:3088–3091. doi: 10.1073/pnas.88.8.3088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford PJ, Gemmell E, Hamlet SM, Hasan A, Walker PJ, West MJ, Cullinan MP, Seymour GJ. Cross-reactivity of GroEL antibodies with human heat shock protein 60 and quantification of pathogens in atherosclerosis. Oral Microbiol Immunol. 2005;20:296–302. doi: 10.1111/j.1399-302X.2005.00230.x. [DOI] [PubMed] [Google Scholar]
- Foteinos G, Afzal AR, Mandal K, Jahangiri M, Xu Q. Anti-heat shock protein 60 autoantibodies induce atherosclerosis in apolipoprotein E-deficient mice via endothelial damage. Circulation. 2005;112:1206–1213. doi: 10.1161/CIRCULATIONAHA.105.547414. [DOI] [PubMed] [Google Scholar]
- George J, Shoenfeld Y, Afek A, Gilburd B, Keren P, Shaish A, Kopolovic J, Wick G, Harats D. Enhanced fatty streak formation in C57BL/6J mice by immunization with heat shock protein-65. Arterioscler Thromb Vasc Biol. 1999;19:505–510. doi: 10.1161/01.ATV.19.3.505. [DOI] [PubMed] [Google Scholar]
- George J, Greenberg S, Barshack I, Goldberg I, Keren G, Roth A. Immunity to heat shock protein 65—an additional determinant in intimal thickening. Atherosclerosis. 2003;168:33–38. doi: 10.1016/S0021-9150(03)00055-8. [DOI] [PubMed] [Google Scholar]
- Grundtman C, Wick G. The autoimmune concept of atherosclerosis. Curr Opin Lipidol. 2011;22:327–334. doi: 10.1097/MOL.0b013e32834aa0c2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundtman C, Kreutmayer SB, Almanzar G, Wick MC, Wick G. Heat shock protein 60 and immune inflammatory responses in atherosclerosis. Arterioscler Thromb Vasc Biol. 2011;31:960–968. doi: 10.1161/ATVBAHA.110.217877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundtman C, Jakic B, Buszko M, Onestingel E, Almanzar G, Demetz E, Dietrich H, Cappellano G, Wick G. Mycobacterial heat shock protein 65 (mbHSP65)-induced atherosclerosis: preventive oral tolerization and definition of atheroprotective and atherogenic mbHSP65 peptides. Atherosclerosis. 2015;242:303–310. doi: 10.1016/j.atherosclerosis.2015.06.044. [DOI] [PubMed] [Google Scholar]
- Harats D, Yacov N, Gilburd B, Shoenfeld Y, George J. Oral tolerance with heat shock protein 65 attenuates Mycobacterium tuberculosis-induced and high-fat-diet-driven atherosclerotic lesions. J Am Coll Cardiol. 2002;40:1333–1338. doi: 10.1016/S0735-1097(02)02135-6. [DOI] [PubMed] [Google Scholar]
- Hoppichler F, Koch T, Dzien A, Gschwandtner G, Lechleitner M. Prognostic value of antibody titre to heat-shock protein 65 on cardiovascular events. Cardiology. 2000;94:220–223. doi: 10.1159/000047320. [DOI] [PubMed] [Google Scholar]
- Huber SA, Sakkinen P, David C, Newell MK, Tracy RP. T helper-cell phenotype regulates atherosclerosis in mice under conditions of mild hypercholesterolemia. Circulation. 2001;103:2610–2616. doi: 10.1161/01.CIR.103.21.2610. [DOI] [PubMed] [Google Scholar]
- Huurman VA, Decochez K, Mathieu C, Cohen IR, Roep BO. Therapy with the hsp60 peptide DiaPep277 in C-peptide positive type 1 diabetes patients. Diabetes Metab Res Rev. 2007;23:269–275. doi: 10.1002/dmrr.691. [DOI] [PubMed] [Google Scholar]
- Huurman VA, van der Meide PE, Duinkerken G, Willemen S, Cohen IR, Elias D, Roep BO. Immunological efficacy of heat shock protein 60 peptide DiaPep277 therapy in clinical type I diabetes. Clin Exp Immunol. 2008;152:488–497. doi: 10.1111/j.1365-2249.2008.03656.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin L, Zhu A, Wang Y, Chen Q, Xiong Q, Li J, Sun Y, Li T, Cao R, Wu J, Liu J. A Th1-recognized peptide P277, when tandemly repeated, enhances a Th2 immune response toward effective vaccines against autoimmune diabetes in nonobese diabetic mice. J Immunol. 2008;180:58–63. doi: 10.4049/jimmunol.180.1.58. [DOI] [PubMed] [Google Scholar]
- Jing H, Yong L, Haiyan L, Yanjun M, Yun X, Yu Z, Taiming L, Rongyue C, Liang J, Jie W, Li Z, Jingjing L. Oral administration of Lactococcus lactis delivered heat shock protein 65 attenuates atherosclerosis in low-density lipoprotein receptor-deficient mice. Vaccine. 2011;29:4102–4109. doi: 10.1016/j.vaccine.2011.03.105. [DOI] [PubMed] [Google Scholar]
- Kanwar RK, Kanwar JR, Wang D, Ormrod DJ, Krissansen GW. Temporal expression of heat shock proteins 60 and 70 at lesion-prone sites during atherogenesis in ApoE-deficient mice. Arterioscler Thromb Vasc Biol. 2001;21:1991–1997. doi: 10.1161/hq1201.100263. [DOI] [PubMed] [Google Scholar]
- Khallou-Laschet J, Tupin E, Caligiuri G, Poirier B, Thieblemont N, Gaston AT, Vandaele M, Bleton J, Tchapla A, Kaveri SV, Rudling M, Nicoletti A. Atheroprotective effect of adjuvants in apolipoprotein E knockout mice. Atherosclerosis. 2006;184:330–341. doi: 10.1016/j.atherosclerosis.2005.04.021. [DOI] [PubMed] [Google Scholar]
- Kleindienst R, Xu Q, Willeit J, Waldenberger FR, Weimann S, Wick G. Immunology of atherosclerosis. Demonstration of heat shock protein 60 expression and T lymphocytes bearing alpha/beta or gamma/delta receptor in human atherosclerotic lesions. Am J Pathol. 1993;142:1927–1937. [PMC free article] [PubMed] [Google Scholar]
- Klingenberg R, Ketelhuth DF, Strodthoff D, Gregori S, Hansson GK. Subcutaneous immunization with heat shock protein-65 reduces atherosclerosis in Apoe(-)/(-) mice. Immunobiology. 2012;217:540–547. doi: 10.1016/j.imbio.2011.06.006. [DOI] [PubMed] [Google Scholar]
- Knoflach M, Kiechl S, Kind M, Said M, Sief R, Gisinger M, van der Zee R, Gaston H, Jarosch E, Willeit J, Wick G. Cardiovascular risk factors and atherosclerosis in young males: ARMY study (atherosclerosis risk-factors in male youngsters) Circulation. 2003;108:1064–1069. doi: 10.1161/01.CIR.0000085996.95532.FF. [DOI] [PubMed] [Google Scholar]
- Knoflach M, Kiechl S, Mayrl B, Kind M, Gaston JS, van der Zee R, Faggionato A, Mayr A, Willeit J, Wick G. T-cell reactivity against HSP60 relates to early but not advanced atherosclerosis. Atherosclerosis. 2007;195:333–338. doi: 10.1016/j.atherosclerosis.2006.09.021. [DOI] [PubMed] [Google Scholar]
- Knoflach M, Kiechl S, Penz D, Zangerle A, Schmidauer C, Rossmann A, Shingh M, Spallek R, Griesmacher A, Bernhard D, Robatscher P, Buchberger W, Draxl W, Willeit J, Wick G. Cardiovascular risk factors and atherosclerosis in young women: atherosclerosis risk factors in female youngsters (ARFY study) Stroke. 2009;40:1063–1069. doi: 10.1161/STROKEAHA.108.525675. [DOI] [PubMed] [Google Scholar]
- Koffeman EC, Genovese M, Amox D, Keogh E, Santana E, Matteson EL, Kavanaugh A, Molitor JA, Schiff MH, Posever JO, Bathon JM, Kivitz AJ, Samodal R, Belardi F, Dennehey C, van den Broek T, van Wijk F, Zhang X, Zieseniss P, Le T, Prakken BA, Cutter GC, Albani S. Epitope-specific immunotherapy of rheumatoid arthritis: clinical responsiveness occurs with immune deviation and relies on the expression of a cluster of molecules associated with T cell tolerance in a double-blind, placebo-controlled, pilot phase II trial. Arthritis Rheum. 2009;60:3207–3216. doi: 10.1002/art.24916. [DOI] [PubMed] [Google Scholar]
- Lamb DJ, El-Sankary W, Ferns GA. Molecular mimicry in atherosclerosis: a role for heat shock proteins in immunisation. Atherosclerosis. 2003;167:177–185. doi: 10.1016/S0021-9150(02)00301-5. [DOI] [PubMed] [Google Scholar]
- Lazar L, Ofan R, Weintrob N, Avron A, Tamir M, Elias D, Phillip M, Josefsberg Z. Heat-shock protein peptide DiaPep277 treatment in children with newly diagnosed type 1 diabetes: a randomised, double-blind phase II study. Diabetes Metab Res Rev. 2007;23:286–291. doi: 10.1002/dmrr.711. [DOI] [PubMed] [Google Scholar]
- Lee HJ, Takemoto N, Kurata H, Kamogawa Y, Miyatake S, O'Garra A, Arai N. GATA-3 induces T helper cell type 2 (Th2) cytokine expression and chromatin remodeling in committed Th1 cells. J Exp Med. 2000;192:105–115. doi: 10.1084/jem.192.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Ding Y, Yi G, Zeng Q, Yang W. Establishment of nasal tolerance to heat shock protein-60 alleviates atherosclerosis by inducing TGF-beta-dependent regulatory T cells. J Huazhong Univ Sci Technolog Med Sci. 2012;32:24–30. doi: 10.1007/s11596-012-0004-z. [DOI] [PubMed] [Google Scholar]
- Lindquist S, Craig EA. The heat-shock proteins. Annu Rev Genet. 1988;22:631–677. doi: 10.1146/annurev.ge.22.120188.003215. [DOI] [PubMed] [Google Scholar]
- Liu P, Yu YR, Spencer JA, Johnson AE, Vallanat CT, Fong AM, Patterson C, Patel DD. CX3CR1 deficiency impairs dendritic cell accumulation in arterial intima and reduces atherosclerotic burden. Arterioscler Thromb Vasc Biol. 2008;28:243–250. doi: 10.1161/ATVBAHA.107.158675. [DOI] [PubMed] [Google Scholar]
- Liu A, Ming JY, Fiskesund R, Ninio E, Karabina SA, Bergmark C, Frostegard AG, Frostegard J. Induction of dendritic cell-mediated T-cell activation by modified but not native low-density lipoprotein in humans and inhibition by annexin a5: involvement of heat shock proteins. Arterioscler Thromb Vasc Biol. 2015;35:197–205. doi: 10.1161/ATVBAHA.114.304342. [DOI] [PubMed] [Google Scholar]
- Lu X, Chen D, Endresz V, Xia M, Faludi I, Burian K, Szabo A, Csanadi A, Miczak A, Gonczol E, Kakkar V. Immunization with a combination of ApoB and HSP60 epitopes significantly reduces early atherosclerotic lesion in Apobtm2SgyLdlrtm1Her/J mice. Atherosclerosis. 2010;212:472–480. doi: 10.1016/j.atherosclerosis.2010.06.007. [DOI] [PubMed] [Google Scholar]
- Lu X, Xia M, Endresz V, Faludi I, Szabo A, Gonczol E, Mundkur L, Chen D, Kakkar V. Impact of multiple antigenic epitopes from ApoB100, hHSP60 and Chlamydophila pneumoniae on atherosclerotic lesion development in Apob(tm2Sgy)Ldlr(tm1Her)J mice. Atherosclerosis. 2012;225:56–68. doi: 10.1016/j.atherosclerosis.2012.07.021. [DOI] [PubMed] [Google Scholar]
- Maron R, Sukhova G, Faria AM, Hoffmann E, Mach F, Libby P, Weiner HL. Mucosal administration of heat shock protein-65 decreases atherosclerosis and inflammation in aortic arch of low-density lipoprotein receptor-deficient mice. Circulation. 2002;106:1708–1715. doi: 10.1161/01.CIR.0000029750.99462.30. [DOI] [PubMed] [Google Scholar]
- Mayr M, Metzler B, Kiechl S, Willeit J, Schett G, Xu Q, Wick G. Endothelial cytotoxicity mediated by serum antibodies to heat shock proteins of Escherichia coli and Chlamydia pneumoniae: immune reactions to heat shock proteins as a possible link between infection and atherosclerosis. Circulation. 1999;99:1560–1566. doi: 10.1161/01.CIR.99.12.1560. [DOI] [PubMed] [Google Scholar]
- Mayr M, Kiechl S, Willeit J, Wick G, Xu Q. Infections, immunity, and atherosclerosis: associations of antibodies to Chlamydia pneumoniae, Helicobacter pylori, and cytomegalovirus with immune reactions to heat-shock protein 60 and carotid or femoral atherosclerosis. Circulation. 2000;102:833–839. doi: 10.1161/01.CIR.102.8.833. [DOI] [PubMed] [Google Scholar]
- McKenzie SL, Henikoff S, Meselson M. Localization of RNA from heat-induced polysomes at puff sites in Drosophila melanogaster. Proc Natl Acad Sci U S A. 1975;72:1117–1121. doi: 10.1073/pnas.72.3.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzler B, Mayr M, Dietrich H, Singh M, Wiebe E, Xu Q, Wick G. Inhibition of arteriosclerosis by T-cell depletion in normocholesterolemic rabbits immunized with heat shock protein 65. Arterioscler Thromb Vasc Biol. 1999;19:1905–1911. doi: 10.1161/01.ATV.19.8.1905. [DOI] [PubMed] [Google Scholar]
- Millonig G, Niederegger H, Rabl W, Hochleitner BW, Hoefer D, Romani N, Wick G. Network of vascular-associated dendritic cells in intima of healthy young individuals. Arterioscler Thromb Vasc Biol. 2001;21:503–508. doi: 10.1161/01.ATV.21.4.503. [DOI] [PubMed] [Google Scholar]
- Millonig G, Niederegger H, Wick G. Analysis of the cellular composition of the arterial intima with modified en face techniques. Lab Invest. 2001;81:639–641. doi: 10.1038/labinvest.3780273. [DOI] [PubMed] [Google Scholar]
- Millonig G, Malcom GT, Wick G. Early inflammatory-immunological lesions in juvenile atherosclerosis from the pathobiological determinants of atherosclerosis in youth (PDAY)-study. Atherosclerosis. 2002;160:441–448. doi: 10.1016/S0021-9150(01)00596-2. [DOI] [PubMed] [Google Scholar]
- Moran L, Mirault ME, Arrigo AP, Goldschmidt-Clermont M, Tissieres A. Heat shock of Drosophila melanogaster induces the synthesis of new messenger RNAs and proteins. Philos Trans R Soc Lond B Biol Sci. 1978;283:391–406. doi: 10.1098/rstb.1978.0044. [DOI] [PubMed] [Google Scholar]
- Mori Y, Kitamura H, Song QH, Kobayashi T, Umemura S, Cyong JC. A new murine model for atherosclerosis with inflammation in the periodontal tissue induced by immunization with heat shock protein 60. Hypertens Res. 2000;23:475–481. doi: 10.1291/hypres.23.475. [DOI] [PubMed] [Google Scholar]
- Mundkur L, Mukhopadhyay R, Samson S, Varma M, Kale D, Chen D, Shivaprasad S, Sivanandan H, Soman V, Lu X, Kakkar VV. Mucosal tolerance to a combination of ApoB and HSP60 peptides controls plaque progression and stabilizes vulnerable plaque in Apob(tm2Sgy)Ldlr(tm1Her)/J mice. PLoS One. 2013;8:e58364. doi: 10.1371/journal.pone.0058364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mundkur LA, Varma M, Shivanandan H, Krishna D, Kumar K, Lu X, Kakkar VV. Activation of inflammatory cells and cytokines by peptide epitopes in vitro: a simple in-vitro screening assay for prioritizing them for in-vivo studies. Inflamm Res. 2013;62:471–481. doi: 10.1007/s00011-013-0599-y. [DOI] [PubMed] [Google Scholar]
- Murshid A, Gong J, Calderwood SK. The role of heat shock proteins in antigen cross presentation. Front Immunol. 2012;3:63. doi: 10.3389/fimmu.2012.00063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohue R, Hashimoto K, Nakamoto M, Furukawa Y, Masuda T, Kitabatake N, Tani F. Bacterial heat shock protein 60, GroEL, can induce the conversion of naive T cells into a CD4 CD25(+) Foxp3-expressing phenotype. J Innate Immun. 2011;3:605–613. doi: 10.1159/000330786. [DOI] [PubMed] [Google Scholar]
- Perschinka H, Mayr M, Millonig G, Mayerl C, van der Zee R, Morrison SG, Morrison RP, Xu Q, Wick G. Cross-reactive B-cell epitopes of microbial and human heat shock protein 60/65 in atherosclerosis. Arterioscler Thromb Vasc Biol. 2003;23:1060–1065. doi: 10.1161/01.ATV.0000071701.62486.49. [DOI] [PubMed] [Google Scholar]
- Pockley AG, Wu R, Lemne C, Kiessling R, de Faire U, Frostegard J. Circulating heat shock protein 60 is associated with early cardiovascular disease. Hypertension. 2000;36:303–307. doi: 10.1161/01.HYP.36.2.303. [DOI] [PubMed] [Google Scholar]
- Prakken BJ, Samodal R, Le TD, Giannoni F, Yung GP, Scavulli J, Amox D, Roord S, de Kleer I, Bonnin D, Lanza P, Berry C, Massa M, Billetta R, Albani S. Epitope-specific immunotherapy induces immune deviation of proinflammatory T cells in rheumatoid arthritis. Proc Natl Acad Sci U S A. 2004;101:4228–4233. doi: 10.1073/pnas.0400061101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintana FJ, Carmi P, Mor F, Cohen IR. Inhibition of adjuvant-induced arthritis by DNA vaccination with the 70-kd or the 90-kd human heat-shock protein: immune cross-regulation with the 60-kd heat-shock protein. Arthritis Rheum. 2004;50:3712–3720. doi: 10.1002/art.20635. [DOI] [PubMed] [Google Scholar]
- Raz I, Elias D, Avron A, Tamir M, Metzger M, Cohen IR. Beta-cell function in new-onset type 1 diabetes and immunomodulation with a heat-shock protein peptide (DiaPep277): a randomised, double-blind, phase II trial. Lancet. 2001;358:1749–1753. doi: 10.1016/S0140-6736(01)06801-5. [DOI] [PubMed] [Google Scholar]
- Raz I, Avron A, Tamir M, Metzger M, Symer L, Eldor R, Cohen IR, Elias D. Treatment of new-onset type 1 diabetes with peptide DiaPep277 is safe and associated with preserved beta-cell function: extension of a randomized, double-blind, phase II trial. Diabetes Metab Res Rev. 2007;23:292–298. doi: 10.1002/dmrr.712. [DOI] [PubMed] [Google Scholar]
- Ritossa FM. A new puffing pattern induced by a temperature shock and DNP in Drosophila. Experientia. 1962;18:571–573. doi: 10.1007/BF02172188. [DOI] [Google Scholar]
- Ritossa FM. Experimental activation of specific loci in polytene chromosomes of Drosophila. Exp Cell Res. 1964;35:601–607. doi: 10.1016/0014-4827(64)90147-8. [DOI] [PubMed] [Google Scholar]
- Rossmann A, Henderson B, Heidecker B, Seiler R, Fraedrich G, Singh M, Parson W, Keller M, Grubeck-Loebenstein B, Wick G. T-cells from advanced atherosclerotic lesions recognize hHSP60 and have a restricted T-cell receptor repertoire. Exp Gerontol. 2008;43:229–237. doi: 10.1016/j.exger.2007.11.009. [DOI] [PubMed] [Google Scholar]
- Schett G, Xu Q, Amberger A, Van der Zee R, Recheis H, Willeit J, Wick G. Autoantibodies against heat shock protein 60 mediate endothelial cytotoxicity. J Clin Invest. 1995;96:2569–2577. doi: 10.1172/JCI118320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seitz CS, Kleindienst R, Xu Q, Wick G. Coexpression of heat-shock protein 60 and intercellular-adhesion molecule-1 is related to increased adhesion of monocytes and T cells to aortic endothelium of rats in response to endotoxin. Lab Invest. 1996;74:241–252. [PubMed] [Google Scholar]
- Shoenfeld Y, Harats D, George J. Heat shock protein 60/65, beta 2-glycoprotein I and oxidized LDL as players in murine atherosclerosis. J Autoimmun. 2000;15:199–202. doi: 10.1006/jaut.2000.0393. [DOI] [PubMed] [Google Scholar]
- Spradling A, Penman S, Pardue ML. Analysis of drosophila mRNA by in situ hybridization: sequences transcribed in normal and heat shocked cultured cells. Cell. 1975;4:395–404. doi: 10.1016/0092-8674(75)90160-9. [DOI] [PubMed] [Google Scholar]
- Sun J, Hartvigsen K, Chou MY, Zhang Y, Sukhova GK, Zhang J, Lopez-Ilasaca M, Diehl CJ, Yakov N, Harats D, George J, Witztum JL, Libby P, Ploegh H, Shi GP. Deficiency of antigen-presenting cell invariant chain reduces atherosclerosis in mice. Circulation. 2010;122:808–820. doi: 10.1161/CIRCULATIONAHA.109.891887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura Y, Torigoe T, Kukita K, Saito K, Okuya K, Kutomi G, Hirata K, Sato N. Heat-shock proteins as endogenous ligands building a bridge between innate and adaptive immunity. Immunotherapy. 2012;4:841–852. doi: 10.2217/imt.12.75. [DOI] [PubMed] [Google Scholar]
- Tanaka S, Kimura Y, Mitani A, Yamamoto G, Nishimura H, Spallek R, Singh M, Noguchi T, Yoshikai Y. Activation of T cells recognizing an epitope of heat-shock protein 70 can protect against rat adjuvant arthritis. J Immunol. 1999;163:5560–5565. [PubMed] [Google Scholar]
- Tanaka K, Namba T, Arai Y, Fujimoto M, Adachi H, Sobue G, Takeuchi K, Nakai A, Mizushima T. Genetic evidence for a protective role for heat shock factor 1 and heat shock protein 70 against colitis. J Biol Chem. 2007;282:23240–23252. doi: 10.1074/jbc.M704081200. [DOI] [PubMed] [Google Scholar]
- Thompson SJ, Butcher PD, Patel VK, Rook GA, Stanford J, van der Zee R, Elson CJ. Modulation of pristane-induced arthritis by mycobacterial antigens. Autoimmunity. 1991;11:35–43. doi: 10.3109/08916939108994706. [DOI] [PubMed] [Google Scholar]
- Tissieres A, Mitchell HK, Tracy UM. Protein synthesis in salivary glands of Drosophila melanogaster: relation to chromosome puffs. J Mol Biol. 1974;84:389–398. doi: 10.1016/0022-2836(74)90447-1. [DOI] [PubMed] [Google Scholar]
- van Herwijnen MJ, Wieten L, van der Zee R, van Kooten PJ, Wagenaar-Hilbers JP, Hoek A, den Braber I, Anderton SM, Singh M, Meiring HD, van Els CA, van Eden W, Broere F. Regulatory T cells that recognize a ubiquitous stress-inducible self-antigen are long-lived suppressors of autoimmune arthritis. Proc Natl Acad Sci U S A. 2012;109:14134–14139. doi: 10.1073/pnas.1206803109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Puijvelde GH, Hauer AD, de Vos P, van den Heuvel R, van Herwijnen MJ, van der Zee R, van Eden W, van Berkel TJ, Kuiper J. Induction of oral tolerance to oxidized low-density lipoprotein ameliorates atherosclerosis. Circulation. 2006;114:1968–1976. doi: 10.1161/CIRCULATIONAHA.106.615609. [DOI] [PubMed] [Google Scholar]
- van Puijvelde GH, van Es T, van Wanrooij EJ, Habets KL, de Vos P, van der Zee R, van Eden W, van Berkel TJ, Kuiper J. Induction of oral tolerance to HSP60 or an HSP60-peptide activates T cell regulation and reduces atherosclerosis. Arterioscler Thromb Vasc Biol. 2007;27:2677–2683. doi: 10.1161/ATVBAHA.107.151274. [DOI] [PubMed] [Google Scholar]
- Wallin RP, Lundqvist A, More SH, von Bonin A, Kiessling R, Ljunggren HG. Heat-shock proteins as activators of the innate immune system. Trends Immunol. 2002;23:130–135. doi: 10.1016/S1471-4906(01)02168-8. [DOI] [PubMed] [Google Scholar]
- Waltner-Romen M, Falkensammer G, Rabl W, Wick G. A previously unrecognized site of local accumulation of mononuclear cells. The vascular-associated lymphoid tissue. J Histochem Cytochem. 1998;46:1347–1350. doi: 10.1177/002215549804601202. [DOI] [PubMed] [Google Scholar]
- Wendling U, Paul L, van der Zee R, Prakken B, Singh M, van Eden W. A conserved mycobacterial heat shock protein (HSP) 70 sequence prevents adjuvant arthritis upon nasal administration and induces IL-10-producing T cells that cross-react with the mammalian self-hsp70 homologue. J Immunol. 2000;164:2711–2717. doi: 10.4049/jimmunol.164.5.2711. [DOI] [PubMed] [Google Scholar]
- Westwood JT, Clos J, Wu C. Stress-induced oligomerization and chromosomal relocalization of heat-shock factor. Nature. 1991;353:822–827. doi: 10.1038/353822a0. [DOI] [PubMed] [Google Scholar]
- Wick G, Kleindienst R, Dietrich H, Xu Q. Is atherosclerosis an autoimmune disease? Trends Food Sci Tech. 1992;3:114–119. doi: 10.1016/0924-2244(92)90154-O. [DOI] [Google Scholar]
- Wick G, Schett G, Amberger A, Kleindienst R, Xu Q. Is atherosclerosis an immunologically mediated disease? Immunol Today. 1995;16:27–33. doi: 10.1016/0167-5699(95)80067-0. [DOI] [PubMed] [Google Scholar]
- Wick G, Knoflach M, Xu Q. Autoimmune and inflammatory mechanisms in atherosclerosis. Annu Rev Immunol. 2004;22:361–403. doi: 10.1146/annurev.immunol.22.012703.104644. [DOI] [PubMed] [Google Scholar]
- Wick G, Jakic B, Buszko M, Wick MC, Grundtman C. The role of heat shock proteins in atherosclerosis. Nat Rev Cardiol. 2014;11:516–529. doi: 10.1038/nrcardio.2014.91. [DOI] [PubMed] [Google Scholar]
- Wieten L, Berlo SE, Ten Brink CB, van Kooten PJ, Singh M, van der Zee R, Glant TT, Broere F, van Eden W. IL-10 is critically involved in mycobacterial HSP70 induced suppression of proteoglycan-induced arthritis. PLoS One. 2009;4:e4186. doi: 10.1371/journal.pone.0004186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigren M, Bengtsson D, Duner P, Olofsson K, Bjorkbacka H, Bengtsson E, Fredrikson GN, Nilsson J. Atheroprotective effects of alum are associated with capture of oxidized LDL antigens and activation of regulatory T cells. Circ Res. 2009;104:e62–e70. doi: 10.1161/CIRCRESAHA.109.196667. [DOI] [PubMed] [Google Scholar]
- Willeit J, Kiechl S. Prevalence and risk factors of asymptomatic extracranial carotid artery atherosclerosis. A population-based study. Arterioscler Thromb. 1993;13:661–668. doi: 10.1161/01.ATV.13.5.661. [DOI] [PubMed] [Google Scholar]
- Xiao Q, Mandal K, Schett G, Mayr M, Wick G, Oberhollenzer F, Willeit J, Kiechl S, Xu Q. Association of serum-soluble heat shock protein 60 with carotid atherosclerosis: clinical significance determined in a follow-up study. Stroke. 2005;36:2571–2576. doi: 10.1161/01.STR.0000189632.98944.ab. [DOI] [PubMed] [Google Scholar]
- Xiong Q, Li J, Jin L, Liu J, Li T. Nasal immunization with heat shock protein 65 attenuates atherosclerosis and reduces serum lipids in cholesterol-fed wild-type rabbits probably through different mechanisms. Immunol Lett. 2009;125:40–45. doi: 10.1016/j.imlet.2009.05.007. [DOI] [PubMed] [Google Scholar]
- Xu Q, Wick G. The role of heat shock proteins in protection and pathophysiology of the arterial wall. Mol Med Today. 1996;2:372–379. doi: 10.1016/S1357-4310(96)10034-4. [DOI] [PubMed] [Google Scholar]
- Xu QB, Oberhuber G, Gruschwit ZM, Wick G. Immunology of atherosclerosis: cellular composition and major histocompatibility complex class II antigen expression in aortic intima, fatty streaks, and atherosclerotic plaques in young and aged human specimens. Clin Immunol Immunopathol. 1990;56:344–359. doi: 10.1016/0090-1229(90)90155-J. [DOI] [PubMed] [Google Scholar]
- Xu Q, Dietrich H, Steiner HJ, Gown AM, Schoel B, Mikuz G, Kaufmann SH, Wick G. Induction of arteriosclerosis in normocholesterolemic rabbits by immunization with heat shock protein 65. Arterioscler Thromb. 1992;12:789–799. doi: 10.1161/01.ATV.12.7.789. [DOI] [PubMed] [Google Scholar]
- Xu Q, Kleindienst R, Waitz W, Dietrich H, Wick G. Increased expression of heat shock protein 65 coincides with a population of infiltrating T lymphocytes in atherosclerotic lesions of rabbits specifically responding to heat shock protein 65. J Clin Invest. 1993;91:2693–2702. doi: 10.1172/JCI116508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Q, Willeit J, Marosi M, Kleindienst R, Oberhollenzer F, Kiechl S, Stulnig T, Luef G, Wick G. Association of serum antibodies to heat-shock protein 65 with carotid atherosclerosis. Lancet. 1993;341:255–259. doi: 10.1016/0140-6736(93)92613-X. [DOI] [PubMed] [Google Scholar]
- Xu Q, Kleindienst R, Schett G, Waitz W, Jindal S, Gupta RS, Dietrich H, Wick G. Regression of arteriosclerotic lesions induced by immunization with heat shock protein 65-containing material in normocholesterolemic, but not hypercholesterolemic, rabbits. Atherosclerosis. 1996;123:145–155. doi: 10.1016/0021-9150(96)05800-5. [DOI] [PubMed] [Google Scholar]
- Xu Q, Kiechl S, Mayr M, Metzler B, Egger G, Oberhollenzer F, Willeit J, Wick G. Association of serum antibodies to heat-shock protein 65 with carotid atherosclerosis: clinical significance determined in a follow-up study. Circulation. 1999;100:1169–1174. doi: 10.1161/01.CIR.100.11.1169. [DOI] [PubMed] [Google Scholar]
- Xu Q, Schett G, Perschinka H, Mayr M, Egger G, Oberhollenzer F, Willeit J, Kiechl S, Wick G. Serum soluble heat shock protein 60 is elevated in subjects with atherosclerosis in a general population. Circulation. 2000;102:14–20. doi: 10.1161/01.CIR.102.1.14. [DOI] [PubMed] [Google Scholar]
- Young RA, Elliott TJ. Stress proteins, infection, and immune surveillance. Cell. 1989;59:5–8. doi: 10.1016/0092-8674(89)90861-1. [DOI] [PubMed] [Google Scholar]
- Zhang X, He M, Cheng L, Chen Y, Zhou L, Zeng H, Pockley AG, Hu FB, Wu T. Elevated heat shock protein 60 levels are associated with higher risk of coronary heart disease in Chinese. Circulation. 2008;118:2687–2693. doi: 10.1161/CIRCULATIONAHA.108.781856. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Xiong Q, Hu X, Sun Y, Tan X, Zhang H, Lu Y, Liu J. A novel atherogenic epitope from Mycobacterium tuberculosis heat shock protein 65 enhances atherosclerosis in rabbit and LDL receptor-deficient mice. Heart Vessels. 2012;27:411–418. doi: 10.1007/s00380-011-0183-8. [DOI] [PubMed] [Google Scholar]