Abstract
Land snails are exposed to conditions of high ambient temperature and low humidity, and their survival depends on a suite of morphological, behavioral, physiological, and molecular adaptations to the specific microhabitat. We tested in six populations of the land snail Theba pisana whether adaptations to different habitats affect their ability to cope with thermal stress and their strategies of heat shock protein (HSP) expression. Levels of Hsp70 and Hsp90 in the foot tissue were measured in field-collected snails and after acclimation to laboratory conditions. Snails were also exposed to various temperatures (32 up to 54 °C) for 2 h and HSP messenger RNA (mRNA) levels were measured in the foot tissue and survival was determined. To test whether the physiological and molecular data are related to genetic parameters, we analyzed T. pisana populations using partial sequences of nuclear and mitochondrial DNA ribosomal RNA genes. We show that populations collected from warmer habitats were more thermotolerant and had higher constitutive levels of Hsp70 isoforms in the foot tissue. Quantitative real-time polymerase chain reaction (PCR) analysis indicated that hsp70 and hsp90 mRNA levels increased significantly in response to thermal stress, although the increase in hsp70 mRNA was larger compared to hsp90 and its induction continued up to higher temperatures. Generally, warm-adapted populations had higher temperatures of maximal induction of hsp70 mRNA synthesis and higher upper thermal limits to HSP mRNA synthesis. Our study suggests that Hsp70 in the foot tissue of T. pisana snails may have important roles in determining stress resistance, while Hsp90 is more likely implicated in signal transduction processes that are activated by stress. In the phylogenetic analysis, T. pisana haplotypes were principally divided into two major clades largely corresponding to the physiological ability to withstand stress, thus pointing to genetically fixed tolerance.
Electronic supplementary material
The online version of this article (doi:10.1007/s12192-015-0652-6) contains supplementary material, which is available to authorized users.
Keywords: HSPs, Land snails, Phylogeny, Heat stress, Environmental stress
Introduction
Terrestrial snails are abundant on land, where conditions of high ambient temperature and low humidity prevail, and are exposed to daily and seasonal changes in temperature and in water availability. Their successful colonization of the terrestrial habitat depends on a range of morphological, physiological, behavioral, and biochemical adaptations for coping with problems of maintaining water, ionic, and thermal balance (Arad 2009; Dittbrenner et al. 2009; Giokas et al. 2005; Riddle 1983; Storey 2002). Temperature is an important environmental factor known to have pronounced effects on physiological performance, survival, and biogeographic distribution in ectothermic animals (McMillan et al. 2011; Parmesan 2006; Portner 2002; Somero 2005). Comparative studies in land snails have revealed that, in general, resistance to heat and aridity is correlated with distribution patterns and with abiotic environmental variation (Cameron 1970; Machin 1967). In a series of studies on water relations and resistance to experimental desiccation of Israeli land snails, Mediterranean snails were less resistant than desert species and populations and the ability to cope with desiccating conditions was correlated with climatic conditions (Arad 2009; Arad et al. 1989; Arad et al. 1992; Arad et al. 1993). In this context, multiple lines of scientific evidence show that the climate system is warming, and further warming is predicted to continue over the following decades (IPCC 2007). The increase in global temperatures is already associated with a wide range of ecological and evolutionary observations, including changes in periodic plant and animal life cycle events in addition to changes in geographical distribution (Parmesan 2006; Parmesan et al. 1999; Visser 2008). The prospective for further warming may be especially important for terrestrial ectotherms, as many of them were found to have limited potential to change their upper thermal limits (Hoffmann et al. 2013).
The land snail Theba pisana (Helicidae) is a bush-dwelling species with a circum-Mediterranean distribution, that is, largely a semelparous species with an annual life cycle (Heller 2009). In Israel, its distribution is limited to a narrow sandy coastal strip along which there is a north-to-south gradient in mean annual rainfall (700 to 300 mm per year) (Jaffe 1988). Recently, we tested populations of T. pisana along a climatic gradient in rainfall and found significant intraspecific differences in resistance to desiccation (Mizrahi et al. 2015). Interestingly, in contradiction to the general assumption that the more arid the habitat, the more adapted to desiccation conditions is the population, the ability of T. pisana snails to cope with desiccating conditions was correlated with habitat temperature but not with the rainfall gradient. We suggested that in T. pisana snails inhabiting the coastal region, temperature is likely to have a major impact on desiccation resistance and, thereby, on species survival and distribution pattern. With the indication for future climate warming, we are interested in better understanding of the physiological and molecular mechanisms by which T. pisana snails cope with temperature changes in their environment.
Most studies on thermal adaptations relate elevated temperatures with traits relevant for thermal stress resistance, including the induction of heat shock proteins (HSPs). HSPs are highly conserved, ubiquitously expressed families of stress response proteins that are induced in diverse organisms by different physiological and environmental stressors (Fabbri et al. 2008; Feder and Hofmann 1999; Lindquist and Craig 1988; Sørensen et al. 2003). HSPs have a critical role in the recovery of cells from stress and in cytoprotection by preventing the irreversible aggregation of stress-denatured proteins and aiding in their refolding into native, functional states. The 70-kDa family is considered the most prominent eukaryotic family of stress proteins and several isoforms exist including the constitutively expressed and the heat-inducible Hsp70, whereas Hsp90 is one of the most abundant cytosolic proteins in eukaryotes (Csermely et al. 1998; Mayer and Bukau 2005; Nollen and Morimoto 2002; Pratt and Toft 2003). It is generally accepted that HSPs protect organisms from the detrimental effects of heat and possibly other environmental stressors including various chemicals, heavy metals, oxidative stress, and desiccation and that stress tolerance depends on the synthesis of HSPs (Bahrndorff et al. 2009; Feder and Hofmann 1999; Kregel 2002; Lindquist 1986; Somero 1995). The expression level of HSPs in each species and population and the stress needed to induce them are strongly related to habitat conditions (Feder and Hofmann 1999). In general, species occupying warmer habitats have higher standing stocks of HSPs and higher thermal threshold for HSP synthesis (Bedulina et al. 2013; Dong et al. 2008; Evgen’ev et al. 2007; Nakano and Iwama 2002; Tomanek and Somero 1999), although evolution in harsh environments may also result in selection for reduced HSP expression, probably due to fitness costs associated with the continuous activation of the HSP machinery (Arad et al. 2010; Mizrahi et al. 2012a; Sørensen et al. 2001; Zatsepina et al. 2001).
Studies in land snails suggest that they use HSPs as part of their survival strategy for coping with environmental stress. Thus, Hsp70 level in the Mediterranean land snail Xeropicta derbentina followed the ambient temperature during diurnal and seasonal variations (Dieterich et al. 2013), and induction of HSPs was demonstrated in different Mediterranean species in response to heat stress (Köhler et al. 2009; Mizrahi et al. 2012b; Reuner et al. 2008; Scheil et al. 2011), short-term experimental aestivation (Brooks and Storey 1995; Ramnanan et al. 2009), and desiccation (Mizrahi et al. 2010). However, comparative studies relating habitat conditions to HSP expression in land snails are sparse. Our studies in two closely related Sphincterochila species occupying different habitats, a desert species Sphincterochila zonata and a Mediterranean-type species Sphincterochila cariosa, demonstrated species-dependent, tissue-specific variation in HSP expression that reflects their habitat conditions (Arad et al. 2010; Mizrahi et al. 2010, 2012b). Recently, a study in the land snail Codringtonia suggested that Codringtonia species adapt to harsher environmental conditions by maintaining higher levels of Hsp70 (Kotsakiozi et al. 2015). Similarly, in our recent study of T. pisana, we found higher levels of Hsp74 in the foot tissue of populations inhabiting warmer habitats together with a delayed and milder response of HSPs to desiccation stress in the warm-adapted, resistant populations compared to the more susceptible populations. This suggests that T. pisana populations from warmer habitats developed distinct strategies of HSP expression for survival (Mizrahi et al. 2015).
The aim of the present study was to improve our knowledge of how adaptations of T. pisana snails to different ecological habitats affect their ability to cope with thermal stress and their strategies of HSP expression. We were especially interested in examining the HSP response to thermal stress at the transcriptional level that can enable the evaluation of the early cellular response. Specifically, we exposed six populations of T. pisana occupying different habitats to various temperatures (32 up to 54 °C) for 2 h and immediately thereafter tested foot immobility as a proxy for mortality and measured hsp70 and hsp90 messenger RNA (mRNA) levels using quantitative real-time polymerase chain reaction (qPCR). In addition, we tested whether HSP expression patterns in the field and after acclimation to laboratory conditions are correlated with thermotolerance and habitat conditions. As part of the study, we performed a phylogenetic analysis of T. pisana populations based on partial sequences of the mitochondrial DNA (mtDNA) 16S ribosomal (r) RNA and nuclear rRNA genes to see whether their genetic characteristics can explain the physiological and molecular data. Understanding the possible mechanisms underlying the stress response as well as the environmental and genetic contributions to variation in stress resistance in T. pisana snails can provide further information regarding their survival strategies and help us evaluate the impact of climatic change in temperature on the species survival.
Materials and methods
Sampling sites of T. pisana snails
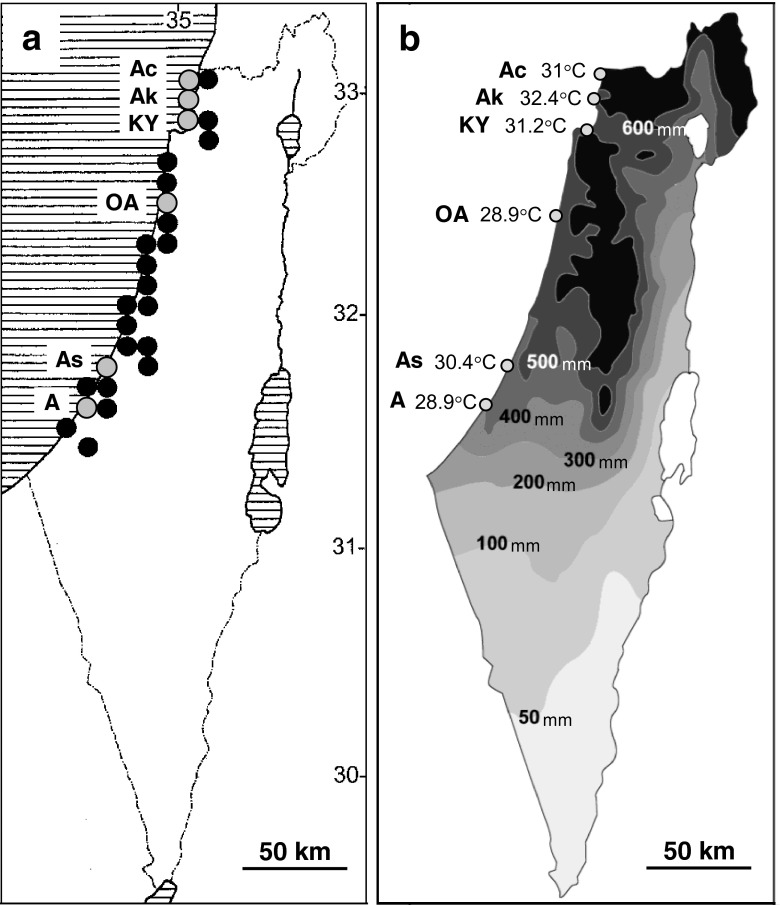
Six populations of adult T. pisana (Muller, 1774) were sampled from different regions along the Mediterranean coast of Israel, broadly covering the distribution of this species: Achziv (600–700 mm annual precipitation; 33.05745° N/35.10540° E), Akko (500–600 mm; 32.95243° N/35.07536° E), Kiryat Yam (500–600 mm; 32.85509° N/35.06676° E), Or Akiva (500–600 mm; 32.48152° N/34.92635° E), Ashdod (400–500 mm; 31.77994° N/34.62200° E), and Ashkelon (400–500 mm; 31.64454° N/34.54939° E) (Fig. 1a, b) (the data for mean annual rainfall were obtained from the Israel Meteorological Service for 1981–2010). The data for maximum daily temperatures in six meteorological stations localized along the coast adjacent to the T. pisana sampling locations were obtained from the Israel Meteorological Service for 2007–2014. In August (the hottest month of the year), the average maximum daily temperatures for 2007–2014 range between 28.9 and 32.4 °C (Fig. 1b). In the northern stations, corresponding to the sampling locations of Achziv, Akko, and Kiryat Yam, temperature measurements were higher compared to all other sites (31.05 ± 0.15, 32.46 ± 0.27, and 31.22 ± 0.40 °C, respectively). The average maximum daily temperature measured near the sampling location of Ashdod was 30.48 ± 0.20 and near Or Akiva and Ashkelon 28.91 ± 0.17 and 28.90 ± 0.17 °C, respectively. Temperature measurements for the summer season (21 of June up to 21 of September for 2007–2014) revealed a similar trend. Average maximum daily temperatures were higher near the sampling locations of Achziv, Akko, and Kiryat Yam (30.32 ± 0.13, 31.68 ± 0.23, and 30.50 ± 0.18 °C, respectively), intermediate near Ashdod (29.85 ± 0.16), and the lowest near Or Akiva and Ashkelon (28.24 ± 0.14 and 28.32 ± 0.12 °C, respectively). ANOVA analysis revealed significant differences among the different sites in temperature measurements for August and for the summer season (P < 0.001). The temperatures measured near the sampling location of Akko population were significantly higher compared to all other sites, whereas temperatures measured near the sampling locations of Ashkelon and Or Akiva were significantly lower compared to all other sites. Temperature analysis for all months of the year revealed a similar trend from April to November.
Fig. 1.
Distribution pattern of Theba pisana in Israel along a climatic gradient. a Distribution map, adapted from Heller and Kadmon (2004). Every black dot represents a sampling area of 25 km2. Our sampling locations are indicated on the map (grey dots): Ac Achziv, Ak Akko, KY Kiryat Yam, OA Or Akiva, As Ashdod, A Ashkelon. b Rainfall (mm per year) and temperature (average maximum daily temperature in August) map, adapted from Kadmon and Heller (1998). Temperature measurements were collected from six meteorological stations (indicated on the map by grey dots) localized along the coast adjacent to the T. pisana sampling locations
Snails of the banded morph (one to four bands) were collected in the field in the fall activity period (October 2011). Snails from Achziv, Akko, and Kiryat Yam were collected on the 26th of October. Maximum temperatures measured near the sampling locations on the date of collection were 25.6, 27.1, and 25.7 °C, respectively. Snails from Or Akiva, Ashdod, and Ashkelon were collected on the 31st of October. Maximum temperatures measured near the sampling locations on the date of collection were 22.4, 23.4, and 22.5 °C, respectively. On both collection dates there was no precipitation. The snails were brought to the laboratory and maintained in aquaria within a temperature-controlled room at 25 ± 0.3 °C (a temperature within the natural range of T. pisana populations investigated in the present study) and a 12L-12D photoperiod. The soil in the aquaria was kept wet and the snails were fed lettuce every other day.
HSP expression in the field samples and after acclimation to laboratory conditions
Sample preparation
Of each population, snails were sampled in the field, brought to the laboratory, and weighed on an analytical balance to the nearest 0.1 mg. For assessment of HSP expression in the field, a group of 10 snails of approximately the same body mass were sacrificed immediately upon arrival, and the foot tissue was dissected out and frozen in liquid nitrogen for later analysis of proteins. A second group was acclimated to laboratory conditions for 3 weeks within a temperature-controlled room at 25 ± 0.3 °C. After the laboratory acclimation period, snails of approximately the same body mass were sacrificed, and the foot tissue was dissected out and frozen in liquid nitrogen (n = 10, except for Kiryat Yam where n = 5). All tissue samples were stored at −80 °C until further processing. Frozen tissues were homogenized using TissueLyser II (Retsch, Qiagen) in ice-cold buffer containing 0.1 M NaCl, 20 mM Tris pH 7.4, 1 mM EDTA, 1 % IGEPAL, 1 mM dithiothreitol (DTT), protease inhibitor cocktail (Sigma, Cat. P-8340), and 1 mM phenylmethylsulfonyl fluoride (PMSF). The homogenate was centrifuged (10 min, 17,000g at 4 °C) and total protein concentration in each supernatant was determined by a standard method (Bradford 1976). The calibration curve of the Bradford assay was created using bovine serum albumin (BSA) standards.
Western blotting and protein quantification
Discontinuous 16 × 16 cm gels, consisting of a 10-% acrylamide/bis-acrylamide separating lower gel and a 4-% acrylamide/bis-acrylamide stacking upper gel (acrylamide/bis-acrylamide 40 % solution (mix ratio 29:1), Sigma, Cat. A7802), were prepared in the laboratory. Equivalent amounts of protein (20 μg) from tissue lysates prepared from individual snails were boiled in sample buffer containing DTT (100 mM DTT, 50 mM Tris pH 6.8, 2 % sodium dodecyl sulfate (SDS), 10 % glycerol, 0.01 % bromophenol blue) and loaded into each well. Proteins were separated by SDS-PAGE for 30 min at 80 V and 180 min at 100 V using the vertical slab electrophoresis instrument PROTEAN II xi cell (Bio-Rad laboratories) and transferred onto nitrocellulose membranes (Pall Gelman Laboratory) by wet electroblotting for 70 min at 100 V using the Trans-Blot Electrophoretic Transfer Cell (Bio-Rad laboratories). The membranes were blocked for 1 h at room temperature in a Tris-buffered saline (TBS) (20 mM Tris-HCl, 0.9 % NaCl, pH 7.6) solution containing 0.05 % Tween-20 (Sigma, Cat. P5927) and 5 % nonfat dry milk (Bio-Rad, Cat. 170-6404). Thereafter, membranes were incubated at room temperature overnight in a primary antibody specific for either Hsp70 protein family members (mouse monoclonal antibody against bovine brain Hsp70 recognizing both the constitutive and the inducible forms of mammalian Hsp70, Sigma, Cat. H-5147, lot 061M4807, dilution 1:5000) or Hsp90 (mouse monoclonal antibody against Hsp90, Sigma, Cat. H-1775, lot 035M4819V, dilution 1:1000). Both antibodies were prepared in TBS solution containing 5 % nonfat dry milk. Thereafter, membranes were washed three times in TBS solution containing 0.05 % Tween-20 (TTBS), then incubated at room temperature for 1.5 h in a secondary antibody (goat anti-mouse IgG conjugated to peroxidase, Sigma, Cat. A-2554, lot 076K4841, dilution 1:25,000 in TTBS containing 5 % nonfat dry milk). After washing three times in TTBS and a final short washing in TBS, the antibody complex was detected by incubating the membranes for 2 min in chemiluminescent peroxidase substrate (WesternBright ECL, Advansta Corporation, USA, Cat. K-12045-D20). The chemiluminescent membranes were imaged by exposure to the camera system ImageQuant LAS 4000 (General Electric Healthcare Life Sciences).
The optical volumes of the individual HSP bands (Hsp72, Hsp74, and Hsp90) were quantified with the densitometry software ImageJ. All samples were quantified relative to a standard (a lysate prepared from the foot tissue of T. pisana), which was run three times on each gel (in the first three wells) to insure comparability among all samples. The intensity of the bands for each individual HSP was related to the mean value of its matching HSP band in the lysate standard. In addition, three samples from each gel were chosen and were simultaneously run on a single gel in order to confirm the correctness of normalization. The samples were analyzed simultaneously on a single Western blot, and their quantification values (expressed as pixel intensity) were found to be positively correlated with the quantification values (relative to standard) of the same samples analyzed on different gels (P < 0.01 for Hsp72, Hsp74, and Hsp90).
Heat shock experiments
Heat exposure
After 1 week of laboratory acclimation, snails from each of the six populations were transferred to plastic containers laid with damp substrate and allowed to hydrate for 24 h. Thereafter, for each population, snails of approximately the same body mass were divided into groups. The control group, referred to as the 25 °C control group, was not submitted to thermal stress, and the snails (n = 5) were sacrificed and the foot tissue was dissected out and frozen in liquid nitrogen for later analysis of RNA. The other groups were placed in plastic containers (n = 10 for each experimental temperature) and transferred into an oven held at either 32, 34, 36, 38, 40, 42, 44, 46, 48, 50, 52, or 54 °C for 2 h at each temperature. Immediately after heat treatment, the immobility of the foot of every snail was recorded after pocking with sharp tweezers, and five snails were sacrificed and the foot tissue was dissected out and frozen in liquid nitrogen for later analysis of RNA. All tissues were stored at −80 °C until further processing. The immobility of the foot was used as a proxy for mortality. The temperature at which 50 % of the snails had died is used as the measure for upper thermal tolerance limit and is referred to as the LT50.
RNA extraction and complementary DNA synthesis
Total RNA was extracted from the foot tissues with TRIzol Reagent (Ambion, Cat. 15596-026, Invitrogen) according to the manufacturer’s instructions. The concentration of RNA was measured by spectrophotometry (NanoDrop ND-1000, Bargal Analytical Instruments), and RNA integrity was analyzed on agarose gel. RNA yield was 0.6–1 μg RNA/1 mg tissue (A260/280 of >1.9). Total RNA was treated with DNase I to remove any contaminating DNA (Ambion DNA-free Kit, Cat. AM1906, Invitrogen) according to the manufacturer’s instructions. Removal of contaminating DNA was assessed by conducting polymerase chain reaction (PCR) with specific primers for reference and target genes (primer design is described in the next section) and analyzing the PCR products on agarose gel. PCR amplifications were carried out in a total volume of 20 μl, consisting of 10 μl of Apex Taq Red Master Mix DNA Polymerase (Genesee Scientific, Cat. 42-137), 50 ng of DNase I-treated RNA, 1 μl of each primer (0.05 μM), and deionized water. The PCR conditions were as follows: an initial denaturation step at 95 °C for 5 min, followed by 40 cycles (94 °C for 30 s, 60 °C for 30 s, and 72 °C for 30 s), and a final extension step at 72 °C for 5 min. First-strand complementary DNA (DNA) synthesis was performed with Thermo Scientific Verso cDNA Synthesis Kit (Cat. AB-1453/B) according to the manufacturer’s instructions. The reaction was carried out in a 20-μl reaction mixture containing RNA-dependent DNA polymerase with a significantly attenuated RNase H activity, anchored oligo-dT as RNA primer, RT enhancer (to remove contaminating DNA), dNTP mix (500 μM each), and 0.5 μg of DNase I-treated RNA. The cDNA synthesis conditions were as follows: an initial synthesis step at 42 °C for 30 min, followed by an inactivation step (to inactivate the RT enhancer) for 2 min at 95 °C.
Primer design, PCR, and cDNA sequencing
We designed specific primers for T. pisana hsp70 and hsp90 and for the reference genes actin and Elongation Factor-1α (EF-1α) (information such as GenBank accession numbers, sequences, and amplicon size (bp) is presented in Table 1 and in Supplementary Table 1). To amplify partial hsp70 and actin sequences from T. pisana, we designed the primers according to hsp70 and actin cDNA of Cantareus apertus (Reuner et al. 2008). For hsp90, the primers were first designed according to hsp84 cDNA of Haliotis tuberculata and then optimized by sequencing the corresponding nucleotide amplificates from T. pisana. For EF-1α, the primers were first designed according to EF-1α cDNA of Baccinum sp. and then optimized by sequencing the corresponding nucleotide amplificates from T. pisana. All PCR amplifications were carried out in a total volume of 20 μl, consisting of 10 μl of Apex Taq Red Master Mix DNA Polymerase, 2 μl cDNA, 1 μl of each primer (0.05 μM), and 6 μl deionized water. The PCR conditions were as follows: an initial denaturation step at 95 °C for 5 min, followed by 40 cycles (94 °C for 30 s, 60 °C for 30 s, and 72 °C for 1 min), and a final extension step at 72 °C for 5 min. In order to ensure that the primers used in this study were amplifying the targeted genes in T. pisana, we extracted PCR products from 1.5 % agarose gel with Wizard SV Gel and PCR Clean-Up system (Promega, Cat. A9281), sequenced them in both directions on a 3500xL Genetic Analyzer (Applied Biosystems), and conducted a BLAST (Basic Local Alignment Search Tool) search against the nonredundant (nr) database of NCBI and the genome sequences of molluscan species in order to verify the homology of the amplified product.
Table 1.
List of primers used for qPCR. Information such as identification, GenBank accession numbers, sequence (5′–3′), and amplicon size (bp) is presented
| Amplicon (bp) | Sequence | GenBank accession no. | Gene name |
|---|---|---|---|
| 100 | Forward 5′-ACTTTGATAACCGCATGGTGAA-3′ Reverse 5′-GTGCGCAGACGTCGGATAG-3′ | EU164851 | Hsp70 |
| 104 | Forward 5′-TGGATCATTCACCGTGATTCA-3′ Reverse 5′-CTTCCAAATACTCCTGCTGATCCT-3′ | AM283515 | Hsp90 |
| 107 | Forward 5′-GCTGGTCGTGACCTCACAGAT-3′ Reverse 5′-AGCTTCTCCTTGATGTCACGAAC-3′ | EU164850 | Actin |
| 100 | Forward 5′-GCCACTGCAGGATGTTTACAAG-3′ Reverse 5′-CGCAAATGTGACCACCATACC-3′ | JN671476 | Elongation Factor 1α |
qPCR analysis
The expression of hsp70 and hsp90 genes in the foot tissue of heat-stressed snails and the control group of each snail population was assessed by quantitative real-time PCR (qPCR). Two reference genes were selected for the analysis: actin and EF-1α. All qPCR reactions were done with the CFX96 real-time system (Bio-Rad) and have been carried out in 96-well plates (BIOplastics, Cat. AB19700, natural color) sealed with optical adhesive film (MicroAmp, Applied Biosystems, Cat. 4311971). Dilution series protocol of pooled cDNAs was used to construct a relative standard curve to determine the PCR efficiencies. PCR efficiency for EF-1α was 96.6 % (R2 = 0.998, slope = −3.406, y-int = 28.291), for actin 102.7 % (R2 = 0.999, slope = −3.259, y-int = 23.244), for hsp70 103.3 % (R2 = 0.997, slope = −3.244, y-int = 22.302), and for hsp90 102.2 % (R2 = 0.998, slope = −3.270, y-int = 25.527).
cDNA samples were diluted 1:10 with deionized water. PCR amplifications were carried out in a total volume of 20 μl, consisting of 10 μl of Perfecta SYBR Green FastMix (Cat. 95072-012, Quanta Biosciences), 4 μl cDNA (from dilution 1:10), 1 μl of each primer (0.05 μM), and 4 μl deionized water. Each reaction of qPCR (corresponding to one individual) was run in triplicate. Since a high number of plates have been run to accommodate all the samples for each population, the control group (n = 5 individuals) was repeated in all plates. Thus, qPCR results from all the different plates connected could be compared among them and analyzed simultaneously. Controls without template cDNA and controls without enzyme were included on PCR plates to ensure the absence of contaminating DNA. The qPCR conditions were as follows: an initial denaturation step at 95 °C for 3 min, followed by 40 cycles (95 °C for 10 s and 60 °C for 45 s), and a final step of melting curve analysis after the amplification where the specificity of the amplification was checked. Data analysis was conducted using the Bio-Rad CFX manager 3.1 software, and the results were expressed as relative quantity (∆Cq, relative to zero). In the present study, thermal treatment had no significant effect on the expression levels of actin and EF-1α, and both were considered appropriate reference genes for examining gene expression. Gene-specific mRNA quantification of hsp70 and hsp90 was performed by normalizing data to the geometric mean of actin and EF-1α mRNA, according to the study of Vandesompele et al. (2002).
DNA analysis
DNA extraction and PCR amplification
For DNA analysis, 10 snails of different banding patterns (one to four bands) in addition to a white shell morph were collected from each sampling site. The snails were brought to the laboratory, and the foot tissue was dissected out and frozen in liquid nitrogen. All tissue samples were stored at −80 °C until further processing. Total DNA was extracted from the foot tissue using DNeasy Blood and Tissue Kit (Qiagen, Cat. 69504) according to the manufacturer’s instructions with the following modification: the lysate at the end of the lysis step was centrifuged through a QIAshredder spin column (Qiagen, Cat. 79654) in order to remove PCR-interfering protein-polysaccharide complexes derived from the mucus of the snail. DNA integrity was analyzed on agarose gel.
A mitochondrial DNA (mtDNA) 16S ribosomal RNA (rRNA) gene fragment of approximately 850 bp was amplified by PCR with primers 16S forward: 5′-AAACATACCTTTTGCATAATGG-3′ and 16S reverse: 5′-CTACGGTCCTTTCGTACTA-3′ (Watanabe and Chiba 2001). Also, an approximately 880-bp region of the nuclear rRNA gene cluster was amplified by PCR, including the 3′ end of the 5.8s gene, the complete internal transcribed spacer (ITS)-2 region, and the 5′ end of the large subunit (LSU; 28s) gene, using the primers LSU1 forward: 5′-CTAGCTGCGAGAATTAATGTGA-3′ and LSU3 reverse: 5′-ACTTTCCCTCACGGTACTTG-3′ (Wade and Mordan 2000). Both PCR reactions were carried out in a total volume of 20 μl, consisting of 10 μl of high-fidelity PrimeSTAR Max DNA Polymerase (Takara Biotechnology, Cat. R045A), 100 ng template DNA, 1 μl of each primer (0.05 μM), and deionized water. The PCR conditions were as follows: an initial denaturation step at 95 °C for 5 min, followed by 40 cycles (94 °C for 30 s, 50 °C annealing for 30 s, and 72 °C extension for 1 min), and a final extension step at 72 °C for 5 min. PCR products were extracted from 1.5 % agarose gel with Wizard SV Gel and PCR Clean-Up system and sequenced in one direction on a 3500xL Genetic Analyzer.
Sequence analysis
Two DNA samples of snail tissue prepared from Theba subdentata (Morocco, N 29° 48.071′/W 09° 38.078′) and Theba sacchii (Morocco, N 28° 08.679′/W 11° 15.814′) were kindly provided by Dr. Carola Greve from the KOENIG museum, Bonn, and were used as outgroups for the phylogenetic and sequence analysis. The final sequence for the 16S rRNA that was used for the phylogenetic and sequence analyses had a length of 791 bp. For the nuclear rRNA gene cluster region, the final sequence had a length of 820 bp, including the 3′ end (61 nucleotides) of the 5.8s gene, the complete ITS-2 region (448 nucleotides), and the 5′ end (311 nucleotides) of the 28s gene. Sequences were aligned using ClustalW 2.0.3 (Dereeper et al. 2008). Phylogenetic relationships were constructed using the maximum likelihood (ML) program PhyML 3.0 (Guindon et al. 2010) using default parameter settings. The neighbor-joining (NJ) starting tree (inferred with BioNJ) was improved by performing either simultaneous nearest neighbor interchanges (NNIs) or by using subtree pruning and regrafting (SPR) topological moves. The resulting ML tree was edited in the program FigTree 1.4.2. For branch support, we used approximate likelihood-ratio test (aLRT) based on a nonparametric, Shimodaira-Hasegawa-like (SH-like) procedure. The aLRT SH-like test is presented as a competitive alternative to nonparametric bootstrap analysis of branch support. The robustness of reconstruction was also assessed by bootstrapping with 1000 replicates. Pairwise-based diversity (number of nucleotide differences within populations), pairwise-based divergence of T. pisana populations from consensus or outgroup sequences, and pairwise-based distance between populations were estimated using DIVEIN (Deng et al. 2010), and their values were presented in percentages. For testing whether genetic parameters of the studied populations significantly reflect molecular or physiological differences, we conducted a correlation analysis between two genetic parameters, mean diversity (within population differences) and mean divergence from consensus sequence (among population differences) and either HSP levels in field-collected or acclimated snails; habitat temperature; thermal tolerance (LT50); or desiccation tolerance (the percentage of total mass loss after 3 weeks of desiccation, adapted from our previous study in T. pisana (Mizrahi et al. 2015)).
Statistics
Statistical analysis of HSP protein expression
The statistical analysis software program SPSS 20.0 was used to check the data for homogeneity of variances (Levene’s test of equality of error variances) and for normality (Kolmogorov-Smirnov and Shapiro-Wilk). Once data were confirmed to fit the validation criteria, SPSS 20.0 was used to perform a one-way ANOVA or a two-way ANOVA, with Tukey or Gabriel’s post hoc tests for multiple comparisons (between the populations). In case of doubt that the population variances are equal, we verified the significance by the Welch F ratio and the Brown-Forsythe F ratio and used the Games-Howell procedure. Otherwise, the significance was verified by independent T test. The SPSS 20.0 program was used to perform correlation analysis. In all cases, P < 0.05 was considered significant.
Statistical analysis of HSP gene expression
SPSS 20.0 was used to perform two-way ANOVA. We describe the first temperature at which the mRNA level was significantly higher than the mRNA level of the 25 °C control group as the onset temperature (Ton) of mRNA synthesis. The temperature of maximal induction of HSP mRNA synthesis was described as Tpeak. The first temperature at which the mRNA level was significantly lower than the mRNA level at Tpeak was described as Toff. The significance was verified by independent T test. In all the cases, P < 0.05 was considered significant.
Results
Thermal tolerance
T. pisana populations were sampled from different regions along the Mediterranean coast, characterized by a north-to-south gradient in mean annual rainfall and relatively small but significant differences in the average maximum daily temperature between the different sites, with the northern populations of Achziv, Akko, and Kiryat Yam occupying warmer habitats compared to all other populations (Fig. 1a, b). All populations survived 2 h of thermal stress up to 48 °C and showed 100 % immobility at 54 °C (Fig. 2a). The six populations differed in their thermotolerance (assessed by examining the withdrawal response of the foot to probing). The more thermotolerant group consisted of the three populations occupying warmer habitats, Achziv, Akko, and Kiryat Yam. Achziv and Kiryat Yam, in particular, succumbed only after exposure to 52 °C. The less thermotolerant group included the populations from the relatively cooler regions of Ashdod, Or Akiva, and Ashkelon. The upper thermal tolerance limit (LT50) was the highest in the population of Kiryat Yam (52.6 °C), Achziv and Akko ranked second (52 °C), Ashdod third (51.6 °C), and Or Akiva and Ashkelon had the lowest LT50s (50.7 and 51 °C, respectively) (Fig. 2b). A positive correlation was found between the LT50 of each population and the average maximum daily temperature in the corresponding habitat.
Fig. 2.
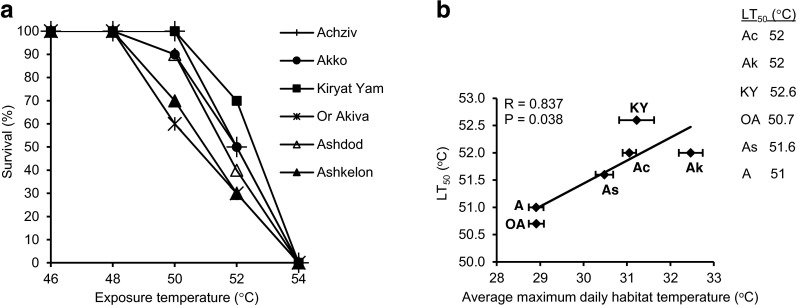
Percentage survival of Theba pisana populations after exposure for 2 h to different temperatures (n = 10 at each temperature). Survival was assessed immediately after heat exposure by examining the withdrawal response of the foot. a Mean percentage survival. LT50—the temperature at which 50 % of the snails had died. LT50 is used as the measure for upper thermal tolerance limit. b Correlation analysis relating habitat temperature (the average maximum daily temperatures in August, means ± SE) to LT50. Population abbreviated names: Ac Achziv, Ak Akko, KY Kiryat Yam, OA Or Akiva, As Ashdod, A Ashkelon
HSP expression in the field and after acclimation to laboratory conditions
To compare the physiological status of T. pisana populations collected from different sites, we measured the levels of Hsp70 and Hsp90 in the foot tissue of snails brought from the field and after a laboratory acclimation period of 3 weeks within a temperature-controlled room. We assumed that whereas HSP level in the field may reflect occasional stress events at the time of sampling, the level of HSPs in snails maintained under stable conditions for a long period may suggest a genetically fixed adaptation.
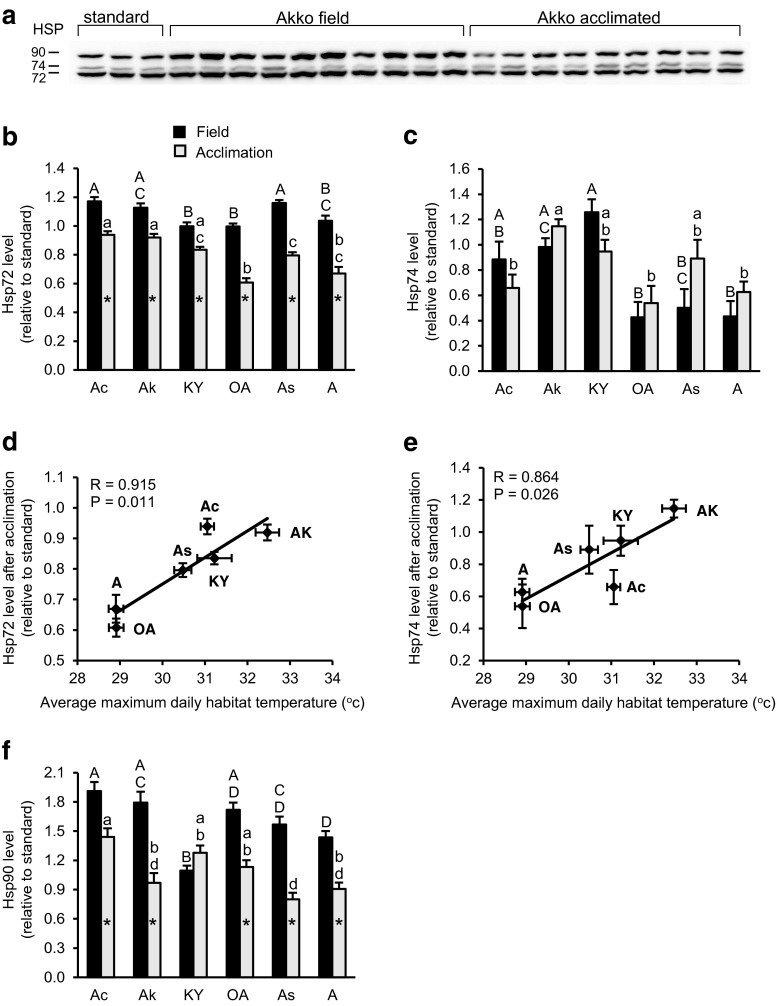
Figure 3a displays a representative Western blot for the effect of long acclimation to laboratory conditions on HSP expression levels in one of the populations (Akko population). As seen, the monoclonal antibody to Hsp70 detected in the foot tissue two forms of Hsp70 of approximately 72 and 74 kDa. Results of two-way ANOVA with population and the state of snails (brought from the field or acclimated) as main effects show that both factors had significant effects on Hsp72 level and the interaction between them was also significant (P < 0.001), whereas for Hsp74, there was a significant effect of population (P < 0.001), but there was no effect of state. The levels of Hsp72 in acclimated snails were significantly lower than in snails brought from the field, suggesting that the snails were exposed to higher stress conditions in their natural habitat (Fig. 3b and Supplementary Table 2a). Post hoc comparisons of Hsp70 isoform levels showed significant differences among populations in snails brought from the field (P < 0.001) and after acclimation (P < 0.001 for Hsp72 and P < 0.01 for Hsp74). In general, populations collected from warmer habitats maintained higher levels of Hsp70 isoforms in the foot tissue. Thus, the populations of Achziv and Akko maintained relatively higher levels of Hsp72 in the field (significantly higher compared to Kiryat Yam and Or Akiva, and in Achziv also compared to Ashkelon) and after acclimation (significantly higher compared to Or Akiva, Ashdod, and Ashkelon) (Fig. 3b and Supplementary Table 2b). In contrast, the populations from the relatively cooler regions of Or Akiva and Ashkelon maintained relatively lower levels of Hsp72 in the field (significantly lower compared to Achziv and Ashdod, and in Or Akiva also compared to Akko) and after acclimation (significantly lower compared to Achziv and Akko, and in Or Akiva also compared to Kiryat Yam and Ashdod). Similarly, Hsp74 levels in the field were significantly higher in the populations of Akko and Kiryat Yam compared to Or Akiva and Ashkelon, and in Kiryat Yam also compared to Ashdod, and after acclimation, the Akko population had significantly higher levels of Hsp74 compared to Achziv, Or Akiva, and Ashkelon (Fig. 3c and Supplementary Table 2c). In correlation analysis, a positive trend was observed between Hsp70 isoform levels in each population and either the corresponding habitat temperature or LT50, though the correlation between Hsp70 isoform levels and habitat temperature was significant only in acclimated snails (Table 2, Fig. 3d, e).
Fig. 3.
HSP levels in populations of Theba pisana in field-collected snails and in acclimated snails. Total protein was extracted from the foot tissue of snails immediately upon arrival from the field (n = 10) and after 3 weeks of acclimation to laboratory conditions at 25 °C (n = 10, except for Kiryat Yam where n = 5) and subjected to Western blotting. a A representative Western blot demonstrating the effect of acclimation on HSP expression levels in one of the populations (Akko population). b Relative levels of Hsp72 in field-collected snails (black columns) and in acclimated snails (grey columns). c Relative levels of Hsp74 in field-collected snails (black columns) and in acclimated snails (grey columns). d Correlation analysis relating habitat temperature (the average maximum daily temperatures in August) to Hsp72 level in acclimated snails (means ± SE). e Correlation analysis relating habitat temperature to Hsp74 level in acclimated snails (means ± SE). f Relative levels of Hsp90 in field-collected snails (black columns) and in acclimated snails (grey columns). The level of each HSP is expressed relative to an internal matching protein standard (means + SE). Different superscript letters above the columns in parts b, c, and f indicate significant differences between populations (post hoc comparisons, P < 0.05; upper case—field collected snails; lower case—acclimated snails). Values sharing the same letter across populations do not differ significantly. Asterisks inside the grey columns denote significant differences between field-collected snails and acclimated snails within each population (independent T test, P < 0.05). Analysis results including P values for each comparison are shown in Supplementary Table 2. Population abbreviated names: Ac Achziv, Ak Akko, KY Kiryat Yam, OA Or Akiva, As Ashdod, A Ashkelon
Table 2.
Correlation analysis between HSP levels in each population and habitat temperature (the average maximum daily temperatures in August) of the corresponding population, between HSP levels in each population and the upper thermal tolerance limit (LT50) of the corresponding population, and between LT50 of each population and habitat temperature
| Habitat temperature | LT50 | |||
|---|---|---|---|---|
| R | P | R | P | |
| Hsp72 in field-collected snails | 0.506 | 0.306 | 0.260 | 0.618 |
| Hsp74 in field-collected snails | 0.796 | 0.058 | 0.935 | 0.006* |
| Hsp90 in field-collected snails | 0.128 | 0.809 | −0.298 | 0.566 |
| Hsp72 in acclimated snails | 0.915 | 0.011* | 0.836 | 0.038* |
| Hsp74 in acclimated snails | 0.864 | 0.026* | 0.696 | 0.125 |
| Hsp90 in acclimated snails | 0.191 | 0.717 | 0.415 | 0.413 |
| LT50 | 0.837 | 0.038* | ||
The significance values (P values) are presented in the column. P<0.05 was considered significant
R Pearson correlation
In resemblance to Hsp72, Hsp90 level in the foot tissue was significantly lower in acclimated snails than in snails brought from the field (in all populations except for Kiryat Yam) (Fig. 3f and Supplementary Table 2a). Results of two-way ANOVA on Hsp90 levels showed that there was a significant effect of both population and state of snail factors, and the interaction between them was also significant (P < 0.001). Post hoc comparisons showed significant differences among populations in Hsp90 levels in the field and after acclimation (P < 0.001); however, no relations were found between Hsp90 levels and habitat temperature nor with LT50 (Table 2). Overall, the Achziv population maintained higher levels of Hsp90 in the field (significantly higher compared to Kiryat Yam, Ashdod, and Ashkelon) and after acclimation (significantly higher compared to Akko, Ashdod, and Ashkelon) (Fig. 3f and Supplementary Table 2d).
HSP mRNA expression in response to thermal stress
We exposed the snails to a 2-h thermal stress (32 up to 52 °C). Immediately after heat treatment, we measured hsp70, hsp90, actin, and EF-1α mRNA levels accumulated over the period of thermal stress. Gene-specific mRNA quantification of hsp70 and hsp90 was performed by normalizing data to the geometric mean of the two reference genes actin and EF-1α mRNA. Our presented results are expressed after first normalizing to the geometric mean of the two reference genes, and then expressed relative to the 25 °C control group.
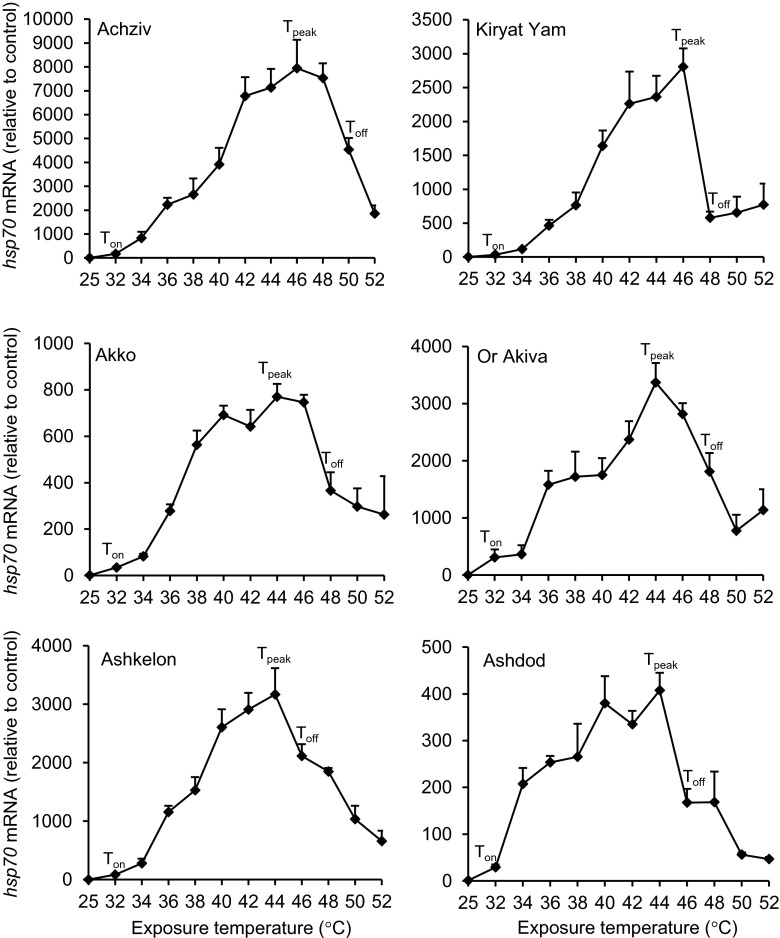
While the monoclonal antibody to Hsp70 protein detected in the foot tissue two forms of Hsp70, cDNA amplification with the primers for T. pisana hsp70 in both heat-exposed and control snail resulted in a single product (100 bp). BLAST search using the sequence of T. pisana hsp70 amplicon against the genome sequences of molluscan species yielded a match to a highly conserved region of HSP70 genes, coding for conserved motifs such as the HSP70 family protein motifs, the HSP70 family motifs of the cytoplasmic members, and the nuclear localization signal (NLS) (Kourtidis et al. 2006). Inside that region, T. pisana hsp70 amplicon was complementary to the highly conserved region coding for NLS, suggesting that the signal observed from qPCR is probably a reflection of the concentration of both HSP70 mRNAs together. Results of two-way ANOVA showed that both temperature and population factors had significant effects on hsp70 mRNA level and the interaction between them was significant (P < 0.001). Hsp70 mRNA levels were significantly upregulated in all populations already at the lowest temperature tested of 32 °C (Fig. 4). Nevertheless, comparison of hsp70 mRNA synthesis pattern at higher temperatures revealed characteristic differences among T. pisana populations that reflected the differences in thermotolerance among them. Among the three populations occupying warmer habitats (Achziv, Akko, and Kiryat Yam), Achziv and Kiryat Yam have the highest temperature of maximal induction of hsp70 mRNA synthesis (Tpeak at 46 °C), 2 °C higher compared to all other populations. In the Akko population, although the peak for hsp70 mRNA induction occurred at 44 °C, high level of synthesis was maintained up to 46 °C, whereas in the populations from the relatively cooler regions of Or Akiva, Ashkelon, and Ashdod, noticeable reduction in hsp70 mRNA level occurred already at 46 °C. The temperature at which hsp70 mRNA synthesis was heat-inactivated was the highest in the Achziv population (Toff at 50 °C), near the upper limit of thermal tolerance (LT50 at 52 °C). In contrast, Ashdod and Ashkelon had the lowest Toff (46 °C), 5.6 and 5 °C lower than their respective thermal tolerance limits.
Fig. 4.
Induction of hsp70 mRNA synthesis in Theba pisana populations during thermal stress. Snails were allowed at least a 1-week acclimation phase to laboratory conditions. The experimental groups of snails (n = 5) were allowed to hydrate for 24 h at 25 °C (control group) and then exposed to various temperatures (32 up to 52 °C) for 2 h at each temperature. Immediately thereafter, hsp70, actin, and Elongation factor-1α (EF-1α) mRNA levels were measured in the foot tissue using qPCR. Our presented results are expressed after first normalizing to the geometric mean of the two reference genes actin and EF-1α, and then expressed relative to the 25 °C control group (means + SE). T on indicates the onset temperature, T peak the temperature of maximal induction, and T off the temperature at which mRNA level was significantly lower than mRNA level at T peak
It is important to note that the intensities of induction of hsp70 mRNA in response to heat stress differed significantly among T. pisana populations (P < 0.001 for peak values), with the Achziv population having the highest value and the Akko and Ashdod populations having the lowest values of maximal hsp70 mRNA induction (Supplementary Table 3a and b). When we compared the levels of hsp70 mRNA in the 25 °C control groups, we found significant differences among populations (P < 0.05). Correlation analysis revealed a negative correlation between the peak value of hsp70 mRNA level of each population and the hsp70 mRNA level of their control group (P = 0.020 and R = −0.883), suggesting that populations with lower levels of hsp70 mRNA at the beginning of the experiment responded to heat stress with higher intensities of induction of hsp70 mRNA.
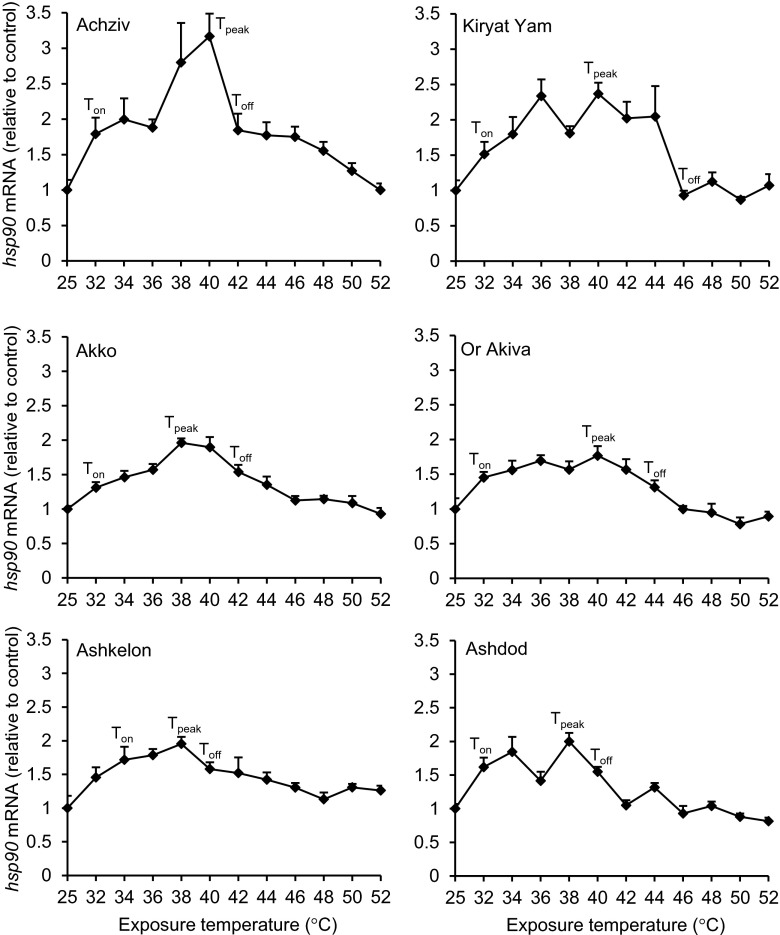
In the case of hsp90 mRNA induction, two-way ANOVA revealed that both temperature and population factors had significant effects on hsp90 mRNA level and the interaction between them was also significant (P < 0.001). However, the expression patterns of hsp90 and hsp70 mRNAs in response to thermal stress were clearly different in terms of magnitude and kinetics. Maximal induction of hsp90 mRNA synthesis was lower by approximately three orders of magnitude compared to hsp70 mRNA and occurred at much lower temperatures (38 to 40 °C) (Fig. 5). Toff occurred at 40 to 44 °C, 2 to 4 °C higher than Tpeak, except for the Kiryat Yam population where Toff occurred at 46 °C, 6 °C higher than Tpeak. In general, our data suggest that the Toff for hsp70 mRNA synthesis is closer to the upper limits of thermal tolerance compared to hsp90 mRNA. Nevertheless, the differences found among T. pisana populations resembled the findings obtained for hsp70 mRNA kinetics. First, the intensities of maximal hsp90 mRNA induction differed significantly among T. pisana populations (P < 0.05), with the Achziv population having the highest value of maximal mRNA induction compared to all other populations (Supplementary Table 3a and c). However, no correlation was found between the peak value of hsp90 mRNA level of each population and the hsp90 mRNA level of their control group. Second, the Ashdod and Ashkelon populations had the lowest Toff values (40 °C) compared to all other populations, supporting their being more sensitive to thermal stress.
Fig. 5.
Induction of hsp90 mRNA synthesis in Theba pisana populations during thermal stress. Snails were allowed at least a 1-week acclimation phase to laboratory conditions. The experimental groups of snails (n = 5) were allowed to hydrate for 24 h at 25 °C (control group) and then exposed to various temperatures (32 up to 52 °C) for 2 h. Immediately thereafter, hsp90, actin, and Elongation factor-1α (EF-1α) mRNA levels were measured in the foot tissue using qPCR. Our presented results are expressed after first normalizing to the geometric mean of the two reference genes actin and EF-1α, and then expressed relative to the 25 °C control group (means + SE). T on indicates the onset temperature, T peak the temperature of maximal induction, and T off the temperature at which mRNA level was significantly lower than mRNA level at T peak
Phylogenetic analysis
Phylogenetic analysis based on mitochondrial and nuclear genes can provide means to examine the roles of history and selection in the origins of biodiversity in land snails (Davison 2002). In the present study, we used partial sequences of nuclear rRNA gene cluster region (820 bp) and mtDNA 16S rRNA gene (791 bp) to infer phylogenetic relationships among T. pisana populations. Two Theba individuals, T. subdentata and T. sacchii, were used as outgroups and were also studied.
The nuclear rRNA gene cluster region comprises a combination of conserved, variable, and highly variable sections. In the present study, the amplified sequence of the ITS-2 region was highly conserved. No variation among populations was found, and only 0.6 % of nucleotides were variable at the genus level. Because the nuclear rRNA region showed extremely high similarity, it was not suitable for examining phylogenetic relationships.
In the present study, alignment analysis of the mtDNA 16S rRNA sequences resulted in a total of 20 haplotypes among T. pisana individuals. Pairwise divergences of T. pisana individuals from outgroups were large, ranging from 23.3 to 24.51 % of sequence divergence from T. sacchii and from 20.5 to 21.64 % from T. subdentata. In contrast, very low levels of divergence from consensus sequence were found among T. pisana haplotypes, ranging from 0.12 to 0.89 % (Table 3). In addition, low levels of diversity were found within populations, ranging from 0.11 % in the Achziv up to 0.69 % in the Ashdod population (Table 4), and the genetic distance between T. pisana populations was small, up to a maximum of 1.66 %. The finding that more than 98 % of nucleotide positions were shared among T. pisana haplotypes strongly suggests that all individuals belonged to the same species.
Table 3.
Genetic analysis of Theba pisana populations based on the 16S rRNA sequence (791 bp). Pairwise-based divergence of T. pisana populations (n = 10 for each population) from consensus sequence
| Population | Mean (%) | STD (%) | Min (%) | Median (%) | Max (%) |
|---|---|---|---|---|---|
| Achziv | 0.2154 | 0.0613 | 0.1266 | 0.2534 | 0.2535 |
| Kiryat Yam | 0.3560 | 0.2872 | 0.1266 | 0.2535 | 0.7643 |
| Akko | 0.2923 | 0.2948 | 0.1266 | 0.1266 | 0.8929 |
| Ashdod | 0.4502 | 0.2212 | 0.1266 | 0.3861 | 0.7629 |
| Or Akiva | 0.5753 | 0.0936 | 0.5075 | 0.5075 | 0.7629 |
| Ashkelon | 0.6771 | 0.1038 | 0.5075 | 0.7051 | 0.7629 |
Table 4.
Genetic analysis of Theba pisana populations based on the 16S rRNA sequence (791 bp). Pairwise-based diversity within T. pisana populations
| Population | Mean (%) | STD (%) | Min (%) | Median (%) | Max (%) |
|---|---|---|---|---|---|
| Achziv | 0.1154 | 0.0928 | 0 | 0.1266 | 0.2533 |
| Kiryat Yam | 0.4762 | 0.4510 | 0 | 0.1266 | 1.0218 |
| Akko | 0.4255 | 0.4922 | 0 | 0.2535 | 1.6995 |
| Ashdod | 0.6193 | 0.4313 | 0 | 0.7629 | 1.1614 |
| Or Akiva | 0.1275 | 0.1154 | 0 | 0.1266 | 0.3885 |
| Ashkelon | 0.2004 | 0.1290 | 0 | 0.1266 | 0.5083 |
In correlation analysis, the genetic parameter of mean divergence of T. pisana populations from consensus sequence (differences among populations) reflected the molecular and physiological differences (Table 5). Correlation analysis revealed significant negative correlations between divergence parameter and either Hsp72 level in acclimated snails (P = 0.006 and R = −0.938) or habitat temperature (P = 0.023 and R = −0.874), a significant positive correlation between divergence and desiccation tolerance (the percentage of total mass loss after 3 weeks of desiccation) (P = 0.005 and R = 0.944), and a negative trend between divergence and the thermal tolerance (LT50) (P = 0.055 and R = −0.801). Neither HSP level, habitat temperature, nor stress resistance were significantly correlated with diversity parameter.
Table 5.
Genetic analysis of Theba pisana populations based on the 16S rRNA sequence (791 bp). Correlation analysis between divergence and diversity and either HSP level, habitat temperature (the average maximum daily temperatures in August), thermal tolerance (LT50), or desiccation tolerance (the percentage of total mass loss after 3 weeks of desiccation, adapted from our previous study in T. pisana (Mizrahi et al. 2015))
| Divergence | Diversity | |||
|---|---|---|---|---|
| R | P | R | P | |
| Hsp72 in acclimated snails | −0.938 | 0.006* | 0.297 | 0.567 |
| Hsp74 in acclimated snails | −0.528 | 0.281 | 0.772 | 0.072 |
| Hsp90 in acclimated snails | −0.556 | 0.809 | −0.507 | 0.304 |
| Hsp72 in field-collected snails | −0.585 | 0.223 | 0.224 | 0.670 |
| Hsp74 in field-collected snails | −0.756 | 0.082 | 0.284 | 0.586 |
| Hsp90 in field-collected snails | −0.312 | 0.547 | −0.439 | 0.383 |
| Thermal tolerance | −0.801 | 0.055 | 0.489 | 0.325 |
| Desiccation tolerance | 0.944 | 0.005* | −0.434 | 0.390 |
| Habitat temperature | −0.874 | 0.023* | 0.477 | 0.338 |
The significance values (P values) are presented in the column. P<0.05 was considered significant
R Pearson correlation
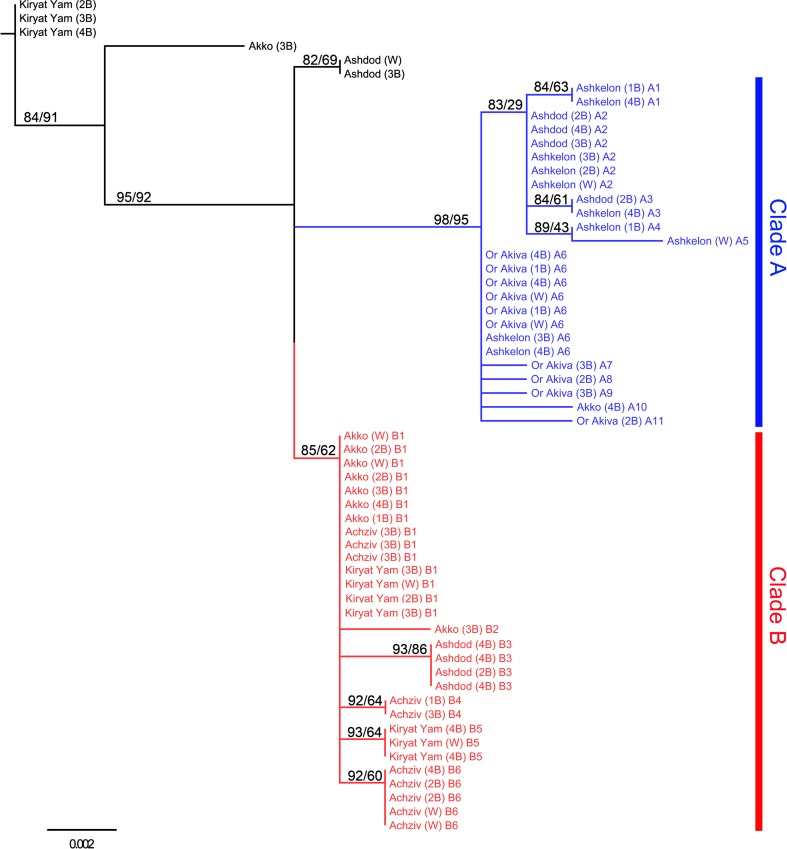
The phylogenetic relationships among the haplotypes were constructed using the maximum likelihood (ML) method. Improvement of the starting NJ tree using either NNI or SPR resulted in a similar ML tree (Fig. 6). Since the two outgroups we used were not suitable for the presentation of the relationships among T. pisana haplotypes, the ML tree is presented unrooted. As indicated in the ML tree, T. pisana haplotypes are principally divided into two major phylogenetic clades that were largely corresponding to the physiological ability to withstand stress. Clade A is well supported in 98 % of aLRT (95 % of bootstrap replicates) and included all individuals belonging to the least stress-resistant populations of Or Akiva and Ashkelon, in addition to 40 % of Ashdod individuals. Inside this clade, the two populations of Ashkelon and Ashdod were very close to each other, suggesting close relationships between them. In addition, clade A contained one individual belonging to the Akko population. Clade B is supported in 85 % of aLRT (62 % of bootstrap replicates) and included the more stress-resistant populations of Achziv (all individuals) Akko, and Kiryat Yam (80 and 70 % of individuals, respectively), in addition to 40 % of Ashdod individuals. T. pisana shell phenotypes seem not to be related to the observed variation in 16S rRNA sequence as snails with different banding patterns had similar haplotypes. It is important to note that sequences from several individuals belonging to the Ashdod, Akko, and Kiryat Yam populations did not cluster together in the phylogenetic tree, suggesting that these populations are polyphyletic. Yet, the evidence from 16S rRNA phylogeny suggest that the ability to withstand stress is related to the population genetic structure.
Fig. 6.
The 16S ribosomal RNA phylogeny for Theba pisana constructed using the maximum likelihood method. The tree is not rooted because there is no suitable outgroup. The haplotypes are principally divided into two major phylogenetic clades (A in blue and B in red). Numbers above nodes are percentage support for individual branches: we used approximate likelihood-ratio test based on a nonparametric SH-like procedure (on the left) or bootstrapping with 1000 replicates (on the right). T. pisana populations are listed by their location name followed by their shell banding patterns in parentheses (number of bands, W indicates white shell) and Haplotype (A1–A11 within clade A, B1–B6 within clade B). Population abbreviated names: Ac Achziv, Ak Akko, KY Kiryat Yam, OA Or Akiva, As Ashdod, A Ashkelon
Discussion
Thermal tolerance
In our previous study, we found that the ability of T. pisana snails to cope with desiccating conditions was correlated with habitat temperature but not with the rainfall gradient (Mizrahi et al. 2015). Our present study in T. pisana demonstrates also a positive correlation between habitat temperature and thermal tolerance, suggesting that temperature plays important roles in determining the ability of T. pisana snails to cope with environmental stress conditions of both heat and aridity. The populations occupying the warmer habitats of Kiryat Yam, Achziv, and Akko were the most heat-tolerant and had higher upper thermal tolerance limits (LT50s of 52.6, 52, and 52 °C, respectively) compared to the populations from the relatively cooler regions of Ashdod, Or Akiva, and Ashkelon (51.6, 50.7, and 51 °C, respectively). Thus, our study is consistent with previous comparative studies of ectothermic species from different habitats demonstrating a direct relationship between habitat temperature and thermotolerance (Sorte and Hofmann 2005; Stillman and Somero 2000). Importantly, our findings for thermal tolerance limits are consistent with the general hierarchy of desiccation resistance, where the populations of Akko, Achziv, and Kiryat Yam were the most desiccation-resistant, the populations of Ashkelon and Or Akiva were the least resistant, and the Ashdod population having an intermediate response to desiccation stress (Mizrahi et al. 2015).
As the terrestrial Mediterranean coastal habitat of T. pisana is characterized by rather mild temperatures, the temperatures of the upper limits in the present study seem to considerably surpass natural heat exposure. However, the extreme maximum temperatures in the coastal habitat are very high, and temperatures up to 46 °C were measured during heat waves in the transition seasons (Jaffe 1988). Thus, the probability that T. pisana snails are exposed to temperatures exceeding 45 °C in nature can explain their ability to survive short-term exposure to temperatures up to 48 °C. The upper thermal limits for survival are of particular interest because they may provide indication on the ability of T. pisana populations to cope with future, further increase in temperature. Comparative studies in aquatic invertebrates investigating the proximity of their LT50 values to current extremes of habitat temperature revealed that the most warm-adapted species may live closer to their thermal tolerance limits and have lower abilities to increase heat tolerance through acclimation than more cold-adapted species (Somero 2005; Stillman and Somero 2000). Moreover, many terrestrial ectotherms were found to have limited potential to change their upper thermal limits (Hoffmann et al. 2013). Although we do not have data on the maximal habitat temperature at the exact site where the snails were sampled for this study, it is possible that the more warm-adapted populations of Achziv, Akko, and Kiryat Yam may live close to their thermal limits, such that small changes in environmental conditions may lead to large changes in distribution and abundance.
HSP expression in field-collected snails and after acclimation
Comparative studies in ectotherms suggest that organisms occupying harsher environments will employ a “preparative defense” strategy involving maintenance of high constitutive levels of Hsp70 in their cells. In thermally challenging environments, this strategy may provide cytoprotection and delay thermal injury without the need for de novo protein synthesis upon thermal stress, thus delaying the induction of HSP synthesis to higher temperatures (Bedulina et al. 2013; Dong et al. 2008; Evgen’ev et al. 2007; Nakano and Iwama 2002; Tomanek and Somero 1999). In this context, a study of the land snail Codringtonia suggested that Codringtonia species adapt to harsher environmental conditions by maintaining higher levels of Hsp70 (Kotsakiozi et al. 2015). In the present study, the expression levels of Hsp70 isoforms in T. pisana differed among populations in both field-collected and in laboratory-acclimated snails. In agreement with the general concept of the preparative defense mechanism discussed above, a positive trend was found between the level of Hsp70 isoforms in each population and either habitat temperature or thermal tolerance, with the least resistant populations of Ashkelon and Or Akiva occupying milder habitats having the lowest levels of Hsp70 isoforms compared to all other populations. These findings suggest that Hsp70 may be important in conferring resistance to thermal stress in T. pisana populations occupying warmer habitats and suggest that they maintain larger constitutive levels of Hsp70 isoforms in the foot tissue as a survival strategy for coping with environmental stress conditions. It is important to note that in our study, the correlation between habitat temperature and Hsp70 isoform level was significant in snails acclimated to laboratory conditions but not in field-collected snails. Our finding that snails expressed higher levels of HSPs in the field suggests that they were exposed to higher stress conditions in their natural habitat thus raise the possibility that for at least some of the populations, the levels of Hsp70 found in field-collected snails are simply a consequence of environmental stress they may have encountered shortly before collection. In contrast, Hsp70 levels in snails acclimated to laboratory conditions for 3 weeks may indicate a genetically fixed adaptation, as that period should have been long enough to allow for the decay of any Hsp70 produced in response to environmental stress (Chapple et al. 1997; Diller 2006; Dong and Dong 2008; Landry et al. 1982).
In contrast to our findings for Hsp70 isoforms, Hsp90 levels in the foot tissue were not related to either habitat temperature or thermal tolerance. Hsp90 is distinguished from other chaperones in that most of its known substrates are signal transduction proteins and in having highly selective functions in normal metabolism (Mayer and Bukau 1999; Pratt and Toft 2003; Young et al. 2001). In our recent study in T. pisana, the level of Hsp74 but not of Hsp90 in the foot tissue was positively correlated with desiccation tolerance, and only Hsp70 isoforms were induced in the foot in response to desiccation stress (Mizrahi et al. 2015). Our findings do not rule out that Hsp90 is acting as a chaperone in the foot tissue of T. pisana snails in response to stress. However, based on our recent study and our present data, we suggest that Hsp90 is more likely implicated in signal transduction processes that are activated by the imposed stress.
HSP mRNA expression in response to thermal stress
Being an external organ, the foot in land snails is an early sensor of environmental changes in temperature. Thus, any change in HSP level may indicate the sensitivity of snails to heat and affect their ability to cope with external heat stress. Accordingly, we hypothesized that the induction pattern of HSPs in T. pisana populations may explain the differences in thermal tolerance observed among them. In response to thermal stress, both hsp70 and hsp90 genes were clearly upregulated in the foot tissue. However, hsp70 showed a massive upregulation of gene expression in response to heat stress, and the temperatures at which hsp70 mRNA synthesis was heat-inactivated were near the upper limits of the thermal tolerance ranges, suggesting that the hsp70 gene has an important role for the survival of T. pisana snails following heat stress. In contrast, the hsp90 gene showed a small increase (approximately three orders of magnitude lower compared to hsp70), and both the peak and the upper thermal limits of hsp90 mRNA synthesis occurred at much lower temperatures compared to hsp70. Likewise, in a study investigating the heat stress-induced expression of HSPs in natural populations of Mediterranean land snails (Köhler et al. 2009), the authors note that T. pisana increased its Hsp70 levels at environmentally relevant temperatures yet kept its Hsp90 levels remarkably low. Moreover, in the study of the tidepool sculpin Oligocottus maculosus, Todgham et al. (2006) found differences in the expression profiles between hsp70 and hsp90 mRNA response to the same thermal conditions and suggested that they likely reflect differences in the mechanisms of HSP regulation among hsp genes and may be a consequence of their differing roles as chaperones. In this context, previous studies in molluscs found several introns in the hsp90 gene (Pantzartzi et al. 2009), which can lead to a lower amount of translatable hsp90 mRNA immediately after thermal stress. The different response of hsp70 and hsp90 genes to thermal stress in T. pisana snails is consistent with our observations at the protein level and further support a direct role for Hsp70 in conferring thermotolerance by stabilizing proteins, while Hsp90 is likely implicated in signal transduction processes accompanying stress conditions.
As discussed above, maintenance of high constitutive levels of HSPs may enable the delayed induction of HSP synthesis in response to higher temperatures. In this context, Köhler et al. (2009) demonstrated heat stress induction of Hsp70 and Hsp90 in Mediterranean populations of X. derbentina, with the more resistant population having a delayed induction and a higher upper thermal limit for Hsp70 synthesis compared to the less resistant population. Thus, we expected that the threshold temperature for hsp70 mRNA synthesis will be higher in the more warm-adapted populations that maintained higher constitutive levels of Hsp70 isoforms. In a previous work studying the response of X. derbentina and T. pisana snails to 8 h of elevated temperatures (25, 33, 38, 40 up to 52 °C), a significant induction of Hsp70 protein was found only after exposure to 38 °C (Köhler et al. 2009). However, in the present study, all T. pisana populations increased HSP mRNA synthesis already at the lowest temperature tested of 32 °C. Nevertheless, despite the similarity in Ton, T. pisana populations differed in two other temperature parameters that are of importance in determining thermal tolerance and distribution patterns, the peak temperatures and the upper thermal limits for HSP mRNA synthesis (Tpeak and Toff). In the present study, the thermal tolerance of T. pisana populations was reflected, in general, in the expression pattern of hsp70 mRNA. Among the three populations occupying warmer habitats, Achziv, Akko, and Kiryat Yam, the peak of hsp70 mRNA induction in Achziv and Kiryat Yam occurred at 46 °C, 2 °C higher compared to all other populations. In addition, although Tpeak occurred at 44 °C in the Akko population, it exhibited an attenuation of the maximal level of hsp70 synthesis up to 46 °C, whereas in the populations from the milder regions, noticeable reduction in hsp70 mRNA level occurred already at 46 °C. Previous observations in marine molluscs and terrestrial insects suggested that differences in Toff for HSP synthesis may play an important role in establishing thermal tolerance limits and, thereby, contribute to the biogeographic distributions (Gehring and Wehner 1995; Sanders et al. 1991; Tomanek and Somero 1999). In agreement, the more warm-adapted, thermoresistant population of Achziv had the highest upper thermal limits of hsp70 mRNA induction. In contrast, two of the populations occupying milder habitats, Ashdod and Ashkelon, that were more sensitive to thermal stress had the lowest upper thermal limits of hsp70 and hsp90 mRNA synthesis, suggesting that the thermal sensitivity of HSP mRNA synthesis by these populations may prevent them from inhabiting warmer habitats.
However, it is important to note that compared to the strong correlation found between the constitutive level of Hsp70 isoforms and thermal tolerance, the patterns of hsp70 mRNA synthesis in response to thermal stress were strongly coupled to thermal tolerance for only some of the T. pisana populations. Thus, while the populations of Or Akiva and Ashkelon were the most susceptible to desiccation and heat stress, Or Akiva continued to induce hsp70 and hsp90 mRNA synthesis up to higher temperatures compared to Ashkelon. Likewise, the study of the Mediterranean land snail X. derbentina (Troschinski et al. 2014) showed that even in similar habitats, within a close range, populations of the same species use different Hsp70 stress response strategies. Although the ability of organisms to express HSPs under different types of stress is an essential mechanism to cope with natural variation in environmental conditions, activation of the HSP machinery is energetically costly and may incur fitness costs on individuals that regularly experience environmental stress, because of reduced energy available for growth and reproduction (Feder 1999; Krebs and Bettencourt 1999; Krebs and Feder 1997; Sørensen et al. 1999). Thus, the expression level of HSPs in each species and population is a balance between costs and benefits, and organisms may exhibit strategies for adaptation to adverse environmental conditions other than HSP expression (Narum et al. 2013; Sørensen et al. 2003; Zatsepina et al. 2001). In this context, our study in the land snail Sphincterochila (Arad et al. 2010; Mizrahi et al. 2012a) suggested that due to the fitness consequences of continuous HSP upregulation, the desert species S. zonata prefers to rely on mechanisms and adaptations for survival other than HSP expression. Our present observations suggest that T. pisana populations occupying similar thermal habitats may differ in their HSP heat stress response strategies. Thus, it is possible that thermotolerance in some T. pisana populations involves other physiological or cellular mechanisms besides HSP expression that affect their ability to cope with thermal stress. In support, in our recent study in T. pisana, we suggested that both early recruitment of water-preserving mechanisms and maintenance of higher constitutive levels of Hsp74 in the foot tissue affect the ability of T. pisana snails to withstand desiccation stress (Mizrahi et al. 2015).
Molecular phylogeny
Ecological speciation can be promoted by adaptation to different thermal habitats (Keller and Seehausen 2012). In order to clarify the relative roles of nonadaptive and adaptive factors in evolution, many studies in land snails used phylogenetic analysis based on mitochondrial and nuclear genes. For example, mtDNA 16S rRNA, mtDNA cytochrome oxidase subunit I (COI), and nuclear ITS 1 phylogenies provided evidence for ecological speciation in species of the land snail Candidula, suggesting that the divergence between the sister species Candidula unifasciata and Candidula rugosiuscula is a result of desiccation-resistant shell characters (Pfenninger et al. 2003). Phylogeography was also implicated in studying historical and speciation events in Theba species. Thus, mitochondrial COI and AFLP phylogenies indicated genotype-environment associations and possibly ecological-driven differentiation in two closely related Theba species (Greve et al. 2012).
In contrast to the high level of divergence found in other studies in land snails in the mtDNA 16S rRNA gene (Davison 2002; Teshima et al. 2003; Watanabe and Chiba 2001), in the present study, we found very low levels of sequence divergence. In addition, the diversity within T. pisana populations and the genetic distance between them were small, up to a maximum of 1.6 %. This extremely low genetic differentiation implies a very recent distribution. Within the genus Theba, T. pisana has the widest distribution; has spread throughout the Mediterranean area and along the European Atlantic coasts; and has been introduced in parts of the USA, South Africa, Australia, and Argentina (Gittenberger and Ripken 1987). Some of the T. pisana introductions occurred relatively recently, probably during the nineteenth and twentieth centuries. Molecular data based on mtDNA and nuclear rRNA sequences strongly supported a Moroccan origin of T. pisana and implied a very recent distribution, apparently mediated by human activities (Daumer et al. 2012; Greve et al. 2010). In line with our present findings of low genetic differentiation within and among T. pisana populations in Israel, T. pisana is a relatively newly introduced species (assumption based on fossil record), arriving only during historic times, some 3000 years ago (Heller 1988). Nevertheless, the molecular phylogeny inferred by 16S rRNA sequences agreed largely with most of the physiological and molecular data, as T. pisana haplotypes were principally divided into a more stress-resistant clade (comprising the populations of Achziv, Akko, and Kiryat Yam, in addition to 40 % of Ashdod individuals) and a less stress-resistant clade (comprising the populations of Ashkelon and Or Akiva, in addition to 40 % of Ashdod individuals). In particular, all individuals belonging to the populations from the milder regions of Ashkelon and Or Akiva, which were the least resistant to desiccation and thermal stress, clustered within one clade. Moreover, the finding that the Ashdod population was included within both clades can explain the intermediate physiological responses of the Ashdod population to stress conditions. Another result that is of particular interest is the close phylogenetic relationship found between Ashdod and Ashkelon populations. The association between them is consistent with their similar pattern of HSP mRNA synthesis in response to thermal stress, as both populations had the lowest upper thermal limits for hsp70 and hsp90 mRNA synthesis compared to all other populations. Correlation analysis confirmed the physiological and molecular data, as the genetic parameter for differences among populations (divergence) could explain the mean differences in Hsp72 level in acclimated snails, habitat temperature, and stress tolerance. This confirmation suggests that the ability of T. pisana snails to withstand stress is related to the population genetic structure, thus pointing to genetically fixed tolerance.
The most straightforward explanation for our findings is that the differences in stress resistance resulted from post-colonization selection in different thermal habitats and have evolved entirely in situ. According to this hypothesis, the higher temperatures in the habitats of the Achziv, Akko, and Kiryat Yam populations directly affected their ability to cope with conditions of heat and aridity, in part by selection for higher constitutive levels of Hsp70 and higher temperatures for hsp70 mRNA synthesis. Similarly, genetic analysis based on mtDNA 16S sequences suggested that the upper thermal tolerance limits in congeneric species of porcelain crabs (genus Petrolisthes) have evolved in response to maximal microhabitat temperature (Stillman and Somero 2000). Moreover, Chu et al. (2014) found latitudinal patterns of population genetic structure and polymorphism in heat stress genes (including Hsp68) in the North Atlantic snail Nucella lapillus and suggested that the divergence between the latitudinal clades may in part stem from adaptation to different thermal environment, and that certain genes associated with heat stress tolerance are under selection for different thermal regimes in the Northwestern Atlantic.
Our findings that sequences from different locations clustered together in the phylogenetic tree suggest that there has been some gene flow among habitats. This possibility is further supported by the presence of identical haplotypes in adjacent locations in both clades. Movement of snails between locations can also explain the apparent polyphyly of individual populations, as sequences from several individuals belonging to the Ashdod, Akko, and Kiryat Yam populations did not cluster together in the phylogenetic tree.
In conclusion, we demonstrated geographic variation in thermal tolerance of T. pisana populations that can be explained by differences in habitat temperature, suggesting an important role for microclimatic temperature selection in this species. The strategies for HSP expression may contribute to the observed difference in thermal tolerance, as the ability to cope with thermal stress was positively correlated with the constitutive levels of Hsp70 isoforms in the foot tissue and was generally reflected in the expression pattern of hsp70 mRNA in response to thermal stress. Our data point to genetically fixed tolerance to environmental stressors of heat and aridity that can contribute to the geographic distributions and abundance of T. pisana snails in Israel and affect their ability to cope with the projected increase in ambient temperatures.
Electronic supplementary material
Amplicon sequences (5′-3′) for T. pisana hsp70, hsp90, actin and Elongation factor 1α (PPTM 40 kb)
Analysis of the effect of acclimation to laboratory conditions on HSP levels. (a) Independent T-test comparisons within each population of HSP levels between field collected snails and acclimated snails. P values are reported for each population. (b-d) Post hoc comparisons between populations of HSP levels. P values are reported for each state: upper p values for field collected snails; lower p values for acclimated snails (PPTM 51 kb)
Analysis of the intensities of induction of hsp70 and hsp90 mRNAs in response to heat stress in T. pisana populations. (a) Peak values for hsp70 and hsp90 mRNA induction (n = 5) (relative to control, means ± SE). (b) Post hoc comparisons between populations of peak values of hsp70 mRNA induction. (c) Post hoc comparisons between populations of peak values of hsp90 mRNA induction (PPTM 49 kb)
Acknowledgments
We wish to express our gratitude to Dr. Carola Greve (KOENIG museum, Bonn, Germany) for providing research material, to Ido Izhaki for his help with the statistical analysis, and to the Life Sciences and Engineering Infrastructure Center for their help with the qPCR analysis. This work was supported by the Israel Science Foundation grant no. 537/11 and the Russell Berrie Nanotechnology Institute.
References
- Arad Z. Resistance to desiccation and heat. In: Heller J, editor. Landsnails of the land of Israel. Sofia and Moscow: Pensoft; 2009. pp. 74–93. [Google Scholar]
- Arad Z, Goldenberg S, Heller J. Resistance to desiccation and distribution patterns in the land snail Sphincterochila. J Zool (Lond.) 1989;218:353–364. doi: 10.1111/j.1469-7998.1989.tb02549.x. [DOI] [Google Scholar]
- Arad Z, Goldenberg S, Heller J. Intraspecific variation in resistance to desiccation and climatic gradients in the distribution of the land snail Xeropicta vestalis. J Zool (Lond.) 1992;226:643–656. doi: 10.1111/j.1469-7998.1992.tb07507.x. [DOI] [Google Scholar]
- Arad Z, Goldenberg S, Heller J. Intraspecific variation in resistance to desiccation and climatic gradients in the distribution of the bush-dwelling land snail Trochoidea simulata. J Zool (Lond.) 1993;229:249–265. doi: 10.1111/j.1469-7998.1993.tb02634.x. [DOI] [Google Scholar]
- Arad Z, Mizrahi T, Goldenberg S, Heller J. Natural annual cycle of heat shock proteins expression in land snails: desert vs. Mediterranean species of Sphincterochila. J Exp Biol. 2010;213:3487–3495. doi: 10.1242/jeb.047670. [DOI] [PubMed] [Google Scholar]
- Bahrndorff S, Marien J, Loeschcke V, Ellers J. Dynamics of heat-induced thermal stress resistance and Hsp70 expression in the springtail, Orchesella cincta. Funct Ecol. 2009;23:233–239. doi: 10.1111/j.1365-2435.2009.01541.x. [DOI] [Google Scholar]
- Bedulina DS, et al. Expression patterns and organization of the hsp70 genes correlate with thermotolerance in two congener endemic amphipod species (Eulimnogammarus cyaneus and E. verrucosus) from Lake Baikal. Mol Ecol. 2013;22:1416–1430. doi: 10.1111/mec.12136. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brooks SP, Storey KB. Evidence for aestivation specific proteins in Otala lactea. Mol Cell Biochem. 1995;143:15–20. doi: 10.1007/BF00925922. [DOI] [PubMed] [Google Scholar]
- Cameron RAD. The survival, weight-loss and behaviour of three species of land snail in conditions of low humidity. J Zool (Lond.) 1970;160:143–157. doi: 10.1111/j.1469-7998.1970.tb02900.x. [DOI] [Google Scholar]
- Chapple JP, Smerdon GR, Hawkins AJS. Stress-70 protein induction in Mytilus edulis: tissue-specific responses to elevated temperature reflect relative vulnerability and physiological function. J Exp Mar Biol Ecol. 1997;217:225–235. doi: 10.1016/S0022-0981(97)00057-9. [DOI] [Google Scholar]
- Chu ND, Kaluziak ST, Trussell GC, Vollmer SV. Phylogenomic analyses reveal latitudinal population structure and polymorphisms in heat stress genes in the North Atlantic snail Nucella lapillus. Mol Ecol. 2014;23:1863–1873. doi: 10.1111/mec.12681. [DOI] [PubMed] [Google Scholar]
- Csermely P, Schnaider T, Soti C, Prohaszka Z, Nardai G. The 90-kDa molecular chaperone family: structure, function, and clinical applications. A comprehensive review. Pharmacol Ther. 1998;79:129–168. doi: 10.1016/S0163-7258(98)00013-8. [DOI] [PubMed] [Google Scholar]
- Daumer C, Greve C, Hutterer R, Misof B, Haase M. Phylogeography of an invasive land snail: natural range expansion versus anthropogenic dispersal in Theba pisana pisana. Biol Invasions. 2012;14:1665–1682. doi: 10.1007/s10530-012-0179-z. [DOI] [Google Scholar]
- Davison A. Land snails as a model to understand the role of history and selection in the origins of biodiversity. Popul Ecol. 2002;44:129–136. doi: 10.1007/s101440200016. [DOI] [Google Scholar]
- Deng W, et al. DIVEIN: a web server to analyze phylogenies, sequence divergence, diversity, and informative sites. Biotechniques. 2010;48:405–408. doi: 10.2144/000113370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dereeper A, et al. Phylogeny.fr: robust phylogenetic analysis for the non-specialist. Nucleic Acids Res. 2008;36:W465–W469. doi: 10.1093/nar/gkn180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieterich A, et al. Daily and seasonal changes in heat exposure and the Hsp70 level of individuals from a field population of Xeropicta derbentina (Krynicki 1836) (Pulmonata, Hygromiidae) in Southern France. Cell Stress Chaperones. 2013;18:405–414. doi: 10.1007/s12192-012-0393-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diller KR. Stress protein expression kinetics. Annu Rev Biomed Eng. 2006;8:403–424. doi: 10.1146/annurev.bioeng.7.060804.100449. [DOI] [PubMed] [Google Scholar]
- Dittbrenner N, Lazzara R, Köhler HR, Mazzia C, Capowiez Y, Triebskorn R. Heat tolerance in Mediterranean land snails: histopathology after exposure to different temperature regimes. J Molluscan Stud. 2009;75:9–18. doi: 10.1093/mollus/eyn033. [DOI] [Google Scholar]
- Dong Y, Dong S. Induced thermotolerance and expression of heat shock protein 70 in sea cucumber Apostichopus japonicus. Fish Sci. 2008;74:573–578. doi: 10.1111/j.1444-2906.2008.01560.x. [DOI] [Google Scholar]
- Dong Y, Miller LP, Sanders JG, Somero GN. Heat-shock protein 70 (Hsp70) expression in four limpets of the genus Lottia: interspecific variation in constitutive and inducible synthesis correlates with in situ exposure to heat stress. Biol Bull. 2008;215:173–181. doi: 10.2307/25470698. [DOI] [PubMed] [Google Scholar]
- Evgen’ev MB, Garbuz DG, Shilova VY, Zatsepina OG. Molecular mechanisms underlying thermal adaptation of xeric animals. J Biosci. 2007;32:489–499. doi: 10.1007/s12038-007-0048-6. [DOI] [PubMed] [Google Scholar]
- Fabbri E, Valbonesi P, Franzellitti S. HSP expression in bivalves. Invertebr Surviv J. 2008;5:135–161. [Google Scholar]
- Feder ME. Organismal, ecological, and evolutionary aspects of heat-shock proteins and the stress response: established conclusions and unresolved issues. Am Zool. 1999;39:857–864. doi: 10.1093/icb/39.6.857. [DOI] [Google Scholar]
- Feder ME, Hofmann GE. Heat-shock proteins, molecular chaperones, and the stress response: evolutionary and ecological physiology. Annu Rev Physiol. 1999;61:243–282. doi: 10.1146/annurev.physiol.61.1.243. [DOI] [PubMed] [Google Scholar]
- Gehring WJ, Wehner R. Heat shock protein synthesis and thermotolerance in Cataglyphis, an ant from Sahara desert. Proc Natl Acad Sci U S A. 1995;92:2994–2998. doi: 10.1073/pnas.92.7.2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giokas S, Pafilis P, Valakos E. Ecological and physiological adaptations of the land snail Albinaria caerulea (pulmonata: Clausiliidae) J Molluscan Stud. 2005;71:15–23. doi: 10.1093/mollus/eyi001. [DOI] [Google Scholar]
- Gittenberger E, Ripken TEJ. The genus Theba (Mollusca: Gastropoda: Helicidae), systematics and distribution. Zool Verh. 1987;241:3–59. [Google Scholar]
- Greve C, Gimnich F, Hutterer R, Misof B, Haase M. Radiating on oceanic islands: patterns and processes of speciation in the land snail genus Theba (Risso 1826) PLoS One. 2012;7 doi: 10.1371/journal.pone.0034339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greve C, Hutterer R, Groh K, Haase M, Misof B. Evolutionary diversification of the genus Theba (Gastropoda: Helicidae) in space and time: a land snail conquering islands and continents. Mol Phylogenet Evol. 2010;57:572–584. doi: 10.1016/j.ympev.2010.08.021. [DOI] [PubMed] [Google Scholar]
- Guindon S, Dufayard JF, Lefort V, Anisimova M, Hordijk W, Gascuel O. New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Syst Biol. 2010;59:307–321. doi: 10.1093/sysbio/syq010. [DOI] [PubMed] [Google Scholar]
- Heller J. The biogeography of the land snails of Israel. In: Yom-Tov Y, Tchernov E, editors. The zoogeography of Israel. Dordrecht: Dr. W. Junk Publishers; 1988. pp. 325–353. [Google Scholar]
- Heller J. Activity. In: Heller J, editor. Land snails of the land of Israel. Sofia and Moscow: Pensoft; 2009. pp. 60–73. [Google Scholar]
- Heller J, Kadmon R. The use of GIS mapping techniques in assessing biodiversity. J Conchol. 2004;3:123–132. [Google Scholar]
- Hoffmann AA, Chown SL, Clusella-Trullas S. Upper thermal limits in terrestrial ectotherms: how constrained are they. Funct Ecol. 2013;27:934–949. doi: 10.1111/j.1365-2435.2012.02036.x. [DOI] [Google Scholar]
- IPCC (2007) Intergovernmental panel on climate change. Cambridge, UK
- Jaffe S. Climate of Israel. In: Yom-Tov Y, Tchernov E, editors. The zoogeography of Israel. Dordrecht: Dr. W. Junk Publishers; 1988. pp. 79–94. [Google Scholar]
- Kadmon R, Heller J. Modelling faunal responses to climatic gradients with GIS: land snails as a case study. J Biogeog. 1998;25:527–539. doi: 10.1046/j.1365-2699.1998.2530527.x. [DOI] [Google Scholar]
- Keller I, Seehausen O. Thermal adaptation and ecological speciation. Mol Ecol. 2012;21:782–799. doi: 10.1111/j.1365-294X.2011.05397.x. [DOI] [PubMed] [Google Scholar]
- Köhler HR, Lazzara R, Dittbrenner N, Capowiez Y, Mazzia C, Triebskorn R. Snail phenotypic variation and stress proteins: do different heat response strategies contribute to Waddington’s widget in field populations? J Exp Zool B Mol Dev Evol. 2009;312:136–147. doi: 10.1002/jez.b.21253. [DOI] [PubMed] [Google Scholar]
- Kotsakiozi P, Parmakelis A, Aggeli IK, Gaitanaki C, Giokas S, Valakos ED. Water balance and expression of heat-shock protein 70 in Codringtonia species: a study within a phylogenetic framework. J Molluscan Stud. 2015;81:24–36. doi: 10.1093/mollus/eyu042. [DOI] [Google Scholar]
- Kourtidis A, Drosopoulou E, Nikolaidis N, Hatzi VI, Chintiroglou CC, Scouras ZG. Identification of several cytoplasmic HSP70 genes from the Mediterranean mussel (Mytilus galloprovincialis) and their long-term evolution in Mollusca and Metazoa. J Mol Evol. 2006;62:446–459. doi: 10.1007/s00239-005-0121-4. [DOI] [PubMed] [Google Scholar]
- Krebs RA, Bettencourt BR. Evolution of thermotolerance and variation in the heat shock protein, Hsp70. Am Zool. 1999;39:910–919. doi: 10.1093/icb/39.6.910. [DOI] [Google Scholar]
- Krebs RA, Feder ME. Deleterious consequences of Hsp70 overexpression in Drosophila melanogaster larvae. Cell Stress Chaperones. 1997;2:60–71. doi: 10.1379/1466-1268(1997)002<0060:DCOHOI>2.3.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kregel KC. Heat shock proteins: modifying factors in physiological stress responses and acquired thermotolerance. J Appl Physiol. 2002;92:2177–2186. doi: 10.1152/japplphysiol.01267.2001. [DOI] [PubMed] [Google Scholar]
- Landry J, Bernier D, Chretien P, Nicole LM, Tanguay RM, Marceau N. Synthesis and degradation of heat shock proteins during development and decay of thermotolerance. Cancer Res. 1982;42:2457–2461. [PubMed] [Google Scholar]
- Lindquist S. The heat-shock response. Annu Rev Biochem. 1986;55:1151–1191. doi: 10.1146/annurev.bi.55.070186.005443. [DOI] [PubMed] [Google Scholar]
- Lindquist S, Craig EA. The heat-shock proteins. Annu Rev Genet. 1988;22:631–677. doi: 10.1146/annurev.ge.22.120188.003215. [DOI] [PubMed] [Google Scholar]
- Machin J. Structural adaptation for reducing water-loss in three species of terrestrial snails. J Zool (Lond.) 1967;152:55–65. doi: 10.1111/j.1469-7998.1967.tb01638.x. [DOI] [Google Scholar]
- Mayer MP, Bukau B. Molecular chaperones: the busy life of Hsp90. Curr Biol. 1999;9:R322–R325. doi: 10.1016/S0960-9822(99)80203-6. [DOI] [PubMed] [Google Scholar]
- Mayer MP, Bukau B. Hsp70 chaperones: cellular functions and molecular mechanism. Cell Mol Life Sci. 2005;62:670–684. doi: 10.1007/s00018-004-4464-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMillan DM, Irschick DJ, Rees BB. Geographic variation in the effects of heat exposure on maximum sprint speed and Hsp70 expression in the western fence lizard Sceloporus occidentalis. Physiol Biochem Zool. 2011;84:573–582. doi: 10.1086/662385. [DOI] [PubMed] [Google Scholar]
- Mizrahi T, Goldenberg S, Heller J, Arad Z. Natural variation in resistance to desiccation and heat shock protein expression in the land snail Theba pisana along a climatic gradient. Physiol Biochem Zool. 2015;88:66–80. doi: 10.1086/679485. [DOI] [PubMed] [Google Scholar]
- Mizrahi T, Heller J, Goldenberg S, Arad Z. Heat shock proteins and resistance to desiccation in congeneric land snails. Cell Stress Chaperones. 2010;15:351–363. doi: 10.1007/s12192-009-0150-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizrahi T, Heller J, Goldenberg S, Arad Z. Heat shock proteins and survival strategies in congeneric land snails (Sphincterochila) from different habitats. Cell Stress Chaperones. 2012;17:523–527. doi: 10.1007/s12192-012-0341-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizrahi T, Heller J, Goldenberg S, Arad Z. The heat shock response in congeneric land snails (Sphincterochila) from different habitats. Cell Stress Chaperones. 2012;17:639–645. doi: 10.1007/s12192-012-0340-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano K, Iwama G. The 70-kDa heat shock protein response in two intertidal sculpins, Oligocottus maculosus and O. snyderi: relationship of hsp70 and thermal tolerance. Comp Biochem Physiol A Mol Integr Physiol. 2002;133:79–94. doi: 10.1016/S1095-6433(02)00115-0. [DOI] [PubMed] [Google Scholar]
- Narum SR, Campbell NR, Meyer KA, Miller MR, Hardy RW. Thermal adaptation and acclimation of ectotherms from differing aquatic climates. Mol Ecol. 2013;22:3090–3097. doi: 10.1111/mec.12240. [DOI] [PubMed] [Google Scholar]
- Nollen EA, Morimoto RI. Chaperoning signaling pathways: molecular chaperones as stress-sensing ‘heat shock’ proteins. J Cell Sci. 2002;115:2809–2816. doi: 10.1242/jcs.115.14.2809. [DOI] [PubMed] [Google Scholar]
- Pantzartzi CN, Kourtidis A, Drosopoulou E, Yiangou M, Scouras ZG. Isolation and characterization of two cytoplasmic hsp90s from Mytilus galloprovincialis (Mollusca: Bivalvia) that contain a complex promoter with a p53 binding site. Gene. 2009;431:47–54. doi: 10.1016/j.gene.2008.10.028. [DOI] [PubMed] [Google Scholar]
- Parmesan C. Ecological and evolutionary responses to recent climate change. Annu Rev Ecol Evol Syst. 2006;37:637–669. doi: 10.1146/annurev.ecolsys.37.091305.110100. [DOI] [Google Scholar]
- Parmesan C, et al. Poleward shifts in geographical ranges of butterfly species associated with regional warming. Nature. 1999;399:579–583. doi: 10.1038/21181. [DOI] [Google Scholar]
- Pfenninger M, Eppenstein A, Magnin F. Evidence for ecological speciation in the sister species Candidula unifasciata (Poiret, 1801) and C. rugosiuscula (Michaud, 1831) (Helicellinae, Gastropoda) Biol J Linn Soc. 2003;79:611–628. doi: 10.1046/j.1095-8312.2003.00212.x. [DOI] [Google Scholar]
- Portner HO. Climate variations and the physiological basis of temperature dependent biogeography: systemic to molecular hierarchy of thermal tolerance in animals. Comp Biochem Physiol A Mol Integr Physiol. 2002;132:739–761. doi: 10.1016/S1095-6433(02)00045-4. [DOI] [PubMed] [Google Scholar]
- Pratt WB, Toft DO. Regulation of signaling protein function and trafficking by the hsp90/hsp70-based chaperone machinery. Exp Biol Med. 2003;228:111–133. doi: 10.1177/153537020322800201. [DOI] [PubMed] [Google Scholar]
- Ramnanan CJ, Allan ME, Groom AG, Storey KB. Regulation of global protein translation and protein degradation in aerobic dormancy. Mol Cell Biochem. 2009;323:9–20. doi: 10.1007/s11010-008-9959-2. [DOI] [PubMed] [Google Scholar]
- Reuner A, Brümmer F, Schill RO. Heat shock proteins (Hsp70) and water content in the estivating Mediterranean Grunt Snail (Cantareus apertus) Comp Biochem Physiol B. 2008;151:28–31. doi: 10.1016/j.cbpb.2008.05.004. [DOI] [PubMed] [Google Scholar]
- Riddle WA. Physiological ecology of land snails and slugs. In: Russell-Hunter WD, editor. The Mollusca. London: Academic; 1983. pp. 431–461. [Google Scholar]
- Sanders BM, Hope C, Pascoe VM, Martin LS. Characterization of the stress protein response in two species of Collisella limpets with different temperature tolerances. Physiol Zool. 1991;64:1471–1489. doi: 10.1086/physzool.64.6.30158225. [DOI] [Google Scholar]
- Scheil AE, Kohler HR, Triebskorn R. Heat tolerance and recovery in Mediterranean land snails after pre-exposure in the field. J Molluscan Stud. 2011;77:165–174. doi: 10.1093/mollus/eyr003. [DOI] [Google Scholar]
- Somero GN. Proteins and temperature. Annu Rev Physiol. 1995;57:43–68. doi: 10.1146/annurev.ph.57.030195.000355. [DOI] [PubMed] [Google Scholar]
- Somero GN. Linking biogeography to physiology: evolutionary and acclimatory adjustments of thermal limits. Front Zool. 2005;2:1–9. doi: 10.1186/1742-9994-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sørensen JG, Dahlgaard J, Loeschcke V. Genetic variation in thermal tolerance among natural populations of Drosophila buzzatii: down regulation of Hsp70 expression and variation in heat stress resistance traits. Funct Ecol. 2001;15:289–296. doi: 10.1046/j.1365-2435.2001.00525.x. [DOI] [Google Scholar]
- Sørensen JG, Kristensen TN, Loeschcke V. The evolutionary and ecological role of heat shock proteins. Ecol Lett. 2003;6:1025–1037. doi: 10.1046/j.1461-0248.2003.00528.x. [DOI] [Google Scholar]
- Sørensen JG, Michalak P, Justesen J, Loeschcke V. Expression of the heat-shock protein HSP70 in Drosophila buzzatii lines selected for thermal resistance. Hereditas. 1999;131:155–164. doi: 10.1111/j.1601-5223.1999.00155.x. [DOI] [PubMed] [Google Scholar]
- Sorte CJB, Hofmann GE. Thermotolerance and heat-shock protein expression in Northeastern Pacific Nucella species with different biogeographical ranges. Mar Biol. 2005;146:985–993. doi: 10.1007/s00227-004-1508-2. [DOI] [Google Scholar]
- Stillman JH, Somero GN. A comparative analysis of the upper thermal tolerance limits of eastern Pacific porcelain crabs, genus Petrolisthes: influences of latitude, vertical zonation, acclimation, and phylogeny. Physiol Biochem Zool. 2000;73:200–208. doi: 10.1086/316738. [DOI] [PubMed] [Google Scholar]
- Storey KB. Life in the slow lane: molecular mechanisms of estivation. Comp Biochem Physiol A Mol Integr Physiol. 2002;133:733–754. doi: 10.1016/S1095-6433(02)00206-4. [DOI] [PubMed] [Google Scholar]
- Teshima H, et al. The evolution of extreme shell shape variation in the land snail Ainohelix editha: a phylogeny and hybrid zone analysis. Mol Ecol. 2003;12:1869–1878. doi: 10.1046/j.1365-294X.2003.01862.x. [DOI] [PubMed] [Google Scholar]
- Todgham AE, Iwama GK, Schulte PM. Effects of the natural tidal cycle and artificial temperature cycling on Hsp levels in the tidepool sculpin Oligocottus maculosus. Physiol Biochem Zool. 2006;79:1033–1045. doi: 10.1086/507664. [DOI] [PubMed] [Google Scholar]
- Tomanek L, Somero GN. Evolutionary and acclimation-induced variation in the heat-shock responses of congeneric marine snails (genus Tegula) from different thermal habitats: implications for limits of thermotolerance and biogeography. J Exp Biol. 1999;202:2925–2936. doi: 10.1242/jeb.202.21.2925. [DOI] [PubMed] [Google Scholar]
- Troschinski S, Di Lellis MA, Sereda S, Hauffe T, Wilke T, Triebskorn R, Kohler HR. Intraspecific variation in cellular and biochemical heat response strategies of Mediterranean Xeropicta derbentina [Pulmonata, Hygromiidae] PLoS One. 2014;9 doi: 10.1371/journal.pone.0086613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002;3 doi: 10.1186/gb-2002-3-7-research0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser ME. Keeping up with a warming world; assessing the rate of adaptation to climate change. Proc Biol Sci. 2008;275:649–659. doi: 10.1098/rspb.2007.0997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade CM, Mordan PB. Evolution within the gastropod molluscs; using the ribosomal RNA gene-cluster as an indicator of phylogenetic relationships. J Molluscan Stud. 2000;66:565–570. doi: 10.1093/mollus/66.4.565. [DOI] [Google Scholar]
- Watanabe Y, Chiba S. High within-population mitochondrial DNA variation due to microvicariance and population mixing in the land snail Euhadra quaesita (Pulmonata: Bradybaenidae) Mol Ecol. 2001;10:2635–2645. doi: 10.1046/j.0962-1083.2001.01388.x. [DOI] [PubMed] [Google Scholar]
- Young JC, Moarefi I, Hartl FU. Hsp90: a specialized but essential protein-folding tool. J Cell Biol. 2001;154:267–273. doi: 10.1083/jcb.200104079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zatsepina OG, et al. A Drosophila melanogaster strain from sub-equatorial Africa has exceptional thermotolerance but decreased Hsp70 expression. J Exp Biol. 2001;204:1869–1881. doi: 10.1242/jeb.204.11.1869. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Amplicon sequences (5′-3′) for T. pisana hsp70, hsp90, actin and Elongation factor 1α (PPTM 40 kb)
Analysis of the effect of acclimation to laboratory conditions on HSP levels. (a) Independent T-test comparisons within each population of HSP levels between field collected snails and acclimated snails. P values are reported for each population. (b-d) Post hoc comparisons between populations of HSP levels. P values are reported for each state: upper p values for field collected snails; lower p values for acclimated snails (PPTM 51 kb)
Analysis of the intensities of induction of hsp70 and hsp90 mRNAs in response to heat stress in T. pisana populations. (a) Peak values for hsp70 and hsp90 mRNA induction (n = 5) (relative to control, means ± SE). (b) Post hoc comparisons between populations of peak values of hsp70 mRNA induction. (c) Post hoc comparisons between populations of peak values of hsp90 mRNA induction (PPTM 49 kb)