Abstract
Heat shock protein 90 (Hsp90), a 90-kDa molecular chaperone, is responsible for biological activities of key signaling molecules (clients) such as protein kinases, ubiquitin ligases, steroid receptors, cell cycle regulators, and transcription factors regulating various cellular processes, including growth, survival, differentiation, and apoptosis. Because Hsp90 is also involved in stabilization of oncogenic ‘client’ proteins, its specific chaperone activity blockers are currently being tested as anticancer agents in advanced clinical trials. Recent in vitro and in vivo studies have shown that Hsp90 is also involved in activation of innate and adaptive cells of the immune system. For these reasons, pharmacological inhibition of Hsp90 has been evaluated in murine models of autoimmune and inflammatory diseases. This mini-review summarizes current knowledge of the effects of Hsp90 inhibitors on autoimmune and inflammatory diseases’ features and is based solely on preclinical studies.
Keywords: Anti-Hsp90 therapy, Hsp70, Autoimmune diseases, Mouse model
Introduction
Heat shock proteins (HSPs) are highly conserved and constitutively expressed molecules in the cell. They are localized in the cytoplasm and various intracellular compartments where they act as molecular chaperones or proteases. HSPs usually constitute about 5–10 % of the total protein in most cells, but their intracellular concentrations can be increased by stressors, e.g., increased temperature (fever), oxidative stress, ethanol, and infection that induce protein unfolding, misfolding, or aggregation. They are classified into several families based on their approximate molecular weights: HSP100, HSP90, HSP70, HSP60, HSP40, and the small heat shock proteins (sHsps) (Pockley 2003; van Eden et al. 2005; Kampinga et al. 2009).
Since abnormal levels of Hsp90 have been observed in malignant cells and inflamed tissues, this chaperone is particularly in the focus of scientific interest in the context of the treatment of cancer and autoimmune/inflammatory diseases (Shukla and Pitha 2012; Li et al. 2013; Tukaj et al. 2013). Hsp90 participates in stabilizing and activating more than 200 ‘client’ proteins, including key signaling molecules, such as nuclear transcription factors (e.g., NF-κB, STATs, and p53) and kinases (e.g., Raf/MEK/ERK, PI3K/AKT, and p38/MAPK). Thus, it regulates crucial cellular processes, e.g., inflammation, growth, survival, differentiation, and apoptosis (Trepel et al. 2010). Many oncoproteins may also belong to the ‘clients’, therefore a therapy based on Hsp90 inhibition is currently carried out in several clinical trials (phase I–III) as a promising strategy for the treatment of patients with different types of cancer (Garcia-Carbonero et al. 2013).
Recently, in the course of preclinical rodent studies, Hsp90 inhibitors have been shown to ameliorate autoimmune encephalomyelitis, rheumatoid arthritis, systemic lupus erythematosus, and epidermolysis bullosa acquisita (Dello Russo et al. 2006; Rice et al. 2008; Yun et al. 2011; Han et al. 2010; Kasperkiewicz et al. 2011; Shimp et al. 2012). Moreover, anti-Hsp90 therapy has been successfully applied in some murine non-autoimmune inflammatory disease models.
This mini-review summarizes current knowledge on the effects of Hsp90 inhibitors on autoimmune and inflammatory diseases that is based solely on preclinical studies.
HSP90: structure, expression, and regulatory functions
Human molecular chaperone Hsp90 family consists of several 90-kDa members localized in different cellular compartments: cytosolic isoforms of inducible HSP90AA1 and HSP90AA2 and constitutively expressed HSP90AB1, as well as HSP90B1 (gp96/grp94) and TRAP1, localized in endoplasmic reticulum (ER) and mitochondria, respectively (Langer et al. 2003; Felts et al. 2000; Mazzarella and Green 1987; Sorger and Pelham 1987; Kampinga et al. 2009). Structurally and functionally conserved from bacteria to human, Hsp90 are ATP-dependent homodimers, and each monomer can be divided into three functionally distinct regions, i.e., (i) N-terminal ATP-binding domain (N-domain), (ii) middle domain (M-domain), and (iii) C-terminal dimerization domain (C-domain). Effective Hsp90 production occurs after transactivation of their corresponding genes by the heat shock factors (HSFs). Under normal, non-stressed cellular conditions, HSF1 exists in a complex with cytoplasmic chaperones, i.e., Hsp40, Hsp70, and Hsp90, in a monomeric form without a DNA-binding activity. Following exposure to heat shock, inflammation, unfolded proteins, or others stresses, HSF1 is released and translocated into the nucleus. Subsequently, HSF1 forms a trimer, becomes phosphorylated, and finally binds to a specific DNA sequence [the so-called heat shock element (HSE)] to activate transcription of the Hsps genes, including Hsp70 and Hsp90 (Barbatis and Tsopanomichalou 2009; Li and Buchner 2013).
The chaperone activity and substrate interactions with Hsp90 is additionally regulated by various co-chaperones (e.g., CDC37, STIP1, PP5, AHA1, p23, CHIP, TAH1, PIH1, SGT1, FKBP51, and FKBP52) and post-translational modifications, i.e., phosphorylation, acetylation, nitrosylation, and methylation (Trepel et al. 2010; Mollapour and Neckers 2012).
Hsp90 inhibition in autoimmune and inflammatory diseases
Generally, autoimmune diseases are a group of chronic inflammatory conditions with no specific available to date cure. Although much progress has been made in revealing the immunologic processes in autoimmune diseases, their therapy remains challenging and in most cases still consists of conventional, unspecific immunosuppressive treatment with corticosteroids and cytostatic agents. Recently, biological therapies for various autoimmune diseases, which are targeted at molecules involved in maintaining chronic inflammation, have been extensively applied as an alternative to the existing treatment methods of immunosuppressive medications. Unfortunately, the application of these drugs is limited due to side effects (Davidson and Diamond 2001; Kasperkiewicz and Schmidt 2009; Rosman et al. 2013). Therefore, research aimed at developing more effective therapies for autoimmune diseases is still highly desirable.
Because Hsp90 plays an important role in activation of innate and adaptive cells of the immune system, including neutrophils, natural killers, macrophages, dendritic cells, and T or B lymphocytes (Srivastava 2002; Kasperkiewicz et al. 2011; Bae et al. 2013; Tukaj et al. 2014a, b, 2015), its pharmacological inhibition has increasingly become the focus of research on autoimmune diseases.
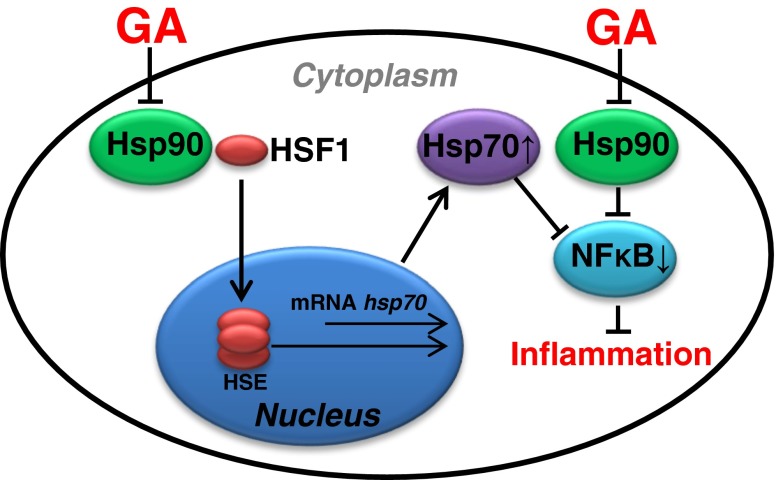
The N-terminal ATP-binding pocket of Hsp90 is a target site for geldanamycin and its semi-synthetic derivatives (anti-Hsp90 therapy). These drugs bind to the ATP-binding pocket with higher affinity than ATP/ADP, and consequently direct Hsp90-dependent ‘client’ proteins to proteasomal degradation (Whitesell and Lindquist 2005). The underlying molecular mechanism responsible for immunoregulatory effects of Hsp90 inhibition still remains unclear. There are at least two mutually non-exclusive explanations. The first is linked to the inhibitory effects of Hsp90 inhibitors on Hsp90-dependent substrate proteins (e.g., NF-κB), which regulate inflammation (Trepel et al. 2010). The second speculates that the anti-inflammatory effects of Hsp90 inhibitors are mediated via release of HSF1, which is known to drive expression of a number of genes, including IL-10 and Hsp70, both of which are known to suppress pro-inflammatory and activate anti-inflammatory genes (Zhang et al. 2012; Collins et al. 2013; Tukaj et al. 2014b) (Fig. 1). The immunosuppressive action of Hsp70 consists of (i) inactivation of antigen presenting cells, (ii) expansion of regulatory T cells, and (iii) blockade of transcription factor NF-kB activity. Moreover, in experimental autoimmune disease models, artificial induction or administration of Hsp70 can prevent or arrest inflammatory damage in an IL-10-dependent way (Stocki and Dickinson 2012; Borges et al. 2012).
Fig. 1.
Hsp90 inhibitors, e.g., geldanamycin (GA), have been shown to bind to the ATP pocket of Hsp90, which disturbs the binding of Hsp90 to HSF1 and alters Hsp70 gene expression. Hsp70 is a potent negative regulator of inflammatory responses through, but not limited to, its negative feedback effect on NF-κB signaling pathway (Stocki and Dickinson 2012; Wieten et al. 2007; Collins et al. 2013; Tukaj et al. 2014b, c)
Interestingly, overexpression of HSF1 is a common feature of numerous cancer types, and its high level correlates with malignancy and mortality. Moreover, numerous data showed that upregulation of HSF1-dependent chaperones, like Hsp90, Hsp70, Hsp40, and Hsp27, plays an important role in cancer cell growth and survival. Unfortunately, the so-called classic Hsp90 inhibitors, like geldanamycin and its derivatives (e.g., 17-DMAG and 17-AAG), are able to activate the HSF1 pathway and in this way support cancer growth. Therefore, to sensitize cancer cells, new therapeutic strategy aimed either to control the expression of Hsp90 (and possibly other chaperone molecules), without HSF1 activation, or to use combined therapies with Hsp90 and HSF1 blockers is more desirable in a cancer therapy (McConnell et al. 2015). On the other hand, ‘classic Hsp90 inhibitors’ seem to be more attractive for the treatment of autoimmune/inflammatory diseases due to activation of the HSF1 signaling pathway.
Encephalomyelitis
First attempts to use anti-Hsp90 therapy in an active mouse model of encephalomyelitis (EAE, MOG-induced C57BL/6 strain), the most commonly employed experimental model for the human inflammatory demyelinating disease like multiple sclerosis (MS) (Constantinescu et al. 2011) revealed that single injection of geldanamycin (GA) at 3 days after immunization reduced the disease onset by over 50 % (Murphy et al. 2002). The same team showed that less toxic GA analogue, 17-AAG, significantly reduced the incidence of the disease when administered early (prophylactic treatment), but also provided therapeutic benefit when administered to mice with established EAE (Dello Russo et al. 2006). Mechanistically, 17-AAG significantly reduced IL-2 production induced in naive T cells by CD3/CD28 mAb, suggesting selective effects of this therapy on the T cell function (Dello Russo et al. 2006). Furthermore, 17-AAG suppressed inducible nitric oxide synthase (NOS2) expression and activity, as well as blocked IL-1β expression. According to the authors, anti-Hsp90 therapy may represent an effective therapeutic strategy of delaying or reducing the clinical development of a demyelinating disease (Dello Russo et al. 2006).
Rheumatoid arthritis
Rheumatoid arthritis (RA) is the most common type of autoimmune arthritis characterized by abnormal infiltration of a range of immune cell types, including macrophages, T and B cells, mast cells, and plasma cells, into synovial tissue leading to joint swelling and cartilage/bone destruction (Firestein 2003). Small molecule inhibitor of Hsp90—SNX-7081 has been tested in two widely used murine models of RA, i.e., rat collagen-induced arthritis (CIA) and adjuvant-induced arthritis (AIA) (Rice et al. 2008). The results revealed that the treatment eliminated ankle and knee swelling in both CIA and AIA with no effects on weight loss (Rice et al. 2008). In addition, histopathological evaluation of joint sections revealed normal synovium, bone, and cartilage in rats treated with SNX-7081 (Rice et al. 2008). Mechanistically, (i) NF-κB activity, (ii) secretion of pro-inflammatory cytokines, (iii) MAP kinases and angiogenic signaling activity, and (iv) NO production were significantly disturbed by SNX-7081 treatment in the cell cultures (Rice et al. 2008).
In addition, a novel synthetic Hsp90 inhibitor—EC144 suppressed the disease development, activation of antigen-specific CD4+ T cells, and the production of antigen-specific antibodies in a mouse model of collagen-induced arthritis (Yun et al. 2011). Converging results obtained for in vitro and in vivo studies using Hsp90 inhibitors support their use in the RA patients.
Systemic lupus erythematosus
Systemic lupus erythematosus (SLE) is an autoimmune inflammatory disease characterized by the presence of skin eruptions, joint pain, recurrent pleurisy, and kidney disease. Elevated expression of Hsp90 in kidneys and serum, as well as higher titer of anti-Hsp90 autoantibodies in the sera of SLE patients, prompted researchers to test the anti-Hsp90 therapy in preclinical studies (Han et al. 2010; Shukla and Pitha 2012; Shimp et al. 2012). The most commonly used mouse model of SLE—MRL/MpJ-Faslpr/J (MRL/lpr) develops an autoimmune disease that reflects pathologies of human SLE, including lymph node enlargement, increased IgG levels, anti-nuclear antibody production, proteinuria, and kidney failure caused by inflammation of the glomeruli (Perry et al. 2011).
Shimp et al. (2012) observed that MRL/lpr mice treated with Hsp90 inhibitor (17-DMAG) had decreased proteinuria and reduced serum anti-dsDNA antibody levels, however, glomerulonephritis and glomerular IgG and C3 were not significantly affected by the treatment. 17-DMAG treatment led to an increase in the number of CD8 positive T cells, reduced the level of double-negative T cells, decreased the CD4/CD8 ratio, and reduced the number of follicular B cells. According to the authors, these studies suggest that Hsp90 may play a significant role in regulating T cell differentiation and activation, and that Hsp90 blockade is a promising therapeutic strategy in lupus (Shimp et al. 2012).
It has been shown that dendritic cells (DC) promote autoimmune responses in SLE (Monrad and Kaplan 2007). Since cell surface gp96 (ER Hsp90 homolog) induced DC activation in SLE, a chemical method which blocks maturation of these cells via gp96 has been proposed as a potential therapeutic approach in treating this disease (Han et al. 2010). The selective suppression of cell surface gp96 via (S)-methyl 2-(4,6-dimethoxypyrimidine-2-yloxy)-3-methylbutanoate reduced the incidence and severity of symptoms associated with SLE, such as glomerulonephritis, proteinuria, and accumulation of anti-nuclear and anti-dsDNA antibodies in the mouse model (Han et al. 2010).
Epidermolysis bullosa acquisita
Kasperkiewicz et al. (2011) demonstrated that treatment with Hsp90 inhibitors is clinically effective in mice with experimental epidermolysis bullosa acquisita (EBA), a chronic subepidermal blistering autoimmune disease characterized by circulating and tissue-bound autoantibodies against the non-collagenous domain 1 of type VII collagen of the dermal–epidermal junction (Ludwig and Zillikens 2011). Both 17-DMAG and the non-toxic peptide derivative TCBL-145 suppressed autoantibody production and reduced dermal neutrophilic infiltrate in EBA mice, as well as inhibited T cell proliferation in ex vivo collagen type VII or CD3/CD28 mAb restimulated EBA lymph node cells (Kasperkiewicz et al. 2011). Detailed in vitro experiments showed that anti-CD3 antibody-stimulated human peripheral blood mononuclear cell cultures in the presence of non-toxic concentrations of 17-DMAG, significantly blocked T cell proliferation, reduced pro-inflammatory IFN-γ and IL-17 expression on CD4+ T lymphocytes, as well as arrested secretion of IFN-γ, TNF-α, and IL-17, cytokines characteristic of Th1 and Th17 cells, respectively (Tukaj et al. 2014a). In addition, type VII collagen-immunized mice, early treated with 17-DMAG, had a reduced total B cell number in spleens, increased splenic regulatory B cell fractions (Breg; CD19+CD1dhiCD5+ and CD19+IL-10+), and higher serum IL-10 concentrations, relative to vehicle-treated immunized mice. Autoantibody production was blunted in isolated and autoantigen-restimulated lymph node cells from immunized mice by either 17-DMAG or purified autologous splenic regulatory B cells (Tukaj et al. 2014b). In vitro experiments confirmed direct inhibitory effects of 17-DMAG on B cells and IgG secretion in cultures of human peripheral B cells from healthy subjects (Tukaj et al. 2014b).
Colitis
Collins et al. (2013) observed that Hsp90 inhibition by 17-AAG attenuates chemically induced acute murine colitis. This inhibitory effect was correlated, inter alia, with induction of CD4+Foxp3+ T regulatory cells in lamina propia, as well as with induction of anti-inflammatory IL-10 and inhibition of pro-inflammatory IL-2, IL-17, and INF-γ cytokines in cultures of colonic explants from dextran sulfate sodium (DSS)-induced colitis.
Uveitis
Anti-inflammatory activity of 17-AAG was also confirmed in lipopolysaccharide (LPS)-induced uveitis (EIU) rat model. This effect was associated with suppression of leukocyte adhesion and blood retinal barrier breakdown through the inhibitory effects on (i) transcription factors, such as NF-κB and HIF-1 and (ii) inflammatory mediators, like VEGF, IL-1β, and TNF-α (Poulaki et al. 2007).
Sepsis
Sepsis is associated with activation of various pro-inflammatory pathways, including NF-κB, and the production of pro-inflammatory cytokines (such as TNF-α, IL-6, IL-8, and IL-1 β) which are critically involved in the initiation and amplification of the inflammatory insult. Blockade of Hsp90 via 17-AAG in a mouse model of severe sepsis induced by LPS confirmed anti-inflammatory potency of this therapy, and additionally prolonged survival and reduced lung injury in the treated mice (Chatterjee et al. 2007).
Alcoholic liver injury
Inhibition of Hsp90 by 17-DMAG prevented alcoholic liver injury, determined by lower serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST), as well as reduced hepatic triglycerides in the treated mice. Anti-Hsp90 therapy decreased alcohol-mediated oxidative stress, reduced serum endotoxin, and decreased levels of inflammatory cells and mediators (Ambade et al. 2014).
Lung inflammation
Non-geldanamycin Hsp90 inhibitor, ganetespib, also suppressed LPS-induced lung inflammation in mice. As expected, inhibitory effects of ganetespib have been observed at cellular and inflammatory mediator levels (Lilja et al. 2015).
Summary
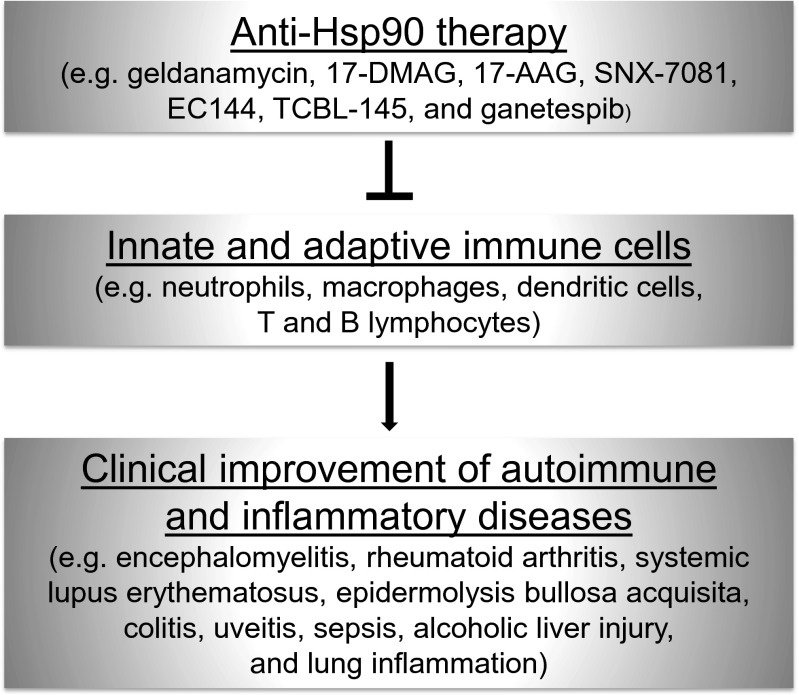
It is believed that the effectiveness of anti-Hsp90 therapy in autoimmune/inflammatory diseases is associated with the selective blockade of innate and adaptive cells of the immune system which plays a key role in the pathogenesis of these disorders (Fig. 2). In parallel, the induction of T and B regulatory cells in response to the therapy seems to additively contribute to the regulation of autoimmune/inflammatory response. Moreover, it is worth to mention that the anti-inflammatory effects of Hsp90 inhibitors may result from both delivery of Hsp90 ‘client’ proteins to the proteasomal degradation and/or activation of HSF1. The latter mechanism is known to drive expression of a number of anti-inflammatory genes, including Hsp70.
Fig. 2.
Overview of studies investigating the effects of Hsp90 inhibitors on innate and adaptive immune cells, in the context of autoimmune and inflammatory disease therapy
The foregoing summary describes the role of Hsp90 in several representative diseases in ways that may be useful for designing experiments to further characterize this chaperone and explore strategies for its utilization as a therapeutic target. This review should add to the growing body of literature on adaptive responses to cellular stress, although it is acknowledged that its scope is not exhaustive of the subject.
References
- Ambade A, Catalano D, Lim A, Kopoyan A, Shaffer SA, Mandrekar P. Inhibition of heat shock protein 90 alleviates steatosis and macrophage activation in murine alcoholic liver injury. J Hepatol. 2014;61:903–911. doi: 10.1016/j.jhep.2014.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae J, Munshi A, Li C, Samur M, Prabhala R, Mitsiades C, Anderson KC, Munshi NC. Heat shock protein 90 is critical for regulation of phenotype and functional activity of human T lymphocytes and NK cells. J Immunol. 2013;190:1360–1371. doi: 10.4049/jimmunol.1200593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbatis C, Tsopanomichalou M. Heat shock proteins in inflammatory bowel disease. Ann Gastroenterol. 2009;22:244–247. [Google Scholar]
- Borges TJ, Wieten L, van Herwijnen MJ, Broere F, van der Zee R, Bonorino C, van Eden W. The anti-inflammatory mechanisms of Hsp70. Front Immunol. 2012;3:95. doi: 10.3389/fimmu.2012.00095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee A, Dimitropoulou C, Drakopanayiotakis F, Antonova G, Snead C, Cannon J, Venema RC, Catravas JD. Heat shock protein 90 inhibitors prolong survival, attenuate inflammation, and reduce lung injury in murine sepsis. Am J Respir Crit Care Med. 2007;176:667–675. doi: 10.1164/rccm.200702-291OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins CB, Aherne CM, Yeckes A, Pound K, Eltzschig HK, Jedlicka P, de Zoeten EF. Inhibition of N-terminal ATPase on HSP90 attenuates colitis through enhanced Treg function. Mucosal Immunol. 2013;6:960–971. doi: 10.1038/mi.2012.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantinescu CS, Farooqi N, O’Brien K, Gran B. Experimental autoimmune encephalomyelitis (EAE) as a model for multiple sclerosis (MS) Br J Pharmacol. 2011;164:1079–1106. doi: 10.1111/j.1476-5381.2011.01302.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson A, Diamond B. Autoimmune diseases. N Engl J Med. 2001;345:340–350. doi: 10.1056/NEJM200108023450506. [DOI] [PubMed] [Google Scholar]
- Dello Russo C, Polak PE, Mercado PR, Spagnolo A, Sharp A, Murphy P, Kamal A, Burrows FJ, Fritz LC, Feinstein DL. The heat-shock protein 90 inhibitor 17-allylamino-17-demethoxygeldanamycin suppresses glial inflammatory responses and ameliorates experimental autoimmune encephalomyelitis. J Neurochem. 2006;99:1351–1362. doi: 10.1111/j.1471-4159.2006.04221.x. [DOI] [PubMed] [Google Scholar]
- Felts SJ, Owen BA, Nguyen P, Trepel J, Donner DB, Toft DO. The hsp90-related protein TRAP1 is a mitochondrial protein with distinct functional properties. J Biol Chem. 2000;275:3305–3312. doi: 10.1074/jbc.275.5.3305. [DOI] [PubMed] [Google Scholar]
- Firestein GS. Evolving concepts of rheumatoid arthritis. Nature. 2003;423:356–361. doi: 10.1038/nature01661. [DOI] [PubMed] [Google Scholar]
- Garcia-Carbonero R, Carnero A, Paz-Ares L. Inhibition of HSP90 molecular chaperones: moving into the clinic. Lancet Oncol. 2013;14:e358–e369. doi: 10.1016/S1470-2045(13)70169-4. [DOI] [PubMed] [Google Scholar]
- Han JM, Kwon NH, Lee JY, Jeong SJ, Jung HJ, Kim HR, Li Z, Kim S. Identification of gp96 as a novel target for treatment of autoimmune disease in mice. PLoS One. 2010;5 doi: 10.1371/journal.pone.0009792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kampinga HH, Hageman J, Vos MJ, Kubota H, Tanguay RM, Bruford EA, Cheetham ME, Chen B, Hightower LE. Guidelines for the nomenclature of the human heat shock proteins. Cell Stress Chaperones. 2009;14:105–111. doi: 10.1007/s12192-008-0068-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasperkiewicz M, Schmidt E. Current treatment of autoimmune blistering diseases. Curr Drug Discov Technol. 2009;6:270–280. doi: 10.2174/157016309789869065. [DOI] [PubMed] [Google Scholar]
- Kasperkiewicz M, Muller R, Manz R, Magens M, Hammers CM, et al. Heat-shock protein 90 inhibition in autoimmunity to type VII collagen: evidence that nonmalignant plasma cells are not therapeutic targets. Blood. 2011;117:6135–6142. doi: 10.1182/blood-2010-10-314609. [DOI] [PubMed] [Google Scholar]
- Langer T, Rosmus S, Fasold H. Intracellular localization of the 90 kDA heat shock protein (HSP90alpha) determined by expression of a EGFP-HSP90alpha-fusion protein in unstressed and heat stressed 3T3 cells. Cell Biol Int. 2003;27:47–52. doi: 10.1016/S1065-6995(02)00256-1. [DOI] [PubMed] [Google Scholar]
- Li J, Buchner J. Structure, function and regulation of the hsp90 machinery. Biomed J. 2013;36:106–117. doi: 10.4103/2319-4170.113230. [DOI] [PubMed] [Google Scholar]
- Li W, Tsen F, Sahu D, Bhatia A, Chen M, Multhoff G, Woodley DT. Extracellular Hsp90 (eHsp90) as the actual target in clinical trials: intentionally or unintentionally. Int Rev Cell Mol Biol. 2013;303:203–235. doi: 10.1016/B978-0-12-407697-6.00005-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilja A, Weeden CE, McArthur K, Nguyen T, Donald A, Wong ZX, Dousha L, Bozinovski S, Vlahos R, Burns CJ, Asselin-Labat ML, Anderson GP. HSP90 inhibition suppresses lipopolysaccharide-induced lung inflammation in vivo. PLoS One. 2015;10 doi: 10.1371/journal.pone.0114975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig RJ, Zillikens D. Pathogenesis of epidermolysis bullosa acquisita. Dermatol Clin. 2011;29:493–501. doi: 10.1016/j.det.2011.03.003. [DOI] [PubMed] [Google Scholar]
- Mazzarella RA, Green M. ERp99, an abundant, conserved glycoprotein of the endoplasmic reticulum, is homologous to the 90-kDa heat shock protein (hsp90) and the 94-kDa glucose regulated protein (GRP94) J Biol Chem. 1987;262:8875–8883. [PubMed] [Google Scholar]
- McConnell JR, Buckton LK, McAlpine SR. Regulating the master regulator: controlling heat shock factor 1 as a chemotherapy approach. Bioorg Med Chem Lett. 2015;25:3409–3414. doi: 10.1016/j.bmcl.2015.06.052. [DOI] [PubMed] [Google Scholar]
- Mollapour M, Neckers L. Post-translational modifications of Hsp90 and their contributions to chaperone regulation. Biochim Biophys Acta. 2012;1823:648–655. doi: 10.1016/j.bbamcr.2011.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monrad S, Kaplan MJ. Dendritic cells and the immunopathogenesis of systemic lupus erythematosus. Immunol Res. 2007;37:135–145. doi: 10.1007/BF02685895. [DOI] [PubMed] [Google Scholar]
- Murphy P, Sharp A, Shin J, Gavrilyuk V, Dello Russo C, Weinberg G, Sharp FR, Lu A, Heneka MT, Feinstein DL (2002) Suppressive effects of ansamycins on inducible nitric oxide synthase expression and the development of experimental autoimmune encephalomyelitis. J Neurosci Res 67:461–470 [DOI] [PubMed]
- Perry D, Sang A, Yin Y, Zheng YY, Morel L. Murine models of systemic lupus erythematosus. J Biomed Biotechnol. 2011;2011:271694. doi: 10.1155/2011/271694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pockley AG. Heat shock proteins as regulators of the immune response. Lancet. 2003;362:469–476. doi: 10.1016/S0140-6736(03)14075-5. [DOI] [PubMed] [Google Scholar]
- Poulaki V, Iliaki E, Mitsiades N, Mitsiades CS, Paulus YN, Bula DV, Gragoudas ES, Miller JW. Inhibition of Hsp90 attenuates inflammation in endotoxin-induced uveitis. FASEB J. 2007;21:2113–2123. doi: 10.1096/fj.06-7637com. [DOI] [PubMed] [Google Scholar]
- Rice JW, Veal JM, Fadden RP, Barabasz AF, Partridge JM, Barta TE, Dubois LG, Huang KH, Mabbett SR, Silinski MA, Steed PM, Hall SE. Small molecule inhibitors of Hsp90 potently affect inflammatory disease pathways and exhibit activity in models of rheumatoid arthritis. Arthritis Rheum. 2008;58:3765–3775. doi: 10.1002/art.24047. [DOI] [PubMed] [Google Scholar]
- Rosman Z, Shoenfeld Y, Zandman-Goddard G. Biologic therapy for autoimmune diseases: an update. BMC Med. 2013;11:88. doi: 10.1186/1741-7015-11-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimp SK, 3rd, Chafin CB, Regna NL, Hammond SE, Read MA, Caudell DL, Rylander M, Reilly CM. Heat shock protein 90 inhibition by 17-DMAG lessens disease in the MRL/lpr mouse model of systemic lupus erythematosus. Cell Mol Immunol. 2012;9:255–266. doi: 10.1038/cmi.2012.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukla HD, Pitha PM. Role of hsp90 in systemic lupus erythematosus and its clinical relevance. Autoimmune Dis. 2012;2012:728605. doi: 10.1155/2012/728605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorger PK, Pelham HR. The glucose-regulated protein grp94 is related to heat shock protein hsp90. J Mol Biol. 1987;194:341–344. doi: 10.1016/0022-2836(87)90380-9. [DOI] [PubMed] [Google Scholar]
- Srivastava P. Roles of heat-shock proteins in innate and adaptive immunity. Nat Rev Immunol. 2002;2:185–194. doi: 10.1038/nri749. [DOI] [PubMed] [Google Scholar]
- Stocki P, Dickinson AM. The immunosuppressive activity of heat shock protein 70. Autoimmune Dis. 2012;2012:617213. doi: 10.1155/2012/617213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trepel J, Mollapour M, Giaccone G, Neckers L. Targeting the dynamic HSP90 complex in cancer. Nat Rev Cancer. 2010;10:537–549. doi: 10.1038/nrc2887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tukaj S, Kleszczyński K, Vafia K, Groth S, Meyersburg D, Trzonkowski P, Ludwig RJ, Zillikens D, Schmidt E, Fischer TW, Kasperkiewicz M. Aberrant expression and secretion of heat shock protein 90 in patients with bullous pemphigoid. PLoS One. 2013;8 doi: 10.1371/journal.pone.0070496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tukaj S, Zillikens D, Kasperkiewicz M. Inhibitory effects of heat shock protein 90 blockade on proinflammatory human Th1 and Th17 cell subpopulations. J Inflamm (Lond) 2014;11:10. doi: 10.1186/1476-9255-11-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tukaj S, Tiburzy B, Manz R, de Castro MA, Orosz A, Ludwig RJ, Zillikens D, Kasperkiewicz M. Immunomodulatory effects of heat shock protein 90 inhibition on humoral immune responses. Exp Dermatol. 2014;23:585–590. doi: 10.1111/exd.12476. [DOI] [PubMed] [Google Scholar]
- Tukaj S, Grüner D, Zillikens D, Kasperkiewicz M. Hsp90 blockade modulates bullous pemphigoid IgG-induced IL-8 production by keratinocytes. Cell Stress Chaperones. 2014;19:887–894. doi: 10.1007/s12192-014-0513-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tukaj S, Hellberg L, Ueck C, Hänsel M, Samavedam U, Zillikens D, Ludwig R, Laskay T, Kasperkiewicz M. Heat shock protein 90 is required for ex vivo neutrophil-driven autoantibody-induced tissue damage in experimental epidermolysis bullosa acquisita. Exp Dermatol. 2015;24:567–571. doi: 10.1111/exd.12760. [DOI] [PubMed] [Google Scholar]
- van Eden W, van der Zee R, Prakken B. Heat-shock proteins induce T-cell regulation of chronic inflammation. Nat Rev Immunol. 2005;5:318–330. doi: 10.1038/nri1593. [DOI] [PubMed] [Google Scholar]
- Wieten L, Broere F, van der Zee R, Koerkamp EK, Wagenaar J, van Eden W. Cell stress induced HSP are targets of regulatory T cells: a role for HSP inducing compounds as anti-inflammatory immuno-modulators? FEBS Lett. 2007;581:3716–3722. doi: 10.1016/j.febslet.2007.04.082. [DOI] [PubMed] [Google Scholar]
- Whitesell L, Lindquist SL (2005) HSP90 and the chaperoning of cancer. Nat Rev Cancer 5:761–772 [DOI] [PubMed]
- Yun TJ, Harning EK, Giza K, Rabah D, Li P, Arndt JW, Luchetti D, Biamonte MA, Shi J, Lundgren K, Manning A, Kehry MR. EC144, a synthetic inhibitor of heat shock protein 90, blocks innate and adaptive immune responses in models of inflammation and autoimmunity. J Immunol. 2011;186:563–575. doi: 10.4049/jimmunol.1000222. [DOI] [PubMed] [Google Scholar]
- Zhang H, Zhang L, Yu F, Liu Y, Liang Q, Deng G, Chen G, Liu M, Xiao X. HSF1 is a transcriptional activator of IL-10 gene expression in RAW264.7 macrophages. Inflammation. 2012;35:1558–1566. doi: 10.1007/s10753-012-9471-4. [DOI] [PubMed] [Google Scholar]