Abstract
Leptin and adiponectin play an essential role in energy metabolism. Leptin has also been proposed as a marker for monitoring training load. So far, no studies have investigated the variability of these hormones in athletes and how they are regulated during cumulative exercise. This study monitored leptin and adiponectin in 15 endurance athletes twice daily in the days before, during and after a 9-day simulated cycling stage race. Adiponectin significantly increased during the race (p = 0.001) and recovery periods (p = 0.002) when compared to the baseline, while leptin decreased significantly during the race (p < 0.0001) and returned to baseline levels during the recovery period. Intra-individual variability was substantially lower than inter-individual variability for both hormones (leptin 34.1 vs. 53.5%, adiponectin 19% vs. 37.2%). With regards to exercise, this study demonstrated that with sufficient, sustained energy expenditure, leptin concentrations can decrease within the first 24 hours. Under the investigated conditions there also appears to be an optimal leptin concentration which ensures stable energy homeostasis, as there was no significant decrease over the subsequent race days. In healthy endurance athletes the recovery of leptin takes 48-72 hours and may even show a supercompensation-like effect. For adiponectin, significant increases were observed within 5 days of commencing racing, with these elevated values failing to return to baseline levels after 3 days of recovery. Additionally, when using leptin and adiponectin to monitor training loads, establishing individual threshold values improves their sensitivity.
Keywords: Leptin, Adiponectin, Cycling Stage Racing, Athletes, Variability
INTRODUCTION
Leptin is a 16-kDa non-glycosylated peptide hormone mainly secreted by adipose tissue, but low levels are also produced in the stomach, intestine, placenta, skeletal muscle, and possibly the brain [1]. Leptin acts via the Ob-Rb receptor on the hypothalamus to regulate food intake and energy expenditure. The concentrations of leptin in adipose tissue and plasma correlate closely with the mass of adipose tissue and adipocyte size and triglyceride content [1]. While in skeletal muscle leptin affects fatty acid oxidation and increases glucose uptake [2, 3], in the liver it regulates gluconeogenesis and increases fatty acid uptake and oxidation [4, 5]. Leptin is also sensitive to energy intake, especially in deficiency [6]. Even without a loss of body fat, leptin can decrease after two to three days of fasting [7]. Reduced IGF-1, thyroid hormones and ACTH have been reported to correlate with decreases in leptin concentrations [8, 9]. In humans a decrease in leptin concentration results in increased food intake and decreased energy expenditure [10].
Adiponectin is a 30-kDa peptide hormone secreted by adipocytes that modulates a number of metabolic processes including glucose regulation and fatty acid oxidation. It circulates in the blood in complexes such as trimers (low molecular weight form, LMW), hexamers (medium molecular weight form, MLW) and higher order multimers (high molecular weight form, HMW), with the HMW form being the most active form in glucose regulation [6]. Adiponectin reacts via its two receptors AdipoR1 and AdipoR2. AdipoR1 can be found in different tissues and is linked to AMPK pathways, whereas AdipoR2 is mainly expressed in the liver and functions via an activation of PPAR-α [11]. Adiponectin decreases glucose production in the liver and improves the uptake of glucose and fatty acid oxidation in skeletal muscle and the heart [12–14]. In comparison to many other hormones, adiponectin circulates in plasma in relatively high concentrations, and its levels are inversely correlated with body fat percentage. Unlike leptin, adiponectin is reduced in obesity, increases in response to fasting, and decreases after feeding [15].
With their high relevance in energy metabolism adipokines play an important role in physical exercise, and leptin has been even proposed as marker to monitor training load in athletes as its depression seems to be associated with training intensity, and thus a delayed recovery might be a sign for overloading the organism [16, 17]. For adiponectin our hypothesis was that an increase of adiponectin by heavy exercise and its time until return to baseline might give valuable information when energy levels would be sufficiently restored. Regarding acute exercise effects, several studies have been carried out for leptin [16, 18–20] and adiponectin [21–24]. From these acute response studies it appears that a decreased leptin concentration is only observed after continuous endurance exercise lasting minimum 30 minutes or following exercise that generates a significant impact on energy expenditure (> 5500 kcal) and that changes in adiponectin concentrations may be dependent on the performance level as well as the time of sample collection.
Several studies have also investigated the response of leptin and adiponectin to non-acute exercise lasting from 2 weeks to 9 months. For both leptin and adiponectin the results are however not consistent, with some studies showing changes while others did not [16, 25–29].
As highlighted above, not many studies exist for leptin and adiponectin in well-trained athletes. This is especially the case for studies looking at the effects of intermediate to long-term exercise with very high energetic demand. There is no information on whether cumulative, heavy exercise could potentiate the increases or decreases of leptin or adiponectin to exercise, and so far no studies have investigated the variability of these hormones in athletes.
Therefore the aims of this study were to:
investigate the regulation of leptin and adiponectin during 9 days of cumulative heavy exercise;
compare the variability of these hormones (intra- vs. inter-individual) over a 15-day period including travel/tapering, race and recovery periods to assess their potential for use as markers for monitoring training load.
MATERIALS AND METHODS
Subjects
Fifteen healthy male Caucasian amateur cyclists (n = 11) and triathletes (n = 4) took part in this study. The athletes were recruited through an advertisement at the university. Written informed consent was obtained from each participant. The study complied with the guidelines set out in the Declaration of Helsinki. Their baseline anthropometric, fitness and hormonal data are presented in Table 1.
TABLE 1.
Anthropometric, fitness and hormonal data of the subjects.
| Age (y) | 28.3 ± 4.5 |
| Body Mass (kg) | 72.4 ± 5.7 |
| Height (cm) | 180.4 ± 6.4 |
| Body Mass Index | 22.3 ± 1.5 |
| Body Fat (%) | 13.5 ± 3.1 |
| VO2max, (ml·kg−1·min−1) | 63.5 ± 5.8 |
| Baseline Adiponectin (ng·mL−1) | 7220 ± 2847 |
| Baseline Leptin (pg·mL−1) | 742 ± 370 |
Notes: values are means ± SD.
The study was approved by the ethics committee of the University of Freiburg, Germany. Written informed consent was obtained from all athletes before the start of the study.
Cycling intervention
To mimic a real life training and competition regime, the subjects performed a three-day resting period (travel and tapering phase) followed by a nine-day cycling stage race simulation and three-day recovery period. The simulated stage race was designed to be as close to a real race as possible, with certain tasks performed by the participants on each day. Special financial incentives for different tasks (e.g. sprints) were offered each day to increase motivation. Table 2 presents the information on the stages of the nine days of exercise.
TABLE 2.
Information about the race stages (day= study day).
| Stage | Stage | Stage | Stage | Stage | Stage | Stage | Stage | Stage | |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | |
| (Day 4) | (Day 5) | (Day 6) | (Day 7) | (Day 8) | (Day 9) | (Day 10) | (Day 11) | (Day 12) | |
| Distance (km) | 124 | 136 | 152 | 152 | 45 | 124 | 147 | 105 | 152 |
| Temperature (°C) | 27 | 24 | 23 | 24 | 25 | 27 | 25 | 25 | 30 |
| Stage Duration (h:min) | 03:22 | 04:00 | 04:14 | 04:12 | 00:49 | 04:05 | 04:21 | 02:51 | 04:22 |
| Stage Speed (km·h−1) | 36.8 | 34 | 35.9 | 36.2 | 55.1 | 30.4 | 33.8 | 36.8 | 34.8 |
| Energy Expenditure (kcal) (Race only) | 3424 | 3299 | 4055 | 4103 | 2592 | 2471 | 3519 | 2911 | 3773 |
Blood sampling
Venous blood samples were collected every morning before breakfast at 8.00 am and every evening at 6.00 pm over the 15-day test period. Samples were taken a minimum of 2 hours after exercise to exclude acute effects of the exercise on leptin and adiponectin concentrations. A total of 11.7 ml of blood was drawn each time (one 2.7 ml EDTA tube (Sarstedt, Numbrecht, Germany) and one 9.0 ml tube with clot activator (S-Monovette, Sarstedt, Numbrecht, Germany)).
Serum variables
Serum samples were centrifuged on-site after clotting and two aliquots were transferred into Eppendorf “protein low binding” tubes. The aliquots were then stored at -20°C until all analyses were performed as a batch. For adiponectin (human total adiponectin) and leptin, Quantikine ELISA kits were used (R&D Systems, Minneapolis, USA). Serum samples were prepared according to the instructions of the manufacturer and then analysed using a plate reader (Tecan, Crailsheim, Germany). The analytical errors expressed as CV% were 4.7% for adiponectin and 3.5% for leptin. All values were corrected for changes in plasma volume.
Haemoglobin mass and plasma volume
To investigate plasma volume effects, haemoglobin mass (Hb mass) was measured using the optimised CO rebreathing method as previously described by Schmidt and Prommer [30]. Vascular volumes were subsequently calculated according to the methods described previously [31]. The analytical error of measurement of the Hb mass measurements calculated from the duplicate measures was 2.3% [32].
Physical measures
Body mass was measured every morning in a fasted state using a calibrated scale (ADE, Hamburg, Germany). Standing height was assessed on the morning of day two using a wall mounted stadiometer (Holtain, UK).
Energy expenditure
The average energy expenditure for each stage (race calories without basic daily energy consumption) was estimated using an online tool (http://www.kreuzotter.de/english/espeed.htm) into which the average body mass and height (Table 1) of the athletes as well as the available data of each stage (Table 2) were entered. The subjects used race bikes with high pressure racing tires. For the subject position we chose the hands on the bottom of the handlebar, although this position likely changed occasionally during the race, which could have resulted in slightly higher values for the estimated energy expenditure.
Statistics
Statistics were performed using IBM SPSS 20. Data were controlled for normal distribution using the Kolmogorov-Smirnov test and dependent on the distribution further processed with a paired t-test or a Wilcoxon test to determine whether changes were significant. Inter- and intra-individual variability was calculated from the mean and standard deviation and expressed as the coefficient of variation percentage (CV%).
The box plots in the figures represent data of all subjects. Whiskers present the 95% confidence interval, the box defines the values within 25-75% of the population, and the line in the box is the median. Outliers are presented as open circles (1.5 x interquartile range) or stars (3 x interquartile range).
RESULTS
Data obtained from the exercise intervention
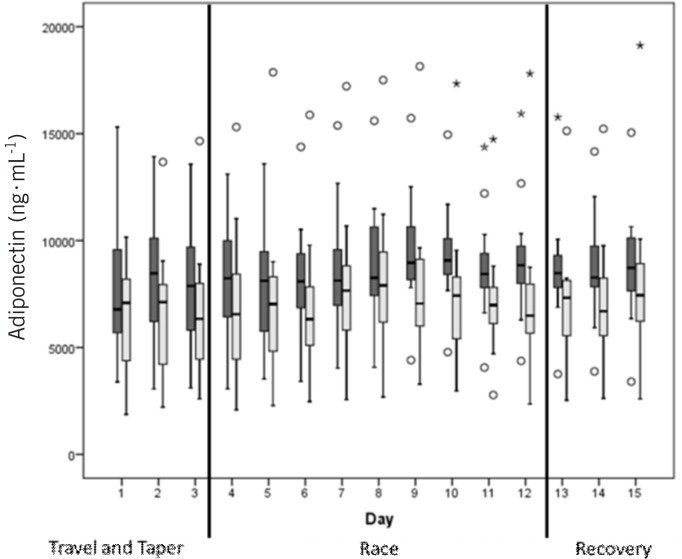
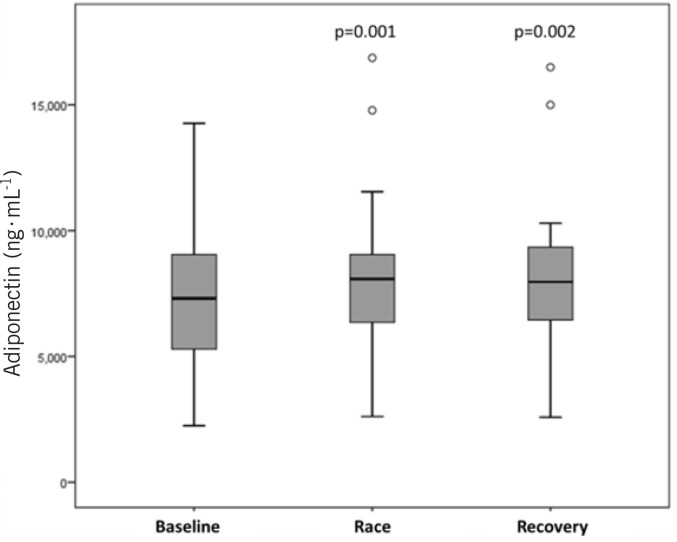
Figure 1 shows the plasma volume corrected morning and evening values of adiponectin over the 15 days of the study period. Adiponectin showed significantly (p = 0.001) increased values (+849±1238 ng · mL−1) when the whole race period was compared to the baseline (Figure 2); however, it seems that the increase was slightly delayed until the fifth race day (study day 8). Adiponectin remained significantly elevated during the recovery period compared to baseline (p = 0.002) (+835 ±1553 ng · mL−1).
FIG. 1.
Adiponectin values (corrected by plasma volume) over the 15-day study period. Dark bars represent morning values, light bars evening values. Circles and stars are outliers. Days 1-3 represent the travel/tapering period, 4 to 12 are the stage race days and 13-15 the recovery days.
FIG. 2.
Plasma-volume-corrected adiponectin concentration at baseline (days 1-3), during the race (days 4-12) and during the recovery period (days 13-15). The concentrations are significantly elevated during the race (p = 0.001) and recovery period (p = 0.002) when compared to the baseline.
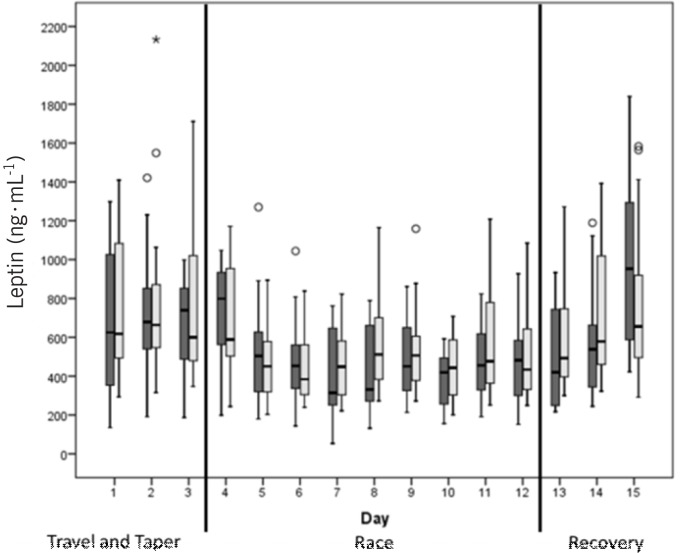
Figure 3 shows the plasma volume corrected morning and evening values of leptin over the complete 15-day study period. Leptin showed an immediate decrease after the first race stage (day 4). After that the levels remained constant throughout the race stages (day 12). During the recovery period (days 13-15) leptin was still decreased until the morning of day 14, and then it started to increase. The third day of the recovery period (day 15) showed a large increase in leptin in the morning which exceeded the baseline values.
FIG. 3.
Leptin values (corrected by the plasma volume) over the 15-day study period. Dark bars represent morning values, light bars evening values. Circles and stars are outliers. Days 1-3 represent the travel/tapering period, 4 to 12 are the stage race days and 13-15 the recovery days.
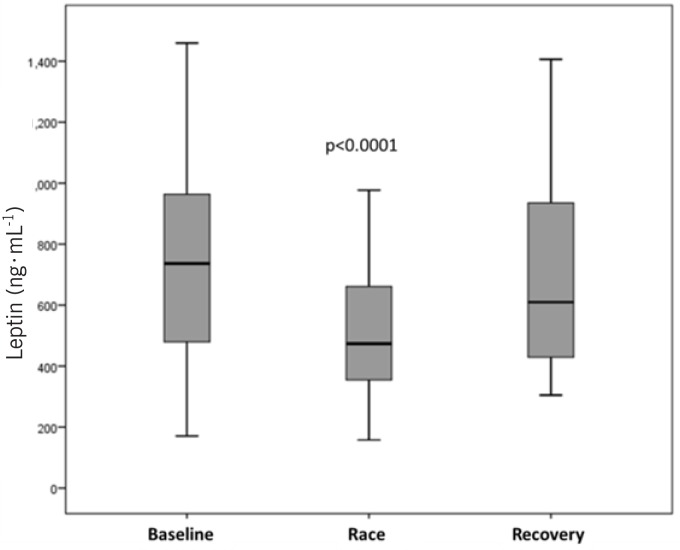
When leptin values were grouped in baseline, race and recovery periods, highly significant differences between the baseline and race period (p = 0.001), and the race period and recovery (p = 0.002) were observed (Figure 4).
FIG. 4.
Plasma-volume-corrected leptin concentration at baseline (days 1-3), during the race (days 4-12) and during the recovery period (days 13-15). The concentrations are significantly decreased (p < 0.0001) during the race period when compared to baseline.
Our study showed that an increase in energy expenditure resulted in a significant decrease in leptin concentrations (p < 0.001) within 24 hours. The levels then remained relatively constant throughout the remainder of the study period despite sustained high metabolic demands.
Data on intra- and inter-individual variability of leptin and adiponectin
The inter-individual variability of leptin expressed as CV% was 53.6%, while the average intra-individual variability was only 34.1% (range 28.7%-41.1%). For adiponectin the inter-individual and intra-individual variability were 37.2% and 19% respectively (range 8.1-32.1%). When reducing the observations to morning values only, the average intra-individual variability could be further reduced for adiponectin to 12% (range 6.4-20.8%), but it did not change substantially for leptin.
DISCUSSION
Data obtained from the exercise intervention
Adiponectin showed a significant increase from the fifth exercise day, which is different from the study of Lombardi et al., who did not find significant increases in adiponectin until after 22 days (20 stages) in professional cyclists during the Giro d'Italia stage race [28]. In their study, nine professional cyclists were tested during the race, but due to limited subject access during a world-class competition, blood was only sampled at three time points (days 1, 12 and 22) [28]. Although significant increases for adiponectin were reported, unfortunately no corrections for changes in the plasma volume were performed, which could be a major confounding factor in the data obtained during this study [33, 34]. Our results also differ from the study of Lakhdar et al. [29], in which eight non-professional cyclists were tested before and after a six-month training intervention. While the authors hypothesized that the resting adiponectin concentrations would increase after the intervention, they actually found a decrease despite a decrease in body fat percentage. Unfortunately changes in plasma volume were also not controlled in this study. Plasma volume would have been expected to increase with training, especially since the subjects undertook this study at the beginning of the season after 1.5 months of holiday. This could help explain their results, since an increase in plasma volume would have decreased the adiponectin concentration. Another explanation could be adaptation of the subjects to the increased metabolic demands of the training, so that the blood testing after 6 months may have been too late to observe any changes. With respect to the regulation of the two hormones, the adiponectin values in our study were significantly higher in the morning when compared to the evening (p = 0.001), revealing a diurnal effect. This is in contrast to a study from Shea et al. [35], who investigated the circadian pattern of adiponectin and leptin. An explanation for our values could be the intermediate fast overnight. Interestingly, we observed an increase of the differences between morning and evening adiponectin values from day 9 (stage 6) to day 12 (stage 9), which was still visible on the recovery days 13 and 14 (Figure 1). As a hypothesis, this may be interpreted as a sign of a reduced recovery capacity of the athletes, with processes of protein synthesis affected by the accumulated exercise.
Leptin decreased directly after the first race stage, after which it remained constant. During the recovery period leptin increased again and even exceeded the baseline values, suggesting that leptin reacts relatively quickly (within 1-3 days) to metabolic changes. The morning values on the last day of the recovery period (day 15) had the highest leptin concentrations of the whole study. This increase may be related not only to fat stores but also to glycogen supercompensation. Our results are different from those of Jurimae et al. [16], who studied the leptin concentrations of highly trained rowers who were tested before and after three weeks of heavy training and following a two-week taper period. Although leptin significantly decreased after the three-week training block, as in our study, it was still significantly lower than baseline after a two-week tapering period. With regard to recovery time, our findings also differed from a study performed by Eriksson et al., who performed a two-week cross-country skiing expedition in which subjects worked approximately 10 hours per day, carrying 25-30 kg and skiing 12-30 km [25]. Additionally, the subjects in that study slept in snow caves which they had to build themselves each night at temperatures between -10 and - 25°C. Leptin and adiponectin measures were taken at baseline, immediately and six weeks after the expedition. Under these extreme conditions, leptin significantly decreased and was still significantly below baseline after six weeks of recovery.
With regard to possible circadian regulation, our data again did not support the findings of Shea et al. [35], who reported higher leptin values in the morning. We did not observe such a pattern in our study – especially during the race period. We do not have an explanation for this and can only speculate that the effect of accumulated exercise in our study resulted in a disturbance of the typical diurnal pattern.
Desgorces et al. [27] studied leptin concentrations in rowers at the beginning and the end of an intense 36-week endurance training period. The authors did not find any significant changes in the baseline concentrations. Unfortunately, in their paper no information is given on training load on the days before testing – thus the leptin concentrations in their subjects might have already returned to baseline. Other reasons could be that these subjects were already at a physical level where leptin could not decrease any further, or the energy expenditure during each training session was too low.
Looking at our results, it seems that an accumulation of physical stress, in the range applied in our study, does not produce an additional decrease in leptin values. This is also supported by the data of Lombardi [28], who did not observe any further decreases from days 12 to 22 in his study. Ara et al. also reported no changes in leptin in their study in which 18 healthy men undertook a six-week individualized strength training programme focusing on the lower body [26]. Despite body fat percentage decreasing and lean body mass increasing, it seems that the metabolic demand imposed in this study was not sufficient, or the time of taking the blood samples (48 to 72 h after training) was too late to observe a decrease.
Data on intra- and inter-individual variability of leptin and adiponectin
According to Harris et al., one of the main requirements for a biological marker in longitudinal follow-ups is that the intra-individual variability is lower than the corresponding inter-individual variability [36]. Our data fulfil this requirement for a population of healthy, highly trained endurance athletes. The reported variability includes the analytical error plus the variability during rest and heavy exercise. Thus, if adiponectin and leptin are to be used as markers for monitoring training load, a personalized baseline should ideally be established for each athlete rather than trying to use a global threshold value. In this context, samples should be always analysed with the same ELISA kits to enhance comparability. Our data include the race period with very high energetic demands which may exceed those of normal training and could therefore affect individual variability by the inclusion of abnormally high or low values. It was not however the aim of this study to establish a model for cut-off thresholds. Such a model would require more data collected over a longer period of time and controlled for interfering factors such as body weight and fat mass.
CONCLUSIONS
With respect to exercise, this study demonstrated that with sufficient, sustained energy expenditure, leptin concentrations can decrease within 24 hours. It seems that under the investigated conditions there is an optimal level for the leptin concentration to ensure stable energy homeostasis, as there was no further significant decrease over the subsequent race days. We observed that in healthy, well-trained endurance athletes the recovery of leptin takes 48–72 hours and may even show a supercompensation-like effect. For adiponectin, 5 continuous days of heavy exercise was enough to induce significantly increased levels, but the recovery period of 3 days was not long enough to observe a return to baseline.
When measuring adipokines to monitor training stress, a longitudinal model which incorporates personalized reference ranges should be used.
Acknowledgements
This project was funded by Anti-Doping Lab Qatar and the Aspire Academy for Sports Excellence. The authors would also like to gratefully acknowledge; Noora Alsowaidi, Mohamed Ibrahim Elzain Elgingo, Felix Klodt and Damiano Nonis for their help in planning and technical assistance.
Conflict of interests
The authors declared no conflict of interests regarding the publication of this manuscript.
REFERENCES
- 1.Ahima RS, Lazar MA. Adipokines and the peripheral and neural control of energy balance. Mol Endocrinol. 2008;22(5):1023–31. doi: 10.1210/me.2007-0529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Suzuki A, Okamoto S, Lee S, Saito K, Shiuchi T, Minokoshi Y. Leptin stimulates fatty acid oxidation and peroxisome proliferator-activated receptor alpha gene expression in mouse C2C12 myoblasts by changing the subcellular localization of the alpha2 form of AMP-activated protein kinase. Mol Cell Biol. 2007;27(12):4317–27. doi: 10.1128/MCB.02222-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kamohara S, Burcelin R, Halaas JL, Friedman J, M.Charron MJ. Acute stimulation of glucose metabolism in mice by leptin treatment. Nature. 1997;389(6649):374–7. doi: 10.1038/38717. [DOI] [PubMed] [Google Scholar]
- 4.Raman P, Donkin SS, Spurlock ME. Regulation of hepatic glucose metabolism by leptin in pig and rat primary hepatocyte cultures. Am J Physiol Regul Integr Comp Physiol. 2004;286(1):R206–16. doi: 10.1152/ajpregu.00340.2003. [DOI] [PubMed] [Google Scholar]
- 5.Huang W, Dedousis N, Bandi A, Lopaschuk GD, O'Doherty RM. Liver triglyceride secretion and lipid oxidative metabolism are rapidly altered by leptin in vivo. Endocrinology. 2006;147(3):1480–7. doi: 10.1210/en.2005-0731. [DOI] [PubMed] [Google Scholar]
- 6.Golbidi S, Laher I. Exercise induced adipokine changes and the metabolic syndrome. J Diabetes Res. 2014;2014:726861. doi: 10.1155/2014/726861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chan JL, Heist K, DePaoli AM, Veldhuis JD, Mantzoros CS. The role of falling leptin levels in the neuroendocrine and metabolic adaptation to short-term starvation in healthy men. J Clin Invest. 2003;111(9):1409–21. doi: 10.1172/JCI17490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chan JL, Mantzoros CS. Leptin and the hypothalamic-pituitary regulation of the gonadotropin-gonadal axis. Pituitary. 2001;4(1-2):87–92. doi: 10.1023/a:1012947113197. [DOI] [PubMed] [Google Scholar]
- 9.Mantzoros CS. The role of leptin and hypothalamic neuropeptides in energy homeostasis: update on leptin in obesity. Growth Horm IGF Res. 2001;11(Suppl A):S85–9. doi: 10.1016/s1096-6374(01)80014-9. [DOI] [PubMed] [Google Scholar]
- 10.Myers MG, Cowley MA, Munzberg H. Mechanisms of leptin action and leptin resistance. Annu Rev Physiol. 2008;70:537–56. doi: 10.1146/annurev.physiol.70.113006.100707. [DOI] [PubMed] [Google Scholar]
- 11.Yamauchi T, Nio Y, Maki T, Kobayashi M, Takazawa T, Iwabu M, Okada-Iwabu M, Kawamoto S, Kubota N, Kubota T, Ito Y, Kamon J, Tsuchida A, Kumagai K, Kozono H, Hada Y, Ogata H, Tokuyama K, Tsunoda M, Ide T, Murakami K, Awazawa M, Takamoto I, Froguel P, Hara K, Tobe K, Nagai R, Ueki K, Kadowaki T. Targeted disruption of AdipoR1 and AdipoR2 causes abrogation of adiponectin binding and metabolic actions. Nat Med. 2007;13(3):332–9. doi: 10.1038/nm1557. [DOI] [PubMed] [Google Scholar]
- 12.Yamauchi T, Kamon J, Minokoshi Y, Ito Y, Waki H, Uchida S, Yamashita S, Noda M, Kita S, Ueki K, Eto K, Akanuma Y, Froguel P, Foufelle F, Ferre P, Carling D, Kimura S, Nagai R, Kahn BB. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med. 2002;8(11):288–95. doi: 10.1038/nm788. [DOI] [PubMed] [Google Scholar]
- 13.Berg AH, Combs TP, Du X, Brownlee M, Scherer PE. The adipocyte-secreted protein Acrp30 enhances hepatic insulin action. Nat Med. 2001;7(8):947–53. doi: 10.1038/90992. [DOI] [PubMed] [Google Scholar]
- 14.Li L, Wu L, Wang C, Liu L, Zhao Y. Adiponectin modulates carnitine palmitoyltransferase-1 through AMPK signaling cascade in rat cardiomyocytes. Regul Pept. 2007;139(1-3):72–9. doi: 10.1016/j.regpep.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 15.Kadowaki T, Yamauchi T. Adiponectin and adiponectin receptors. Endocr Rev. 2005;26(3):439–51. doi: 10.1210/er.2005-0005. [DOI] [PubMed] [Google Scholar]
- 16.Jurimae J, Maestu J, Jurimae T. Leptin as a marker of training stress in highly trained male rowers? Eur J Appl Physiol. 2003;90(5-6):533–8. doi: 10.1007/s00421-003-0879-2. [DOI] [PubMed] [Google Scholar]
- 17.Bouassida A, Chamari K, Zaouali M, Feki Y, Zbidi A, Tabka Z. Review on leptin and adiponectin responses and adaptations to acute and chronic exercise. Br J Sports Med. 2010;44(9):620–30. doi: 10.1136/bjsm.2008.046151. [DOI] [PubMed] [Google Scholar]
- 18.Jurimae J, Jurimae T. Leptin responses to short term exercise in college level male rowers. Br J Sports Med. 2005;39(1):6–9. doi: 10.1136/bjsm.2003.008516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zaccaria M, Ermolao A, Roi GS, Englaro P, Tegon G, Varnier M. Leptin reduction after endurance races differing in duration and energy expenditure. Eur J Appl Physiol. 2002;87(2):108–11. doi: 10.1007/s00421-002-0606-4. [DOI] [PubMed] [Google Scholar]
- 20.Zafeiridis A, Smilios I, Considine RV, Tokmakidis SP. Serum leptin responses after acute resistance exercise protocols. J Appl Physiol. 2003;94(2):591–7. doi: 10.1152/japplphysiol.00330.2002. [DOI] [PubMed] [Google Scholar]
- 21.Ferguson MA, White LJ, McCoy S, Kim HW, Petty T, Wilsey J. Plasma adiponectin response to acute exercise in healthy subjects. Eur J Appl Physiol. 2004;91(2-3):324–9. doi: 10.1007/s00421-003-0985-1. [DOI] [PubMed] [Google Scholar]
- 22.Roupas ND, Mamali I, Maragkos S, Leonidou L, Armeni AK, Markantes GK, Tsekouras A, Sakellaropoulos GC, Markou KB, Georgopoulos NA. The effect of prolonged aerobic exercise on serum adipokine levels during an ultra-marathon endurance race. Hormones (Athens) 2013;12(2):275–82. doi: 10.14310/horm.2002.1411. [DOI] [PubMed] [Google Scholar]
- 23.Jurimae J, Hofmann P, Jurimae T, Maestu J, Purge P, Wonisch M, Pokan R, von Duvillard SP. Plasma adiponectin response to sculling exercise at individual anaerobic threshold in college level male rowers. Int J Sports Med. 2006;27(4):272–7. doi: 10.1055/s-2005-865661. [DOI] [PubMed] [Google Scholar]
- 24.Jurimae J, Purge P, Jurimae T. Adiponectin is altered after maximal exercise in highly trained male rowers. Eur J Appl Physiol. 2005;93(4):502–5. doi: 10.1007/s00421-004-1238-7. [DOI] [PubMed] [Google Scholar]
- 25.Eriksson M, Johnson O, Boman K, Hallmans G, Hellsten G, Nilsson TK, Soderberg S. Improved fibrinolytic activity during exercise may be an effect of the adipocyte-derived hormones leptin and adiponectin. Thromb Res. 2008;122(5):701–8. doi: 10.1016/j.thromres.2008.01.010. [DOI] [PubMed] [Google Scholar]
- 26.Ara I, Perez-Gomez J, Vicente-Rodriguez G, Chavarren J, Dorado C, Calbet JA. Serum free testosterone, leptin and soluble leptin receptor changes in a 6-week strength-training programme. Br J Nutr. 2006;96(6):1053–9. doi: 10.1017/bjn20061956. [DOI] [PubMed] [Google Scholar]
- 27.Desgorces FD, Chennaoui M, Gomez-Merino D, Drogou C, Bonneau D, Guezennec CY. Leptin, catecholamines and free fatty acids related to reduced recovery delays after training. Eur J Appl Physiol. 2004;93(1-2):153–8. doi: 10.1007/s00421-004-1190-6. [DOI] [PubMed] [Google Scholar]
- 28.Lombardi G, Lanteri P, Graziani R, Colombini A, Banfi G, Corsetti R. Bone and Energy Metabolism Parameters in Professional Cyclists during the Giro d'Italia 3-Weeks Stage Race. PLoS One. 2012;7(7):e42077. doi: 10.1371/journal.pone.0042077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lakhdar N, Ben Saad H, Denguezli M, Zaouali M, Zbidi A, Tabka Z, Bouassida A. Effects of intense cycling training on plasma leptin and adiponectin and its relation to insulin resistance. Neuro Endocrinol Lett. 2013;34(3):229–35. [PubMed] [Google Scholar]
- 30.Schmidt W, Prommer N. The optimised CO-rebreathing method: a new tool to determine total haemoglobin mass routinely. Eur J Appl Physiol. 2005;95(5-6):486–95. doi: 10.1007/s00421-005-0050-3. [DOI] [PubMed] [Google Scholar]
- 31.Voss SC, Robinson N, Alsayrafi M, Bourdon PC, Schumacher YO, Saugy M, Giraud S. The effect of a period of intense exercise on the marker approach to detect growth hormone doping in sports. Drug Test Anal. 2014;6(6):582–6. doi: 10.1002/dta.1666. [DOI] [PubMed] [Google Scholar]
- 32.Hopkins WG. Measures of reliability in sports medicine and science. Sports Med. 2000;30(1):1–15. doi: 10.2165/00007256-200030010-00001. [DOI] [PubMed] [Google Scholar]
- 33.Voss SC, Alsayrafi M, Bourdon PC, Klodt F, Nonis D, Hopkins WG, Schumacher YO. Variability of Serum Markers of Erythropoiesis during 6 Days of Racing in Highly Trained Cyclists. Int J Sports Med. 2014;35(2):89–94. doi: 10.1055/s-0033-1345177. [DOI] [PubMed] [Google Scholar]
- 34.Kargotich S, Goodman C, Keast D, Fry RW, Garcia-Webb P, Crawford PM, Morton AR. Influence of exercise-induced plasma volume changes on the interpretation of biochemical data following high-intensity exercise. Clin J Sport Med. 1997;7(3):185–91. doi: 10.1097/00042752-199707000-00006. [DOI] [PubMed] [Google Scholar]
- 35.Shea SA, Hilton MF, Orlova C, Ayers RT, Mantzoros CS. Independent circadian and sleep/wake regulation of adipokines and glucose in humans. J Clin Endocrinol Metab. 2005;90(5):2537–44. doi: 10.1210/jc.2004-2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Harris EK. Effects of intra- and interindividual variation on the appropriate use of normal ranges. Clin Chem. 1974;20(12):1535–42. [PubMed] [Google Scholar]