Graphical abstract
Bioassays with synergists can provide a quick and easy basis for initial characterization of resistant mosquito populations, without the need of preserved specimens, expensive equipment and substrates or specialized expertise.
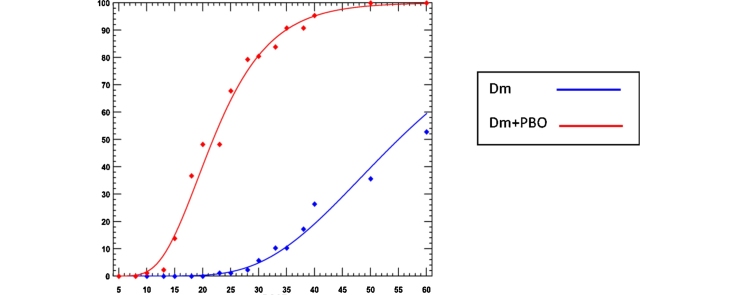
Pictograme: Knock down time responses of a resistant Anopheles gambiae population from Tiassalé to deltamethrin. A significant reduction in knockdown time was observed after 20 min pre-exposure to the P450 inhibitor (PBO). KDT50 shifted from 63.32 min for deltamethrin alone to 21.86 min for deltamethrin + PBO.
Abbreviations: P450s, cytochrome P450 oxidases; P-gp, permeable-glycoprotein; PBO, piperonyl butoxide; COE, carboxylesterase; GST, glutathion S-transferase; KD, knock down; KDT, knock down time; MDR, multi drug resistance
Keywords: Anopheles gambiae, Insecticide resistance, Metabolic resistance, P450s, Esterases, P-glycoprotein efflux pump
Highlights
-
•
Resistance to deltamethrin was confirmed in the mosquito population from Tiassale, Ivory Coast.
-
•
Pre-exposure to esterase synergists yielded an increase mortality and knockdown.
-
•
Pre-exposure to oxidase synergists yielded an increase mortality and knockdown.
-
•
Pre-exposure to a P-gps inhibitor caused no significant increase in mortality and knockdown.
-
•
Use of synergists is a convenient method for investigating potential metabolic resistance mechanisms in field mosquitoes.
Abstract
Metabolic resistance and the potential role of permeability-glycoprotein (P-gp) efflux pumps were investigated in a pyrethroid-resistant wild Anopheles gambiae s.l. Tiassalé population, using WHO susceptibility assays with deltamethrin (0.05%), with and without pre-exposure to synergists. The synergists used included an inhibitor of P-glycoprotein efflux pumps (verapamil), an inhibitor of esterases (EN 16-5), and an inhibitor of P450s and esterases (piperonyl butoxide). Pre-exposure to verapamil followed by deltamethrin led to a slight but non-significant (P = 0.59) increase in mortality relative to exposure to deltamethrin alone (64.5% versus 69.2%). Similarly, pre-exposure to EN 16-5 yielded a non-significant increase in mortality (to 76.6%; P = 0.85) but a significant increase in the knock down rate (from 48.3% to 78.7%; P < 0.01). Pre-exposure with PBO caused a significant increase in mortality (to 93.1%; P < 0.001) and knockdown rate (100%; P < 0.001), which related to a 2.9 fold decrease in the resistance level. The results provide evidence that metabolic resistance mechanisms are present within the assessed mosquito population. The decrease in time to knock down of this population with deltamethrin following exposure to EN16-5 and PBO is of particular relevance to vector control, where quick knock down is a highly desired characteristic. The suspected resistance mechanisms present in this population merit further investigation through biochemical and molecular analyses for full resistance profile characterization. Bioassays with synergists can provide a quick and easy basis for initial characterization of resistant mosquito populations, without the need of preserved specimens, expensive equipment and substrates or specialized expertise.
1. Introduction
The basic mechanisms underlying insecticide resistance include insecticide target-site mutations such as changes in the sodium channel that leads to so-called ‘knockdown resistance’, and increased metabolic detoxification of the insecticide through overproduction or elevated enzymatic activity (Hemingway and Ranson, 2000). Three enzyme families are primarily involved in insecticide detoxification: the carboxylesterases (COEs), glutathione-S-transferases (GSTs) and cytochrome P450s (P450s). Even though the detoxification gene families involved in insecticide resistance have been identified, little is known about how many genes in each family are directly involved in conferring resistance. The molecular mechanisms involved in insecticide resistance in general, and the functioning of up-regulated P450 genes, GSTs, and esterases in particular, are not yet clear. Insects and other eukaryotes can also become resistant to xenobiotics through the activity of permeability glycoprotein (P-gp) that actively removes xenobiotics from the cell before it can cause harm. Also known as multidrug resistance protein or ATP-binding cassette, P-gp is an ATP-dependent transporter which transports a wide variety of substrates across extra- and intra-cellular membranes. The pump mechanism attaches a protein label to the xenobiotic and removes it by exocytosis (Sarkadi et al., 2006). Apart from its role in multidrug resistance in mammalian tumour cell lines, there is considerable evidence that insect P-gps may play an important role in insecticide resistance (Lanning et al., 1994, Buss and Callaghan, 2008). The pattern of cross-resistance observed in malaria vectors is similar to multidrug resistance seen in tumour cell lines where insects develop resistance to structurally unrelated insecticides. This has stimulated our interest in investigating this mechanism in malaria vectors through the use of synergists. The use of microarrays has been crucial to detect up-regulated genes, including ATP-binding cassette transporters, but requires sophisticated equipment and training. Synergists can be used in bioassays as a relatively simple way of investigating the role of different enzymes in insecticide metabolism. Furthermore, we investigated the role of both P450s and COEs on a wild Anopheles gambiae s.l. population from Tiassalé (southern Côte d’Ivoire) where resistance to almost all the families of insecticides used in agriculture and public health has been described (Chouaibou et al., 2012, Edi et al., 2012).
2. Material and methods
An. gambiae s.l. mosquitoes were collected as pre-imaginal stages (L1 to L4 instars) from the village of Tiassalé in Côte d’Ivoire in 2010. Larvae were fed with powdered TetraFin® fish food and reared to adults under insectary conditions of 25–28 °C and 70%–80% relative humidity. Unfed adult female An. gambiae s.l. at 1 to 3 days post-emergence were used in WHO susceptibility tests (WHO, 1998). Four different serials of susceptibility tests were performed. The first one was conducted with deltamethrin-treated papers alone (0.05%) in standard assays performed at 25 °C and 70%–80% relative humidity. The three other serials of tests were done by exposing mosquitoes to a synergist before exposure to deltamethrin. Three different synergists were used: (1) verapamil (0.01%), a calcium blocker known to be a potent inhibitor of P-gps (Podsiadlowski et al., 1998); (2) EN 16-5 (0.1%), an analogue of PBO known to inhibit esterases (Moores et al., 2009), and (3) PBO (0.1%), an inhibitor of P450s and esterases (Khot et al., 2008). For each of the three susceptibility tests, mosquitoes were pre-exposed to a specific synergist for 20 min prior to 60 min exposure to deltamethrin. Control batches were exposed to synergist only. During the 60 min exposure period, the knockdown rate (KD) was recorded at 5 min intervals. Unfed female adults of 1–3 days post-emergence from a susceptible An. gambiae s.s. (Kisumu) laboratory strain were used as a reference to validate the quality of treated papers. Following exposure, mosquitoes were supplied with 10% honey solution and kept overnight under insectary conditions with mortality noted at 24 h post-exposure. Results were analyzed using EpiInfo Version 6 to test for any significant difference in mortalities between the different groups via Mentel–Haenszel Khi square analyses. The time at which 50% of the test population were knocked down (KDT50) was determined using PoloPlus software, via log-probit analysis. The resistance reduction was determined by dividing the KDT50 obtained for deltamethrin by the KDT50 obtained for deltamethrin plus the synergist.
3. Results and discussion
The An. gambiae s.s. Kisumu reference strain exhibited full susceptibility to deltamethrin (100% mortality) in standard WHO susceptibility tests, confirming bioefficacy of the treated papers used. For the wild Tiassalé strain, phenotypic resistance to deltamethrin was confirmed based on 64.37% mortality and 48.35% knock down (Fig. 1). A similar mortality (69.23%; P = 0.58) and knockdown (52.87%; P = 0.59) were observed when the population was pre-exposed to verapamil (P = 0.59) (Fig. 1). Pre-exposure to EN 16-5 also yielded no significant increase in mortality (76.6%; P = 0.85), although a significant increase in the knock down rate was observed (from 48.3% to 78.7%; P < 0.01) (Fig. 1). The required time for 50% of the mosquitoes to be knocked down (KDT50) shifted from 63.32 min for deltamethrin alone to 56.36 and 36.31 min where there had been pre-exposure to verapamil or EN16-5, respectively (Fig. 2, Fig. 3, Table 1). PBO pre-exposure followed by deltamethrin resulted in 93.10% mortality and 100% knock down, which was significantly higher than for deltamethrin alone, deltamethrin + verapamil and deltamethrin + EN16-5 (P < 0.001, for all). Mortality in all control groups was consistently below 5%, and thus no correction was required.
Fig. 1.
Knock down time and mortality rates of a wild resistant Anopheles gambiae population from Tiassalé. Increase in mortality to deltamethrin was observed following 20 min pre-exposure to several synergists (verapamil = inhibitor of P-gps, EN 16-5 = COE inhibitor, PBO = P450 inhibitor).
Fig. 2.
Knock down time responses of a resistant An. gambiae population from Tiassalé to deltamethrin. A slight reduction in KDT was observed after 20 min pre-exposure to the inhibitor of P-gps (verapamil).
Fig. 3.
Knock down time responses of a resistant An. gambiae population from Tiassalé to deltamethrin. A reduction in KDT was observed after 20 min pre-exposure to the inhibitor of COE (EN 16-5). KDT50 shifted from 63.32 min for deltamethrin alone to 36.31 min for deltamethrin + EN 16-5.
Table 1.
Time necessary for 50% of wild resistant An. gambiae mosquitoes from Tiassalé to be knocked down and magnitude of resistance reduction following exposure to deltamethrin-treated papers in standard WHO susceptibility tests (WHO, 1998) with and without pre-exposure to synergist-treated papers.
| KDT50 (min) | IC95 (%) | Resistance reduction fold | |
|---|---|---|---|
| Delta | 63.32 | [57.26–75.31] | – |
| Delta + ver | 56.36 | [53.43–61.02] | 1.1 |
| Delta + EN | 36.31 | [34.87–38.34] | 1.7 |
| Delta + PBO | 21.86 | [21.21–22.64] | 2.9 |
A reduction in the time to knock down houseflies, using PBO with pyrethrins, has previously been observed at Rothamsted (Khambay, pers comm). This has been attributed to the enhancement of pyrethroid penetration due to the surfactant properties of synergists such as PBO and EN16-5 (Unpublished internal report, Dept. University of Florence, 2007). Although the pre-exposure in this study was of a short duration (20 min) instead of 1 h (Nwane et al., 2013), the level of resistance was reduced by around 3-fold with PBO which was accompanied by a shift in KDT50 from 63.32 min to 21.86 min (Fig. 4), and for 1.7 fold with EN 16-5 (Table 1). This suggests that both P450s and COEs play an important role in the deltamethrin resistance observed in this vector population. Temporal synergism in this population should be further explored, using various pre-exposure times to verify if this can affect outcomes (Moores et al., 2009).
Fig. 4.
Knock down time responses of a resistant An. gambiae population from Tiassalé to deltamethrin. A significant reduction in knockdown time was observed after 20 min pre-exposure to the P450 inhibitor (PBO). KDT50 shifted from 63.32 min for deltamethrin alone to 21.86 min for deltamethrin + PBO.
PBO is primarily referred as a synergist of cytochrome P450s, which belong to a superfamily of enzymes (Feyereisen, 1999) that are well known for their ability to metabolize a wide range of compounds (David et al., 2013). Elevated levels of P450 activity have been observed in pyrethroid-resistant malaria vectors in Africa, particularly in Anopheles funestus from southern Africa (Brooke et al., 2001, Wondji et al., 2009, Wondji et al., 2012). The role of PBO in COE inhibition has also been demonstrated (Khot et al., 2008). COEs can confer resistance to organophosphates, carbamates and pyrethroids which are rich with ester-bonds. Elevated COEs have been detected in An. culicifacies resistant to malathion in India (Malcolm and Boddington, 1989). GSTs are dimeric multifunctional enzymes that play a role in detoxification of a large range of xenobiotics, and are likely important in detoxification of DDT. Although we did not investigate the role of GSTs, their involvement merits further investigation using the synergist diethyl maleate (Nwane et al., 2013) as these could also contribute to the high level of DDT resistance previously observed in Tiassalé An. gambiae s.s. population (Chouaibou et al., 2012, Edi et al., 2012). The target site mutations that confer cross-resistance to DDT and pyrethroids (kdr), and those implicated in resistance to carbamates and organophosphates (modified acetyl-cholinesterase, Ace-IR) have also been previously detected in this population (Chouaibou et al., 2012, Edi et al., 2012, Alou et al., 2010).
Only a low and non-significant activity of P-gps was seen in this study, although it is important to note that recent reports indicate that verapamil can inhibit some P450s. Our observations may be due to short pre-exposure time insufficient to allow full inhibition to occur (20 min). Furthermore, since only a single pyrethroid (deltamethrin) was used in this study, there was a reduced probability of observing any possible role of efflux pumps that may have an effect against other types or classes of insecticides. There is substantial evidence to indicate that the presence of the kdr mutation may not always produce a resistance phenotype that is of significance to operational control (Brooke, 2008). A number of studies have reported high-level insecticide resistance associated with the kdr mutation in populations from farm fields in West Africa (Diabaté et al., 2002, Tripet et al., 2007, Chouaibou et al., 2012, Edi et al., 2012). For these examples, it is highly likely that the resistance phenotype may be influenced by metabolic mechanisms that were not tested for because of the lack of common diagnostic tools.
Microarray technology has provided evidence of several putative genes involvement in metabolic resistance. However, the implementation of this technology is very expensive and its use is technically complex requiring specialized expertise. Furthermore, the process of handling and conservation of mosquito specimens following field collections can also affect the quality of results. Therefore, given the speed with which resistance phenomena occur and their geographic spread, microarray technology is currently not suitable for prompt and timely data to enable evidence-based decision making to guide vector control interventions. Bioassays remain the simplest and quickest method to assess metabolic resistance in field mosquitoes. The use of synergists in bioassays should be implemented along with standard bioassay monitoring procedures and investments in promoting understanding of how to read and report results from these tests is needed. Ideally, synergist-impregnated papers should be made available and accessible through the same channel as the WHO insecticide-treated papers. Only then can we understand which insecticides will likely work with resistant populations and appropriately design and deploy new tools. From this stage, a more complete characterization including biochemical and molecular analyzes can be undertaken ultimately to build up specific rapid diagnostic tools better adapted for the sub-Saharan African context.
The effort to overcome the insecticide resistance problem in malaria vectors should take into account all putative sources of resistance and mechanisms involved. The results herein provide evidence that metabolic resistance mechanisms are present within the mosquito population investigated. These mechanisms warrant further investigation through biochemical and molecular analyses, in order to fully characterise the resistance profile of the test population. The reduction in time to knock down following pre-exposure to EN16-5 and PBO for the Tiassalé population is an interesting finding and of particular relevance to vector control tools, where quick knock down is a highly desirable characteristic. Bioassays with synergists can provide a quick and easy basis for initial characterization of resistant mosquito populations, without the need for correctly-preserved specimens, sophisticated equipment and specialized training of laboratory personnel.
Acknowledgements
We acknowledge Nestor Kesse, Louis N’Dri and Joseph Chabi for their technical assistance, and the administration of the Centre Suisse de Recherches Scientifiques. We thank the community and the authorities of Tiassalé for facilitating this study. This work was financially supported by Vestergaard Frandsen. The synergist EN 16-5 was kindly provided by Endura S.p.A (Bologna, Italy). The manuscript writing received the support from the Afrique One consortium funded by the Wellcome Trust.
References
- Alou L., Koffi A., Adja M., Tia M., Kouassi P., Koné M., Chandre F. Distribution of ace-1R and resistance to carbamates and organophosphates in Anopheles gambiae s.s. populations from Côte d’Ivoire. Malar. J. 2010;9:167. doi: 10.1186/1475-2875-9-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooke B.D. kdr: can a single mutation produce an entire insecticide resistance phenotype? Trans. R. Soc. Trop. Med. Hyg. 2008;102:524–525. doi: 10.1016/j.trstmh.2008.01.001. [DOI] [PubMed] [Google Scholar]
- Brooke B.D., Kloke G., Hunt R.H., Koekemoer L.L., Temu E.A., Taylor M.E., Small G., Hemingway J., Coetzee M. Bioassay and biochemical analyses of insecticide resistance in southern African Anopheles funestus (Diptera: Culicidae) Bull. Entomol. Res. 2001;91:265–272. doi: 10.1079/ber2001108. [DOI] [PubMed] [Google Scholar]
- Buss D.S., Callaghan A. Interaction of pesticides with p-glycoprotein and other ABC proteins: a survey of the possible importance to insecticide, herbicide and fungicide resistance. Pest. Biochem. Physiol. 2008;90:141–153. [Google Scholar]
- Chouaibou M.S., Chabi J., Bingham G.V., Knox T.B., N’Dri L., Kesse N., Bonfoh B., Pates Jamet H. Increase in susceptibility to insecticides with aging of wild Anopheles gambiae mosquitoes from Côte d’Ivoire. BMC Infect. Dis. 2012;12:214. doi: 10.1186/1471-2334-12-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David J-P., Ismail H.M., Chandor-Proust A., Paine M.J.I. Role of cytochrome P450s in insecticide resistance: impact on the control of mosquito-borne diseases and use of insecticides on Earth. Philos. Trans. R. Soc. London, Ser. B. 2013;368:20120429. doi: 10.1098/rstb.2012.0429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diabaté A., Baldet T., Chandre F., Akogbeto M., Guiguemde R., Darriet F., Brengues C., Guillet P., Hemingway J., Graham J., Hougard J.M. The role of agricultural use of insecticides in resistance to pyrethroids in Anopheles gambiae s.l. in Burkina Faso. Am. J. Trop. Med. Hyg. 2002;67(6):617–622. doi: 10.4269/ajtmh.2002.67.617. [DOI] [PubMed] [Google Scholar]
- Edi V.A., Koudou G.B., Jones C.M., Weetman D., Ranson H. Multiple-insecticide resistance in Anopheles gambiae mosquitoes, southern Côte d’Ivoire. Emerg. Infect. Dis. 2012;18(9) doi: 10.3201/eid1809.120262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feyereisen R. Insect P450 enzymes. Annu. Rev. Entomol. 1999;44:507–533. doi: 10.1146/annurev.ento.44.1.507. [DOI] [PubMed] [Google Scholar]
- Hemingway J., Ranson H. Insecticide resistance in insect vectors of human disease. Annu. Rev. Entomol. 2000;45:371–391. doi: 10.1146/annurev.ento.45.1.371. [DOI] [PubMed] [Google Scholar]
- Khot A.C., Bingham G., Field L.M., Moores G.D. A novel assay reveals the blockade of esterases by piperonyl butoxide. Pest. Manage. Sci. 2008;64:1139–1142. doi: 10.1002/ps.1603. [DOI] [PubMed] [Google Scholar]
- Lanning C.L., Corcoran J.J., Ayad H.M., Fine R.L., Abou-Donia M.B. A novel mechanism for insecticide-induced resistance. Proc. Annu. Meeting Soc. Toxicol. 1994;14:393. [Google Scholar]
- Malcolm C.A., Boddington R.G. Malathion resistance conferred by a carboxylesterase in Anopheles culicifacies Giles (species B) (Diptera: Culicidae) Bull. Entomol. Res. 1989;79:193–199. [Google Scholar]
- Moores G.D., Philippou D., Borzatta V., Trincia P., Jewess P., Gunning R., Bingham G. An analogue of piperonyl butoxide facilitates the characterisation of metabolic resistance. Pest. Manag. Sci. 2009;65:150–154. doi: 10.1002/ps.1661. [DOI] [PubMed] [Google Scholar]
- Nwane P., Etang J., Chouaïbou M., Toto J.C., Koffi A., Mimpfoundi R., Simard F. Multiple insecticide resistance mechanisms in Anopheles gambiae s.l. populations from Cameroon, Central Africa. Parasites Vectors. 2013;6:41. doi: 10.1186/1756-3305-6-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podsiadlowski L., Matha V., Vilcinskas A. Detection of a P-glycoprotein related pump in Chironomus larvae and its inhibition by verapamil and cyclosporin A. Comp. Biochem. Physiol. B: Biochem. Mol. Biol. 1998;121(4):443–450. doi: 10.1016/s0305-0491(98)10137-2. [DOI] [PubMed] [Google Scholar]
- Sarkadi B., Homolya L., Szakacs G., Varad A. Human multidrug resistance ABCB and ABCG transporters: participation in a chemoimmunity defense system. Physiol. Rev. 2006;86:1179–1236. doi: 10.1152/physrev.00037.2005. [DOI] [PubMed] [Google Scholar]
- Tripet F., Wright J., Cornel A., Fofana A., Mcabee R., Meneses C., Reimer L., Slotman M., Thieman T., Guimigo D., Traoré S., Lanzaro G. Longitudinal survey of knock down resistance to pyrethroid kdr in Mali, West Africa, and evidence of its emergence in the Bamako form of Anopheles gambiae s.s. Am. J. Trop. Med. Hyg. 2007;76(1):81–87. [PubMed] [Google Scholar]
- WHO . Report of the WHO Informal Consultation, Geneva. 1998. Tests Procedures for insecticide resistance monitoring in malaria vectors, bioefficacy and persistence of insecticides on treated surfaces; p. 43. WHO/MAL/98. [Google Scholar]
- Wondji C.S., Irving H., Morgan J., Lobo N.F., Collins F.H., Hunt R.H., Coetzee M., Hemingway J., Ranson H. Two duplicated P450 genes are associated with pyrethroid resistance in Anopheles funestus, a major malaria vector. Genome Res. 2009;19:452–459. doi: 10.1101/gr.087916.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wondji C.S., Coleman M., Kleinschmidt I., Mzilahowa T., Irving H., Ndula M., Rehman A., Morgan J., Barnes K.G., Hemingway J. Impact of pyrethroid resistance on operational malaria control in Malawi. PNAS. 2012;109:19063–19070. doi: 10.1073/pnas.1217229109. [DOI] [PMC free article] [PubMed] [Google Scholar]