Abstract
Neutrophil infiltration is a hallmark of alcoholic steatohepatitis; however, the underlying mechanisms remain unclear. We previously reported that chronic-plus-binge ethanol feeding synergistically induces hepatic recruitment of neutrophils, which contributes to liver injury. In this paper, we investigated the roles of invariant natural killer T (iNKT) cells in chronic-plus-binge ethanol feeding-induced hepatic neutrophil infiltration and liver injury. Wild-type and two strains of iNKT cell-deficient mice (CD1d- and Jα18-deficient mice) were subjected to chronic-plus-binge ethanol feeding. Liver injury and inflammation were examined. Chronic-plus-binge ethanol feeding synergistically increased the number of hepatic iNKT cells and induced their activation, compared with chronic feeding or binge alone. iNKT cell-deficient mice were protected from chronic-plus-binge ethanol-induced hepatic neutrophil infiltration and liver injury. Moreover, chronic-plus-binge ethanol feeding markedly upregulated the hepatic expression of several genes associated with inflammation and neutrophil recruitment in wild-type mice, but induction of these genes was abrogated in iNKT cell-deficient mice. Importantly, several cytokines and chemokines (e.g., MIP-2, MIP-1, IL-4, IL-6 and osteopontin) involved in neutrophil infiltration were upregulated in hepatic NKT cells isolated from chronic-plus-binge ethanol-fed mice compared to pair-fed mice. Finally, treatment with CD1d blocking antibody, which blocks iNKT cell activation, partially prevented chronic-plus-binge ethanol-induced liver injury and inflammation. Chronic-plus-binge ethanol feeding activates hepatic iNKT cells, which play a critical role in the development of early alcoholic liver injury, in part by releasing mediators that recruit neutrophils to the liver, and thus, iNKT cells represent a potential therapeutic target for the treatment of alcoholic liver disease.
Keywords: alcoholic hepatitis, CD1d, inflammation, liver
Introduction
Alcoholic liver disease (ALD) is one of the leading causes of mortality worldwide. Excessive alcohol consumption induces steatosis, and prolonged ingestion leads to steatohepatitis, fibrosis, cirrhosis and hepatocellular carcinoma.1,2,3,4 Several risk factors that contribute to ALD have been identified, including pattern of drinking, type of alcohol consumed, female gender and nutrition;4,5,6,7,8,9 however, the underlying mechanisms contributing to disease progression are only partially understood. In recent years, the role of inflammation in driving ALD pathogenesis has begun to be appreciated.10,11 Several reports suggest that chronic inflammation, which is marked by elevated serum levels of pro-inflammatory cytokines/chemokines such as tumor necrosis factor alpha (TNF-α), IL-6, IL-8 and monocyte chemoattractant protein-1, enhances disease progression owing to the recruitment of neutrophils, monocytes and macrophages.10,11,12,13 Although a role for Kupffer cells and neutrophils in the progression of ALD is acknowledged,10,11,13 there is much to be understood regarding how various immune cells interact and contribute to alcohol-induced liver injury.
The liver is enriched with invariant natural killer T (iNKT) cells, which have been implicated in host defense against pathogen infection and tumor transformation and in the pathogenesis of liver injury, inflammation and fibrosis;14,15,16 however, the role of iNKT cells in alcohol-induced liver injury remains obscure. NKT cells are a heterogeneous group of lymphocytes expressing markers of NK cells as well as a T-cell receptor (TCR) that recognizes glycolipid antigens presented by the non-classical major histocompatibility complex class I-like molecule CD1d.17,18 Several subsets of NKT cells have been proposed; however, the best characterized and most predominant subset is type I or iNKT cells, which express an invariant TCR-α and account for 95% of liver NKT cells. iNKT cells can be activated by various mechanisms: direct activation via TCR-mediated recognition of lipid antigens (e.g., α-galactosylceramide (α-Galcer)) presented by CD1d, indirect activation via Toll-like receptors (e.g., TLR4, TLR9) or cytokines (e.g., IL-18, IL-12) or a combination of these mechanisms.17,18 Activation of iNKT cells results in the rapid release of a variety of cytokines and chemokines that modify the host immune response.17,18 Accumulating evidence indicates that different modes of iNKT cell activation result in different signaling outcomes due to the induction of varying sets of cytokines: α-Galcer-mediated activation of iNKT cells produces IFN-γ, IL-4, IL-17 and other cytokines, whereas activation by IL-18 plus IL-12 leads to the predominant production of IFN-γ.19 Owing to their ability to produce a wide variety of inflammatory mediators, iNKT cells play complex roles in the pathogenesis of liver diseases.14,20,21 The role of iNKT cells in chronic alcoholic liver injury was previously investigated in a murine model of intragastric ethanol infusion.22 In this model, chronic feeding of mice with an ethanol diet for 4 weeks sensitized the hepatocytes to killing by α-Galcer-activated NKT cells via Fas and TNF-α-mediated mechanisms.22 Neither the role of NKT cells in alcohol-induced liver inflammation and injury nor the ability of alcohol to induce NKT cell activation were addressed in this study.22 Here, we investigated the role of iNKT cells in alcoholic liver injury in a mouse model of chronic-plus-binge ethanol feeding. Our data suggest that iNKT cells are activated after chronic-plus-binge ethanol feeding and contribute to hepatic neutrophil recruitment and liver injury.
Materials and methods
Mice
Ten- to twelve-week-old female mice were used in this study. Mice were housed in a temperature-controlled environment with a 12-h dark/12-h light cycle. C57BL/6J, IL-4−/−, IFN-γ−/− and CD1d−/− mice on a C57BL/6J background were purchased from the Jackson Laboratory (Bar Harbor, MI, USA). CD1d−/− mice were further backcrossed in our facility for at least three generations. Mice deficient in Vα14 NKT cells (Jα18−/− mice) on a C57BL/6 background were kindly provided by Dr Taniguchi (Yokohama, Kanagawa, Japan) and were further backcrossed onto a C57BL/6J background for at least five generations in our facility. So both CD1d−/− and Jα18−/− mice were backcrossed onto a C57BL/6J background for more than 14 generations. To minimize environmental effects on experiments, C57BL/6J, CD1d−/− and Jα18−/− mice were bred at the same time at the NIAAA FLAC facility. All animals were cared for in accordance with National Institutes of Health guidelines, and all animal experiments were approved by the National Institute on Alcohol Abuse and Alcoholism Animal Care and Use Committee.
Chronic-plus-binge ethanol feeding
Mice were subjected to chronic (10 days) feeding plus a single binge of ethanol as previously described.23 Briefly, mice were acclimated to a liquid diet for 5 days with ad libitum feeding of a control diet (Bio-Serv, Frenchtown, NJ, USA). Following acclimation, the mice were either fed a 5% ethanol-containing diet or pair-fed with an isocaloric control diet (Bio-Serv) for 10 days. On the morning of day 11, ethanol-fed and pair-fed mice were gavaged with a single dose of ethanol (5 g/kg b.w.) or isocaloric maltodextrin, respectively, and were killed 3, 6 or 9 h later.
Isolation of liver leukocytes and flow cytometric analyses
Hepatic leukocytes were isolated by pressing liver tissue through a 70-µm mesh and collected in a 50-ml tube with PBS. Cell suspensions were centrifuged at 50 × g for 5 min to pellet the cellular debris. The supernatants were then centrifuged at 50 × g for 10 min at 4 °C to pellet cells. The cell pellets were resuspended in cold PBS and centrifuged again at 700 × g for 10 min at 4 °C. The resulting cell pellet was resuspended in 15 ml of 35% Percoll solution (room temperature) and overlaid on 10 ml of 70% Percoll solution. The gradient was spun at room temperature for 30 min at 700 × g. Liver leukocytes were isolated from the interface, washed twice in PBS and resuspended in FACS buffer (PBS containing 2 mM EDTA and 0.5% BSA). Leukocytes were stained for Gr-1 and CD11b (eBioscience, San Diego, CA, USA) to identify neutrophils or for CD3, CD69, NK1.1, APC (eBioscience) and CD1d-tetramer (NIH Core Facility) to identify iNKT cells and were analyzed on a FACSCanto II machine (BD Biosciences, San Jose, CA, USA).
Isolation of liver iNKT cells
Hepatic leukocytes from four mice were pooled, and iNKT cells were purified from isolated liver leukocytes using the NK1.1+ iNKT cell isolation kit (MiltenyiBiotec, Ashburn, CA, USA) according to the manufacturer's protocol. Briefly, purification of iNKT cells is a two-step procedure involving negative selection to remove unwanted cells followed by positive selection to collect NK1.1+ iNKT cells.
Treatment of mice with an anti-mouse CD1d blocking monoclonal antibody
To prevent CD1d-dependent activation of iNKT cells, mice were intraperitoneally injected with 50 µg of monoclonal anti-CD1d blocking antibody (BD Biosciences, San Diego, CA, USA) 6 days, 3 days and 12 h prior to the ethanol gavage. Mice that were intraperitoneally injected with 50 µg of isotype-matched antibody (BD Biosciences, San Diego, CA, USA) served as controls. Antibodies were diluted in sterile PBS prior to injection.
Statistical analysis
The data are shown as mean±s.e.m. of three independent experiments with 4–10 mice per treatment group. Data from two groups were compared with an unpaired t-test. Data from three or more groups were analyzed with one-way ANOVA with Tukey's multiple comparisons test. Significance was based on P values less than 0.05.
Results
Hepatic iNKT cells are increased in number and activated in response to chronic-plus-binge ethanol feeding
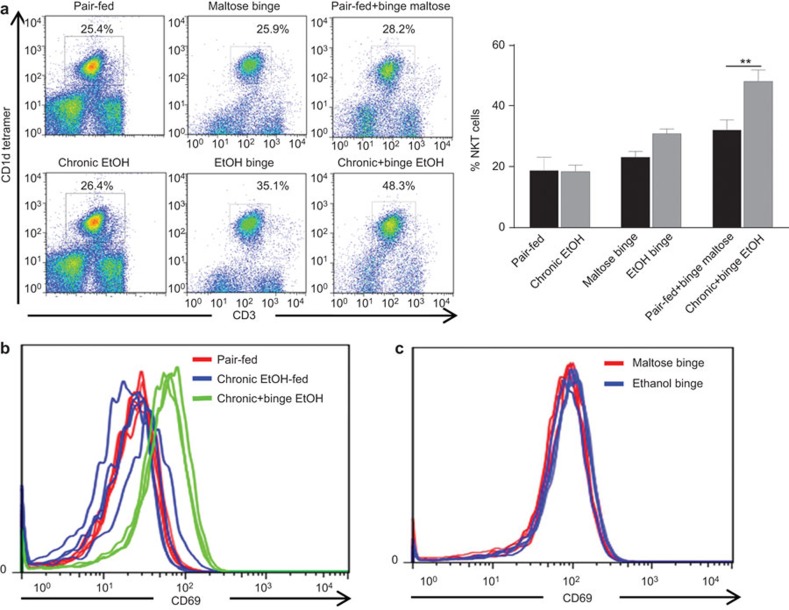
The pattern of alcohol consumption is a major risk factor for the progression of alcohol-induced liver injury, and a history of chronic alcohol consumption plus recent episodes of binge drinking is associated with increased risk of ALD.2,9 We analyzed the effects of various ethanol feeding patterns (binge, chronic and chronic-plus-binge) on hepatic iNKT cell accumulation in C57BL/6J (wild-type (WT)) mice. As illustrated in Figure 1a, the percentages of iNKT cells were comparable between pair-fed and chronically ethanol-fed mice. Mice administered a single binge of ethanol (5 g/kg, oral gavage) exhibited an increase of approximately 8% in the percentage of hepatic iNKT cells compared to maltose-gavaged controls, which suggests that binge alcohol consumption induces hepatic iNKT cell recruitment. Importantly, mice that received chronic-plus-binge ethanol demonstrated an average 18% increase in the percentage of iNKT cells compared to pair-fed plus binge maltose mice, thus suggesting a synergism between chronic and binge ethanol feeding. Furthermore, FACS analysis revealed that iNKT cells from chronic-plus-binge ethanol-fed mice had higher levels of CD69 expression than those isolated from pair-fed or chronic ethanol-fed mice (Figure 1b). In contrast, L-selectin (CD62L) expression was decreased on liver iNKT cells from chronic-plus-binge ethanol-fed mice compared to those from pair-fed or chronic ethanol-fed mice (data not shown). Increased expression of CD69 with a corresponding decrease in CD62L is an indicator of NKT cell activation.24 Interestingly, ethanol binge alone did not upregulate the expression of CD69 (Figure 1c), further suggesting that iNKT cell activation is a result of chronic-plus-binge ethanol feeding. Finally, the percentage of splenic iNKT cells was slightly but not significantly higher in chronic-plus-binge ethanol-fed mice than in pair-fed mice (Supplementary Figure 1).
Figure 1.
Chronic-plus-binge ethanol feeding increases hepatic iNKT cell numbers and induces iNKT activation in C57BL/6J mice. Liver lymphocytes were isolated from various groups of mice. Pair-fed: mice were pair-fed a control diet for 10 days; chronic EtOH: mice were fed an ethanol diet for 10 days; maltose binge: mice were gavaged with a single dose of maltose; EtOH binge: mice were gavaged with a single oral dose of ethanol (5 g/kg body weight); pair-fed+binge maltose: mice were pair-fed a control diet for 10 days followed by gavage of a single dose of maltose; chronic+binge EtOH: mice were fed an ethanol diet for 10 days followed by gavage of a single dose of ethanol. Liver lymphocytes were analyzed to determine the percentage of iNKT cells (a, representative figure, n=9 mice per group), and iNKT cells were analyzed for expression of the activation marker CD69 (b and c, n=3 mice per group). **P<0.01. iNKT: invariant natural killer T.
iNKT cell-deficient mice are protected from chronic-plus-binge ethanol-induced liver injury
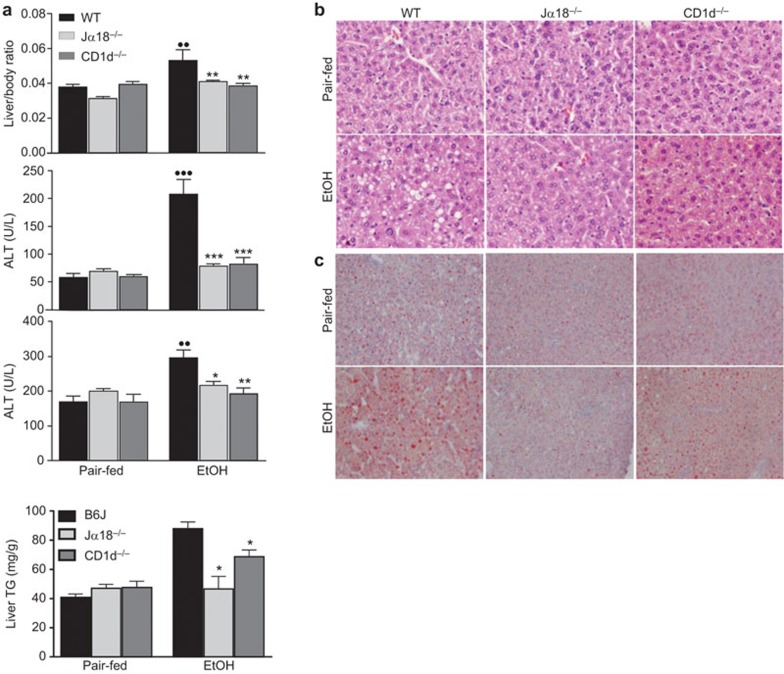
To examine the impact of iNKT cells on chronic-plus-binge ethanol-induced liver injury, WT and iNKT cell-deficient mice (Jα18−/− and CD1d−/− mice) were subjected to chronic-plus-binge ethanol feeding. As illustrated in Figure 2a, chronic-plus-binge ethanol feeding markedly increased the liver-to-body weight ratio and elevated serum levels of ALT and AST in WT mice but not in Jα18−/−or CD1d−/− mice. Hematoxylin and eosin (H&E) staining revealed that the steatosis induced by chronic-plus-binge ethanol feeding was attenuated in Jα18−/− and CD1d−/− mice compared to WT mice (Figure 2b). Finally, ethanol-fed Jα18−/−and CD1d−/− mice showed less hepatic fat accumulation than WT mice as demonstrated by Oil Red O staining (Figure 2c) and a hepatic TG assay (Figure 2d).
Figure 2.
iNKT cell-deficient mice are protected from chronic-plus-binge ethanol-induced liver injury and steatosis. iNKT cell-deficient (Jα18−/− and CD1d−/−) and WT mice were subjected to chronic-plus-binge ethanol (EtOH) feeding or pair-feeding (pair-fed). (a) Livers were collected, and the liver-to-body weight ratios were recorded. Serum was collected and analyzed for ALT and AST (n=6–20 mice per group). (b) Formalin-fixed liver tissues were subjected to H&E staining. (c) Frozen liver tissues were subjected to Oil Red O staining. (d) Hepatic TG levels were measured, and the difference between EtOH-fed WT and Jα18−/− or CD1d−/− mice was analyzed by Student's t-test (n=3 randomly selected samples per group). ••P<0.01, •••P<0.001 vs. pair-fed WT. *P<0.05, **P<0.01, ***P<0.0001 vs. EtOH-fed WT. ALT, alanine aminotransferase; H&E, hematoxylin and eosin; iNKT: invariant natural killer T; TG, triglyceride; WT, wild-type.
To ensure the observed effects were not due to differences in ethanol metabolism between the WT and iNKT cell-deficient mice, we analyzed several parameters involved in the metabolism of ethanol. First, we examined the expression of the ethanol metabolizing enzyme Cyp2E1 by performing immunohistochemical staining. As illustrated in Supplementary Figure 2a, hepatic expression of the Cyp2E1 protein was comparable in ethanol-fed WT, Jα18−/− and CD1d−/− mice. This is in agreement with a previous study that found that the hepatic expression of the Cyp2E1 protein was comparable in naive non-starved WT and iNKT cell-deficient mice, while higher Cyp2E1 expression was only found in starved iNKT cell-deficient mice compared with starved WT mice.25 Next, we analyzed ALDH2 activity and found no significant differences between WT and iNKT cell-deficient mice in response to pair- or chronic-plus-binge ethanol feeding (Supplementary Figure 2b). Finally, blood alcohol concentration, which was measured 1 h post-binge of a single gavage of ethanol (5 g/kg body weight), was comparable in WT and iNKT cell-deficient mice (Supplementary Figure 2c); therefore, it is unlikely that the differences in liver injury were due to alterations in ethanol metabolism in iNKT cell-deficient mice.
Hepatic expression of pro-inflammatory and fibrogenic genes is upregulated in chronic-plus-binge ethanol-fed WT mice but not in iNKT cell-deficient mice
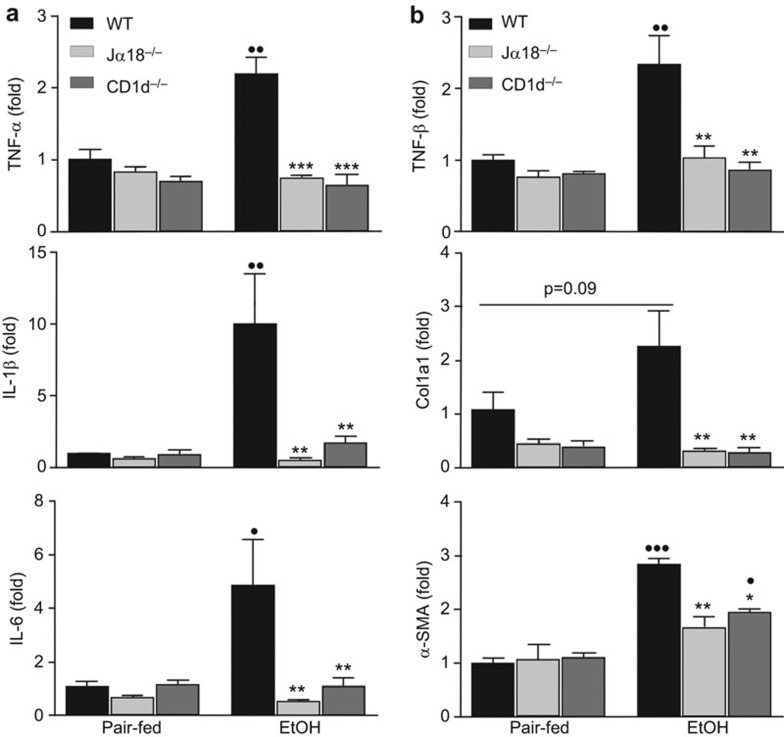
Inflammation is known to be a key factor in the progression of ALD, and persistent inflammation can lead to fibrosis.10 Therefore, we analyzed the induction of several pro-inflammatory genes in chronic-plus-binge ethanol-fed WT and iNKT cell-deficient mice to determine whether iNKT cells impact inflammatory signaling pathways. As expected, induction of mRNA expression of TNF-α, IL-1β and IL-6 was significantly elevated in ethanol-fed WT mice compared to their pair-fed controls; however, the induction of these genes was reduced in ethanol-fed Jα18−/− and CD1d−/− mice (Figure 3a).
Figure 3.
iNKT cell-deficient mice are protected from chronic-plus-binge ethanol-induced activation of pro-inflammatory and fibrogenic genes compared to WT mice. iNKT cell-deficient (Jα18−/− and CD1d−/−) and WT mice were subjected to chronic-plus-binge ethanol feeding (EtOH) or pair-feeding. Liver RNA was reverse transcribed, and the cDNA was subjected to real-time PCR analyses of pro-inflammatory gene expression (TNF-α, IL-1β and IL-6) (a) and fibrogenic gene expression (TGF-β, Col1a1 and α-SMA) (b). •P<0.05, ••P<0.01, •••P<0.001 vs. pair-fed WT. *P<0.05, **P<0.01, ***P<0.001 vs. EtOH-fed WT. n=3–10 mice per group. iNKT: invariant natural killer T; PCR, polymerase chain reaction; WT, wild-type.
Sirius red staining did not detect significant liver fibrosisin chronic-plus-binge ethanol fed-mice (data not shown); however, real-time polymerase chain reaction (PCR) analyses revealed that the hepatic expression of several genes associated with fibrogenesis, such as TGF-β and α-SMA, was significantly upregulated in ethanol-fed mice (Figure 3b). The upregulation of these fibrogenic genes was suppressed in both strains of iNKT cell-deficient mice (Figure 3b).
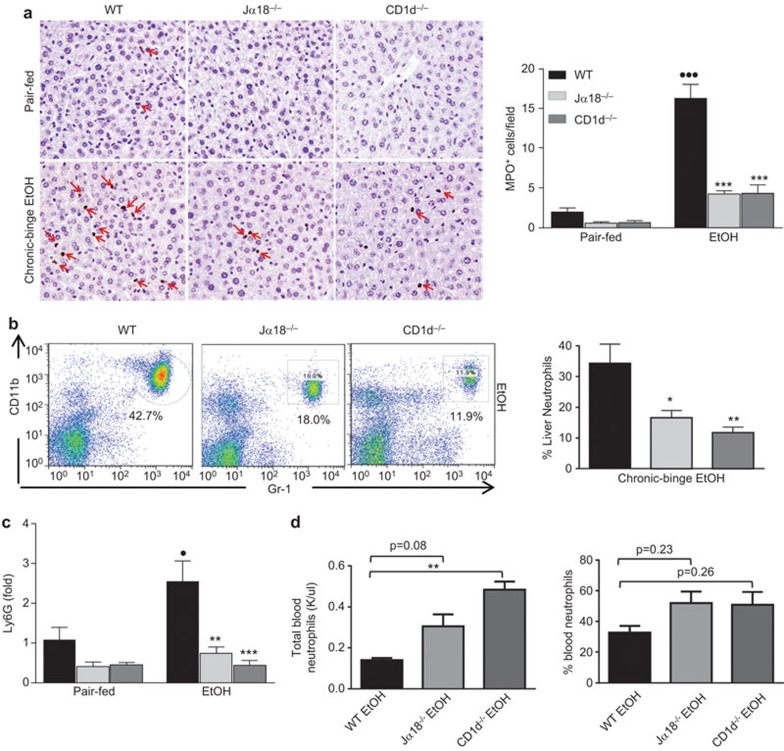
Hepatic neutrophil recruitment is blocked in chronic-plus-binge ethanol-fed iNKT cell-deficient mice
Neutrophil numbers are elevated in the livers of alcoholic hepatitis patients and are thought to correlate with disease progression and severity.26 Importantly, a recent report from our lab points to a correlation between hepatic neutrophils and liver injury in chronic-plus-binge ethanol-fed mice.23 To determine whether the reduced liver injury in ethanol-fed iNKT cell-deficient mice was due to reduced hepatic neutrophil infiltration, we performed immunohistochemical staining for myeloperoxidase (MPO). As illustrated in Figure 4a, the number of MPO+ neutrophils was significantly higher in chronic-plus-binge ethanol-fed WT mice than in pair-fed WT mice, but this elevation was reduced in ethanol-fed Jα18−/− and CD1d−/− mice. Furthermore, FACS analysis of liver polymorphonuclear cells revealed fewer CD11b+Gr1high neutrophils in Jα18−/− and CD1d−/− mice than in WT mice following chronic-plus-binge ethanol feeding (Figure 4b). In line with these findings, ethanol-fed iNKT cell-deficient mice had significantly lower hepatic mRNA expression levels of the neutrophil marker Ly6G than did ethanol-fed WT mice (Figure 4c). Interestingly, blood from ethanol-fed iNKT cell-deficient mice contained higher total numbers of circulating neutrophils than that from ethanol-fed WT mice, although the percentage was comparable between these groups (Figure 4d).
Figure 4.
Chronic-plus-binge ethanol-induced hepatic neutrophil accumulation is attenuated in iNKT cell-deficient mice. NKT cell-deficient (Jα18−/− and CD1d−/−) and WT mice were subjected to chronic-plus-binge ethanol feeding (EtOH) or pair-feeding. (a) Liver tissues were stained for neutrophils with an anti-MPO antibody. (b) Liver leukocytes were isolated and subjected to FACS analyses. Representative FACS analysis of liver neutrophils and quantification. (c) Liver mRNA was subjected to real-time PCR analyses of Ly6G, a neutrophil marker. (d) Analysis of blood neutrophil numbers and percentage. •P<0.05, •••P<0.001 vs. pair-fed WT. *P<0.05, **P<0.01, ***P<0.001 vs. EtOH-fed WT. n=6–10 mice per group. iNKT, invariant natural killer T; MPO, myeloperoxidase; WT, wild-type.
IL-4 and IFN-γ do not significantly contribute to chronic-plus-binge ethanol-induced liver injury
The hallmark of TCR-mediated iNKT cell activation is the production of IL-4 and IFN-γ.17,18 Surprisingly, the hepatic expression of IL-4 and IFN-γ mRNAs was lower in chronic-plus-binge ethanol-fed mice than in pair-fed mice (Supplementary Figure 3), and the serum levels of IL-4 and IFN-γ were not elevated after chronic-plus-binge ethanol feeding (data not shown). We used IL-4−/− and IFN-γ−/− mice to explore whether these cytokines contribute to chronic-plus-binge ethanol-induced liver injury. As illustrated in Supplementary Figure 4a, IL-4−/−and IFN-γ−/− mice on the chronic-plus-binge ethanol feeding regimen demonstrated a trend toward lower ALT levels compared to WT mice, but this difference did not reach statistical significance. Interestingly, MPO staining of liver sections revealed significantly less positive staining in IL-4−/− mice but not in IFN-γ−/− mice compared to WT mice (Supplementary Figure 4b).
iNKT cells contribute to chronic-plus-binge ethanol-induced hepatic neutrophil recruitment by producing several neutrophil infiltration-associated cytokines and chemokines
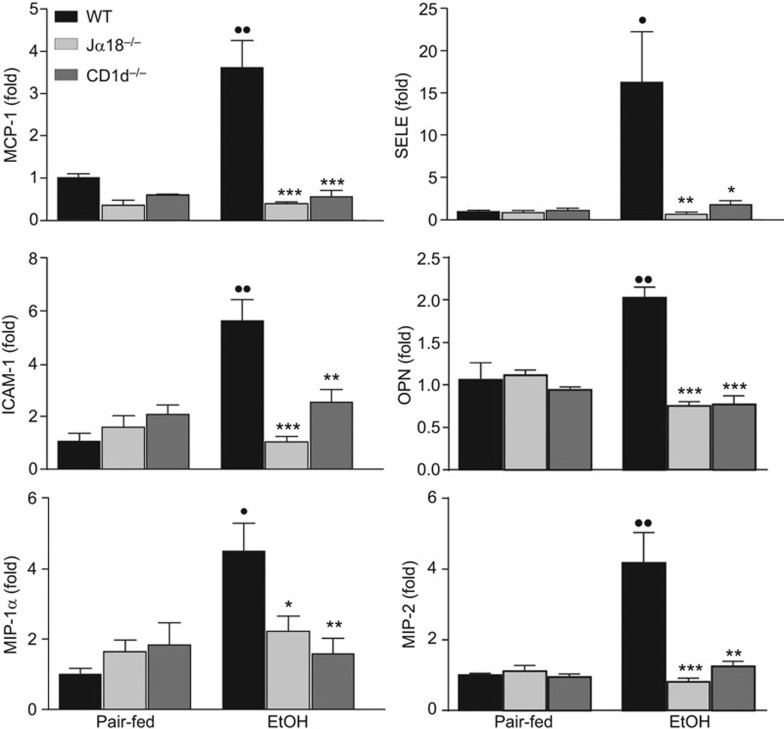
The above data suggest that IL-4 partially contributes to the induction of hepatic neutrophil infiltration after chronic-plus-binge ethanol feeding. To determine whether other factors also contribute to hepatic neutrophil recruitment in this model, real-time PCR assays were performed to analyze the expression of several genes that are associated with neutrophil infiltration. As illustrated in Figure 5, chronic-plus-binge ethanol feeding upregulated the hepatic expression of MCP-1, ICAM-1, E-selectin, MIP-1α, MIP-2 and osteopontin (OPN) in WT mice. Strikingly, these increases were significantly blunted in both Jα18−/− and CD1d−/− mice (Figure 5).
Figure 5.
iNKT cell-deficient mice are protected from ethanol-induced activation of genes associated with neutrophil infiltration. iNKT cell-deficient (Jα18−/− and CD1d−/−) and WT mice were subjected to chronic-plus-binge ethanol feeding (EtOH) or pair-feeding. Liver mRNA was subjected to real-time PCR analyses for chemokines and adhesion molecules. •P<0.05, ••P<0.01 vs. pair-fed WT. *P<0.05, **P<0.01, ***P<0.001 vs. EtOH-fed WT. n=6–10 mice per group. iNKT, invariant natural killer T; WT, wild-type.
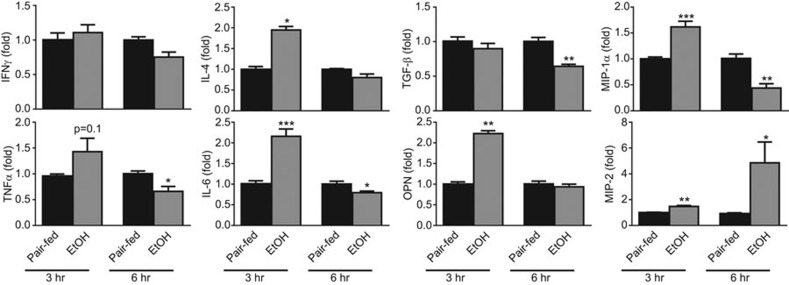
As iNKT cells are known to release a variety of pro-inflammatory cytokines as well as chemokines that may play a role in the recruitment of neutrophils,27,28 we further examined the expression of these mediators in iNKT cells isolated from chronic-plus-binge ethanol fed mice. As illustrated in Figure 6, real-time PCR analysis showed that IFN-γ mRNA was not elevated in hepatic iNKT cells from chronic-plus-binge ethanol-fed mice; however, IL-4 showed a twofold increase 3 h post-binge in these mice. There were also significant increases in the mRNA levels of IL-6, MIP-1α and OPN 3 h post-binge. Interestingly, the expression of MIP-2 was significantly increased 6 h post-binge, whereas the expression of TGF-β and TNF-α remained unchanged.
Figure 6.
Hepatic iNKT cellular expression of multiple genes associated with neutrophil infiltration is upregulated after chronic-plus-binge ethanol feeding. RNA was extracted from purified iNKT cells pooled from four chronic-plus-binge ethanol- or pair-fed C57BL6J mice and subjected to real-time PCR analysis of genes associated with inflammation or neutrophil infiltration. Data are represented as mean±s.e.m. from three independent experiments. *P<0.05, **P<0.01, ***P<0.001 vs. pair-fed. iNKT, invariant natural killer T; PCR, polymerase chain reaction.
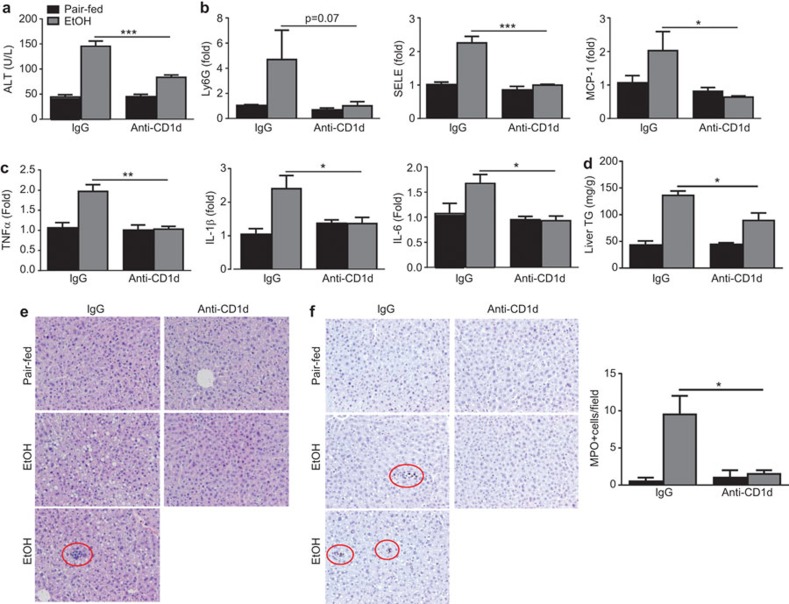
Treatment with an anti-mouse CD1d blocking monoclonal antibody protects WT mice from chronic-plus-binge ethanol-induced liver injury and inflammation
iNKT cells can be activated by TCR-mediated recognition of lipid antigens presented by CD1d. To determine whether this activation mechanism is involved in hepatic iNKT cell activation after chronic-plus-binge ethanol feeding, mice were treated with an anti-CD1 blocking antibody. As illustrated in Figure 7, chronic-plus-binge ethanol feeding significantly elevated serum ALT levels and hepatic expression of several genes associated with inflammation and neutrophil recruitment in the control antibody IgG groups. However, these elevations were markedly blunted in the anti-CD1d blocking antibody-treated groups. Furthermore, hepatic triglyceride assay, H&E staining and MPO staining of liver tissue revealed that treatment with anti-CD1d markedly reduced steatosis, accumulation of inflammatory foci and infiltration of MPO+ neutrophils induced by chronic-plus-binge ethanol feeding (Figure 7d–f).
Figure 7.
Treatment with an anti-CD1d antibody protects against chronic-plus-binge ethanol-induced liver injury and inflammation. C57BL6/J mice were subjected to a chronic-plus-binge feeding regimen or pair-feeding and received 500 µg anti-CD1d antibody or control IgG via i.p. injection. The mice were euthanized 9 h post-gavage. (a) Serum ALT levels were determined. (b, c) Liver RNA was isolated and subjected to real-time PCR analyses. (d) Hepatic TG assay. (e) Representative H&E staining. Inflammatory foci in IgG treated chronic-plus-binge ethanol-fed mice is circled in red. (f) Representative MPO staining. MPO+ cells are circled in red. The number of MPO+ cells was quantified and is shown on the right. *P<0.05, **P<0.01, ***P<0.001 (EtOH-fed anti-CD1d vs. EtOH-fed IgG group). n=4–8 mice per group. ALT, alanine aminotransferase; H&E, hematoxylin and eosin; MPO, myeloperoxidase; PCR, polymerase chain reaction; TG, triglyceride.
Discussion
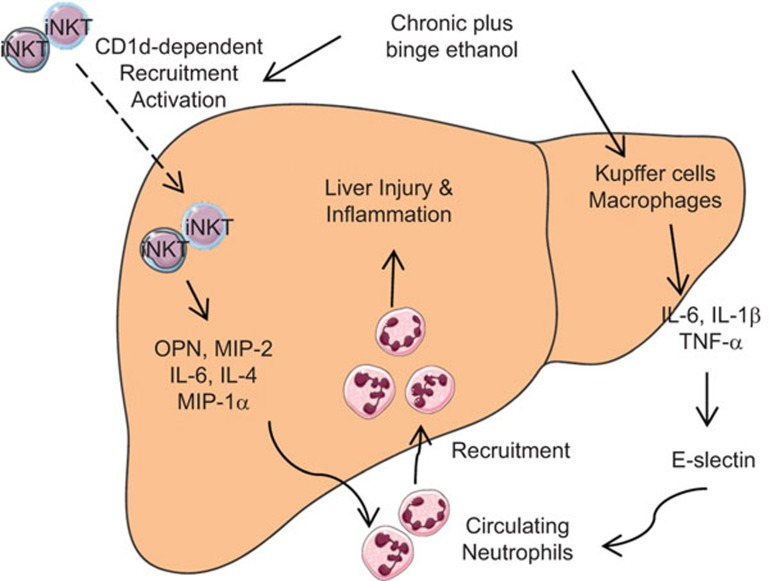
Several novel findings are presented in this study: (i) chronic-plus-binge ethanol feeding leads to an increase in the number and activation of hepatic iNKT cells; (ii) iNKT cell deficiency prevents chronic-plus-binge ethanol feeding-induced liver injury and inflammation; and (iii) iNKT cell activation promotes hepatic neutrophil recruitment by upregulating the expression of several genes associated with neutrophil infiltration. We have integrated these findings into a model shown in Figure 8.
Figure 8.
A model depicting the potential role of iNKT cells in chronic-plus-binge ethanol-induced neutrophil recruitment and liver injury. Ethanol exposure recruits iNKT cells to the liver and induces the activation of these cells via a CD1d-dependent mechanism. Activated iNKT cells release a variety of cytokines and chemokines that lead to the recruitment of neutrophils from the circulation. In addition, chronic-plus-binge ethanol feeding induces the activation of Kupffer cells/macrophages to produce cytokines, which upregulate the expression of E-selectin and subsequently promote hepatic neutrophil infiltration. Infiltrating neutrophils contribute to liver injury and inflammation. Servier Medical Art was used to produce this figure (http://www.servier.com). iNKT, invariant natural killer T.
CD1d-dependent hepatic iNKT cell activation after chronic-plus-binge ethanol feeding
Here, we report that chronic-plus-binge ethanol feeding significantly increased the number of hepatic iNKT cells and caused an activation of these cells, evident by their increased expression of CD69 as well as their production of mediators that trigger inflammation and recruitment of other leukocytes. The mechanisms underlying iNKT cell activation in this model are not clear. It has been proposed that iNKT cells can be activated by several mechanisms: CD1d-mediated lipid antigen-driven, antigen plus cytokine-driven, and cytokine-driven.17,18,19 These various modes of activation introduce complexity to the study of iNKT activation in the context of different disease states. In thec urrent study, we show that treatment with an anti-CD1d blocking monoclonal antibody abrogated liver injury and inflammation in chronic-plus-binge ethanol-fed C57BL6/J mice, suggesting that the CD1d-mediated lipid antigen-driven mechanism contributes to chronic-plus-binge ethanol feeding-induced iNKT cell activation and liver injury and that ethanol has no direct effect on iNKT cell activation. However, we cannot rule out the possibility that the cytokine-driven mechanism might also contribute to iNKT cell activation after chronic-plus-binge ethanol feeding. Presently, the identity of the endogenous lipid antigens that contribute to chronic-plus-binge ethanol-induced hepatic iNKT cell activation is unknown. It is known that chronic ethanol feeding induces gut bacterial overgrowth and increases gut permeability,29,30,31 which may result in elevated hepatic levels of bacterial products, such as glycosphingolipids, which can induce CD1d-dependent NKT cell activation.32 Interestingly, a recent study reported that incubation with physiological concentrations of ethanol stimulated NKT cell activation by enhancing CD1d glycolipid loading.33 Thus, it is reasonable to speculate that chronic ethanol feeding increases gut bacterial overgrowth and elevates hepatic bacterial products, including glycosphingolipids, and a subsequent ethanol binge enhances glycolipid loading onto CD1d and induces hepatic iNKT cell activation.
Hepatic iNKT cell activation contributes to chronic-plus-binge ethanol-induced liver injury by promoting hepatic neutrophil infiltration
Chronic-plus-binge ethanol feeding activated iNKT cells and increased liver injury and inflammation in WT mice, but these effects were abrogated in two strains of iNKT cell-deficient mice, suggesting that iNKT cells play a critical role in promoting chronic-plus-binge ethanol-induced liver injury. The pathological role of iNKT cells may be mediated via either direct iNKT cell killing of hepatocytes or indirect regulation of other types of immune cells. Indeed, direct NKT cell killing of hepatocytes has been implicated in several models of liver injury, including an intragastric chronic ethanol feeding model, via FasL-mediated cytotoxicity.22 However, in our study, we did not observe increased FasL expression on hepatic NKT cells after chronic-plus-binge ethanol feeding, suggesting that FasL may not contribute to liver injury in our model. A possible explanation for the discrepancy is the different feeding models used: we used a 10-day chronic-plus-binge model, whereas Minagawa et al.22 used intragastric chronic feeding for up to 4 weeks. Furthermore, in the current study, we provided evidence suggesting that activation of iNKT cells promotes chronic-plus-binge ethanol-induced liver injury by recruiting hepatic neutrophils. First, we found that iNKT cell-deficient mice (Jα18−/− and CD1d−/− mice) had markedly less infiltrating neutrophils in the liver and exhibited reduced liver injury after chronic-plus-binge ethanol feeding compared to WT mice. Second, the pathogenic functions of infiltrating neutrophils in this model have been demonstrated in our previous studies; specifically the reduction of hepatic neutrophils either by antibody-mediated depletion or via the disruption of E-selectin markedly prevented chronic-plus-binge ethanol-induced liver injury.23
Activation of iNKT cells promotes hepatic neutrophil infiltration via the production of cytokines and chemokines after chronic-plus-binge ethanol feeding
In the current study, we found that compared to WT mice, iNKT cell-deficient mice had fewer hepatic neutrophils but more circulating neutrophils in the blood in response to chronic-plus-binge ethanol feeding (Figure 4). These results indicate that the lack of hepatic neutrophil accumulation in chronic-plus-binge ethanol-fed iNKT cell-deficient mice is attributable to the retention of neutrophils in the blood, suggesting that iNKT cells play an important role in promoting hepatic neutrophil recruitment. It was previously reported that iNKT cells attenuates hepatocellular damage and subsequently reduces injury-associated hepatic neutrophil infiltration in a murine model of intragastric ethanol infusion plus α-Galcer treatment,34 suggesting that iNKT cells indirectly promoting hepatic neutrophil infiltration by inducing liver injury. Although we cannot rule out this mechanism in our model, we provided several lines of evidence suggesting an additional mechanism that iNKT cells can directly recruit neutrophil infiltration via the production of chemokines. First, in our chronic-plus-binge ethanol feeding model, activation of iNKT cells and hepatic neutrophil infiltration occurred 3 h post-binge, which was much earlier than the peak of liver injury (9 h post-binge) (unpublished data). Second, iNKT cells have been shown to recruit neutrophils to the liver via the induction of several cytokines and chemokines (e.g., IL-4, OPN and MIP-2) in various models of liver injury.27,35,36 Interestingly, deletion of the IL-4 gene only partially reduced hepatic neutrophil infiltration, but did not affect elevation of serum ALT levels (Supplementary Figure 4). This suggests that other chemokines rather than IL-4 may play a more important role in promoting hepatic neutrophil infiltration and injury induced by chronic-plus-binge ethanol feeding. Indeed, in addition to IL-4, several other important inflammatory mediators that are associated with neutrophil recruitment, such as OPN and MIP-2,27,37 were markedly elevated in hepatic iNKT cells after chronic-plus-binge ethanol feeding (Figure 6). Deletion of the OPN gene markedly diminished hepatic neutrophil infiltration and injury in the chronic-plus-binge ethanol feeding model,37 although this was not observed in a more severe injury model of Tsukamoto-hybrid ethanol feeding38 or in a mouse model with long-term (7-week) ethanol feeding.39 Furthermore, the concanavalin-A-induced T-cell hepatitis model was used to demonstrate that release of OPN from iNKT cells led to the subsequent release of MIP-2, both of which aid in the recruitment of neutrophils into the liver and contribute to hepatic injury.27 It is possible that the same process occurs in our model, as OPN gene expression in hepatic iNKT cells was increased at 3 h, whereas MIP-2 mRNA was subsequently elevated 6 h post-binge (Figure 6). Finally, we have previously demonstrated that chronic-plus-binge ethanol feeding synergistically upregulates the hepatic expression of E-selectin, which plays an important role in promoting hepatic neutrophil infiltration.23 In the current paper, we demonstrated that the hepatic expression of E-selectin was highly elevated in WT mice but not in iNKT cell-deficient mice after chronic-plus-binge ethanol feeding (Figure 5). This finding suggests that the activation of iNKT cells plays an important role in upregulating the expression of E-selectin. However, the mechanisms linking iNKT cells and E-selectin expression remain unknown. It is possible that activated iNKT cells produce cytokines (such as IL-6) that directly upregulate the hepatic expression of E-selectin or that iNKT cells indirectly activate Kupffer cells to produce cytokines and subsequently upregulate hepatic expression of E-selectin (Figure 8).
In summary, we have demonstrated that chronic-plus-binge ethanol feeding promotes hepatic neutrophil infiltration via the activation of hepatic iNKT cells in mice. Many patients with alcoholic hepatitis report years of chronic drinking combined with more recent episodes of bingeing (chronic-plus-binge drinking pattern).5,6,7,8,9 Therefore, it is plausible to speculate that hepatic iNKT cells are activated and subsequently promote hepatic neutrophil infiltration and liver injury in alcoholic hepatitis patients. Further studies are needed to examine this hypothesis and investigate iNKT cells as potential therapeutic targets for the treatment of alcoholic hepatitis.
Authors' contributions
SM made research design, performed experiments, analyzed the data and drafted the manuscript; DF performed flow cytometry analysis; IM, CJ and VK analyzed the data and edited the manuscript; BG made research design, analyzed the data and edited the manuscript.
Acknowledgments
This work was supported by the intramural program of NIAAA, NIH (BG), U01AA021723 (to CJ and BG) and R01 AA020864 &R01 CA100660 (VK).
There is no conflict of interest to disclose for all authors.
Footnotes
Supplementary Information accompanies the paper on Cellular & Molecular Immunology's website. (http://www.nature.com/cmi).
Supplementary Information
References
- Gao B, Bataller R. Alcoholic liver disease: pathogenesis and new therapeutic targets. Gastroenterology 2011; 141: 1572–1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Shea RS, Dasarathy S, McCullough AJ, Practice Guideline Committee of the American Association for the Study of Liver D, the Practice Parameters Committee of the American College of G. Alcoholic liver disease. Hepatology 2010; 51: 307–328.20034030 [Google Scholar]
- Lucey MR, Mathurin P, Morgan TR. Alcoholic hepatitis. N Engl J Med 2009; 360: 2758–2769. [DOI] [PubMed] [Google Scholar]
- Williams JA, Manley S, Ding WX. New advances in molecular mechanisms and emerging therapeutic targets in alcoholic liver diseases. World J Gastroenterol 2014; 20: 12908–12933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellentani S, Saccoccio G, Costa G, Tiribelli C, Manenti F, Sodde M et al. Drinking habits as cofactors of risk for alcohol induced liver damage. Gut 1997; 41: 845–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stranges S, Freudenheim JL, Muti P, Farinaro E, Russell M, Nochajski TH et al. Differential effects of alcohol drinking pattern on liver enzymes in men and women. Alcohol Clin Exp Res 2004; 28: 949–956. [DOI] [PubMed] [Google Scholar]
- Zakhari S, Li TK. Determinants of alcohol use and abuse: impact of quantity and frequency patterns on liver disease. Hepatology 2007; 46: 2032–2039. [DOI] [PubMed] [Google Scholar]
- Shukla SD, Pruett SB, Szabo G, Arteel GE. Binge ethanol and liver: new molecular developments. Alcohol Clin Exp Res 2013; 37: 550–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrio E, Tome S, Rodriguez I, Gude F, Sanchez-Leira J, Perez-Becerra E et al. Liver disease in heavy drinkers with and without alcohol withdrawal syndrome. Alcohol Clin Exp Res 2004; 28: 131–136. [DOI] [PubMed] [Google Scholar]
- Wang HJ, Gao B, Zakhari S, Nagy LE. Inflammation in alcoholic liver disease. Annu Rev Nutr 2012; 32: 343–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo G, Petrasek J, Bala S. Innate immunity and alcoholic liver disease. Dig Dis 2012; 30 Suppl 1: 55–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandrekar P, Ambade A, Lim A, Szabo G, Catalano D. An essential role for monocyte chemoattractant protein-1 in alcoholic liver injury: regulation of proinflammatory cytokines and hepatic steatosis in mice. Hepatology 2011; 54: 2185–2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan J, Benkdane M, Teixeira-Clerc F, Bonnafous S, Louvet A, Lafdil F et al. M2 Kupffer cells promote M1 Kupffer cell apoptosis: a protective mechanism against alcoholic and nonalcoholic fatty liver disease. Hepatology 2014; 59: 130–142. [DOI] [PubMed] [Google Scholar]
- Gao B, Radaeva S, Park O. Liver natural killer and natural killer T cells: immunobiology and emerging roles in liver diseases. J Leukoc Biol 2009; 86: 513–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar V. NKT-cell subsets: promoters and protectors in inflammatory liver disease. J Hepatol 2013; 59: 618–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santodomingo-Garzon T, Swain MG. Role of NKT cells in autoimmune liver disease. Autoimmun Rev 2011; 10: 793–800. [DOI] [PubMed] [Google Scholar]
- Kronenberg M. Toward an understanding of NKT cell biology: progress and paradoxes. Annu Rev Immunol 2005; 23: 877–900. [DOI] [PubMed] [Google Scholar]
- Bendelac A, Savage PB, Teyton L. The biology of NKT cells. Annu Rev Immunol 2007; 25: 297–336. [DOI] [PubMed] [Google Scholar]
- Brigl M, Brenner MB. How invariant natural killer T cells respond to infection by recognizing microbial or endogenous lipid antigens. Semin Immunol 2010; 22: 79–86. [DOI] [PubMed] [Google Scholar]
- Mattner J. Natural killer T (NKT) cells in autoimmune hepatitis. Curr Opin Immunol 2013; 25: 697–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain MG. Natural killer T cells within the liver: conductors of the hepatic immune orchestra. Dig Dis 2010; 28: 7–13. [DOI] [PubMed] [Google Scholar]
- Minagawa M, Deng Q, Liu ZX, Tsukamoto H, Dennert G. Activated natural killer T cells induce liver injury by Fas and tumor necrosis factor-α during alcohol consumption. Gastroenterology 2004; 126: 1387–1399. [DOI] [PubMed] [Google Scholar]
- Bertola A, Park O, Gao B. Chronic plus binge ethanol feeding synergistically induces neutrophil infiltration and liver injury in mice: a critical role for E-selectin. Hepatology 2013; 58: 1814–1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberl G, Lees R, Smiley ST, Taniguchi M, Grusby MJ, MacDonald HR. Tissue-specific segregation of CD1d-dependent and CD1d-independent NK T cells. J Immunol 1999; 162: 6410–6419. [PubMed] [Google Scholar]
- Martin-Murphy BV, Kominsky DJ, Orlicky DJ, Donohue TM Jr, Ju C. Increased susceptibility of natural killer T-cell-deficient mice to acetaminophen-induced liver injury. Hepatology 2013; 57: 1575–1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramaiah SK, Jaeschke H. Role of neutrophils in the pathogenesis of acute inflammatory liver injury. Toxicol Pathol 2007; 35: 757–766. [DOI] [PubMed] [Google Scholar]
- Diao H, Kon S, Iwabuchi K, Kimura C, Morimoto J, Ito D et al. Osteopontin as a mediator of NKT cell function in T cell-mediated liver diseases. Immunity 2004; 21: 539–550. [DOI] [PubMed] [Google Scholar]
- Matsuda JL, Mallevaey T, Scott-Browne J, Gapin L. CD1d-restricted iNKT cells, the ‘Swiss-Army knife' of the immune system. Curr Opin Immunol 2008; 20: 358–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnabl B, Brenner DA. Interactions between the intestinal microbiome and liver diseases. Gastroenterology 2014; 146: 1513–1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann P, Chen P, Wang HJ, Wang L, McCole DF, Brandl K et al. Deficiency of intestinal mucin-2 ameliorates experimental alcoholic liver disease in mice. Hepatology 2013; 58: 108–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao R. Endotoxemia and gut barrier dysfunction in alcoholic liver disease. Hepatology 2009; 50: 638–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinjo Y, Wu D, Kim G, Xing GW, Poles MA, Ho DD et al. Recognition of bacterial glycosphingolipids by natural killer T cells. Nature 2005; 434: 520–525. [DOI] [PubMed] [Google Scholar]
- Buschard K, Hansen AK, Jensen K, Lindenbergh-Kortleve DJ, de Ruiter LF, Krohn TC et al. Alcohol facilitates CD1d loading, subsequent activation of NKT cells, and reduces the incidence of diabetes in NOD mice. PLoS One 2011; 6: e17931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubes P, Mehal WZ. Sterile inflammation in the liver. Gastroenterology 2012; 143: 1158–1172. [DOI] [PubMed] [Google Scholar]
- Wang H, Feng D, Park O, Yin S, Gao B. Invariant NKT cell activation induces neutrophil accumulation and hepatitis: opposite regulation by IL-4 and IFN-gamma. Hepatology 2013; 58: 1474–1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng L, You Q, Yin H, Holt MP, Ju C. Involvement of natural killer T cells in halothane-induced liver injury in mice. Biochem Pharmacol 2010; 80: 255–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales-Ibanez O, Dominguez M, Ki SH, Marcos M, Chaves JF, Nguyen-Khac E et al. Human and experimental evidence supporting a role for osteopontin in alcoholic hepatitis. Hepatology 2013; 58: 1742–1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazaro R, Wu R, Lee S, Zhu NL, Chen CL, French SW et al. Osteopontin deficiency does not prevent but promotes alcoholic neutrophilic hepatitis in mice. Hepatology 2015; 69: 129–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge X, Leung TM, Arriazu E, Lu Y, Urtasun R, Christensen B et al. Osteopontin binding to lipopolysaccharide lowers tumor necrosis factor-alpha and prevents early alcohol-induced liver injury in mice. Hepatology 2014; 59: 1600–1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.