Abstract
Interleukin 4 (IL-4) has a variety of immune functions, including helper T-cell (Th-cell) differentiation and innate immune-response processes. However, the impact of IL-4 on gamma delta (γδ) T cells remains unclear. In this study, we investigate the effects of IL-4 on the activation and proliferation of γδ T cells and the balance between variable delta 1 (Vδ1) and Vδ2 T cells in humans. The results show that IL-4 inhibits the activation of γδ T cells in the presence of γδ T-cell receptor (TCR) stimulation in a STAT6-dependent manner. IL-4 promoted the growth of activated γδ T cells and increased the levels of Vδ1 T cells, which in turn inhibited Vδ2 T-cell growth via significant IL-10 secretion. Vδ1 T cells secreted significantly less interferon gamma (IFNγ) and more IL-10 relative to Vδ2. Furthermore, Vδ1 T cells showed relatively low levels of Natural Killer Group 2D (NKG2D) expression in the presence of IL-4, suggesting that Vδ1 T cells weaken the γδ T cell-mediated anti-tumor immune response. For the first time, our findings demonstrate a negative regulatory role of IL-4 in γδ T cell-mediated anti-tumor immunity.
Keywords: cytokines, signal transduction, T-cell receptors, T cells, tumor immunity
Introduction
T lymphocytes bearing gamma delta T-cell receptors (γδ TCRs) represent a minor population of T lymphocytes in human peripheral blood, and most exhibit the CD3+CD4−CD8− phenotype.1 γδ T cells directly recognize and bind antigens in a manner independent of the major histocompatibility complex and play an important role in immune surveillance and regulation in both innate and adaptive immunity.2,3,4,5 The γδ T cells kill a variety of epithelial tumor cells via secretion of interferon gamma (IFNγ), tumor necrosis factor alpha (TNFα), perforin, and granzymes. The γδ T cells are also involved in immune surveillance of infection caused by numerous viruses, such as human immunodeficiency virus, Epstein–Barr virus and hepatitis B virus.6,7
In humans, γδ T cells in peripheral blood are characterized as either Vδ1 or Vδ2 T cells depending on the δ chain used. Of these two subsets, Vδ2 T cells respond to inflammation/infection by producing inflammatory cytokines and inducing cytotoxicity in infected host cells. Vδ1 cells also regulate the immune response8,9 in addition to exerting anti-tumor10,11,12,13,14 and anti-viral effects.15,16,17 For example, a dominant Vδ1 T-cell population in tumor-infiltrating lymphocytes triggers potent immunosuppression via toll-like receptor 8 signaling.18 Therefore, the ratio of Vδ1 to Vδ2 T cells increases in the peripheral blood of tolerant recipients after liver transplantation and in cases of accepted grafts. In contrast, the ratio decreases in cases of chronically rejected grafts and graft recipients unable to cease immunosuppression therapy.19,20,21 In mice, Vγ1 γδ T cells suppress Vγ4 γδ T cell-mediated anti-tumor functions through Interleukin-4 (IL-4) production independent of cell–cell contacts.22 These results indicate that Vδ1 T cells have inhibitory effects on the immune response. The distinct roles of these two subsets of γδ T cells have also been demonstrated in autoimmune disease models and infection and immunity.23,24,25,26,27
IL-4 is a glycosylated, type-I cytokine primarily produced by T cells, natural killer T cells, mast cells and eosinophils. IL-4 initiates signal transduction through either the type I or type II receptor. IL-4 signaling is required for the differentiation of helper T 2 (Th2) and Th9 cells and regulates immunoglobulin class switching in B cells.28,29 IL-4 also plays a central role in the development of allergic inflammation and asthma by enhancing the expression of the high-affinity IgE receptor Fc epsilon RI on B cells, mast cells and basophils, promoting mast-cell survival and proliferation and inducing chemotaxis in mast cells, basophils and eosinophils.
In humans, IL-4 levels are usually elevated in the microenvironment of tumors, including renal cell cancer, non-small cell lung cancer, prostate cancer, colon cancer and breast cancer. In fact, production of IL-4 may be closely related to the stage and grade of malignancy in cancer patients.30 IL-4 receptor (IL-4R) is expressed at higher levels in situ in lung, ovarian, breast and pancreatic tumor samples compared with normal tissues.31,32,33,34 γδ T cells have also been identified in many types of tumors.6,35 It has been suggested that tumor-derived γδ T cells have regulatory effects in addition to typical anti-tumor effects.36 Breast tumor-derived γδ T regulatory cells were shown to induce immunosenescence in targeted naive and effector T cells and dendritic cells.37 However, it was also reported that patients exhibiting increased circulating Vδ1T lymphocytes, high levels of serum IL-4 and high expression of UL16 binding protein (ULBP) showed stable disease in a 1-year follow-up, in contrast to disease progression seen in patients with low circulating Vδ1T cells and undetectable IL-4 or ULBPs.38
It is essential to understand the role of tumor-infiltrating γδ T cells in order to effectively design immunotherapies. However, the exact function(s) of the subsets of γδ T cells in tumors are largely unknown, especially regarding the potentially suppressive effects of γδ T cells. In this study, we assess the effects of IL-4 on the human γδ T cell-mediated immune response in order to investigate the relationship between IL-4 and γδ T cells in tumor microenvironments.
Materials and methods
Antibodies and reagents
Purified anti-human γδ-TCR mAb (IMMU 510), anti-human Vδ2-TCR fluorescein isothiocyanate (FITC)-conjugated mAb (IMMU 389) and anti-human Natural Killer Group 2A (NKG2A) phycoerythrin (PE)-conjugated mAb (IM329IU) were obtained from ImmunoTech, Beckman Coulter, Fullerton, CA, USA. Purified anti-TCR Vδ1 mAb (TS 8.2) and anti-human Vδ1-TCR-FITC mAb (TS 8.2) were from Thermo Scientific, Waltham, MA, USA. Fluorescence-conjugated mAbs to CD212, CD210, CD124, CD152, CD27, NKG2D, CD94, T-bet, Gata3, Foxp3, Stat6, PLC-γ1 (pY783), SLP-76 (pY128), ERK1/2 (pT202/pY204), Akt (pS473), IRS-1 (pY896) and PKCθ (27/PKCθ) were from BD Pharmingen, San Diego, CA, USA. Fluorescence-conjugated mAbs to Vδ2-TCR, IFNγ, IL-10, CD3, CD45, Tim-3, TNFα, IL17A, IL-4, MICA/B and LEAF purified anti-human IL-4, IL-10 and IL-13 mAb were obtained from BioLegend, San Diego, CA, USA. Neutralizing anti-human TGF-β mAb was purchased from Abcam, Cambridge, MA, USA. Bender Instant ELISA kits for IL-4, IL-10, IFNγ and TGFβ were obtained from eBioscience, San Diego, CA, USA. Milliplex human Th17 multiplex panel was from Millipore, Bedford, MA, USA. The CytoTox96 Non-Radioactive Cytotoxicity Assay kit was obtained from Promega. Recombinant human IL-4, IL-10 and IL-13 were purchased from PeproTech, Rocky Hill, NJ. The BD Phosflow T-cell activation kit was obtained from BD Pharmingen, San Diego, CA, USA. PE anti-human CD159a was from Beckman Coulter, Fullerton, CA, USA. APC anti-human IL-13Rα1 was from R&D Systems, Minneapolis, MN, USA. TCR γ/δ+ T-cell isolation kit (human), anti-FITC MicroBeads and anti-PE MicroBeads were from Miltenyi Biotec, Bergisch Gladbach, Germany. SignalSilence Stat6 siRNA II was from Cell Signaling Technology, Danvers, MA, USA. CellTrace carboxyfluorescein succinimidyl ester (CFSE) Cell Proliferation Kit and Fluo-4 was from Invitrogen, Carlsbad, CA, USA. Zoledronic acid was purchased from Sigma. Pamidronate disodium was from Novartis, Basel, Switzerland.
Expansion of γδ T cells in vitro
Peripheral blood mononuclear cells (PBMCs) from healthy adult donors were isolated by Ficoll-Hypaque (TBD, Tianjin, China) centrifugation. The expansion of both Vδ1 T cells and Vδ2 T cells was performed as previously described.39,40 Briefly, PBMCs were cultured in complete RPMI 1640 medium supplemented with 2 mM L-glutamine, 10 mM HEPES, 10% FCS and 100 IU/ml rhIL-2 in 48- or 24-well culture plates pre-coated with immobilized anti-human γδ-TCR mAb (1 µg/ml) for 12–14 d. In some groups, rIL-4 (5 ng/ml), IL-10 or IL-13 was added at the beginning of the culture and replenished every 2 days. To selectively expand Vδ1 T cells, PBMCs were expanded with purified anti-TCR Vδ1 mAb and cultured in the same medium as described above. To selectively expand Vδ2 T cells, PBMCs were stimulated with zoledronic acid (5 µM) or pamidronate disodium (10 µM) and cultured in the same medium as described above.41 Cell viability was assayed by Trypan Blue exclusion method.
Cell sorting
After 2 weeks in culture, the γδ T cells that were expanded with anti-human TCR γδ mAb were collected and purified by negative selection using a human TCRγ/δ+ T Cell Isolation Kit (Miltenyi Biotec). The purified γδ T cells were then divided into two parts and labeled with FITC-conjugated anti-Vδ1 TCR or PE-conjugated anti-Vδ2 TCR antibody. Anti-FITC MicroBeads or anti-PE MicroBeads were added (Miltenyi Biotec) and magnetically separated using MS columns (Miltenyi Biotec) according to the manufacturer's protocol. We positively selected Vδ1 T cells or Vδ2 T cells, and negatively enriched Vδ2 T cells or Vδ1 T cells by the method described above. After 72 h of rest, isolated γδ T cells were collected and used for cytotoxicity assays or detection of phosphorylated signaling molecules.
Cytokine detection in supernatants
Purified Vδ1 T cells (>90%) and Vδ2 T cells (>92%) were obtained through magnetic-bead sorting technology and were cultured for 12, 24 and 48 h in 24-well plates. Cell-free supernatants were collected to detect the levels of IFNγ, TNFα, IL-4, IL-10, IL-6 and IL-17A using the human ELISA Immunoassay Kit (eBioscience) or the Milliplex human Th17 multiplex panel (Millipore) following the appropriate manufacturer's instructions.
Flow cytometric analysis
γδ T cells were stained at 4 °C for 10–15 min with the appropriate antibodies. Stained cells were analyzed by flow cytometry on a BD Accuri C6 flow cytometer (Becton Dickinson) using FlowJo Software (Tree Star Inc.).
Intracellular cytokine staining
Cells were activated with phorbol myristate acetate (20 ng/ml) plus ionomycin (1 µg/ml) for 4 h, and monensin was added in the last 2 h of cell-culture activation. Cell-surface staining was performed using FITC-conjugated anti-Vδ1 or anti-Vδ2 antibody. Subsequently, intracellular IFNγ, TNFα, IL-4, IL-10 or IL-17A was stained according to the Intracellular Cytokine Staining protocol (BioLegend).
Detection of phosphorylation
γδ T cells (>92%) were maintained for 24 h in RPMI 1640 medium containing 0.1% serum. To activate γδ-TCR signaling, γδ T cells were stimulated with immobilized anti-human γδ-TCR mAb (1 µg/ml) for 5 min in RPMI 1640 medium at 37 °C. To activate cytokine-induced signaling, γδ T cells were transferred to RPMI 1640 containing IL-2 (100 ng/ml) or IL-4 (100 ng/ml), or IL-2 plus IL-4 for 10 to 45 min at 37 °C. At the end of the treatment, one volume of the warmed BD Phosflow Fix Buffer I was immediately mixed with one volume of the PBMC suspension from treated and untreated samples and mixed well, and the tubes were incubated in a 37 °C water bath for 10 min. After fixation and washes, cells were permeabilized by slowly adding cold BD Phosflow Perm Buffer III while vortexing. Cells were incubated on ice for 30 min, washed and resuspended in 100 µl Stain Buffer (FBS) at 1×107 cells/ml. The treated and untreated γδ T cells were stained with antibody for 1 h. Stained cells were acquired by flow cytometry on a BD Accuri C6 flow cytometer (Becton Dickinson) and analyzed using FlowJo Software (Tree Star Inc.).
Ca2+ flux analysis
γδ T cells (>92%) were maintained for 24 h in RPMI 1640 medium containing 0.1% serum and were then loaded with 2 µM Fluo-4 AM (Invitrogen) for 45 min at room temperature in HBSS. After washing and resuspending in 200 µL HBSS, γδ T cells were seeded on Lab-Tek glass chamber slides (Nunc, Roskilde, Denmark) pre-coated with immobilized anti-human TCR γδ mAb (1 µg/ml). Changes in fluorescence are shown as a function of time.
Cytotoxicity assay
The cytotoxicity activity of Vδ1 T cells and Vδ2 T cells was quantitatively measured by CytoTox 96 Non-Radioactive Cytotoxicity Assay (Promega) based on the colorimetric detection of the released lactate dehydrogenase. MGC 803, K562 or G401 was co-cultured with γδ T cells at ratios of effector cells to target cells (E/T) of 20∶1 and 40∶1. After 6 h, the culture supernatant was used to detect lactate dehydrogenase activity according to the manufacturer's instructions.
CFSE assay
Autologous fresh PBMCs were labeled with CFSE and used as the responder cells, which were cultured in the lower chamber of a Transwell plate pre-coated with immobilized anti-human TCR γδ mAb (1 µg/ml). Purified Vδ1 T cells (>90%) and Vδ2 T cells (>92%) obtained through magnetic bead sorting were used as effector cells and placed in the upper chamber. The ratio of effector cells to responder cells was 3∶7. After a 10-day incubation, the percentage of Vδ2 T cells was analyzed by flow cytometry.
RNA interference and DNA transfection
For RNA interference, γδ T cells were transfected with 300 pmol of SignalSilence Stat6 siRNAII (Cell Signaling) using an Amaxa Nucleofector system. A total of 2×107 cells were resuspended in 100 µl of Amaxa Kit solution V, mixed with siRNA and immediately transfected using program I-24. Cells were incubated for 48 h at 37 °C and 5% CO2, with the last 24 h used for resting before the assays were performed as indicated. The negative siRNA control was obtained from Invitrogen.
Statistical analysis
The results are expressed as the mean±s.d. Statistical significance was analyzed by two-tailed unpaired Student's t-test using GraphPad Prism 5 for Windows (GraphPad, San Diego, CA, USA). Throughout the text, figures, and legends, the following terminology is used to show statistical significance: *P<0.05, **P<0.01 and ***P<0.001.
Results
IL-4 inhibits the activation of both Vδ1 and Vδ2 T cells in vitro
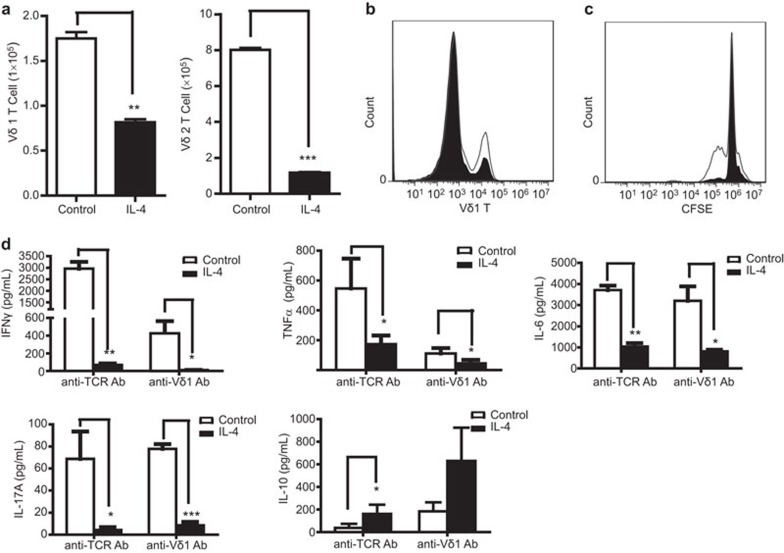
When naive Vδ1 T cells are activated by anti-TCR Vδ1 mAb or naive Vδ2 T cells are activated by zoledronic acid, IL-4 demonstrates a regulatory effect on the activation of both Vδ1 and Vδ2 T cells. The absolute number of Vδ1T cells (Figure 1a, left) or Vδ2 T cells (Figure 1a, right) and the relative ratio of Vδ1 T cells (Figure 1b) significantly decreased in the presence of IL-4 (5 ng/ml). Similarly, inhibitory effects of IL-4 on the activation of Vδ1 T cells were also demonstrated by the CFSE assay (Figure 1c). These results suggest that activation-induced proliferation of Vδ1 T cells, or Vδ2 T cells, is greatly suppressed by IL-4. In addition, IL-4 significantly inhibited the secretion of pro-inflammatory cytokines, including IFNγ, TNFα, IL-6 and IL-17A, from activated γδ T cells (Figure 1d). IFNγ was significantly reduced from approximately 3 ng/ml to <100 pg/ml after IL-4 treatment. However, the level of IL-10, an anti-inflammatory cytokine, was higher than in the absence of IL-4 treatment (Figure 1d). These results, taken together, suggest that IL-4 inhibits the activation of both Vδ1 and Vδ2 T cells in vitro.
Figure 1.
IL-4 inhibits the activation of both Vδ1 and Vδ2 T cells in vitro. Vδ1T cells or Vδ2 T cells in fresh PBMCs were activated by immobilized anti-TCR Vδ1 mAb or zoledronic acid and cultured in RPMI 1640 medium with 100 IU IL-2 in the presence or absence of IL-4 (5 ng/ml). (a) The absolute numbers of Vδ1 T cells (left) or Vδ2 T cells (right) after 5 days were analyzed by flow cytometry. One representative experiment of three independent experiments is shown. **P<0.01 and ***P<0.001. (b) The relative ratio of Vδ1 T cells with (shaded histogram) and without (open histogram) IL-4 treatment (5 ng/ml) was analyzed by flow cytometry. One representative of three independent experiments is shown. (c) The fresh PBMCs were labeled with CFSE and expanded by anti-Vδ1 mAb. The data demonstrate the proliferation of Vδ1 T cells with (shaded histogram) and without (open histogram) IL-4 treatment at 5.5 days. One representative of three independent experiments is shown. (d) γδ T cells were activated by anti-human TCR PAN γδ mAb or anti-human Vδ1 mAb and cultured for 7 days. The cytokines IFNγ, TNFα, IL-6, IL-17A and IL-10 in the culture supernatant were assayed by ELISA or the Milliplex method. Flow cytometry was performed on a BD Accuri C6 flow cytometer system. *P<0.05, **P<0.01 and ***P<0.001. IFN, interferon; PBMC, peripheral blood mononuclear cell; TCR, T-cell receptor; TNF, tumor necrosis factor.
IL-4 inhibits γδ T-cell activation by suppressing the phosphorylation of SLP76, PLCγ1 and Erk1/2 and by suppressing Ca2+ release
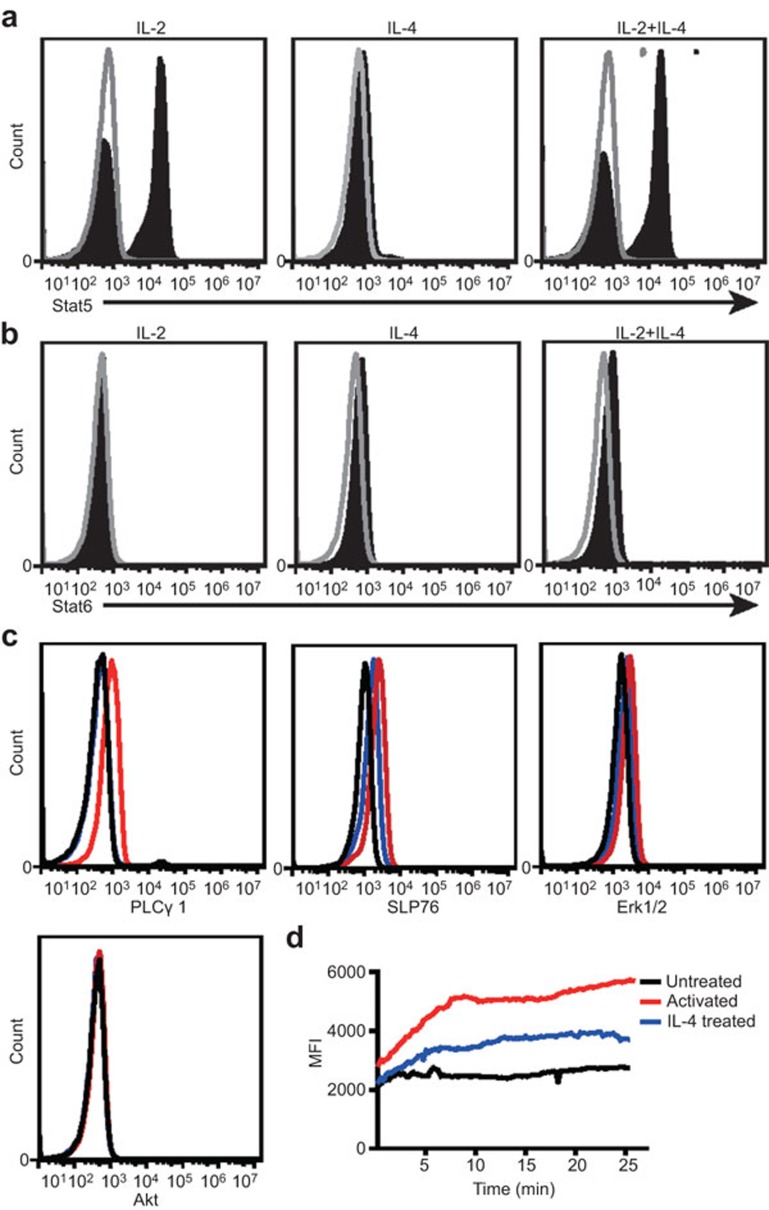
We studied the mechanism of the inhibitory effect of IL-4 on γδ T-cell activation. Given that IL-13 has little effect on the activation of γδ T cells (data not shown), we speculated that IL-4 function is mediated by type I IL-4 receptor but not type II receptor. The former consists of the two subunits IL-4Rα1 and γc chain, whereas the latter consists of the two subunits IL-4Rα1 and IL-13Rα1. Therefore, this study focused on the signals mediated by type I IL-4 receptor. We detected the effect of IL-4 on the phosphorylation status of important signaling molecules for γδ T-cell activation. The data show that IL-4 triggered phosphorylation of Stat5 and Stat6 (Figure 2a and b), but not P38 MAPK, Erk1/2, Stat1, Stat3, PKCθ, Akt, PLCγ1, SLP76 or IRS-1 (data not shown). This result is consistent with previous studies showing IL-4Rα1 mediates the phosphorylation of Stat6, and γc chain (also a subunit of IL-2R) mediates the phosphorylation of Stat5. To further investigate the mechanism of the inhibitory effect of IL-4 on γδ T cells, we analyzed the phosphorylation status of important TCR signaling molecules using the BD Phosflow assay, and we measured the levels of Ca2+ by laser confocal microscopy when γδ T cells were activated in the presence or absence of IL-4. The results show that TCR stimulation quickly triggered the phosphorylation of key signaling molecules, including SLP76, PLCγ1 and ERK1/2, and triggered the release of Ca2+ (Figure 2c and d). However, IL-4 significantly inhibited the phosphorylation of SLP76, PLCγ1 and Erk1/2 and the release of Ca2+ (Figure 2c and d).
Figure 2.
IL-4 inhibits the γδ T-cell activation by suppressing γδ TCR signal transduction. γδ T cells were treated with IL-2 or IL-4 or IL-2 plus IL-4 (shaded histogram) for 15 min. The untreated cells were used as a control (open histograms). The cells were fixed (BD Cytofix buffer) for 10 min at 37 °C, permeabilized (BD Phosflow Perm Buffer III) on ice for 30 min, blocked with normal mouse immunoglobulin and then stained with Stat5 (pY694) (a) or Stat6 (pY641) (b) for 1 h. The data represent one of three independent experiments. (c) γδ T cells were either treated with (blue line) or without IL-4 (red line) for 4 min before anti-human TCR PAN γδ mAb stimulation. The cells were then fixed, permeabilized and blocked as described above, and then stained with Akt (pS473), PLCγ1 (pY783), SLP76 (pY128) or Erk1/2 (pT202/pY204) mAb. Black line: rested γδ T cells; red line: γδ T cells activated by anti-human TCR PAN γδ blue line: γδ T cells activated by anti-human TCR PAN γδ mAb and treated with IL-4. Flow cytometry was performed on a BD Accuri C6 flow cytometer system. The data represent one of three independent experiments. (d) γδ T cells were loaded with Fluo-4 and monitored for changes in intracellular Ca2+ levels by laser confocal microscopy for the indicated acquisition time. Black line: rested γδ T cells; red line: γδ T cells activated by anti-human TCR PAN γδ blue line: γδ T cells activated by anti-human TCR PANγδ mAb and treated with IL-4. The data represent one of three independent experiments. TCR, T-cell receptor.
IL-4 promotes the proliferation of activated γδ T cells
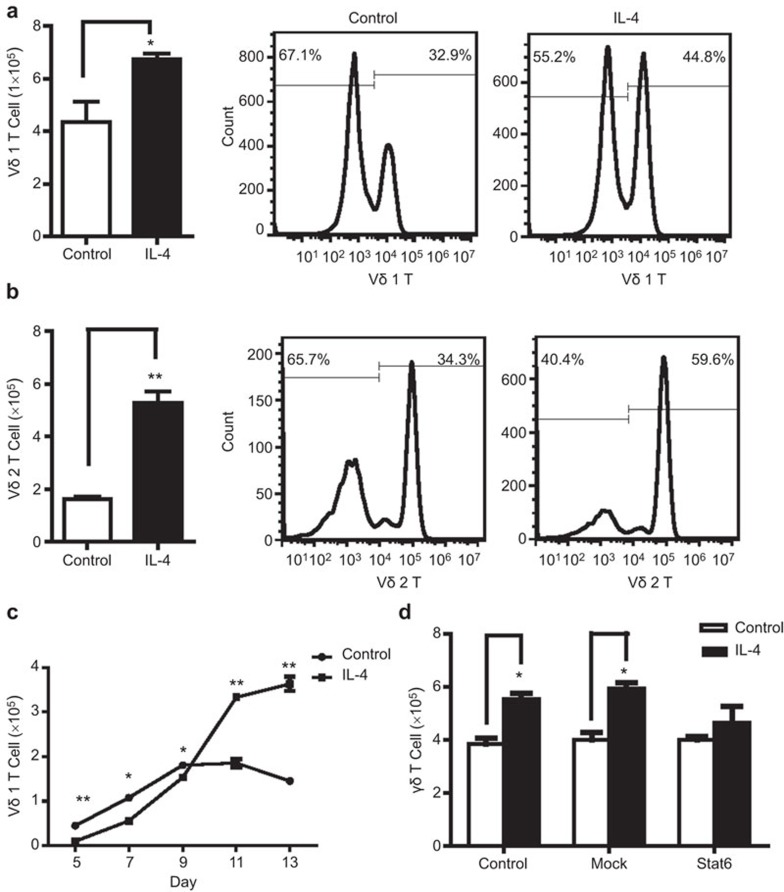
To determine the effect of IL-4 on the subsets of activated γδ T cells, Vδ1 T cells or Vδ2 T cells were activated by immobilized anti-TCR Vδ1 mAb or zoledronic acid, respectively. After 5 days in culture, the activated Vδ1 T cells and Vδ2 T cells were transferred to new wells and were cultured in the presence or absence of IL-4 (5 ng/ml). The absolute number and the relative ratio of Vδ1 T to Vδ2 T cells were analyzed after 5 further days of culture by flow cytometry. Interestingly, IL-4 treatment significantly increased the numbers of both activated Vδ1 T and Vδ2 T cells, demonstrating a stimulatory effect on the proliferation of activated γδ T cells (Figure 3a and b).
Figure 3.
IL-4 promotes the proliferation of activated γδ T cells. Vδ1 T cells or Vδ2T cells were activated first by immobilized anti-TCR Vδ1 mAb or zoledronic acid and then cultured with or without IL-4 for 5 days. The absolute number and relative ratio of Vδ1 T cells (a) or Vδ2 T cells (b) after a further 5 days of culture were analyzed by flow cytometry. The data represent one of three independent experiments. *P<0.05, **P<0.01. (c) Vδ1T cells in fresh PBMCs were activated by immobilized anti-TCR Vδ1 mAb and continuously cultured with or without IL-4 (5 ng/ml) for 13 days. The absolute number of Vδ1 T cells at different time points were counted by flow cytometry. *P<0.05, **P<0.01. (d) The activated γδ T cells were successfully transfected with Stat6 RNAi or Mock RNAi and cultured with or without IL-4 (5 ng/ml) for 3 days. The absolute number of γδ T cells was counted by flow cytometry. *P<0.05. TCR, T-cell receptor.
IL-4 inhibits the activation of naive Vδ1 T cells and promotes the proliferation of activated Vδ1 T cells. During the early stage (approximately 9 days) of Vδ1 T-cell activation by immobilized anti-TCR Vδ1 mAb, the number of Vδ1 T cells after IL-4 treatment was significantly less compared to the case without IL-4 treatment (Figure 3c). After 9 days and IL-4 treatment, the level of Vδ1 T cells was significantly higher compared to the case without IL-4 treatment (Figure 3c). We then verified whether IL-4 promoted the proliferation of γδ T cells through the Stat6 pathway. We inhibited Stat6 expression by RNA interference in γδ T cells to analyze the effect of IL-4 on the proliferation of Stat6− γδ T cells. The results show that the IL-4-induced proliferation of γδ T cells was partially blocked by the inhibition of Stat6 expression (Figure 3d).
IL-4 increased the absolute number and ratio of Vδ1 to Vδ2 T cells
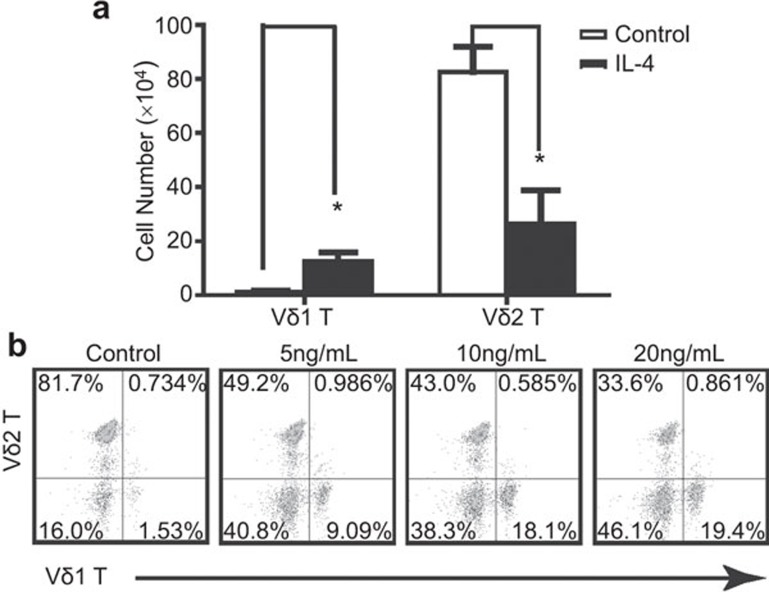
To further examine the effect of IL-4 on the simultaneous activation of Vδ1 and Vδ2 T cells, both subsets were activated by immobilized anti-human TCR PAN γδ mAb and cultured in RPMI 1640 medium with 100 IU IL-2 in the presence or absence of IL-4. The absolute numbers and the relative ratio of Vδ1 to Vδ2 T cells after 12 days were analyzed by flow cytometry. IL-4 treatment significantly decreased the number and ratio of Vδ2 T cells and significantly increased the number and ratio of Vδ1 T cells (Figure 4a and b). Furthermore, the effect of IL-4 was dose-dependent (Figure 4b). These results taken together indicate IL-4 promotes the proliferation of Vδ1 T cells.
Figure 4.
IL-4 increased the number and ratio of Vδ1 T cells in γδ T cells. (a) Both Vδ1 T cells and Vδ2 T cells were activated by immobilized anti-human TCR PAN γδ mAb and cultured with or without IL-4 for 12 days. The absolute number of Vδ1 T cells or Vδ2 T cells after 12 days culture was analyzed by flow cytometry. *P<0.05. (b) γδ T cells were activated by anti-human TCR PANγδ mAb with 0, 5 ng/ml, 10 ng/ml and 20 ng/ml IL-4 treatment (from left panel to right panel). The relative ratio of Vδ1 T cells to Vδ2 T cells was analyzed using a BD Accuri C6 flow cytometer. The data represent one of three independent experiments. TCR, T-cell receptor.
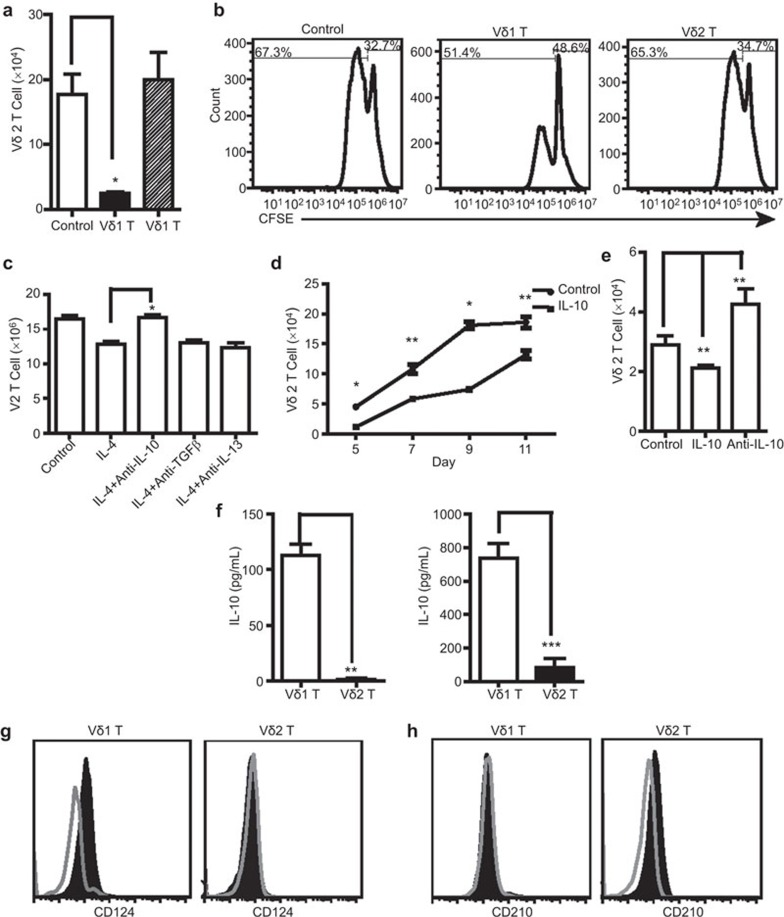
IL-4 induced Vδ1 T-cell bias via IL-10
The above results show that IL-4 promoted Vδ1 T cell growth and inhibited Vδ2 T-cell growth, resulting in a shift in the cellular balance toward Vδ1 T cells. However, this result contradicts other results in our study that show that IL-4 promoted the proliferation of activated Vδ2 T cells. In order to explain this phenomenon, we proposed a hypothesis that Vδ1T cells directly inhibit the proliferation of Vδ2T cells. Thus, we performed a Transwell assay and the results confirm that Vδ1T cells were directly inhibited by the proliferation of Vδ2T cells. This inhibitory effect was not dependent on direct cell–cell contact, but rather, was mediated by certain soluble factors (Figure 5a). The CFSE assay results demonstrate that Vδ1 T cells in the upper chamber inhibited Vδ2 T-cell growth in the lower chamber (Figure 5b). In order to investigate which factors play an inhibitory role, anti-IL-10, anti-IL-13 and anti-TGFβ antibodies were detected in our experimental system. The results of cytokine blocking experiments show that only the addition of anti-IL-10 antibody blocked IL-4-mediated Vδ1-T-cell bias; anti-IL-13 and anti-TGFβ antibody had no effect (Figure 5c). As expected, we observed that IL-10 inhibited the activation and proliferation of Vδ2 T cells (Figure 5d and e), while the effect of IL-10 on Vδ1 T cells was very weak (data not shown). To determine if Vδ1 T cells are the main source of inhibitory cytokine IL-10, Vδ1 and Vδ2 T cells were sorted by magnetic beads to examine IL-10 secretion. The results show that IL-10 levels in the rested and activated Vδ1 T cells were significantly higher compared with Vδ2 T cells (Figure 5f). Furthermore, IL-4 induced CD210 expression in activated Vδ2 T cells, while CD210 expression on activated Vδ1 T cells did not change significantly after IL-4 treatment. IL-4 induced expression of CD124 on activated Vδ1 T cells. However, CD124 expression on activated Vδ2T cells was not significantly affected after IL-4 treatment (Figure 5g and h). Together, these results highlight the differential effects of IL-4 on the CD124 and CD210 expression of Vδ1 and Vδ2 T cells and the contribution to the balance of γδ T cells and to the Vδ1 T cell bias.
Figure 5.
IL-4 induced the Vδ1 T-cell bias through IL-10 production by Vδ1 T cells. (a) Purified Vδ1 T cells (>90%) and Vδ2 T cells (>92%) were obtained through magnetic bead sorting and used as effector cells, which were placed in the upper chamber. Fresh PBMCs were expanded by immobilized anti-human TCR PAN γδ mAb in the lower chamber. The ratio of effector to responder cells was 3∶7. After 10-day incubation, the Vδ2 T cells in the lower chamber were counted by flow cytometry. The data represent one of three independent experiments. *P<0.05. (b) The transwell assay was performed as described above except that fresh PBMCs in the lower chamber were labeled with CFSE. The data demonstrate the growth of Vδ2 T cells under the effect of Vδ1 T cells or Vδ2 T cells in the upper chamber. The data represent one of three independent experiments. (c) Anti-TGF-β mAb, anti-IL-13 mAb or anti-IL-10 mAb was added into the system described in Figure 4a. The absolute number of Vδ2 T cells after 10 days culture was analyzed by flow cytometry. *P<0.05. (d) Vδ2 T cells were activated by pamidronate disodium and treated with IL-10 (10 ng/ml). Vδ2 T cells were counted by flow cytometry at different time points. The data represent one of three independent experiments. *P<0.05, **P<0.01. (e) Vδ2 T cells were activated by pamidronate disodium and treated with IL-10 (10 ng/ml) or anti-IL-10 mAb. Vδ2 T cells were counted at the fifth day by flow cytometry. The data represent one of three independent experiments. *P<0.05, **P<0.01. (f) Vδ1 T cells and Vδ2 T cells were expanded by anti-TCR γδ mAb in the presence of IL-4 and IL-2. Purified Vδ1 T cells (>90%) and Vδ2 T cells (>92%) were obtained through magnetic beads sorting and cultured in RPMI 1640 without IL-2 and IL-4. The secretion levels of IL-10 from the rested (left panel) or activated (right panel) Vδ1 T cells or Vδ2 T cells at 24 h were examined by ELISA or Milliplex method. **P<0.01 and ***P<0.001. Vδ1 T cells were activated by anti-TCR PAN γδ antibody and cultured with or without IL-4. Vδ2 T cells were activated by zoledronic acid and cultured with or without IL-4. CD 124 staining (g) and CD 210 staining (h) on Vδ1 T cells and Vδ2 T cells were performed. Open histograms represented the activated Vδ1 T cells or Vδ2 T cells. Shaded histogram represented the activated Vδ1 T cells or Vδ2 T cells treated with IL-4. PBMC, peripheral blood mononuclear cell; TCR, T-cell receptor.
IL-4-induced Vδ1 T-cell bias weakened the overall immune response of γδ T cells
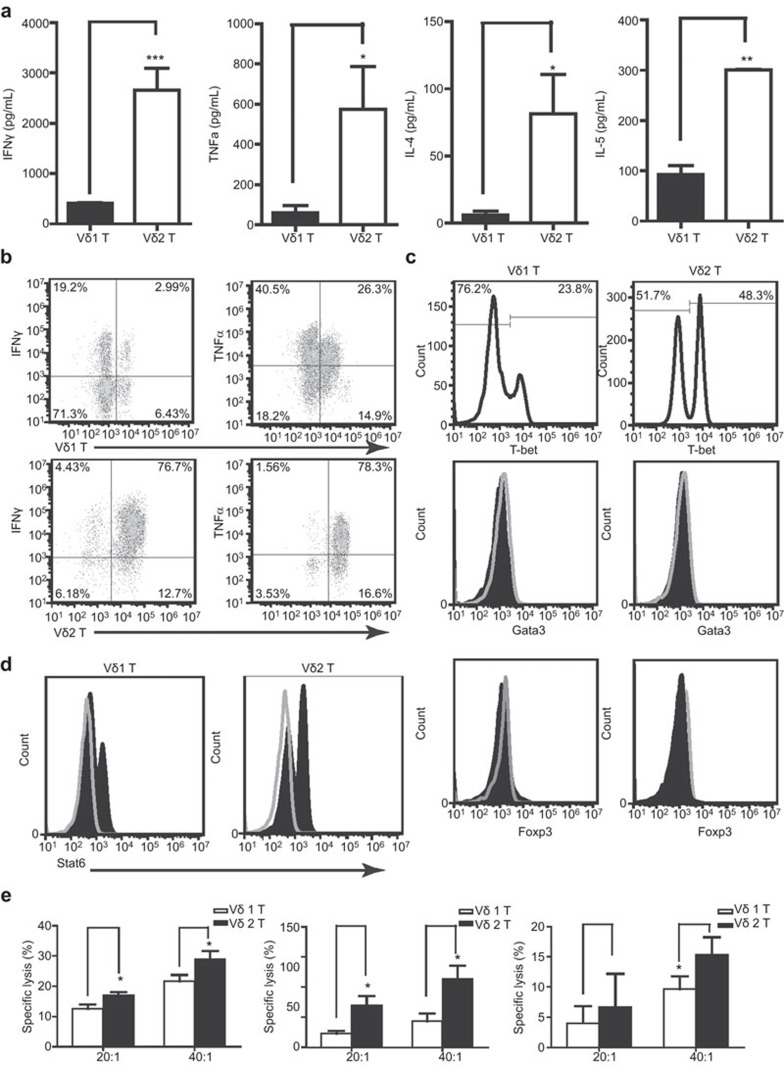
To confirm the biological significance of Vδ1 T-cell bias, we analyzed the cytokines, transcription factors and killing function of these two cell populations. The results show significant differences in the cytokine secretion spectrum between Vδ1 and Vδ2 T cells. Of the 10 cytokines detected, Vδ1 T cells secreted a high level of IL-10 and a small amount of IFNγ (approximately 400 pg/ml) and TNFα (approximately 70 pg/ml). Other cytokines were all below the detection limit. Vδ2 T cells secreted high levels of IFNγ (3 ng/ml) and TNFα (approximately 600 pg/ml), a normal amount of IL-4 (90 pg/ml) and IL-5 (300 pg/ml), but nearly no IL-10 was detected (Figure 5f and 6a). The intracellular staining signal of IFNγ and TNFα in Vδ2 T cells was stronger than that of Vδ1 T cells (Figure 6b). In order to determine the characteristic transcription factor expression patterns for Vδ1 T cells and Vδ2 T cells, three specific T-cell transcription factors (T-bet, Gata3 and Foxp3) were detected by the BD Phosflow method. The data show that both cell types expressed T-bet, although Vδ2T cells expressed a higher level of T-bet (Figure 6c). Neither cell type expressed Gata3 or Foxp3 (Figure 6d). In addition, Stat6 was detected in both Vδ1 T cells and Vδ2 T cells, which indicates that IL-4 acts on γδ T cells and causes biological effects (Figure 6d). To compare the anti-tumor effects of Vδ1 T cells and Vδ2 T cells, we examined the cytolysis effect on MGC803, K562 and G501 at 20∶1 and 40∶1 E/T ratios. The data show Vδ1 T cells exert a significantly weaker effect compared with Vδ2 T cells in killing tumor cells MGC803, K562 and G401 (Figure 6e). Furthermore, all tumor cells expressed MICA, MICB, ULBP1, ULBP2, ULBP3 and ULBP4 (data not shown), all of which can be recognized by NKG2D.11,42,43,44
Figure 6.
IL-4-induced Vδ1 T-cell bias weakened the overall immune response of γδ T cells. Vδ1 T cells and Vδ2 T cells were expanded by anti-TCR γδ mAb in the presence of IL-4. (a) Purified Vδ1 T cells (>90%) and Vδ2 T cells (>92%) were obtained through magnetic bead sorting and cultured in vitro. IFNγ, TNFα, IL-4 and IL-5 levels in the culture supernatant were analyzed by ELISA or Milliplex method. **P<0.01 and ***P<0.001. (b) Intracellular staining for IFNγ and TNFα in activated Vδ1 T cells and Vδ2 T cells was performed. The data represent one of three independent experiments. (c) Purified Vδ1 T cells (>90%) and Vδ2 T cells (>92%) were stained with anti-Gata3 mAb (L50-823), anti T-bet mAb (O4-46) anti Foxp3 (236a) and isotype mAb. The data represent one of three independent experiments. Open histograms represent the isotype and shaded histograms represent Gata3 or Foxp3. The data represent one of three independent experiments. (d) Purified Vδ1 T cells (>90%) and Vδ2 T cells (>92%) were stained with anti-Stat6 mAb (23/Stat6) or isotype mAb. (e) Purified Vδ1 T cells (>90%) and Vδ2 T cells (>92%) were prepared as described above. MGC 803, K562 or G401 was cocultured with γδ T cells at a ratio of effector cells to target cells (E/T) of 20∶1 and 40∶1. After 6 h, culture supernatant was used to detect LDH activity according to the manufacturer's instructions. *P<0.05. IFN, interferon; LDH, lactate dehydrogenase; TCR, T-cell receptor; TNF, tumor necrosis factor.
IL-4 inhibited the expression of NKG2D on Vδ1 T cells
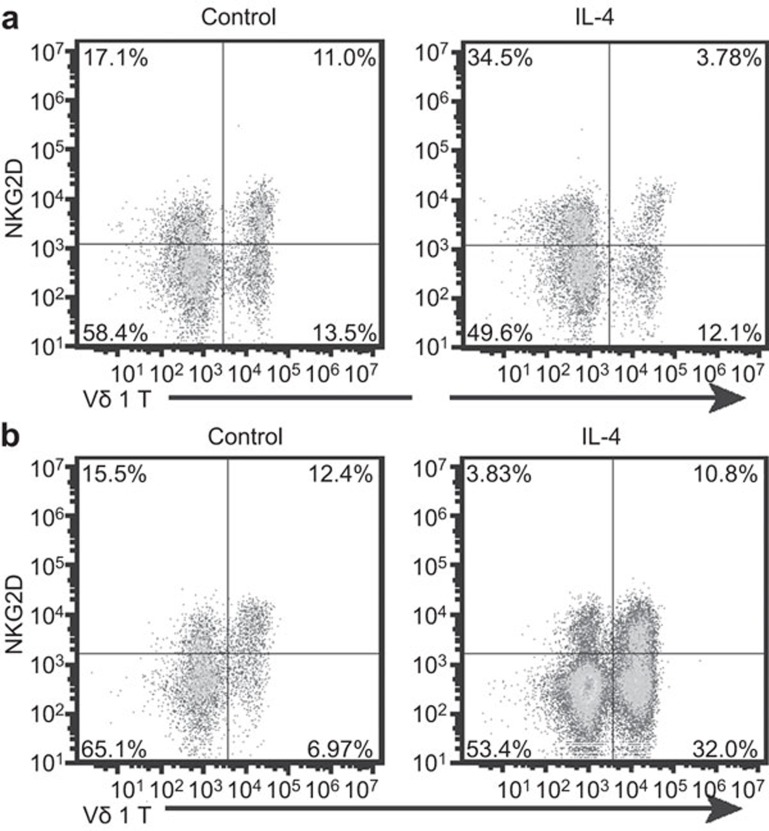
NKG2D receptor plays an important role in protecting the host from infection and cancer. NKG2D provides a powerful co-stimulus for the activation of γδ T cells.42,43,45,46,47,48 Here, NKG2D expression on γδ T cells was detected by flow cytometry. The results show that nearly all activated Vδ2 T cells expressed the NKG2D receptor. In comparison, some Vδ1 T cells were NKG2D− after activation. Furthermore, IL-4 significantly inhibited the expression of NKG2D on Vδ1 T cells both in the activation and proliferative stages (Figure 7). Therefore, we hypothesize that the inhibition of NKG2D by IL-4 reduced the anti-tumor function of Vδ1 T cells.
Figure 7.
IL-4 inhibited the expression of NKG2D on Vδ1 T cells. (a) Vδ1T cells in fresh PBMCs were activated by immobilized anti-TCR Vδ1 mAb and cultured with or without IL-4 (5 ng/ml). After 6 days of culture, NKG2D on Vδ1 T cells was stained and analyzed by flow cytometry. (b) Activated Vδ1 T cells were cultured with or without IL-4. After 5 days of culture, NKG2D on Vδ1 T cells was stained and analyzed by flow cytometry. The data represent one of three independent experiments. NKG2D, Natural Killer Group 2D; PBMC, peripheral blood mononuclear cell; TCR, T-cell receptor.
Discussion
IL-4 promotes Th2 and Th9 differentiation, B-cell proliferation, and mast cell survival and proliferation. γδ T cells play important roles in targeting a broad spectrum of tumors, including major histocompatibility complex-unrestricted recognition, abundant IFNγ secretion and potent cytotoxicity.6 In this study, we revealed a critical negative regulatory role of IL-4 in the γδ T cell-mediated immune response.
One of the most striking findings in this study was that IL-4 significantly inhibited the phosphorylation of the key signaling molecules SLP76, PLCγ1 and Erk1/2 in the TCR signaling pathway and inhibited the release of Ca2+ in γδ T cells, thereby inhibiting the TCR activation signaling of γδ T cells. It should be noted that IL-4 inhibited the activation of both Vδ1 T cells and Vδ2 T cells, which differs from the effect of IL-4 on Th1 cells and Th2 cells. To our knowledge, our results demonstrate, for the first time, that IL-4 confers an inhibitory effect on the activation of naive γδ T cells.
It was unexpected that IL-4 promoted the proliferation of the activated Vδ1 and Vδ2 T cells. Our previous understanding was that IL-4Rα1 activation blocks the γδ TCR activation signals when naive γδ T cells receive both TCR and IL-4 signals. IL-4 promotes proliferation when the activated γδ T cells receive the IL-4 signal alone. Therefore, the effects of IL-4 on γδ T cells include two aspects: (i) inhibition of TCR signaling; and (ii) promotion of proliferation. Although the molecular mechanism is not clear, the differential effects of IL-4 on resting γδ versus activated γδ T cells remain an intriguing aspect of this study. Collectively, our results support the view that IL-4 effects are dependent on the activation status of γδ T cells.
IL-10 is a broad inhibitory cytokine. In our study, IL-10 conferred an inhibitory effect on γδ T cells, particularly on Vδ2 T cells. Furthermore, IL-4 increased the CD124 expression on activated Vδ1 T cells and increased CD210 expression on activated Vδ2 T cells. CD124 expression on activated Vδ2 T cells and CD210 expression on Vδ1 T cells after IL-4 treatment were not significantly altered. These changes in IL-4R and IL-10R amplified the effect of IL-4 on Vδ1 T cells and the effect of IL-10 on Vδ2 T cells and subsequently led to a bias toward Vδ1 T cells.
IL-4 also suppressed NKG2D expression on Vδ1 T cells. This finding is similar to previous results showing that IL-4 downregulated NKG2D expression on human CD8+ T and natural killer cells and suppressed anti-tumor immune responses.49,50,51 In our previous study, we demonstrated that CD27+CD25high Vδ1 T cells have a regulatory function.40 We plan to further compare the functions of NKG2D− Vδ1 T cells and NKG2D+ Vδ1 T cells to examine whether the NKG2D− Vδ1 T cells have regulatory effects.
We compared characteristic cytokines, surface molecules, transcription factors and the cytolysis functions of Vδ1 T cells and Vδ2 T cells to examine Vδ1 T cell bias. Vδ1 T cells characteristically produced high levels of IL-10 and only a small amount of IFNγ and TNFα. Vδ2 T cells secreted high levels of IFNγ and TNFα, as well as IL-4 and IL-5, although nearly no IL-10 was detected. For the cell-surface phenotypes, almost all activated Vδ2T cells expressed NKG2D receptors. However, there was still a substantial population of NKG2D-negative Vδ1 T cells after activation. In addition, Vδ2 T cells expressed significantly higher CD94/NKG2A and TIM-3 molecules than Vδ1 T cells (data not shown).
Both human CD94/NKG2A and TIM-3 are inhibitory receptors expressed by natural killer cells or a subset of T cells and provide a negative feedback signal to suppress excessive immune responses. In this regard, our understanding is that Vδ2 T cells have a strong immune function via expression of inhibitory receptors that initiate a negative feedback to avoid excessive immunization. For characteristic transcription factors, both cells expressed T-bet, although more was expressed in Vδ2 T cells. Neither cell type expressed Gata3 or Foxp3. However, He et al.52 detected the expression of Foxp3, which suggests that IL-4 may have a pro-inflammatory effect on Vδ1 T cells. The molecular basis for the secretion of IFNγ by γδ T cells, especially Vδ2 T cells, is potentially due to a high level of T-bet expression and absence of GATA-3. The paucity of IL-4 synthesis in these cells is likely secondary to the low level of GATA-3 expression. These results indicate that Vδ2 T cells represent common γδ T cells with strong immune function, while Vδ1 T cells may have inhibitory effects on immune response. TCR and NKG2D are involved in tumor recognition of γδ T cells. IFNγ is the critical mediator in the protective immune response and has been widely used in many anti-cancer therapies. Considering Vδ1 T cells secreted much less IFNγ and more IL-10, and only part of Vδ1 T cells were NKG2D+ Vd1 T cells, it was expected that Vδ1 T cells were significantly weaker than Vδ2 T cells in killing tumor cells.
In summary, IL-4 directly inhibited the activation of γδ T cells at the beginning of the immune response and then increased the number and ratio of the Vδ1 T cell subset and decreased the number and ratio of the Vδ2 T cell subset in all γδ T cells. Finally, IL-4 continued to play an indirect inhibitory role on γδ T cells through Vδ1 T cells. Therefore, IL-4 suppressed the immune function of γδ T cells from the activation phase through the effector phase, resulting in compromised specific cytotoxicity and IFNγ secretion in vitro.
IL-4 and IL-2 have been used previously to stimulate the activation and proliferation of γδ T cells. It should be noted that these proliferated cells were used as efficient effectors against tumor targets both in vitro and in vivo. For example, Dokouhaki et al.53 cultured CD4−CD8− depleted PBMC from 14 healthy individuals in the presence of rhIL-2 and at a low concentration of rhIL-4 (0.1 ng/ml) plus anti-CD3 mAb for 10–14 days. The ex vivo expanded γδ T cells expressed high levels of TRAIL, NKG2D, perforin and granzyme B and were induced to produce TNFα and IFNγ. Furthermore, these γδ T cells had potent anti-tumor effects in vitro against cell lines of both hematological and epithelial origin and significantly inhibited the growth of lung cancer in a mouse xenograft model.53 This method involves the expansion of both Vδ1 and Vδ2 T cells. However, it is not clear whether the anti-tumor effect results primarily from the Vδ1 or Vδ2 T-cell subset, or both. Siegers et al.54,55 showed that fresh peripheral blood γδ T cells treated with Concanavalin A and 10 ng/ml of human IL-2 and IL4 yielded higher percentages of Vδ1 compared with Vδ2 T cells in mixed cultures independent of day 0 subset levels in the blood. Although this result was attributed to the duration of Concanavalin A exposure (leading to the apoptosis of Vδ2 cells), the result demonstrates that the inhibitory effect of IL-4 can be surpassed by IL-2 stimulation, as these Vδ1 cell-enriched cultures were highly cytotoxic against MEC-1, a human CLL cell line, and PC-3M, a human prostate cancer cell line. These data support IL-4 as a proliferator of γδ T cells. In addition, IL-4 has a ‘protective' role on Vδ1 T cells by preventing activation-induced cell death.55 A previous study showed that after being challenged by neuroblastoma cells, propagating Vδ1 but not Vδ2 T cells supported an anti-tumor response by the secretion of pro-inflammatory cytokines. Furthermore, Vδ1 T cells did not sustain a growth-promoting or tolerogenic microenvironment in contrast to other cell types.56
Tumor microenvironment includes multiple innate and adaptive immune cells and soluble factors that, together with tumor and tissue components, regulate tumor formation, progression and metastasis. Cytokines are the critical soluble factors that influence anti-tumor immunity.57 Among these cytokines, IL-4 has both protective as well as suppressive effects on anti-tumor immune responses depending on its sources, phases and doses, as well as the molecular and cellular environments. Fundamentally understanding the relationship between IL-4 and the compromised immune response of γδ T cells is critical for the development of effective strategies for anti-tumor immunotherapy. This article describes the mechanism of action of IL-4 regulation of the γδ T-cell immune response. This subject is not only conducive to a comprehensive understanding of the biological role of γδ T cells, but it is also helpful to guide potential γδ T cell-based biological therapies.
Acknowledgments
We thank Dr Mingxiao He (Duke University for Immunology Research) for critical reading of this manuscript. This work was supported by the Research Special Fund for Public Welfare Industry of Health (Grant Nos. 201202004 and 201302018), the National Program for Key Basic Research Projects, Ministry of Science and Technology, China (2013CB530503 and 2014CB542103), the Beijing Natural Science Foundation (Grant No. 5122031), the National Natural Science Foundation of China (Grant No. 30972776) and the National High Technology Research and Development Program (863 Program) (Grant No. 2007AA021109). We thank Dr Austin Cape for careful reading and feedback.
The authors declare no competing financial interests.
References
- Kabelitz D, Wesch D, He W. Perspectives of gammadelta T cells in tumor immunology. Cancer Res 2007; 67: 5–8. [DOI] [PubMed] [Google Scholar]
- Thedrez A, Sabourin C, Gertner J, Devilder MC, Allain-Maillet S, Fournie JJ et al. Self/non-self discrimination by human gammadelta T cells: simple solutions for a complex issue? Immunol Rev 2007; 215: 123–135. [DOI] [PubMed] [Google Scholar]
- Hayday AC. [gamma][delta] cells: a right time and a right place for a conserved third way of protection. Annu Rev Immunol 2000; 18: 975–1026. [DOI] [PubMed] [Google Scholar]
- Willcox CR, Pitard V, Netzer S, Couzi L, Salim M, Silberzahn T et al. Cytomegalovirus and tumor stress surveillance by binding of a human gammadelta T cell antigen receptor to endothelial protein C receptor. Nat Immunol 2012; 13: 872–879. [DOI] [PubMed] [Google Scholar]
- Gober HJ, Kistowska M, Angman L, Jeno P, Mori L, de Libero G. Human T cell receptor gammadelta cells recognize endogenous mevalonate metabolites in tumor cells. J Exp Med 2003; 197: 163–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonneville M, O'Brien RL, Born WK. Gammadelta T cell effector functions: a blend of innate programming and acquired plasticity. Nat Rev Immunol 2010; 10: 467–478. [DOI] [PubMed] [Google Scholar]
- Champagne E. gammadelta T cell receptor ligands and modes of antigen recognition. Arch Immunol Ther Exp (Warsz) 2011; 59: 117–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H, Knight JF, Alexander SI. Regulatory gamma delta T cells in Heymann nephritis express an invariant Vgamma6/Vdelta1 with a canonical CDR3 sequence. Eur J Immunol 2004; 34: 2322–2230. [DOI] [PubMed] [Google Scholar]
- Kuhl AA, Pawlowski NN, Grollich K, Blessenohl M, Westermann J, Zeitz M et al. Human peripheral gammadelta T cells possess regulatory potential. Immunology 2009; 128: 580–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correia DV, Fogli M, Hudspeth K, da Silva MG, Mavilio D, Silva-Santos B. Differentiation of human peripheral blood Vdelta1+ T cells expressing the natural cytotoxicity receptor NKp30 for recognition of lymphoid leukemia cells. Blood 2011; 118: 992–1001. [DOI] [PubMed] [Google Scholar]
- Groh V, Steinle A, Bauer S, Spies T. Recognition of stress-induced MHC molecules by intestinal epithelial gammadelta T cells. Science 1998; 279: 1737–1740. [DOI] [PubMed] [Google Scholar]
- Knight A, Mackinnon S, Lowdell MW. Human Vdelta1 gamma-delta T cells exert potent specific cytotoxicity against primary multiple myeloma cells. Cytotherapy 2012; 14: 1110–1118. [DOI] [PubMed] [Google Scholar]
- Maeurer MJ, Martin D, Walter W, Liu K, Zitvogel L, Halusczcak K et al. Human intestinal Vdelta1+ lymphocytes recognize tumor cells of epithelial origin. J Exp Med 1996; 183: 1681–1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poggi A, Venturino C, Catellani S, Clavio M, Miglino M, Gobbi M et al. Vdelta1 T lymphocytes from B-CLL patients recognize ULBP3 expressed on leukemic B cells and up-regulated by trans-retinoic acid. Cancer Res 2004; 64: 9172–9179. [DOI] [PubMed] [Google Scholar]
- Dechanet J, Merville P, Lim A, Retiere C, Pitard V, Lafarge X et al. Implication of gammadelta T cells in the human immune response to cytomegalovirus. J Clin Invest 1999; 103: 1437–1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenoglio D, Poggi A, Catellani S, Battaglia F, Ferrera A, Setti M et al. Vdelta1 T lymphocytes producing IFN-gamma and IL-17 are expanded in HIV-1-infected patients and respond to Candida albicans. Blood 2009; 113: 6611–6618. [DOI] [PubMed] [Google Scholar]
- Halary F, Pitard V, Dlubek D, Krzysiek R, de la Salle H, Merville P et al. Shared reactivity of Vδ2− γδ T cells against cytomegalovirus-infected cells and tumor intestinal epithelial cells. J Exp Med 2005; 201: 1567–1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng G, Wang HY, Peng W, Kiniwa Y, Seo KH, Wang RF. Tumor-infiltrating gammadelta T cells suppress T and dendritic cell function via mechanisms controlled by a unique Toll-like receptor signaling pathway. Immunity 2007; 27: 334–348. [DOI] [PubMed] [Google Scholar]
- Li Y, Koshiba T, Yoshizawa A, Yonekawa Y, Masuda K, Ito A et al. Analyses of peripheral blood mononuclear cells in operational tolerance after pediatric living donor liver transplantation. Am J Transplant 2004; 4: 2118–2125. [DOI] [PubMed] [Google Scholar]
- Martinez-Llordella M, Puig-Pey I, Orlando G, Ramoni M, Tisone G, Rimola A et al. Multiparameter immune profiling of operational tolerance in liver transplantation. Am J Transplant 2007; 7: 309–319. [DOI] [PubMed] [Google Scholar]
- Zhao X, Li Y, Ohe H, Nafady-Hego H, Uemoto S, Bishop GA et al. Intragraft Vdelta1 gammadelta T cells with a unique T-cell receptor are closely associated with pediatric semiallogeneic liver transplant tolerance. Transplantation 2013; 95: 192–202. [DOI] [PubMed] [Google Scholar]
- Hao J, Dong S, Xia S, He W, Jia H, Zhang S et al. Regulatory role of Vgamma1 gammadelta T cells in tumor immunity through IL-4 production. J Immunol 2011; 187: 4979–4986. [DOI] [PubMed] [Google Scholar]
- Wen L, Peakman M, Mieli-Vergani G, Vergani D. Elevation of activated gamma delta T cell receptor bearing T lymphocytes in patients with autoimmune chronic liver disease. Clin Exp Immunol 1992; 89: 78–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajan AJ, Asensio VC, Campbell IL, Brosnan CF. Experimental autoimmune encephalomyelitis on the SJL mouse: effect of gamma delta T cell depletion on chemokine and chemokine receptor expression in the central nervous system. J Immunol 2000; 164: 2120–2130. [DOI] [PubMed] [Google Scholar]
- Huber SA, Stone JE, Wagner DH Jr, Kupperman J, Pfeiffer L, David C et al. gamma delta+ T cells regulate major histocompatibility complex class II(IA and IE)-dependent susceptibility to coxsackievirus B3-induced autoimmune myocarditis. J Virol 1999; 73: 5630–5636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agrati C, Castilletti C, de Santis R, Cimini E, Bordi L, Malkovsky M et al. Interferon-gamma-mediated antiviral immunity against orthopoxvirus infection is provided by gamma delta T cells. J Infect Dis 2006; 193: 1606–1607; author reply 1607–1608. [DOI] [PubMed] [Google Scholar]
- Petermann F, Rothhammer V, Claussen MC, Haas JD, Blanco LR, Heink S et al. gammadelta T cells enhance autoimmunity by restraining regulatory T cell responses via an interleukin-23-dependent mechanism. Immunity 2010; 33: 351–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veldhoen M, Uyttenhove C, van Snick J, Helmby H, Westendorf A, Buer J et al. Transforming growth factor-beta ‘reprograms' the differentiation of T helper 2 cells and promotes an interleukin 9-producing subset. Nat Immunol 2008; 9: 1341–1346. [DOI] [PubMed] [Google Scholar]
- Dardalhon V, Awasthi A, Kwon H, Galileos G, Gao W, Sobel RA et al. IL-4 inhibits TGF-beta-induced Foxp3+ T cells and, together with TGF-beta, generates IL-9+ IL-10+ Foxp3− effector T cells. Nat Immunol 2008; 9: 1347–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onishi T, Ohishi Y, Imagawa K, Ohmoto Y, Murata K. An assessment of the immunological environment based on intratumoral cytokine production in renal cell carcinoma. BJU Int 1999; 83: 488–492. [DOI] [PubMed] [Google Scholar]
- Kawakami M, Kawakami K, Stepensky VA, Maki RA, Robin H, Muller W et al. Interleukin 4 receptor on human lung cancer: a molecular target for cytotoxin therapy. Clin Cancer Res 2002; 8: 3503–3511. [PubMed] [Google Scholar]
- Kioi M, Takahashi S, Kawakami M, Kawakami K, Kreitman RJ, Puri RK. Expression and targeting of interleukin-4 receptor for primary and advanced ovarian cancer therapy. Cancer Res 2005; 65: 8388–8396. [DOI] [PubMed] [Google Scholar]
- Leland P, Taguchi J, Husain SR, Kreitman RJ, Pastan I, Puri RK. Human breast carcinoma cells express type II IL-4 receptors and are sensitive to antitumor activity of a chimeric IL-4–Pseudomonas exotoxin fusion protein in vitro and in vivo. Mol Med 2000; 6: 165–178. [PMC free article] [PubMed] [Google Scholar]
- Kawakami K, Kawakami M, Husain SR, Puri RK. Targeting interleukin-4 receptors for effective pancreatic cancer therapy. Cancer Res 2002; 62: 3575–3580. [PubMed] [Google Scholar]
- Yi Y, He HW, Wang JX, Cai XY, Li YW, Zhou J et al. The functional impairment of HCC-infiltrating gammadelta T cells, partially mediated by regulatory T cells in a TGFbeta- and IL-10-dependent manner. J Hepatol 2013; 58: 977–983. [DOI] [PubMed] [Google Scholar]
- Seo N, Tokura Y, Takigawa M, Egawa K. Depletion of IL-10- and TGF-beta-producing regulatory gamma delta T cells by administering a daunomycin-conjugated specific monoclonal antibody in early tumor lesions augments the activity of CTLs and NK cells. J Immunol 1999; 163: 242–249. [PubMed] [Google Scholar]
- Ye J, Ma C, Hsueh EC, Eickhoff CS, Zhang Y, Varvares MA et al. Tumor-derived gammadelta regulatory T cells suppress innate and adaptive immunity through the induction of immunosenescence. J Immunol 2013; 190: 2403–2414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catellani S, Poggi A, Bruzzone A, Dadati P, Ravetti JL, Gobbi M et al. Expansion of Vdelta1 T lymphocytes producing IL-4 in low-grade non-Hodgkin lymphomas expressing UL-16-binding proteins. Blood 2007; 109: 2078–2085. [DOI] [PubMed] [Google Scholar]
- Kang N, Zhou J, Zhang T, Wang L, Lu F, Cui Y et al. Adoptive immunotherapy of lung cancer with immobilized anti-TCRgammadelta antibody-expanded human gammadelta T-cells in peripheral blood. Cancer Biol Ther 2009; 8: 1540–1549. [DOI] [PubMed] [Google Scholar]
- Li X, Kang N, Zhang X, Dong X, Wei W, Cui L et al. Generation of human regulatory gammadelta T cells by TCRgammadelta stimulation in the presence of TGF-beta and their involvement in the pathogenesis of systemic lupus erythematosus. J Immunol 2011; 186: 6693–6700. [DOI] [PubMed] [Google Scholar]
- Kabelitz D, Wesch D, Pitters E, Zoller M. Characterization of tumor reactivity of human V gamma 9V delta 2 gamma delta T cells in vitro and in SCID mice in vivo. J Immunol 2004; 173: 6767–6776. [DOI] [PubMed] [Google Scholar]
- Kong Y, Cao W, Xi X, Ma C, Cui L, He W. The NKG2D ligand ULBP4 binds to TCRgamma9/delta2 and induces cytotoxicity to tumor cells through both TCRgammadelta and NKG2D. Blood 2009; 114: 310–317. [DOI] [PubMed] [Google Scholar]
- Dai Y, Chen H, Mo C, Cui L, He W. Ectopically-expressed human tumor biomarker MutS homologue 2 is a novel endogenous ligand that is recognized by human gammadelta T cells to induce innate anti-tumor/virus immunity. J Biol Chem 2012; 287: 16812–16819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanca T, Correia DV, Moita CF, Raquel H, Neves-Costa A, Ferreira C et al. The MHC class Ib protein ULBP1 is a nonredundant determinant of leukemia/lymphoma susceptibility to gammadelta T-cell cytotoxicity. Blood 2010; 115: 2407–2411. [DOI] [PubMed] [Google Scholar]
- Rincon-Orozco B, Kunzmann V, Wrobel P, Kabelitz D, Steinle A, Herrmann T. Activation of V gamma 9V delta 2 T cells by NKG2D. J Immunol 2005; 175: 2144–2151. [DOI] [PubMed] [Google Scholar]
- Girardi M, Oppenheim DE, Steele CR, Lewis JM, Glusac E, Filler R et al. Regulation of cutaneous malignancy by gammadelta T cells. Science 2001; 294: 605–609. [DOI] [PubMed] [Google Scholar]
- Das H, Groh V, Kuijl C, Sugita M, Morita CT, Spies T et al. MICA engagement by human Vgamma2Vdelta2 T cells enhances their antigen-dependent effector function. Immunity 2001; 15: 83–93. [DOI] [PubMed] [Google Scholar]
- Lu J, Aggarwal R, Kanji S, Das M, Joseph M, Pompili V et al. Human ovarian tumor cells escape gammadelta T cell recognition partly by down regulating surface expression of MICA and limiting cell cycle related molecules. PLoS One 2011; 6: e23348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YJ, Han MK, Broxmeyer HE. 4-1BB regulates NKG2D costimulation in human cord blood CD8+ T cells. Blood 2008; 111: 1378–1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady J, Carotta S, Thong RP, Chan CJ, Hayakawa Y, Smyth MJ et al. The interactions of multiple cytokines control NK cell maturation. J Immunol 2010; 185: 6679–6688. [DOI] [PubMed] [Google Scholar]
- Ventre E, Brinza L, Schicklin S, Mafille J, Coupet CA, Marcais A et al. Negative regulation of NKG2D expression by IL-4 in memory CD8 T cells. J Immunol 2012; 189: 3480–3489. [DOI] [PubMed] [Google Scholar]
- He W, Hao J, Dong S, Gao Y, Tao J, Chi H et al. Naturally activated V gamma 4 gamma delta T cells play a protective role in tumor immunity through expression of eomesodermin. J Immunol 2010; 185: 126–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dokouhaki P, Han M, Joe B, Li M, Johnston MR, Tsao MS et al. Adoptive immunotherapy of cancer using ex vivo expanded human gammadelta T cells: a new approach. Cancer Lett 2010; 297: 126–136. [DOI] [PubMed] [Google Scholar]
- Siegers GM, Dhamko H, Wang XH, Mathieson AM, Kosaka Y, Felizardo TC et al. Human Vdelta1 gammadelta T cells expanded from peripheral blood exhibit specific cytotoxicity against B-cell chronic lymphocytic leukemia-derived cells. Cytotherapy 2011; 13: 753–764. [DOI] [PubMed] [Google Scholar]
- Siegers GM, Ribot EJ, Keating A, Foster PJ. Extensive expansion of primary human gamma delta T cells generates cytotoxic effector memory cells that can be labeled with Feraheme for cellular MRI. Cancer Immunol Immunother 2013; 62: 571–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilbach K, Frommer K, Meier S, Handgretinger R, Eyrich M. Immune response of human propagated gammadelta-T-cells to neuroblastoma recommend the Vdelta1+ subset for gammadelta-T-cell-based immunotherapy. J Immunother 2008; 31: 896–905. [DOI] [PubMed] [Google Scholar]
- Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell 2010; 140: 883–899. [DOI] [PMC free article] [PubMed] [Google Scholar]