Abstract
Increasing numbers of clinical trials and animal experiments have shown that probiotic bacteria are promising tools for allergy prevention. Here, we analyzed the immunomodulatory properties of three selected lactobacillus strains and the impact of their mixture on allergic sensitization to Bet v 1 using a gnotobiotic mouse model. We showed that Lactobacillus (L.) rhamnosus LOCK0900, L. rhamnosus LOCK0908 and L. casei LOCK0919 are recognized via Toll-like receptor 2 (TLR2) and nucleotide-binding oligomerization domain-containing protein 2 (NOD2) receptors and stimulate bone marrow-derived dendritic cells to produce cytokines in species- and strain-dependent manners. Colonization of germ-free (GF) mice with a mixture of all three strains (Lmix) improved the intestinal barrier by strengthening the apical junctional complexes of enterocytes and restoring the structures of microfilaments extending into the terminal web. Mice colonized with Lmix and sensitized to the Bet v 1 allergen showed significantly lower levels of allergen-specific IgE, IgG1 and IgG2a and an elevated total IgA level in the sera and intestinal lavages as well as an increased transforming growth factor (TGF)-β level compared with the sensitized GF mice. Splenocytes and mesenteric lymph node cells from the Lmix-colonized mice showed the significant upregulation of TGF-β after in vitro stimulation with Bet v 1. Our results show that Lmix colonization improved the gut epithelial barrier and reduced allergic sensitization to Bet v 1. Furthermore, these findings were accompanied by the increased production of circulating and secretory IgA and the regulatory cytokine TGF-β. Thus, this mixture of three lactobacillus strains shows potential for use in the prevention of increased gut permeability and the onset of allergies in humans.
Keywords: allergic sensitization, germ-free, intestinal barrier, Lactobacillus, probiotics
Introduction
Humans, like all vertebrates, are essentially born germ-free (GF). This GF status changes rapidly during and after delivery, and subsequent interactions between the host and colonizing microbiota plays crucial roles in the development and function of the immune system as well as the maintenance of intestinal homeostasis.1,2 Perturbations in colonizing microbiota lead to the breakdown of the equilibrium between commensal and pathogenic microbes. This dysbiosis has been linked to the increased permeability of the epithelium3,4 and the development of chronic inflammatory diseases, such as allergies and inflammatory bowel disease.5,6,7
Allergies have become a serious health burden in developed countries. In accordance with the general hypothesis of Strachan8 that the rapid increase in allergic diseases in humans is dependent on microbial deprivation early in life, reduced bacterial diversity and lower counts of lactobacilli and bifidobacteria have been detected in the gut of allergic children.9,10 This finding has been the rationale for the administration of probiotic bacteria for the prevention and/or treatment of allergies.11,12,13
Probiotic lactobacilli and bifidobacteria are non-invasive and non-pathogenic Gram-positive bacteria possessing immunomodulatory properties that are strictly strain-dependent.14 They have been documented to compete with pathogens and toxins for adherence to the intestinal epithelium and to promote intestinal epithelial cell survival, enhance barrier function and directly interact with cells of the immune system, such as dendritic cells (DCs).15 Through the engagement of innate receptors, such as Toll-like receptors (TLRs), nucleotide-binding oligomerization domains (NODs) or C-type lectin receptors, probiotic lactobacilli and bifidobacteria induce distinct innate responses and cytokine profiles that subsequently shape T-helper cell responses.16,17,18 There is accumulating evidence that certain strains possess intrinsic Th1-type immunomodulatory properties,18,19 while others are able to induce regulatory responses.17,20,21
Transforming growth factor (TGF)-β is present at high concentrations in the intestine and has a crucial involvement in modulating the immune response.22 It has been shown to inhibit the proliferation and differentiation of both B and T cells,23 and altered TGF-β signaling has been linked to the development of allergic disease.24 Furthermore, TGF-β is an initial trigger for the production of mucosal IgA, which has a role in regulating mucosal integrity.25 Along these lines, we have previously shown that Lactobacillus paracasei stimulates the production of the regulatory cytokine TGF-β from bone marrow-derived DCs in a TLR2/4-dependent manner.21
Among the inhalant allergens, the pollen of the white birch (Betula verrucosa) is one of the most important sources responsible for eliciting allergic symptoms.26 In an experimental model, we have shown that the oral application of L. paracasei to pregnant mothers prevents the development of allergies in their offspring in a mouse model of birch pollen allergy.21 Similarly, intranasal application of probiotic bacteria reduces allergic poly-sensitization in adult mice.27 Although the majority of studies use single strains, supplementation with probiotic mixtures might have a greater efficacy.28
Germ-free animals represent a unique tool to study the interactions of hosts with specific probiotic strains or with defined probiotic mixtures and to investigate their impacts on the development of the immune system.6,29 Using a mouse model of allergic sensitization to the major birch pollen allergen Bet v 1, we have previously shown that neonatal colonization of GF mice with Bifidobacterium longum is able to prevent allergic sensitization,20 but the underlying mechanism of the host–bacteria interaction in gnotobiotic models is still far from being elucidated.
Recently, we have selected three lactobacillus strains, L. rhamnosus LOCK0900, L. rhamnosus LOCK0908 and L. casei LOCK0919, out of 24 strains isolated from stool samples obtained from healthy infants.30 These selected strains showed properties required for probiotic bacteria, e.g., resistance to gastric acids and bile salts and inhibitory activities against bacterial pathogens.30 Moreover, the mixture of these strains (Lmix) showed synergistic effects in the induction of anti-allergic Th1-type cytokines and regulatory cytokine TGF-β in human whole blood cell cultures compared with the levels induced by each single strain alone.31 Our pilot study showed that the supplementation of children presenting the first symptoms of allergy (atopic dermatitis) with the Lmix reduced serum levels of IgE and IL-5 and diminished the severity of the disease (Cukrowska, unpublished data).
Based on these observed effects, the aims of this study were to further characterize the immunomodulatory properties of the individual lactobacillus strains L. rhamnosus LOCK0900, L. rhamnosus LOCK0908 and L. casei LOCK0919, as well as their mixture Lmix, in vitro and to investigate the effects of Lmix on the development of allergic sensitization to the allergen Bet v 1 in a gnotobiotic mouse model.
Materials and methods
Bacterial strains
L. rhamnosus LOCK0900,32 L. rhamnosus LOCK090833 and L. casei LOCK091934 were obtained from the Pure Culture Collection of the Technical University of Lodz, Poland (LOCK). Overnight cultures in MRS broth (Oxoid, Basingstoke, UK) were centrifuged and washed in sterile phosphate-buffered saline (PBS), and their concentrations were adjusted to 109 CFU/ml. For the in vitro experiments, single bacterial strains were inactivated with 1% formaldehyde-PBS for 3 h at room temperature, washed twice with sterile saline (PBS) and stored at −40 °C.
Stimulation of HEK293 cells stably transfected with TLR2, NOD2 and TLR4
The human embryonic kidney cell line HEK293 stably transfected with a plasmid carrying the human (h)TLR2/CD14 gene was kindly provided by M. Yazdanbakhsh (Leiden, The Netherlands), cells transfected with hTLR4/MD2/CD14 were a gift from B. Bohle (Vienna, Austria), and cells transfected with hNOD2 were purchased from InvivoGen (InvivoGen, Toulouse, France). The cells were stimulated with the formalin-inactivated L. rhamnosus LOCK0900, L. rhamnosus LOCK0908, L. casei LOCK0919 or their equal-part mixture (Lmix) at a concentration of 107 CFU/ml. TLR2 ligand Pam3CSK4 (PAM3; 1 µg/ml; InvivoGen), NOD2 ligand muramyl dipeptide (100 ng/ml; InvivoGen) and TLR4 ligand ultrapure LPS-EB (LPS; 1 µg/ml, InvivoGen, Toulouse, France) were used as positive controls. After a 20-h incubation period, culture supernatants were harvested, and human IL-8 concentrations were analyzed by Enzyme-Linked Immunosorbent Assay (Thermo Fisher Scientific, Waltham, MA, USA) according to the manufacturer's instructions.
Preparation and activation of bone marrow-derived DCs (BM-DCs)
Mouse BM-DCs were prepared as previously described.21 Briefly, bone marrow precursors were isolated from the femurs and tibias of conventional (CV) BALB/c mice. Cells were cultured at 4×105/ml in bacteriological Petri dishes in 10 ml of culture medium with GM-CSF (20 ng/ml; Sigma-Aldrich, Saint-Louis, MO, USA). Fresh medium was added on days 3 and 6, and the BM-DCs were used on day 8 of culture. The BM-DCs (106 cells/ml) were stimulated with 107 CFU/ml of inactivated L. rhamnosus LOCK0900, L. rhamnosus LOCK0908, L. casei LOCK0919 or their equal-part mixture (Lmix) for 18 h. BM-DCs incubated with Pam3CSK4 (PAM3; 1 µg/ml) or ultrapure LPS-EB (LPS, 1 µg/ml) were used as controls. Levels of IL-10, TGF-β and TNF-α in the culture supernatants were determined by ELISA Ready-Set-Go! Kits (eBioscience, San Diego, CA, USA) according to manufacturer's instructions. IL-12p70 levels were measured with matched antibody pairs (BD Biosciences, San Jose, CA, USA).
Animals
GF inbred BALB/c mice were born and housed under sterile conditions and fed a sterile standard pellet diet (ST1; Bergman, Kocanda, Czech Republic; 59 kGy irradiated for 30 min) and were provided sterile water ad libitum. The animals were kept in a room with a 12 h light–dark cycle at 22 °C. Fecal samples were evaluated weekly for the presence of aerobic and anerobic bacteria, molds and yeast by standard microbiological methodologies. CV BALB/c mice (n=5) were fed the same sterile diet as their GF counterparts. The animal experiments were approved by the Committee for the Protection and Use of Experimental Animals of the Institute of Microbiology v.v.i., Academy of Sciences of the Czech Republic (approval ID: 50/2013).
Experimental design
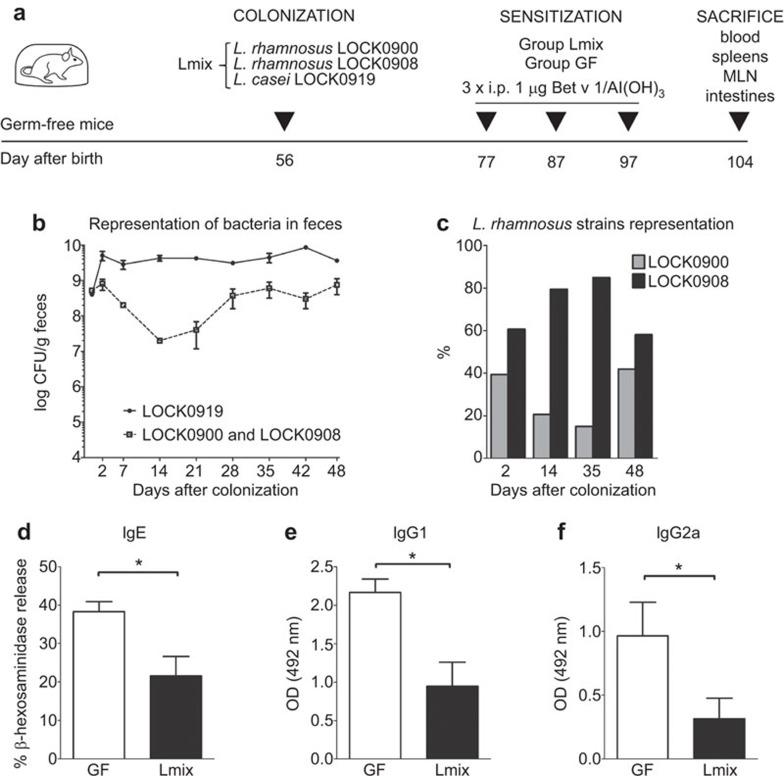
Eight-week-old GF mice (n=12) were divided into two groups. The mice were colonized by intragastric tubing with 2×108 CFU of equal parts of overnight cultures of L. rhamnosus LOCK0900, L. rhamnosus LOCK0908 and L. casei LOCK0919 in 0.2 ml of sterile PBS (group 1). The second group served as a GF control. Three weeks after colonization, the Lactobacillus-colonized mice and GF controls were intraperitoneally immunized three times with 1 µg of the recombinant birch pollen allergen Bet v 1 (Biomay, Vienna, Austria) adsorbed to 2 mg aluminium hydroxide (Serva, Heidelberg, Germany) at 10-day intervals, as previously described.35 The mice were euthanized at seven days after the last immunization by cervical dislocation (Figure 4a). Blood was collected, and serum samples were stored at −40 °C until analysis. Terminal ileum samples were removed for immunohistochemistry, Western blot and electron microscopy analysis, and the rest of the small intestine was excised for the determination of total IgA, with lavages performed as previously described.36 Mesenteric lymph nodes (MLNs, pooled per group) and the spleen were aseptically removed and prepared for in vitro cytokine assays. Briefly, after gentle crushing, straining through a 70-µm cell strainer (BD Biosciences, San Jose, CA, USA) and the lysis of red blood cells (180 mM NH4Cl and 17 mM Na2EDTA, pH 7.3; Sigma-Aldrich, Saint-Louis, MO, USA), mononuclear cells were resuspended in complete RPMI 1640 medium (Sigma-Aldrich, Saint-Louis, MO, USA) containing 10% fetal calf serum, 2 mM glutamine, 100 U penicillin and 100 µg/ml streptomycin.
Figure 4.
Sensitization of GF and Lmix-colonized mice with the major birch pollen allergen Bet v 1. (a) The experimental design was as follows: 8-week-old GF mice (n=12) were divided into two groups. The first group (Lmix) received an equal-part mixture (2×108 CFU/ml) of L. rhamnosus LOCK0900, L. rhamnosus LOCK0908 and L. casei LOCK0919 by intragastric tubing. The second group was kept GF. Mice were sensitized three times intraperitoneally with recombinant Bet v 1 (1 µg in alum) on days 77, 87 and 97. One week after the last immunization (day 104), tissue samples were collected for further analyses. Bacterial colonization of the Lmix-colonized mice was evaluated on the first 2 days and then at weekly intervals throughout the experiment. At the species level, bacteria were distinguished based on colony morphology by the cultivation of appropriate serial dilutions of the fecal samples. (b) L. casei LOCK0919 (full circles, solid line), L. rhamnosus LOCK0900 and LOCK0908 (open squares, dotted line). L. rhamnosus strain-specific discrimination was performed by qPCR using DNA isolated from the fecal samples at the indicated time points (c) L. rhamnosus LOCK0900 (gray bars) and L. rhamnosus LOCK0908 (black bars). The data are shown as a percentage of each strain out of all detected L. rhamnosus bacteria on the indicated day after colonization. Bet v 1-specific antibodies were measured in the sera of GF (white bars) and Lmix-colonized mice (black bars). IgE was measured by Bet v 1-mediated β-hexosaminidase release from rat basophil leukemia cells (d). Levels of IgG1 (e) and IgG2a (f) were evaluated by ELISA and expressed as OD units. The data are shown as the mean±s.e.m. One representative out of two experiments is shown; n=6/group. *P<0.05 and **P<0.01. GF, germ-free; Ig, immunoglobulin; i.p., intraperitoneally; Lmix, Lactobacillus mixture; OD, optical density.
Bacterial colonization
The bacterial colonization of the mice was evaluated on the first 2 days and then at weekly intervals throughout the experiment. The fecal samples were pooled for each group, diluted (1∶9, w/v) in sterile PBS and exhaustively vortexed with sterile glass beads. Volumes of 1 ml at the appropriate 10-fold dilution were plated onto MRS agar (Oxoid, Basingstoke, UK) and cultivated in triplicate at 37 °C for 48 h. At the species level, bacteria were distinguished on the basis of colony morphology. The strain L. casei LOCK0919 formed small, white, non-mucosal colonies, whereas the strains L. rhamnosus LOCK0900 and LOCK0908 formed larger white-gray-colored mucosal colonies. To distinguish between L. rhamnosus strains, we isolated DNA from the feces of colonized mice and performed strain-specific qPCR (Supplementary Information).
Immunohistochemical detection of IgA-producing cells
Segments of the terminal ileum were embedded in Tissue-Tek (Sakura Finetec Europe B.V., Netherlands) and frozen in liquid nitrogen. Cryosections (5 μm thick) of acetone-fixed colon were used for immunocytochemistry. Immunostaining was performed with a goat anti-mouse IgA-FITC antibody (Thermo Fisher Scientific, Waltham, MA, USA). Samples were viewed under an Olympus BX 40 microscope equipped with an Olympus DP 70 digital camera. Photographs were taken with a Camedia Master 2.5 and DP-Soft (Olympus Corporation, Tokyo, Japan).
Transmission electron microscopy
The ileum tissues were cut into small pieces (1×1 mm) and immediately fixed in 2.5% glutaraldehyde in PBS for 90 min. After fixation in 1% osmium tetroxide (Sigma-Aldrich, Saint-Louis, MO, USA) for 1 h and washing in 0.1 M cacodylate buffer, the samples were successively dehydrated in 35%, 70%, 96% and 100% ethanol and propylene oxide (EMS, Hatfield, PA, USA). Subsequently, they were embedded in Epon resin (EMS, Hatfield, PA, USA). Selected semi-thin sections of ileum were cut into 65 nm ultra-thin sections by Leica Ultracut Uct52 (Leica Microsystems, Wetzlar, Germany), stained with uranyl acetate and lead citrate, and examined by electron microscopy (Jem 1011; Jeol, Peabody, MA, USA). Images of the ultrastructural features of the ileal structures and junctions were visualized at magnifications ranging from ×3000 to ×100 000. Specimens were obtained from five mice from each group. The widths and lengths of the intracellular junctions were measured using the morphometric iTEM program (Olympus Corporation, Tokyo, Japan) at a magnification of ×100 000. For each specimen, 10–15 measurements were performed, and the results are presented in nm.
Western blot analysis of ZO-1 and occludin
The terminal ileum was homogenized on ice in protein extract buffer with a protease inhibitor cocktail (Thermo Fisher Scientific, Waltham, MA, USA) for 10 min and sonicated. Samples were centrifuged at 10,000 rpm for 10 min at 4 °C and stored at −80 °C until use. Protein concentrations were measured using a BCA Protein Assay Kit (Thermo Fisher Scientific, Waltham, MA, USA). Western blotting was performed as previously described.37 The membranes were blocked with 2% (w/v) dry milk in 0.05% PBS-Tween-20 for 1 h at room temperature and incubated overnight at 4 °C with antibodies against occludin (1∶1000) ZO-1 (1∶1000) (Thermo Fisher Scientific, Waltham, MA, USA) and β-actin (1∶5000) (Abcam, Cambridge, UK). After incubation with the respective primary antibodies, secondary staining was conducted using horseradish peroxidase-conjugated species-specific antibodies (1∶1000) (Thermo Fisher Scientific, Waltham, MA, USA) for 1 h at room temperature. The reactions were developed using a SuperSignal West Femto Maximum Sensitivity Substrate (Thermo Fisher Scientific, Waltham, MA, USA), and the signal intensities were measured with a G∶BOX (Syngene, Cambridge, UK) and processed with the ImageJ program.38
Allergen-specific antibody responses: ELISA and basophil release assay
Allergen-specific serum IgG1, IgG2a and IgA levels were determined by ELISA as previously described.39 Briefly, 96-well microtiter plates were coated with Bet v 1 (2 µg/ml). Serum samples were diluted 1∶10000 for IgG1, 1∶100 for IgG2a and 1∶10 for IgA. Rat anti-mouse IgG1, IgG2a and IgA antibodies (1 µg/ml; BD Biosciences, San Jose, CA, USA) were applied, followed by peroxidase-conjugated mouse anti-rat IgG antibodies (1∶1000; Jackson ImmunoResearch Laboratories, West Grove, PA, USA) for detection. Antibody levels were reported as optical densities. Allergen-specific IgE levels in the sera were quantified by the degranulation of rat basophil leukemia (RBL-2H3) cells as previously described.40 RBL-2H3 cells were plated in 96-well tissue culture plates (4×104 cells per well) and passively sensitized by incubation with mouse sera at a final dilution of 1∶30 for 2 h. After washing, Bet v 1 (0.3 µg/ml) was added for 30 min at 37 °C to induce degranulation. Supernatants were incubated with 4-methylumbelliferyl-N-acetyl-β-d-glucosaminide (Sigma-Aldrich, Saint-Louis, MO, USA) for analysis of β-hexosaminidase using an Infinite M200 (Tecan Group, Männedorf, Switzerland) fluorescence microplate reader (λex: 360 nm/λem: 465 nm). The results are reported as the percentage of total β-hexosaminidase release from the cells after disruption with 1% Triton X-100.
Total IgA and IgE responses
Total IgA and IgE were measured in the sera and gut lavages (IgA only) with a mouse IgA and IgE ELISA quantification kit (Bethyl, Montgomery, TX, USA) according to manufacturer's instructions. The serum samples were diluted 1∶400 for the IgA and 1∶10 for the IgE measurements, and for IgA determination in the gut lavages, a 1∶2500 dilution was used. Antibody levels are reported as µg/ml for the sera and as µg/g for the gut lavages.
Cytokine production
Spleen cells and pooled MLN cell suspensions were cultured in 48-well flat-bottom plates at a concentration of 5×106 cells in 500 µl of complete RPMI 1640 medium. Cells were cultivated with/without Bet v 1 (10 µg/well) restimulation at 37 °C under 5% CO2 for 48 h. After cultivation, supernatants were collected and stored at −40 °C until analysis. IL-4, IL-5, IL-10 and interferon (IFN)-γ levels were determined by a Mouse Cytokine/Chemokine Multiplex Immunoassay (Millipore, Billerica, Ma, USA) according to the manufacturer's instructions and analyzed with a Luminex 200 System (Bio-Rad Laboratories, Hercules, CA, USA) at sensitivities of <0.3 pg/ml for IL-4, <0.3 pg/ml for IL-5, <10.3 pg/ml for IL-10 and <0.7 pg/ml for IFN-γ. TGF-β was measured in the culture supernatants and in 1∶10 diluted serum samples with an ELISA kit (R&D Duoset Systems, Minneapolis, MN, USA) according to the manufacturer's instructions, with a detection limit of <4 pg/ml.
Statistical analyses
The non-parametric Mann–Whitney test was used for comparisons between two groups, and for comparisons between multiple groups, ANOVA with Tukey's multiple comparison test was performed with the GraphPad Prism 5.02 software. Values of P<0.05 were considered significantly different. All data are expressed as the mean±standard error of the mean (s.e.m.) unless stated otherwise.
Results
TLR2 and NOD2 but not TLR4 are involved in the recognition of all three investigated Lactobacillus strains
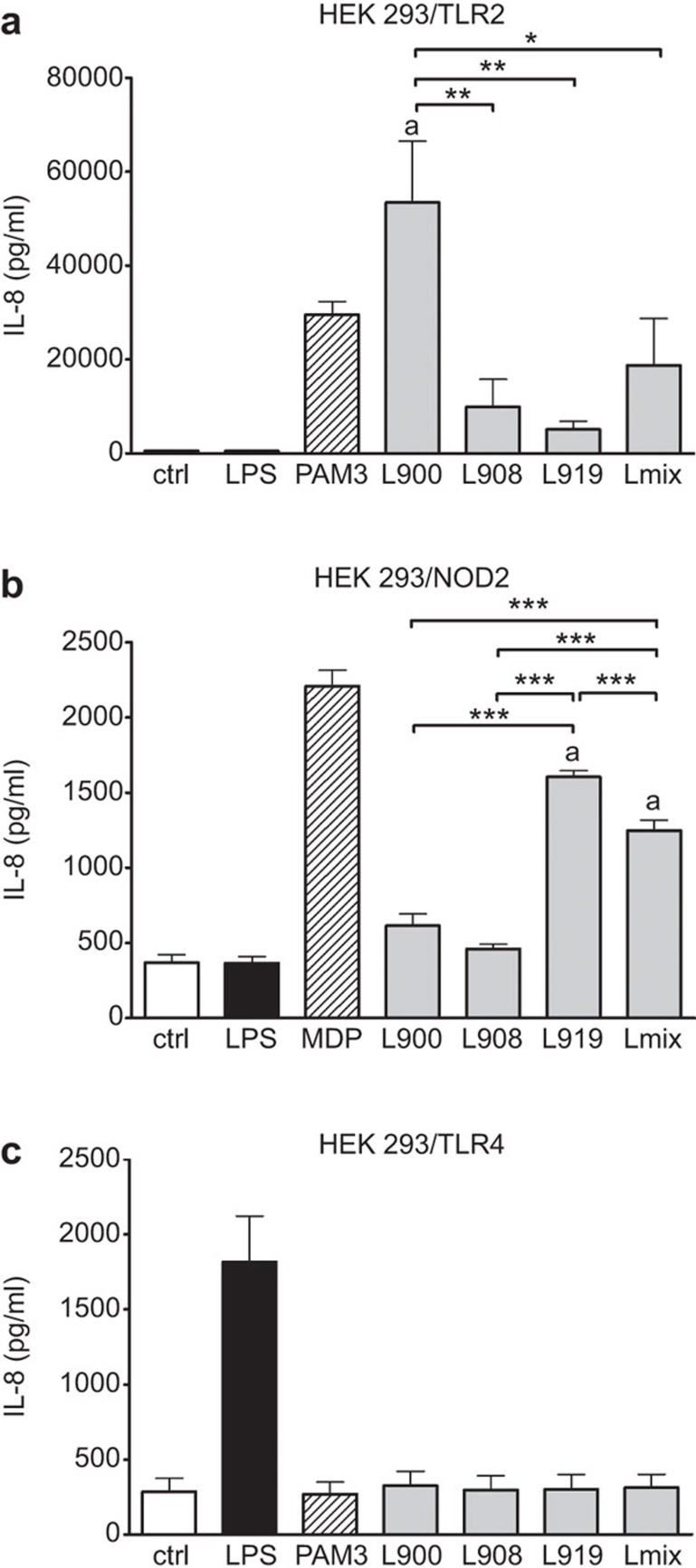
To specify pattern recognition receptors involved in Lactobacillus signaling pathways, the single strains L. rhamnosus LOCK0900, L. rhamnosus LOCK0908, L. casei LOCK0919 or their equal-part mixture (Lmix) were incubated with HEK293 cells transfected either with TLR2, TLR4 or NOD2. The cytokine IL-8 level was measured as an indicator of cell stimulation via a specific receptor, and it was found to be significantly increased in the supernatants of the HEK293/TLR2 cells incubated with L. rhamnosus LOCK0900 and in the HEK/NOD2 cells exposed to L. casei LOCK0919 and Lmix (Figure 1a and b). There was no IL-8 stimulation detected in the HEK293/TLR4 cells incubated with any single lactobacillus strain or Lmix (Figure 1c).
Figure 1.
Stimulation of HEK293 TLR2-, NOD2- and TLR4-transfected cells with Lactobacillus strains. Human embryonic kidney cells (HEK293) stably transfected with an expression vector for human TLR2 (293-hTLR2) (a), NOD2 (pUNO-hNOD2) (b) and TLR4 (293-hTLR4/MD2/CD14) (c) were cultured for 20 h with 107 CFU/ml of formalin-inactivated L. rhamnosus LOCK0900 (L900), L. rhamnosus LOCK0908 (L908), L. casei LOCK0919 (L919) or an equal-part mixture of these strains (Lmix). PAM3 (1 µg/ml), MDP (10 µg/ml) and ultrapure lipopolysaccharide from E. coli (LPS; 1 µg/ml) were used as positive controls for TLR2, NOD2 and TLR4, respectively. Unstimulated cells (ctrl) were used as negative controls. Stimulation was evaluated by the measurement of IL-8 production. The results are expressed as the mean±s.e.m. Pooled values of at least three experiments are shown. PAM3, MDP and LPS served as positive or negative stimulated controls and were not included in statistical analysis. aSignificantly different from unstimulated control; *P<0.05, **P<0.01 and ***P<0.001. Ctrl, unstimulated cells; HEK293, human embryonic kidney cell line 293; MDP, muramyl dipeptide; NOD2, nucleotide-binding oligomerization domain-containing protein 2; PAM3, Pam3CSK4; TLR, Toll-like receptor.
Strain-specific profile of cytokines produced by stimulated BM-DCs
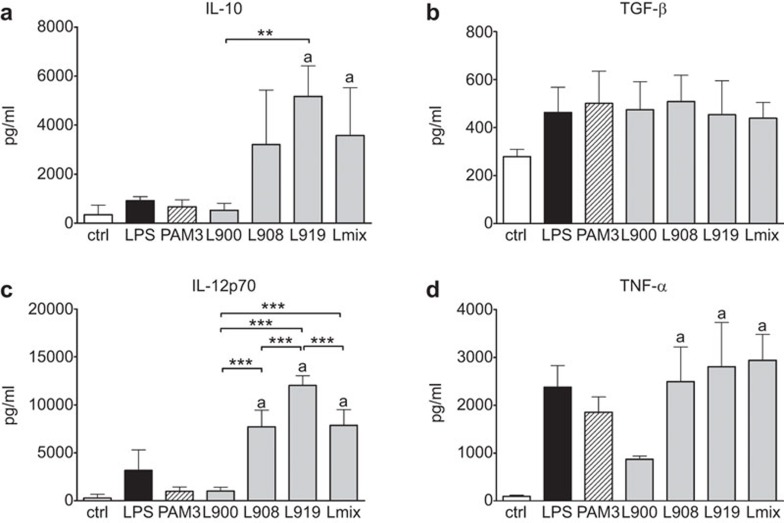
Activation of BM-DCs with the single strains L. rhamnosus LOCK0900, L. rhamnosus LOCK0908 and L. casei LOCK0919 or Lmix showed a trend toward the increased induction of the regulatory cytokine TGF-β independent of the applied bacterial strain. However, the production of IL-10, IL-12p70 and TNF-α was strictly species- and strain-dependent, and the stimulation of cytokine production by Lmix corresponded with the average of the cytokine concentrations induced by individually applied bacterial strains (Figure 2).
Figure 2.
Stimulation of bone marrow-derived dendritic cells with Lactobacillus strains. BM-DCs were cultured with 107 CFU/ml of formalin-inactivated L. rhamnosus LOCK0900 (L900), L. rhamnosus LOCK0908 (L908), L. casei LOCK0919 (L919) strains or an equal-part mixture of these strains (Lmix) for 18 h. As positive controls, PAM3 (1 µg/ml) or ultrapure lipopolysaccharide from E. coli (LPS; 1 µg/ml) were applied. Ctrl served as negative controls. The levels of IL-10, TGF-β, IL-12p70 and TNF-α in the culture supernatants were determined by ELISA and expressed as the mean±s.e.m. Pooled values of at least three experiments are shown. PAM3 and LPS served as stimulated controls and were not included in statistical analysis. aSignificantly different from unstimulated control; *P<0.05, **P<0.01 and ***P<0.001. BM-DC, bone marrow-derived dendritic cells; ctrl, unstimulated cell; Lmix, Lactobacillus mixture; PAM3, Pam3CSK4; TGF, transforming growth factor; TNF, tumor necrosis factor.
Colonization with Lmix improves the intestinal barrier
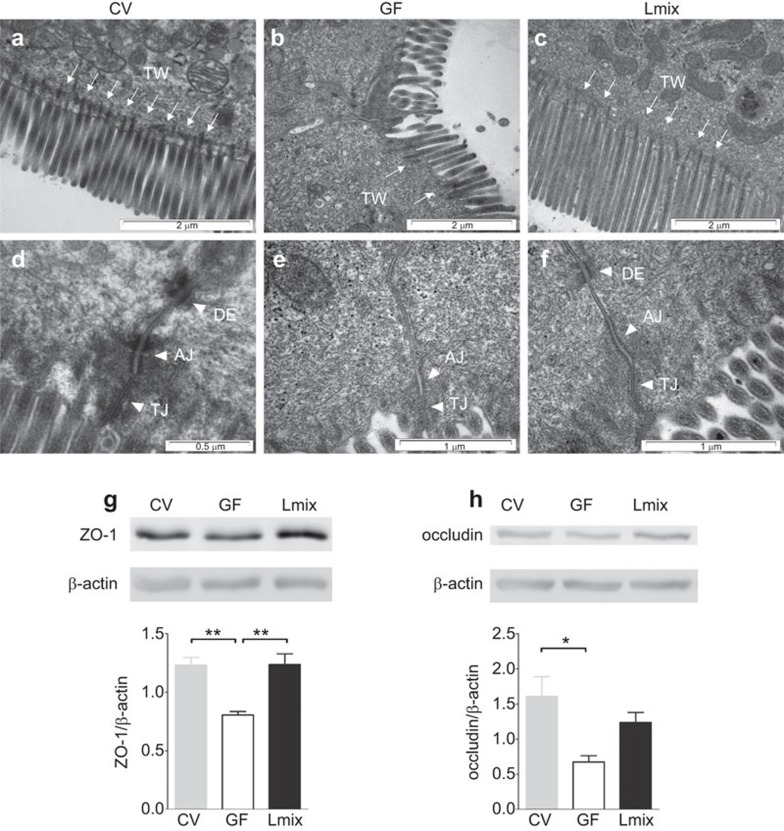
To evaluate the effect of Lmix colonization on the intestinal barrier, ultrastructural analyses of the apical portion of ileal enterocytes were performed. In mice reared under conventional conditions, the brush borders were regular, straight and contained microfilaments extending into the terminal web (TW) (Figure 3a). The apical junctional complex, including the tight junction, adherens junction (AJ) and desmosome, were well organized. In contrast, the enterocyte brush borders of the GF mice were irregularly arranged and exhibited decreased numbers of cytoskeletal microfilaments and a lack of elongation into the TW. As shown in Table 1, the AJ region was significantly broader and shorter in the GF mice compared with the CV and Lmix-colonized mice. Interestingly, incomplete apical junctional complexes lacking desmosomes (DE) were observed in approximately 30% of the enterocytes of the GF mice (Figure 3b). Lmix colonization of the GF mice led to a more organized arrangement of enterocyte microvilli with cytoskeletal microfilaments anchored in the TW, similar to the CV mice (Figure 3c). In these mice, DEs were detected in each apical junctional complex in contrast with the GF mice. Moreover, the AJs in the Lmix-colonized mice were significantly elongated and narrow compared with the GF mice, resembling those found in the CV mice (Table 1). Western blot analysis of the terminal ileum further confirmed the electron microscopic findings. The levels of ZO-1 (Figure 3g) were significantly increased in the CV and Lmix-colonized mice compared with the GF controls. Concomitantly, the occludin level was significantly higher in the CV mice, and there was a trend toward its increase in the Lmix-colonized mice (Figure 3h).
Figure 3.
The effects of Lmix colonization on the architecture of the apical junctional complex of enterocytes and the production of ZO-1 and occludin. Electron microscopy micrographs of the apical surfaces of ileal enterocytes in CV, GF and Lmix-colonized mice (Lmix). The epithelial surface is covered by microvilli. Microfilaments extend from the microvilli into the apical cytoplasm and filamentous TW, which was deficient in the GF animals and was restored in the Lmix-colonized mice (a–c). The epithelial cell junctional complex contains the TJs, AJs and DEs. DEs were absent in 30% of the junctional complexes in the GF mice (d–f). Representative micrographs were obtained from 10–15 measurements per sample; n=5 samples per group. Western blot analysis of ZO-1 (g) and occludin (h) in the ileum. A representative mouse from each group is shown (3–4 mice per group were analyzed). Quantification of the signals was performed using ImageJ. The data are expressed as the mean±s.e.m. of 3–4 mice per group. *P<0.05 and **P<0.01. AJ, adherens junction; DE, desmosome; GF, germ-free; Lmix, Lactobacillus mixture; TJ, tight junction; TW, TW, terminal web; ZO-1, zonulin-1.
Table 1. Effects of bacterial colonization on the width (W) and length (L) of the apical intracellular junction in the ileum of CV, GF and Lmix-colonized mice.
| Tight junctions (nm) | Adherens junctions (nm) | |||
|---|---|---|---|---|
| Group | W | L | W | L |
| CV | 10±1 | 336±40 | 30±2 | 226±50 |
| GF | 10±1 | 203±50* | 40±10* | 181±40* |
| Lmix | 11±3 | 236±80 | 30±7 | 234±70 |
Abbreviations: CV, conventional; GF, germ-free; Lmix, Lactobacillus mixture.
The values are expressed as the mean±s.e.m. (nm) and were obtained from 10–15 measurements per sample, and n=5 samples were assessed per group.
P<0.05, significant difference of the GF group versus the Lmix and CV groups.
Colonization of GF mice with Lmix
The stability of colonization with Lmix was evaluated throughout the experiment. By plating the fecal samples on MRS agar, we were able to distinguish the bacteria at the species level. As shown in Figure 4b, starting from the second day after colonization, the concentration of L. casei reached 3.3–5.0×109 CFU/g of feces, while L. rhamnosus strains were detected at concentrations ranging from 0.2–8.0×108 CFU/g. To distinguish between the two L. rhamnosus strains, we isolated the DNA from the stool samples and showed that the LOCK0908 strain was more abundant compared with the LOCK0900 strain by qPCR (Figure 4c).
Colonization by Lmix suppresses Bet v 1-specific antibody production
To analyze the effect of Lmix colonization on allergic sensitization, our recently published mouse model20,41 was applied, and the production of specific antibodies and cytokines were evaluated. Lmix-colonized and GF mice were immunized intraperitoneally with the recombinant birch pollen allergen Bet v 1 at 10-day intervals starting at 3 weeks after bacterial colonization (Figure 4a). Colonization with Lmix significantly reduced Bet v 1-specific IgE (P<0.03), IgG1 (P<0.03) and IgG2a (P<0.03) serum antibodies compared with the age-matched Bet v 1-sensitized GF controls (Figure 4d–f). No differences were found in Bet v 1-specific IgA antibodies between both groups (GF: 0.187±0.44 OD and Lmix: 0.167±0.027 OD; P=0.857).
Colonization with Lmix reduces systemic IgE and induces systemic and local IgA production
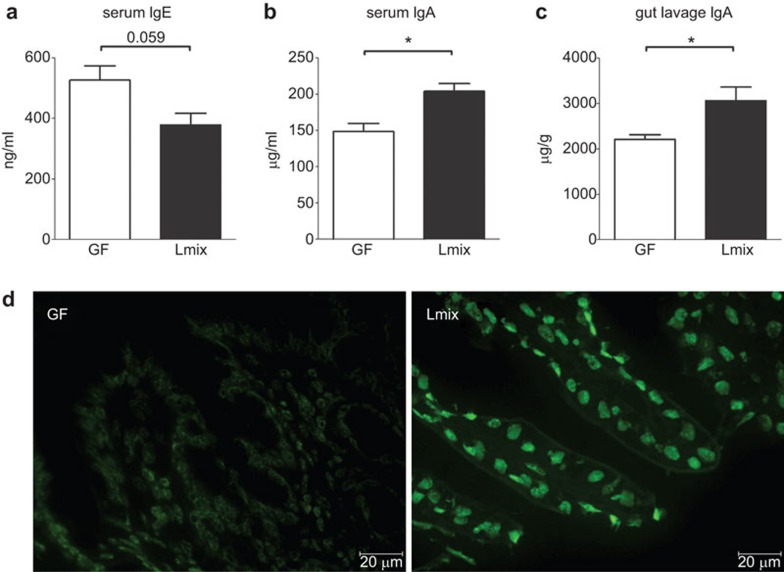
Colonization of the GF mice with Lmix induced a decreasing trend in the level of total IgE in the serum samples (Figure 5a), while the total levels of IgA in the serum samples (P<0.013) and small intestinal lavages were significantly increased (P<0.04) in comparison with the Bet v 1-senzitized GF controls (Figure 5b and c). In the Lmix-colonized group, the induction of activated IgA-secreting plasma cells in the lamina propria of the terminal ileum was confirmed by immunofluorescence staining (Figure 5d). However, no IgA-producing cells were found in the age-matched GF controls (Figure 5d).
Figure 5.
Local and systemic humoral responses in sensitized GF and Lmix-colonized mice. Levels of total IgE (a) and total IgA (b) in the sera and total IgA (c) in the gut lavage were measured by ELISA. Germ-free mice (GF, white bars) and Lmix-colonized mice (Lmix, black bars). The data are shown as the mean±s.e.m. One representative out of two experiments is shown; n=6/group. *P<0.05 and **P<0.01. IgA-positive plasmocytes in the lamina propria of the terminal ileum were visualized with an FITC-labeled anti-IgA antibody (d). GF, germ-free; Ig, immunoglobulin; Lmix, Lactobacillus mixture.
Lmix colonization reduces Bet v 1-specific IL-4 and IL-5 cytokine production
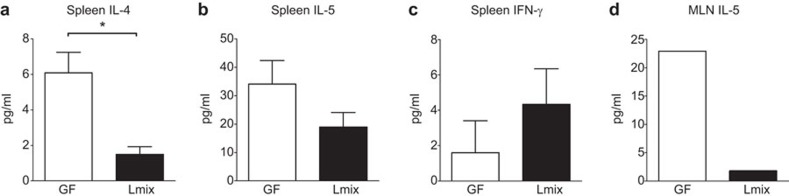
To investigate the impact of Lmix on Th1 and Th2 cytokine production, splenocytes and pooled MLN cells from Bet v 1-sensitized mice were cocultured with Bet v 1 in vitro. We observed the significantly reduced secretion of the Th2 cytokine IL-4, a trend toward a reduction in IL-5 and a slight increase in the level of the Th1-type cytokine IFN-γ in spleen cell supernatants from the Lmix-colonized mice compared with the GF controls (Figure 6a–c). No IL-4 production was detected in the pooled MLN cell cultures, and the levels of both IL-5 (Figure 6d) and IFN-γ (GF: 5.56 pg/ml and Lmix: 1.30 pg/ml) were lower in the supernatants from the Lmix-colonized mice compared with the GF controls.
Figure 6.
The effect of Lmix colonization on cytokine production in vitro. Spleen and pooled MLN cells from Bet v 1-sensitized GF (white bars) and Lmix-colonized mice (black bars) were restimulated with Bet v 1 (10 µg/well) for 48 h. The levels of IL-4 (a), IL-5 (b) and IFN-γ (c) in the spleen cell cultures and IL-5 (d) in the pooled MLN cells were determined by ELISA. The results are the values obtained following the subtraction of the cytokine levels measured in the supernatants of the non-stimulated cell cultures. One representative out of two experiments is shown; n=6/group. *P<0.05. GF, germ-free; MLN, mesenteric lymph node.
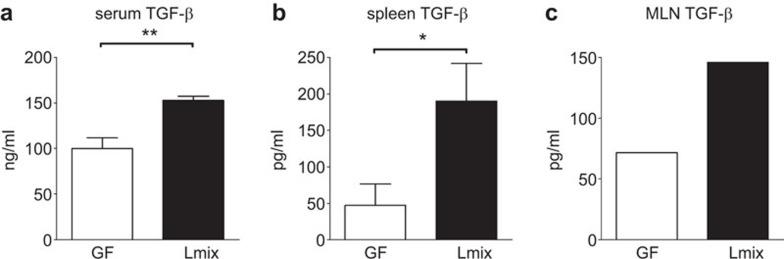
Colonization with Lmix stimulates TGF-β production
To evaluate the effects of Lmix colonization on the regulatory cytokine response, the level of TGF-β was determined in serum samples and supernatants from spleen or MLN cells co-cultured with Bet v 1 in vitro. A significant upregulation of TGF-β in the sera was detected in the mice colonized with Lmix compared with the GF controls (P<0.009) (Figure 7a). We observed a significant increase in the TGF-β level in supernatants of the Bet v 1-stimulated splenocyte cultures of the Lmix-colonized mice compared with the GF controls (Figure 7b). A similar tendency was detected in supernatants of the MLN cells isolated from the Lmix-colonized mice (Figure 7c). There was no difference between the Lmix-colonized and GF control groups in IL-10 production in any of the cell culture supernatants (data not shown).
Figure 7.
The effect of Lmix colonization on systemic and local TGF-β production. The level of TGF-β in Bet v 1-sensitized GF (white bars) and Lmix-colonized mice (black bars) in the sera (a) and supernatants from Bet v 1-restimulated spleen cell (b) or pooled MLN cell (c) cultures was determined by ELISA. The results are expressed after the subtraction of the cytokines measured in the supernatants of the non-stimulated cell cultures. One representative out of two experiments is shown; n=6/group. **P<0.01 and *P<0.05. GF, germ-free; MLN, mesenteric lymph node; TGF, transforming growth factor.
Discussion
In the present study, we aimed to investigate the ability of Lmix, a mixture of the three lactobacillus strains L. rhamnosus LOCK0900, L. rhamnosus LOCK0908 and L. casei LOCK0919, to modulate allergic sensitization in a gnotobiotic mouse model. We showed that colonization with Lmix ameliorated Bet v 1-specific allergic responses at both the humoral and cellular levels. Furthermore, Lmix colonization improved the barrier structure of the gut, which was immature in the GF mice.
The modulation of immune responses by single bacterial strains or by mixtures of different probiotic strains has been documented in mouse models as well as in human trials.42,43 This modulation occurs either by the promotion of Th1-type responses44 or by the induction of regulatory cells and cytokines.20,45 Using a gnotobiotic mouse model, we showed that colonization with Lmix reduced the serum levels of the Th2-related Bet v 1-specific IgE and IgG1 antibodies as well as the Th1-related IgG2a antibody, implicating the involvement of regulatory mechanisms. These findings were further supported by the significantly higher serum level of TGF-β. After the in vitro restimulation of splenocytes or MLN cells with Bet v 1, we observed an alteration in Th2/T regulatory (Treg) cytokine production. We detected the downregulation of the Th2-associated cytokines IL-4 and IL-5 and the upregulation of TGF-β production in the Lmix-colonized group, suggesting that Lmix colonization induced immunoregulatory mechanisms. Previously, Feleszko et al.45 have demonstrated that the oral delivery of probiotic bacteria leads to the suppression of allergic sensitization and airway inflammation by TGF-β-producing Treg cells, which can be found in MLNs. It has also been shown that the peripheral conversion of CD4+ T cells to Treg cells occurs primarily in gut-associated lymphoid tissue in the presence of TGF-β and retinoic acid.46 In accordance with these findings, we suggest that colonization with a lactobacillus mixture induces the upregulation of TGF-β production in the intestine and the generation of Treg cells.
In correlation with the increased production of TGF-β, we found a significant increase in the gut and serum IgA levels in the Lmix-colonized mice. Secretory IgA has been shown to play a crucial role in maintaining bacterial homeostasis in the gut (reviewed in Ref. 47). These results are in accordance with previous findings that the colonization of GF mice with probiotic bacteria induces the activation of IgA production and that a mixture of probiotic strains is more effective in the development of plasmablasts in the gut compared with single strains.48
The intestinal barrier is immature in GF mice,49 and Lmix significantly improves this condition. We found that the enterocyte brush borders of the GF mice were irregularly arranged and exhibited a decreased number of cytoskeletal microfilaments and a lack of elongation into the terminal web. The adherens junctions in the Lmix-colonized mice were significantly elongated and narrow compared with those in the GF mice and resembled those found in CV mice. This fortification of the intestinal barrier was further evident from the increased levels of the ZO-1 and occludin proteins in the Lmix-colonized and CV mice. To our knowledge, this is the first report documenting the effect of lactobacillus colonization on the ultrastructure of brush border and apical junctional complexes of enterocytes in gnotobiotic mice. Along these lines, increased gut permeability has been found in children with food allergies50 and it has also been recently detected in asthmatic patients.51 The homeostasis of the intestinal epithelium is maintained by a complex interplay of multiple regulatory mechanisms.52 In vitro studies have indicated that the pro-allergic cytokine IL-4 contributes to barrier impairment in contrast with TGF-β, which enhances the barrier function and activates the expression of proteins comprising intercellular junctions.53 In our study, the improvement of the gut barrier in the Lmix-colonized mice was accompanied by the reduced secretion of pro-allergic cytokines and the significant enhancement of TGF-β.
There is increasing evidence that probiotic bacteria can exert their functions by directly interacting with pattern recognition receptors. In this study, we showed that TLR2 played an important role in the recognition of L. rhamnosus LOCK0900 and that NOD2 participated in the recognition of L. casei LOCK0919. In contrast, L. rhamnosus LOCK0908 was poorly recognized by both of these receptors. Interestingly, a significant feature of the L. rhamnosus LOCK0908 strain is its high level of exopolysaccharide (EPS) production.33 Fanning et al.54 have recently shown that bifidobacterial strain-producing surface EPS fail to elicit a strong immune response compared with EPS-deficient variants. Thus, it is tempting to speculate that the lack of TLR2, TLR4 and NOD2 activation by L. rhamnosus LOCK0908 may be caused by EPS covering the bacterial surface and masking bioactive components, which play a role in binding to pattern recognition receptors.
We have previously shown in human blood cell cultures that the application of L. rhamnosus LOCK0900, L. rhamnosus LOCK0908 and L. casei LOCK0919 strains together as a mixture has synergistic effects on the induction of anti-allergic Th1-type cytokines compared with the levels induced by each single strain alone.31 We were not able to confirm these findings using mouse BM-DCs, and we did not observe any synergistic effects on cytokine production. This discrepancy may be explained by the different manners of bacterial inactivation (heating vs. formalin inactivation)55 and also by the different donor species and cell types used.
By evaluating bacterial colonization, we were able to show that all three bacterial strains were detectable in the fecal samples until the end of the experiment. Two days after colonization, L. casei LOCK0919 became the dominant strain in the feces of the colonized mice. This finding can be related to a recent analysis of the complete genomic sequence of L. casei LOCK0919, which has revealed the presence of factors relevant to its colonization and persistence in the human gut, including proteins with roles in adhesion to host cell structures.34 However, further experiments are needed to determine whether the effects observed in vivo can be achieved by the colonization of mice by L. casei LOCK0919 alone. Although the L. rhamnosus strains represented a minority of the strains present in the feces of the colonized mice, we cannot exclude that they may play an important role in the immunomodulatory activity of the mixture and that they are necessary for the successful reduction of allergic sensitization. This argument is supported by our recent finding that EPS produced by the L. rhamnosus LOCK0900 strain can modulate the cytokine production of BM-DCs induced by another bacteria.56
In conclusion, we have shown that the three lactobacillus strains in Lmix, L. rhamnosus LOCK0900, L. rhamnosus LOCK0908 and L. casei LOCK0919, were able to reduce sensitization to Bet v 1. The specific serum IgE and IgG levels as well as the production of the pro-allergic cytokines IL-4 and IL-5 by splenocytes and MLN cells were also reduced. This suppression was accompanied by the upregulation of the regulatory cytokine TGF-β and the improvement of the epithelial gut barrier. These results clearly demonstrate the beneficial roles of the selected lactobacillus strains in the process of allergic sensitization and support their uses in the early prevention of allergies.
Author contributions
HK, MS and BC designed the experiments. HK, MS, DS, IS and PH performed the experiments and analyzed the data. EC, IR and BC performed and analyzed the electron microscopy micrographs. ZZ performed and analyzed the western blot experiments. TA-P and AK-B performed and analyzed the qPCR experiments. TH performed and analyzed the immunohistochemistry experiments. HK, MS, LT, IS, HT-H and BC wrote the manuscript.
Acknowledgments
The excellent technical assistance of J Jarkovska, A Smolova, I Grimova and D Drasnarova is gratefully acknowledged. This research was supported by grant NR12-0101-10/2011 of the Republic of Poland, grants P304/11/1252 and 303/09/0449 of the Czech Science Foundation, grants CZ.3.22/2.1.00/09.01574 and CZ.3.22/2.1.00/13.03892, grant SFB F46 from the Austrian Science Fund. and Institutional Research Concept RVO 61388971.
There is no conflict of interest to disclose for all authors.
Footnotes
Supplementary Information accompanies the paper on Cellular & Molecular Immunology's website (http://www.nature.com/cmi).
Supplementary Information
References
- Maloy KJ, Powrie F. Intestinal homeostasis and its breakdown in inflammatory bowel disease. Nature 2011; 474: 298–306. [DOI] [PubMed] [Google Scholar]
- Tlaskalova-Hogenova H, Sterzl J, Stepankova R, Dlabac V, Veticka V, Rossmann P et al. Development of immunological capacity under germfree and conventional conditions. Ann NY Acad Sci 1983; 409: 96–113. [DOI] [PubMed] [Google Scholar]
- Perrier C, Corthesy B. Gut permeability and food allergies. Clin Exp Allergy 2011; 41: 20–28. [DOI] [PubMed] [Google Scholar]
- van Ree R, Hummelshoj L, Plantinga M, Poulsen LK, Swindle E. Allergic sensitization: host-immune factors. Clin Transl Allergy 2014; 4: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artis D. Epithelial-cell recognition of commensal bacteria and maintenance of immune homeostasis in the gut. Nat Rev Immunol 2008; 8: 411–420. [DOI] [PubMed] [Google Scholar]
- Tlaskalova-Hogenova H, Stepankova R, Kozakova H, Hudcovic T, Vannucci L, Tuckova L et al. The role of gut microbiota (commensal bacteria) and the mucosal barrier in the pathogenesis of inflammatory and autoimmune diseases and cancer: contribution of germ-free and gnotobiotic animal models of human diseases. Cell Mol Immunol 2011; 8: 110–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penders J, Gerhold K, Stobberingh EE, Thijs C, Zimmermann K, Lau S et al. Establishment of the intestinal microbiota and its role for atopic dermatitis in early childhood. J Allergy Clin Immunol 2013; 132: 601–607. [DOI] [PubMed] [Google Scholar]
- Strachan DP. Hay fever, hygiene, and household size. BMJ 1989; 299: 1259–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalliomaki M, Kirjavainen P, Eerola E, Kero P, Salminen S, Isolauri E. Distinct patterns of neonatal gut microflora in infants in whom atopy was and was not developing. J Allergy Clin Immunol 2001; 107: 129–134. [DOI] [PubMed] [Google Scholar]
- Abrahamsson TR, Jakobsson HE, Andersson AF, Bjorksten B, Engstrand L, Jenmalm MC. Low gut microbiota diversity in early infancy precedes asthma at school age. Clin Exp Allergy 2014; 44: 842–850. [DOI] [PubMed] [Google Scholar]
- Gourbeyre P, Denery S, Bodinier M. Probiotics, prebiotics, and synbiotics: impact on the gut immune system and allergic reactions. J Leukoc Biol 2011; 89: 685–695. [DOI] [PubMed] [Google Scholar]
- Lodinova-Zadnikova R, Cukrowska B, Tlaskalova-Hogenova H. Oral administration of probiotic Escherichia coli after birth reduces frequency of allergies and repeated infections later in life (after 10 and 20 years). Int Arch Allergy Immunol 2003; 131: 209–11. [DOI] [PubMed] [Google Scholar]
- Forsberg A, Abrahamsson TR, Bjorksten B, Jenmalm MC. Pre- and post-natal Lactobacillus reuteri supplementation decreases allergen responsiveness in infancy. Clin Exp Allergy 2013; 43: 434–442. [DOI] [PubMed] [Google Scholar]
- Borchers AT, Selmi C, Meyers FJ, Keen CL, Gershwin ME. Probiotics and immunity. J Gastroenterol 2009; 44: 26–46. [DOI] [PubMed] [Google Scholar]
- Vanderpool C, Yan F, Polk DB. Mechanisms of probiotic action: implications for therapeutic applications in inflammatory bowel diseases. Inflamm Bowel Dis 2008; 14: 1585–1596. [DOI] [PubMed] [Google Scholar]
- Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N et al. Conversion of peripheral CD4+CD25− naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med 2003; 198: 1875–1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konieczna P, Groeger D, Ziegler M, Frei R, Ferstl R, Shanahan F et al. Bifidobacterium infantis 35624 administration induces Foxp3 T regulatory cells in human peripheral blood: potential role for myeloid and plasmacytoid dendritic cells. Gut 2012; 61: 354–366. [DOI] [PubMed] [Google Scholar]
- Mileti E, Matteoli G, Iliev ID, Rescigno M. Comparison of the immunomodulatory properties of three probiotic strains of Lactobacilli using complex culture systems: prediction for in vivo efficacy. PLoS One 2009; 4: e7056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigaux P, Daniel C, Hisbergues M, Muraille E, Hols P, Pot B et al. Immunomodulatory properties of Lactobacillus plantarum and its use as a recombinant vaccine against mite allergy. Allergy 2009; 64: 406–414. [DOI] [PubMed] [Google Scholar]
- Schwarzer M, Srutkova D, Schabussova I, Hudcovic T, Akgun J, Wiedermann U et al. Neonatal colonization of germ-free mice with Bifidobacterium longum prevents allergic sensitization to major birch pollen allergen Bet v 1. Vaccine 2013; 31: 5405–5412. [DOI] [PubMed] [Google Scholar]
- Schabussova I, Hufnagl K, Tang ML, Hoflehner E, Wagner A, Loupal G et al. Perinatal maternal administration of Lactobacillus paracasei NCC 2461 prevents allergic inflammation in a mouse model of birch pollen allergy. PLoS One 2012; 7: e40271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li MO, Flavell RA. Contextual regulation of inflammation: a duet by transforming growth factor-beta and interleukin-10. Immunity 2008; 28: 468–476. [DOI] [PubMed] [Google Scholar]
- Akdis M, Akdis CA. Mechanisms of allergen-specific immunotherapy: multiple suppressor factors at work in immune tolerance to allergens. J Allergy Clin Immunol 2014; 133: 621–631. [DOI] [PubMed] [Google Scholar]
- Frischmeyer-Guerrerio PA, Guerrerio AL, Oswald G, Chichester K, Myers L, Halushka MK et al. TGFbeta receptor mutations impose a strong predisposition for human allergic disease. Sci Transl Med 2013; 5: 195ra94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West CE, Jenmalm MC, Prescott SL. The gut microbiota and its role in the development of allergic disease: a wider perspective. Clin Exp Allergy 2014; doi: 10.1111/cea.12332. [DOI] [PubMed]
- Wiedermann U. Hitting the mucosal road in tolerance induction. Nestle Nutr Workshop Ser Pediatr Program 2009; 64: 63–72. [DOI] [PubMed] [Google Scholar]
- Schabussova I, Hufnagl K, Wild C, Nutten S, Zuercher AW, Mercenier A et al. Distinctive anti-allergy properties of two probiotic bacterial strains in a mouse model of allergic poly-sensitization. Vaccine 2011; 29: 1981–1990. [DOI] [PubMed] [Google Scholar]
- Chapman CM, Gibson GR, Rowland I. Health benefits of probiotics: are mixtures more effective than single strains? J Nutr 2011; 50: 1–17. [DOI] [PubMed] [Google Scholar]
- Hansen CH, Nielsen DS, Kverka M, Zakostelska Z, Klimesova K, Hudcovic T et al. Patterns of early gut colonization shape future immune responses of the host. PLoS One 2012; 7: e34043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cukrowska B, Motyl I, Kozakova H, Schwarzer M, Gorecki RK, Klewicka E et al. Probiotic Lactobacillus strains: in vitro and in vivo studies. Folia Microbiol 2009; 54: 533–537. [DOI] [PubMed] [Google Scholar]
- Cukrowska B, Rosiak I, Klewicka E, Motyl I, Schwarzer M, Libudzisz Z et al. Impact of heat-inactivated Lactobacillus casei and Lactobacillus paracasei strains on cytokine responses in whole blood cell cultures of children with atopic dermatitis. Folia Microbiol 2010; 55: 277–280. [DOI] [PubMed] [Google Scholar]
- Aleksandrzak-Piekarczyk T, Koryszewska-Baginska A, Bardowski J. Genome sequence of the probiotic strain Lactobacillus rhamnosus (formerly Lactobacillus casei) LOCK900. Genome Announc 2013; 1: 00640-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koryszewska-Baginska A, Bardowski J, Aleksandrzak-Piekarczyk T. Genome sequence of the probiotic strain Lactobacillus rhamnosus (formerly Lactobacillus casei) LOCK908. Genome Announc 2014; 2: 00120-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koryszewska-Baginska A, Aleksandrzak-Piekarczyk T, Bardowski J. Complete genome sequence of the probiotic strain Lactobacillus casei (formerly Lactobacillus paracasei) LOCK919. Genome Announc 2013; 1: e00758-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repa A, Kozakova H, Hudcovic T, Stepankova R, Hrncir T, Tlaskalova-Hogenova H et al. Susceptibility to nasal and oral tolerance induction to the major birch pollen allergen Bet v 1 is not dependent on the presence of the microflora. Immunol Lett 2008; 117: 50–56. [DOI] [PubMed] [Google Scholar]
- Daniel C, Repa A, Wild C, Pollak A, Pot B, Breiteneder H et al. Modulation of allergic immune responses by mucosal application of recombinant lactic acid bacteria producing the major birch pollen allergen Bet v 1. Allergy 2006; 61: 812–819. [DOI] [PubMed] [Google Scholar]
- Cinova J, de Palma G, Stepankova R, Kofronova O, Kverka M, Sanz Y et al. Role of intestinal bacteria in gliadin-induced changes in intestinal mucosa: study in germ-free rats. PloS One 2011; 6: e16169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods 2012; 9: 671–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiedermann U, Jahn-Schmid B, Bohle B, Repa A, Renz H, Kraft D et al. Suppression of antigen-specific T- and B-cell responses by intranasal or oral administration of recombinant bet v 1, the major birch pollen allergen, in a murine model of type I allergy. The J Allergy Clin Immunol 1999; 103: 1202–1210. [DOI] [PubMed] [Google Scholar]
- Wiedermann U, Herz U, Baier K, Vrtala S, Neuhaus-Steinmetz U, Bohle B et al. Intranasal treatment with a recombinant hypoallergenic derivative of the major birch pollen allergen Bet v 1 prevents allergic sensitization and airway inflammation in mice. Int Arch Allergy Immunol 2001; 126: 68–77. [DOI] [PubMed] [Google Scholar]
- Schwarzer M, Repa A, Daniel C, Schabussova I, Hrncir T, Pot B et al. Neonatal colonization of mice with Lactobacillus plantarum producing the aeroallergen Bet v 1 biases towards Th1 and T-regulatory responses upon systemic sensitization. Allergy 2011; 66: 368–375. [DOI] [PubMed] [Google Scholar]
- Kalliomaki M, Antoine JM, Herz U, Rijkers GT, Wells JM, Mercenier A. Guidance for substantiating the evidence for beneficial effects of probiotics: prevention and management of allergic diseases by probiotics. J Nutr 2010; 140: 713S–721S. [DOI] [PubMed] [Google Scholar]
- Lim LH, Li HY, Huang CH, Lee BW, Lee YK, Chua KY. The effects of heat-killed wild-type Lactobacillus casei Shirota on allergic immune responses in an allergy mouse model. Int Arch Allergy Immunol 2009; 148: 297–304. [DOI] [PubMed] [Google Scholar]
- Hisbergues M, Magi M, Rigaux P, Steuve J, Garcia L, Goudercourt D et al. In vivo and in vitro immunomodulation of Der p 1 allergen-specific response by Lactobacillus plantarum bacteria. Clin Exp Allergy 2007; 37: 1286–1295. [DOI] [PubMed] [Google Scholar]
- Feleszko W, Jaworska J, Rha RD, Steinhausen S, Avagyan A, Jaudszus A et al. Probiotic-induced suppression of allergic sensitization and airway inflammation is associated with an increase of T regulatory-dependent mechanisms in a murine model of asthma. Clin Exp Allergy 2007; 37: 498–505. [DOI] [PubMed] [Google Scholar]
- Sun CM, Hall JA, Blank RB, Bouladoux N, Oukka M, Mora JR et al. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. J Exp Med 2007; 204: 1775–1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corthesy B, Gaskins HR, Mercenier A. Cross-talk between probiotic bacteria and the host immune system. J Nutr 2007; 137: 781S–790S. [DOI] [PubMed] [Google Scholar]
- Cebra JJ. Influences of microbiota on intestinal immune system development. Am J Clin Nutr 1999; 69: 1046S–1051S. [DOI] [PubMed] [Google Scholar]
- Ukena SN, Singh A, Dringenberg U, Engelhardt R, Seidler U, Hansen W et al. Probiotic Escherichia coli Nissle 1917 inhibits leaky gut by enhancing mucosal integrity. PLoS One 2007; 2: e1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvinen KM, Konstantinou GN, Pilapil M, Arrieta MC, Noone S, Sampson HA et al. Intestinal permeability in children with food allergy on specific elimination diets. Pediatr Allergy Immunol 2013; 24: 589–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker J, Dieleman L, Mah D, Park K, Meddings J, Vethanayagam D. High prevalence of abnormal gastrointestinal permeability in moderate-severe asthma. Clin Invest Med 2014; 37: E53–E57. [DOI] [PubMed] [Google Scholar]
- Jeon MK, Klaus C, Kaemmerer E, Gassler N. Intestinal barrier: molecular pathways and modifiers. World J Gastrointest Pathophysiol 2013; 4: 94–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe KL, Reardon C, Wang A, Nazli A, McKay DM. Transforming growth factor-beta regulation of epithelial tight junction proteins enhances barrier function and blocks enterohemorrhagic Escherichia coli O157:H7-induced increased permeability. Am J Pathol 2005; 167: 1587–1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanning S, Hall LJ, Cronin M, Zomer A, MacSharry J, Goulding D et al. Bifidobacterial surface-exopolysaccharide facilitates commensal-host interaction through immune modulation and pathogen protection. Proc Natl Acad Sci USA 2012; 109: 2108–2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taverniti V, Guglielmetti S. The immunomodulatory properties of probiotic microorganisms beyond their viability (ghost probiotics: proposal of paraprobiotic concept). Genes Nutr 2011; 6: 261–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorska S, Schwarzer M, Jachymek W, Srutkova D, Brzozowska E, Kozakova H et al. Distinct immunomodulation of bone marrow-derived dendritic cell responses to Lactobacillus plantarum WCFS1 by two different polysaccharides isolated from Lactobacillus rhamnosus LOCK 0900. Appl Environ Microbiol 2014; 80: 6506–6516. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.