Abstract
While there is mounting evidence that interleukin (IL)-23-IL-17 axis plays a critical role in the pathogenesis of various autoimmune diseases, much remains to be elucidated on how IL-23 is induced in the pathological processes. IL-23 is a heterodimer composed of p19 and p40, the latter being shared with IL-12. We previously reported that prostaglandin (PG) E2 promotes CD40-mediated induction of Il23a (p19) expression through its E receptor subtype 4 (EP4) receptor in splenic dendritic cells (DCs). Here, we have analyzed signaling pathways regulating Il23a induction in the cross talk between EP4 and CD40 in bone marrow-derived DCs. We found that PGE2 synergistically induced Il23a transcription with CD40 signaling. An EP4 agonist, but not agonists of EP1, EP2, or EP3, reproduced this action. Stimulation of CD40 with an agonist antibody evoked biphasic induction of Il23a expression, with the early phase peaking at 1 h and the late phase peaking at 12 h and lasting up to 36 h after stimulation, whereas induction by lipopolysaccharide or tumor necrosis factor-α was transient. The early phase induction by CD40 stimulation was absent in DCs derived from Nfkb1-deficient mice, and the late phase induction was eliminated by RNA interference of nuclear factor-kappa B (NF-κB) p100 subunit. Further, cAMP response element-binding protein (CREB) depletion completely eliminated the induction of Il23a by CD40 stimulation. The addition of the EP4 agonist amplified the induction in both phases through the cAMP-protein kinase A (PKA) pathway. These results suggest that Il23a expression in DCs is synergistically triggered by the PG E2-EP4-cAMP-PKA pathway and canonical/non-canonical NF-κB pathways and CREB activated by CD40 stimulation.
Keywords: dendritic cell, IL-23, p19, prostaglandin E2, EP4
Introduction
A group of lymphoid cell populations that produce interleukin (IL)-17 and are positively regulated by IL-23, including Th17 cells,1 γδT cells2 and RORγ+ group 3 innate lymphoid (ILC-3) cells,3,4,5 have emerged as critical cell populations in autoimmune diseases. Their involvement in human diseases has been strongly indicated by the clinical efficacy of anti-IL-17 monoclonal antibodies such as secukinumab in psoriasis,6 rheumatoid arthritis,7 and ankylosing spondylitis8 of anti-IL12/IL23 p40 antibodies such as ustekinumab in psoriasis9,10 and Crohn's disease;11 and of anti-IL-23 p19 antibodies such as tildrakizumab in psoriasis.12 IL-23 is a heterodimer composed of two subunits, p19 and p40,13 the latter being shared withIL-12, which is composed of p35 and p40. These cytokines are produced by antigen-presenting cells, mainly activated dendritic cells (DCs), with IL-12 driving the differentiation of Th1 cells and IL-23-stabilizing and expanding Th17 cells. It is possible that various inflammatory molecules activate DCs in conjunction with their antigen uptake and that the composition of these stimulating substances determines the profile of cytokines they produce. Indeed, in vitro experiments have shown that stimulation with a single substance is not sufficient to produce IL-12 family cytokines. Combined stimulation with different Toll-like receptor (TLR) ligands, other cytokines such as IFN-γ and IL-4, or CD40L binding to CD40 is required for proper biosynthesis. One molecule with DC-stimulating activity is prostaglandin (PG) E2. PGs are arachidonate metabolites produced by cyclooxygenase (COX) and respective synthases in response to various, often noxious, stimuli.14 PGE2 is a PG with versatile actions in various inflammatory processes. PGE2 exerts its actions through four subtypes of G-protein-coupled receptors, PG E receptor (EP) 1 to 4.15,16 Previously, Ganea and collaborators reported that simultaneous treatment with PGE2 and either granulocyte-macrophage colony-stimulating factor (GM-CSF) or various TLR ligands such as lipopolysaccharide (LPS), poly I:C, CpG, or proteoglycan promoted p19 gene (Il23a) expression and suppressed p35 expression in bone marrow-derived DCs (BMDCs).17 They further found that BMDCs induced to differentiate in the presence of PGE2 produced more p19 than p35 in response to LPS. Comparing the effects of various PGE analogs, they suggested that these actions of PGE2 are mediated by EP2 and EP4 receptors. We found a similar promoting effect of PGE2 on Il23a expression in splenic DCs (sDCs) treated with an agonistic anti-CD40 antibody.18 This action was reproduced by an EP4-selective agonist and eliminated by the addition of an EP4 antagonist. Interestingly, the addition of the EP4 antagonist not only eliminated the enhancing effect of PGE2 but also suppressed p19 production almost to a negligible level, which was mimicked by indomethacin. These results suggest that during CD40 stimulation, PGE2 is formed endogenously and amplifies the CD40-mediated induction of Il23a. Because CD40-CD40L is a co-stimulatory pathway and significant amounts of CD40L are expressed in activated T cells, the enhancement of a CD40-mediated Il23a expression by PGE2 could be relevant to physiological actions of IL-23, which acts on primed Th17 cells to stabilize and expand this Th subset. However, this synergy of PGE2-EP4 and CD40 signaling has not been characterized in detail, and its mechanism remains to be studied. In the present study, we have examined signaling pathways and transcription factors in this cross talk between EP4 and CD40 signaling that mediates the induction of Il23a gene expression synergistically. We found that CD40 signaling evokes biphasic, long-lasting induction of Il23a expression in BMDCs through the canonical nuclear factor-kappa B (NF-κB) pathway in the early phase and the non-canonical NF-κB pathway in the late phase combined with cAMP response element-binding protein (CREB) activation, which is synergistically amplified by the PGE2-EP4 signaling through the cAMP-protein kinase A (PKA) pathway.
Materials and methods
Mice
C57BL/6NCrSlc mice were purchased from Japan SLC Inc. (Shizuoka, Japan) and Nfkb1 (p50)-deficient mice were purchased from the Jackson Laboratory (Bar Harbor, ME, USA). All procedures were in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and approved by the Committee on Animal Research of Kyoto University Faculty of Medicine.
Preparation of BMDCs
BMDCs were prepared according to the previous study with some modifications.19 Femurs and tibiae were dissected from 8- to 12-week-old female mice and bone marrow cells were collected. Cells were cultured in RPMI-1640 containing 10% fetal bovine serum and 10 ng mL−1 GM-CSF and harvested on the eighth day. The purity of the DCs, as determined by fluorescence-activated cell sorting (FACS) analysis as CD11c-positive cells on FACS Calibur (Becton, Dickinson and Company, San Jose, CA, USA), was 80.9% (n = 3).
Quantitative real-time PCR (quantitative RT-PCR)
Purified DCs were stimulated as indicated in each experiment. Total RNA was purified from stimulated cells using an RNeasy Mini kit (QIAGEN, Hilden, Germany) and reverse-transcribed to cDNA by a high-capacity cDNA Reverse Transcription kit (Life Technologies Corporation, Carlsbad, CA, USA), according to manufacturers' instructions. PCR quantification was performed with a LightCycler 480 system using a LightCyclerTaqman Master kit with a Universal Probe Library Set (Probe number 47) (Roche Molecular Diagnostics, Pleasanton, CA, USA)and a SYBR Premix Ex Taq II kit (Takara Bio Inc., Shiga, Japan) with GAPDH as an internal control.
Enzyme-linked immunosorbent assay (ELISA)/enzyme immunoassay (EIA)
DCs were stimulated as described in the figure legends in the presence of 100 μM of indomethacin. The concentration of IL-23 in supernatant of cultured cells or the content of cAMP in cell lysate was determined using a Mouse IL-23 Immunoassay (R&D Systems Inc., Minneapolis, MN, USA) and a Cyclic AMP EIA kit (Cayman Chemical, Ann Arbor, MI, USA), respectively, according to the manufacturers' instructions.
Western blot analysis
DCs were stimulated with an anti-CD40 antibody (10 μg mL−1), an EP4 agonist (ONO-AE1-329, 1 μM) or both for 15 min in the presence of 100 μM of indomethacin. In some experiments, cells were stimulated with either an anti-CD40 antibody (10 μg mL−1), LPS (1 μg mL−1), or tumor necrosis factor-α (TNF-α) (100 ng mL−1) for the indicated period. In some experiments, the nuclear and the cytoplasmic fractions were separately purified using NE-PER Nuclear and Cytoplasmic Extraction Reagents (Thermo Fisher Scientific, Waltham, MA, USA) from stimulated cells. Extracted proteins from stimulated cells were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis and transferred to a polyvinylidene fluoride membrane (Immobilon-P, Merck, Darmstadt, Germany). After blocking with an ECL Blocking Agent (GE Healthcare, Piscataway, NJ, USA), membranes were incubated with primary antibodies, followed by incubation with secondary antibodies conjugated with horseradish peroxidase (GE Healthcare). Signals were detected using an ECL Prime Western Blotting Detection Reagent (GE Healthcare).
RNA interference (RNAi) in BMDCs
RNAi in BMDCs was achieved by electroporation using the Neon Transfection System (1700 V, 30 ms, 1 pulse) (Life Technologies Corporation) and a pre-designed small interfering RNA (siRNA) purchased from Life Technologies Corporation (Nfkb2 siRNA, ID# s70545; Creb1 siRNA, ID# MSS236242) according to the manufacturer's instructions. After electroporation, cells were cultured in RPMI-1640 for additional 48 h and used for experiments. Knockdown efficiency was determined by RT-PCR analysis.
Chromatin immunoprecipitation (ChIP) assay
ChIP assay was performed with a SimpleChIPPlus Enzymatic Chromatin IP kit (Cell Signaling Technology Inc., Beverly, MA, USA) according to the manufacturer's instructions. In brief, cells were treated as indicated. Then, treated cells were fixed with paraformaldehyde for cross-linking transcription factors bound to promoter regions with chromatin and the chromatin was enzymatically sheared. Sheared chromatin was subjected to immunoprecipitation by an anti-CREB antibody or a control normal rabbit IgG. The content of the chromatin fragment corresponding to the promoter and enhancer regions of the Il23a gene in each sample after immunoprecipitation was assessed by quantitative RT-PCR analysis. Relative enrichment was calculated as a ratio of corresponding immunoprecipitation to normal IgG isotypes.
Statistical analysis
All data are shown as the mean ± SEM. Statistical analysis was performed by the unpaired two-tailed Student's t-test and statistical significance was defined as p < 0.05.
Results
Induction of IL-23 in BMDCs by PGE2-EP4 signaling
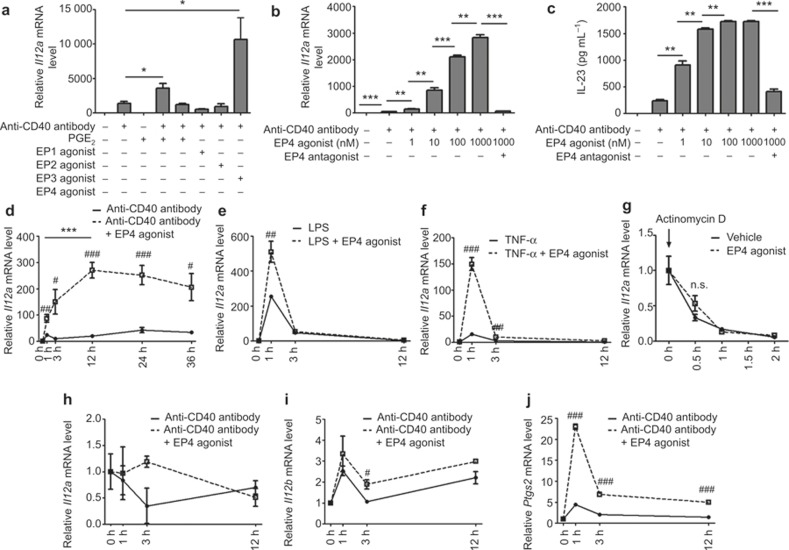
We previously found that PGE2-EP4 signaling enhanced IL-23 p19 production in sDCs stimulated with an anti-CD40 antibody.18 Because we used BMDCs instead of sDCs to confirm this mechanism through transfection experiments, we first confirmed the action of PGE2 and various EP agonists in BMDCs. BMDCs were stimulated with an anti-CD40 antibody either alone or in combination with PGE2 or various EP agonists in the presence of 100 μM of indomethacin, a broad COX inhibitor, for 24 h. Similar to sDCs, PGE2 tripled the CD40-mediated expression of Il23a mRNA, and this action was reproduced selectively by an EP4 agonist, ONO-AE1-329,which increased Il23a expression in a concentration-dependent manner (Figure 1a and 1b). Consistently, the ONO-AE1-329 treatment augmented the production of the IL-23 protein, assessed by ELISA, in a similar concentration-dependent manner (Figure 1c). The addition of a selective EP4 antagonist, ONO-AE3-208, suppressed these actions of ONO-AE1-329 to the level obtained by CD40 stimulation alone (Figure 1b and 1c), verifying that the actions of PGE2 and ONO-AE1-329 were through the EP4 receptor. To characterize the effect of ONO-AE1-329 on CD40-mediated induction of Il23a, we next compared the duration of this effect with those of Il23a induction by LPS, which acts on TLR4, and TNF-α, which acts on a TNF receptor structurally similar to CD40. Notably, in the stimulation of the CD40-evoked biphasic induction of Il23a expression, the early phase peaked at 1 h and the late phase peaked at 24 h and lasted up to 36 h after the stimulation (Figure 1d). The addition of the EP4 agonist enhanced the expression in both phases by up to ten-fold, increasing almost linearly for12 h before plateauing (Figure 1d). In contrast, stimulation with either LPS or TNF-α evoked a sharp increase of Il23a mRNA, which peaked at 1 h and returned almost to the basal level at 3 h; the EP4 stimulation enhanced the expression at the peak (Figure 1e and 1f). Thus, the EP4 stimulation induced a prolonged enhancement of Il23a mRNA expression in CD40-stimulated BMDCs. To verify that this effect of EP4 stimulation was due to its effect on transcription and not a post-transcriptional mechanism, and because a previous report showed that IFN-γ stimulation can stabilize Il23a mRNA in DCs,20 we examined the stability of Il23a mRNA. We first incubated BMDCs with 10 μg mL−1 of an anti-CD40 antibody and then treated the cells with 10 μg mL−1 of actinomycin D in the presence or absence of 100 nM of ONO-AE1-329. We found that the treatment with ONO-AE1-329 did not influence the decay of Il23a mRNA under these conditions (Figure 1g), suggesting that PGE2-EP4 signaling does regulate the transcription of Il23a. Because IL-23 and IL-12 belong to the IL-12 cytokine family and share one subunit, p40, we next examined the specificity of the above actions of CD40 and EP4 by analyzing the expression of Il12a encoding p35 of IL-12 and Il12b encoding p40. CD40 stimulation did not induce expression of Il12a and the EP4 agonist did not enhance it (Figure 1h). The Il12b expression was induced by CD40 stimulation, and this induction was enhanced by the addition of the EP4 agonist (Figure 1i), although the extent of enhancement was not as high as was observed for Il23a expression. We further found that induction of the expression of Ptgs2 (COX-2) mRNA by CD40 stimulation and ONO-AE1-329 synergistically enhanced this induction (Figure 1j). Given that COX-2 is an inducible form of PG synthase that produces PGE2 in various inflammatory settings, this induction may form a positive feedback loop to amplify the PGE2-EP4-COX-2 signaling. These results suggest that PGE2-EP4 signaling regulates the IL-23 production synergistically with CD40 signaling in DCs through the persistent augmentation of mRNA expression.
Figure 1.
Enhancement of CD40-induced persistent expression of the Il23a gene by PGE2-EP4 signaling in BMDCs. (a) Selective enhancement of CD40-induced Il23a expression by an EP4 agonist. Il23a mRNA expression was assessed by quantitative RT-PCR analysis in BMDCs treated with an anti-CD40 antibody (10 μg mL−1) in the presence of 100 nM of PGE2 or an agonist selective to each EP subtype for 24 h. ONO-D1-004, ONO-AE1-259, ONO-AE-248, and ONO-AE1-329 were used as EP1, EP2, EP3, and EP4 agonists, respectively. n = 3 in each group. *p < 0.05. All bars indicate mean ± SEM. (b, c) Concentration-dependent effects of the EP4 agonist on CD40-induced expression of Il23a mRNA (b) and IL-23 protein (c). Il23a mRNA expression or IL-23 protein content in supernatant was assessed by quantitative RT-PCR analysis or ELISA in BMDCs stimulated with an anti-CD40 antibody (10 μg mL−1) in the presence of various concentrations of ONO-AE1-329 for 24 h. A selective EP4 antagonist, ONO-AE3-208, was used at 10 μM to confirm the EP4 specificity of the effects. n = 3 in each group. **p < 0.01, ***p < 0.001. All bars indicate mean ± SEM. (d–f) Duration of induction of Il23a mRNA expression by an anti-CD40 antibody (d, 10 μg mL−1), LPS (e, 1 μg mL−1), and TNF-α (f, 100 ng mL−1) and enhancement by the EP4 agonist (ONO-AE1-329, 100 nM). Il23a mRNA was measured by quantitative RT-PCR analysis. n = 5 in each group. #p < 0.05, ##p < 0.01, and ###p < 0.001, compared to the groups treated with an anti-CD40 antibody alone (d), LPS alone (e), or TNF-α alone (f). ***p < 0.001 between groups as indicated. All bars indicate mean ± SEM. (g) Effect of the EP4 agonist on the stability of Il23a mRNA. BMDCs were first stimulated with an anti-CD40 antibody (10 μg mL−1) for 1 h actinomycin D (10 μg mL−1) was then added to the medium with either vehicle or 100 nM ONO-AE1-329. Il23a mRNA was measured by quantitative RT-PCR analysis. n = 3 in each group. n.s. = not statistically significant. All bars indicate mean ± SEM. (h–j) Time-dependent induction of Il12a (h), Il12b (i), and Ptgs2 (j) mRNA in BMDCs stimulated with an anti-CD40 antibody (10 μg mL−1) in the absence or presence of the EP4 agonist (ONO-AE1-329, 100 nM). n = 3 in each group. #p < 0.05, ###p < 0.001, compared to the groups treated with an anti-CD40 antibody alone. All bars indicate mean ± SEM.
Involvement of NF-κB in the induction of Il23a mRNA expression under the CD40 signaling in BMDCs
Because the transcription factor NF-κB is activated downstream of CD40 signaling,21 we examined whether NF-κB was essential for the induction of Il23a mRNA expression in CD40-stimulated BMDCs and the synergistic action of EP4 signaling. Consistent with the previous report, the treatment with an anti-CD40 antibody augmented nuclear translocation of the NF-κB p65 subunit (Figure 2a), and increased phosphorylation of the NF-κB p65 subunit at serine 536 residue (Figure 2b). Because p65 functions in a complex with the p50 subunit of NF-κB in the nucleus, we examined the role of this canonical NF-κB signaling by using BMDCs derived from mice deficient in p50. The p50 deficiency suppressed the canonical NF-κB pathway as evidenced by the inhibition of nuclear translocation of the p65 subunit in BMDCs under CD40 stimulation (Figure 2a), and significantly suppressed Il23a mRNA induction at 1 h by the CD40 stimulation and the stimulation of CD40 and EP4 signaling together (Figure 2c). Nonetheless, there was significant enhancement of Il23a expression by EP4 stimulation with ONO-AE1-329 compared to that achieved by CD40 stimulation alone, suggesting that the augmenting effect of EP4 stimulation is still present without the p50 of NF-κB (Figure 2c). Because the CD40 stimulation evoked biphasic Il23a induction and EP4 augmented the induction in both phases, we next examined the effects of a p50 deficiency on the duration of Il23a induction by CD40 simulation with or without the stimulation of EP4 signaling. Notably, the p50 deficiency eliminated the early phase response but not the late phase response in BMDCs stimulated with an anti-CD40 antibody alone and significantly suppressed Il23a induction in the early phase and kept the late phase induction intact in BMDCs simultaneously stimulated with an anti-CD40 antibody and the EP4 agonist (Figure 2d). These results suggest that the response to CD40 stimulation and synergistic action with EP4 stimulation in the late phase was independent of a canonical NF-κB pathway. Because CD40 signaling can activate a non-canonical NF-κB pathway as well, we examined whether CD40 stimulation activated a non-canonical NF-κB pathway in BMDCs at 12 h. We found that treatment with an anti-CD40 antibody increased the content of non-canonical NF-κB subunits, p52 and RelB, in the nucleus, accompanied by the decrease of p52 precursor p100 in the cytoplasm (Figure 2e). To test if p52 was involved in Il23a mRNA induction by CD40 stimulation, we decreased the p100 by RNAi in BMDCs and examined Il23a mRNA expression at 12 h after stimulation with an anti-CD40 antibody with or without the EP4 agonist. Depletion of the p100 by 89.6% (n = 3), as examined for its mRNA content by quantitative RT-PCR analysis, almost completely eliminated the induction of Il23a mRNA expression by CD40 stimulation, and the enhancement by EP4 stimulation was similarly suppressed (Figure 2f). These results suggest that EP4 stimulation synergizes with both canonical and non-canonical NF-κB signaling to amplify CD40-mediated Il23a mRNA expression in the phase-dependent manner.
Figure 2.
Canonical and non-canonical NF-κB pathways mediate Il23a mRNA expression in succession in CD40-stimulated BMDCs. (a) Nuclear accumulation of NF-κB p65 in C57BL/6NCrSlc mice (wild type) and Nfkb1 (p50)-deficient BMDCs simulated with an anti-CD40 antibody, LPS, or TNF-α. BMDCs were stimulated with an anti-CD40 antibody (10 μg mL−1), LPS (1 μg mL−1), or TNF-α (100 ng mL−1) for the indicated periods and nuclear extracts were prepared for Western blot analysis for NF-κB p65. Lamin B was used as a loading control. Representative images from three independent experiments were shown. (b) Western blot analysis for Ser536-phosphorylated NF-κB p65 in BMDCs treated with either an anti-CD40 antibody (10 μg mL−1), the EP4 agonist (ONO-AE1-329, 1 μM) or both for 15 min. Western blot analysis for the total NF-κB p65 subunit was shown as an internal control. Representative images from three independent experiments were shown. (c) Quantitative RT-PCR analysis for Il23a mRNA induction in wild type or Nfkb1-deficient BMDCs treated with an anti-CD40 antibody (10 μg mL−1), the EP4 agonist (ONO-AE1-329, 100 nM), or both for 1 h. n = 9 in each group. **p < 0.01, ***p < 0.001 between groups as indicated. All bars indicate mean ± SEM. (d) Duration of Il23a mRNA expression in wild type or Nfkb1-deficient BMDCs treated with 10 μg mL−1 of an anti-CD40 antibody alone or an anti-CD40 antibody and 100 nM ONO-AE1-329, the EP4 agonist. Il23a mRNA expression was assessed by quantitative RT-PCR analysis. n = 3 in each group. ***p < 0.001 compared to the expression in wild-type mice. All bars indicate mean ± SEM. (e) Increased nuclear accumulation of NF-κB p52 subunit and RelB in CD40-stimulated BMDCs. BMDCs were stimulated with an anti-CD40 antibody (10 μg mL−1) for 24 h and nuclear or cytoplasmic extract was prepared for Western blot analysis for RelB, p52, or its precursor, p100, subunits of NF-κB, respectively. Lamin B and α-tubulin were used as loading controls. Representative images from three independent experiments were shown. (f) Effect of NF-κB p100 depletion on Il23a mRNA expression in BMDCs stimulated with an anti-CD40 antibody with or without the EP4 agonist. BMDCs were treated with siRNA for p100 or scrambled siRNA for 48 h, and then stimulated with an anti-CD40 antibody (10 μg mL−1) and/or the EP4 agonist (ONO-AE1-329, 100 nM) for an additional 12 h. Total RNA from stimulated cells was prepared for quantitative RT-PCR analysis. n = 3 in each group. **p < 0.01 between groups as indicated. All bars indicate mean ± SEM.
Involvement of the cAMP-PKA pathway in the EP4-mediated amplification of Il23a mRNA expression in BMDCs
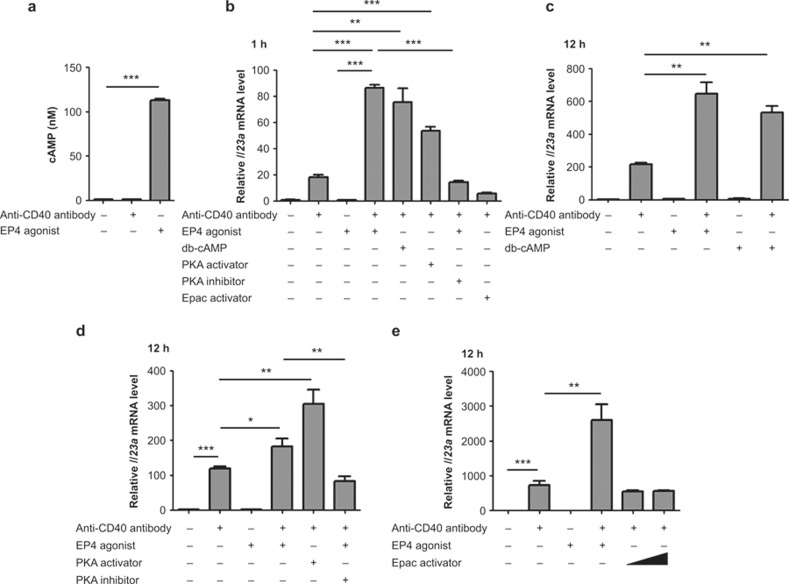
We next examined the downstream machinery of PGE2-EP4 signaling to amplify Il23a mRNA expression in BMDCs. We first focused on the actions of EP4 in the early phase. Because EP4 activates the cAMP-PKA signaling pathway,22 we examined the involvement of this pathway in the transcriptional regulation of the Il23a gene under PGE2-EP4 signaling. Indeed, a significant increase in the intracellular content of the cAMP was observed in BMDCs treated with 100 nM ONO-AE1-329 for 10 min (Figure 3a), and the amplification of the CD40-induced Il23a mRNA expression was reproduced by the addition of a cAMP analogue, dibutyryl cAMP (db-cAMP), as well as by a PKA activator, N6-Bnz-cAMP (Figure 3b). Notably, an exchange protein directly activated by cAMP (Epac) activator, 8-pCPT-2′-O-Me-cAMP, failed to show the augmentation (Figure 3b), indicating involvement of a cAMP-PKA, rather than a cAMP-Epac, signaling PGE2-EP4-mediated augmentation of Il23a mRNA expression in BMDCs. Consistently, treatment with10 μM H-89, a PKA inhibitor, cancelled the ONO-AE1-329 action on Il23a mRNA expression (Figure 3b). While PGE2-EP4 signaling can also activate the PI3K pathway,22 treatment with PI3K inhibitors, LY-294002 (5 μM) or wortmannin (1 μM) did not affect the ONO-AE1-329-induced expression of Il23a mRNA (data not shown), excluding the role of the PI3K pathway in the present action of PGE2-EP4 signaling. Similar involvement of the cAMP-PKA pathway in the PGE2-EP4 signaling was also observed in augmentation in the late phase. The augmented Il23a expression by the EP4 agonist was again mimicked by db-cAMP and N6-Bnz-cAMP, but not by 8-pCPT-2′-O-Me-cAMP, and was inhibited by H-89 (Figure 3c–3e). The EP4 stimulation induced phosphorylation of neither p38 nor ERK (Supplementary Figure 1). Thus, the cAMP-PKA pathway mediates the augmenting action of EP4 stimulation on CD40-induced Il23a expression in both early and late phases.
Figure 3.
cAMP-PKA signaling amplified both early and late phases of Il23a mRNA induction downstream of EP4 in CD40-stimulated BMDCs. (a) EP4-mediated increase in cAMP in BMDCs. BMDCs were treated with either an anti-CD40 antibody (10 μg mL−1) or ONO-AE1-329 (100 nM) for 10 min. Whole cell lysates were prepared and cAMP content was measured by EIA. n = 3 for each group. ***p < 0.001. All bars indicate mean ± SEM. (b) Involvement of cAMP-PKA signaling in EP4-induced amplification in the early phase Il23a mRNA expression in CD40-stimulated BMDCs. BMDCs were treated with 100 nM ONO-AE1-329, 100 μM db-cAMP, 100 μM N6-Bnz-cAMP (a PKA activator), 10 μM H-89 (a PKA inhibitor), or 100 μM 8-pCPT-2′-O-Me-cAMP (an Epac activator) for 1 h, and Il23a mRNA was measured by quantitative RT-PCR analysis. n = 3 for each group. **p < 0.01, ***p < 0.001 between groups as indicated. All bars indicate mean ± SEM. (c–e) Involvement of cAMP-PKA signaling in EP4-induced amplification in the late phase Il23a mRNA expression in CD40-stimulated BMDCs. BMDCs were treated for 12 h with 100 nM ONO-AE1-329 or 100 μM db-cAMP either alone or in combination with 10 μg mL−1 of an anti-CD40 antibody (c), 100 μM N6-Bnz-cAMP, or 100 nM ONO-AE1-329 with or without 100 μM H-89 in combination with 10 μg mL−1 of an anti-CD40 antibody (d) and 100 and 500 μM 8-pCPT-2′-O-Me-cAMP, an Epac activator, in combination with 10 μg mL−1 of an anti-CD40 antibody (e). Il23a mRNA was measured by quantitative RT-PCR analysis. n = 5 for each group. *p < 0.05, **p < 0.01, ***p < 0.001 between groups as indicated. All bars indicate mean ± SEM.
Involvement of CREB in synergistic induction of Il23a mRNA by CD40 and EP4
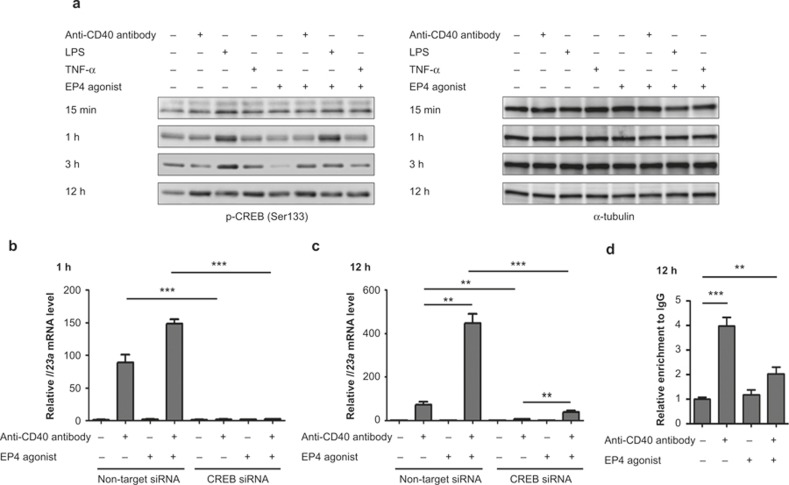
Because CREB is a major transcription factor downstream of the cAMP-PKA pathway, we examined its involvement in synergistic induction of Il23a expression by CD40 and EP4. We first examined the level of CREB phosphorylated at Ser133 in BMDCs treated with either an anti-CD40 antibody, LPS, and TNF-α, an EP4 agonist or an EP4 agonist combined with one other stimulant (Figure 4a). Indeed, Ser133-phopsphorylated CREB increased with EP4 stimulation at 15 min, but this effect was weak at 1 h and 3 h. CD40 stimulation evoked a robust increase in the level of Ser133-phosphorylated CREB at 12 h. To examine the involvement of this response in Il23a induction, we conducted RNAi for CREB. BMDCs were transfected with siRNA for CREB and used for experiment after a 48-h culture. The knockdown efficiency was 97.0% as examined for its mRNA content by quantitative RT-PCR analysis. When these CREB-depleted cells were stimulated, induction of Il23a was significantly suppressed at 1 h and 12 h (Figure 4b and 4c). Notably, RNAi for CREB suppressed the induction by CD40 alone at both 1 h and 12 h, and there remained a significant difference between Il23a induction by CD40 stimulation alone and that by CD40 and EP4 stimulation together at 12 h. These results indicate that, although CREB is involved in the induction, it is used mostly downstream of CD40 stimulation and the EP4-cAMP-PKA pathway amplifies the induction independent of CREB, at least in the late phase. Consistently, ChIP analysis revealed significant enrichment of CREB binding to the known CRE site (−982 to −972 bp) in the Il23a promoter only after CD40 stimulation at 12 h (Figure 4d). We did not obtain a significant level of CREB binding to these sites in the cells stimulated for 15 min, probably because of weak stimulation for this short time period.
Figure 4.
Transcription factor CREB is involved in Il23a induction in BMDC. (a) Increase of the amount of the phosphorylated form of CREB at Ser133 residue in BMDCs stimulated with an anti-CD40 antibody, LPS, TNF-α, or the EP4 agonist alone and in combination with the EP4 agonist. BMDCs were stimulated with an anti-CD40 antibody (10 μg mL−1), LPS (1 μg mL−1), TNF-α (100 ng mL−1), or the EP4 agonist (ONO-AE1-329, 1 μM) for indicated periods and total cell lysates were prepared for Western blot analysis for the phosphorylated form of CREB (Ser133). α-tubulin was used as a loading control. Representative images from three independent experiments were shown. (b, c) Effect of the depletion of the Creb1 gene by RNAi on the induction of Il23a mRNA in BMDCs stimulated with an anti-CD40 antibody (10 μg mL−1) and the EP4 agonist (ONO-AE1-329, 100 nM) for 1 h (b) or 12 h (c) examined by quantitative RT-PCR analysis. n = 3 in each group. **p < 0.01, ***p < 0.001 between groups as indicated. All bars indicate the SEM. (d) Increase of CREB binding on the promoter region of the Il23a gene in BMDCs stimulated with an anti-CD40 antibody by ChIP assay. BMDCs were stimulated with an anti-CD40 antibody (10 μg mL−1), the EP4 agonist (ONO-AE1-329, 1 μM), or both for 12 h and CREB bound to the promoter region of the Il23a gene was immunoprecipitated. Relative enrichment of chromatin fragment immunoprecipitated with anti-CREB antibody was examined by quantitative RT-PCR analysis using immunoprecipitated chromatin by IgG isotype as a control. n = 3 in each group. **p < 0.01, ***p < 0.001 between groups as indicated. All bars indicate mean ± SEM.
Impaired induction of IL-23 by PGE2-EP2 signaling in BMDCs
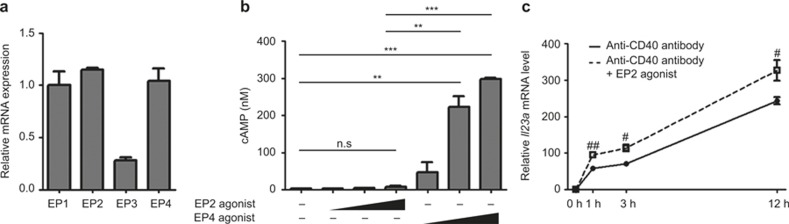
One puzzling question in this study involved impaired Il23a induction by EP2 stimulation (Figure 1a) because EP2 is a Gs-coupled receptor-like EP4, which increases the intracellular cAMP level as a second messenger.22 We examined the expression of each PGE receptor subtype mRNA in BMDCs by quantitative RT-PCR analysis and found that Ptger2 (EP2) and Ptger4 (EP4) mRNAs were expressed in similar levels in these cells (Figure 5a). However, stimulation of these cells with an EP2 agonist, ONO-AE1-259, increased the intracellular cAMP content marginally compared to that achieved by the EP4 agonist, ONO-AE1-329 (Figure 5b). Consistently, ONO-AE1-259 augmented the CD40-stimulated Il23a mRNA expression significantly but only marginally (Figure 5c). Together, these results suggest that EP2 is expressed on BMDCs but is not functionally coupled to cAMP through an unknown mechanism.
Figure 5.
Expression and function of EP2 in BMDCs. (a) Expression of EPs in BMDCs. RNA was prepared from unstimulated BMDCs, and Ptger1 (EP1), Ptger2 (EP2), Ptger3 (EP3), and Ptger4 (EP4) mRNA were measured by quantitative RT-PCR analysis. All bars indicate mean ± SEM. n = 3 in each group. (b) cAMP response to EP2 and EP4 stimulation in BMDCs. BMDCs were treated with 10 nM, 100 nM, and 500 nM of either ONO-AE1-259, an EP2 agonist or ONO-AE1-329, an EP4 agonist, for 10 min and cell lysates were prepared. cAMP was measured by EIA. n = 3 in each group. **p < 0.01, ***p < 0.001 between groups as indicated. n.s. = statistically not significant. All bars indicate mean ± SEM. (c) Little enhancement of CD40-induced Il23a mRNA expression by EP2 stimulation. BMDCs were treated with 10 μg mL−1 of an anti-CD40 antibody with or without 500 nM ONO-AE1-259, an EP2 agonist for indicated time, and Il23a mRNA was measured by quantitative RT-PCR analysis. n = 3 in each group. #p < 0.05 or ##p < 0.01, compared to the anti-CD40 antibody-treated groups. All bars indicate mean ± SEM.
Discussion
We used BMDCs and characterized IL-23 p19 production by the stimulation of CD40 with an agonistic anti-CD40 antibody. We found that CD40 stimulation evoked biphasic and sustained induction of Il23a (p19) expression, with peaks at 1 and 12 to 24 h; significant induction remained at 36 h. In contrast, stimulation with LPS or TNF-α evoked only transient induction of Il23a expression with a peak at 1 h and returning to the basal level at 3 h, which corresponded to the early peak in the CD40-stimulated cells. Our analysis using BMDCs from NF-κB NFkb1 (p50)-deficient mice or mice subjected to p100 RNAi revealed that the early phase of induction was mediated by the p50-dependent canonical NF-κB pathway and the late phase of induction was mediated by the non-canonical RelB/p52 pathway. A previous study by Mise-Omata et al. showed that the canonical NF-κB pathway for p19 induction in macrophages was mediated by both p65/RelA and c-Rel in an interdependent manner.23 Our finding of phosphorylation and nuclear translocation of p65 supports its involvement, and our study does not exclude the involvement of c-Rel, which was shown to be essential in TLR-induced p19 expression in BMDCs.24 Thus, the sustained Il23a induction in CD40-stimulated BMDCs is apparently supported by switching from a canonical to a non-canonical NF-κB pathway during stimulation. Intriguingly, PGE2 enhanced both phases of the induction in CD40-stimulated cells through EP4, thus amplifying the production of Il23a mRNA up to ten-fold. This effect of EP4 is through the cAMP-PKA pathway in both phases because it was reproduced by db-cAMP and PKA agonists and inhibited by H-89. Interestingly, although phosphorylation of CREB was observed in BMDCs at 15 min and 12 h after stimulation, the PKA pathway contributed mainly to the early phosphorylation, CD40 stimulation contributed to phosphorylation at both early and late phases, and CREB binding to the known CRE site in the Il23a promoter was enhanced only after the CD40 stimulation at 12 h. Thus, the cAMP-PKA pathway appears to amplify the CD40-induced Il23a expression not through CREB but through an unidentified mechanism. Whatever this mechanism is, EP4 mobilizes only the PKA pathway to amplify Il23a induction. It is different than the recent report by the Ganea's group, which found in their BMDCs that PGE2 amplifies LPS-induced Il23a expression by mobilizing both cAMP-PKA-CREB and cAMP-Epac-C/EBPβ pathways.25 Another difference between the studies is that Ganea's group consistently found that the PGE2 action was exerted through both EP2 and EP4 receptors in their BMDCs, whereas our BMDCs utilize only EP4. As shown in the RESULTS section, our BMDCs expressed EP2 mRNA at a level comparable to EP4 but did not respond to an EP2 agonist that showed potent actions in T cells in an EP2-dependent manner.26 This is consistent with our previous finding that only the EP4 agonist and not the EP1, EP2, and EP3 agonists amplified CD40-induced Il23a expression in sDCs from C57BL/6.18 At present, we cannot provide an adequate explanation for this discrepancy. One possibility is that the EP2 receptor was desensitized in our BMDCs. This may have been because of mouse strain or preparation procedures; we used C57BL/6 mice as our source and Ganea's group used B10.A mice.25 Previously, Nishigaki et al. found different sensitivities of EP2 and EP4 to PGE2-induced desensitization.27 Although that paper reported that EP4 undergoes desensitization more quickly than does EP2, which was the opposite of our finding, it showed that different sensitivities to desensitization do occur between EP2 and EP4. Further, Poloso et al. found that in monocyte-derived DCs of humans low concentrations (0.1 nM–10 nM) of PGE2 promoted IL-23 production via EP4, while higher concentrations (>50 nM) of PGE2 suppressed IL-23 via EP2.28 Kalim and Groettrup reported that PGE2 blocks the production of IL-23 in LPS-stimulated monocytes in a cAMP-dependent manner through down-regulation of the p40 subunit of IL-23, while it has the opposite effect in DCs.29 Thus, the effect of PGE2 and the action of EP receptors could differ depending on cell types.
In the present study, we used an anti-CD40 antibody to induce Il23a expression in DCs. CD40 is a co-stimulatory receptor expressed on antigen-presenting cells, including DCs,30 and its binding to CD40L expressed on the surface of CD4+ T cells facilitates activation and maturation of T cells. Reciprocally, the binding of CD40L on T cells to CD40 on DCs facilitates activation and maturation of DCs, for example, by increasing the expression of CD58, CD80, CD86, and MHC-II.31,32 This bidirectional signaling appears to make a positive feedback cycle because CD40L is induced both transcriptionally and by mRNA stabilization with T-cell activation. Here we found that induction of the PG-synthesizing enzyme, COX-2, in BMDCs stimulated with an anti-CD40 antibody was boosted by the addition of the EP4 agonist. This result suggests that this COX-2 induction might form another positive feedback cycle for Il23a induction. Although we did not verify this hypothesis in the present study, which is probably due to the low and transient COX-2 induction, we found that the addition of indomethacin significantly diminished Il23a induction by CD40 stimulation in sDCs.18 Given that IL-23 does not induce differentiation of naïve T cells from Th17 cells but acts on primed Th17 cells to stabilize and expand this T-cell subset, induction of Il23a in BMDCs by CD40 ligation is likely to occur in a physiological setting of Th17 expansion. In this context, PGE2 production by DCs, if it occurs, operates not only to amplify the IL-23 production by DCs but also to amplify the IL-23 action on Th17 cells. We previously found that PGE2 facilitates the IL-23-induced Th17 expansion in an EP2/EP4-dependent manner.18
The present study shows that PGE2-EP4 signaling strongly amplifies the production of IL-23 by DCs and suggests that the inhibition of this pathway might be therapeutically beneficial in IL-23–IL-17 axis-driven inflammatory immune diseases such as psoriasis, and could be involved in immune diseases in which this axis plays an important role, such as multiple sclerosis, rheumatoid arthritis, and Crohn's disease. Indeed, we previously demonstrated that the treatment with an EP4 antagonist or EP4 deficiency effectively suppressed the development of EAE,33 an animal model of multiple sclerosis to which both Th1 and Th17 cells contribute, and ameliorated the transfer colitis. Other authors demonstrated the significant inhibition of the incidence of collagen-induced arthritis through the oral administration of an EP4 antagonist.34 Consistently, recent genome-wide association studies (GWAS) identified the Ptger4 locus, the human EP4 gene, as one of the disease susceptibility loci in immune diseases, including Crohn's disease,35 multiple sclerosis,36 and allergies.37 Furthermore, a recent study revealed that candidate causal single nucleotide polymorphism related to multiple sclerosis, Crohn's disease, allergies, and ulcerative colitis are enriched with acetylated H3K27-labled active enhancer regions at the Ptger4 locus and are well matched with expression of this gene as evidenced by the RNA sequence.38
Introduction of therapeutic antibodies such as secukinumab, ustekinumab, and tildrakizumab to IL-17 and the two chains of IL-23 have greatly improved therapeutic outcomes for patients suffering from IL-23–IL-17 axis-driven immune diseases such as psoriasis,6 rheumatoid arthritis,7 ankylosing spondylitis,8 and Crohn's disease.11 However, a high cost associated with these antibody treatments could be problematic. Here, we showed that PGE2-EP4 signaling strongly amplifies IL-23 production by DCs and that treatment with a selective EP4 antagonist shuts off this amplification. These results suggest that EP4 can be a potential therapeutic target and EP4 antagonists can be a cost-effective alternative to the above antibodies for these autoimmune diseases.
Acknowledgments
This work was supported by Core Research for Evolutional Science and Technology (CREST) from the Japan Science and Technology Agency (JST) (Shuh Narumiya). We thank Ono Pharmaceutical Co., Ltd., for EP agonists and antagonists, N. Asamoto for technical assistance, and T. Arai and A. Washimi for secretarial assistance. Tomohiro Aoki and Shuh Narumiya were supported by Coordination Fund from JST and Astellas Pharma Inc.
Footnotes
Supplementary Information of this article can be found on Cellular & Molecular Immunology website: http://www.nature.com/cmi.
Supplementary Information
References
- Langrish CL, Chen Y, Blumenschein WM, Mattson J, Basham B, Sedgwick JD et al. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med 2005; 201: 233–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton CE, Lalor SJ, Sweeney CM, Brereton CF, Lavelle EC, Mills KH. Interleukin-1 and IL-23 induce innate IL-17 production from γδ T cells, amplifying Th17 responses and autoimmunity. Immunity 2009; 31: 331–341. [DOI] [PubMed] [Google Scholar]
- Spits H, Cupedo T. Innate lymphoid cells: emerging insights in development, lineage relationships, and function. Annu Rev Immunol 2012; 30: 647–675. [DOI] [PubMed] [Google Scholar]
- Spits H, Artis D, Colonna M, Diefenbach A, Di Santo JP, Eberl G et al. Innate lymphoid cells – a proposal for uniform nomenclature. Nat Rev Immunol 2013; 13: 145–149. [DOI] [PubMed] [Google Scholar]
- Tait Wojno ED, Artis D. Innate lymphoid cells: balancing immunity, inflammation, and tissue repair in the intestine. Cell Host Microbe 2012; 12: 445–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt J, Rosumeck S, Thomaschewski G, Sporbeck B, Haufe E, Nast A. Efficacy and safety of systemic treatments for moderate-to-severe psoriasis: meta-analysis of randomized controlled trials. Br J Dermatol 2013; 170: 274–303. [DOI] [PubMed] [Google Scholar]
- Genovese MC, Durez P, Richards HB, Supronik J, Dokoupilova E, Mazurov V et al. Efficacy and safety of secukinumab in patients with rheumatoid arthritis: a phase II, dose-finding, double-blind, randomised, placebo controlled study. Ann Rheum Dis 2013; 72: 863–869. [DOI] [PubMed] [Google Scholar]
- Baeten D, Baraliakos X, Braun J, Sieper J, Emery P, van der Heijde D et al. Anti-interleukin-17A monoclonal antibody secukinumab in treatment of ankylosing spondylitis: a randomised, double-blind, placebo-controlled trial. Lancet 2013; 382: 1705–1713. [DOI] [PubMed] [Google Scholar]
- Leonardi CL, Kimball AB, Papp KA, Yeilding N, Guzzo C, Wang Y et al. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 76-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 1). Lancet 2008; 371: 1665–1674. [DOI] [PubMed] [Google Scholar]
- Papp KA, Langley RG, Lebwohl M, Krueger GG, Szapary P, Yeilding N et al. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 52-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 2). Lancet 2008; 371: 1675–1684. [DOI] [PubMed] [Google Scholar]
- Sandborn WJ, Gasink C, Gao LL, Blank MA, Johanns J, Guzzo C et al. Ustekinumab induction and maintenance therapy in refractory Crohn's disease. N Engl J Med 2012; 367: 1519–1528. [DOI] [PubMed] [Google Scholar]
- Kopp T, Riedl E, Bangert C, Bowman EP, Greisenegger E, Horowitz A et al. Clinical improvement in psoriasis with specific targeting of interleukin-23. Nature 2015; 521: 222–226. [DOI] [PubMed] [Google Scholar]
- Oppmann B, Lesley R, Blom B, Timans JC, Xu Y, Hunte B et al. Novel p19 protein engages IL-12p40 to form a cytokine, IL-23, with biological activities similar as well as distinct from IL-12. Immunity 2000; 13: 715–725. [DOI] [PubMed] [Google Scholar]
- Tilley SL, Coffman TM, Koller BH. Mixed messages: modulation of inflammation and immune responses by prostaglandins and thromboxanes. J Clin Invest 2001; 108: 15–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narumiya S, Furuyashiki T. Fever, inflammation, pain and beyond: prostanoid receptor research during these 25 years. FASEB J 2011; 25: 813–818. [DOI] [PubMed] [Google Scholar]
- Hirata T, Narumiya S. Prostanoid receptors. Chem Rev 2011; 111: 6209–6230. [DOI] [PubMed] [Google Scholar]
- Sheibanie AF, Tadmori I, Jing H, Vassiliou E, Ganea D. Prostaglandin E2 induces IL-23 production in bone marrow-derived dendritic cells. FASEB J 2004; 18: 1318–1320. [DOI] [PubMed] [Google Scholar]
- Yao C, Sakata D, Esaki Y, Li Y, Matsuoka T, Kuroiwa K et al. Prostaglandin E2-EP4 signaling promotes immune inflammation through Th1 cell differentiation and Th17 cell expansion. Nat Med 2009; 15: 633–640. [DOI] [PubMed] [Google Scholar]
- Inaba K, Inaba M, Romani N, Aya H, Deguchi M, Ikehara S et al. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J Exp Med 1992; 176: 1693–1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian X, Ning H, Zhang J, Hoft DF, Stumpo DJ, Blackshear PJ et al. Posttranscriptional regulation of IL-23 expression by IFN-γ through tristetraprolin. J Immunol 2011; 186: 6454–6464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Z, Jin R, Yu S, Rivet JJ, Smyth SS, Nanda A et al. CD40 is essential in the upregulation of TRAF proteins and NF-κB-dependent proinflammatory gene expression after arterial injury. PLoS One 2011; 6: e23239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimoto Y, Narumiya S. Prostaglandin E receptors. J Biol Chem 2007; 282: 11613–11617. [DOI] [PubMed] [Google Scholar]
- Mise-Omata S, Kuroda E, Niikura J, Yamashita U, Obata Y, Doi TS. A proximal κB site in the IL-23 p19 promoter is responsible for RelA- and c-Rel-dependent transcription. J Immunol 2007; 179: 6596–6603. [DOI] [PubMed] [Google Scholar]
- Carmody RJ, Ruan Q, Liou HC, Chen YH. Essential roles of c-Rel in TLR-induced IL-23 p19 gene expression in dendritic cells. J Immunol 2007; 178: 186–191. [DOI] [PubMed] [Google Scholar]
- Kocieda VP, Adhikary S, Emig F, Yen JH, Toscano MG, Ganea D. Prostaglandin E2-induced IL-23p19 subunit is regulated by cAMP-responsive element-binding protein and C/AATT enhancer-binding protein beta in bone marrow-derived dendritic cells. J Biol Chem 2012; 287: 36922–36935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao C, Hirata T, Soontrapa K, Ma X, Takemori H, Narumiya S. Prostaglandin E2 promotes Th1 differentiation via synergistic amplification of IL-12 signalling by cAMP and PI3-kinase. Nat Commun 2013; 4: 1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishigaki N, Negishi M, Ichikawa A. Two Gs-coupled prostaglandin E receptor subtypes, EP2 and EP4, differ in desensitization and sensitivity to the metabolic inactivation of the agonist. Mol Pharmacol 1996; 50: 1031–1037. [PubMed] [Google Scholar]
- Poloso NJ, Urquhart P, Nicolaou A, Wang J, Woodward DF. PGE2 differentially regulates monocyte-derived dendritic cell cytokine responses depending on receptor usage (EP2/EP4). Mol Immunol 2013; 54: 284–295. [DOI] [PubMed] [Google Scholar]
- Kalim KW, Groettrup M. Prostaglandin E2 inhibits IL-23 and IL-12 production by human monocytes through down-regulation of their common p40 subunit. Mol Immunol 2013; 53: 274–282. [DOI] [PubMed] [Google Scholar]
- Mackey MF, Barth RJ, Jr., Noelle RJ. The role of CD40/CD154 interactions in the priming, differentiation, and effector function of helper and cytotoxic T cells. J Leukoc Biol 1998; 63: 418–428. [DOI] [PubMed] [Google Scholar]
- Caux C, Massacrier C, Vanbervliet B, Dubois B, Van Kooten C, Durand I et al. Activation of human dendritic cells through CD40 cross-linking. J Exp Med 1994; 180: 1263–1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallusto F, Lanzavecchia A. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alpha. J Exp Med 1994; 179: 1109–1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esaki Y, Li Y, Sakata D, Yao C, Segi-Nishida E, Matsuoka T et al. Dual roles of PGE2-EP4 signaling in mouse experimental autoimmune encephalomyelitis. Proc Natl Acad Sci USA 2010; 107: 12233–12238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, Muramoto K, Masaaki N, Ding Y, Yang H, Mackey M et al. A novel antagonist of the prostaglandin E2 EP4 receptor inhibits Th1 differentiation and Th17 expansion and is orally active in arthritis models. Br J Pharmacol 2010; 160: 292–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glas J, Seiderer J, Czamara D, Pasciuto G, Diegelmann J, Wetzke M et al. PTGER4 expression-modulating polymorphisms in the 5p13.1 region predispose to Crohn's disease and affect NF-κB and XBP1 binding sites. PLoS One 2012; 7: e52873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- International Multiple Sclerosis Genetics C, Wellcome Trust Case Control C, Sawcer S, Hellenthal G, Pirinen M, Spencer CC et al. Genetic risk and a primary role for cell-mediated immune mechanisms in multiple sclerosis. Nature 2011; 476: 214–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinds DA, McMahon G, Kiefer AK, Do CB, Eriksson N, Evans DM et al. A genome-wide association meta-analysis of self-reported allergy identifies shared and allergy-specific susceptibility loci. Nat Genet 2013; 45: 907–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farh KK, Marson A, Zhu J, Kleinewietfeld M, Housley WJ, Beik S et al. Genetic and epigenetic fine mapping of causal autoimmune disease variants. Nature 2015; 518: 337–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.