Abstract
N-Acetylglucosaminyltransferase III (GnT-III), which catalyzes the addition of the bisecting GlcNAc branch on N-glycans, is usually described as a metastasis suppressor. Overexpression of GnT-III inhibited migration in multiple types of tumor cells. However, these results seem controversial to the clinical observations for the increased expression of GnT-III in human hepatomas, glioma, and ovarian cancers. Here, we present evidence that these inconsistencies are mainly attributed to the different expression pattern of cell sialylation. In detail, we show that overexpression of GnT-III significantly inhibits α2,3-sialylation but not α2,6-sialylation. The migratory ability of cells without or with a low level of α2,6-sialylation is consistently suppressed after GnT-III overexpression. In contrast, the effects of GnT-III overexpression are variable in tumor cells that are highly α2,6-sialylated. Overexpression of GnT-III promotes the cell migration in glioma cells U-251 and hepatoma cells HepG2, although it has little influence in human breast cancer cell MDA-MB-231 and gastric cancer cell MKN-45. Interestingly, up-regulation of α2,6-sialylation by overexpressing β-galactoside α2,6-sialyltranferase 1 in the α2,6-hyposialylated HeLa-S3 cells abolishes the anti-migratory effects of GnT-III. Conversely, depletion of α2,6-sialylation by knock-out of β-galactoside α2,6-sialyltranferase 1 in α2,6-hypersialylated HepG2 cells endows GnT-III with the anti-migratory ability. Taken together, our data clearly demonstrate that high expression of α2,6-sialylation on the cell surface could affect the anti-migratory role of GnT-III, which provides an insight into the mechanistic roles of GnT-III in tumor metastasis.
Keywords: cell migration, glycosyltransferase, integrin, N-linked glycosylation, sialyltransferase, bisecting GlcNAc
Introduction
Alterations in N-linked glycosylation have been observed in various malignant diseases such as cancer. Certain glycan structures resulting from the dysregulated glycosyltransferases and glycosidases are well known tumor markers and closely associated with the malignant progression (1). As examples, the α1,6-fucosylated α-fetoprotein catalyzed by α1,6-fucosyltransferase has been developed as a biomarker for primary hepatoma by Abelev (2). An increase in β1,6-GlcNAc branching of N-glycans as a consequence of enhanced expression of N-acetylglucosaminyltransferase V (GnT-V)2 is convincingly correlated with the increased frequency of tumor metastasis (3–6).N-Acetylglucosaminyltransferase III (GnT-III) is one more important glycosyltransferase that has been receiving much attention for its involvement in the biology of tumor (7, 8). It catalyzes the transfer of N-acetylglucosamine (GlcNAc) in a β1,4 linkage to mannose on N-glycans forming a bisecting GlcNAc structure. This step plays critical roles in determining the structure of N-glycans because introduction of the bisecting GlcNAc residue could preclude further processing and elongation of N-glycans, such as the formation of β1,6-GlcNAc branching structures (9). Considering also that overexpression of GnT-III inhibited the metastasis of multiple types of carcinomas (10, 11) and decreased expression of GnT-III was observed in the renal cancer (12), GnT-III has been usually regarded as a tumor metastasis suppressor (1, 13, 14). However, in fact, there also exist some inconsistent results. It has been reported for decades that GnT-III expression was enhanced more than 100 times in human hepatomas (15). Also, recently, the expression of GnT-III, as well as α2,6-galactoside sialylatransferase 1 (ST6GAL1), which primarily catalyzes α2,6-linked sialic acids on N-glycans, was found up-regulated in the ovarian cancer (16), and an ovarian cancer cell line, which carries an abundance of bisecting GlcNAc glycans, displays highly metastatic property (17). These results indicate that there may exist one or several independent factors that overwhelm the effects of GnT-III and affect tumor metastasis.
Sialic acid is the most abundant terminal monosaccharide of glycoconjugates and known to be linked via an α2,3 or α2,6 bond to Gal/GalNAc or an α2,8 bond to sialic acid in proteins through a group of sialyltransferases. Altered sialylation has been reported in almost all types of carcinomas and has long been associated with metastatic cell behaviors (18–22). Recently, accumulating information is obtained on the molecular details of how distinct sialylated structures or sialylated carrier proteins regulate cell signaling to control responses such as cell adhesion and migration (1, 23). In contrast, there is limited knowledge regarding the regulatory mechanisms of sialylation on the cell surface. Several reports have indicated that some transcription factors were involved in the dysregulated expression of some sialyltransferases and neuraminidases (24–28). However, the altered transcription of these enzymes in tumor cells could not explain all the changes in sialylation. Given the outermost position of sialic acids on glycans and the branch specificities of different sialyltransferases, the amount and distribution of sialylation on the cell surface are also supposed to be affected by the altered expression of other glycosyltransferases responsible for branch formation in the N-glycan biosynthetic pathway. Nevertheless, the mechanisms in this respect are poorly understood.
Given the important role of GnT-III in N-glycan biosynthesis and tumor cell behaviors, we considered there exists an interplay between GnT-III and sialyltransferases in the expression of their enzymatic products and also their functions in tumor metastasis. In this work, we present evidence that overexpression of GnT-III in different tumor cells significantly inhibits α2,3-sialylation, but not α2,6-sialylation. Further, GnT-III exerts anti-migratory functions only in cells without or with a low level of α2,6-sialylation. Knock-out of ST6GAL1 in the α2,6-hypersialylated HepG2 cells endows GnT-III with the anti-migratory role. Together, these findings implicate GnT-III as a tumor migration suppressor under an α2,6-hyposialylated context, but this role could be impaired by high expression of ST6GAL1, which provides a plausible explanation for the increased expression of GnT-III during tumor progression.
Experimental Procedures
Cell Lines and Cell Culture
The 293T and HeLa S3 cell lines were provided by the RIKEN Cell Bank (Tsukuba, Japan). The MDA-MB-231 cell line was purchased from ATCC. The CHO-K1 and the U-251MG cell lines were gifted by Dr. Hideyoshi Higashi (Tohoku Pharmaceutical University, Sendai, Japan) and Prof. Jun Nakayama (Graduate School of Medicine, Shinshu University, Matsumoto, Japan), respectively. All cell lines above, as well as human hepatoma HepG2 cells, were maintained in high glucose DMEM with 2 mm l-glutamine and 10% FBS, for CHO-K1 cells also with nonessential amino acids. Two other previously established MKN-45 cell lines transfected with GnT-III-expressing or mock vectors were cultured at 37 °C in RPMI 1640 medium with 10% FBS (9). Pro-5 cells were gifts from Dr. Kitazume Shinobu (RIKEN) and cultured in α-MEM with 10% FBS. All cells were cultured under a humidified atmosphere containing 5% CO2 at 37 °C.
Western Blot Analysis and Immunoprecipitation
Cells were washed with PBS and then lysed with lysis buffer (10 mm Tris-HCl, 1% Triton X-100, 150 mm NaCl). Insoluble materials were removed by centrifugation at 15,000 rpm for 10 min at 4 °C. Equal amounts of protein were separated using 7.5% SDS-PAGE, transferred to PVDF, and probed with the appropriate antibodies as indicated or with biotinylated erythro-agglutinating phytohemagglutinin (E4-PHA), biotinylated Sambucus nigra lectin (SNA), Maackia amurensis agglutinin (MAA), Datura stramonium agglutinin (DSA), and concanavalin A (ConA) lectins (Seikagaku Kogyo Inc., Tokyo, Japan). Immunoreactive bands were visualized using a Vectastain ABC kit (Vector Laboratories) and an ECL kit (Amersham Biosciences). Monoclonal antibodies against α3 integrin, αv integrin, and β1 integrin were purchased from BD Biosciences, and the anti-α-tubulin antibody was from Sigma. For immunoprecipitation, the supernatant (2 mg of protein) was incubated for 1 h at 4 °C with ConA-agarose or MAM (M. amurensis) agarose or Sambucus sieboldiana agarose (J-Oil Mill). After washing three times with lysis buffer, the immunoprecipitates were subjected to 7.5% SDS-PAGE, and the separated proteins were transferred to a nitrocellulose membrane. The membrane was incubated with a lectin for lectin blot analysis or with an antibody for immunoblot analysis. Immunoblotting was carried out as described previously (29). Proteins were separated by SDS-PAGE. Samples were incubated at 4 °C overnight with appropriate primary antibodies. Proteins were visualized by chemiluminescence (ECL, Pierce Biotechnology or Immobilon Western, Millipore).
The Establishment of GnT-III-overexpressing Cell Lines
For the GnT-III overexpression in tumor cells used in the present study, we utilized the doxycycline (DOX)-inducible shRNA expression system (Invitrogen) as previously described (30). Briefly, human GnT-III gene was inserted into the Gateway Entry vectors pENTR-d-Topo (31). GnT-III gene on the resultant plasmid was subsequently introduced into the overexpression vector CSIV-TRE-RfA-CMV-KT we constructed previously by the Gateway Conversion System (Invitrogen) (30). The obtained lentiviral vectors were transfected into 293T cells with packaging plasmids by the calcium phosphate for the preparation of viruses. The obtained viruses were then incubated with different cell lines for 72 h. The infected cells were selected by the Kusabira Orange marker using FACS Aria II (BD Bioscience). The expression of GnT-III was induced by addition of 1 μg/ml DOX in the established cell line, and the cells cultured under DOX-free medium were used as the control in the present study.
Construction of ST6GAL1 Knock-out Clones
The sgRNAs (pSpCas9-ST6GAL1-For, CACCGATGATCATGACGCAGTCCTG; and pSpCas9 ST6GAL1-Rev, AAACCAGGACTGCGTCATGATCATC) for ST6GAL1 knock-out were designed based on previously described design rules and inserted into the plasmid pX458 (32, 33). The obtained plasmid were confirmed by DNA sequencing and transformed into DH5α competent cells for amplification. The constructed plasmids were transfected into different tumor cells by Cell Line NucleofectorTM kits (Lonza). The transfected cells were then incubated for 72 h and selected by the GFP marker using FACS Aria II (BD Bioscience). Finally, further negative selection for the obtained cells by SNA lectin was performed by using FACS Aria II to remove the cells expressing α2,6-sialylation.
Flow Cytometry Analysis of Cells
Cells were grown to ∼90% confluency, detached using trypsin containing 1 mm EDTA at 37 °C, and washed three times with cold PBS. Then cells were stained with anti-β1 integrin antibody (P5D2) or 10 μg/ml biotinylated SNA or MAA for 30 min on ice, followed by incubation with streptavidin conjugate Alexa Fluor 647 (Invitrogen) for 30 min on ice. Finally, cells were washed three times with PBS and analyzed by flow cytometry (BD Biosciences).
RT-PCR for mRNA Expression Analysis
Total RNA was prepared with TRI reagent (Invitrogen), and 1 μg of total RNA was reverse-transcribed using a PrimeScript RT reagent kit with a gDNA Eraser (Takara) according to the manufacturer's instructions. The sequences of the primers used for the PCR amplification were designed previously (29). The GAPDH mRNA was used as a control in PCR runs, and the reaction products obtained were subjected to electrophoresis using 2% agarose gels containing ethidium bromide.
Cell Migration Assay
Cell migration was examined with Transwells (BD BioCoatTM control inserts, 8.0-mm inserts; BD Biosciences) as described previously (31). Transwells were coated only on the bottom side with 10 μg/ml fibronectin (FN) at 4 °C overnight. Cells cultured for 4 days were starved in serum-free medium for 12 h, trypsinized, and suspended with 0.5 mg/ml trypsin inhibitor (Nacalai Tesque) in DMEM. The suspended cells were centrifuged, and the supernatants were removed. The resulted cell pellets were resuspended with assay medium (serum-free DMEM) and diluted to 1 × 105 cells/ml. To each FN-coated Transwell, 500-μl aliquots of the cell suspension were added; the cells were then incubated at 37 °C for appropriate time. After incubation, cells on the upper side were removed by scraping with a cotton swab. The membranes in the Transwells were fixed with 4% paraformaldehyde and stained with 0.5% crystal violet for 1 h. The cells that had migrated to the lower side were counted using a phase contrast microscope.
Pyridiaminated (PA) Oligosaccharide Preparation and Glycosidase Digestion
Cells were harvested from three dishes (15 cm) of subconfluent cultures. The PA oligosaccharides purified were prepared as described previously (34–36), and the glycosidase digestion of the PA oligosaccharides was performed as previously indicated (37).
Reversed Phase HPLC for Oligosaccharide Structural Analysis
The prepared PA oligosaccharides were subjected to an HPLC system equipped with a TSK-gel 80TM column (4.6 × 150 mm; Tosoh). Elution was performed at a flow rate of 1 ml/min at 40 °C using 20 mm ammonium acetate buffer (pH 4.0) as solvent A and the same buffer containing 1% 1-butanol as solvent B. The column was pre-equilibrated with 10% solvents B, and after injection of a sample, the PA oligosaccharides were separated by a linear gradient of 10–25% of solvent B for 60 min. The eluted PA oligosaccharides were monitored by a fluorescence detector at excitation and emission wavelengths of 320 and 400 nm, respectively.
Quantitative Analysis of Sialylated Glycans by Anion Exchange HPLC
The prepared PA oligosaccharides treated with or without neuraminidases were subjected to an HPLC system equipped with a TSKgel DEAE-5PW column (7.5 × 75 mm; Tosoh) and analyzed as described previously (34).
Mass Spectrometry
Membrane fractions of cells were prepared as described previously (34–36). Pellets were dissolved in 8 m urea in PBS, and then the aliquots (300 μg of protein) were reduced in 20 mm DTT, followed by alkylation with 40 mm iodoacetamide. After dilution with distilled water to 2 m urea solution, 6 μg of trypsin from bovine pancreas (Wako Pure Chemical Industries) was added to the solution. The mixtures were incubated at 37 °C for 18 h and then dialyzed against distilled water by using a mini dialysis kit (1-kDa cutoff; GE Healthcare). After inactivation of the trypsin by heating at 95 °C for 5 min followed by lyophilization, the mixtures were dissolved in 50 mm ammonium hydrogen carbonate. Glycopeptidase F (1 milliunit; Takara Bio Inc.) was added to the solution, incubated at 37 °C for 20 h, and then lyophilized. Obtained residues were dissolved in distilled water (100 μl) and passed through cation exchange cartridge OASIS MCX (1 ml; Waters) and solid phase extraction cartridge SepPak C18 (1 ml; Waters), and then the cartridges were washed with 1 ml of distilled water. The pass-through fraction and washings were combined and lyophilized. For α2,3-neuraminidase digestion, the lyophilized residues were dissolved in 40 μl of 50 mm sodium phosphate buffer (pH 6.0). α2,3-Neuraminidases from Streptococcus pneumoniae (2 μl; Sigma) was added to the solution and incubated at 37 °C for 20 h, then passed through solid phase extraction cartridge SepPak C18 (1 ml, Waters), and lyophilized. Obtained residues were permethylated as reported previously (37). Mass measurements were performed with a MALDI quadrupole ion trap TOF instrument (AXIMA-QIT; Shimadzu Corp.). All of the spectra were obtained using MS mode with high mass range (over m/z 2000) and were the result of signal averaging of 200 laser shots. For sample preparation, 0.5 μl of 2,5-dihydroxybenzoic acid solution (10 mg/ml in 30% ethanol) was deposited on the target plate and allowed to dry. Then 0.5 μl of analyte dissolved in 50% acetonitrile was used to cover the matrix on the target plate and allowed to dry. Finally, the dried materials were recrystallized by adding 0.15 μl of 99.5% ethanol to the matrix-analyte mixture on the target plate.
Statistical Analysis
Statistical analyses were performed using either an one-tail unpaired t test or one-way analysis of variance using GraphPad Prism5.
Results
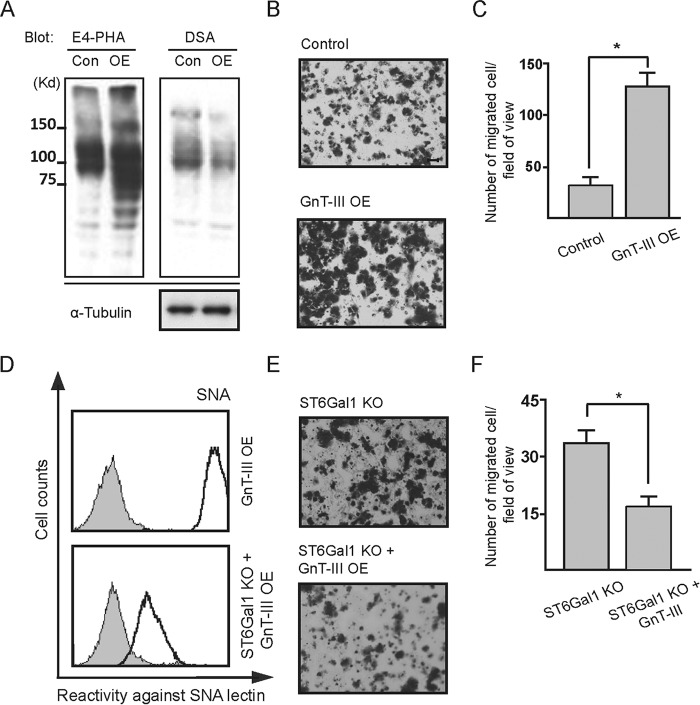
Overexpression of GnT-III Dramatically Inhibits α2,3-Sialylation, but Not α2,6-Sialylation via Post-transcriptional Mechanisms
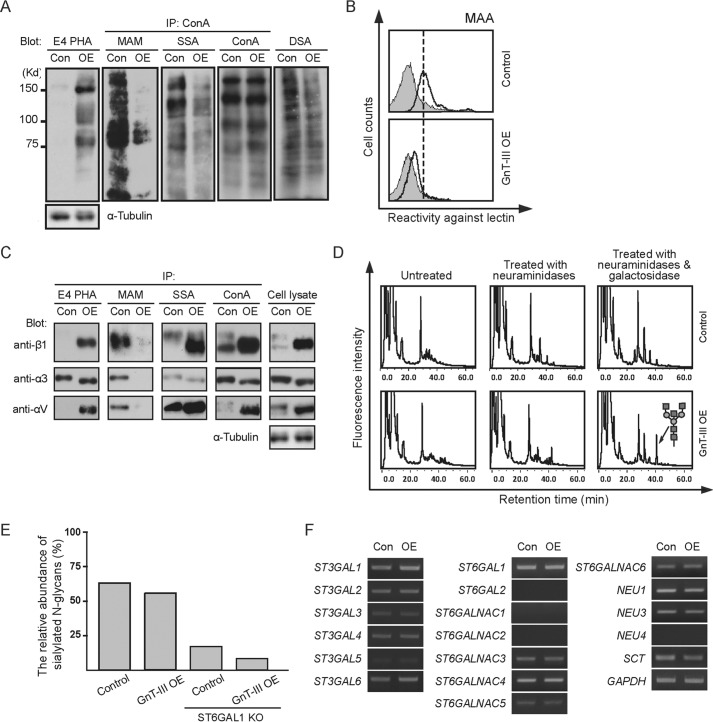
Alterations in early branch points of the normal N-glycan biosynthetic pathway can markedly affect the relative amount of one class of structure while allowing the dominance of another. One striking example is that the introduction of bisecting structure by overexpression of GnT-III leads to an inhibition of further processing and elongation of N-glycans (9). Considering the outmost position of sialic acids on N-Glycans, we investigated the effects of GnT-III on the cell surface sialylation by using the human breast cancer cell line MDA-MB-231. Reversed phase HPLC analysis, as well as lectin blot with E4-PHA lectin, which mainly recognizes the bisected N-glycans produced by GnT-III, showed efficient overexpression of this gene in MDA-MB-231 cells (Fig. 1, A and D). As expected, the binding of DSA (recognizing the multiantennary N-glycans) was clearly suppressed after GnT-III overexpression as compared with the control cells (Fig. 1A). To determine the effect of GnT-III overexpression on sialylation, we first used ConA-agarose to pull down N-glycans, followed by blotting with ConA, MAA, and SNA lectins. MAA and SNA lectins specifically recognize α2,3- and α2,6-sialylated glycans, respectively. As shown by the immunoprecipitation results, forced expression of GnT-III led to a dramatic reduction of α2,3-sialylation but not α2,6-sialylation (Fig. 1A). The decreased expression of α2,3-sialylation was further corroborated by FACS analysis (Fig. 1B). Consistently, similar changes in sialylation were also observed on specific membrane proteins including α3, αv, and β1 integrins (Fig. 1C). To analyze the effect of GnT-III on sialylation quantitatively, we next performed anion exchange HPLC by using the N-glycans treated with or without neuraminidases from the control and GnT-III-overexpressing cells. In contrast to a small decrease in the amount of sialylated N-glycans after GnT-III overexpression in MDA-MB-231 cells, a significant reduction was observed when we overexpressed GnT-III in ST6GAL1 knock-out cells (Fig. 1E). One explanation for these observations is that MDA-MB-231 cells majorly express α2,6-linked sialic acids because our data above have shown that GnT-III dramatically suppressed the α2,3-sialylation but not α2,6-sialylation. Consistent with this idea, our MS spectrometry analysis of N-glycans showed that α2,3-sialylated N-glycans were lowly expressed as no big difference in the peak pattern of MDA-MB-231 control cells was observed after the treatment with α2,3-neuraminidases (Fig. 2A and Table 1). Given that α2,3-sialyltransferases and α2,6-sialyltransferases share a common set of N-glycan substrates, MS spectrometry analysis of N-glycans (Fig. 2 and Table 1) was also performed to confirm the effects of the GnT-III on α2,3-sialylation using the ST6GAL1 knock-out cells with or without GnT-III overexpression. As expected, ST6GAL1 knock-out resulted in a significant increase in the glycans with α2,3-linked sialic acids as their corresponding peaks were decreased markedly after digestion with α2,3-neuraminidases (Fig. 2B). Further overexpression of GnT-III led to a clear enhancement in the glycans with bisecting GlcNAc structures (peaks of 7, 9, 12, and 14) but dramatically reduced the amount of α2,3-sialylated glycans (Fig. 2C). It is worth mentioning that α2,6-sialylation still could be detected in ST6GAL1 knock-out cells according to the MS spectrometry analysis, but it is largely decreased as compared with control cells. The α2,6-linked sialic acids in ST6GAL1 knock-out cells should be due to the low expression of ST6GAL2, which has also been reportedly involved in α2,6-sialylation on N-glycans (38). Notably, the sialic acids on bisected N-glycans were almost in an α2,6-linkage because treatment with α2,3-neuraminidases exerted little effect on the pattern of their corresponding peaks. All the results above suggest that GnT-III overexpression could suppress the α2,3-sialylation. Then we were wondering how GnT-III affected the sialylation. To address this question, RT-PCR analysis was performed. Little difference in the expression level of sialyltransferases and neuraminidases involved in N-glycan sialylation was observed after GnT-III overexpression, indicating that GnT-III regulates the sialylation at the post-transcriptional level (Fig. 1F).
FIGURE 1.
Overexpression of GnT-III in MDA-MB-231 cells significantly inhibited the α2,3-sialylation, but not α2,6-sialylation, at a post-transcriptional level. A, cell lysates from control and GnT-III transfected MDA-MB-231 cells were immunoblotted with E4-PHA and DSA lectins or immunoprecipitated (IP) with ConA-agarose and blotted with ConA, MAA, and SNA lectins. B, to confirm the effect of GnT-III expression on cell sialylation, cells transfected with or without GnT-III were incubated with (bold line) or without (gray shading) biotin-conjugated MAA (recognizing α2,3-sialylated proteins), followed by incubation with streptavidin Alexa Fluor 647 conjugate and subjected to FACS analysis. C, to determine the changes in the N-glycans on specific proteins after GnT-III overexpression, the cell lysates from control and GnT-III transfected MDA-MB-231 cells were also immunoprecipitated by ConA, E4-PHA, MAM (recognizing α2,3-sialylated proteins), and S. sieboldiana (recognizing α2,6-sialylated proteins) agaroses and probed with antibodies against α3 integrin, αv integrin, and β1 integrin separately. D, PA oligosaccharides treated with or without glycosidases from those cells were analyzed by reversed phase HPLC. E, PA oligosaccharides treated with or without neuraminidases from the control, GnT-III transfected MDA-MB-231 cells, as well as their ST6GAL1 knock-out counterparts, were analyzed by anion exchange HPLC to quantify the amount of sialylated N-glycans. F, RT-PCR using total RNA extracted from the control and GnT-III transfected MDA-MB-231 cells was carried out to examine the expression levels of genes involved in protein sialylation. The expression level of Gapdh was used as a loading control. SCT, sialic acid transporter; St3gal, β-galactoside α2,3-sialyltransferase; St6galnac, α-N-acetylgalactosaminide α2,6-sialyltransferase; NEU, neuraminidase. Con, control (DOX-inducible GnT-III-overexpressing cells without DOX treatment); OE, DOX inducible GnT-III-overexpressing cells treated with DOX; ST6GAL1 KO, ST6GAL1 knock-out cells.
FIGURE 2.
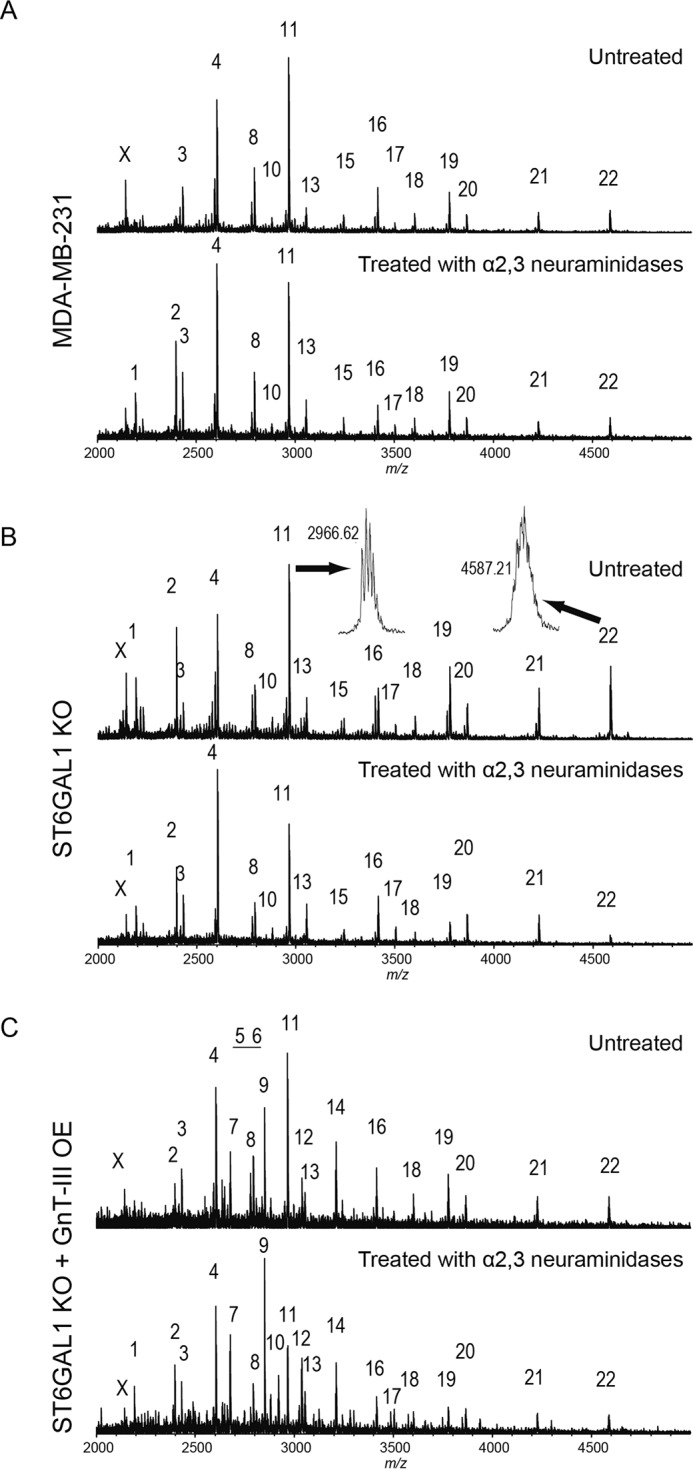
MALDI MS spectra of N-glycans of membrane fractions from MDA-MB-231 cells and their genetically modified cells. Each bottom panel shows the MS spectrum after α2,3-neuraminidase digestion. Observed glycan peaks are numbered and summarized in Table 1. The peaks indicated with X are assigned to in-source decay fragments of glycans. Representative examples of peak resolution are shown in B. A, MDA-MB-231 cells. B, ST6GAL1 knock-out MDA-MB-231 cells (ST6GAL1 KO). C, GnT-III-overexpressing ST6GAL1 knock-out MDA-MB-231 cells (ST6GAL1 KO + GnT-III OE).
TABLE 1.
Summary of peaks in the MS spectra
| Peak No. | Observed m/za | Calculated m/z | Monosaccharide composition |
|---|---|---|---|
| 1 | 2192.25 | 2192.08 | (Hex)5 + (Man)3(GlcNAc)2 |
| 2 | 2396.42 | 2396.18 | (Hex)6 + (Man)3(GlcNAc)2 |
| 3 | 2431.44 | 2431.21 | (Hex)2(HexNAc)2(NeuAc)1 + (Man)3(GlcNAc)2 |
| 4 | 2605.48 | 2605.30 | (Hex)2(HexNAc)2(Deoxyhexose)1(NeuAc)1 + (Man)3(GlcNAc)2 |
| 5 | 2635.49 | 2635.31 | (Hex)3(HexNAc)2(NeuAc)1 + (Man)3(GlcNAc)2 |
| 6 | 2646.60 | 2646.33 | (Hex)1(HexNAc)3(Deoxyhexose)1(NeuAc)1 + (Man)3(GlcNAc)2 |
| 7 | 2676.54 | 2676.34 | (Hex)2(HexNAc)3(NeuAc)1 + (Man)3(GlcNAc)2 |
| 8 | 2792.65 | 2792.38 | (Hex)2(HexNAc)2(NeuAc)2 + (Man)3(GlcNAc)2 |
| 9 | 2850.68 | 2850.43 | (Hex)2(HexNAc)3(Deoxyhexose)1(NeuAc)1 + (Man)3(GlcNAc)2 |
| 10 | 2880.62 | 2880.44 | (Hex)3(HexNAc)3(NeuAc)1 + (Man)3(GlcNAc)2 |
| 11 | 2966.62 | 2966.47 | (Hex)2(HexNAc)2(Deoxyhexose)1(NeuAc)2 + (Man)3(GlcNAc)2 |
| 12 | 3037.73 | 3037.51 | (Hex)2(HexNAc)3(NeuAc)2 + (Man)3(GlcNAc)2 |
| 13 | 3054.67 | 3054.53 | (Hex)3(HexNAc)3(Deoxyhexose)1(NeuAc)1 + (Man)3(GlcNAc)2 |
| 14 | 3211.79 | 3211.60 | (Hex)2(HexNAc)3(Deoxyhexose)1(NeuAc)2 + (Man)3(GlcNAc)2 |
| 15 | 3241.79 | 3241.61 | (Hex)3(HexNAc)3(NeuAc)2 + (Man)3(GlcNAc)2 |
| 16 | 3415.79 | 3415.70 | (Hex)3(HexNAc)3(Deoxyhexose)1(NeuAc)2 + (Man)3(GlcNAc)2 |
| 17 | 3503.77 | 3503.75 | (Hex)4(HexNAc)4(Deoxyhexose)1(NeuAc)1 + (Man)3(GlcNAc)2 |
| 18 | 3602.90 | 3602.78 | (Hex)3(HexNAc)3(NeuAc)3 + (Man)3(GlcNAc)2 |
| 19 | 3776.78 | 3776.87 | (Hex)3(HexNAc)3(Deoxyhexose)1(NeuAc)3 + (Man)3(GlcNAc)2 |
| 20 | 3864.90 | 3864.92 | (Hex)4(HexNAc)4(Deoxyhexose)1(NeuAc)2 + (Man)3(GlcNAc)2 |
| 21 | 4226.10 | 4226.10 | (Hex)4(HexNAc)4(Deoxyhexose)1(NeuAc)3 + (Man)3(GlcNAc)2 |
| 22 | 4587.21 | 4587.27 | (Hex)4(HexNAc)4(Deoxyhexose)1(NeuAc)4 + (Man)3(GlcNAc)2 |
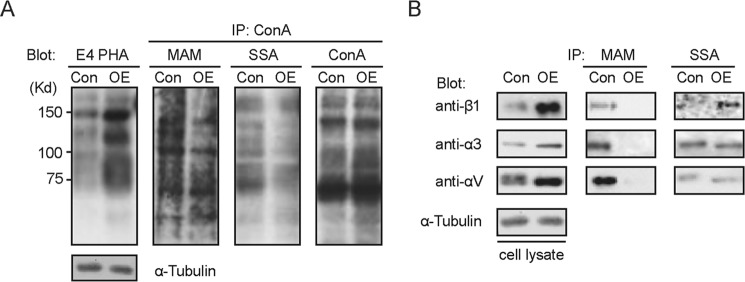
To decide whether GnT-III exerts similar effects in other cells, we replicated the experiments above in glioma cells U-251. Like in MDA-MB-231 cells, similar influences were observed in U-251 cells (Fig. 3, A and B). Taken together, the results above demonstrate that GnT-III is capable of inhibiting α2,3-sialylation, but not α2,6-sialylation.
FIGURE 3.
Overexpression of GnT-III in U-251 cells also led to a significant inhibition of the α2,3-sialylation but not α2,6-sialylation. A, cell lysates from the control and GnT-III-overexpressing U-251 cells were immunoblotted with E4-PHA lectins or immunoprecipitated (IP) with ConA-agarose and blotted with ConA, MAA, and SNA lectins. B, to determine the changes in the N-glycans on specific proteins after GnT-III overexpression, the cell lysates were also immunoprecipitated by MAM and S. sieboldiana agaroses and probed with antibodies against α3 integrin, αv integrin, and β1 integrin separately. Con, control; OE, GnT-III-overexpressing cells.
High Expression of ST6GAL1 Antagonizes the Anti-migratory Role of GnT-III in MDA-MB-231 Cells
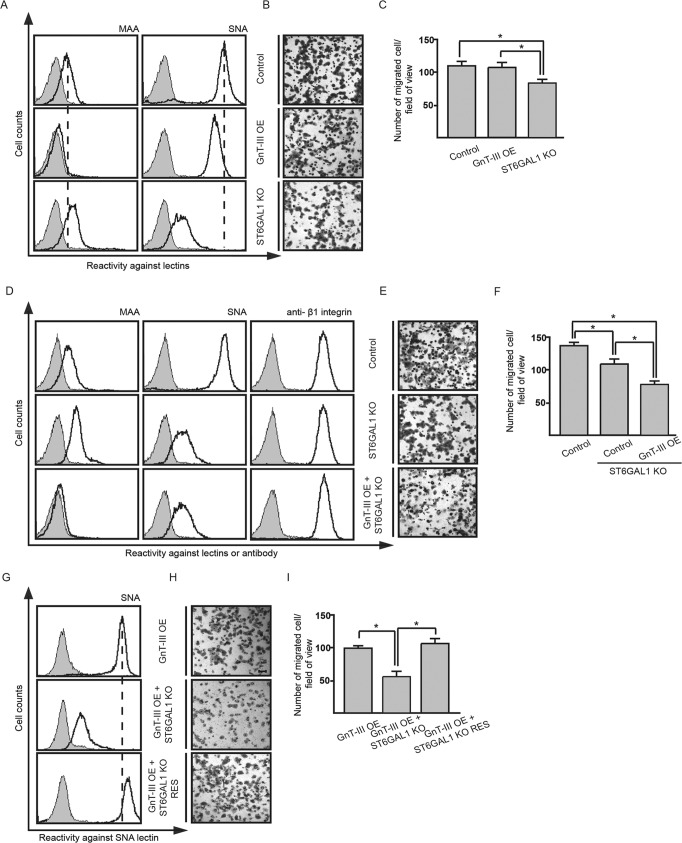
Next, to check the effect of GnT-III overexpression on cell migration in MDA-MB-231, Boyden chamber analysis was carried out. The control and GnT-III-overexpressing cells showed comparable migratory ability (Fig. 4, B and C). This is not so surprising because we have shown previously that overexpression of GnT-III had little effect on the cell migration in MKN-45 cells either, although GnT-III is usually considered as a tumor metastasis suppressor. One possibility for these different effects is that there exist one or several independent factors that neutralize or overwhelm the effects of GnT-III and affect tumor metastasis. Recently, several groups, including ours, showed that ST6GAL1-mediated α2,6 hypersialylation correlated with the increased cell migration of multiple tumor cells (30, 39, 40). Considering also our data above that GnT-III had little effect on α2,6-sialylation, we hypothesized that the terminal α2,6-linked sialic acids on N-glycans might affect the role of bisected GlcNAc in tumor metastasis. To test this idea, ST6GAL1 was knocked out in MDA-MB-231 cells, which was confirmed by FACS analysis using SNA (Fig. 4D). ST6GAL1 knock-out clearly inhibited cell migration as compared with parent cells (Fig. 4, E and F), which is consistent with the effects of ST6GAL1 knockdown we reported previously (29). Overexpression of GnT-III in this knock-out cells led to a further decrease in cell motility (Fig. 4, D–F). To exclude the possibility that this influence is due to the off target effects of designed sequence for ST6GAL1 knock-out, rescue experiments were performed by overexpressing ST6GAL1 in GnT-III-overexpressing ST6GAL1 knock-out cells (Fig. 4G). After overexpression of ST6GAL1, little difference in cell migration was observed (Fig. 4, H and I). Taken together, the results clearly indicate that high expression of α2,6-linked sialic acids may affect the role of GnT-III in cell migration.
FIGURE 4.
Knock-out of ST6GAL1 endowed GnT-III with an anti-migratory role in MDA-MB-231 cells. The MDA-MB-231 derivative cell lines as indicated were incubated with (bold line) or without (gray shading) biotin-conjugated MAA, biotin-conjugated SNA, or β1 integrin antibody followed by incubation with appropriate Alexa Fluor 647 conjugate and subjected to FACS analysis (A, D, and G). Their cell migration toward FN was determined by Transwell assay. Cells that migrated through the Transwell membrane were stained with 0.5% crystal violet. Shown are representative examples recorded by phase contrast microscopy (B, E, and H). Scale bar, 200 μm. The migrated cells were counted under a microscope. The quantitative data were obtained from three independent experiments (C, F, and I). The p values were calculated using one-tail unpaired t test. Error bars indicate standard derivation. *, p < 0.01. ST6GAL1 KO RES, ST6GAL1 knock-out cells rescued by overexpression of ST6GAL1.
Forced Expression of GnT-III Exerts Inhibitory Effects on Cell Migration in the Cells without or with a Low Level of α2,6-Sialylation
Then, the question arises regarding whether α2,6-sialylation also affects the function of GnT-III in other cells. To address it, we overexpressed GnT-III in different cells and checked its effects on cell motility (Fig. 5). Fig. 5B showed the sialylation patterns in these cells. Interestingly, the migratory ability of cells (HeLa-S3, CHO-K1 and Pro-5) without or with a low level of α2,6-sialylation was consistently suppressed after overexpression of GnT-III (Fig. 5A). In contrast, overexpression of GnT-III exerted varying effects on cell migration in tumor cells that are α2,6-hypersialylated. Like in MDA-MB-231 (Fig. 5A), little influence was observed in human gastric cancer cell line MKN-45 as reported previously (9). However, surprisingly, cell motility of glioma cells U-251 and hepatoma cells HepG2 was significantly enhanced. Taken together, the results indicate that GnT-III plays an anti-migratory role in tumor cells without or with a low level of α2,6-linked sialic acids but not in those α2,6-hypersialylated.
FIGURE 5.
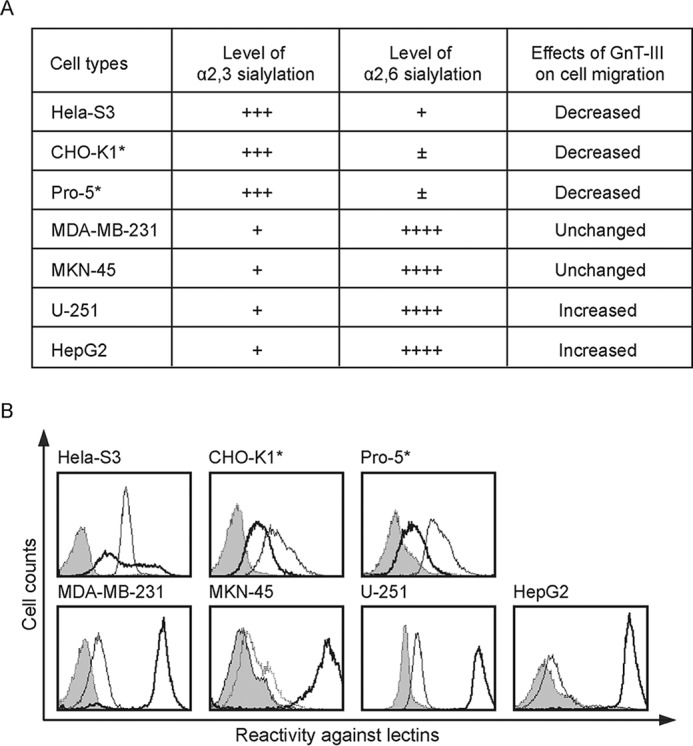
The migratory ability of cells without or with a low level of α2,6-sialylation was inhibited after overexpression of GnT-III. A, the effects of GnT-III overexpression on the fibronectin-mediated migration in different cells. ±, undetectable; +, less; ++, normal; +++, much; ++++, very much. B, the level of sialylation is based on the results of FACS analysis using MAA or SNA. Cell lines as indicated were incubated with biotin-conjugated MAA (thin line) or biotin-conjugated SNA (bold line) or without biotin-conjugated MAA and SNA lectins (gray shading), followed by incubation with streptavidin Alexa Fluor 647 conjugate and subjected to FACS analysis to examine the cell sialylation status. *, it has been reported that CHO-K1 and Pro-5 express no an α2,6-linked sialic acids (64). The peak shift in those SNA-stained cells should be due to nonspecific reaction.
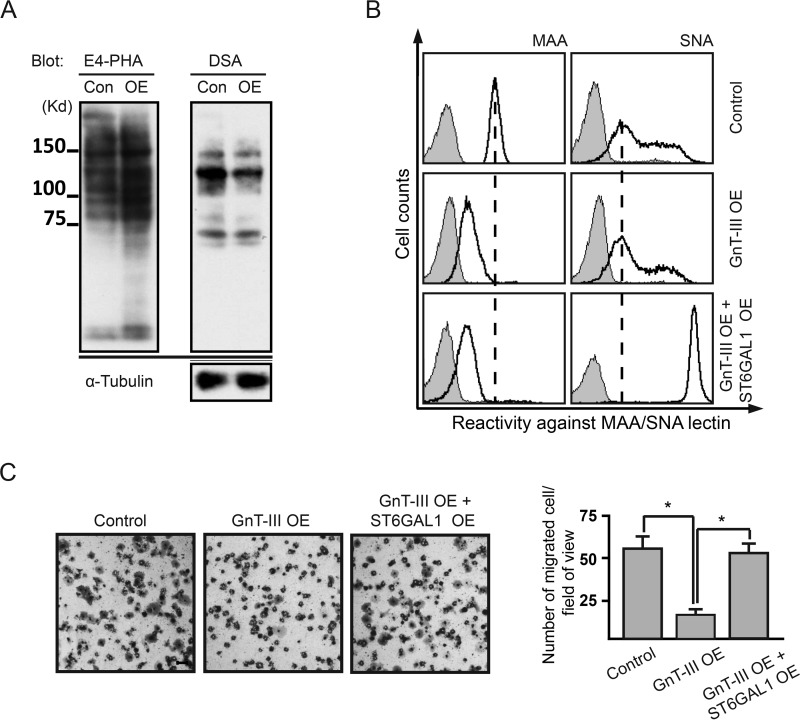
Forced Expression of ST6GAL1 Could Impair the Anti-migratory Role of GnT-III in HeLa-S3 Cells
To examine whether cell surface α2,6-linked sialic acids could affect the anti-migratory role of GnT-III in cells that are α2,6-hyposialylated, we overexpressed ST6GAL1 gene in GnT-III-overexpressing HeLa-S3 cells. Lectin blot and FACS analyses showed the efficient expression of GnT-III and ST6GAL1 (Fig. 6, A and B). It is worth mentioning that the inhibitory effects of GnT-III on α2,3-sialylation was also clearly reflected in this cell line because the reactivity with MAA lectin was markedly decreased after overexpression of GnT-III. As shown by Boyden chamber analysis (Fig. 6, C and D), GnT-III overexpression led to a significant inhibition of the cell motility in HeLa-S3, but these effects were totally abolished by further expression of ST6GAL1, suggesting that enhancing α2,6-linked sialic acids in α2,6-hyposialylated cells is capable of impairing the anti-migratory role of GnT-III.
FIGURE 6.
Overexpression of ST6GAL1 was able to neutralize the anti-migratory role of GnT-III in HeLa cells. A, cell lysates from the control and GnT-III-overexpressing HeLa cells were immunoblotted with E4-PHA and DSA lectins to check the overexpression efficiency of GnT-III. B, these two cells together with ST6GAL1 and GnT-III-overexpressing HeLa cells were incubated with (bold line) or without (gray shading) biotin-conjugated MAA or biotin-conjugated SNA, followed by incubation with streptavidin Alexa Fluor 647 conjugate and subjected to FACS analysis. C, their cell migration toward FN was determined by Transwell assay. Cells that migrated through the Transwell membrane were stained with 0.5% crystal violet. Shown is a representative example recorded by phase contrast microscopy. Scale bar, 200 μm. The migrated cells were counted under a microscope. The quantitative data were obtained from three independent experiments. The p values were calculated using one-tail unpaired t test. Error bars indicate standard derivation. *, p < 0.01. ST6GAL1 OE, ST6GAL1-overexpressing cells; Con, control; OE, GnT-III-overexpressing cells.
Depletion of α2,6-Sialylation by Knock-out of ST6GAL1 Endows GnT-III with Inhibitory Roles in Cell Migration of HepG2 Cells
Forced expression of GnT-III in glioma U-251 and hepatoma HepG2 resulted in a clear increase in cell migration as shown in Figs. 4A and 7 (A–C). Therefore, we further asked whether this pro-migratory role of GnT-III is dependent on α2,6-sialylation. To test it, ST6GAL1 gene was knocked out in GnT-III-overexpressing HepG2 cells. FACS analysis showed that knock-out of ST6GAL1 almost abolished the reactivity with SNA lectin (Fig. 7D). Like in MDA-MB-231 cells, ST6GAL1 knock-out in HepG2 cells also significantly decreased cell motility as compared with the control cells (data not shown). In contrast to the increased cell migration after GnT-III overexpression in HepG2 cells, forced expression of GnT-III in the ST6GAL1 knock-out cells clearly suppressed cell motility (Fig. 7, E and F). These data further confirmed that GnT-III may act as a tumor suppressor under an α2,6-hyposialylated context, but this role could be impaired by the high expression of ST6GAL1.
FIGURE 7.
GnT-III overexpression in HepG2 cells significantly increased cell migration, but knock-out of ST6GAL1 in this cell line conferred GnT-III with the opposite effect on cell motility. Overexpression of ST6GAL1 was able to neutralize the anti-migratory role of GnT-III in HeLa cells. A, cell lysates from the control and GnT-III-overexpressing HepG2 cells were immunoblotted with E4-PHA and DSA lectins to check the overexpression efficiency of GnT-III. B, their cell migration toward FN was determined by Transwell assay. Cells that migrated through the Transwell membrane were stained with 0.5% crystal violet. Shown is a representative example recorded by phase contrast microscopy. Scale bar, 100 μm. C, the migrated cells were counted under a microscope. The quantitative data were obtained from three independent experiments. D, ST6GAL1 knock-out HepG2 cells were incubated with (bold line) or without (gray shading) biotin-conjugated SNA, followed by incubation with streptavidin Alexa Fluor 647 conjugate and subjected to FACS analysis. E, the migratory ability of cells indicated toward FN was determined by Transwell assay. Cells that migrated through the Transwell membrane were stained with 0.5% crystal violet. Shown is a representative example recorded by phase contrast microscopy. Scale bar, 100 μm. F, the migrated cells were counted under a microscope. The quantitative data were obtained from three independent experiments. The p values were calculated using one-tail unpaired t test. Error bars indicate standard derivation. *, p < 0.01. Con, control; OE, GnT-III-overexpressing cells.
Discussion
GnT-III plays critical roles in defining the ultimate structure of hybrid and complex N-Glycans as the addition of a bisecting GlcNAc suppresses further processing and elongation of N-glycans to form branching structures, such as the β1,6-GlcNAc branching structures catalyzed by GnT-V. Because GnT-V has long been linked to the increased metastasis in tumors and overexpression of GnT-III in some cancer cells like HeLa-S3 and B16 reduced the rate of metastasis (11, 41), GnT-III has been usually regarded as a metastasis suppressor. However, there is also evidence that GnT-III overexpression had little influence in the human gastric cancer cell line MKN-45, and the expression of GnT-III was significantly increased in hepatoma, glioma, and ovarian cancer (9, 12, 15, 16). This raised the question as to the mechanistic roles of GnT-III in regulating the metastatic cell behaviors. Our results here demonstrate that the effects of GnT-III on cell migration are dependent on the cell sialylation pattern. GnT-III plays an anti-migratory role under an α2,6-hyposialylated context. High expression of α2,6-sialylation could antagonize this role and endow the cells with high migratory ability.
We have clearly demonstrated that overexpression of GnT-III dramatically suppresses α2,3-sialylation, but not α2,6-sialylation with a combination of assays including HPLC, Western blot, and FACS. These effects are exerted at a post-transcriptional level, because RT-PCR analysis revealed little difference in the expression of sialyltransferases and neuraminidases between the control and GnT-III-overexpressing cells. DSA blotting showed that the introduction of bisecting structures suppresses the formation of multiantennary sugar chains. Therefore, it is reasonable to consider that the decrease in α2,3-linked sialic acids is, at least partially, due to the reduced branches of N-glycans. However, this seems not the main mechanism because the decreased extent of α2,3-sialylation either overall or on specific integrins is more dramatic than that of multiantennary glycans as shown by DSA lectin. Given that each sialyltransferase possesses a distinct preference for one or two branches and has a low or intermediate affinity for the others (42–44), it is possible that the down-regulated α2,3-sialylation after GnT-III overexpression could be also attributed to the branch specificities of sialyltransferases. In agreement with this hypothesis, previous reports showed that a low degree of branching favors α2,6-sialylation but does not favor α2,3-sialylation (42, 45). Further, our mass spectrometry analysis showed that the sialic acids on bisected N-glycans were almost in an α2,6-linkage. Consistently, a recent study on the N-glycan profiling of a GnT-III highly expressed colorectal cancer cell line using the method of porous graphitized carbon LC-ESI-MS/MS indicated that the bisecting GlcNAc structures accounted for 38% of total relative abundance of complex and hybrid glycans, but no α2,3-linked sialic acids were detected on the bisected N-glycans (17). All these results suggest that the biantennary or hybrid bisecting structures may not favor the subsequent transfer of α2,3-linked sialic acids as compared with the nonbisecting chains, but it does not mean that α2,3-sialyltransferases could not use the bisecting structures as substrates at all because traces of the bisecting N-glycans that carry α2,3-linked sialic acids have been detected recently in ovarian cancer cell lines, although they are much less than α2,6-sialylated bisecting N-glycans (16). In contrast to branch specificities of α2,3-sialyltransferase, in vitro enzymatic activity study of ST6GAL1 has shown that introduction of bisecting GlcNAc residue does not affect the subsequent α2,6-sialylation (37), which is consistent with the predominant or exclusive existence of α2,6-sialylated bisecting N-glycans in tumor cells as we discussed above. In addition to branch specificities, we could not exclude the possibility for the decrease in α2,3-sialylation resulting from the substrate competition. One clear reason is that α2,3-sialyltransferases and α2,6-sialyltransferase share a common pool of sugar chains, but in fact, such competition is not limited to an interaction with one or the same terminal sugar residue. Mutual exclusion has been also observed for the α2,6-sialylation of the galactose and α1,3-fucosylation of the GlcNAc in the N-acetyllactosamine unit (46). Therefore, it is tempting to speculate that the addition of α2,6-linked sialic acids on specific N-glycans may not favor the further sialylation in an α2,3-linkage. Furthermore, the decreased α2,3-sialylation is also possibly due to the sialylatransferase relocation, although no evidence has been shown so far that the expression of one glycosyltransferase could affect the localization of the others. Taken together, GnT-III could regulate the α2,3-sialylation through many mechanisms. Interestingly, a recent glycomic analysis of gastric carcinoma cells showed that overexpression of ST3GAL4, which could produce the α2,3-linked sialic acids on N-glycans, significantly suppressed the expression of the bisected N-glycans (47). Considering also our data here, there may exist a negative interplay between the expression of α2,3-sialylation and bisecting structures, so it is reasonable to speculate that altered expression in one glycosyltransferase may affect the enzymatic products of the others, thereby having a major impact in the glycoproteome of cancer cells.
Our lectin blot and FACS analyses showed that overexpression of GnT-III in MDA-MB-231 cells decreased the level of α2,6-sialylation to a small extent, whereas little effect was observed on the amount of α2,6-sialylated N-glycans as indicated by HPLC. These data suggest that the decrease in α2,6-sialylation is mainly due to the reduced antennae of N-glycans after GnT-III overexpression. Consistent with this idea, our immunoprecipitation analysis suggests that the amount of α2,6-sialylated integrins is not decreased in GnT-III-overexpressing cells. Furthermore, a previous enzymatic activity assay showed that ST6GAL1 could catalyze the transfer of sialic acids to the bisecting biantennary glycans as efficiently as to the nonbisecting counterparts (37).
Sialic acids play important roles in cancer cell behaviors (18, 20, 21, 23). Alterations in sialylation in many glycoconjugates may have dramatic impact in the biology of cancer cells (48, 49). In recent years, ST6GAL1 is receiving more and more attention as the up-regulated expression of ST6GAL1 was observed in carcinomas of the colon, breast, cervix, choriocarcinomas, acute myeloid leukemias, and some malignancies of the brain as well (19). Several groups, including ours, have shown that increased α2,6-sialylation catalyzed by ST6GAL1 contributes to tumor metastasis and invasion (30, 39, 40). Considering also the location of sialic acids at the outmost reaches of the cell surface and their relatively strong electronegative charge, it is not surprising that high expression of ST6GAL1 as shown in the present study is able to impair the anti-migratory role of GnT-III. However, the underlying molecular mechanisms seem complicated. In vitro studies have suggested that ST6GAL1 promoted cell migration and invasion, at least in part, by the increased α2,6-sialylation of the β1 integrin (40, 50, 51). The α5β1 integrins were also required for suppressive role of GnT-III in fibronectin-mediated cell migration. Therefore, it is reasonable to postulate that further modification with α2,6-sialylation on bisecting GlcNAc structures may affect the cell signaling mediated by α5β1 integrins. In addition, considering that α5 integrin is rarely expressed in HepG2 cells, other mediators should exist. Among these, αvβ3 and αvβ6 could be the targets because both of them act as the important receptors for fibronectin, and both of them are deeply implicated in the promotion of the cancer cell metastasis (52–55). One more important molecule that could not be ignored is E-cadherin, which plays critical roles in cell-cell adhesion. The disruption of E-cadherin-mediated cell adhesion appears to be a central event in the transition from noninvasive to invasive carcinomas (56). Our lab has reported that the presence of bisecting structures on E-cadherin may prolong its retention at the cell border (31), whereas overexpression of ST6GAL1 exerts the opposing effects (29). Further, knockdown of ST6GAL1 induced the expression of E-cadherin in several tumor cell lines (29). Therefore, it is conceivable that ST6GAL1 may counteract the anti-metastatic role of GnT-III also through E-cadherin. The formation of galectin-glycan complex is highly implicated in cancer metastatic behavior, and different glycan structures show different affinity with certain glycans (57, 58). Introduction of both sialic acids and bisected GlcNAc structures are able to affect the binding with the galectins, but they have distinctive effects on the binding with some different galectins (59, 60), so galectin recognition could be also a possible explanation for the mask effect of ST6GAL1 on the function of GnT-III in cell migration. Taken together, the counteractive effect of ST6GAL1 on GnT-III in cell motility could be mediated by a number of molecules involved in cell adhesion and migration. The different expression pattern of these molecules in each cell system may serve as a possible explanation for why overexpression of GnT-III promoted the cell migration in some α2,6-hypersialylated cells but not in others. Detailed characterization of how α2,6-sialylated N-glycans and bisected N-glycans affect the signaling pathways mediated by these molecules is required for better understanding of the interplay between ST6GAL1 and GnT3 in regulating the tumor metastasis.
Glycosylation is involved in fundamental molecular and cell biology processes occurring in cancer (1). The increased expression of GnT-III and bisecting GlcNAc glycans has long been observed in hepatoma and glioma in mammals (12, 15). Our study showed, for the first time, that GnT-III overexpression could stimulate the migratory properties of α2,6-hypersialylated glioma cells U-251 and hepatoma cells HepG2, which indicates a clear biological significance of GnT-III in the malignancy of gliomas and hepatomas. However, the pro-migratory role of GnT-III seems α2,6-sialylation-dependent because GnT-III overexpression in ST6GAL1 knock-out HepG2 cells led to a suppression of cell motility. Consistent with this idea, in addition to GnT-III, ST6GAL1 is also highly expressed in both gliomas and hepatomas according to the human protein atlas database (12). Recently, the expression of both GnT-III and ST6GAL1 was found up-regulated in the human ovarian cancer and knockdown of ST6GAL1 significantly inhibited cell migration (16, 40). One group last year showed a highly metastatic colorectal cancer cell line, which contained an abundance of bisecting GlcNAc glycans (17). This made them somewhat surprised because GnT-III is usually considered to be a tumor metastasis suppressor. Now, it becomes conceivable because their RT-PCR result showed that ST6GAL1 was also expressed at a high level in that cell. All these results indicate that the cell glycosylation patterns are very relevant for the “functional” outcome in cancer cells, and certain cells alterations in N-glycans may have different outcomes depending on sialylation. Then a question arises regarding how this strategy benefits the tumor progression. One possibility for the case of GnT-III and ST6GAL1 could be that it favors the status transition of tumor cells between metastasis and proliferation. In detail, under the metastatic status, tumor cells may express both GnT-III and ST6GAL1 to endow itself with migratory potential, but after they reach the secondary sites, they could easily transit to the adhesion mode by down-regulating the α2,6-sialylation and prepare for subsequent proliferation because GnT-III has been reported to contribute to cell-cell contacts. Clearly, further evidence is needed for this hypothesis.
Like GnT-III, GnT-V expression could also affect the cell sialylation. We found that overexpression of GnT-V in HepG2 cells and MKN-45 cells led to a clear increase in α2,3-sialylation of β1 integrin, while decreasing its α2,6-sialylation (data not shown). This is in agreement with the previous observation that knock-out of GnT-V in MEF cells up-regulated α2,6-sialylation but down-regulated α2,3-sialylation (45). Because both GnT-V and ST6GAL1 have been correlated with the increased metastasis and invasion in tumor progression, it has been postulated that ST6GAL1 mediates the pro-metastatic role of GnT-V (61). However, this seems not to be the case. Overexpression of GnT-V in either HepG2 parent cells or its ST6GAL1 knock-out cells promoted the cell migration, although GnT-V-overexpressing cells exhibited higher metastatic ability than the GnT-V-overexpressing ST6GAL1 knock-out cells (data not shown). Further, it has been shown that GnT-V plays a pro-metastatic role, at least partially, via interacting with galectin-3 (62), whereas the α2,6-sialylation but not α2,3-sialylation of N-glycan is able to inhibit its binding to galectin-3 (60, 63). Therefore, it is reasonable to consider that ST6GAL1 and GnT-V contributes to the malignant progression by different mechanisms.
Tumor cells exhibit significant changes in cell surface glycosylation. Certain glycan structures are closely associated with the metastatic cell behaviors including invasion and enhanced cell survival, which provide important sources of markers for tumor progression. Here, we showed that GnT-III plays anti-migratory roles in the α2,6-hyposialylated cells, but high expression of ST6GAL1 may impair this role and endow the cells with strong metastatic potential, suggesting that high expression of GnT-III, together with ST6GAL1 or not, may serve as different markers for tumor malignancy.
Author Contributions
J. L. performed all the experiments with the help of T. I., T. F., and S. I., and J. L., S. I., and T. I. constructed the virus expression and knock-out vectors. A. K analyzed N-glycans by using mass spectrometry. J. G. designed the experiment. J. L. and J. G. analyzed the data, prepared the figures, and wrote the manuscript. All authors discussed the results and commented on the manuscript.
This work was supported in part by Grant-in-Aid for Scientific Research 15H04354 (to J. G.), by Challenging Exploratory Research Grant 15K14408 (to J. G.) from the Japan Society for the Promotion of Science, and by a Strategic Research Foundation Grant-aided Project for Private Universities from the Ministry of Education, Culture, Sports, Science and Technology of Japan. The authors declare that they have no conflicts of interest with the contents of this article.
- GnT-V
- N-acetylglucosaminyltransferase V
- ConA
- concanavalin A
- DSA
- D. stramonium agglutinin
- E4-PHA
- erythro-agglutinating phytohemagglutinin
- FN
- fibronectin
- GnT-III
- N-acetylglucosaminyltransferase III
- MAA
- M. amurensis agglutinin
- SNA
- S. nigra lectin
- ST6GAL1
- β-galactoside α2,6-sialyltranferase 1
- DOX
- doxycycline
- PA
- pyridiaminated.
References
- 1. Pinho S. S., and Reis C. A. (2015) Glycosylation in cancer: mechanisms and clinical implications. Nat. Rev. Cancer 15, 540–555 [DOI] [PubMed] [Google Scholar]
- 2. Abelev G. I. (1971) α-Fetoprotein in ontogenesis and its association with malignant tumors. Adv. Cancer Res. 14, 295–358 [DOI] [PubMed] [Google Scholar]
- 3. Dennis J. W., Laferté S., Waghorne C., Breitman M. L., and Kerbel R. S. (1987) β1–6 branching of Asn-linked oligosaccharides is directly associated with metastasis. Science 236, 582–585 [DOI] [PubMed] [Google Scholar]
- 4. Lau K. S., and Dennis J. W. (2008) N-Glycans in cancer progression. Glycobiology 18, 750–760 [DOI] [PubMed] [Google Scholar]
- 5. Guo H. B., Randolph M., and Pierce M. (2007) Inhibition of a specific N-glycosylation activity results in attenuation of breast carcinoma cell invasiveness-related phenotypes: inhibition of epidermal growth factor-induced dephosphorylation of focal adhesion kinase. J. Biol. Chem. 282, 22150–22162 [DOI] [PubMed] [Google Scholar]
- 6. Lagana A., Goetz J. G., Cheung P., Raz A., Dennis J. W., and Nabi I. R. (2006) Galectin binding to Mgat5-modified N-glycans regulates fibronectin matrix remodeling in tumor cells. Mol. Cell. Biol. 26, 3181–3193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Brockhausen I., Narasimhan S., and Schachter H. (1988) The biosynthesis of highly branched N-glycans: studies on the sequential pathway and functional role of N-acetylglucosaminyltransferases I, II, III, IV, V and VI. Biochimie 70, 1521–1533 [DOI] [PubMed] [Google Scholar]
- 8. Schachter H. (1986) Biosynthetic controls that determine the branching and microheterogeneity of protein-bound oligosaccharides. Biochem. Cell Biol. 64, 163–181 [DOI] [PubMed] [Google Scholar]
- 9. Zhao Y., Nakagawa T., Itoh S., Inamori K., Isaji T., Kariya Y., Kondo A., Miyoshi E., Miyazaki K., Kawasaki N., Taniguchi N., and Gu J. (2006) N-Acetylglucosaminyltransferase III antagonizes the effect of N-acetylglucosaminyltransferase V on α3β1 integrin-mediated cell migration. J. Biol. Chem. 281, 32122–32130 [DOI] [PubMed] [Google Scholar]
- 10. Song Y., Aglipay J. A., Bernstein J. D., Goswami S., and Stanley P. (2010) The bisecting GlcNAc on N-glycans inhibits growth factor signaling and retards mammary tumor progression. Cancer Res. 70, 3361–3371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yoshimura M., Nishikawa A., Ihara Y., Taniguchi S., and Taniguchi N. (1995) Suppression of lung metastasis of B16 mouse melanoma by N-acetylglucosaminyltransferase-III gene transfection. Proc. Natl. Acad. Sci. U.S.A. 92, 8754–8758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Uhlén M., Björling E., Agaton C., Szigyarto C. A., Amini B., Andersen E., Andersson A. C., Angelidou P., Asplund A., Asplund C., Berglund L., Bergström K., Brumer H., Cerjan D., Ekstrom M., et al. (2005) A human protein atlas for normal and cancer tissues based on antibody proteomics. Mol. Cell. Proteomics 4, 1920–1932 [DOI] [PubMed] [Google Scholar]
- 13. Isaji T., Kariya Y., Xu Q., Fukuda T., Taniguchi N., and Gu J. (2010) Functional roles of the bisecting GlcNAc in integrin-mediated cell adhesion. Methods Enzymol. 480, 445–459 [DOI] [PubMed] [Google Scholar]
- 14. Gu J., Sato Y., Kariya Y., Isaji T., Taniguchi N., and Fukuda T. (2009) A mutual regulation between cell-cell adhesion and N-glycosylation: implication of the bisecting GlcNAc for biological functions. J. Proteome Res. 8, 431–435 [DOI] [PubMed] [Google Scholar]
- 15. Nishikawa A., Gu J., Fujii S., and Taniguchi N. (1990) Determination of N-acetylglucosaminyltransferase-III, N-acetylglucosaminyltransferase-IV and N-acetylglucosaminyltransferase-V in normal and hepatoma tissues of rats. Biochim. Biophys. Acta 1035, 313–318 [DOI] [PubMed] [Google Scholar]
- 16. Anugraham M., Jacob F., Nixdorf S., Everest-Dass A. V., Heinzelmann-Schwarz V., and Packer N. H. (2014) Specific glycosylation of membrane proteins in epithelial ovarian cancer cell lines: glycan structures reflect gene expression and DNA methylation status. Mol. Cell. Proteomics 13, 2213–2232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sethi M. K., Thaysen-Andersen M., Smith J. T., Baker M. S., Packer N. H., Hancock W. S., and Fanayan S. (2014) Comparative N-glycan profiling of colorectal cancer cell lines reveals unique bisecting GlcNAc and α2,3-linked sialic acid determinants are associated with membrane proteins of the more metastatic/aggressive cell lines. J. Proteome Res. 13, 277–288 [DOI] [PubMed] [Google Scholar]
- 18. Lu J., and Gu J. (2015) Significance of β-Galactoside α2,6 sialyltranferase 1 in cancers. Molecules 20, 7509–7527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dall'Olio F., and Chiricolo M. (2001) Sialyltransferases in cancer. Glycoconj. J. 18, 841–850 [DOI] [PubMed] [Google Scholar]
- 20. Büll C., den Brok M. H., and Adema G. J. (2014) Sweet escape: sialic acids in tumor immune evasion. Biochim. Biophys. Acta 1846, 238–246 [DOI] [PubMed] [Google Scholar]
- 21. Büll C., Stoel M. A., den Brok M. H., and Adema G. J. (2014) Sialic acids sweeten a tumor's life. Cancer Res. 74, 3199–3204 [DOI] [PubMed] [Google Scholar]
- 22. Glavey S. V., Huynh D., Reagan M. R., Manier S., Moschetta M., Kawano Y., Roccaro A. M., Ghobrial I. M., Joshi L., and O'Dwyer M. E. (2015) The cancer glycome: carbohydrates as mediators of metastasis. Blood Rev. 29, 269–279 [DOI] [PubMed] [Google Scholar]
- 23. Schultz M. J., Swindall A. F., and Bellis S. L. (2012) Regulation of the metastatic cell phenotype by sialylated glycans. Cancer Metastasis Rev. 31, 501–518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wang P. H., Li Y. F., Juang C. M., Lee Y. R., Chao H. T., Ng H. T., Tsai Y. C., and Yuan C. C. (2002) Expression of sialyltransferase family members in cervix squamous cell carcinoma correlates with lymph node metastasis. Gynecol. Oncol. 86, 45–52 [DOI] [PubMed] [Google Scholar]
- 25. Wang X., O'Hanlon T. P., Young R. F., and Lau J. T. (1990) Rat β-galactoside α2,6-sialyltransferase genomic organization: alternate promoters direct the synthesis of liver and kidney transcripts. Glycobiology 1, 25–31 [DOI] [PubMed] [Google Scholar]
- 26. López-Morales D., Velázquez-Márquez N., Valenzuela O., Santos-López G., Reyes-Leyva J., and Vallejo-Ruiz V. (2009) Enhanced sialyltransferases transcription in cervical intraepithelial neoplasia. Invest. Clin. 50, 45–53 [PubMed] [Google Scholar]
- 27. Milflores-Flores L., Millán-Pérez L., Santos-López G., Reyes-Leyva J., and Vallejo-Ruiz V. (2012) Characterization of P1 promoter activity of the β-galactoside α2,6-sialyltransferase I gene (siat 1) in cervical and hepatic cancer cell lines. J. Biosci. 37, 259–267 [DOI] [PubMed] [Google Scholar]
- 28. Dalziel M., Dall'Olio F., Mungul A., Piller V., and Piller F. (2004) Ras oncogene induces β-galactoside α2,6-sialyltransferase (ST6Gal I) via a RalGEF-mediated signal to its housekeeping promoter. Eur. J. Biochem. 271, 3623–3634 [DOI] [PubMed] [Google Scholar]
- 29. Lu J., Isaji T., Im S., Fukuda T., Hashii N., Takakura D., Kawasaki N., and Gu J. (2014) β-Galactoside α2,6-sialyltranferase 1 promotes transforming growth factor-β-mediated epithelial-mesenchymal transition. J. Biol. Chem. 289, 34627–34641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Isaji T., Im S., Gu W., Wang Y., Hang Q., Lu J., Fukuda T., Hashii N., Takakura D., Kawasaki N., Miyoshi H., and Gu J. (2014) An oncogenic protein Golgi phosphoprotein 3 up-regulates cell migration via sialylation. J. Biol. Chem. 289, 20694–20705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Xu Q., Isaji T., Lu Y., Gu W., Kondo M., Fukuda T., Du Y., and Gu J. (2012) Roles of N-acetylglucosaminyltransferase III in epithelial-to-mesenchymal transition induced by transforming growth factor β1 (TGF-β1) in epithelial cell lines. J. Biol. Chem. 287, 16563–16574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ran F. A., Hsu P. D., Wright J., Agarwala V., Scott D. A., and Zhang F. (2013) Genome engineering using the CRISPR-Cas9 system. Nat. Protoc. 8, 2281–2308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Shalem O., Sanjana N. E., Hartenian E., Shi X., Scott D. A., Mikkelsen T. S., Heckl D., Ebert B. L., Root D. E., Doench J. G., and Zhang F. (2014) Genome-scale CRISPR-Cas9 knockout screening in human cells. Science 343, 84–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Natsuka S., Hirohata Y., Nakakita S., Sumiyoshi W., and Hase S. (2011) Structural analysis of N-glycans of the planarian Dugesia japonica. FEBS J. 278, 452–460 [DOI] [PubMed] [Google Scholar]
- 35. Hase S., Ikenaka T., and Matsushima Y. (1978) Structure analyses of oligosaccharides by tagging of the reducing end sugars with a fluorescent compound. Biochem. Biophys. Res. Commun. 85, 257–263 [DOI] [PubMed] [Google Scholar]
- 36. Kuraya N., and Hase S. (1992) Release of O-linked sugar chains from glycoproteins with anhydrous hydrazine and pyridylamination of the sugar chains with improved reaction conditions. J. Biochem. 112, 122–126 [DOI] [PubMed] [Google Scholar]
- 37. Koyota S., Ikeda Y., Miyagawa S., Ihara H., Koma M., Honke K., Shirakura R., and Taniguchi N. (2001) Down-regulation of the α-Gal epitope expression in N-glycans of swine endothelial cells by transfection with the N-acetylglucosaminyltransferase III gene: modulation of the biosynthesis of terminal structures by a bisecting GlcNAc. J. Biol. Chem. 276, 32867–32874 [DOI] [PubMed] [Google Scholar]
- 38. Krzewinski-Recchi M. A., Julien S., Juliant S., Teintenier-Lelièvre M., Samyn-Petit B., Montiel M. D., Mir A. M., Cerutti M., Harduin-Lepers A., and Delannoy P. (2003) Identification and functional expression of a second human β-galactoside α2,6-sialyltransferase, ST6Gal II. Eur. J. Biochem. 270, 950–961 [DOI] [PubMed] [Google Scholar]
- 39. Zhao Y., Li Y., Ma H., Dong W., Zhou H., Song X., Zhang J., and Jia L. (2014) Modification of sialylation mediates the invasive properties and chemosensitivity of human hepatocellular carcinoma. Mol. Cell. Proteomics 13, 520–536 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 40. Christie D. R., Shaikh F. M., Lucas J. A. 4th, Lucas J. A. 3rd, and Bellis S. L. (2008) ST6Gal-I expression in ovarian cancer cells promotes an invasive phenotype by altering integrin glycosylation and function. J. Ovarian Res. 1, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Isaji T., Gu J., Nishiuchi R., Zhao Y., Takahashi M., Miyoshi E., Honke K., Sekiguchi K., and Taniguchi N. (2004) Introduction of bisecting GlcNAc into integrin α5β1 reduces ligand binding and down-regulates cell adhesion and cell migration. J. Biol. Chem. 279, 19747–19754 [DOI] [PubMed] [Google Scholar]
- 42. Joziasse D. H., Bergh M. L., ter Hart H. G., Koppen P. L., Hooghwinkel G. J., and Van den Eijnden D. H. (1985) Purification and enzymatic characterization of CMP-sialic acid: β-galactosyl 1–3-N-acetylgalactosaminide α2–3-sialyltransferase from human placenta. J. Biol. Chem. 260, 4941–4951 [PubMed] [Google Scholar]
- 43. Joziasse D. H., Schiphorst W. E., Van den Eijnden D. H., Van Kuik J. A., Van Halbeek H., and Vliegenthart J. F. (1987) Branch specificity of bovine colostrum CMP-sialic acid: Gal β 1–4GlcNAc-R α2–6-sialyltransferase. Sialylation of bi-, tri-, and tetraantennary oligosaccharides and glycopeptides of the N-acetyllactosamine type. J. Biol. Chem. 262, 2025–2033 [PubMed] [Google Scholar]
- 44. Joziasse D. H., Schiphorst W. E., van den Eijnden D. H., van Kuik J. A., van Halbeek H., and Vliegenthart J. F. (1985) Branch specificity of bovine colostrum CMP-sialic acid: N-acetyllactosaminide α2–6-sialyltransferase. Interaction with biantennary oligosaccharides and glycopeptides of N-glycosylproteins. J. Biol. Chem. 260, 714–719 [PubMed] [Google Scholar]
- 45. Guo H. B., Nairn A., Harris K., Randolph M., Alvarez-Manilla G., Moremen K., and Pierce M. (2008) Loss of expression of N-acetylglucosaminyltransferase Va results in altered gene expression of glycosyltransferases and galectins. FEBS Lett. 582, 527–535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Paulson J. C., Prieels J. P., Glasgow L. R., and Hill R. L. (1978) Sialyl- and fucosyltransferases in the biosynthesis of asparaginyl-linked oligosaccharides in glycoproteins: mutually exclusive glycosylation by β-galactoside α2 goes to 6 sialyltransferase and N-acetylglucosaminide α1 goes to 3 fucosyltransferase. J. Biol. Chem. 253, 5617–5624 [PubMed] [Google Scholar]
- 47. Mereiter S., Magalhaes A., Adamczyk B., Jin C., Almeida A., Drici L., Ibanez-Vea M., Gomes C., Ferreira J. A., Afonso L. P., Santos L. L., Larsen M. R., Kolarich D., Karlsson N. G., and Reis C. A. (2015) Glycomic analysis of gastric carcinoma cells discloses glycans as modulators of RON receptor tyrosine kinase activation in cancer. Biochim. Biophys. Acta 10.1016/j.bbagen.2015.12.016 [DOI] [PubMed] [Google Scholar]
- 48. Cazet A., Bobowski M., Rombouts Y., Lefebvre J., Steenackers A., Popa I., Guérardel Y., Le Bourhis X., Tulasne D., and Delannoy P. (2012) The ganglioside G(D2) induces the constitutive activation of c-Met in MDA-MB-231 breast cancer cells expressing the G(D3) synthase. Glycobiology 22, 806–816 [DOI] [PubMed] [Google Scholar]
- 49. Gomes C., Osório H., Pinto M. T., Campos D., Oliveira M. J., and Reis C. A. (2013) Expression of ST3GAL4 leads to SLe (x) expression and induces c-Met activation and an invasive phenotype in gastric carcinoma cells. PLoS One 8, e66737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Seales E. C., Jurado G. A., Brunson B. A., Wakefield J. K., Frost A. R., and Bellis S. L. (2005) Hypersialylation of β1 integrins, observed in colon adenocarcinoma, may contribute to cancer progression by up-regulating cell motility. Cancer Res. 65, 4645–4652 [DOI] [PubMed] [Google Scholar]
- 51. Shaikh F. M., Seales E. C., Clem W. C., Hennessy K. M., Zhuo Y., and Bellis S. L. (2008) Tumor cell migration and invasion are regulated by expression of variant integrin glycoforms. Exp. Cell Res. 314, 2941–2950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Chen Q., Manning C. D., Millar H., McCabe F. L., Ferrante C., Sharp C., Shahied-Arruda L., Doshi P., Nakada M. T., and Anderson G. M. (2008) CNTO 95, a fully human anti αv integrin antibody, inhibits cell signaling, migration, invasion, and spontaneous metastasis of human breast cancer cells. Clin. Exp. Metastasis 25, 139–148 [DOI] [PubMed] [Google Scholar]
- 53. Thomas G. J., Lewis M. P., Hart I. R., Marshall J. F., and Speight P. M. (2001) αvβ6 integrin promotes invasion of squamous carcinoma cells through up-regulation of matrix metalloproteinase-9. Int. J. Cancer 92, 641–650 [DOI] [PubMed] [Google Scholar]
- 54. Thomas G. J., Lewis M. P., Whawell S. A., Russell A., Sheppard D., Hart I. R., Speight P. M., and Marshall J. F. (2001) Expression of the αvβ6 integrin promotes migration and invasion in squamous carcinoma cells. J. Invest. Dermatol. 117, 67–73 [DOI] [PubMed] [Google Scholar]
- 55. Sloan E. K., Pouliot N., Stanley K. L., Chia J., Moseley J. M., Hards D. K., and Anderson R. L. (2006) Tumor-specific expression of αvβ3 integrin promotes spontaneous metastasis of breast cancer to bone. Breast Cancer Res. 8, R20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Hirohashi S. (1998) Inactivation of the E-cadherin-mediated cell adhesion system in human cancers. Am. J. Pathol. 153, 333–339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Elola M. T., Blidner A. G., Ferragut F., Bracalente C., and Rabinovich G. A. (2015) Assembly, organization and regulation of cell-surface receptors by lectin-glycan complexes. Biochem. J 469, 1–16 [DOI] [PubMed] [Google Scholar]
- 58. Blidner A. G., Méndez-Huergo S. P., Cagnoni A. J., and Rabinovich G. A. (2015) Re-wiring regulatory cell networks in immunity by galectin-glycan interactions. FEBS Lett. 589, 3407–3418 [DOI] [PubMed] [Google Scholar]
- 59. Miwa H. E., Song Y., Alvarez R., Cummings R. D., and Stanley P. (2012) The bisecting GlcNAc in cell growth control and tumor progression. Glycoconj. J. 29, 609–618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Hirabayashi J., Hashidate T., Arata Y., Nishi N., Nakamura T., Hirashima M., Urashima T., Oka T., Futai M., Muller W. E., Yagi F., and Kasai K. (2002) Oligosaccharide specificity of galectins: a search by frontal affinity chromatography. Biochim. Biophys. Acta 1572, 232–254 [DOI] [PubMed] [Google Scholar]
- 61. Ranjan A., and Kalraiya R. D. (2013) α2,6 sialylation associated with increased β 1,6-branched N-oligosaccharides influences cellular adhesion and invasion. J. Biosci. 38, 867–876 [DOI] [PubMed] [Google Scholar]
- 62. Partridge E. A., Le Roy C., Di Guglielmo G. M., Pawling J., Cheung P., Granovsky M., Nabi I. R., Wrana J. L., and Dennis J. W. (2004) Regulation of cytokine receptors by Golgi N-glycan processing and endocytosis. Science 306, 120–124 [DOI] [PubMed] [Google Scholar]
- 63. Zhuo Y., and Bellis S. L. (2011) Emerging role of α2,6-sialic acid as a negative regulator of galectin binding and function. J. Biol. Chem. 286, 5935–5941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Xu X., Nagarajan H., Lewis N. E., Pan S., Cai Z., Liu X., Chen W., Xie M., Wang W., Hammond S., Andersen M. R., Neff N., Passarelli B., Koh W., Fan H. C., et al. (2011) The genomic sequence of the Chinese hamster ovary (CHO)-K1 cell line. Nat. Biotechnol. 29, 735–741 [DOI] [PMC free article] [PubMed] [Google Scholar]