Abstract
Mammary gland development is induced by the actions of various hormones to form a structure consisting of collecting ducts and milk-secreting alveoli, which comprise two types of epithelial cells known as luminal and basal cells. These cells adhere to each other by cell adhesion apparatuses whose roles in hormone-dependent mammary gland development remain largely unknown. Here we identified a novel cell adhesion apparatus at the boundary between the luminal and basal cells in addition to desmosomes. This apparatus was formed by the trans-interaction between the cell adhesion molecules nectin-4 and nectin-1, which were expressed in the luminal and basal cells, respectively. Nectin-4 of this apparatus further cis-interacted with the prolactin receptor in the luminal cells to enhance the prolactin-induced prolactin receptor signaling for alveolar development with lactogenic differentiation. Thus, a novel nectin-mediated cell adhesion apparatus regulates the prolactin receptor signaling for mammary gland development.
Keywords: cell adhesion, cell-cell interaction, Janus kinase (JAK), mammary gland, prolactin, nectin-1, nectin-4
Introduction
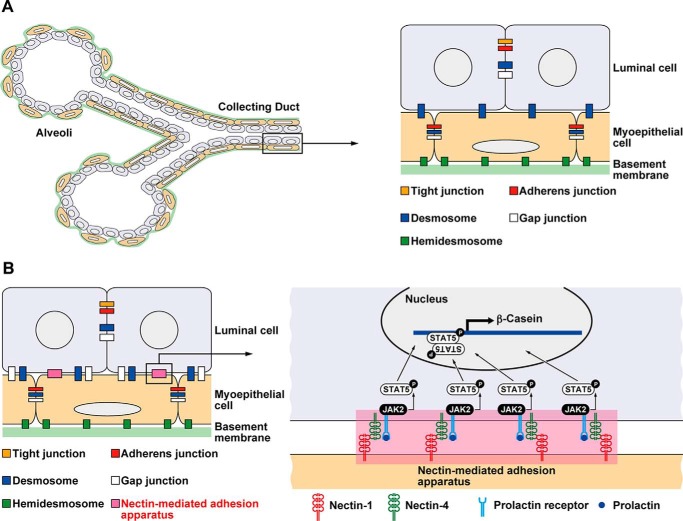
The adult mammary gland, consisting of collecting ducts and alveoli, is arranged in a bi-layer structure of inner luminal cells surrounded by basal myoepithelial cells, with the outermost side ensheathed by the basement membrane (1) (Fig. 1A). The luminal cells form a sheet by adhering to adjacent cells through cell adhesion apparatuses, including tight junctions (TJs),3 adherens junctions (AJs), desmosomes, and gap junctions (2, 3). TJs are located at the most apical side of the lateral membranes of luminal cells, and AJs are located at the basal side of TJs. Desmosomes and gap junctions are randomly located along the basolateral plasma membranes. The basal cells attach to each other by AJs, desmosomes, and gap junctions. The luminal and basal cells attach to each other through desmosomes. The presence of gap junctions between the luminal and basal cells is controversial (4). In addition, the basal cells attach to the basement membrane through hemidesmosomes (2).
FIGURE 1.
The novel cell adhesion apparatus mediated by nectin-1 and nectin-4 as a platform for cellular signaling in the mammary gland. A, schematic of the mammary gland. The adult mammary gland, consisting of collecting ducts and alveoli, is composed of a bilayer structure of inner luminal cells surrounded by outer basal myoepithelial cells with the outermost side ensheathed by the basement membrane. B, schematic of the findings. A novel cell adhesion apparatus mediated by nectins was found at the boundary between the luminal and basal cells in addition to desmosomes. The nectin-mediated adhesion apparatus identified here may serve as a platform for cellular signaling.
These cell adhesion apparatuses are composed of cell adhesion molecules (CAMs) and peripheral membrane proteins, which connect CAMs with cytoskeletal proteins, such as actin filaments (F-actin), intermediate filaments, and microtubules (5, 6). The major CAMs at AJs are classical cadherins and nectins (5, 6). Cadherins directly bind β-catenin, which interacts with α-catenin and is associated with F-actin, whereas nectins bind afadin and are associated with F-actin. The major CAMs at TJs are claudins, occludin, and junctional adhesion molecules (7). These CAMs bind zonula occludens (ZO)-1, ZO-2, and ZO-3 and are associated with F-actin. The major CAMs at desmosomes are desmosomal cadherins, which bind to plakoglobin and plakophilin and are associated with keratin intermediate filaments through desmoplakin (8). Gap junctions consist of connexons made up of six connexin proteins (9). In the mammary gland, these CAMs and their associated molecules have been identified and characterized (10), but nectins and afadin have not yet been investigated.
The mammary gland develops from a thickening in the ventral skin during embryogenesis that grows into a rudimentary ductal tree before birth (11–13). The female mammary gland continues to develop in a hormone-independent manner until puberty following which hormones induce ductal elongation and branching. Elongation and branching of ducts along with alveolar development with lactogenic differentiation are induced by pregnancy. This development of the mammary gland during puberty and pregnancy are regulated by hormones, including estrogen, progesterone, and prolactin, and growth factors and cytokines, such as growth hormone, insulin-like growth factor 1, EGF, FGF, Wnt, and receptor activator of nuclear factor κ-B ligand (12, 14, 15). It is known that each stage of ductal morphogenesis depends on a specific extracellular signaling pathway; however, the roles of the cell adhesion apparatuses in the regulation of mammary gland development remain largely unknown.
We previously generated nectin-1 (also know as Pvrl1) knock-out (nectin-1 KO) mice (16). In the brain, nectin-1 and nectin-3 are localized at the presynaptic and postsynaptic sides of hippocampal mossy fiber synapses, respectively (17), and the heterophilic trans-interaction between nectin-1 and nectin-3 is involved in the selective interaction of an axon with dendrites (18). In the nectin-1 KO brain, the hippocampal mossy fiber synapses exhibit abnormal morphology (19). Mutations in nectin-1 cause cleft lip/palate-ectodermal dysplasia and ectodermal dysplasia-syndactyly syndrome 1 (20). Nectin-1 expression has also been shown to be down-regulated or up-regulated in a variety of cancers (21–24). In addition, nectin-1 serves as the entry receptor for herpes simplex virus type 1 (6).
After backcrossing nectin-1 KO mice, which we previously generated (16), into the genetic background of C57BL/6 mice from the 129/Sv-C57BL/6 mixed background, we noticed that the backcrossed female mice often failed to breastfeed their pups. Analysis of pregnant nectin-1 KO female mice revealed that their mammary glands were functionally impaired because of insufficient mammary gland development. Therefore, in this study, we examined the role, localization, and mode of action of nectin-1 in mammary gland development.
Experimental Procedures
Mice
The nectin-1+/− mice were described previously (16). nectin-1−/− mice on the 129/Sv-C57BL/6 mixed background were fertile and nursed their pups. nectin-1−/− mice on the C57BL/6 background were fertile, but the nectin-1−/− female mice rarely nursed nectin-1−/− pups, which were generated by intercrossing of nectin-1−/− mice, to adulthood. In contrast, nectin-1+/− female mice nursed ∼40% of nectin-1−/− pups as well as most nectin-1+/− and wild-type pups, which were generated by intercrossing of nectin-1+/− mice, to adulthood. The mutant and control samples were prepared from the same litter. The morning after coitus and the day of birth were defined as E0.5 and P0, respectively. All animal experiments were performed in strict accordance with the guidelines of the institution and approved by the Administrative Panel on Laboratory Animal Care of Kobe University, Japan. The protocol was approved by the Committee on the Ethics of Animal Experiments of Kobe University Graduate School of Medicine (Permit Numbers P111109 and P141206). All efforts were made to minimize suffering.
Tissue Preparation
The animals were euthanized, and the inguinal mammary glands were harvested from 8–12-week-old non-pregnant virgin, pregnancy day 18.5 (P18.5), or lactating day 1 (L1) female mice. The mammary glands were frozen in O.C.T. Compound (Sakura Finetek USA, Inc., Torrance, CA) for immunofluorescence microscopy and H&E staining. Otherwise, the mammary glands were fixed in 4% paraformaldehyde in PBS for whole-mount carmine alum staining. Protein lysates of mammary epithelium were prepared from 8–14-week-old non-pregnant virgin or P18.5 female mice. The mammary glands were digested with 200 units/ml collagenase (Worthington) and 10 Kunitz units/ml DNase I (Sigma-Aldrich), and the cells were lysed in lysis buffer A (20 mm Tris-HCl at pH 7.5, 150 mm NaCl, 0.5% Nonidet P-40, 5% glycerol, 1 mm EDTA, 10 mm NaF, 1 mm Na3VO4, 10 μg/ml leupeptin, and 1 mm PMSF). Protein concentrations were determined using the Bio-Rad protein assay.
Antibodies (Abs)
Rabbit anti-α-actinin polyclonal antibody (pAb) (sc-15335, Santa Cruz Biotechnology, Santa Cruz, CA), mouse anti-actin mAb (MAB1501, Merck Millipore, Billerica, MA), rabbit anti-afadin pAb (A0349, Sigma-Aldrich), goat anti-β-casein pAb (sc-17969, Santa Cruz Biotechnology), rabbit anti-β-catenin pAb (C22006, Sigma-Aldrich), rat phycoerythrin-conjugated anti-CD24 mAb (12-0242, eBioscience, San Diego, CA), rat allophycocyanin-conjugated anti-CD49f mAb (FAB13501, R&D Systems, Inc., Minneapolis, MN), mouse anti-connexin 43 mAb (MAB3068, Merck Millipore), mouse anti-desmoplakin 1/2 mAb (651109, PROGEN Biotechnik, Heidelberg, Germany), mouse anti-FLAG mAb (for immunoprecipitation; F3165, Sigma-Aldrich), rabbit anti-FLAG pAb (for immunoblotting and immunofluorescence staining; F7425, Sigma-Aldrich), rabbit anti-GFP pAb (for immunoblotting; 598, MBL International, Nagoya, Japan), rat anti-GFP mAb (for immunofluorescence staining; GF090R, Nacalai Tesque, Kyoto, Japan), mouse anti-HA mAb (for immunoblotting; MMS-101P, BioLegend, San Diego, CA), rabbit anti-HA pAb (for immunoprecipitation; H6908, Sigma-Aldrich), rabbit anti-nectin-1 pAb (for immunoblotting; sc-28639, Santa Cruz Biotechnology), rat anti-nectin-1 mAb (for immunofluorescence staining; D146-3, MBL International), rat anti-nectin-2 mAb (D083-3, MBL International), rabbit anti-nectin-3 pAb (for immunoblotting; sc-28637, Santa Cruz Biotechnology), rat anti-nectin-3 mAb (for immunofluorescence staining; D084-3, MBL International), goat anti-nectin-4 pAb (AF2659, R&D Systems, Inc.), rabbit anti-occludin pAb (for immunoblotting; 71-1500, Thermo Fisher Scientific, Waltham, MA), rat anti-occludin mAb (for immunofluorescence staining; MOC37, Sanko Junyaku, Tokyo, Japan), mouse FITC-conjugated anti-smooth muscle actin mAb (F3777, Sigma-Aldrich), rabbit anti-STAT5 pAb (9363, Cell Signaling Technology, Danvers, MA), rabbit anti-STAT5a pAb (sc-1081, Santa Cruz Biotechnology), rabbit anti-phospho-STAT5 (Tyr-694) mAb (4322, Cell Signaling Technology), mouse anti-phosphotyrosine mAb (clone 4G10, 05-321, Merck Millipore), mouse anti-vinculin mAb (V9131, Sigma-Aldrich), mouse anti-ZO-1 mAb (for immunofluorescence staining; 33-9100, Thermo Fisher Scientific), and rabbit anti-ZO-1 pAb (for immunoblotting; 61-7300, Thermo Fisher Scientific) were purchased from the indicated suppliers. Rat anti-E-cadherin mAb (ECCD2) was a kind gift from Dr. M. Takeichi (Center for Developmental Biology, RIKEN). Alexa Fluor 488-, 555-, and 647-conjugated and Cy3-conjugated goat secondary Abs were purchased from Thermo Fischer Scientific and Merck Millipore, respectively. An HRP-conjugated secondary Ab was purchased from GE Healthcare.
Cell Culture
Human embryonic kidney (HEK) 293E cells were maintained in DMEM supplemented with 10% FBS, 100 units/ml penicillin, and 100 μg/ml streptomycin and cultured at 5% CO2 at 37 °C. Mouse mammary epithelial EpH4 cells were maintained in DMEM/F-12 supplemented with 2% FBS, 5 μg/ml insulin, and 50 μg/ml gentamycin and cultured at 5% CO2 at 37 °C.
Plasmid Constructions
To obtain the C-terminally HA-tagged prolactin receptor, the cDNA of mouse prolactin receptor was purchased from Sino Biological Inc. (catalogue number MG50457-M-M; Beijing, China). The cDNA encoding the prolactin receptor without the stop codon was amplified by PCR and inserted into the modified pcDNA3.1 vector containing a 3×HA epitope sequence. The plasmids encoding N-terminally FLAG-tagged human nectin-1, mouse nectin-2, mouse nectin-3, and mouse nectin-4 were constructed as described previously (25). The plasmids encoding N-terminally FLAG-tagged nectin-4 mutants (FLAG-nectin-4ΔCP (deletion of the signaling peptide and the cytoplasmic region, corresponding to amino acids 1–29 and 375–508, respectively) and FLAG-nectin-4ΔEC (deletion of the extracellular region, corresponding to amino acids 1–332)) were constructed by PCR using the FLAG-tagged full-length nectin-4 as a template. The cDNAs encoding FLAG-nectins were introduced into the retrovirus vector pCX4-puro. The cDNAs encoding JAK2 was kindly provided by Dr. T. Naka (National Institute of Biomedical Innovation) by courtesy of Dr. T. Kishimoto (Osaka University), and the expression vector for GFP-tagged JAK2 was constructed. The 21-base targeting sequences for mouse nectin-1 were 5′-GGTGAACGACTCCATGTATGG-3′ (for shNectin-1 #1) and 5′-GGCAGAGTACCAGGAGATC-3′ (for shNectin-1 #2).
Immunofluorescence Microscopy
The frozen tissues were sliced into 10-μm-thick sections using a cryostat. The sections were air-dried, fixed in acetone for 5 min, and blocked with 3% BSA for 1 h at room temperature. After being washed with PBS, the sections were incubated with the appropriate Abs at room temperature for 2 h and then incubated with the corresponding secondary Abs with DAPI at room temperature for 1 h. The secondary Abs were anti-goat, anti-mouse, or anti-rabbit Alexa Fluor 488 Abs and anti-rat Cy3 Ab. The images were acquired using a confocal laser-scanning microscope (LSM 700, Carl Zeiss, Jena, Germany) with a Plan-Apochromat 64×/1.4 numerical aperture oil immersion objective lens (Carl Zeiss) at room temperature under the control of Zen 2009 software (Carl Zeiss). The images were processed using Zen 2009.
FACS and Semiquantitative Real Time PCR
Mammary glands from 8–14-week-old virgin female mice were digested with 200 units/ml collagenase (Worthington) and 10 Kunitz units/ml DNase I (Sigma-Aldrich) and filtered through a 40-μm mesh to obtain a single cell suspension. A CD49fhighCD24+ population and a CD49fmedCD24+ population, corresponding to basal cells and luminal cells, respectively, were collected using FACSAriaIII (BD Biosciences). The cells were lysed with TRIzol (Life Technologies), and total RNAs were extracted following the manufacturer's protocol. Reverse transcription was performed using SuperScript III reverse transcriptase (Life Technologies). The abundance of each mRNA was measured using a thermal cycler Dice real time PCR system (Takara Bio, Otsu, Japan). The following primers were used for the semiquantitative real time PCR: nectin-1, 5′-CTTCTTCCTCCCAGGCACT-3′ and 5′-CCATGTGACCTGGGTGATT-3′; nectin-4, 5′-AGGAGACACTCTGGGCTTTC-3′ and 5′-ACCTGAGAATCCCTCGAAGA-3′; and gapdh, 5′-AACTTTGGCATTGTGGAAGG-3′ and 5′-CACATTGGGGGTAGGAACAC-3′.
Western Blotting
Protein lysates were mixed with SDS sample buffer (60 mm Tris-HCl at pH 6.7, 3% SDS, 2% 2-mercaptoethanol, and 5% glycerol) and boiled for 5 min. Then the samples were separated by SDS-PAGE and transferred to PVDF membranes (Merck Millipore). After being blocked with 2% skim milk or 3% BSA in Tris-buffered saline plus 0.05% Tween 20, the membranes were incubated with the indicated Abs. After being washed with Tris-buffered saline plus 0.05% Tween 20 three times, the membranes were incubated with peroxidase-conjugated anti-rabbit, anti-mouse, or anti-goat IgG Ab. The signals for the proteins were detected using Immobilon Western Chemiluminescent HRP Substrate (Merck Millipore).
Carmine Alum Staining
The whole mammary glands fixed in 4% paraformaldehyde in PBS were stained overnight in a carmine alum solution at room temperature. The samples were then cleared in xylene and flat mounted on slide glass. The images were acquired using AXIO Scope A1 (Carl Zeiss) with an A-Plan 5×/0.12 numerical aperture objective lens (Carl Zeiss) and Axio CamMRC (Carl Zeiss) at room temperature under the control of Axio Vision (Carl Zeiss). The images were processed using Photoshop CS4 (Adobe, San Jose, CA).
H&E Staining
The frozen tissues were sliced into 10-μm-thick sections using a cryostat, and the sections were subjected to H&E staining. The images were acquired using AXIO Scope A1 with an A-Plan 5×/0.12 numerical aperture objective lens and Axio CamMRC at room temperature under the control of Axio Vision. The images were processed using Photoshop CS4.
Transfection and Retrovirus Infection
For transient expression of various constructs in HEK293E cells, Lipofectamine 2000 reagent was used according to the manufacturer's protocol. To generate the retroviral supernatant, HEK293E cells were co-transfected with pGP, pE-Ampho, and either pCX4-puro or pSIREN-retroQ harboring a hygromycin resistance gene. After a 48-h culture period, the viral supernatant was added to the EpH4 cells in the presence of 8 μg/ml Polybrene. The cells were cultured in the presence of 5 μg/ml puromycin or 500 μg/ml hygromycin for 1 week and then used for the experiments.
Immunoprecipitation Assay
The suspension-cultured co-immunoprecipitation assay was performed as described previously (26). Briefly, HEK293E cells were transfected with various combinations of plasmids, cultured for 48 h, detached with 0.05% trypsin and 0.53 mm EDTA, and treated with a trypsin inhibitor. Then the cells were cultured in suspension with DMEM containing 0.5% fatty acid-free BSA for 30 min, collected by centrifugation, washed once with PBS, and lysed with lysis buffer B (20 mm Tris-HCl at pH 8.0, 150 mm NaCl, 1 mm CaCl2, 1 mm MgCl2, 10% glycerol, 1% Nonidet P-40, 10 mm NaF, 1 mm Na3VO4, 10 μg/ml leupeptin, 2 μg/ml aprotinin, and phosphatase inhibitor mixture 3). The lysates were rotated for 30 min and subjected to centrifugation at 12,000 × g for 15 min. The supernatant was precleared with protein A-Sepharose 4 Fast Flow beads (GE Healthcare) at 4 °C for 1 h. The precleared lysates were incubated with an anti-FLAG M2 mAb overnight and collected with protein A-Sepharose beads at 4 °C for 4 h. After the beads were extensively washed with lysis buffer B, bound proteins were eluted by boiling the beads in SDS sample buffer for 5 min and subjected to SDS-PAGE followed by Western blotting using the indicated Abs.
Bead-Cell Contact Assay
The bead-cell contact assay was performed as previously described (27, 28) with some modifications. The extracellular fragment of nectin-1 fused to the human IgG Fc (Nef-1) was purified from the culture supernatant of HEK293E cells expressing Nef-1. For preparation of the Nef-1-coated beads, purified Nef-1 was adsorbed onto latex-sulfate beads (5-μm diameter; Interfacial Dynamics, Portland, OR) precoated with Fc-specific goat anti-human IgG pAb (Jackson ImmunoResearch Laboratories). HEK293E cells expressing the GFP-tagged prolactin receptor together with FLAG-tagged nectin-4 or FLAG alone were detached from culture dishes, and the cells were mixed with Nef-1-coated beads or concanavalin A-coated beads in suspension at 37 °C for 30 min. The cells were then spread on glass coverslips, fixed with 2% paraformaldehyde in PBS for 2 min, blocked with 1% BSA in PBS, permeabilized with 0.1% Triton X-100 in PBS, and immunostained with rat anti-GFP and rabbit anti-FLAG Abs. To minimize the cross-reactivity between the secondary Abs, anti-FLAG Ab was prelabeled with the Zenon Alexa Fluor 555 rabbit IgG labeling kit (Life Technologies). After the incubation with the first Ab, the cells were incubated for 1 h with donkey anti-rat IgG conjugated with Alexa Fluor 488 and then mounted with FluorSave Reagent (Merck Millipore). The images were acquired using a Nikon C2 confocal system (Nikon, Inc., Tokyo, Japan) with a Plan Apo 60×/1.2 numerical aperture water immersion objective lens (Nikon, Inc) with 2× digital zoom at room temperature under the control of NIS-Elements AR Analysis software 4.20 64-bit (Nikon, Inc.) The images were processed using ImageJ 1.49a 64-bit software.
Prolactin-induced STAT5 Activation Assay
STAT5 tyrosine phosphorylation in EpH4 cells was assayed as described previously (29). Briefly, EpH4 cells, plated at a density of 2 × 104 cells/cm2 on dishes coated with Matrigel, were cultured for 16–24 h, and the cells were stimulated with prolactin by exchanging with fresh DMEM/F-12 containing 2% Matrigel (v/v), 5 μg/ml insulin, 50 μg/ml gentamycin, 1 μg/ml hydrocortisone, and 3 μg/ml prolactin for the indicated periods. The cells were washed with ice-cold PBS three times and lysed with lysis buffer B. Protein concentrations were determined using the Bio-Rad protein assay. The lysates were then boiled in SDS sample buffer for 5 min. Twenty-five micrograms of proteins, including Matrigel, were loaded and subjected to SDS-PAGE followed by Western blotting using the indicated Abs. The band intensity of the phosphorylated STAT5 was normalized to that of the total STAT5.
Results
A Novel Type of Cell Adhesion Apparatus Mediated by Nectin-1 and Nectin-4
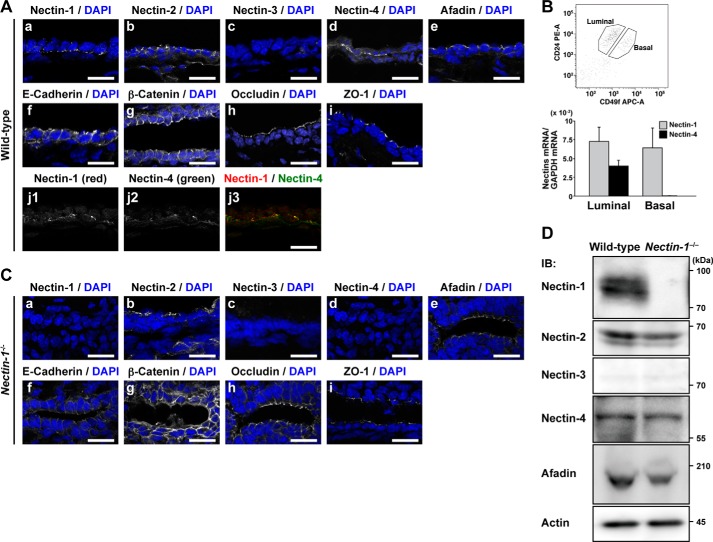
We first examined the expression and localization of nectin-1 in comparison with the components of AJs and TJs in the mammary epithelium. The immunofluorescence signals for nectin-1 and nectin-4 were concentrated as dots or short lines at the boundary between the luminal and basal cells, which overlapped with each other (Fig. 2A, panels a, d, and j). In contrast, the signals for nectin-2 and afadin were concentrated at the apical side of the lateral membrane at the boundary between the adjacent luminal cells (Fig. 2A, panels b and e), whereas nectin-3 was not observed in any region (Fig. 2A, panel c). The signals for E-cadherin, β-catenin, occludin, and ZO-1 were also concentrated at the apical side of the lateral membrane at the boundary between the adjacent luminal cells (Fig. 2A, panels f–i), consistent with previous observations (30–32). These results indicate that nectin-1 and nectin-4 are localized at the boundary between the luminal and basal cells; nectin-2, afadin, E-cadherin, and β-catenin are localized at AJs between adjacent luminal cells; and occludin and ZO-1 are localized at TJs between adjacent luminal cells. None of these signals was observed at AJs between the adjacent basal cells presumably because AJs at this region are much smaller than those between adjacent luminal cells and are too faint to be detected with the immunofluorescence microscopy used here.
FIGURE 2.
Localization of nectin-1 and nectin-4 at the boundary between the luminal and basal cells. A, panels a–j, localization of cell adhesion components in the mammary epithelium of pregnancy day 18.5 wild-type mice. The sections were stained with the indicated Abs. Nuclei were counterstained with DAPI. A, panel j, co-localization of nectin-1 and nectin-4 at the boundary between the luminal and basal cells. Scale bars, 20 μm. B, nectin-1 and nectin-4 mRNA expression in the luminal and basal cells. RNAs extracted from the sorted luminal cells, a CD49fmedCD24+ population, and basal cells, a CD49fhighCD24+ population, were subjected to semiquantitative real time PCR. Error bars, S.E. C, panels a–i, localization of cell adhesion components in the mammary epithelium of pregnancy day 18.5 nectin-1−/− mice. The sections were stained with the indicated Abs. Nuclei were counterstained with DAPI. Scale bars, 20 μm. D, expression levels of nectin-1, nectin-2, nectin-3, nectin-4, and afadin in the mammary epithelium of pregnancy day 18.5 wild-type and nectin-1−/− mice. The lysates (10 μg of protein each) of the mammary epithelium from pregnancy day 18.5 wild-type and nectin-1−/− mice were subjected to Western blotting using their respective Abs. Actin was used as a loading control. Results are representative of three independent experiments. PE, phycoerythrin; APC, allophycocyanin; IB, immunoblotting.
To identify in which cell types, the luminal or basal cells, nectin-1 and nectin-4 are expressed, we isolated the luminal cells, a CD49fmedCD24+ population, or basal cells, a CD49fhighCD24+ population, by FACS and quantified the mRNA levels of nectin-1 and nectin-4 in each cell by quantitative real time PCR. The nectin-1 mRNA was expressed in both the luminal and basal cells to roughly similar extents, whereas the nectin-4 mRNA was exclusively expressed in the luminal cells (Fig. 2B). The expression levels of the nectin-1 and nectin-4 mRNAs in the luminal cells were roughly 3:2. On the assumption that these molar ratios of the mRNAs reflect those of the proteins, these results indicate that both nectin-1 and nectin-4 are expressed in the luminal cells with a rough ratio of 3:2, whereas only nectin-1 is expressed in the basal cells, and that the expression levels of nectin-4 in the luminal cells and nectin-1 in the basal cells were roughly 2:3. In the mammary glands obtained from nectin-1 KO female mice (nectin-1 KO mammary gland), the signals for nectin-1 and nectin-4 were undetectable, whereas the levels of the other components of AJs and TJs between adjacent luminal cells were unchanged (Fig. 2C). By Western blotting analysis, nectin-1, nectin-2, nectin-4, and afadin were detected in the wild-type mammary epithelium, but in the nectin-1 KO mammary epithelium, nectin-1 was undetectable, whereas nectin-2, nectin-4, and afadin were unchanged (Fig. 2D). The discrepancy between the immunofluorescence staining and the Western blotting analysis is presumably due to the lack of the concentration of nectin-4 at specific sites in the immunofluorescence staining. It was previously shown that nectin-1 and nectin-4 not only undergo homophilic interactions but also undergo a heterophilic trans-interaction with each other (33, 34); the dissociation constant values for nectin-1/nectin-4, nectin-1/nectin-1, and nectin-4/nectin-4 are 0.1, 17.5, and 153 μm, respectively (34, 35). On the assumption that the molar ratios of the mRNAs expressed in the luminal and basal cells reflect those of the proteins, it is likely that the trans-interaction between nectin-4 in the luminal cells and nectin-1 in the basal cells mainly forms a cell adhesion apparatus at the boundary between the luminal and basal cells. All the nectin-1 molecules in the basal cells are likely to be trans-interacted with the nectin-4 molecules in the luminal cells, whereas all the nectin-1 molecules in the luminal cells are likely to have no trans-interacting partner in the basal cells and to be free.
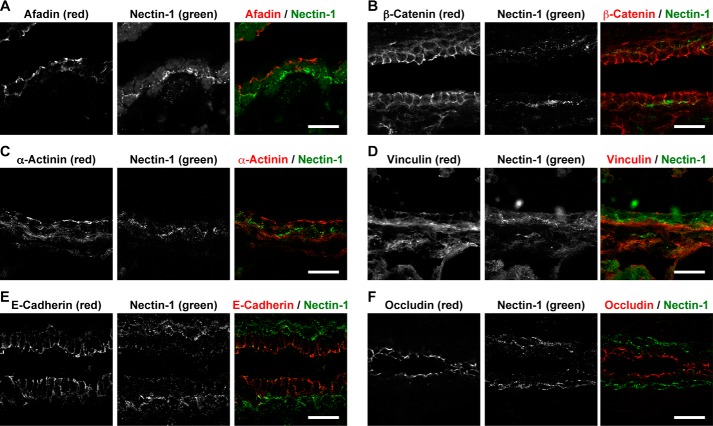
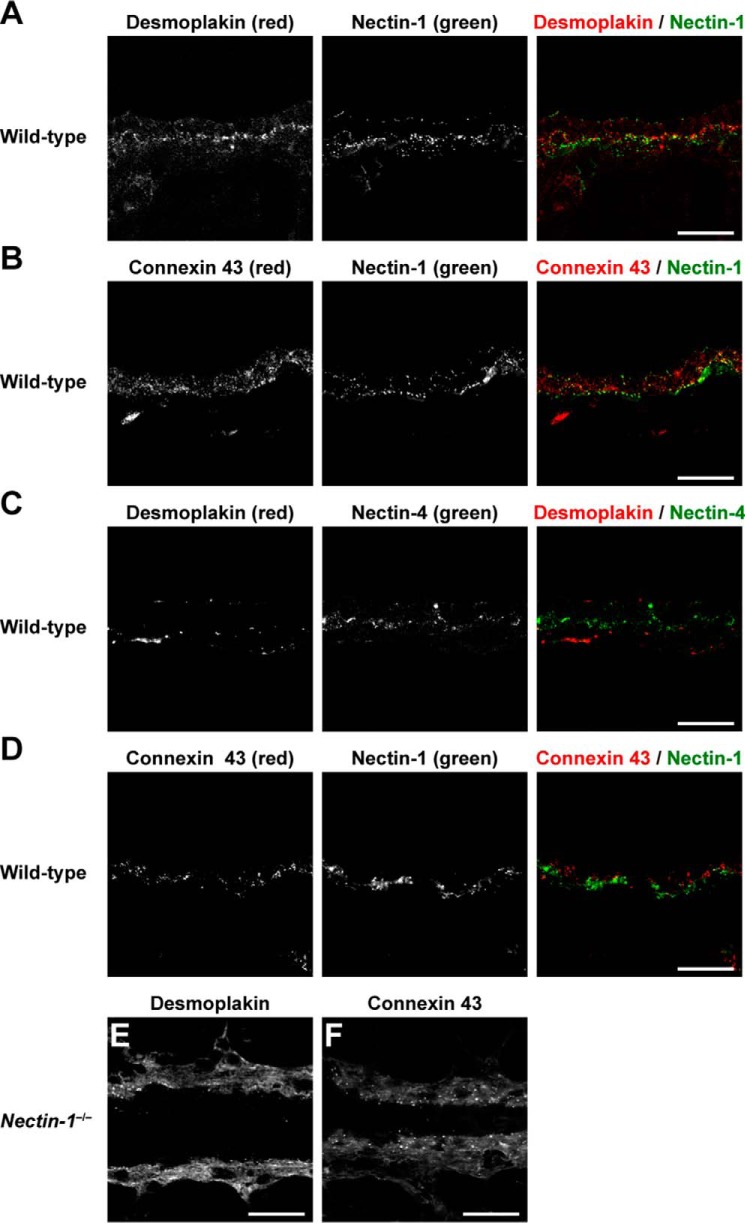
None of the signals for the components of AJs and TJs, such as nectin-2, afadin, E-cadherin, β-catenin, occludin, and ZO-1, was concentrated at the boundary between the luminal and basal cells. None of the signals for other F-actin-binding proteins that directly or indirectly bind to nectins and E-cadherin was co-localized with the signal for nectin-1 (Fig. 3). Moreover, neither the signal for desmoplakin, a component of desmosome, nor the signal for connexin 43, a component of gap junctions, was co-localized with the signal for nectin-1 or nectin-4 (Fig. 4, A–D). These results indicate that nectin-1 and nectin-4 constitute a novel type of cell adhesion apparatus that is different from AJs, TJs, desmosomes, and gap junctions.
FIGURE 3.
A novel type of cell adhesion apparatus mediated by nectin-1 and nectin-4 different from thus far known cell adhesion apparatus. A–F, localization of cell adhesion components and nectin-1 in the mammary gland of wild-type mice. Each component (red) was co-immunostained with nectin-1 (green). A, afadin and nectin-1; B, β-catenin and nectin-1; C, α-actinin and nectin-1; D, vinculin and nectin-1; E, E-cadherin and nectin-1; and F, occludin and nectin-1. Scale bars, 20 μm. Results are representative of five independent experiments.
FIGURE 4.
Localization of nectin-1 and nectin-4 at the boundary between the luminal and basal cells distinct from desmosomes and gap junctions. A and C, localization of desmoplakin in the mammary gland of wild-type mice. Desmoplakin (red) was co-immunostained with nectin-1 or nectin-4 (green). B and D, localization of connexin 43 in the mammary gland of wild-type mice. Connexin 43 (red) was co-immunostained with nectin-1 or nectin-4 (green). E and F, localization of desmoplakin and connexin 43 in the mammary gland of nectin-1−/− mice. Desmoplakin and connexin 43 was immunostained with the indicated Abs (red). Scale bars, 20 μm. Results are representative of five independent experiments.
The signals for the components of these cell adhesion apparatuses (i.e. nectin-2, afadin, E-cadherin, β-catenin, occludin, ZO-1, desmoplakin, and connexin 43) in nectin-1 KO mammary glands were similar to those in the wild-type mammary glands; however, the localization and intensity of the signals for desmoplakin and connexin 43 in the nectin-1 KO mammary gland were slightly different from those in the wild-type mammary gland possibly because of the morphological changes in the ducts and alveoli induced by nectin-1 knock-out (Fig. 4, E and F). These results indicate that this novel adhesion apparatus does not affect the formation and/or maintenance of other cell adhesion apparatuses.
Morphological and Functional Impairments in the Pregnancy-induced Alveolar Development in the Nectin-1 KO Mammary Gland
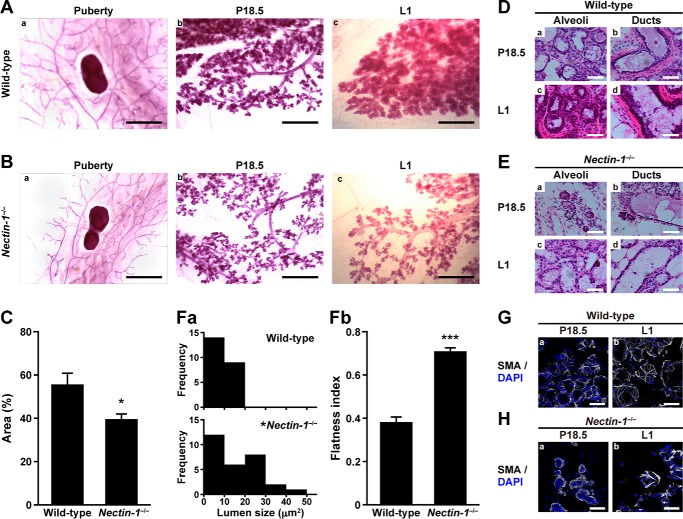
Next we examined whether the novel adhesion apparatus mediated by nectin-1 and nectin-4 plays a role in the mammary gland development. The wild-type and nectin-1 KO mammary glands at various developmental stages were histologically analyzed. In the whole-mount mammary glands stained with carmine alum, the sizes of mammary ductal trees were apparently indistinguishable between the wild-type and nectin-1 KO mice during puberty (Fig. 5, A, panel a, and B, panel a). At P18.5 and L1, the numbers of first and second branches were reduced in the nectin-1 KO mammary gland, and the number of alveoli was reduced compared with those in the wild-type mammary tissue (Fig. 5, A, panels b and c, and B, panels b and c). To quantify the pregnancy-induced alveolar development of the mammary gland, the percentage of carmine alum-stained area at the distal site from the lymph node was quantified (Fig. 5C). The cell densities of the luminal cells in the alveoli were reduced in the nectin-1 KO mammary gland at P18.5 and L1 (Fig. 5, D, panels a and c, and E, panels a and c). The morphologies of the luminal cells in the alveoli were also changed in the nectin-1 KO mammary gland at these stages: they were columnar with secretary granules in the wild-type mammary gland, but they became flattened and were absent from secretary granules in the nectin-1 KO mammary gland. Overall, alveoli with large size lumens were more frequently observed in the nectin-1 KO mammary gland than in the wild type (Fig. 5F, panel a). The morphologies of the luminal cells in the ducts were flatter in the nectin-1 KO mammary gland compared with those in the wild type at P18.5 and L1 (Fig. 5, D, panels b and d; E, panels b and d; and F, panel b). When the basal cells in the alveoli were visualized by staining with smooth muscle actin at P18.5 and L1, the numbers of the long thin processes that radiated from the cell body and of contact sites between the processes were reduced in the nectin-1 KO mammary gland compared with those in the wild type (Fig. 5, G and H). Thus, the basket-like structure of the basal cells in the alveoli was more sparse in the nectin-1 KO mammary gland compared with that in the wild type, suggesting that the alveolus contraction ability of the basal cells was impaired in the nectin-1 KO mammary gland. These results indicate that nectin-1 is required for the pregnancy-induced alveolar development in the mammary gland and suggest that the novel adhesion apparatus is involved in this developmental process.
FIGURE 5.
Morphological impairments in pregnancy-induced alveolar development in the nectin-1−/− mammary gland. A and B, carmine alum staining. A, panels a–c, the wild-type mammary gland; B, panels a–c, the nectin-1−/− mammary gland. A, panel a, and B, panel a, puberty; A, panel b, and B, panel b, P18.5; A, panel c, and B, panel c, L1. Scale bars, A, panel a, and B, panel a, 5 mm; A, panels b and c, and B, panels b and c, 1 mm. C and D, H&E staining. C, percentage of carmine alum-stained area at the distal site from the lymph node. For the quantification, three independent animals of wild type and nectin-1−/− at P18.5 were examined. *, p = 0.037 versus wild type as determined by Student's t test. Error bars, S.E. D, panels a–d, the wild-type mammary gland; E, panels a–d, the nectin-1−/− mammary gland. D, panel a, and E, panel a, alveoli of P18.5 mice; D, panel c, and E, panel c, alveoli of L1 mice; D, panel b, and E, panel b, ducts of P18.5 mice; D, panel d, and E, panel d, ducts of L1 mice. Scale bars, 50 μm. F, quantification of the mammary gland morphologies. F, panel a, lumen size distribution. The lumen size of 23 alveoli from three independent animals of wild type and 29 alveoli from three independent animals of nectin-1−/− at P18.5 were examined. *, p = 0.024 versus wild type as determined by χ2 test. F, panel b, cell flatness. The flatness index was obtained by dividing cell width by cell height. At least 10 cells per mouse from three independent animals of wild type and nectin-1−/− at P18.5 were examined. ***, p = 3.3 × 10−4 versus wild type as determined by Student's t test. G and H, basal cell morphologies. G, panels a and b, the wild-type mammary gland; H, panels a and b, the nectin-1−/− mammary gland. The specimens were stained with the FITC-conjugated anti-smooth muscle actin (SMA) Ab (shown in gray). Nuclei were counterstained with DAPI. G, panel a, and H, panel a, P18.5; and G, panel b, and H, panel b, L1. Scale bars, 50 μm. Results are representative of three independent experiments.
Alveolar development with lactogenic differentiation during pregnancy is mainly induced by prolactin through its receptor and downstream signaling molecules, such as JAK and STAT5 (36–38). We examined the effect of the genetic ablation of nectin-1 on this pregnancy-induced functional alveolar development by measuring STAT5 tyrosine phosphorylation and β-casein synthesis. The STAT5a tyrosine phosphorylation observed in the wild-type pregnant mammary gland (39) was not observed in the nectin-1 KO pregnant mammary gland (Fig. 6A). The increase in total β-casein levels in the wild-type pregnant mammary gland was not observed in the nectin-1 KO pregnant mammary gland (Fig. 6B). These results indicate that nectin-1 is required for the STAT5a phosphorylation, which leads to alveolar development with lactogenic differentiation during pregnancy, and suggest that the novel adhesion apparatus is involved in this reaction.
FIGURE 6.
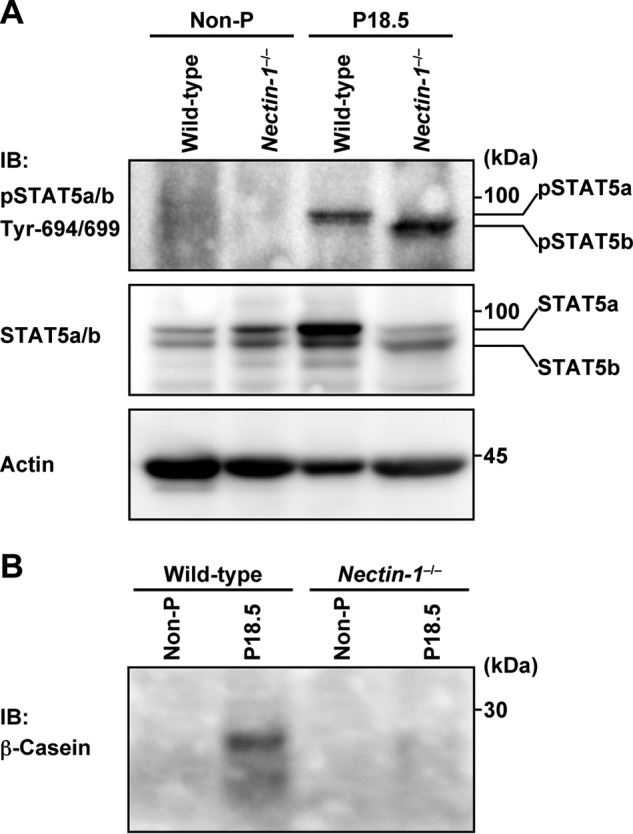
Functional impairments in pregnancy-induced alveolar development in nectin-1−/− mammary gland. A, STAT5 tyrosine phosphorylation. The lysates (10 μg of protein each) of the mammary epithelium from non-pregnant (Non-P) and P18.5 wild-type and nectin-1−/− mice were subjected to Western blotting using an anti-phospho-STAT5 (pSTAT5) Ab. Actin was used as a loading control. B, β-casein synthesis. The lysates (10 μg of protein each) of the mammary epithelium from non-pregnant and P18.5 wild-type and nectin-1−/− mice were subjected to Western blotting using an anti-β-casein Ab. Results are representative of three independent experiments. IB, immunoblotting.
Physical cis-Interactions of Nectin-1 and Nectin-4 with the Prolactin Receptor
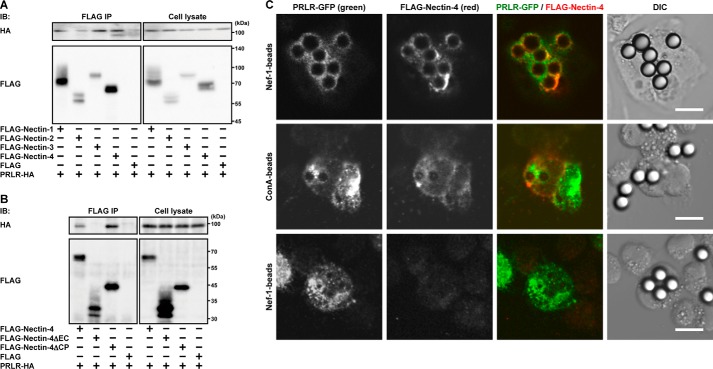
It was shown that the prolactin receptor is expressed in the luminal cells (40). We therefore examined whether nectin-1, nectin-2, and nectin-4 as well as nectin-3 cis-interact with the prolactin receptor. FLAG-tagged nectin-1, nectin-2, nectin-3, or nectin-4 or the FLAG tag alone was co-expressed with the HA-tagged prolactin receptor in HEK293E cells, and the cells were cultured in suspension. The suspension culture was used to enable the detection of a possible cis-interaction between each FLAG-tagged nectin and the prolactin receptor on the plasma membrane. When each FLAG-tagged nectin or the FLAG tag alone was immunoprecipitated from each cell lysate using an anti-FLAG mAb, the HA-tagged prolactin receptor was co-immunoprecipitated with FLAG-tagged nectin-1, nectin-3, and nectin-4 to similar extents but not with nectin-2 or the FLAG tag alone (Fig. 7A). These results indicate that nectin-1, nectin-3, and nectin-4, but not nectin-2, are able to cis-interact directly or indirectly with the prolactin receptor in vitro. The interaction of these nectins with the prolactin receptor was mediated through their extracellular regions as analyzed by the same method, and the result for nectin-4 is shown as a representative (Fig. 7B).
FIGURE 7.
cis-Interaction of nectin-4 with the prolactin receptor. A, co-immunoprecipitation of the prolactin receptor (PRLR) with the nectin family members. HEK293E cells were transfected with various combinations of the indicated plasmids and cultured in suspension. Each FLAG-tagged nectin family member was immunoprecipitated with an anti-FLAG Ab. B, cis-interaction of nectin-4 with the PRLR through its extracellular region. HEK293E cells were transfected with various combinations of the indicated plasmids and cultured in suspension. FLAG-tagged full-length nectin-4 or deletion mutants of nectin-4 were immunoprecipitated with an anti-FLAG Ab. Results are representative of three independent experiments. C, co-localization of GFP-tagged PRLR and FLAG-tagged nectin-4 at the boundary between HEK293E cells expressing both the GFP-tagged PRLR and FLAG-tagged nectin-4 and the Nef-1-coated microbeads. HEK293E cells expressing both the GFP-tagged PRLR and FLAG-tagged nectin-4 or HEK293E cells expressing GFP-tagged PRLR alone were mixed with Nef-1- or concanavalin A (ConA)-coated (control) microbeads and incubated in suspension followed by fixation and immunofluorescence microscopy. Scale bars, 10 μm. Results are representative of three independent experiments. IP, immunoprecipitation; IB, immunoblotting; DIC, differential interference contrast.
We further confirmed the in vitro cis-interaction of nectin-4 with the prolactin receptor by another method. Microbeads precoated with Nef-1 or concanavalin A as a control were mixed with a suspension of HEK293E cells expressing both the GFP-tagged prolactin receptor and FLAG-tagged nectin-4 or expressing the GFP-tagged prolactin receptor alone, and the co-localization of these molecules was examined. Nef-1 used herein contained the extracellular region of nectin-1 fused to the Fc portion of IgG (27). The immunofluorescence signals for both the GFP-tagged prolactin receptor and FLAG-tagged nectin-4 were concentrated at the boundary between the surface of the cells expressing both proteins and the Nef-1-coated microbeads but not the microbeads coated with concanavalin A (Fig. 7C). The signal for the GFP-tagged prolactin receptor in the cells expressing it alone was not concentrated at the boundary between the surface of the cells and the Nef-1-coated microbeads. These results are consistent with the results in the co-immunoprecipitation assay and further indicate that nectin-4, which trans-interacts with nectin-1, is able to cis-interact with the prolactin receptor in vitro.
Functional cis-Interactions of Nectin-1 and Nectin-4 with the Prolactin Receptor
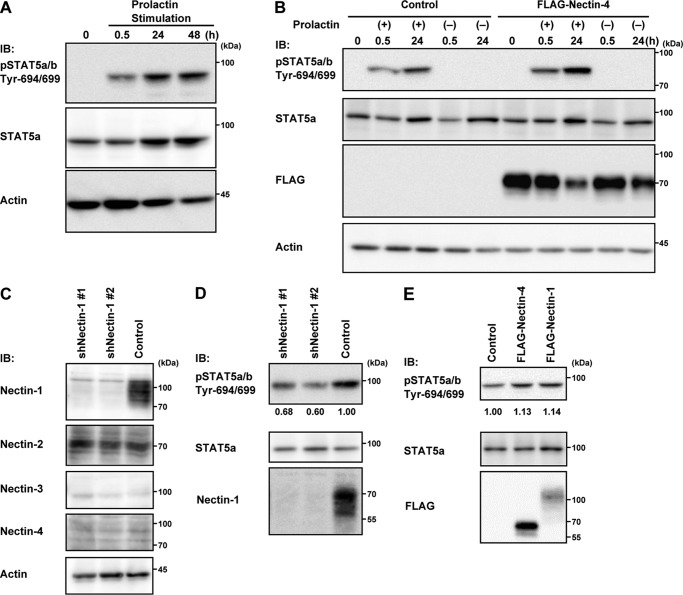
It was previously shown that EpH4 mammary epithelial cells respond to prolactin to induce STAT5 tyrosine phosphorylation (29). We first confirmed that prolactin indeed stimulates the STAT5 tyrosine phosphorylation in EpH4 cells (Fig. 8A). Because nectin-4 was not expressed in EpH4 cells (Fig. 8C), we expressed FLAG-tagged nectin-4 to examine its effect on this reaction. The expression of FLAG-tagged nectin-4 enhanced the STAT5 tyrosine phosphorylation in the presence of prolactin but not in the absence of prolactin (Fig. 8B). Nectin-1 was then knocked down in EpH4 cells using shRNA because nectin-1 was expressed in this cell line (Fig. 8C) and nectin-1 cis-interacted with the prolactin receptor (Fig. 7A). By Western blotting, nectin-1 was indeed down-regulated, whereas nectin-2 was unaffected (Fig. 8C). The knockdown of nectin-1 reduced the prolactin-induced STAT5 tyrosine phosphorylation (Fig. 8D). In addition, the expression of FLAG-tagged nectin-1 enhanced the prolactin-induced STAT5 tyrosine phosphorylation (Fig. 8E). These results indicate that nectin-1 and nectin-4 are able to enhance the prolactin-induced STAT5 tyrosine phosphorylation in vitro.
FIGURE 8.
Nectin-1- and nectin-4-mediated enhancement of the prolactin-induced STAT5 tyrosine phosphorylation in EpH4 cells. A, the prolactin-induced STAT5 tyrosine phosphorylation. EpH4 cells cultured on Matrigel-coated dishes were stimulated with 3 μg/ml prolactin for the indicated periods. The samples (25 μg of protein each, including Matrigel) were subjected to Western blotting using the indicated Abs. Actin was used as a loading control. B, nectin-4-mediated enhancement of the prolactin-induced STAT5 tyrosine phosphorylation. EpH4 cells stably expressing the empty or FLAG-nectin-4 vector were stimulated with 3 μg/ml prolactin for 0.5 or 24 h. The samples (25 μg of protein each, including Matrigel) were subjected to Western blotting using the indicated Abs. Actin was used as a loading control. C, expression of nectin-1 and nectin-2 in EpH4 cells and the effect of the knockdown of nectin-1 on the expression of nectin-2. EpH4 cells were infected with a retrovirus encoding the control shRNA (Control) or the shRNA sequence targeting nectin-1 (shNectin-1 #1 or shNectin-1 #2). The samples (25 μg of protein each, including Matrigel) were subjected to Western blotting using the indicated Abs. Actin was used as a loading control. D, reduction of the prolactin-induced STAT5 tyrosine phosphorylation following nectin-1 knockdown. EpH4 cells stably expressing the control or nectin-1 shRNAs were stimulated with 3 μg/ml prolactin for 30 min. The samples (25 μg of protein each, including Matrigel) were subjected to Western blotting using the indicated Abs. The values indicate relative STAT5 tyrosine phosphorylation when control was set at 1.0. E, nectin-1- and nectin-4-mediated enhancement of the STAT5 tyrosine phosphorylation. EpH4 cells stably expressing the empty, FLAG-nectin-1, or FLAG-nectin-4 vector were stimulated with 3 μg/ml prolactin for 30 min. The samples were subjected to Western blotting using the indicated Abs. The values indicate relative STAT5 tyrosine phosphorylation when control was set at 1.0. Results are representative of three independent experiments. IB, immunoblotting; pSTAT5, phospho-STAT5.
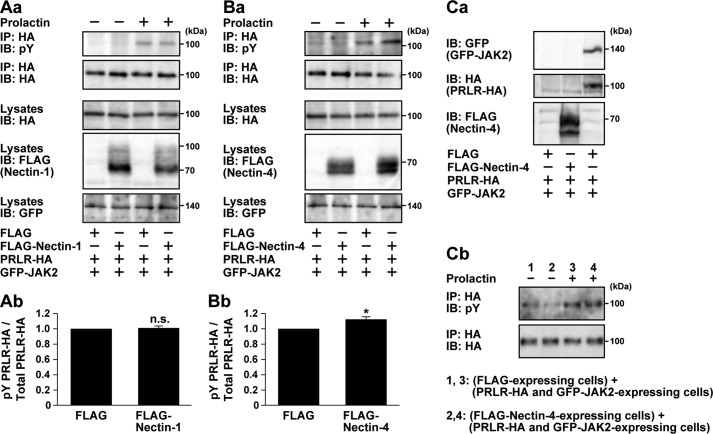
It was also previously shown that prolactin induced the JAK2-mediated prolactin receptor tyrosine phosphorylation (41). Thus, we examined whether nectin-1 and nectin-4 enhanced this reaction. HA-tagged prolactin receptor and GFP-tagged JAK2 were co-expressed with FLAG-tagged nectin-1 or nectin-4 or FLAG tag alone in HEK293E cells. The cells were incubated in the presence and absence of prolactin under the suspension culture condition. HA-tagged prolactin receptor was immunoprecipitated with an anti-HA pAb followed by Western blotting with an anti-HA mAb and an anti-phosphotyrosine mAb. The HA-tagged prolactin receptor tyrosine phosphorylation was enhanced in the presence of prolactin as described previously (41), and this prolactin-induced reaction was further enhanced by co-expression with FLAG-tagged nectin-4 but not nectin-1 or FLAG tag alone (Fig. 9, A and B). We further examined whether the trans-interaction between nectin-4 and the prolactin receptor modulates receptor signaling. HEK293E cells expressing FLAG-nectin-4 and HEK293E cells expressing the HA-tagged prolactin receptor and GFP-tagged JAK2 were co-cultured on a dish to induce the trans-interaction between nectin-4 and the prolactin receptor, if any, and stimulated with prolactin. The HA-tagged prolactin receptor tyrosine phosphorylation was not enhanced when the HEK293E cells expressing both the HA-tagged prolactin receptor and GFP-tagged JAK2 were attached to the HEK293E cells expressing FLAG-nectin-4 (Fig. 9C). These results indicate that the cis-interaction, not the trans-interaction, of nectin-4 with the prolactin receptor is able to enhance the prolactin-induced JAK2-mediated prolactin receptor tyrosine phosphorylation in vitro. The reason for the inability of the cis-interaction of nectin-1 with the prolactin receptor to enhance this reaction is unknown even though nectin-1 enhanced the prolactin-induced STAT5 tyrosine phosphorylation in EpH4 cells.
FIGURE 9.
Nectin-4-mediated enhancement of the prolactin-induced prolactin receptor tyrosine phosphorylation. A, no effect of nectin-1 on the prolactin-induced prolactin receptor tyrosine phosphorylation. A, panel a, HEK293E cells were co-transfected with various combinations of the indicated plasmids. The cells were serum-starved for 24 h and stimulated with 3 μg/ml prolactin for 10 min. The HA-tagged prolactin receptor (PRLR-HA) was immunoprecipitated with an anti-HA Ab, and the samples were subjected to Western blotting using the indicated Abs. Results are representative of three independent experiments. A, panel b, the band intensity of the phosphorylated PRLR-HA (pY PRLR-HA) was normalized to that of the total PRLR-HA, and the normalized value of the control FLAG-transfected cells stimulated with prolactin was set as 1.0. n.s., not significant. Error bars, S.E. B, nectin-4-mediated enhancement of the prolactin-induced prolactin receptor tyrosine phosphorylation. B, panel a, HEK293E cells were co-transfected with various combinations of the indicated plasmids. The cells were serum-starved for 24 h and stimulated with 3 μg/ml prolactin for 10 min. The HA-tagged prolactin receptor was immunoprecipitated with an anti-HA Ab, and the samples were subjected to Western blotting using the indicated Abs. Results are representative of three independent experiments. B, panel b, the band intensity of the phosphorylated PRLR-HA was normalized to that of the total PRLR-HA, and the normalized value of the control FLAG-transfected cells stimulated with prolactin was set as 1.0. n.s., not significant. *, p = 0.014 versus control as determined by Student's t test. Error bars, S.E. C, no enhancement of the prolactin receptor tyrosine phosphorylation when nectin-4 and the prolactin receptor were engaged in trans. C, panel a, preparation of the cells expressing FLAG alone, FLAG-nectin-4 alone, and both the PRLR-HA and GFP-JAK2. The cells were transfected with various combinations of the indicated plasmids, and the samples were subjected to Western blotting using the indicated Abs. C, panel b, no effect of nectin-4 on the prolactin-induced prolactin receptor tyrosine phosphorylation when both molecules were engaged in trans. HEK293E cells expressing FLAG-nectin-4 and HEK293E cells expressing PRLR-HA and GFP-JAK2 were co-cultured on a dish to induce trans-interaction between nectin-4 and the prolactin receptor. The cells were serum-starved for 24 h and stimulated with 3 μg/ml prolactin for 10 min. The PRLR-HA was immunoprecipitated with an anti-HA Ab, and the samples were subjected to Western blotting using the indicated Abs. Results are representative of three independent experiments. IP, immunoprecipitation; IB, immunoblotting; pY, phosphotyrosine.
Discussion
In this study, we found a novel type of cell adhesion apparatus mediated by nectin-1 and nectin-4 at the boundary between the luminal and basal cells in the mammary gland as shown in the schematic in Fig. 1B. This novel apparatus was mainly formed and/or maintained by the trans-interaction between nectin-4 in the luminal cells and nectin-1 in the basal cells. At this boundary, only desmosomes were previously known to be located (2, 3), although the presence of gap junctions has been controversial (4). However, the present results indicate the presence of gap junctions at this boundary. Therefore, at least three types of cell adhesion apparatuses are located at this boundary. Because desmosomes were not disrupted in the absence of the novel apparatus, this apparatus is not required for this heterotypic cell attachment. The results, indicating that other apparatuses, such as AJs, TJs, gap junctions, and desmosomes, were not affected in the absence of this novel apparatus, indicate that it is not required for the formation and/or maintenance of these other apparatuses.
The pregnant nectin-1 KO mammary gland showed insufficient alveolar development, including a suppression of alveolar development, abnormal morphologies of the ducts and alveoli, and reduced de novo synthesis of the milk protein, indicating that nectin-1 is involved in alveolar development with lactogenic differentiation. This impairment in lactation explains why the nectin-1 KO female mice failed to breastfeed their pups. A major hormone involved in the pregnancy-induced alveolar development with lactogenic differentiation is prolactin (38). Prolactin binds to its receptor in the luminal cells (40) and induces activation of JAK2 tyrosine kinase, which tyrosine phosphorylates the prolactin receptor. In addition, JAK2 tyrosine phosphorylates STAT5, which induces the transcription of many genes, including β-casein, which is necessary for alveolar development. Herein we showed that both nectin-1 and nectin-4 could cis-interact with the prolactin receptor to enhance its signaling for alveolar development with lactogenic differentiation in vitro. However, the prolactin receptor is expressed in the luminal cells (40), and nectin-4, but not nectin-1, in the luminal cells was mainly involved in the formation of a novel apparatus by trans-interacting with nectin-1 in the basal cells at the boundary between these two cell types in vivo. In the luminal cells, free nectin-1, which does not trans-interact with nectin-1 in the basal cells, may be present, but the affinity of the prolactin receptor for this free nectin-1 outside the apparatus is speculated to be lower than that for nectin-4 of the apparatus. Therefore, nectin-4 of the novel apparatus is most likely to cis-interact with the prolactin receptor in the luminal cells in vivo. We attempted to show this cis-interaction of the apparatus with the prolactin receptor in vivo but did not succeed because there is currently no available Ab that detects the receptor in the mammary gland in vivo. Instead of this approach, we used a cell culture system to show that nectin-4 of this apparatus was able to cis-interact with the prolactin receptor. Furthermore, the result that the genetic ablation of nectin-1 disrupted the novel apparatus and reduced alveolar development indicates that the trans-interaction between nectin-1 in the basal cells and nectin-4 in the luminal cells is essential for the formation and/or maintenance of this apparatus and alveolar development. Taken together, it is likely that nectin-1 in the basal cells is involved in the formation of a novel apparatus by trans-interacting with nectin-4 in the luminal cells and that nectin-4 of this apparatus cis-interacts with the prolactin receptor in the luminal cells and regulates its signaling for alveolar development with lactogenic differentiation as shown in the schematic in Fig. 1B. In the nectin-1 KO mice mammary gland, it is likely that not only nectin-4 but also the prolactin receptor in the luminal cells is no longer concentrated at the novel apparatus, and thereby sufficient signaling for alveolar development with lactogenic differentiation could not be transduced. Thus, this apparatus may serve as a platform for the prolactin receptor signaling. The reason why there are phenotypes only on the C57BL/6 background but not on the 129/Sv-C57BL/6 mixed background still remains unknown. Further studies are required for better understanding of the underlying mechanisms.
It is unknown whether the interaction between nectin-4 and the prolactin receptor is direct or indirect because we just observed the interaction by a co-immunoprecipitation assay. We previously showed physical and functional interactions between the nectin and nectin-like molecule (Necl) family proteins and receptors, such as nectin-3 and the PDGF receptor, Necl-5 and the PDGF receptor, Necl-2 and ErbB3, Necl-4 and ErbB3, Necl-4 and the VEGF receptor, and Necl-5 and the VEGF receptor (26, 42–46). Most of these studies were done in transfected cell lines overexpressing these proteins, and it still remains unknown whether these interactions are direct or indirect. The direct interaction was reported only between nectin-1 and the FGF receptor (47). In this report, it was shown that the third Ig module of nectin-1 directly interacts with the FGF receptor as estimated by surface plasmon resonance. Because it is of importance to examine whether the interaction between nectin-4 and the prolactin receptor is direct or indirect, we attempted to address this issue using the purified recombinant protein FLAG-nectin-4 or FLAG-nectin-4ΔCP and the extracellular fragment of the prolactin receptor fused to the human IgG Fc, but we have not yet succeeded in showing the direct interaction between these proteins under the conditions thus far examined (data not shown). One possibility is that the interaction between these molecules is indirect so that the interaction could not be reconstituted by using the recombinant proteins without unidentified interaction-mediating factor(s). Another possibility is that the affinity between the whole extracellular region of nectin-4 and the prolactin receptor was not high enough to detect their direct interaction. To better our understanding of the regulatory mechanisms of receptors by the nectin and Necl family proteins, future detailed examination of whether the interactions not only between nectin-4 and the prolactin receptor but also between other nectin and Necl family proteins and receptors are direct or indirect is needed.
Prolactin regulates cell proliferation and differentiation in not only the mammary gland but also the ovary and prostate (48, 49) and is involved in development of breast, prostate, and colorectal cancers (50–53). Nectin-4 is expressed in many organs (33), and mutations in nectin-4 cause ectodermal dysplasia-syndactyly syndrome 1 (54–57). The expression of nectin-4 is up-regulated in several cancers, including breast cancer (24, 58–65). Nectin-4 serves as the entry receptor for measles virus (66, 67). In addition, nectin-1 and nectin-3 cis-interact with the FGF receptor and the PDGF receptor, respectively (42, 47). Therefore, the novel cell adhesion apparatus identified here may also serve as a platform for cellular signaling and play physiological and pathological roles not only in the mammary gland but also in other organs.
Author Contributions
Y. T. conceived the research project. K. Mizutani, Y. S., K. Mandai, and Y. T. designed the experiments. M. K., K. Mizutani, M. M., S. S., Y. U., K. Mandai, and Y. S. performed the experiments. M. K., K. Mizutani, M. M., K. Mandai, Y. S., T. K., and Y. T. analyzed the data. M. K., K. Mizutani, M. M., Y. S, and Y. T. wrote the paper.
Acknowledgments
We thank Dr. S. Kurita (Shiga University of Medical Science) for technical assistance, Drs. T. Kishimoto (Osaka University), T. Naka (National Institute of Biomedical Innovation), and M. Takeichi (Center for Developmental Biology, RIKEN) for generous gifts of reagents, and Dr. H. Takahashi (Kobe University) for helpful discussions.
This work was supported by Grants-in-aid for Scientific Research 21227005 and 26251013 (to Y. T.), 23501268 (to Y. S.), and 26860190 (to K. Mizutani) from the Japan Society for the Promotion of Science; Grants-in-aid for Scientific Research on Innovative Areas 26114007 (to Y. T.) and 23130510 and 25130707 (to Y. S.) from the Ministry of Education, Culture, Sports, Science, and Technology; and the Japan Foundation for Applied Enzymology (to Y. T.). The authors declare no competing financial interests.
- TJ
- tight junction
- AJ
- adherens junction
- CAM
- cell adhesion molecule
- F-actin
- actin filament
- ZO
- zonula occludens
- Ab
- antibody
- pAb
- polyclonal antibody
- Nef-1
- the extracellular fragment of nectin-1 fused to the human IgG Fc
- Necl
- nectin-like molecule
- L1
- lactating day 1
- P18.5
- pregnancy day 18.5.
References
- 1. Muschler J., and Streuli C. H. (2010) Cell-matrix interactions in mammary gland development and breast cancer. Cold Spring Harb. Perspect. Biol. 2, a003202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pitelka D. R., Hamamoto S. T., Duafala J. G., and Nemanic M. K. (1973) Cell contacts in the mouse mammary gland. I. Normal gland in postnatal development and the secretory cycle. J. Cell Biol. 56, 797–818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Monaghan P., Perusinghe N., Carlile G., and Evans W. H. (1994) Rapid modulation of gap junction expression in mouse mammary gland during pregnancy, lactation, and involution. J. Histochem. Cytochem. 42, 931–938 [DOI] [PubMed] [Google Scholar]
- 4. McLachlan E., Shao Q., and Laird D. W. (2007) Connexins and gap junctions in mammary gland development and breast cancer progression. J. Membr. Biol. 218, 107–121 [DOI] [PubMed] [Google Scholar]
- 5. Oda H., and Takeichi M. (2011) Evolution: structural and functional diversity of cadherin at the adherens junction. J. Cell Biol. 193, 1137–1146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Shimono Y., Rikitake Y., Mandai K., Mori M., and Takai Y. (2012) Immunoglobulin superfamily receptors and adherens junctions. Subcell. Biochem. 60, 137–170 [DOI] [PubMed] [Google Scholar]
- 7. Balda M. S., and Matter K. (2008) Tight junctions at a glance. J. Cell Sci. 121, 3677–3682 [DOI] [PubMed] [Google Scholar]
- 8. Garrod D. R., Merritt A. J., and Nie Z. (2002) Desmosomal cadherins. Curr. Opin. Cell Biol. 14, 537–545 [DOI] [PubMed] [Google Scholar]
- 9. Sáez J. C., Retamal M. A., Basilio D., Bukauskas F. F., and Bennett M. V. (2005) Connexin-based gap junction hemichannels: gating mechanisms. Biochim. Biophys. Acta 1711, 215–224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lanigan F., O'Connor D., Martin F., and Gallagher W. M. (2007) Molecular links between mammary gland development and breast cancer. Cell. Mol. Life Sci. 64, 3159–3184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Brisken C., and O'Malley B. (2010) Hormone action in the mammary gland. Cold Spring Harb. Perspect. Biol. 2, a003178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Watson C. J., and Khaled W. T. (2008) Mammary development in the embryo and adult: a journey of morphogenesis and commitment. Development 135, 995–1003 [DOI] [PubMed] [Google Scholar]
- 13. Cowin P., and Wysolmerski J. (2010) Molecular mechanisms guiding embryonic mammary gland development. Cold Spring Harb. Perspect. Biol. 2, a003251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Macias H., and Hinck L. (2012) Mammary gland development. Wiley Interdiscip. Rev. Dev. Biol. 1, 533–557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hung M. C. (2012) On mammary gland growth factors: roles in normal development and in cancer. Cold Spring Harb. Perspect. Biol. 4, a013532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Inagaki M., Irie K., Ishizaki H., Tanaka-Okamoto M., Morimoto K., Inoue E., Ohtsuka T., Miyoshi J., and Takai Y. (2005) Roles of cell-adhesion molecules nectin 1 and nectin 3 in ciliary body development. Development 132, 1525–1537 [DOI] [PubMed] [Google Scholar]
- 17. Mizoguchi A., Nakanishi H., Kimura K., Matsubara K., Ozaki-Kuroda K., Katata T., Honda T., Kiyohara Y., Heo K., Higashi M., Tsutsumi T., Sonoda S., Ide C., and Takai Y. (2002) Nectin: an adhesion molecule involved in formation of synapses. J. Cell Biol. 156, 555–565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Togashi H., Miyoshi J., Honda T., Sakisaka T., Takai Y., and Takeichi M. (2006) Interneurite affinity is regulated by heterophilic nectin interactions in concert with the cadherin machinery. J. Cell Biol. 174, 141–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Honda T., Sakisaka T., Yamada T., Kumazawa N., Hoshino T., Kajita M., Kayahara T., Ishizaki H., Tanaka-Okamoto M., Mizoguchi A., Manabe T., Miyoshi J., and Takai Y. (2006) Involvement of nectins in the formation of puncta adherentia junctions and the mossy fiber trajectory in the mouse hippocampus. Mol. Cell. Neurosci. 31, 315–325 [DOI] [PubMed] [Google Scholar]
- 20. Suzuki K., Hu D., Bustos T., Zlotogora J., Richieri-Costa A., Helms J. A., and Spritz R. A. (2000) Mutations of PVRL1, encoding a cell-cell adhesion molecule/herpesvirus receptor, in cleft lip/palate-ectodermal dysplasia. Nat. Genet. 25, 427–430 [DOI] [PubMed] [Google Scholar]
- 21. Matsushima H., Utani A., Endo H., Matsuura H., Kakuta M., Nakamura Y., Matsuyoshi N., Matsui C., Nakanishi H., Takai Y., and Shinkai H. (2003) The expression of nectin-1α in normal human skin and various skin tumours. Br. J. Dermatol. 148, 755–762 [DOI] [PubMed] [Google Scholar]
- 22. Guzman G., Oh S., Shukla D., and Valyi-Nagy T. (2006) Nectin-1 expression in the normal and neoplastic human uterine cervix. Arch. Pathol. Lab. Med. 130, 1193–1195 [DOI] [PubMed] [Google Scholar]
- 23. Kälin M., Cima I., Schiess R., Fankhauser N., Powles T., Wild P., Templeton A., Cerny T., Aebersold R., Krek W., and Gillessen S. (2011) Novel prognostic markers in the serum of patients with castration-resistant prostate cancer derived from quantitative analysis of the pten conditional knockout mouse proteome. Eur. Urol. 60, 1235–1243 [DOI] [PubMed] [Google Scholar]
- 24. Ballester M., Gonin J., Rodenas A., Bernaudin J. F., Rouzier R., Coutant C., and Daraï E. (2012) Eutopic endometrium and peritoneal, ovarian and colorectal endometriotic tissues express a different profile of nectin-1, -3, -4 and nectin-like molecule 2. Hum. Reprod. 27, 3179–3186 [DOI] [PubMed] [Google Scholar]
- 25. Sakamoto Y., Ogita H., Hirota T., Kawakatsu T., Fukuyama T., Yasumi M., Kanzaki N., Ozaki M., and Takai Y. (2006) Interaction of integrin αvβ3 with nectin. Implication in cross-talk between cell-matrix and cell-cell junctions. J. Biol. Chem. 281, 19631–19644 [DOI] [PubMed] [Google Scholar]
- 26. Kawano S., Ikeda W., Kishimoto M., Ogita H., and Takai Y. (2009) Silencing of ErbB3/ErbB2 Signaling by immunoglobulin-like Necl-2. J. Biol. Chem. 284, 23793–23805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Honda T., Shimizu K., Kawakatsu T., Yasumi M., Shingai T., Fukuhara A., Ozaki-Kuroda K., Irie K., Nakanishi H., and Takai Y. (2003) Antagonistic and agonistic effects of an extracellular fragment of nectin on formation of E-cadherin-based cell-cell adhesion. Genes Cells 8, 51–63 [DOI] [PubMed] [Google Scholar]
- 28. Kurita S., Ogita H., and Takai Y. (2011) Cooperative role of nectin-nectin and nectin-afadin interactions in formation of nectin-based cell-cell adhesion. J. Biol. Chem. 286, 36297–36303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Xu R., Nelson C. M., Muschler J. L., Veiseh M., Vonderhaar B. K., and Bissell M. J. (2009) Sustained activation of STAT5 is essential for chromatin remodeling and maintenance of mammary-specific function. J. Cell Biol. 184, 57–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Daniel C. W., Strickland P., and Friedmann Y. (1995) Expression and functional role of E- and P-cadherins in mouse mammary ductal morphogenesis and growth. Dev. Biol. 169, 511–519 [DOI] [PubMed] [Google Scholar]
- 31. Blackman B., Russell T., Nordeen S. K., Medina D., and Neville M. C. (2005) Claudin 7 expression and localization in the normal murine mammary gland and murine mammary tumors. Breast Cancer Res. 7, R248–R255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Akhtar N., and Streuli C. H. (2013) An integrin-ILK-microtubule network orients cell polarity and lumen formation in glandular epithelium. Nat. Cell Biol. 15, 17–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Reymond N., Fabre S., Lecocq E., Adelaïde J., Dubreuil P., and Lopez M. (2001) Nectin4/PRR4, a new afadin-associated member of the nectin family that trans-interacts with nectin1/PRR1 through V domain interaction. J. Biol. Chem. 276, 43205–43215 [DOI] [PubMed] [Google Scholar]
- 34. Harrison O. J., Vendome J., Brasch J., Jin X., Hong S., Katsamba P. S., Ahlsen G., Troyanovsky R. B., Troyanovsky S. M., Honig B., and Shapiro L. (2012) Nectin ectodomain structures reveal a canonical adhesive interface. Nat. Struct. Mol. Biol. 19, 906–915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Fabre S., Reymond N., Cocchi F., Menotti L., Dubreuil P., Campadelli-Fiume G., and Lopez M. (2002) Prominent role of the Ig-like V domain in trans-interactions of nectins. Nectin3 and nectin4 bind to the predicted C-C′-C″-D β-strands of the nectin1 V domain. J. Biol. Chem. 277, 27006–27013 [DOI] [PubMed] [Google Scholar]
- 36. Gouilleux F., Wakao H., Mundt M., and Groner B. (1994) Prolactin induces phosphorylation of Tyr694 of Stat5 (MGF), a prerequisite for DNA binding and induction of transcription. EMBO J. 13, 4361–4369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Liu X., Robinson G. W., Gouilleux F., Groner B., and Hennighausen L. (1995) Cloning and expression of Stat5 and an additional homologue (Stat5b) involved in prolactin signal transduction in mouse mammary tissue. Proc. Natl. Acad. Sci. U.S.A. 92, 8831–8835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Oakes S. R., Rogers R. L., Naylor M. J., and Ormandy C. J. (2008) Prolactin regulation of mammary gland development. J. Mammary Gland Biol. Neoplasia 13, 13–28 [DOI] [PubMed] [Google Scholar]
- 39. Liu X., Robinson G. W., and Hennighausen L. (1996) Activation of Stat5a and Stat5b by tyrosine phosphorylation is tightly linked to mammary gland differentiation. Mol. Endocrinol. 10, 1496–1506 [DOI] [PubMed] [Google Scholar]
- 40. Bera T. K., Hwang S. I., Swanson S. M., Guzman R. C., Edery M., and Nandi S. (1994) In situ localization of prolactin receptor message in the mammary glands of pituitary-isografted mice. Mol. Cell. Biochem. 132, 145–149 [DOI] [PubMed] [Google Scholar]
- 41. Lebrun J. J., Ali S., Sofer L., Ullrich A., and Kelly P. A. (1994) Prolactin-induced proliferation of Nb2 cells involves tyrosine phosphorylation of the prolactin receptor and its associated tyrosine kinase JAK2. J. Biol. Chem. 269, 14021–14026 [PubMed] [Google Scholar]
- 42. Kanzaki N., Ogita H., Komura H., Ozaki M., Sakamoto Y., Majima T., Ijuin T., Takenawa T., and Takai Y. (2008) Involvement of the nectin-afadin complex in PDGF-induced cell survival. J. Cell Sci. 121, 2008–2017 [DOI] [PubMed] [Google Scholar]
- 43. Amano H., Ikeda W., Kawano S., Kajita M., Tamaru Y., Inoue N., Minami Y., Yamada A., and Takai Y. (2008) Interaction and localization of Necl-5 and PDGF receptor β at the leading edges of moving NIH3T3 cells: implications for directional cell movement. Genes Cells 13, 269–284 [DOI] [PubMed] [Google Scholar]
- 44. Sugiyama H., Mizutani K., Kurita S., Okimoto N., Shimono Y., and Takai Y. (2013) Interaction of Necl-4/CADM4 with ErbB3 and integrin α6β4 and inhibition of ErbB2/ErbB3 signaling and hemidesmosome disassembly. Genes Cells 18, 519–528 [DOI] [PubMed] [Google Scholar]
- 45. Yamana S., Tokiyama A., Mizutani K., Hirata K., Takai Y., and Rikitake Y. (2015) The cell adhesion molecule Necl-4/CADM4 serves as a novel regulator for contact inhibition of cell movement and proliferation. PLoS One 10, e0124259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kinugasa M., Amano H., Satomi-Kobayashi S., Nakayama K., Miyata M., Kubo Y., Nagamatsu Y., Kurogane Y., Kureha F., Yamana S., Hirata K., Miyoshi J., Takai Y., and Rikitake Y. (2012) Necl-5/poliovirus receptor interacts with VEGFR2 and regulates VEGF-induced angiogenesis. Circ. Res. 110, 716–726 [DOI] [PubMed] [Google Scholar]
- 47. Bojesen K. B., Clausen O., Rohde K., Christensen C., Zhang L., Li S., Køhler L., Nielbo S., Nielsen J., Gjørlund M. D., Poulsen F. M., Bock E., and Berezin V. (2012) Nectin-1 binds and signals through the fibroblast growth factor receptor. J. Biol. Chem. 287, 37420–37433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ahonen T. J., Härkönen P. L., Laine J., Rui H., Martikainen P. M., and Nevalainen M. T. (1999) Prolactin is a survival factor for androgen-deprived rat dorsal and lateral prostate epithelium in organ culture. Endocrinology 140, 5412–5421 [DOI] [PubMed] [Google Scholar]
- 49. Russell D. L., and Richards J. S. (1999) Differentiation-dependent prolactin responsiveness and stat (signal transducers and activators of transcription) signaling in rat ovarian cells. Mol. Endocrinol. 13, 2049–2064 [DOI] [PubMed] [Google Scholar]
- 50. Tworoger S. S., Eliassen A. H., Sluss P., and Hankinson S. E. (2007) A prospective study of plasma prolactin concentrations and risk of premenopausal and postmenopausal breast cancer. J. Clin. Oncol. 25, 1482–1488 [DOI] [PubMed] [Google Scholar]
- 51. Harvey P. W., Everett D. J., and Springall C. J. (2008) Adverse effects of prolactin in rodents and humans: breast and prostate cancer. J. Psychopharmacol. 22, 20–27 [DOI] [PubMed] [Google Scholar]
- 52. Harbaum L., Pollheimer M. J., Bauernhofer T., Kornprat P., Lindtner R. A., Schlemmer A., Rehak P., and Langner C. (2010) Clinicopathological significance of prolactin receptor expression in colorectal carcinoma and corresponding metastases. Mod. Pathol. 23, 961–971 [DOI] [PubMed] [Google Scholar]
- 53. Neradugomma N. K., Subramaniam D., Tawfik O. W., Goffin V., Kumar T. R., Jensen R. A., and Anant S. (2014) Prolactin signaling enhances colon cancer stemness by modulating Notch signaling in a Jak2-STAT3/ERK manner. Carcinogenesis 35, 795–806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Brancati F., Fortugno P., Bottillo I., Lopez M., Josselin E., Boudghene-Stambouli O., Agolini E., Bernardini L., Bellacchio E., Iannicelli M., Rossi A., Dib-Lachachi A., Stuppia L., Palka G., Mundlos S., Stricker S., Kornak U., Zambruno G., and Dallapiccola B. (2010) Mutations in PVRL4, encoding cell adhesion molecule nectin-4, cause ectodermal dysplasia-syndactyly syndrome. Am. J. Hum. Genet. 87, 265–273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Jelani M., Chishti M. S., and Ahmad W. (2011) Mutation in PVRL4 gene encoding nectin-4 underlies ectodermal-dysplasia-syndactyly syndrome (EDSS1). J. Hum. Genet. 56, 352–357 [DOI] [PubMed] [Google Scholar]
- 56. Fortugno P., Josselin E., Tsiakas K., Agolini E., Cestra G., Teson M., Santer R., Castiglia D., Novelli G., Dallapiccola B., Kurth I., Lopez M., Zambruno G., and Brancati F. (2014) Nectin-4 mutations causing ectodermal dysplasia with syndactyly perturb the rac1 pathway and the kinetics of adherens junction formation. J. Invest. Dermatol. 134, 2146–2153 [DOI] [PubMed] [Google Scholar]
- 57. Raza S. I., Nasser Dar R., Shah A. A., and Ahmad W. (2015) A novel homozygous nonsense mutation in the PVRL4 gene and expansion of clinical spectrum of EDSS1. Ann. Hum. Genet. 79, 92–98 [DOI] [PubMed] [Google Scholar]
- 58. Fabre-Lafay S., Garrido-Urbani S., Reymond N., Gonçalves A., Dubreuil P., and Lopez M. (2005) Nectin-4, a new serological breast cancer marker, is a substrate for tumor necrosis factor-α-converting enzyme (TACE)/ADAM-17. J. Biol. Chem. 280, 19543–19550 [DOI] [PubMed] [Google Scholar]
- 59. Fabre-Lafay S., Monville F., Garrido-Urbani S., Berruyer-Pouyet C., Ginestier C., Reymond N., Finetti P., Sauvan R., Adélaïde J., Geneix J., Lecocq E., Popovici C., Dubreuil P., Viens P., Gonçalves A., Charafe-Jauffret E., Jacquemier J., Birnbaum D., and Lopez M. (2007) Nectin-4 is a new histological and serological tumor associated marker for breast cancer. BMC Cancer 7, 73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Takano A., Ishikawa N., Nishino R., Masuda K., Yasui W., Inai K., Nishimura H., Ito H., Nakayama H., Miyagi Y., Tsuchiya E., Kohno N., Nakamura Y., and Daigo Y. (2009) Identification of nectin-4 oncoprotein as a diagnostic and therapeutic target for lung cancer. Cancer Res. 69, 6694–6703 [DOI] [PubMed] [Google Scholar]
- 61. Derycke M. S., Pambuccian S. E., Gilks C. B., Kalloger S. E., Ghidouche A., Lopez M., Bliss R. L., Geller M. A., Argenta P. A., Harrington K. M., and Skubitz A. P. (2010) Nectin 4 overexpression in ovarian cancer tissues and serum: potential role as a serum biomarker. Am. J. Clin. Pathol. 134, 835–845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Athanassiadou A. M., Patsouris E., Tsipis A., Gonidi M., and Athanassiadou P. (2011) The significance of survivin and nectin-4 expression in the prognosis of breast carcinoma. Folia Histochem. Cytobiol. 49, 26–33 [DOI] [PubMed] [Google Scholar]
- 63. Pavlova N. N., Pallasch C., Elia A. E., Braun C. J., Westbrook T. F., Hemann M., and Elledge S. J. (2013) A role for PVRL4-driven cell-cell interactions in tumorigenesis. Elife 2, e00358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Lattanzio R., Ghasemi R., Brancati F., Sorda R. L., Tinari N., Perracchio L., Iacobelli S., Mottolese M., Natali P. G., and Piantelli M. (2014) Membranous nectin-4 expression is a risk factor for distant relapse of T1-T2, N0 luminal-A early breast cancer. Oncogenesis 3, e118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Nishiwada S., Sho M., Yasuda S., Shimada K., Yamato I., Akahori T., Kinoshita S., Nagai M., Konishi N., and Nakajima Y. (2015) Nectin-4 expression contributes to tumor proliferation, angiogenesis and patient prognosis in human pancreatic cancer. J. Exp. Clin. Cancer Res. 34, 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Noyce R. S., Bondre D. G., Ha M. N., Lin L. T., Sisson G., Tsao M. S., and Richardson C. D. (2011) Tumor cell marker PVRL4 (nectin 4) is an epithelial cell receptor for measles virus. PLoS Pathog. 7, e1002240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Mühlebach M. D., Mateo M., Sinn P. L., Prüfer S., Uhlig K. M., Leonard V. H., Navaratnarajah C. K., Frenzke M., Wong X. X., Sawatsky B., Ramachandran S., McCray P. B. Jr., Cichutek K., von Messling V., Lopez M., and Cattaneo R. (2011) Adherens junction protein nectin-4 is the epithelial receptor for measles virus. Nature 480, 530–533 [DOI] [PMC free article] [PubMed] [Google Scholar]