Abstract
Saccharomyces cerevisiae Pif1, an SF1B helicase, has been implicated in both mitochondrial and nuclear functions. Here we have characterized the preference of Pif1 for RNA:DNA heteroduplexes in vitro by investigating several kinetic parameters associated with unwinding. We show that the preferential unwinding of RNA:DNA hybrids is due to neither specific binding nor differences in the rate of strand separation. Instead, Pif1 is capable of unwinding RNA:DNA heteroduplexes with moderately greater processivity compared with its duplex DNA:DNA counterparts. This higher processivity of Pif1 is attributed to slower dissociation from RNA:DNA hybrids. Biologically, this preferential role of the helicase may contribute to its functions at both telomeric and nontelomeric sites.
Keywords: DNA binding protein, DNA helicase, molecular motor, pre-steady state kinetics, RNA, yeast
Introduction
Helicases are a diverse group of motor proteins that convert the chemical energy associated with ATP hydrolysis into the mechanical energy required for separation of double-stranded nucleic acids (1). They participate in many different cellular events, ranging from DNA replication to chromatin remodeling (2). Many helicases contain two conserved RecA-like helicase domains with an ATP-binding site at the interface of the core domains (3). Saccharomyces cerevisiae helicase Pif1 is the prototypical member of the Pif1 family of DNA helicases and is conserved from yeast to mammals (4, 5). It was originally discovered in a genetic screen as a gene responsible for the maintenance of yeast mitochondrial DNA (6–8). In its absence, yeast cells are sensitive to UV light and lose mitochondrial DNA at high rates generating respiratory-deficient petite cells (9, 10).
There are two known isoforms of eukaryotic Pif1 helicase: nuclear Pif1 and mitochondrial Pif1, which are crucial for the maintenance of nuclear and mitochondrial genomes, respectively (5). In yeast, Pif1 helicase is suggested to be involved in mitochondrial DNA recombination, repair, and replication (6, 11). Some of its nuclear functions include Okazaki fragment processing (12, 13), inhibition of rDNA replication (14), unwinding of G-quadruplex structures (15–18), telomere regulation (19–21), and repair of double-stranded DNA breaks (22–25). S. cerevisiae Pif1 belongs to the superfamily (SF)2 1B class of helicases. Pif1 is an Escherichia coli RecD homolog and possesses ssDNA-dependent ATPase and 5′ → 3′ directed helicase activities (8). Pif1-like helicases contain a Pif1 family signature sequence (DKLeXvARaiRkqXkPFGGIQ) (5) in addition to the conserved helicase motifs. It is a degenerate sequence located between motifs II and III with a currently unknown function. Other members of the SF1B class include yeast Rrm3, human Pif1 and HelB, E. coli RecD and TraI, and bacteriophage T4 Dda.
Pif1 has been reported to displace telomerase from telomeric DNA in response to DNA damage (20), and telomere length is regulated negatively by Pif1 helicase. It uses its helicase activity to reduce telomerase processivity and releases it from the DNA end (20). This Pif1-mediated telomerase inhibition is believed to prevent aberrant telomere addition to broken DNA ends, thereby promoting DNA repair and genome integrity. The exact biochemical mechanism of telomerase displacement by Pif1 helicase is poorly understood. Studies have reported that RNA:DNA heteroduplexes are unwound preferentially by yeast Pif1 relative to their DNA:DNA counterparts (17, 26). Because telomerase RNA forms a hybrid with telomeric DNA during telomere elongation, the preferential unwinding by Pif1 of RNA:DNA hybrids could possibly explain the phenomenon of telomerase displacement. In the present study, we have investigated the details of unwinding kinetics of DNA:DNA and RNA:DNA duplexes by yeast Pif1 using pre-steady state kinetic studies. We sought to determine the specific kinetic events that lead to preference of RNA:DNA hybrids by Pif1 helicase.
Experimental Procedures
Materials
HEPES, NaCl, MgCl2, EDTA, 2 mm β-mercaptoethanol, BSA, acrylamide, bisacrylamide, KOH, SDS, formamide, xylene cyanol, bromphenol blue, urea, and glycerol were purchased from Fisher Scientific. ATP (disodium salt), and Sephadex G-25 were obtained from Sigma. [γ-32P]ATP was purchased from PerkinElmer Life Sciences. T4 polynucleotide kinase was from Promega. DNA oligonucleotides were purchased from Integrated DNA Technologies, and RNA was from Dharmacon. Oligonucleotides were purified and radiolabeled as described (27). Wild type Pif1 was overexpressed and purified as described (28).
Helicase-mediated Unwinding Assay
Unwinding assays were performed with a KinTek rapid chemical quench flow instrument maintained at 25 °C with a circulating water bath. The reaction buffer consisted of 20 mm Tris, pH 7.5, 50 mm NaCl, 0.1 mg/ml BSA, and 2 mm DTT. The 5′-radiolabeled partial duplex substrate (1 nm) was preincubated with 60 nm Pif1 and the reaction was initiated by addition of 4 mm ATP, 5 mm MgCl2 and a 30-fold excess DNA trap (30 nm) complementary to the unlabeled strand to prevent reannealing of the duplex after unwinding. A protein trap (100 μm T50 in nucleotides) was also added at the initiation of the reaction to prevent rebinding of the enzyme to the substrate after dissociation under single-turnover condition. For multiturnover experiments, the protein trap was excluded from the reaction mixture. Also, the reannealing DNA trap was placed in the quench vial for multiturnover reactions. All concentrations listed are final, i.e. after initiation of the reaction, unless otherwise stated. The reactions were quenched with 400 mm EDTA at increasing times. Nuclease-free reagents were used for the unwinding of RNA substrates to minimize RNase contamination. The duplex substrate and ssDNA product in each reaction were resolved by 20% native PAGE. The gels were exposed to a phosphor screen, imaged using a Typhoon Trio phosphorimaging device (GE Healthcare), and the results were quantified using ImageQuant software. Unwinding data were fit to the scheme in Fig. 1A or 2A using KinTek Global Kinetic Explorer (KinTek Corporation, Austin, TX) (29). The values for various kinetic parameters were obtained and are listed in Tables 2 and 3.
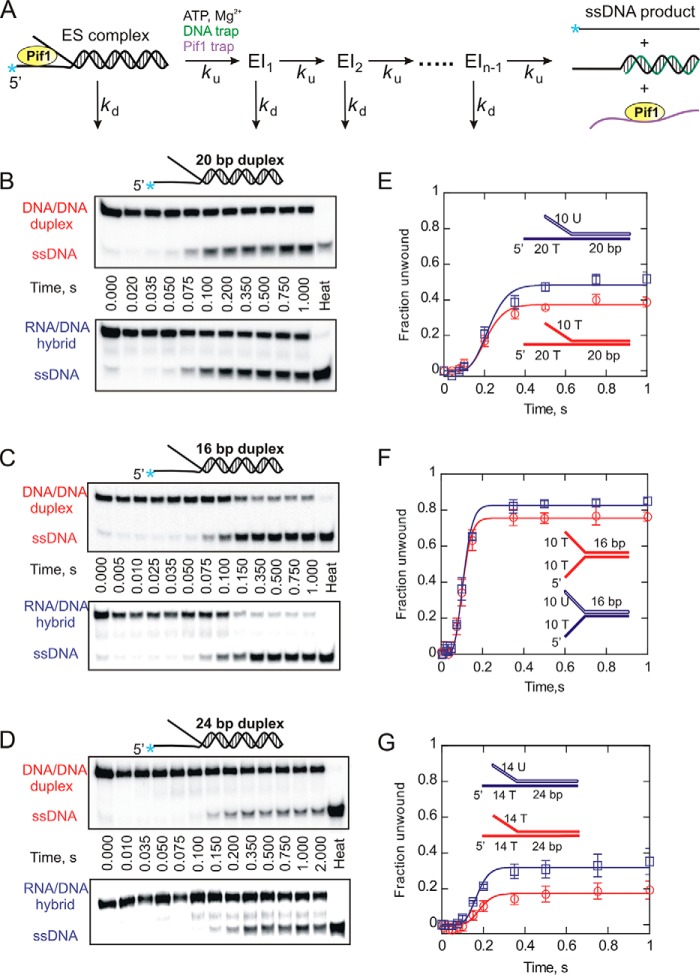
FIGURE 1.
Helicase-catalyzed unwinding kinetics for DNA:DNA (red circles) and RNA:DNA (blue squares) forked duplexes under single turnover (single cycle) conditions. A, a schematic representation of a single cycle unwinding reaction. Pif1 takes a series of n sequential steps to separate duplex strands. ES, enzyme-substrate complex; EI, intermediate. Each step is defined by an unwinding rate constant (ku) and a dissociation rate constant (kd). Unwinding reactions were performed under excess enzyme conditions as described under “Experimental Procedures.” B–D, the representative 20% native PAGE gels for 20- (B), 16- (C), and 24-bp (D) forked substrates show the separation of ssDNA product from forked duplexes. Product formation was plotted against time using Kaleidagraph software. E–G, shown are the reaction progress curves for 20- (E), 16- (F), and 24-bp (G) forked substrates. The data were fit to the scheme shown in A using Kintek Explorer (29). Error bars indicate the standard deviation of 3 independent experiments. Kinetic constants are shown in Tables 2 and 3.
TABLE 2.
Values for unwinding rate constant, ku (s−1) obtained from data fitting using KinTek Global Kinetic Explorer
| Substrate | DNA:DNA | RNA:DNA | Chimera I | Chimera II |
|---|---|---|---|---|
| 16-bp forked duplex | 71.2 ± 8.0a | 74.0 ± 4.3a | NDb | ND |
| 20-bp forked duplex | 53.7 ± 2.4a | 54.2 ± 4.5a | 61.1 ± 7.6a | 66.1 ± 1.4a |
| 16-bp tailed duplex | 68.8 ± 1.5a | 72.5 ± 1.5a | ND | ND |
| 20-bp tailed duplex | ND | 56.4 ± 0.5a | ND | ND |
| 24-bp forked duplex | 65.7 ± 8.0a | 66.8 ± 5.0a | ND | ND |
a Standard deviation of three independent experiments.
b ND, not determined.
TABLE 3.
Values for dissociation rate constant, kd (s−1) obtained from data fitting using KinTek Global Kinetic Explorer
| Substrate | DNA:DNA | RNA:DNA | Chimera I | Chimera II |
|---|---|---|---|---|
| 16-bp forked duplex | 2.8 ± 0.8a | 2.0 ± 0.3a | NDb | ND |
| 20-bp forked duplex | 4.5 ± 0.4a | 3.0 ± 0.2a | 3.8 ± 0.7a | 8.6 ± 0.7a |
| 16-bp tailed duplex | 11.2 ± 0.1a | 8.3 ± 1.3a | ND | ND |
| 20-bp tailed duplex | ND | 6.9 ± 0.4a | ND | ND |
| 24-bp forked duplex | 8.7 ± 1.3a | 6.3 ± 0.6a | ND | ND |
a Standard deviation of three independent experiments.
b ND, not determined.
Fluorescence Anisotropy Binding Assay
To determine the binding affinity of yeast helicase Pif1, fluorescence anisotropy of FAM-labeled partial duplex substrates was measured in buffer (20 mm Tris, pH 7.5, 10 mm NaCl, 0.1 mg/ml BSA, 2 mm DTT, and 5 mm MgCl2). This reaction was performed both in the absence of a nucleotide, as well as in the presence of the nonhydrolyzable ATP analog, AMP-PNP (5 mm). A solution containing fluorescently labeled, 1 nm forked or tailed substrates (5′ F-10T/20T-20bp DNA:DNA fork, 5′ F-10U/20T-r20bp RNA:DNA fork, 5′ F-20T-20bp DNA:DNA tail, and 5′ F 20T-r20bp RNA:DNA tail) was incubated with increasing concentrations of Pif1 (0–500 nm) for 1 h. Each of these duplexes was made with 5′-FAM labeled loading strands. Prior to performing the binding experiments, the duplex substrates were gel purified to eliminate any single-stranded DNA that might interfere with the analysis. Subsequently, fluorescence polarization was measured using a PerkinElmer Life Sciences Victor3 V 1420 with excitation and emission wavelengths set to 485 and 535 nm, respectively. Anisotropy was calculated and plotted against Pif1 concentration using KaleidaGraph software. The data were fit to the quadratic equation to obtain the KD value for each substrate.
ATPase Activity Assay
The ATPase activity of Pif1 was measured in the presence of ssDNA or dsDNA substrates at 25 °C, using a coupled spectrophotometric method (30). The reaction buffer consisted of 5 mm ATP, 10 mm MgCl2, 25 mm HEPES, pH 7.5, 50 mm NaCl, 0.1 mg/ml BSA, 1 mm β-mercaptoethanol, 4 mm phosphoenolpyruvate, 10 units/ml pyruvate kinase, 15 units/ml lactate dehydrogenase, and 0.9 mm NADH. 90 nm Pif1 was added to this reaction mixture. The change in absorbance corresponding to the conversion of NADH to NAD+ was monitored at 380 nm upon addition of 5 μm ssT50, 10T/20T-20bp dsDNA fork, 20T-20bp tailed duplex, 10U/20T-20bp RNA:DNA forked hybrid, or 20T-20bp RNA:DNA tailed substrate. The rate of ATP hydrolysis is linked directly with the NADH oxidation rate. The ATPase activity (s−1) for each substrate was plotted using KaleidaGraph software.
Helicase Dissociation Assay
Pif1 (450 nm) was preincubated with 20-bp forked or tailed duplex substrates (150 nm) in reaction buffer containing 25 mm HEPES, pH 7.5, 50 mm NaCl, 0.1 mm EDTA, 2 mm β-mercaptoethanol, and 10 mm MgCl2. The reactions were initiated in the presence of saturating concentrations of ATP (5 mm) and heparin (2 mg/ml) by rapid mixing in the SX.18MV stopped flow reaction analyzer (Applied Photophysics) at 25 °C. The change in tryptophan fluorescence as the enzyme dissociates over time was monitored using a 320-nm cutoff filter (Newport Optical Filter #FSQ-WG320) with excitation at 280 nm through 1-mm slits.
Results
Single-turnover Unwinding Kinetics of Pif1 Helicase
Yeast Pif1 was previously shown to unwind RNA:DNA heteroduplexes preferentially over dsDNA duplexes (17, 26). To characterize the difference in unwinding kinetics of these substrates by Pif1, we initiated pre-steady state helicase assays under single-turnover conditions. Because Pif1 exhibits a preference for unwinding forked structures (26, 31, 32), we designed two partial duplex substrates each consisting of a 20-bp duplex region with a 20-nucleotide 5′-overhang on the loading strand and a 10-nucleotide 3′-overhang on the displaced strand (Table 1). In the case of the RNA:DNA hybrid, the displaced strand is made up of ribonucleic acid. These substrates are similar to those that have been used in a previous report (26). Helicase unwinding of these substrates was performed under single turnover conditions (Fig. 1B) as described under “Experimental Procedures.” The fraction of substrate unwound was plotted as a function of time (Fig. 1E). The data were fit to an n-step sequential model (Fig. 1A) using KinTek Global Kinetic Explorer (29), and the kinetic parameters obtained are listed in Tables 2 and 3. Interestingly, the results indicate that Pif1 unwinds the 10T/20T-20 bp DNA:DNA fork (ku = 53.7 ± 2.4 s−1) and the 10U/20T-r20 bp RNA:DNA hybrid (ku = 54.2 ± 4.5 s−1) at similar rates. However, a small difference was observed in the amplitudes of the product formation curves with RNA:DNA hybrid fork having the greater amplitude (Fig. 1D).
TABLE 1.
Sequences of partial duplex substrates and single-stranded oligonucleotides
RNA strand is shown in lowercase letters. Uppercase letters indicate DNA strand.
| Substrate | Use | Oligonucleotide sequence |
|---|---|---|
| 10T/10T-16bp DNA:DNA forked duplex | Figs. 1, 2, and 9 | 5′-(T)10CGCTGATGTCGCCTGG-3′ |
| 3′-(T)10GCGACTACAGCGGACC-5′ | ||
| 10U/10T-r16bp RNA:DNA forked duplex | Figs. 1, 2, and 9 | 5′-(T)10CGCTGATGTCGCCTGG-3′ |
| 3′-(u)10gcgacuacagcggacc-5′ | ||
| 10T/20T-20bp DNA:DNA forked duplex | Figs. 1, 2, 3, 4a, 5a, 7, and 8 | 5′-(T)20GCACTCGGATCACCATGGCG-3′ |
| 3′-(T)10CGTGAGCCTAGTGGTACCGC-5′ | ||
| 10U/20T-r20bp RNA:DNA forked duplex | Figs. 1, 2, 3, 4a, 5a, 7, and 8 | 5′-(T)20GCACTCGGATCACCATGGCG-3′ |
| 3′-(u)10cgugagccuagugguaccgc-5′ | ||
| 20T-20bp DNA:DNA tailed duplex | Figs. 4a, 5a, 6, 7, and 8 | 5′-(T)20GCACTCGGATCACCATGGCG-3′ |
| 3′-CGTGAGCCTAGTGGTACCGC-5′ | ||
| 20T-r20bp RNA:DNA tailed duplex | Figs. 4a, 5a, 6, 7, and 8 | 5′-(T)20GCACTCGGATCACCATGGCG-3′ |
| 3′-cgugagccuagugguaccgc-5′ | ||
| 20T-16bp DNA:DNA tailed duplex | Fig. 6 | 5′-(T)20CGCTGATGTCGCCTGG-3′ |
| 3′-GCGACTACAGCGGACC-5′ | ||
| 20T-r16bp RNA:DNA tailed duplex | Fig. 6 | 5′-(T)20CGCTGATGTCGCCTGG-3′ |
| 3′-gcgacuacagcggacc-5′ | ||
| Chimera fork I (10T/20T-r20bp forked duplex) | Fig. 3 | 5′-(T)20GCACTCGGATCACCATGGCG-3′ |
| 3′-(T)10cgugagccuagugguaccgc-5′ | ||
| Chimera fork II (10U/20T-20bp forked duplex) | Fig. 3 | 5′-(T)20GCACTCGGATCACCATGGCG-3′ |
| 3′-(u)10CGTGAGCCTAGTGGTACCGC-5′ | ||
| ssT50 | Fig. 7 | 5′-(T)50-3′ |
| 14T/14T-24bp DNA:DNA forked duplex | Figs. 1 and 2 | 5′-(T)14CGCTGATGTCGCCTGGTACGTCGC-3′ |
| 3′-(T)14GCGACTACAGCGGACCATGCAGCG-5′ | ||
| 14U/14T-24bp RNA:DNA forked duplex | Figs. 1 and 2 | 5′-(T)14CGCTGATGTCGCCTGGTACGTCGC-3′ |
| 3′-(u)14gcgacuacagcggaccaugcagcg-5′ |
To further investigate whether the unwinding rates are similar, we designed two additional forked duplexes. Each substrate consisted of a 16-bp duplex region with a 10-nucleotide overhang on both strands as shown in Table 1 (10T/10T-16 bp DNA:DNA fork, 10U/10T-r16 bp RNA:DNA fork.) The displaced strand in case of the RNA:DNA hybrid is made up of ribonucleic acid. These substrates have shorter duplex length compared with those used in Fig. 1B, being reduced from 20 to 16 bp for accurate measurement of the unwinding rate because nonprocessive helicases unwind shorter duplexes with greater efficiency (32). The length of the loading strand overhang was reduced from 20 to 10 nucleotides to reduce the available initial binding sites for Pif1. Helicase-mediated unwinding of these substrates was performed under single cycle conditions (Fig. 1C), and the fraction of substrate unwound was plotted as a function of time (Fig. 1F). The data were fit to the n-step sequential model shown in Fig. 1A using KinTek Global Kinetic Explorer (29), and the kinetic parameters are listed in Tables 2 and 3. The results indicate that Pif1 unwinds the 10T/10T-16 bp DNA:DNA fork (ku = 71.2 ± 8.0 s−1) and the 10U/10T-r16 bp RNA:DNA hybrid (ku = 74.0 ± 4.3 s−1) at a similar rate as to the 20-bp forked duplexes. Also, there is an overall decrease in the lag phase and an overall increase in the amplitude of the reaction progress curves for 16-bp substrates (Fig. 1F) compared with 20-bp forks (Fig. 1E) because of their shorter duplex length. To extend our analysis to a wider window of duplex lengths, we considered performing Pif1-catalyzed unwinding of yet another set of substrates that consisted of a 24-bp duplex region with 14 nucleotide overhang on either strand (Table 1). Because of the longer duplex length of this set of substrates and the nonprocessive nature of Pif helicase, the overall amplitude of product formation was reduced compared with the 16- and 20-bp duplexes. However, the single-turnover experiments again revealed that both the 24-bp DNA:DNA (ku = 65.7 ± 8.0 s−1) and RNA:DNA (ku = 66.8 ± 5.0 s−1) forked substrates are unwound at essentially the same rate, shown in Fig. 1 (D and G). Thus, our results show that the measured unwinding rate constants for both RNA:DNA hybrids and dsDNA duplexes are similar (Table 2), and small differences in the amplitude of the product formation curves were observed with the RNA:DNA substrate being unwound more efficiently.
Multiple Turnover Unwinding Kinetics of Pif1 Helicase
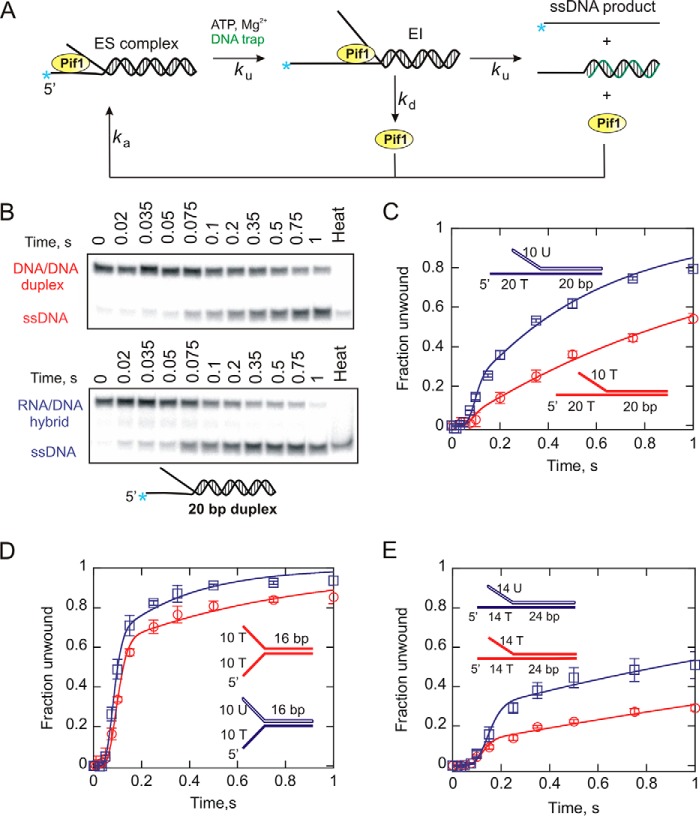
We further investigated Pif1-catalyzed unwinding of the forked dsDNA and RNA:DNA duplexes in the absence of a protein trap. In this case, if the enzyme dissociates from its substrate before completion of an unwinding reaction, it can reassociate to form enzyme-DNA complex and reinitiate unwinding, as shown in Fig. 2. The n-step sequential mechanism for multiple turnover unwinding shown in Fig. 2A was used to fit the data under these conditions. The results for the multiturnover unwinding of the 20-bp forked substrates indicate greater product formation with the 10U/20T-20bp RNA:DNA hybrid fork compared with the DNA:DNA fork (Fig. 2, B and C). The difference in the amount of product formation appears more pronounced with an increased number of catalytic cycles over time. We also examined the sets of 16 bp (Fig. 2D) and 24 bp (Fig. 2E) duplex substrates under multiturnover conditions and found a similar trend in product formation for dsDNA and RNA:DNA hybrids. Overall, these results suggest that yeast Pif1 shows a preference for unwinding RNA:DNA heteroduplexes compared with their DNA:DNA counterparts. These results emphasize the difference in processivity for unwinding of the two substrates.
FIGURE 2.
Helicase-catalyzed unwinding kinetics for DNA:DNA (red circles) and RNA:DNA (blue squares) forked substrates under multiple turnover conditions. A, a schematic representation of a multiple cycle unwinding reaction. ES, enzyme-substrate complex; EI, intermediate. The rate constants for Pif1 unwinding, dissociation, and reassociation are indicated by ku, kd, and ka, respectively. B, unwinding reactions were performed under excess enzyme conditions as described under “Experimental Procedures.” Duplex substrates and ssDNA products were separated by electrophoresis on 20% native polyacrylamide gels. Representative gel images indicate the unwinding of 20-bp forks under multiturnover condition. C–E, shown are the reaction progress curves for 20- (C), 16- (D), and 24-bp (E) forked duplexes. The data were fit to the scheme given in A using Kintek Explorer (29). Error bars indicate the standard deviation of three independent experiments. Kinetic constants are shown in Tables 2 and 3.
Pif1-catalyzed Unwinding of a Chimera Substrate
A possible explanation for the differences in the product formation could be differences in the reassociation event (ka) of Pif1 with substrate under multiple turnover conditions. A previous report indicated that Pif1 is unable to unwind a duplex with the loading strand made up of RNA (20), and another indicates that the ATP hydrolysis by Pif1 is not stimulated by RNA (31), suggesting that Pif1 either does not interact with RNA or interacts with RNA differently than with DNA. In case of the RNA:DNA hybrid under multiturnover conditions, Pif1 can rebind only to the DNA strand of the fork, productively leading to the formation of product. However, because Pif1 can bind both strands of the dsDNA fork, it can bind nonproductively with the displaced strand overhang of the DNA:DNA duplex, thereby reducing its unwinding efficiency. This was previously shown to occur with Dda helicase (33). To test this hypothesis, a forked chimera substrate was designed that consisted of a RNA:DNA hybrid in the duplex region with the two arms or single-stranded overhangs made up of DNA (Fig. 3A). This substrate was expected to behave the same as the DNA:DNA fork when unwound by Pif1 helicase because both arms of the fork are DNA.
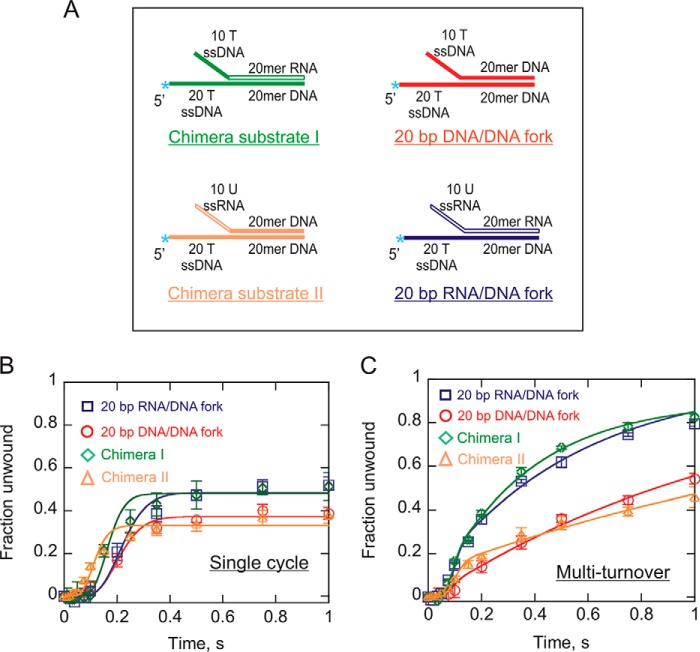
FIGURE 3.
Pif1-catalyzed unwinding of forked chimera substrates. A, shown are the chimera substrate I and II constructed by the hybridization of a radiolabeled 40-mer DNA loading strand to a 30-mer unlabeled displaced strand, resulting in a duplex region (20 bp) consisting of an RNA:DNA hybrid, but the two arms of the fork are DNA or the duplex being dsDNA, and the displaced strand overhang is ssRNA. 20-bp dsDNA and RNA:DNA forked duplexes are also indicated for comparison. B and C, single turnover (B) and multiple turnover (C) results for Pif1-catalyzed unwinding of the forked chimera substrates (green diamonds and yellow triangles). Plots for 20-bp DNA:DNA and RNA:DNA forked duplexes (from Figs. 1E and 2C) have also been shown here for direct comparison with the chimera forks under both reaction conditions. The data were fit using Kintek Explorer (29) to the scheme given in Fig. 1A for single cycle reactions and the scheme in Fig. 2A for multiturnover reactions. Error bars indicate the standard deviation of three independent experiments. Kinetic constants are shown in Tables 2 and 3.
Pif1-catalyzed unwinding of the chimera was performed under single-turnover (Fig. 3B) and multiple-turnover (Fig. 3C) conditions, and the results were compared directly to those of the 20-bp DNA:DNA and RNA:DNA forked duplexes by overlaying the plots from Figs. 1E and 2C. Surprisingly, the results indicate that the chimera fork is unwound by Pif1 similarly to the RNA:DNA fork under both conditions (Fig. 3, B and C). This suggests that the differences in the amplitude of the product formation curves are due to the identity of the displaced strand itself, not differences in association with the single-stranded overhang.
We designed another chimera fork that consisted of dsDNA in the 20-bp duplex region and ssRNA in the 10-nucleotide displaced strand overhang. Pif1 unwinding kinetics for this substrate (Fig. 3) was tested under both single and multiturnover conditions. The unwinding curves have been included in the same plots (Fig. 3, B and C) as the other chimera, and the results indicate that this substrate is unwound very similarly to the DNA:DNA forked duplex. It suggests that the pre-existing ssRNA in the displaced strand overhang does not contribute to unwinding of DNA:DNA duplex. Again, the product formation depends on the identity of the displaced strand within the duplex region.
Measurement of the Binding Affinity of Pif1 Helicase for Partial Duplex Substrates
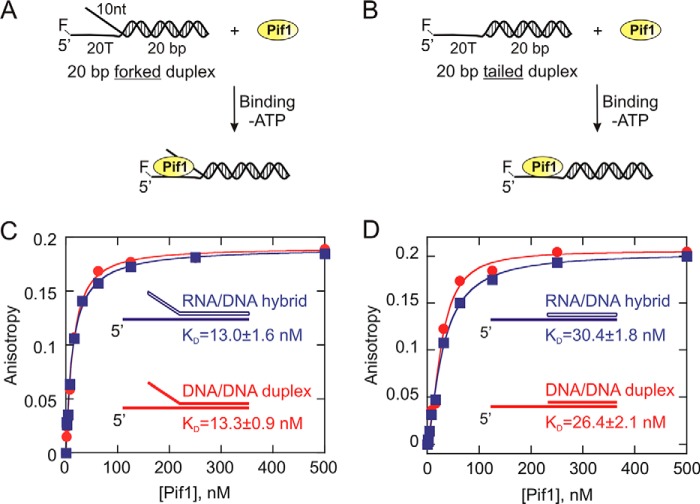
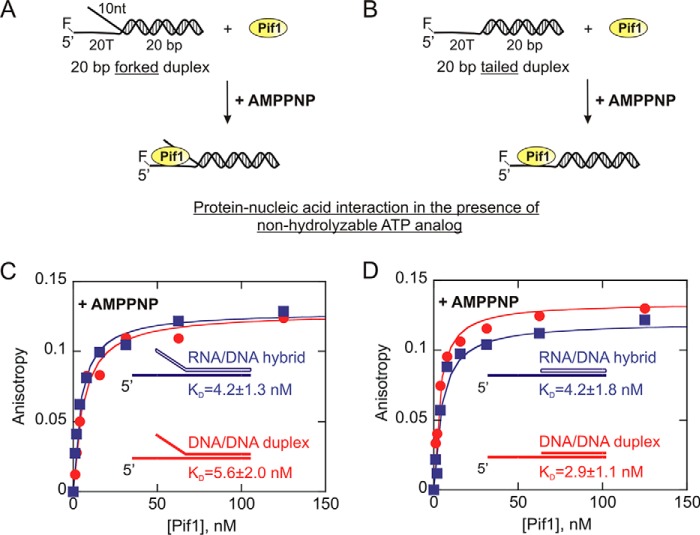
The differences observed in the product formation for DNA:DNA and RNA:DNA duplexes could possibly be explained by differential binding affinity of Pif1 for these substrates. To test this possibility, helicase interaction with fluorescently labeled 20-bp forked substrates (Table 1) were investigated, and the equilibrium dissociation constants (KD values) were obtained. The results indicate that Pif1 binds to the 20-bp forked DNA:DNA (KD = 13.3 ± 0.9 nm) and RNA:DNA (KD = 13.0 ± 1.6 nm) duplexes with similar affinity (Fig. 4C). Simultaneously, we also examined Pif1 interaction with 20-bp tailed substrates that essentially lack the displaced strand overhang as opposed to a fork (Table 1). The KD values for 5′F 20T-20bp DNA:DNA tailed duplex and 5′F 20T-r20bp RNA:DNA tailed hybrid are 26.4 ± 2.1 and 30.4 ± 1.8 nm, respectively. The binding results clearly indicate that the tailed RNA:DNA hybrid and dsDNA duplex also exhibit similar binding affinity (Fig. 4D) much like their forked counterparts. However, these values do indicate that yeast Pif1 shows a higher binding affinity for the forked duplexes compared with their tailed counterparts, which is consistent with the forked substrates being unwound preferentially by Pif1 (26, 31, 32).
FIGURE 4.
Measurement of the equilibrium dissociation constants (KD) for Pif1 interaction with DNA:DNA (red circles) and RNA:DNA (blue squares) partial duplex substrates. A and B, 1 nm 5′-FAM-labeled forked (A) and tailed (B) duplexes were titrated with Pif1 protein in the absence of ATP. C and D, the anisotropy values for each substrate are plotted against Pif1 concentration using Kaleidagraph software, and the data were fit to the quadratic equation to obtain the values for the dissociation constant (KD). The KD values for 10T/20T-20bp DNA:DNA forked duplex, 10U/20T-r20bp RNA:DNA forked duplex, 20T-20bp DNA:DNA tailed duplex, and 20T-r20bp RNA:DNA tailed duplex are 13.3 ± 0.9, 13.0 ± 1.6, 26.4 ± 2.1, and 30.4 ± 1.8 nm, respectively. The errors indicate the standard error of the fit.
Because these binding assays were carried out in the absence of a nucleotide, we subsequently examined Pif1 interaction with these substrates in the presence of AMP-PNP (a nonhydrolyzable ATP analog). These additional binding assays allowed us to test whether the affinity of Pif1 for these substrates changes in the nucleotide-bound state. However, the results in Fig. 5 indicate that even in the presence of an ATP analog, Pif1 is able to interact with the DNA:DNA duplex and the RNA:DNA hybrids very similarly. Pif1 bound to the 20-bp forked dsDNA duplex and RNA:DNA hybrid with the KD values of 5.6 ± 2.0 and 4.2 ± 1.3 nm, respectively (Fig. 5C). Similarly, the KD values for Pif1 interaction with the tailed dsDNA and RNA:DNA duplexes under these conditions are 2.9 ± 1.1 and 4.2 ± 1.8 nm, respectively (Fig. 5D). Thus, the overall results from the binding assay suggest that the preferential unwinding by Pif1 of RNA:DNA hybrids over DNA:DNA duplexes cannot be attributed to its binding affinity, because it binds to both substrates equally well.
FIGURE 5.
Measurement of the equilibrium dissociation constants (KD) for Pif1 interaction with DNA:DNA (red circles) and RNA:DNA (blue squares) partial duplex substrates in the presence of an ATP analog. A and B, 1 nm 5′-FAM-labeled forked (A) and tailed (B) duplexes were titrated with Pif1 protein in the presence of 5 mm AMP-PNP. C and D, the anisotropy values for each substrate are plotted against Pif1 concentration using Kaleidagraph software, and the data were fit to the quadratic equation to obtain the values for the dissociation constant (KD). The KD values for 10T/20T-20bp DNA:DNA forked duplex, 10U/20T-r20bp RNA:DNA forked duplex, 20T-20bp DNA:DNA tailed duplex, and 20T-r20bp RNA:DNA tailed duplex are 5.6 ± 2.0, 4.2 ± 1.3, 2.9 ± 1.1, and 4.2 ± 1.8 nm, respectively. The errors indicate the standard error of the fit.
Measurement of the Kinetic Parameters for Pif1-catalyzed Unwinding of Tailed Duplexes
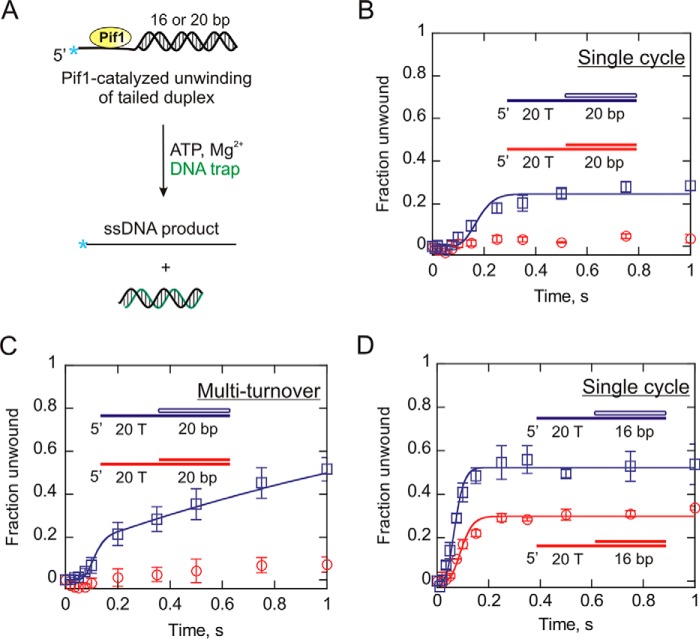
The intriguing results from the analysis of the forked substrates prompted us to investigate Pif1-catalyzed unwinding of the tailed duplexes. Substrates containing a 20-bp duplex region with a 20-nucleotide 5′-ssDNA overhang were used in a Pif1 unwinding assay (20T-20bp DNA:DNA tail and 20T-r20bp RNA:DNA tail). Unwinding of these substrates under single-turnover conditions in the presence of ATP and Mg2+ was measured. Similar to the results with the forked duplexes, the amplitude of the product formation curve was higher for the RNA:DNA tailed hybrid than the DNA:DNA tailed substrate (Fig. 6B). The RNA:DNA hybrid was unwound with a rate constant (ku) of 56.4 ± 0.5 s−1 (Table 2), although with a reduced processivity compared with its forked counterpart shown in Fig. 1E. However, Pif1 was unable to unwind the DNA:DNA tailed substrate to a level that was quantifiable.
FIGURE 6.
Measurement of the unwinding kinetics of dsDNA (red circles) and RNA:DNA (blue squares) tailed duplexes. A, shown is the schematic representation of Pif1-catalyzed melting of tailed substrates in the presence of ATP. B and C, 20-bp tailed dsDNA duplex and RNA:DNA hybrid each with a 20-nucleotide single-stranded overhang was unwound under single cycle (B) and multiturnover (C) conditions. The fraction of substrate unwound is plotted as a function of time using Kaleidagraph software. D, shown are the reaction progress curves for 16-bp tailed substrates with a 20-nucleotide overhang under single cycle condition. The data were fit to the scheme given in Fig. 1A for single cycle reactions and the scheme in Fig. 2A for multiturnover reactions using Kintek Explorer (29). The error bars indicate the standard deviation of three independent experiments.
We hypothesized that dsDNA tailed duplex may not be able to produce enough product in a single binding event and possibly needs multiple rounds of helicase association for duplex melting. Therefore, we carried out the unwinding of the tailed substrates under multiturnover conditions (Fig. 6C) in an attempt to obtain measureable amounts of product. The results, however, indicated very little product formation for DNA:DNA tailed duplex, suggesting that it is not a preferred substrate for Pif1. On the other hand, Pif1 was capable of unwinding the 20-bp RNA:DNA tailed substrate under multiple cycle condition (Fig. 6C). Furthermore, the difference in unwinding efficiency for both substrates, unlike single cycle reaction, becomes exaggerated when the enzyme binds its substrate multiple times after dissociation. This leads to a more pronounced difference in the product formation because of each catalytic turnover producing more product with the RNA:DNA hybrid compared with a dsDNA duplex (Fig. 6C).
We examined the unwinding of a shorter duplex with a 5′ tail. Hence, a 16-bp dsDNA and a RNA:DNA heteroduplex were selected with a 20-nt ssDNA overhang on the 5′ end (Table 1). The single-turnover unwinding of these substrates resulted in measureable amounts of product formation, because of their shorter duplex length (Fig. 6D). The kinetic parameters for unwinding are listed in Tables 2 and 3. As observed with the forked substrates, unwinding of the tailed dsDNA and RNA:DNA duplexes occurred at similar rates. However, the 16-bp RNA:DNA tailed hybrid was unwound more productively by Pif1 compared with its dsDNA counterpart, similar to what was observed for the forked duplexes. However, the difference in the amplitudes of the product formation curves in case of tailed substrates appears to be noticeably greater relative to the forked duplexes. Overall, the results indicate that the displaced strand overhang has a higher impact on the unwinding of the DNA:DNA duplex compared with the RNA:DNA hybrid. The results with the two DNA:DNA substrates in Fig. 6 illustrate that the low processivity of Pif1 has a major impact on product formation. Simply adding 4 bp to the substrate leads to differences in product formation that appear as “all or nothing.” The 20-bp substrate is unwound very little, whereas the 16-bp substrate results in easily measurable product formation.
ATPase Activity of Pif1 on Forked and Tailed Duplex Substrates
The steady state rate for ATP hydrolysis was previously shown to correspond to the rate constants for dsDNA unwinding and translocation on ssDNA (32). Therefore, we investigated the rate of ATP hydrolysis by Pif1 on a short ssDNA (50 nt in length), 20-bp forked and tailed RNA:DNA hybrid, as well as DNA:DNA duplexes (Fig. 7). The ATPase activity of Pif1 was measured at saturating concentrations of each of these substrates. The results indicate that the ATP hydrolysis rates on ssT50, forked dsDNA substrate, and tailed dsDNA duplex are 84.2 ± 7.6, 78.1 ± 3.3, and 68.5 ± 8.3 s−1, respectively. This indicates that the forked and tailed duplexes stimulate the ATPase activity of Pif1 similarly as observed with ssDNA. We found similar results with the forked and tailed RNA:DNA hybrids with ATP hydrolysis rates of 66. 6 ± 9.4 s−1 and 62. 8 ± 4.8 s−1, respectively. Another important point to be noted is that the steady state rate of ATP hydrolysis for hybrid substrates appears to be very similar to the dsDNA duplexes. Also, these rates are consistent with the previously published kinetics of ATP hydrolysis by yeast Pif1 (32).
FIGURE 7.
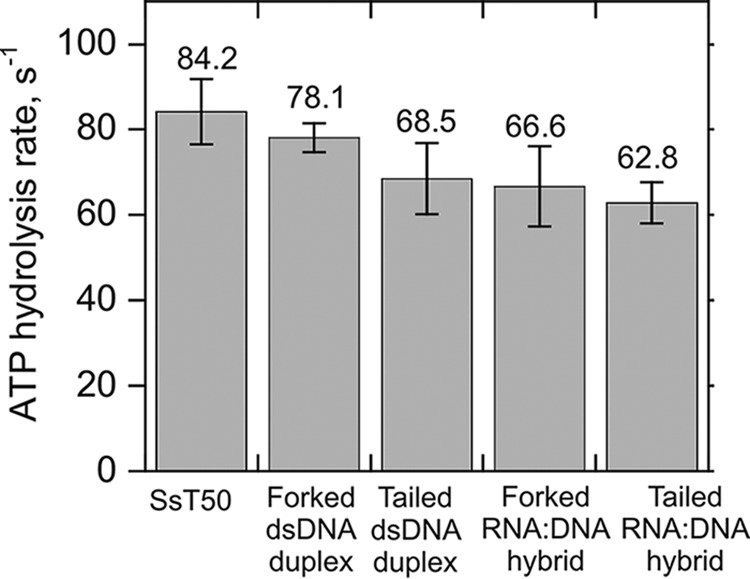
Stimulation of ATP hydrolysis of Pif1 (90 nm) by ssDNA, forked, and tailed dsDNA as well as RNA:DNA hybrids. The rate of ATP hydrolysis by Pif1 was measured on ssT50 (84.2 ± 7.6 s−1), 20-bp dsDNA forked duplex (78.1 ± 3.3 s−1), 20-bp dsDNA tailed duplex (68.5 ± 8.3 s−1), 20-bp RNA:DNA forked hybrid (66. 6 ± 9.4 s−1), and 20-bp RNA:DNA tailed hybrid (62. 8 ± 4.8 s−1) each at a concentration of 5 μm. Error bars indicate the standard deviation of three independent experiments.
Measurement of the Rate Constant for Helicase Dissociation from Duplex Substrates
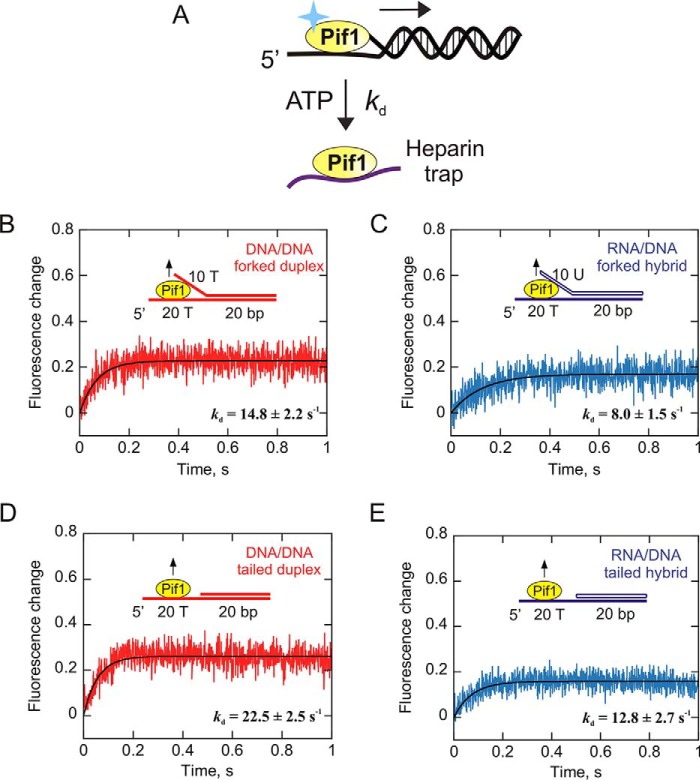
To explain the unwinding results, we further investigated enzyme dissociation from forked and tailed duplexes and sought to determine its spontaneous dissociation rate. Pif1 was preincubated with the 20-bp forked RNA:DNA hybrid or dsDNA duplex, and the reaction was initiated by rapid mixing with heparin in the presence of saturating amounts of ATP. Heparin served as an effective protein trap in the assay because the fluorescence of Pif1 is significantly different when bound to DNA versus heparin. The assay was performed in the presence of ATP because we intended to measure Pif1 dissociation while it unwinds the DNA. Pif1 dissociation from the tailed dsDNA and RNA:DNA substrates was also measured under the same conditions. The change in fluorescence as the enzyme dissociates from its substrate was plotted as a function of time, and the data were fit to a single exponential. The observed rate constants for dissociation of Pif1 from DNA:DNA and RNA:DNA forked duplexes are 14.8 ± 2.2 and 8.0 ± 1.5 s−1, respectively (Fig. 8, B and C). Pif1 dissociates from DNA:DNA tailed duplex and RNA:DNA tailed hybrid at a rate of 22.5 ± 2.5 and 12.8 ± 2.7 s−1, respectively (Fig. 8, D and E). These results indicate that Pif1 dissociates from RNA:DNA hybrids slower than DNA:DNA duplexes. The ability of Pif1 to remain bound to RNA:DNA hybrids longer than its dsDNA counterpart during unwinding may lead to the modest increase in product formation with RNA:DNA heteroduplexes.
FIGURE 8.
Measurement of the helicase dissociation rate from RNA:DNA versus DNA:DNA duplexes. A, an illustration of enzyme dissociation from nucleic acids is shown. Pif1 translocates unidirectionally (5′ to 3′) on DNA to unwind it, or it can dissociate from its substrate. A change in tryptophan fluorescence is associated with Pif1 dissociation. The dissociation of Pif1 from 20-bp forked and tailed RNA:DNA and DNA:DNA duplexes was measured in the presence of ATP by rapidly mixing with heparin. B–E, the intrinsic change in protein fluorescence as it dissociates from DNA:DNA duplexes (B and D) and RNA:DNA hybrids (C and E) is plotted as a function of time. The plots represent the averages of three independent experiments. The observed rate constants for enzyme dissociation obtained from single-exponential fits are indicated in the plots.
Unwinding of dsDNA Duplex and RNA:DNA Heteroduplex in the Presence of Heparin Trap
The values of the measured off rates for Pif1 dissociation from 20-bp duplexes appear to be larger than the values that can be used to refit the unwinding data. On the basis of the results from the dissociation experiments, we decided to examine the effect of heparin on the unwinding kinetics of Pif1. We hypothesized that heparin is capable of enhancing the off rate of Pif1 as the enzyme translocates along the DNA, thereby increasing the apparent dissociation rate constant. To test this hypothesis, we carried out Pif1-catalyzed single turnover unwinding of substrates in the presence of heparin as a protein trap as opposed to a 50-mer (T50). We chose the 16-bp forked substrate for this assay over the 20-bp forked duplex because under conditions where Pif1 dissociation is elevated, a shorter duplex would still result in product formation to a level that would most likely be quantifiable. Unwinding of both the 16-bp dsDNA duplex and the RNA:DNA hybrid was performed under these conditions (Fig. 9, A and B). The results indicate a 4-fold decrease in the amplitudes of product formation in the presence of heparin trap (Fig. 9) compared with a 50-mer protein trap, suggesting an increase in enzyme dissociation. The unwinding data were fit, and the dissociation rate constants were obtained for the 16-bp dsDNA duplex and RNA:DNA hybrid in the presence of heparin were 14.6 ± 0.6 and 13.8 ± 1.0 s−1, respectively, compared with 2.8 ± 0.8 and 2.0 ± 0.3 s−1 observed with a T50 protein trap. This supports the hypothesis that heparin sulfate, in addition to serving as a trap for free enzyme, also enhances the off rate of the bound Pif1, which causes it to dissociate from the duplex during unwinding, leading to the formation of decreased ssDNA product. A similar observation was made previously for a monomer of another SF1 DNA helicase, E. coli UvrD (34).
FIGURE 9.
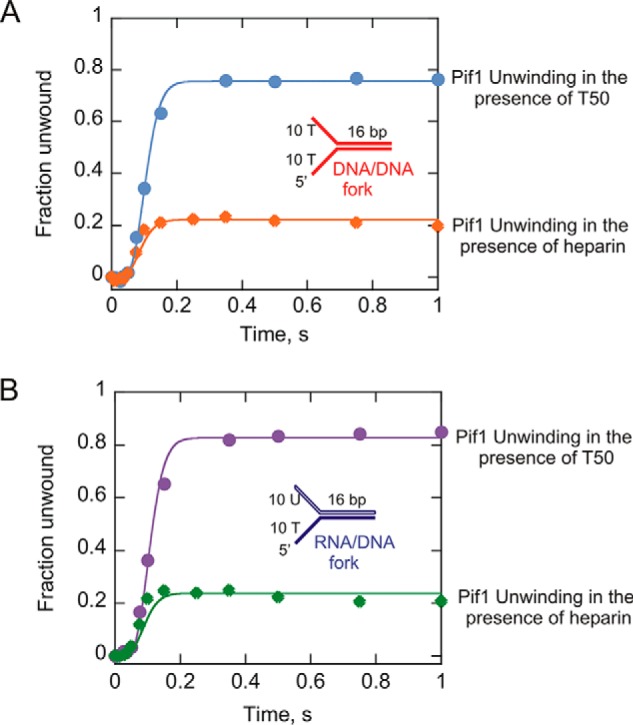
Single turnover unwinding of the 16bp DNA:DNA (A) and RNA:DNA (B) forked duplexes performed in the presence of heparin trap. The plot from Fig. 1F showing the unwinding of the same substrates in the presence of T50 has been overlaid with the unwinding curves obtained in the presence of heparin for direct comparison. The unwinding data were fit using Kintek Explorer (29) to the scheme given in Fig. 1A for single cycle reactions. The dissociation rate constants obtained for the dsDNA duplex and RNA:DNA hybrid in the presence of heparin were 14.6 ± 0.6 and 13.8 ± 1.0 s−1, respectively, compared with 2.8 ± 0.8 and 2.0 ± 0.3 s−1 observed with a T50 protein trap. The errors indicate the standard error of the fit.
Discussion
It has been shown previously that Pif1 exhibits a preference for unwinding RNA:DNA hybrids relative to DNA:DNA duplexes (26), which was proposed as a possible explanation for the displacement of telomerase holoenzyme from DNA ends (20, 26, 35). A recent report indicated using smFRET analysis that a monomeric form of Pif1 can unwind RNA:DNA hybrids but not dsDNA (17). Another SF1 helicase, E. coli UvrD, also shows a preference for unwinding RNA:DNA hybrids over DNA:DNA partial duplexes (36). The report indicated that the rate of UvrD-catalyzed unwinding of a RNA:DNA duplex was more than an order of magnitude faster than unwinding of a dsDNA duplex of similar length.
The goal of this study was to explore various kinetic parameters associated with the unwinding of RNA:DNA hybrids to gain insight into the mechanism of this preferential unwinding by yeast Pif1 helicase. Multiple processes are involved in unwinding a duplex, including the forward rate for base pair melting (ku), dissociation (kd) from the substrate, and reassociation (ka), which is the rate at which an enzyme rebinds if it dissociates before completion of unwinding. The rate of base pair melting is best measured under single-turnover conditions because this eliminates the effects of association with the substrate. Our results indicate that Pif1 unwinds RNA:DNA hybrids and DNA:DNA duplexes at essentially the same rate (Fig. 1 and Table 2). The differences in the amplitudes of product formation, however, reveal that RNA:DNA heteroduplexes are unwound more productively than DNA:DNA duplexes (Fig. 2). These results suggest that yeast Pif1 favors unwinding of RNA:DNA hybrids compared with its dsDNA counterparts, even though the actual rates of unwinding are similar for both substrates. We tested the binding affinity of Pif1 for both substrates using fluorescence anisotropy in the absence (Fig. 4), as well as in the presence of an ATP analog (Fig. 5). The KD values, however, suggested that Pif1 binds to both RNA:DNA and DNA:DNA duplexes with similar affinity, which eliminates the possibility of the preferential binding to the RNA:DNA duplex as an explanation for preferential unwinding. This is also in agreement with a previous report in which it was shown by mobility shift assay that Pif1 binds to DNA:DNA and RNA:DNA substrates equally well (26).
We also investigated the possibility of a difference in the multiturnover reassociation event affecting the overall product formation. Chimeric substrates in which the single-stranded overhang of the displaced strand was DNA and the duplex region of the displaced strand was RNA (chimera I) and a second chimeric substrate in which the single-stranded overhang of the displaced strand was RNA and the duplex region of the displaced strand was DNA were utilized (Fig. 3). Chimera substrate I (with a RNA:DNA hybrid duplex region) was unwound similarly to the RNA:DNA forked substrate (Fig. 3), and chimera substrate II (with a dsDNA duplex region) was unwound similarly to the DNA:DNA forked substrate. These results indicate that association with the substrate and potential nonproductive interaction of Pif1 with the RNA tail on the displaced strand does not affect the rate or amplitude of unwinding of the duplex. The increased amplitude of the product formation curve is somehow due to the RNA:DNA within the duplex region.
Thus, after ruling out the differences in unwinding rates, association of Pif1 with the displaced strand overhang, and the binding affinity of the helicase for the two different duplex substrates, the remaining explanation for the enhanced unwinding of RNA:DNA hybrids is relatively slow dissociation of the enzyme from this substrate compared with a dsDNA duplex. This analysis is supported by the values for dissociation rate constants, kd (Table 3) that were obtained from fitting the unwinding data for each substrate. We found that the rate of Pif1 dissociation from RNA:DNA hybrids is slower by ∼1.5-fold compared with DNA:DNA duplexes. We also examined the kinetics of Pif1 dissociation directly and found that the off rate from the DNA:DNA duplex is 1.8-fold faster than from the RNA:DNA hybrid (Fig. 8). This small difference contributes to modestly enhanced processivity and an overall increase in product formation with RNA:DNA hybrids. In light of the results presented in this report, Pif1 appears to remain bound to the A-form of nucleic acid (RNA:DNA hybrids) longer than the B-form (DNA:DNA duplexes) during unwinding. Because of this small difference in the dissociation rate, Pif1 is able to unwind more base pairs as it moves along an RNA:DNA hybrid compared with a dsDNA duplex. This disparity tends to become more prominent under multiple-turnover conditions where Pif1 can bind its substrate multiple times after dissociation. This effect is especially visible in Fig. 6C in which the DNA:DNA substrate is essentially not unwound, whereas the RNA:DNA substrate is poorly unwound. The cumulative effect of several catalytic turnovers during multiple rounds of unwinding produces a greater difference in the amplitudes of product formation between the two substrates. However, the kinetic constants for each substrate are actually very similar, but one is just within the threshold of unwinding, whereas the other is just outside of the threshold for unwinding.
Similar to the forked substrates, investigation of the tailed duplexes (both 16 and 20 bp) under the same unwinding conditions resulted in a similar trend for comparing RNA:DNA to DNA:DNA substrates (Fig. 6, B and D). Because of the nonprocessive nature of Pif1 helicase and preference for a forked substrate, unwinding of a longer dsDNA duplex was not favored in the absence of a fork. However, Pif1 was still capable of unwinding an RNA:DNA tailed hybrid of the same length, because of its preferential activity on hybrid structures (Fig. 6B). Additionally, we noted that the dissociation rate (Table 3 and Fig. 8) of Pif1 from a tailed duplex is faster (∼2–4-fold) compared with a forked duplex, which is consistent with the tailed substrates being unwound less productively by Pif1. Thus, interaction of Pif1 with the displaced strand of both the DNA:DNA duplex and the RNA:DNA hybrid appears to affect primarily the unwinding processivity.
Several studies have reported that helicases not only interact with the tracking strand but are also capable of binding to the displaced strand of a duplex. Bacteriophage T4 Dda has been shown to interact with each strand of a dsDNA (37) and that its simultaneous interaction with the displaced strand, loading strand, and duplex region enhances unwinding of a DNA fork (33). It has also been reported to exhibit strand switching events during its course of unwinding, implying interaction with each strand (38).
Similarly, NS3 helicase domain from the hepatitis C virus has been shown to interact specifically with the ss/dsDNA junction that facilitates duplex melting with a directional bias in the absence of ATP (39). E. coli UvrD shows specificity for binding 5′-ss/dsDNA junctions (40) and is capable of switching strands during translocation upon encountering a DNA fork (41). E. coli RecBC has been reported to consist of primary and secondary translocase activities that facilitate its translocation along both strands of a duplex simultaneously (42, 43). These studies indicated that the helicase provides a second channel for the excluded strand, such that it moves through the enzyme at the same rate as the tracking strand. A recent report on structural studies of bacterial RecQ helicases indicates their interaction with the 3′-ss/dsDNA junction that induces a tight bend in the DNA and melts a portion of the duplex (44). All these pieces of evidence highlight the significance of SF1 and SF2 helicase interaction with the duplex and the displaced strand of their substrates. This idea extends to the archaeal homohexameric MCM helicase, which unwinds a duplex by encircling the loading strand, whereas the displaced ssDNA tail wraps around the exterior of the MCM complex to provide stability and contribute to unwinding (45).
The ability of a helicase to interact with the two strands of a duplex during unwinding could potentially allow it to encounter several DNA modifications. Helicase action on damaged DNA substrates may be affected by the location of the DNA lesion in a strand-specific manner. Previous studies have reported that for T4 Dda, disruption/modification of the tracking strand disrupts unwinding much more so than disruptions in the displaced strand (37, 46, 47). However, there is clearly interaction of Dda with the displaced strand as well (33). For some other helicases, the interaction with the displaced strand can result in sensitivity to DNA damage. For example, RECQ1, WRN, and other helicases are impeded by DNA adducts in the nontranslocating strand (48, 49).
The results presented here for the preferential unwinding of RNA:DNA hybrids by Pif1 are quantitatively different from those that have been reported previously but follow the same trends and provide qualitatively similar conclusions. Our current work and previous work (17, 26) both indicate that the RNA:DNA heteroduplex is a preferred substrate over dsDNA duplex. These conclusions are supported by both ensemble and previously published single-molecule studies. However, ensemble experiments may underestimate processivity possibly because of the effect of the trapping strand, when compared with single-molecule assays. For example, T4 Dda (an SF1 helicase) was shown to have 2-fold greater processivity at the single-molecule level compared with ensemble (38). The presence of the protein trap in ensemble assays may facilitate the off rate of the enzyme, possibly to a lesser degree compared with heparin.
The ability of Pif1 to preferentially unwind RNA:DNA hybrids can have numerous biological implications. A simple model in Fig. 10 illustrates potential roles on biological substrates. Pif1 encounters and recognizes several DNA structures in cells, including G4 DNA, ss/dsDNA junctions, DNA forks, and RNA:DNA hybrids. Pif1 suppresses telomere addition to dsDNA breaks by binding preferentially to the 3′-ss/ds junction, thereby inhibiting telomerase at the 3′ end (20, 50). Because telomerase RNA can form a hybrid with telomeric DNA, the ability of Pif1 to unwind RNA:DNA hybrid structures more processively may help in the removal of the telomerase holoenzyme. On the other hand, the reduced processivity of Pif1 on DNA:DNA duplex may prevent unwinding of broken DNA ends, thereby promoting dsDNA break repair (Fig. 10A).
FIGURE 10.
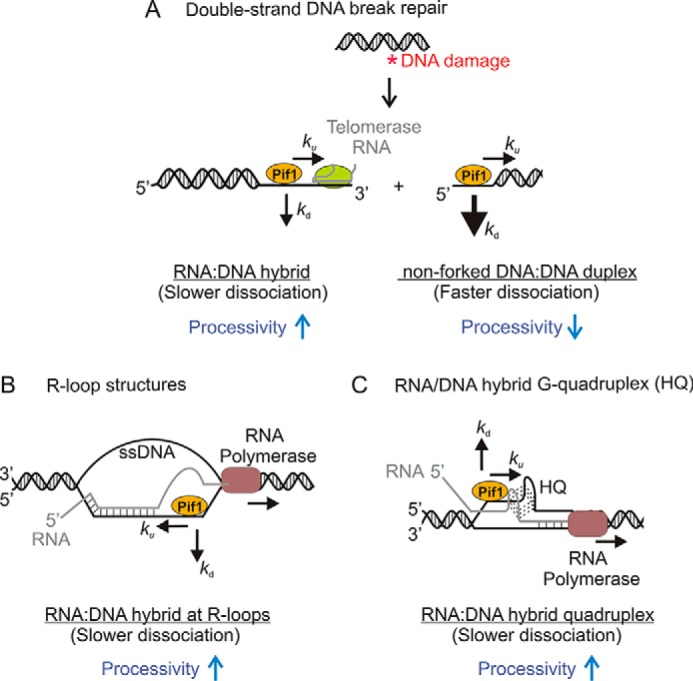
Model depicting the possible impact on Pif1 activity of the difference in processivity of Pif1 for unwinding DNA:DNA duplexes and RNA:DNA hybrids in various cellular activities. A, double-stranded DNA break repair. Pif1 prevents aberrant telomere addition at double strand breaks. Its enhanced processivity on RNA:DNA hybrids in addition to its ability to displace proteins from DNA may contribute to its role in displacing the telomerase holoenzyme. On the other hand, decreased processivity of Pif1 on dsDNA duplexes may prevent unwinding of broken DNA ends. This model is similar to previously proposed ideas (20, 50). B, R-loop resolution. RNA:DNA hybrids or R-loops arising from transcription have been proposed to contribute to the formation of double-stranded breaks (51). Pif1 has been speculated to resolve these R-loops (52, 53). The increased processivity of Pif1 on hybrid substrates may help in removing these structures. C, resolution of RNA/DNA hybrid quadruplex. Pif1 is capable of binding and unwinding G-quadruplex structures (15–17, 56). Its enhanced processivity on RNA:DNA hybrids may facilitate the resolution of hybrid quadruplexes. DNA strands are indicated in black, whereas the RNA strand is shown in gray. The unwinding and the dissociation rate constants are indicated by ku and kd, respectively. As Pif1 translocates during substrate unwinding, it tends to dissociate from dsDNA duplexes faster than RNA:DNA hybrids.
RNA:DNA hybrids have also been proposed to form in various other cellular pathways. These hybrid structures may present a barrier to moving replication forks. Two such secondary structures are RNA/DNA hybrid quadruplex and R-loops that are essentially transcription byproducts (Fig. 10, B and C). The formation of R-loops in cells can result in DNA breaks and cause genome instability (51). Pif1 helicase has been speculated to play a role at these regions and resolve the R-loop structures to allow replication fork progression (52, 53). Recent studies reported the formation of RNA/DNA hybrid G-quadruplexes during transcription that may modulate gene expression (54, 55). Eukaryotic Pif1 has already been shown to bind and unwind G4 DNA structures (15–17, 56). Its additional ability to unwind RNA:DNA structures may facilitate the resolution of the hybrid quadruplexes. Additional experiments are needed to test this idea and understand the mechanism by which these secondary structures are processed.
Author Contributions
All authors participated in designing experiments, data interpretation, and preparation of the manuscript. The experiments were performed by S. C.
Acknowledgment
We acknowledge Dr. Robert L. Eoff for suggesting the RNA:DNA chimera assay.
This work was supported by National Institutes of Health Grant R01 GM098922 (to K. D. R.). The authors declare that they have no conflicts of interest with the contents of this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
- SF
- superfamily
- ssDNA
- single-stranded DNA
- AMP-PNP
- adenosine 5′-(β,γ-imino)triphosphate.
References
- 1. Raney K. D., Byrd A. K., and Aarattuthodiyil S. (2013) Structure and mechanisms of SF1 DNA helicases. Adv. Exp. Med. Biol. 767, 17–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lohman T. M., Tomko E. J., and Wu C. G. (2008) Non-hexameric DNA helicases and translocases: mechanisms and regulation. Nat. Rev. Mol. Cell Biol. 9, 391–401 [DOI] [PubMed] [Google Scholar]
- 3. Singleton M. R., Dillingham M. S., and Wigley D. B. (2007) Structure and mechanism of helicases and nucleic acid translocases. Annu. Rev. Biochem. 76, 23–50 [DOI] [PubMed] [Google Scholar]
- 4. Chung W. H. (2014) To peep into Pif1 helicase: multifaceted all the way from genome stability to repair-associated DNA synthesis. J. Microbiol. 52, 89–98 [DOI] [PubMed] [Google Scholar]
- 5. Bochman M. L., Sabouri N., and Zakian V. A. (2010) Unwinding the functions of the Pif1family helicases. DNA Repair 9, 237–249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Foury F., and Dyck E. V. (1985) A PIF-dependent recombinogenic signal in the mitochondrial DNA of yeast. EMBO J. 4, 3525–3530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Foury F., and Lahaye A. (1987) Cloning and sequencing of the PIF gene involved in repair and recombination of yeast mitochondrial DNA. EMBO J. 6, 1441–1449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lahaye A., Stahl H., Thines-Sempoux D., and Foury F. (1991) PIF1: a DNA helicase in yeast mitochondria. EMBO J. 10, 997–1007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Foury F., and Kolodynski J. (1983) pif mutation blocks recombination between mitochondrial rho+ and rho− genomes having tandemly arrayed repeat units in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. U.S.A. 80, 5345–5349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Van Dyck E., Foury F., Stillman B., and Brill S. J. (1992) A single-stranded DNA binding protein required for mitochondrial DNA replication in S. cerevisiae is homologous to E. coli SSB. EMBO J. 11, 3421–3430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cheng X., Dunaway S., and Ivessa A. S. (2007) The role of Pif1p, a DNA helicase in Saccharomyces cerevisiae, in maintaining mitochondrial DNA. Mitochondrion 7, 211–222 [DOI] [PubMed] [Google Scholar]
- 12. Budd M. E., Reis C. C., Smith S., Myung K., and Campbell J. L. (2006) Evidence suggesting that Pif1 helicase functions in DNA replication with the Dna2 helicase/nuclease and DNA polymerase delta. Mol. Cell. Biol. 26, 2490–2500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rossi M. L., Pike J. E., Wang W., Burgers P. M., Campbell J. L., and Bambara R. A. (2008) Pif1 helicase directs eukaryotic Okazaki fragments toward the two-nuclease cleavage pathway for primer removal. J. Biol. Chem. 283, 27483–27493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ivessa A. S., Zhou J. Q., and Zakian V. A. (2000) The Saccharomyces Pif1p DNA helicase and the highly related Rrm3p have opposite effects on replication fork progression in ribosomal DNA. Cell 100, 479–489 [DOI] [PubMed] [Google Scholar]
- 15. Ribeyre C., Lopes J., Boulé J. B., Piazza A., Guédin A., Zakian V. A., Mergny J. L., and Nicolas A. (2009) The yeast Pif1 helicase prevents genomic instability caused by G-quadruplex-forming CEB1 sequences in vivo. PLoS Genet. 5, e1000475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Byrd A. K., and Raney K. D. (2015) A parallel quadruplex DNA is bound tightly but unfolded slowly by Pif1 helicase. J. Biol. Chem. 290, 6482–6494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhou R., Zhang J., Bochman M. L., Zakian V. A., and Ha T. (2014) Periodic DNA patrolling underlies diverse functions of Pif1 on R-loops and G-rich DNA. eLife 3, e02190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Paeschke K., Bochman M. L., Garcia P. D., Cejka P., Friedman K. L., Kowalczykowski S. C., and Zakian V. A. (2013) Pif1 family helicases suppress genome instability at G-quadruplex motifs. Nature 497, 458–462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Schulz V. P., and Zakian V. A. (1994) The Saccharomyces PIF1 DNA helicase inhibits telomere elongation and de novo telomere formation. Cell 76, 145–155 [DOI] [PubMed] [Google Scholar]
- 20. Boulé J. B., Vega L. R., and Zakian V. A. (2005) The yeast Pif1p helicase removes telomerase from telomeric DNA. Nature 438, 57–61 [DOI] [PubMed] [Google Scholar]
- 21. Zhou J., Monson E. K., Teng S. C., Schulz V. P., and Zakian V. A. (2000) Pif1p helicase, a catalytic inhibitor of telomerase in yeast. Science 289, 771–774 [DOI] [PubMed] [Google Scholar]
- 22. Wilson M. A., Kwon Y., Xu Y., Chung W. H., Chi P., Niu H., Mayle R., Chen X., Malkova A., Sung P., and Ira G. (2013) Pif1 helicase and Pol[dgr] promote recombination-coupled DNA synthesis via bubble migration. Nature 502, 393–396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Myung K., Chen C., and Kolodner R. D. (2001) Multiple pathways cooperate in the suppression of genome instability in Saccharomyces cerevisiae. Nature 411, 1073–1076 [DOI] [PubMed] [Google Scholar]
- 24. Saini N., Ramakrishnan S., Elango R., Ayyar S., Zhang Y., Deem A., Ira G., Haber J. E., Lobachev K. S., and Malkova A. (2013) Migrating bubble during break-induced replication drives conservative DNA synthesis. Nature 502, 389–392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chung W. H., Zhu Z., Papusha A., Malkova A., and Ira G. (2010) Defective resection at DNA double-strand breaks leads to de novo telomere formation and enhances gene targeting. PLoS Genet. 6, e1000948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Boulé J. B., and Zakian V. A. (2007) The yeast Pif1p DNA helicase preferentially unwinds RNA DNA substrates. Nucleic Acids Res. 35, 5809–5818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Morris P. D., Tackett A. J., Babb K., Nanduri B., Chick C., Scott J., and Raney K. D. (2001) Evidence for a functional monomeric form of the bacteriophage T4 DdA helicase: Dda does not form stable oligomeric structures. J. Biol. Chem. 276, 19691–19698 [DOI] [PubMed] [Google Scholar]
- 28. Ramanagoudr-Bhojappa R., Blair L. P., Tackett A. J., and Raney K. D. (2013) Physical and functional interaction between yeast Pif1 helicase and Rim1 single-stranded DNA binding protein. Nucleic Acids Res. 41, 1029–1046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Johnson K. A. (2009) Fitting enzyme kinetic data with KinTek Global Kinetic Explorer. Methods Enzymol. 467, 601–626 [DOI] [PubMed] [Google Scholar]
- 30. Raney K. D., and Benkovic S. J. (1995) Bacteriophage T4 Dda helicase translocates in a unidirectional fashion on single-stranded DNA. J. Biol. Chem. 270, 22236–22242 [DOI] [PubMed] [Google Scholar]
- 31. Lahaye A., Leterme S., and Foury F. (1993) PIF1 DNA helicase from Saccharomyces cerevisiae: biochemical characterization of the enzyme. J. Biol. Chem. 268, 26155–26161 [PubMed] [Google Scholar]
- 32. Ramanagoudr-Bhojappa R., Chib S., Byrd A. K., Aarattuthodiyil S., Pandey M., Patel S. S., and Raney K. D. (2013) Yeast Pif1 helicase exhibits a one base pair stepping mechanism for unwinding duplex DNA. J. Biol. Chem. 288, 16185–16195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Aarattuthodiyil S., Byrd A. K., and Raney K. D. (2014) Simultaneous binding to the tracking strand, displaced strand and the duplex of a DNA fork enhances unwinding by Dda helicase. Nucleic Acids Res. 42, 11707–11720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Fischer C. J., Maluf N. K., and Lohman T. M. (2004) Mechanism of ATP-dependent translocation of E. coli UvrD monomers along single-stranded DNA. J. Mol. Biol. 344, 1287–1309 [DOI] [PubMed] [Google Scholar]
- 35. Boulé J. B., and Zakian V. A. (2006) Roles of Pif1-like helicases in the maintenance of genomic stability. Nucleic Acids Res. 34, 4147–4153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Matson S. W. (1989) Escherichia coli DNA helicase II (uvrD gene product) catalyzes the unwinding of DNA·RNA hybrids in vitro. Proc. Natl. Acad. Sci. U.S.A. 86, 4430–4434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Eoff R. L., and Raney K. D. (2006) Intermediates revealed in the kinetic mechanism for DNA unwinding by a monomeric helicase. Nat. Struct. Mol. Biol. 13, 242–249 [DOI] [PubMed] [Google Scholar]
- 38. Byrd A. K., Matlock D. L., Bagchi D., Aarattuthodiyil S., Harrison D., Croquette V., and Raney K. D. (2012) Dda helicase tightly couples translocation on single-stranded DNA to unwinding of duplex DNA: Dda is an optimally active helicase. J. Mol. Biol. 420, 141–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Reynolds K. A., Cameron C. E., and Raney K. D. (2015) Melting of duplex DNA in the absence of ATP by the NS3 helicase domain through specific interaction with a single-strand/double-strand junction. Biochemistry 54, 4248–4258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Tomko E. J., Jia H., Park J., Maluf N. K., Ha T., and Lohman T. M. (2010) 5′-Single-stranded/duplex DNA junctions are loading sites for E. coli UvrD translocases. EMBO J. 29, 3826–3839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Dessinges M. N., Lionnet T., Xi X. G., Bensimon D., and Croquette V. (2004) Single-molecule assay reveals strand switching and enhanced processivity of UvrD. Proc. Natl. Acad. Sci. U.S.A. 101, 6439–6444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wu C. G., Bradford C., and Lohman T. M. (2010) Escherichia coli RecBC helicase has two translocase activities controlled by a single ATPase motor. Nat. Struct. Mol. Biol. 17, 1210–1217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wu C. G., Xie F., and Lohman T. M. (2012) The primary and secondary translocase activities within E. coli RecBC helicase are tightly coupled to ATP hydrolysis by the RecB motor. J. Mol. Biol. 423, 303–314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Manthei K. A., Hill M. C., Burke J. E., Butcher S. E., and Keck J. L. (2015) Structural mechanisms of DNA binding and unwinding in bacterial RecQ helicases. Proc. Natl. Acad. Sci. U.S.A. 112, 4292–4297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Graham B. W., Schauer G. D., Leuba S. H., and Trakselis M. A. (2011) Steric exclusion and wrapping of the excluded DNA strand occurs along discrete external binding paths during MCM helicase unwinding. Nucleic Acids Res. 39, 6585–6595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Maine I. P., Sun D., Hurley L. H., and Kodadek T. (1992) The antitumor agent CC-1065 inhibits helicase-catalyzed unwinding of duplex DNA. Biochemistry 31, 3968–3975 [DOI] [PubMed] [Google Scholar]
- 47. Tackett A. J., Morris P. D., Dennis R., Goodwin T. E., and Raney K. D. (2001) Unwinding of unnatural substrates by a DNA helicase. Biochemistry 40, 543–548 [DOI] [PubMed] [Google Scholar]
- 48. Khan I., Sommers J. A., and Brosh R. M. Jr. (2015) Close encounters for the first time: helicase interactions with DNA damage. DNA Repair 33, 43–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Suhasini A. N., Sommers J. A., Yu S., Wu Y., Xu T., Kelman Z., Kaplan D. L., and Brosh R. M. (2012) DNA repair and replication fork helicases are differentially affected by alkyl phosphotriester lesion. J. Biol. Chem. 287, 19188–19198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Bochman M. L., Paeschke K., Chan A., and Zakian V. A. (2014) Hrq1, a homolog of the human RecQ4 helicase, acts catalytically and structurally to promote genome integrity. Cell Reports 6, 346–356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Houlard M., Artus J., Léguillier T., Vandormael-Pournin S., and Cohen-Tannoudji M. (2011) DNA-RNA hybrids contribute to the replication dependent genomic instability induced by Omcg1 deficiency. Cell Cycle 10, 108–117 [DOI] [PubMed] [Google Scholar]
- 52. Hamperl S., and Cimprich K. A. (2014) The contribution of co-transcriptional RNA:DNA hybrid structures to DNA damage and genome instability. DNA Repair 19, 84–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Aguilera A., and García-Muse T. (2012) R Loops: from transcription byproducts to threats to genome stability. Mol. Cell 46, 115–124 [DOI] [PubMed] [Google Scholar]
- 54. Zheng K. W., Xiao S., Liu J. Q., Zhang J. Y., Hao Y. H., and Tan Z. (2013) Co-transcriptional formation of DNA:RNA hybrid G-quadruplex and potential function as constitutional cis element for transcription control. Nucleic Acids Res. 41, 5533–5541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Xiao S., Zhang J. Y., Zheng K. W., Hao Y. H., and Tan Z. (2013) Bioinformatic analysis reveals an evolutional selection for DNA:RNA hybrid G-quadruplex structures as putative transcription regulatory elements in warm-blooded animals. Nucleic Acids Res. 41, 10379–10390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Sanders C. M. (2010) Human Pif1 helicase is a G-quadruplex DNA-binding protein with G-quadruplex DNA-unwinding activity. Biochem. J. 430, 119–128 [DOI] [PubMed] [Google Scholar]