Abstract
Receptor-interacting protein kinase 3 (RIPK3) is a serine/threonine kinase with essential function in necroptosis. The activity of RIPK3 is controlled by phosphorylation. Once activated, RIPK3 phosphorylates and activates the downstream effector mixed lineage kinase domain-like (MLKL) to induce necroptosis. In certain situations, RIPK3 has also been shown to promote apoptosis or cytokine expression in a necroptosis and kinase-independent manner. The ubiquitin-proteasome system is the major pathway for selective degradation of cellular proteins and thus has a critical role in many cellular processes such as cell survival and cell death. Clinically, proteasome inhibition has shown promise as an anti-cancer agent. Here we show that the proteasome inhibitors MG132 and bortezomib activate the RIPK3-MLKL necroptotic pathway in mouse fibroblasts as well as human leukemia cells. Unlike necroptosis induced by classical TNF-like cytokines, necroptosis induced by proteasome inhibitors does not require caspase inhibition. However, an intact RIP homotypic interaction motif (RHIM) is essential. Surprisingly, when recruitment of MLKL to RIPK3 is restricted, proteasome inhibitors induced RIPK3-dependent apoptosis. Proteasome inhibition led to accumulation of K48-linked ubiquitinated RIPK3, which was partially reduced when Lys-264 was mutated. Taken together, these results reveal the ubiquitin-proteasome system as a novel regulatory mechanism for RIPK3-dependent necroptosis.
Keywords: anticancer drug, apoptosis, cell death, necrosis (necrotic death), proteasome, ubiquitylation (ubiquitination)
Introduction
Tissue homeostasis is maintained through dynamic balance between cell survival and cell death. A number of diseases such as cancer can be attributed to perturbation of this balance. Caspase-dependent apoptosis is a key cell death module with important functions in development and certain disease pathologies. In addition to apoptosis, recent evidence shows that a form of regulated necrosis, termed necroptosis, is significantly associated with a number of common clinical diseases such as viral infection, ischemia-reperfusion injury, myocardial infarction, atherosclerosis, and inflammatory bowel diseases (1, 2). Necroptosis is driven by the cytosolic serine/threonine kinase receptor-interacting protein kinase 3 (RIPK3)2 (3). A wide variety of stimuli including tumor necrosis factor (TNF) superfamily death ligands have been reported to induce necroptosis (1). Ligation of TNF to TNF receptor (TNFR) 1 causes formation of receptor-associated complex I, which comprises of TNFR1-associated via death domain (TRADD), TNFR-associated factor 2 (TRAF2), cellular inhibitor of apoptosis 1 (cIAP1), cIAP2, linear ubiquitin chain assembly complex (LUBAC), and RIPK1. cIAPs and LUBAC promotes RIPK1 ubiquitination through Lys-11, Lys-63, and linear ubiquitin linkages, which promotes activation of NF-κB and cell survival in part by limiting the formation of the cytosolic death-inducing signaling complex (DISC, aka complex IIa). Removal of the ubiquitin chains on RIPK1 by the de-ubiquitinase cylindromatosis (CYLD) promotes Complex IIa formation and apoptosis (4). When caspase 8 activity is blocked, RIPK3 is recruited to the complex to promote necroptosis. Besides RIPK1 and RIPK3, caspase 8 and its adaptor Fas-associated via death domain (FADD) are also major components of Complex IIa and the RIPK3-associated Complex IIb (aka the necrosome).
RIPK3 possesses an active kinase domain in the N terminus and a unique protein-protein interaction domain called the RIP homotypic interaction motif (RHIM) at the C terminus (5). Phosphorylation of RIPK1 and RIPK3 in their kinase domains facilitates RHIM-mediated interaction between RIPK1 and RIPK3 and formation of an amyloid-like filamentous signaling complex (6, 7). Although RIPK1 is required for TNF-induced RIPK3 activation, it is not a prerequisite in toll-like receptor 3 (TLR3) or interferon-induced necroptosis (8). Phosphorylation of RIPK3 at Thr-231/Ser-232 (Ser-232 in human RIPK3) stimulates recruitment and phosphorylation of the downstream substrate mixed lineage kinase-domain like (MLKL). Activated MLKL undergoes oligomerization and translocates to intracellular and plasma membranes, which eventually leads to membrane lesions through a yet-to-be-defined mechanism (9–15).
The proteasome is a large multimeric complex responsible for selective degradation of proteins that are covalently modified by K48- or K11-linked polyubiquitin chains (16). The expression of many proteins with important functions in cell cycle or cell death is often controlled by the ubiquitin-proteasome system (UPS). As a result, defects in proteasomal degradation can lead to various disorders (17). In cancers, targeting the UPS has proved to be an effective therapeutic approach (18). For example, a first-in-class proteasome inhibitor, bortezomib, has been successfully used for treatment of multiple myeloma and mantle cell lymphoma (19). The success of bortezomib has led to efforts to develop second-generation proteasome inhibitors with improved safety and efficiency (20).
Despite the importance of the UPS in biology, relatively little is known about its role in necroptosis. Here, we show that proteasome inhibitors can potently induce RIPK3-dependent necroptosis. Proteasome inhibitor-induced necroptosis requires an intact RHIM in RIPK3 and downstream activation of MLKL. Surprisingly, when recruitment of or access to MLKL is impeded, proteasome inhibitors promote RIPK3-dependent apoptosis instead. Mechanistically, proteasome inhibitors triggered the accumulation of K48-linked ubiquitinated RIPK3. Mutation of Lys-264 in the kinase domain of RIPK3 significantly reduced proteasome-induced RIPK3 ubiquitination and necroptosis. These results reveal an important function of the UPS in the regulation of necroptosis and suggest that targeting this pathway may be a useful strategy in cancer therapy.
Experimental Procedures
Reagents
z-VAD-fmk, z-IETD-fmk, MG132, staurosporine, and necrostatin-1 were obtained from Enzo Lifesciences. Doxycycline, cycloheximide, 5-FU, oxaliplatin, irinotecan, doxorubicin, and etoposide were obtained from Sigma. Bortezomib was obtained from ApexBio. Anti-mouse Fas agonistic Jo2 antibody was obtained from BD Biosciences. Anti-human Fas agonistic CH11 antibody was obtained from Millipore. Anti-mouse TNF blocking antibody was obtained from R&D systems. GSK′840 and GSK′843 were kindly provided by J. Bertin and P. Gough. BV6 was kindly provided by D. Vucic.
Cell Culture
Ripk3−/− 3T3, Ripk1−/−3T3, and 293T cells were cultured in DMEM medium. T cell leukemia cell lines Jurkat and H9 were cultured in RPMI medium. Ten percent fetal calf serum, 2 mm glutamine, 100 units/ml penicillin, and 100 μg/ml streptomycin were added to the medium.
Plasmids, Viruses, Transfections, and Transductions
Wild type mouse Ripk3 genes were cloned into a modified lentiviral tet-on pTRIPZ/Puro vector. Wild type mouse Ripk3 and Mlkl genes were also cloned into a retroviral pMSCV/Hyg vector. HA and FLAG tags were introduced at the amino and carboxyl termini of RIPK3 by PCR cloning, respectively. FLAG tag was fused at the carboxyl terminus of MLKL. Each mutant Ripk3 expression vector was generated by site-directed mutagenesis. For RHIM mutant, the tetra core sequence of RHIM, VQIG, of mouse RIPK3, was mutated to AAAA. The sequence of all the genes inserted was confirmed by sequence analysis. pGIPZ/puro vector carrying shRNA against mouse Mlkl (Open Biosystems, V3LMM_485516) was used to silence MLKL expression. pGIPZ vector carrying non-silencing scrambled shRNA was used as negative control (Open Biosystems, RHS4346). Lentivirus was generated by transfecting the virus vectors into 293T cells with pMD2.G and psPAX2 vectors. After 24 h, culture media were replaced and the cells were further cultured for 24 h. Retrovirus was generated by transfection in 293T cells using VSV-G and Gag/Pol packaging vectors. Culture medium was collected, filtered, and used for transduction with 10 μg/ml polybrene. After transduction, the cells were selected by hygromycin B (300 μg/ml) or puromycin (2 μg/ml). RIPK3 expression was induced by 1 μg/ml doxycycline.
Western Blot and Immunoprecipitation (IP)
Whole cell extracts were prepared in RIPA lysis buffer and resolved on 4–20% polyacrylamide gels from Invitrogen or GenScript. To detect MLKL oligomers, lysates were heated at 70 °C for 10 min in SDS loading buffer without DTT. After transferring proteins to nitrocellulose membrane, immunoblot analysis was performed with the following antibodies: Anti-mouse RIPK3 (2283, Prosci), human RIPK3 (generated in our own laboratory), mouse RIPK1 (38/RIP, BD Biosciences), mouse caspase 8 (1G12, Enzo Lifesciences), human caspase 8 (12F5, Enzo Lifesciences), human cleaved PARP (9541, Cell Signaling Technology), mouse caspase 3 (46, Santa Cruz Biotechnology), human/mouse MLKL (3H1, Millipore), phospho human MLKL (EPR9514, Abcam), human/mouse cIAP1 (AF818, R&D Systems), ubiquitin (Ubi-1, Sigma), K48 ubiquitin (Apu2, Millipore), K63 ubiquitin (Apu3, Millipore), and mouse FADD (kindly provided by A. Winoto at the University of California, Berkeley) antibodies. Anti-β-actin (3779, Prosci) and HSP90 (68/Hsp90, BD Biosciences) antibodies were used as loading controls. For IP, RIPA lysates were pre-cleared by Sepharose 6B (Sigma) for 1 h at 4 °C, followed by incubation with anti-mouse RIPK3 antibody and anti-rabbit IgG conjugated agarose beads (Sigma) at 4 °C overnight. After washes in RIPA buffer (5×), the resulting immune complex was resolved on polyacrylamide gel. For denaturing IP, cells were lysed with denaturing IP buffer (10 mm Tri-HCl, 150 mm NaCl, 2% SDS) and subsequently boiled at 95 °C for 10 min (21). After sonication, lysates were diluted with dilution buffer (10 mm Tris-HCl, 150 mm NaCl, 2 mm EDTA, 1% Nonidet P-40) to reduce the SDS concentration to 0.2%. After rotation for 45 min at 4 °C and centrifugation at 13,000 rpm for 30 min, lysates were subjected to IP using anti-RIPK3 antibody and anti-rabbit IgG-conjugated agarose beads (Sigma). Normal rabbit IgG (sc-2027, Santa Cruz) was used as control.
Cell Death Assay
Cell death was determined by CellTiter-Glo Luminescent Cell Viability Assay (Promega), CytoTox 96® Non-Radioactive Cytotoxicity Assay (Promega), or FACS using propidium iodide (PI) (Sigma). All cell death assays were performed in triplicates.
Statistical Analysis
Results shown are mean ± S.E. p values were calculated by unpaired t test with Welch's correction. p values less than 0.05 were considered statistically significant.
Results
Proteasome Inhibition Causes RIPK3- and RHIM-dependent Cell Death
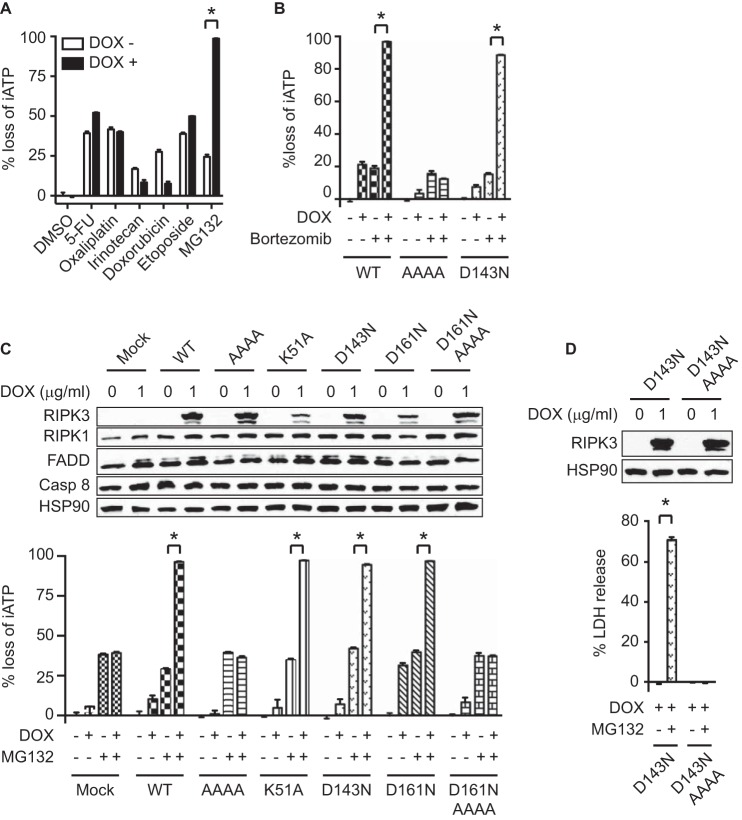
Proteasome inhibitors have shown promise as sensitizing agents that induce cancer cell death (19). RIPK3 was reported to be modified by K48-linked polyubiquitin chain (22). We therefore examined if proteasome inhibition could impact RIPK3-dependent necroptosis. Indeed, we found that the proteasome inhibitor MG132 significantly enhanced cell death in Ripk3−/− 3T3 cells with doxycycline-induced RIPK3 expression (Fig. 1A). In contrast, doxycycline-induced RIPK3 expression did not enhance cell death induced by 5-FU, oxaliplatin, irinotecan, doxorubicin, or etoposide as we previously reported (Fig. 1A) (23). In addition, wild type RIPK3-expressing cells were highly sensitive to cell death induced by bortezomib, a clinically approved proteasome inhibitor (Fig. 1B). To delineate the underlying mechanism, we generated a series of RIPK3 mutants and introduced them into Ripk3−/− 3T3 cells under the control of doxycycline-inducible promoter. MG132 and bortezomib induced comparable cell death in cells expressing wild type RIPK3 as well as the kinase inactive RIPK3 mutants K51A, D143N, and D161N (Fig. 1, B and C). In contrast, cells that express RIPK3 carrying tetra-alanine mutation in the core sequence of RHIM were resistant to MG132 and bortezomib (Fig. 1, B–D). These results indicate that proteasome inhibitor-induced cell death requires an intact RIPK3 RHIM.
FIGURE 1.
MG132 induces RIPK3-dependent cell death through the RHIM. Wild type and mutant RIPK3 tagged by HA and FLAG at the N and C termini, respectively (see “Experimental Procedures”), were expressed in Ripk3−/− 3T3 cells under the control of doxycycline-inducible promoter. A, cells were treated with 1 μg/ml doxycycline (DOX) for 2 h, followed by 100 μm 5-FU, 50 μm oxaliplatin, 50 μm irinotecan, 5 μm doxorubicin, 50 μm etoposide, or 2 μm MG132 for 24 h. B, after induction with DOX for 14 h, Ripk3−/− 3T3 cells expressing wild type or mutant RIPK3 were treated with 12 nm bortezomib. C, after DOX induction, cells were treated with 2 μm MG132 for 19 h. D, after DOX induction for 14 h, cells were treated with 2 μm MG132 for 8.5 h. Induction of RIPK3 expression was confirmed by Western blot after treatment with doxycycline for 17 h (C, D). Cell death was determined by measuring loss of intracellular ATP (iATP) (A–C) or LDH release (D). *, p < 0.05.
MG132 Induces Necroptosis in Wild Type RIPK3-expressing Cells
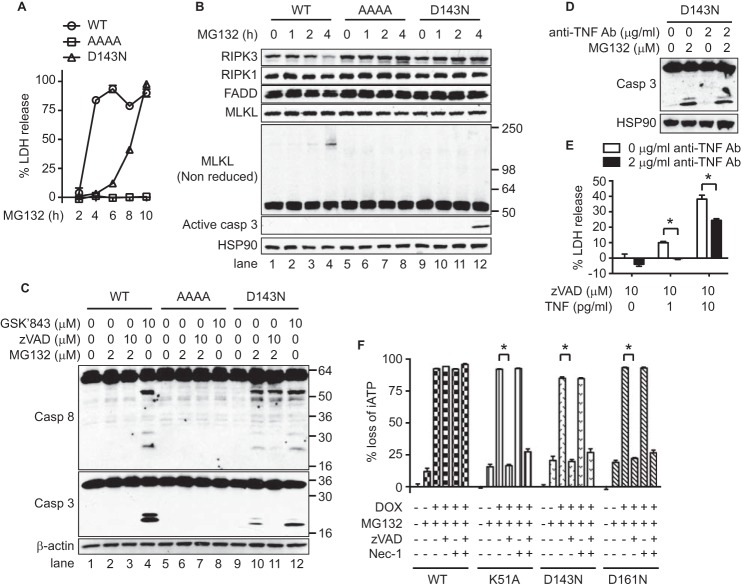
The kinase activity of RIPK3 is indispensable for necroptosis (7). In addition, RIPK3 can also induce apoptosis in a kinase activity-independent manner (24, 25). To determine the cell death mechanism induced by MG132, we first examined the cell death kinetics. Wild type RIPK3-expressing cells underwent rapid cell death within 4 h after MG132 treatment (Fig. 2A). In contrast, cell death in RIPK3-D143N-expressing cells was much slower (Fig. 2A). During necroptosis induction, RIPK3 phosphorylates its downstream substrate MLKL. Activated MLKL undergoes oligomerization and this process can be visualized on non-reducing SDS-PAGE (11–14). In wild type RIPK3-expressing cells, MG132-induced MLKL oligomerization was first detected at 2 h, and was further enhanced at 4 h (Fig. 2B, lanes 3 and 4). Strikingly, MLKL oligomerization was absent in RHIM or D143N mutant-expressing cells (Fig. 2B, lanes 5–12). Instead, we detected cleaved and activated caspase 3 and 8 in D143N mutant-expressing cells at 4 h, but not in wild type RIPK3-expressing cells (Fig. 2, B, lane 12 and C, lane 10). The caspase 3 activation was not blocked by anti-TNF blocking antibody, which significantly blocked TNF-induced necroptosis, indicating that autocrine TNF production by MG132 treatment is not involved in the activation (Fig. 2, D and E). These results suggest that wild type RIPK3 and kinase inactive RIPK3-D143N mediates distinct forms of cell death in response to MG132. Consistent with the biochemical results, the pancaspase inhibitor z-VAD-fmk blocked MG132-induced caspase activation (Fig. 2C, lanes 10–11) and cell death in RIPK3-D143N-expressing cells (Fig. 2F). In contrast, z-VAD-fmk did not inhibit cell death in wild type RIPK3-expressing cells (Fig. 2F).
FIGURE 2.
MG132 induces necroptosis independent of RIPK3 kinase activity. A, after induction of RIPK3 expression by doxycycline (DOX) for 14 h, the cells were treated with 2 μm MG132 for the indicated time. B and C, RIPK3 expression was induced by DOX for 14 h before treatment with 2 μm MG132 for the indicated time (B). In C, cells were treated with 10 μm zVAD for 1 h, followed by MG132 for 4 h or GSK′843 for 2.5 h. Whole cell extracts were analyzed by Western blot. D and E, after treatment with DOX for 14 h, the D143N mutant-expressing cells were pretreated with anti-TNF blocking antibody for 1 h. Subsequently, cells were treated with MG132 for 5 h (D). In E, necroptosis was induced by zVAD and TNF. F, cells were treated with 2 μm MG132, 1 μg/ml DOX, 10 μm z-VAD-fmk (zVAD), and 10 μm necrostatin-1 (Nec-1) for 16 h as indicated. Cell death was determined by measuring loss of intracellular ATP (iATP) (F) or LDH release (A, E). *, p < 0.05.
As recently reported (25), high concentration of the RIPK3 kinase inhibitor GSK'843 induced activation of caspase 3 and 8 in wild type RIPK3 and D143N mutant-expressing cells (Fig. 2C, lanes 4 and 12). Hence, the lack of caspase 3 and 8 activation in MG132-treated wild type RIPK3-expressing cells was not due to intrinsic defect in caspase activation. The RIPK1 kinase inhibitor necrostatin-1 (Nec-1) did not block MG132-induced cell death in wild type and kinase dead mutant-expressing cells (Fig. 2F), indicating that RIPK1 kinase activity is not required. These results indicate that MG132 primarily induces necroptosis when RIPK3 kinase activity is intact, but that inhibition of RIPK3 kinase activity switches the cell death mode from necroptosis to apoptosis.
Availability of MLKL Determines the Cell Death Mode Induced by Proteasome Inhibition
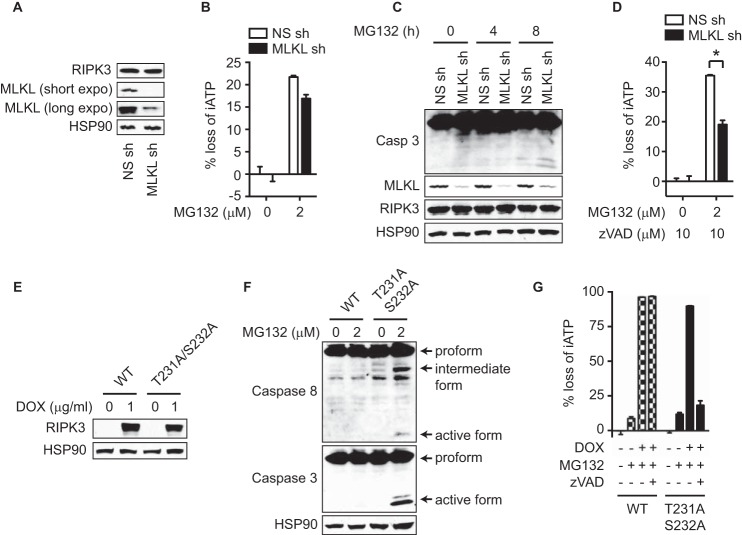
To evaluate the role of MLKL in MG132-induced cell death in wild type RIPK3-expressing cells, we silenced MLKL expression by shRNA (Fig. 3A). Interestingly, knockdown of MLKL did not suppress MG132-induced cell death (Fig. 3B). Previous studies showed that knockdown or knock-out of MLKL switched cell death from necroptosis to apoptosis (26, 27). Consistent with these reports, MG132 induced caspase 3 activation in cells whose MLKL expression was silenced (Fig. 3C). In addition, z-VAD-fmk inhibited MG132-induced cell death in MLKL-knockdown cells (Fig. 3D). The residual cell death in MLKL-knockdown cells was likely due to incomplete silencing of MLKL expression (Fig. 3, A, C, D).
FIGURE 3.
MLKL availability determines the cell death mode in response to MG132. A–D, Ripk3−/− 3T3 cells were transduced with retrovirus expressing wild type HA-RIPK3-FLAG and lentivirus expressing non-silencing (NS) or MLKL shRNA. In A, knockdown of MLKL expression was confirmed by Western blot. In B and D, cell death was determined after 2 μm MG132 treatment for 16 h. In C, active caspase 3 was measured by Western blot after MG132 treatment for the indicated time. E–G, Ripk3−/− 3T3 cells expressing wild type or T231A/S232A RIPK3 under the control of doxycycline-inducible promoter were used. Induction of RIPK3 expression was confirmed by Western blot after treatment with doxycycline (DOX) for 14 h (E). After induction of RIPK3 expression, cells were treated with MG132 for 4 h (F). The cells were treated with DOX and 10 μm zVAD for 1 h prior to treatment with 2 μm MG132 for 16 h (G). Cell death was determined by measuring loss of intracellular ATP (iATP) (B, D, G). *, p < 0.05.
Our results suggest that the availability of MLKL, but not the RIPK3 kinase activity per se, determines whether cells undergo necroptosis or apoptosis. To further test this model, we generated RIPK3 T231A/S232A mutant-expressing cells (Fig. 3E). Since phosphorylation of Thr-231 and Ser-232 is essential for recruitment of MLKL to RIPK3, RIPK3-T231A/S232A cannot activate MLKL despite retention of intact kinase activity (10). Consistent with our model, MG132 induced activation of caspase 8 and 3 in T231A/S232A mutant-expressing cells (Fig. 3F), similar to RIPK3 kinase dead mutant-expressing cells (Fig. 2, B and C). Moreover, z-VAD-fmk inhibited MG132-induced cell death in RIPK3-T231A/S232A cells, but not wild type RIPK3-expressing cells (Fig. 3G). Collectively, these results show that the availability of MLKL determines the cell death mode in response to proteasome inhibition.
Proteasome Inhibition Induces Apoptosis through the Ripoptosome
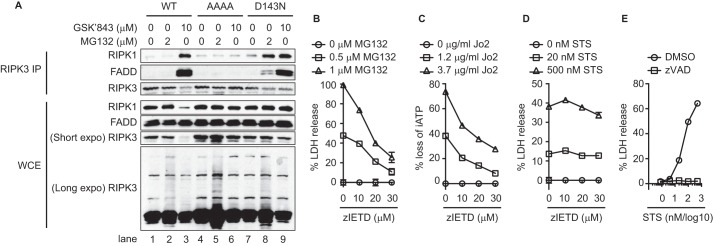
During TNF-induced necroptosis, RIPK3 interacts with RIPK1 to form the necrosome or ripoptosome, which also consists of FADD and caspase 8 (7). In wild type RIPK3-expressing cells, MG132 did not induce ripoptosome formation (Fig. 4A, lane 2). This is in contrast to treatment with the RIPK3 kinase inhibitor GSK'843, which induced strong RIPK1-RIPK3-FADD interaction as previously reported (Fig. 4A, lane 3) (25). Ripoptosome assembly required an intact RHIM, since tetra-alanine RIPK3 RHIM mutant abolished GSK′843-induced ripoptosome assembly (Fig. 4A, lane 6). We also did not detect interaction between MLKL and RIPK3 in wild type RIPK3-expressing cells after MG132 treatment, suggesting that MLKL only transiently interacts with RIPK3 to be activated (data not shown). In D143N mutant-expressing cells, MG132 and GSK′843 both induced formation of the ripoptosome (Fig. 4A, lanes 8–9). Ripoptosome-induced apoptosis in D143N mutant-expressing cells was inhibited by the caspase 8 inhibitor z-IETD-fmk (Fig. 4B). z-IETD-fmk inhibited cell death induced by the anti-Fas agonistic Jo2 antibody (Fig. 4C), but not apoptosis induced by the intrinsic apoptosis inducer staurosporine (STS) (Fig. 4D). By contrast, the pan-caspase inhibitor z-VAD-fmk completely blocked STS-induced cell death (Fig. 4E). These results indicate that when RIPK3 kinase activity is inhibited, MG132 induces ripoptosome formation, caspase 8 activation, and apoptosis.
FIGURE 4.
Inhibition of RIPK3 kinase activity triggers MG132-induced ripoptosome formation. A, after induction with doxycycline for 14 h, cells were treated with MG132 for 4 h or GSK′843 for 2 h. Whole cell extracts were subjected to immunoprecipitation and Western blot analysis. B–E, cells were treated with DOX for 14 h, followed by z-IETD-fmk (zIETD) for 1 h, and MG132 for 8 h (B). In C–E, cells were treated with DOX for 1 h. z-IETD or zVAD were added 1 h before addition of Jo-2 antibody (14 h) or staurosporine (STS, 16 h). Cell death was determined by measuring loss of intracellular ATP (iATP) (C) or LDH release (B, D, E).
RIPK1 Is Required for MG132-induced Apoptosis, but Not Necroptosis
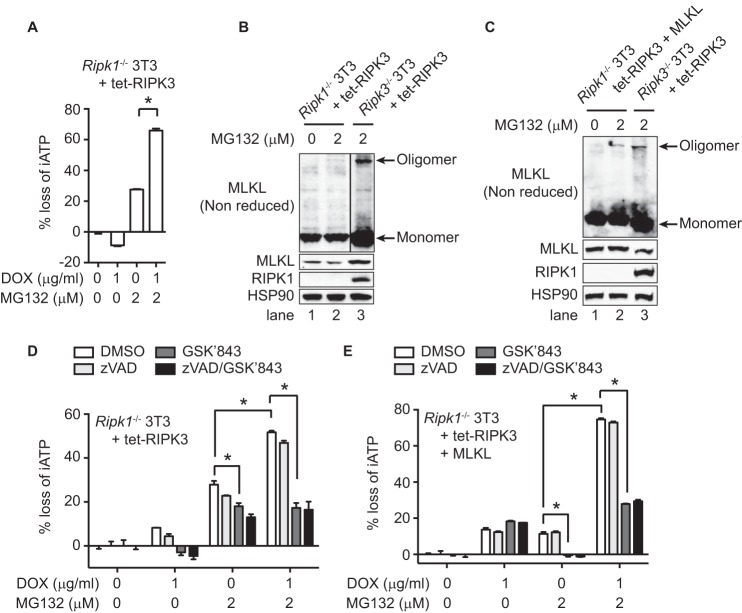
Although the RIPK1 kinase inhibitor Nec-1 did not inhibit RIPK3-dependent necroptosis and apoptosis in response to MG132 (Fig. 2F), RIPK1 was still recruited to the RIPK3-associated ripoptosome when apoptosis was induced in the D143N mutant (Fig. 4A). To examine whether RIPK1 might promote RIPK3-dependent cell death through its scaffolding function (28), we expressed wild type RIPK3 in a doxycycline-inducible manner in Ripk1−/− 3T3 cells. In these cells, RIPK3 expression similarly enhanced MG132-induced cell death (Fig. 5A). However, when compared with Ripk3−/− cells, MG132 induced weak MLKL oligomerization in Ripk1−/− cells (Fig. 5B, lanes 2–3), which was probably due to lower expression of MLKL in Ripk1−/− 3T3 cells (Fig. 5B). Indeed, when we overexpressed MLKL in Ripk1−/− 3T3 cells, MLKL oligomerization became much more prominent (Fig. 5C, lane 2). Interestingly, the RIPK3 kinase inhibitor GSK′843 strongly inhibited MG132-induced cell death in Ripk1−/− cells (Fig. 5, D and E). This is in contrast to RIPK1-sufficient cells, which switched the cell death mode from necroptosis to apoptosis when RIPK3 kinase activity was inhibited (Fig. 2F). MG132 also induced RIPK3 kinase activity-dependent cell death in Ripk1−/− 3T3 cells in the absence of RIPK3 overexpression (Fig. 5, D and E). These results indicate that RIPK1 is an integral component of the MG132-induced ripoptosome and subsequent apoptosis. However, an intact RIPK1 is dispensable for MG132-induced, RIPK3-mediated necroptosis.
FIGURE 5.
RIPK1 is dispensable for MG132-induced necroptosis. Ripk1−/− 3T3 cells expressing wild type RIPK3 under the control of doxycycline-inducible promoter were used in A, B, and D. C and E, wild-type MLKL was stably transfected in these cells. Ripk3−/− 3T3 cells expressing wild type RIPK3 under the control of doxycycline-inducible promoter were used as wild type control for the Ripk1−/− cells. In A, after induction with DOX for 3 h, cells were treated with MG132 for 14 h. In B–C, after DOX treatment for 14 h, they were stimulated with MG132 for 5 h. Whole cell extracts were subjected to Western blot analysis. D–E, after DOX induction for 3 h, cells were treated with 10 μm z-VAD-fmk (zVAD) and/or 2 μm GSK′843 for 1 h, followed by 2 μm MG132 for 14 h (D) or 5 h (E). Cell death was determined by measuring loss of intracellular ATP (iATP) (A, D, E). *, p < 0.05.
Bortezomib Activates Necroptotic Pathway in Human Cancer Cells
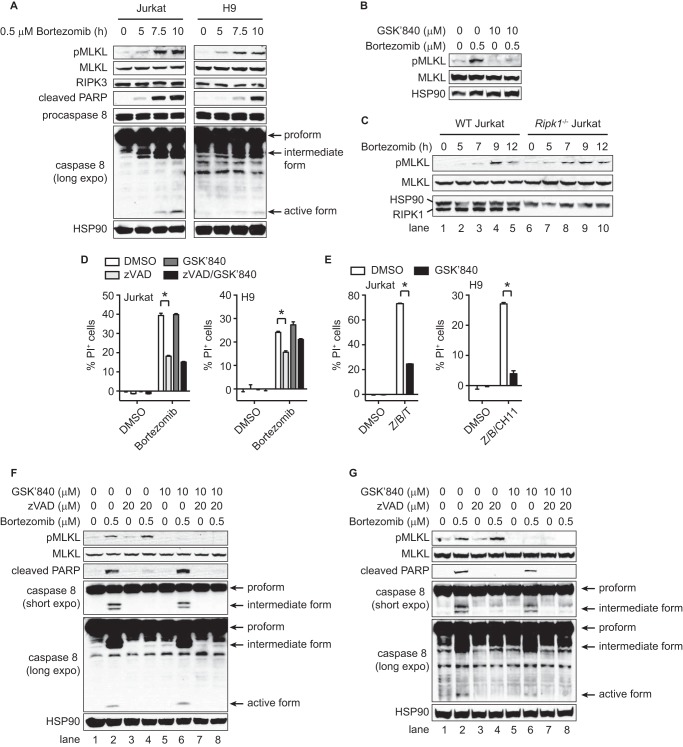
The proteasome inhibitor bortezomib is clinically approved for the treatment of multiple myeloma and mantle cell lymphoma (19). We therefore tested whether bortezomib might activate the necroptotic pathway in human lymphoma. The human lymphoma cell lines Jurkat and H9 both expressed endogenous RIPK3 (23). However, since RIPK3 expression in these cells were lower than that in doxycycline-induced 3T3 cells, we used a higher concentration of bortezomib to induce cell death in these experiments. In these cells, bortezomib induced phosphorylation of MLKL (Fig. 6A, top panel). This phosphorylation was blocked by the human-specific RIPK3 kinase inhibitor GSK′840, indicating that bortezomib activated the RIPK3-MLKL necroptotic pathway (Fig. 6B). Unlike in wild type RIPK3-expressing 3T3 cells, bortezomib also activated the apoptotic pathway in Jurkat and H9 cells, since caspase 8 activation and PARP cleavage was also observed (Fig. 6A). In RIPK1-deficient Jurkat cells, bortezomib also induced MLKL phosphorylation, indicating that RIPK1 is dispensable for proteasome inhibitor-induced MLKL activation (Fig. 6C, lanes 6–10).
FIGURE 6.
Bortezomib activates the RIPK3-MLKL necroptotic pathway in human cancer cells. A–C, in A and C, whole cell extracts from wild type and Ripk1−/− Jurkat, and H9 cells treated with 0.5 μm bortezomib for the indicated time were subjected to Western blot. In B, H9 cells were treated with GSK′840 for 1 h and bortezomib for 7 h. D, cells pretreated with 20 μm z-VAD-fmk (zVAD) and/or 10 μm GSK'840 for 1 h were treated with 0.5 μm bortezomib for 10 h (Jurkat cells) or 7 h (H9 cells). E, Jurkat and H9 cells pretreated with 20 μm zVAD (Z), 2 μm BV6 (B), and 10 μm GSK′840 for 1 h were treated with 100 ng/ml TNF (T) for 10 h or 100 ng/ml anti-Fas agonistic CH11 antibody for 7 h. F–G, cells pretreated with zVAD and/or GSK′840 for 1 h were treated with bortezomib for 9 h (Jurkat cells) or 7 h (H9 cells). Cell death was determined by FACS using PI (D and E). *, p < 0.05.
Apoptosis is often dominant over necroptosis because caspase 8 cleaves and inactivates RIPK1 and RIPK3 (29–31). Indeed, z-VAD-fmk reduced bortezomib-induced cell death by ∼50% in both Jurkat and H9 cells (Fig. 6D). In contrast, GSK′840, which potently inhibited TNF- and Fas-induced necroptosis (Fig. 6E), did not inhibit bortezomib-induced cell death either alone or together with z-VAD-fmk (Fig. 6D). The lack of protection by GSK′840 was not due to incomplete inhibition of RIPK3, since MLKL phosphorylation was completely inhibited (Fig. 6, F and G, lanes 5–8). These results indicate that although bortezomib activates the necroptotic pathway, it mainly induces caspase-dependent apoptosis and caspase-independent non-necroptotic cell death in these lymphoma cell lines.
Proteasome Inhibitors Induce K48-linked RIPK3 Ubiqutination
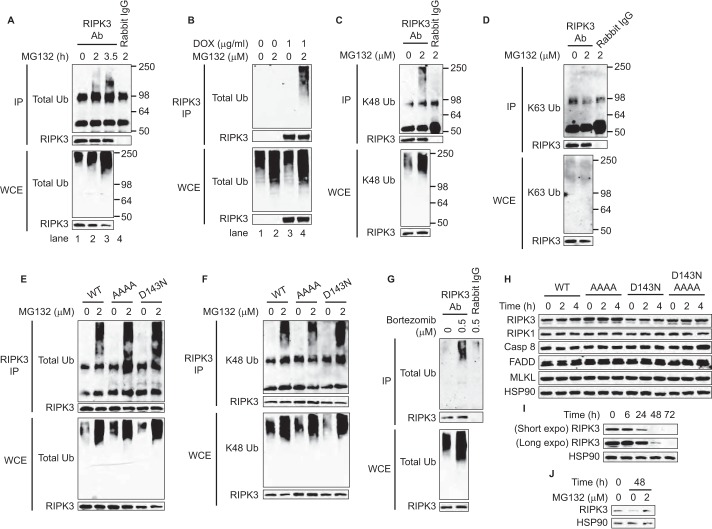
We detected higher molecular weight species in RIPK3 immunoblot when RIPK3-expressing 3T3 cells were treated with MG132 (Fig. 4A, lanes 2, 5, and 8). In contrast, GSK'843 did not induce these higher molecular weight species (Fig. 4A, lanes 3, 6, and 9). To directly test whether these high molecular weight species represent polyubiquitinated RIPK3, we isolated RIPK3 by immunoprecipitation (IP) under denaturing condition to remove other interacting proteins. Indeed, we found that ubiquitinated RIPK3 appeared by 2 h after MG132 treatment in wild type RIPK3 expressing cells (Fig. 7A, lane 2). The high molecular weight species represented RIPK3 since the signal was absent without doxycycline induction (Fig. 7B, compare lanes 2 and 4). The ubiquitinated RIPK3 contained Lys-48, but not Lys-63 linkages (Fig. 7, C and D). MG132-induced RIPK3 ubiquitination was also observed in cells expressing RHIM or D143N RIPK3 mutant (Fig. 7, E and F) and in bortezomib-treated Jurkat cells (Fig. 7G).
FIGURE 7.
Proteasome inhibition induces accumulation of K48-linked polyubiquitinated RIPK3. A–F, after induction of RIPK3 with DOX for 14 h, cells were treated with 2 μm MG132 for the indicated time (A) or for 2.5 h (B–F). G, Jurkat cells were treated with 0.5 μm bortezomib for 7 h. Cell lysates were subjected to denaturing IP as described under “Experimental Procedures” (A–G). H–J, after induction of RIPK3 expression by DOX for 14 h, DOX was removed, and the cells were treated with 2 μg/ml cycloheximide for the indicated time. Cycloheximide was removed after 24 h in I due to its toxicity. In J, Ripk3−/− 3T3 cells expressing tetra-alanine RHIM RIPK3 mutant were used since wild type or kinase dead mutant RIPK3-expressing cells were killed by MG132 much earlier than 24 h (Fig. 2A).
K48-linked polyubiquitin chain is a principal signal for proteasome-mediated degradation (16). As such, MG132 might promote RIPK3-dependent cell death by stabilizing expression of RIPK3 and other ripoptosome components. However, the expression level of RIPK3, RIPK1, FADD, caspase 8, and MLKL was not increased by MG132 treatment after 4 h when MLKL or caspase 8 were fully active (Fig. 2, B and C). In addition, blocking de novo protein synthesis with cycloheximide for up to 4 h did not reduce the expression of the ripoptosome components (Fig. 7H). RIPK3 expression was not changed at 6 h, but was significantly reduced after 24 h of cycloheximide treatment (Fig. 7I). MG132 reversed the loss of RIPK3 expression by cycloheximide (Fig. 7J). These results indicate that a small fraction of RIPK3 constantly undergoes K48-linked polyubiquitination and proteasomal degradation. Our results also suggest that polyubiquitination regulates RIPK3 activation in apoptosis and necroptosis.
K264 Is a Key Ubiquitin Acceptor Site on RIPK3
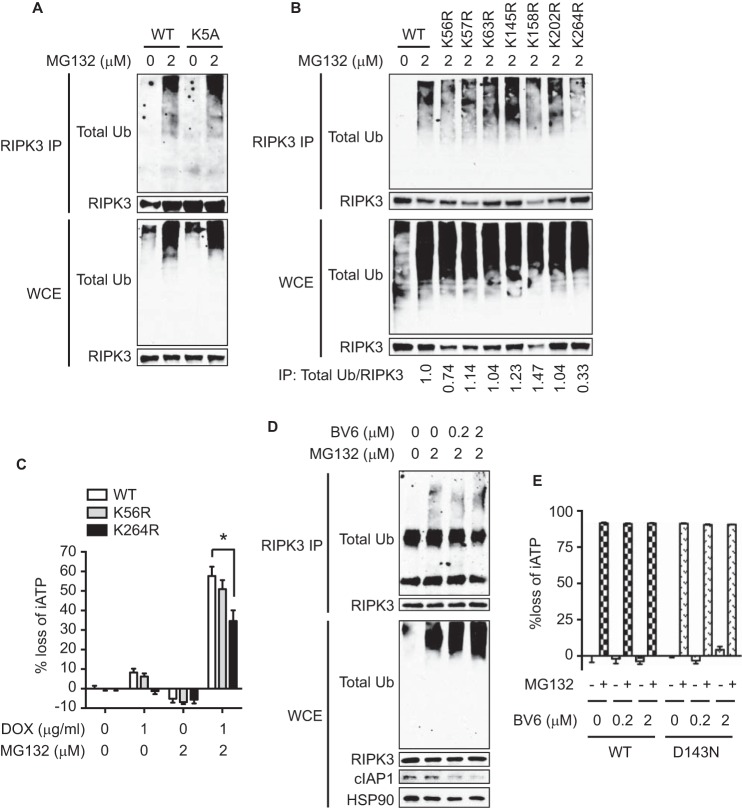
A recent report showed that mouse RIPK3 is modified by K63-linked ubiquitination at Lys-5 in A20-deficient cells during TNF-induced necroptosis (32). In contrast to this report, MG132 induced normal ubiquitination of RIPK3-K5A mutant (Fig. 8A). Sequence alignment of RIPK3 from different species revealed several conserved lysine residues. We introduced arginine substitution at these lysine residues (Lys-56, Lys-57, Lys-63, Lys-145, Lys-158, Lys-202, and Lys-264) and found that none of them affected MG132-induced RIPK3 ubiquitination except the K264R mutant and to a lesser extent the K56R mutant (Fig. 8B). MG132-induced, RIPK3-dependent cell death was partially reduced in cells expressing RIPK3-K264R, but not RIPK3-K56R (Fig. 8C). Similar results were observed with cells expressing RIPK3-K264A (data not shown). The E3 ligases cIAP1, cIAP2, and XIAP were implicated in RIPK3 ubiquitination in LPS-stimulated bone marrow-derived macrophages (33). However, the SMAC mimetic BV6, which induces IAP degradation (34), did not alter MG132-induced RIPK3 ubiquitination or cell death (Fig. 8, D and E). These results indicate that ubiquitination of RIPK3 at Lys-264 by a yet-to-be-identified E3 ligase regulates RIPK3-dependent necroptosis and apoptosis.
FIGURE 8.
Lysine 264 in the kinase domain of RIPK3 is a key ubiquitin acceptor residue. A–D, after induction of RIPK3 expression by DOX for 14 h, the cells were treated with MG132 for 2.5 h (A-B, D) or for 4 h (C). In D, cells were pretreated with BV6 for 1 h before MG132 treatment. Whole cell extracts were subjected to denaturing IP. The ratio of the band intensity quantified by Image J is shown at the bottom in B. E, cells pretreated with BV6 and DOX for 1 h prior to 2 μm MG132 for 14 h. Cell death was determined by measuring loss of intracellular ATP (iATP) (C, E). *, p < 0.05.
Discussion
In this study, we found that proteasome inhibitors activate the RIPK3-MLKL necroptotic pathway in a RHIM-dependent manner. In contrast to TNF-induced necroptosis, which requires inhibition of caspase 8, proteasome inhibitors induced RIPK3 and MLKL activation without caspase 8 inhibition. The differential requirement for caspase 8 inhibition suggests that proteasome inhibitors and TNF-like death cytokines stimulate necroptosis through distinct mechanisms. Our previous study indicates that the RHIM of RIPK3 is sterically masked by the kinase domain to prevent RHIM-mediated oligomerization. Phosphorylation of the RIPK3 kinase domain relieves this steric hindrance and exposes the RHIM to provide a feed-forward signal for further phosphorylation and oligomerization of RIPK3 (6). In wild type RIPK3-expressing 3T3 cells, MG132 treatment induced rapid MLKL oligomerization with a concomitant increase in K48-linked ubiquitinated RIPK3 within 2 h. Mutation of Lys-264, which is located in the kinase domain of RIPK3, partially reduced RIPK3 ubiquitination and necroptosis. It is tempting to speculate that K48-linked RIPK3 polyubiquitination at Lys-264 may similarly expose the RHIM to promote RIPK3 oligomerization and activation (Fig. 9). This model predicts that the proteasome critically regulates a tonic low level of active RIPK3 and perturbation of this process can lead to MLKL activation and necroptosis. However, we cannot exclude the possibility that certain unidentified labile proteins that regulate RIPK3 activation are the real targets of this proteasome-regulated response.
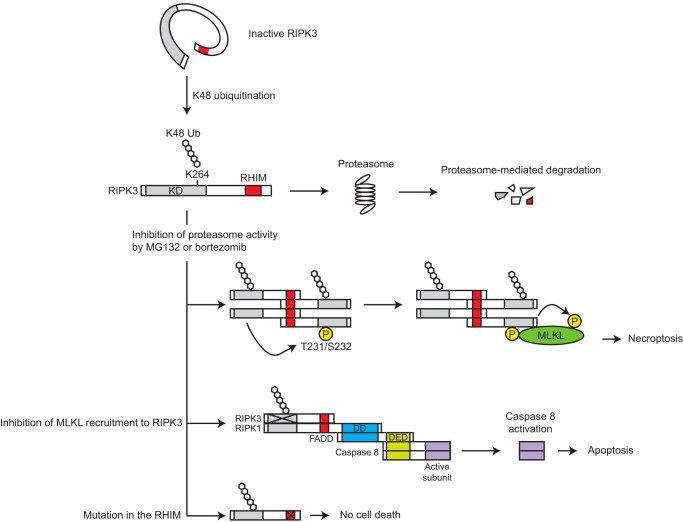
FIGURE 9.
The model of ubiquitination-mediated RIPK3 activation. A majority of intracellular RIPK3 is in an inactive state because the kinase domain sterically masks the RHIM. Lys-48-linked ubiquitination at Lys-264, which is observed in only a small fraction of the total cellular pool, allows the inactive RIPK3 to make a transition to an active form by releasing the kinase domain-mediated steric constraint. Such a small amount of active RIPK3 is eliminated by proteasome and thereby does not induce cell death in a steady state condition. When the proteasome activity is blocked, the active RIPK3 forms RHIM-dependent homooligomer, which in turn recruits and activates MLKL. When MLKL recruitment to RIPK3 is inhibited by 1) inhibition of the RIPK3 kinase activity, 2) mutation of RIPK3 Thr-231/Ser-232, or 3) MLKL knockdown, RIPK3 forms a complex with RIPK1, FADD, and caspase 8. Within this complex, caspase 8 is activated and subsequently induces apoptosis. RHIM is critical for both types of death pathway. KD, kinase domain; DD, death domain; DED, death effector domain; P, phosphorylation.
The turnover of many cell death mediators have been reported to be regulated by the UPS. For instance, TRAF2 facilitates K48-linked polyubiquitination of caspase 8, which functions as a shutoff timer for caspase 8 activation (35). In addition, MDM2 oncoprotein facilitates proteasomal degradation of p53 (36–38). These are some of the examples by which the UPS protects cells from apoptosis. Our study indicates that the UPS also protects cells from spontaneous necroptosis. Intriguingly, in Jurkat and H9 cells, we unexpectedly observed a low level of spontaneous MLKL phosphorylation. We do not know at present why such phosphorylation does not result in necroptosis. Given the results presented here, it is tempting to speculate that similar UPS-mediated degradative mechanism may regulate necroptosis downstream of MLKL phosphorylation.
In cells expressing wild type RIPK3, MG132 induced MLKL activation without RIPK3 binding to other ripoptosome components such as RIPK1 and FADD. Hence, we speculate that RIPK3 likely forms homo-oligomers through the RHIM and recruit MLKL in response to MG132 treatment. Interestingly, when MLKL recruitment to RIPK3 is impeded by inhibition of RIPK3 kinase activity, knockdown of MLKL, or mutation of the MLKL binding site on RIPK3 (Thr-231/Ser-232), RIPK3 recruits RIPK1 and FADD to form the ripoptosome, a caspase 8-activating complex. Cook et al. previously reported a similar observation that forced dimerization of RIPK3 induces necroptosis or apoptosis depending on the availability of MLKL and caspase 8 (27). Thus, while the precise mechanism that regulates this molecular decision is unknown at present, it is clear that RIPK3-dependent apoptosis and necroptosis are regulated by events distal to RIPK3 activation.
RIPK3 modifications that resemble ubiquitination was first observed during TNF-induced necroptosis (7). In addition, recent reports show that Lys-63 ubiquitination of RIPK3 at Lys-5 is critical for TNF-induced necroptosis in A20-deficient mouse embryonic fibroblasts (32). RIPK3 polyubiquitination by the E3 ligases cIAP1/2 has also been detected in LPS-stimulated bone marrow-derived macrophages (33). In contrast to these reports, we found that ubiquitnation of RIPK3 in response to proteasome inhibitors does not involve Lys-5 or the cellular IAPs. While we cannot rule out contribution from other lysine residues, our results suggest that Lys-264 is a key ubiquitin acceptor site on RIPK3 in response to proteasome inhibitors. Importantly, only a small fraction of the total cellular pool of RIPK3 participates in this ubiquitination event, an observation that is consistent with recent proteomic analysis that shows RIPK1, but not RIPK3, as the predominant ubiquitinated species during necroptosis (4, 39).
Bortezomib is a clinically proven proteasome inhibitor in the treatment of multiple myeloma and mantle cell lymphoma (19). Mechanisms such as endoplasmic reticulum stress (40) and inhibition of NF-κB-mediated pro-survival gene expression (41, 42) have been proposed to mediate the cytotoxic effects of bortezomib. Our results demonstrate the RIPK3-MLKL necroptotic pathway as a novel signaling module that can be activated by bortezomib. Curiously, despite clear RIPK3-dependent phosphorylation of MLKL, RIPK3 kinase inhibitor did not rescue bortezomib-induced cell death in the human leukemia cell lines Jurkat and H9. The inability of RIPK3 kinase inhibitor to block bortezomib-induced cell death may be attributed to the multiple compensatory cell death pathways induced by bortezomib. Nonetheless, since proteasome inhibition has the capacity to activate RIPK3 and MLKL in leukemia cell lines, it will be interesting to determine whether bortezomib can induce necroptosis in primary leukemia cells. RIPK3 expression was found to be decreased in various human cancer cell lines (23, 43) as well as in primary colon cancer (23, 44), breast cancer (45), and acute myeloid leukemia (46). Development of drugs to re-activate expression of RIPK3 in cancer cells may therefore synergize with proteasome inhibitors to enhance cancer cell death and treatment efficacy.
Author Contributions
K. M. conceived the project, conducted all the experiments, analyzed the results, and wrote the paper. F. C. supervised the project and wrote the paper.
Acknowledgments
We thank P. Gough and J. Bertin (GlaxoSmithKline) for GSK′840 and ′843 and D. Vucic (Genentech) for BV6.
This work was supported by National Institutes of Health Grant AI083497. The authors declare that they have no conflicts of interest with the contents of this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
- RIPK
- receptor-interacting protein kinase
- RHIM
- receptor interacting protein homotypic interaction motif
- MLKL
- mixed lineage kinase domain-like
- TNF
- tumor necrosis factor
- TRIF
- Toll/IL-1 receptor domain-domain-containing adaptor-inducing interferon β
- T
- threonine
- S
- serine
- K
- lysine
- XIAP
- X-linked inhibitor of apoptosis
- SMAC
- second mitochondria-derived activator of caspases
- IP
- immunoprecipitation.
References
- 1. Chan F. K., Luz N. F., and Moriwaki K. (2015) Programmed necrosis in the cross talk of cell death and inflammation. Annu. Rev. Immunol. 33, 79–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Linkermann A., and Green D. R. (2014) Necroptosis. Necroptosis. N. Engl. J. Med. 370, 455–465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Moriwaki K., and Chan F. K. (2013) RIP3: a molecular switch for necrosis and inflammation. Genes Dev. 27, 1640–1649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Moquin D. M., McQuade T., and Chan F. K. (2013) CYLD deubiquitinates RIP1 in the TNFα-induced necrosome to facilitate kinase activation and programmed necrosis. PloS one 8, e76841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sun X., Yin J., Starovasnik M. A., Fairbrother W. J., and Dixit V. M. (2002) Identification of a novel homotypic interaction motif required for the phosphorylation of receptor-interacting protein (RIP) by RIP3. J. Biol. Chem. 277, 9505–9511 [DOI] [PubMed] [Google Scholar]
- 6. Li J., McQuade T., Siemer A. B., Napetschnig J., Moriwaki K., Hsiao Y. S., Damko E., Moquin D., Walz T., McDermott A., Chan F. K., and Wu H. (2012) The RIP1/RIP3 necrosome forms a functional amyloid signaling complex required for programmed necrosis. Cell 150, 339–350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cho Y. S., Challa S., Moquin D., Genga R., Ray T. D., Guildford M., and Chan F. K. (2009) Phosphorylation-driven assembly of the RIP1-RIP3 complex regulates programmed necrosis and virus-induced inflammation. Cell 137, 1112–1123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dillon C. P., Weinlich R., Rodriguez D. A., Cripps J. G., Quarato G., Gurung P., Verbist K. C., Brewer T. L., Llambi F., Gong Y. N., Janke L. J., Kelliher M. A., Kanneganti T. D., and Green D. R. (2014) RIPK1 blocks early postnatal lethality mediated by caspase-8 and RIPK3. Cell 157, 1189–1202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sun L., Wang H., Wang Z., He S., Chen S., Liao D., Wang L., Yan J., Liu W., Lei X., and Wang X. (2012) Mixed lineage kinase domain-like protein mediates necrosis signaling downstream of RIP3 kinase. Cell 148, 213–227 [DOI] [PubMed] [Google Scholar]
- 10. Chen W., Zhou Z., Li L., Zhong C. Q., Zheng X., Wu X., Zhang Y., Ma H., Huang D., Li W., Xia Z., and Han J. (2013) Diverse sequence determinants control human and mouse receptor interacting protein 3 (RIP3) and mixed lineage kinase domain-like (MLKL) interaction in necroptotic signaling. J. Biol. Chem. 288, 16247–16261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wang H., Sun L., Su L., Rizo J., Liu L., Wang L. F., Wang F. S., and Wang X. (2014) Mixed lineage kinase domain-like protein MLKL causes necrotic membrane disruption upon phosphorylation by RIP3. Mol. Cell 54, 133–146 [DOI] [PubMed] [Google Scholar]
- 12. Chen X., Li W., Ren J., Huang D., He W. T., Song Y., Yang C., Zheng X., Chen P., and Han J. (2014) Translocation of mixed lineage kinase domain-like protein to plasma membrane leads to necrotic cell death. Cell Res. 24, 105–121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cai Z., Jitkaew S., Zhao J., Chiang H. C., Choksi S., Liu J., Ward Y., Wu L. G., and Liu Z. G. (2014) Plasma membrane translocation of trimerized MLKL protein is required for TNF-induced necroptosis. Nat. Cell Biol. 16, 55–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dondelinger Y., Declercq W., Montessuit S., Roelandt R., Goncalves A., Bruggeman I., Hulpiau P., Weber K., Sehon C. A., Marquis R. W., Bertin J., Gough P. J., Savvides S., Martinou J. C., Bertrand M. J., and Vandenabeele P. (2014) MLKL compromises plasma membrane integrity by binding to phosphatidylinositol phosphates. Cell Rep. 7, 971–981 [DOI] [PubMed] [Google Scholar]
- 15. Zhao J., Jitkaew S., Cai Z., Choksi S., Li Q., Luo J., and Liu Z. G. (2012) Mixed lineage kinase domain-like is a key receptor interacting protein 3 downstream component of TNF-induced necrosis. Proc. Natl. Acad. Sci. U.S.A. 109, 5322–5327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bhattacharyya S., Yu H., Mim C., and Matouschek A. (2014) Regulated protein turnover: snapshots of the proteasome in action. Nat. Rev. Mol. Cell Biol. 15, 122–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Schwartz A. L., and Ciechanover A. (1999) The ubiquitin-proteasome pathway and pathogenesis of human diseases. Annu. Rev. Med. 50, 57–74 [DOI] [PubMed] [Google Scholar]
- 18. Bedford L., Lowe J., Dick L. R., Mayer R. J., and Brownell J. E. (2011) Ubiquitin-like protein conjugation and the ubiquitin-proteasome system as drug targets. Nat. Rev. Drug. Discov. 10, 29–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Richardson P. G., Mitsiades C., Hideshima T., and Anderson K. C. (2006) Bortezomib: proteasome inhibition as an effective anticancer therapy. Annu. Rev. Med. 57, 33–47 [DOI] [PubMed] [Google Scholar]
- 20. Dick L. R., and Fleming P. E. (2010) Building on bortezomib: second-generation proteasome inhibitors as anti-cancer therapy. Drug. Discov. Today 15, 243–249 [DOI] [PubMed] [Google Scholar]
- 21. Choo Y. S., and Zhang Z. (2009) Detection of protein ubiquitination. J. Vis. Exp 30, 1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bertrand M. J., Lippens S., Staes A., Gilbert B., Roelandt R., De Medts J., Gevaert K., Declercq W., and Vandenabeele P. (2011) cIAP1/2 are direct E3 ligases conjugating diverse types of ubiquitin chains to receptor-interacting proteins kinases 1 to 4 (RIP1–4). PloS one 6, e22356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Moriwaki K., Bertin J., Gough P. J., Orlowski G. M., and Chan F. K. (2015) Differential roles of RIPK1 and RIPK3 in TNF-induced necroptosis and chemotherapeutic agent-induced cell death. Cell Death Dis. 6, e1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Newton K., Dugger D. L., Wickliffe K. E., Kapoor N., de Almagro M. C., Vucic D., Komuves L., Ferrando R. E., French D. M., Webster J., Roose-Girma M., Warming S., and Dixit V. M. (2014) Activity of protein kinase RIPK3 determines whether cells die by necroptosis or apoptosis. Science 343, 1357–1360 [DOI] [PubMed] [Google Scholar]
- 25. Mandal P., Berger S. B., Pillay S., Moriwaki K., Huang C., Guo H., Lich J. D., Finger J., Kasparcova V., Votta B., Ouellette M., King B. W., Wisnoski D., Lakdawala A. S., DeMartino M. P., Casillas L. N., Haile P. A., Sehon C. A., Marquis R. W., Upton J., Daley-Bauer L. P., Roback L., Ramia N., Dovey C. M., Carette J. E., Chan F. K., Bertin J., Gough P. J., Mocarski E. S., and Kaiser W. J. (2014) RIP3 induces apoptosis independent of pronecrotic kinase activity. Mol. Cell 56, 481–495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Remijsen Q., Goossens V., Grootjans S., Van den Haute C., Vanlangenakker N., Dondelinger Y., Roelandt R., Bruggeman I., Goncalves A., Bertrand M. J., Baekelandt V., Takahashi N., Berghe T. V., and Vandenabeele P. (2014) Depletion of RIPK3 or MLKL blocks TNF-driven necroptosis and switches towards a delayed RIPK1 kinase-dependent apoptosis. Cell Death Dis. 5, e1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cook W. D., Moujalled D. M., Ralph T. J., Lock P., Young S. N., Murphy J. M., and Vaux D. L. (2014) RIPK1- and RIPK3-induced cell death mode is determined by target availability. Cell Death Differ. 21, 1600–1612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Weinlich R., and Green D. R. (2014) The two faces of receptor interacting protein kinase-1. Mol. Cell 56, 469–480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Feng S., Yang Y., Mei Y., Ma L., Zhu D. E., Hoti N., Castanares M., and Wu M. (2007) Cleavage of RIP3 inactivates its caspase-independent apoptosis pathway by removal of kinase domain. Cell Signal. 19, 2056–2067 [DOI] [PubMed] [Google Scholar]
- 30. Lin Y., Devin A., Rodriguez Y., and Liu Z. G. (1999) Cleavage of the death domain kinase RIP by caspase-8 prompts TNF-induced apoptosis. Genes Dev. 13, 2514–2526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chan F. K., Shisler J., Bixby J. G., Felices M., Zheng L., Appel M., Orenstein J., Moss B., and Lenardo M. J. (2003) A role for tumor necrosis factor receptor-2 and receptor-interacting protein in programmed necrosis and antiviral responses. J. Biol. Chem. 278, 51613–51621 [DOI] [PubMed] [Google Scholar]
- 32. Onizawa M., Oshima S., Schulze-Topphoff U., Oses-Prieto J. A., Lu T., Tavares R., Prodhomme T., Duong B., Whang M. I., Advincula R., Agelidis A., Barrera J., Wu H., Burlingame A., Malynn B. A., Zamvil S. S., and Ma A. (2015) The ubiquitin-modifying enzyme A20 restricts ubiquitination of the kinase RIPK3 and protects cells from necroptosis. Nat. Immunol. 16, 618–627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lawlor K. E., Khan N., Mildenhall A., Gerlic M., Croker B. A., D'Cruz A. A., Hall C., Kaur Spall S., Anderton H., Masters S. L., Rashidi M., Wicks I. P., Alexander W. S., Mitsuuchi Y., Benetatos C. A., Condon S. M., Wong W. W., Silke J., Vaux D. L., and Vince J. E. (2015) RIPK3 promotes cell death and NLRP3 inflammasome activation in the absence of MLKL. Nat. Commun. 6, 6282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Varfolomeev E., Blankenship J. W., Wayson S. M., Fedorova A. V., Kayagaki N., Garg P., Zobel K., Dynek J. N., Elliott L. O., Wallweber H. J., Flygare J. A., Fairbrother W. J., Deshayes K., Dixit V. M., and Vucic D. (2007) IAP antagonists induce autoubiquitination of c-IAPs, NF-κB activation, and TNFα-dependent apoptosis. Cell 131, 669–681 [DOI] [PubMed] [Google Scholar]
- 35. Gonzalvez F., Lawrence D., Yang B., Yee S., Pitti R., Marsters S., Pham V. C., Stephan J. P., Lill J., and Ashkenazi A. (2012) TRAF2 Sets a threshold for extrinsic apoptosis by tagging caspase-8 with a ubiquitin shutoff timer. Mol. Cell 48, 888–899 [DOI] [PubMed] [Google Scholar]
- 36. Haupt Y., Maya R., Kazaz A., and Oren M. (1997) Mdm2 promotes the rapid degradation of p53. Nature 387, 296–299 [DOI] [PubMed] [Google Scholar]
- 37. Kubbutat M. H., Jones S. N., and Vousden K. H. (1997) Regulation of p53 stability by Mdm2. Nature 387, 299–303 [DOI] [PubMed] [Google Scholar]
- 38. Honda R., Tanaka H., and Yasuda H. (1997) Oncoprotein MDM2 is a ubiquitin ligase E3 for tumor suppressor p53. FEBS Lett. 420, 25–27 [DOI] [PubMed] [Google Scholar]
- 39. de Almagro M. C., Goncharov T., Newton K., and Vucic D. (2015) Cellular IAP proteins and LUBAC differentially regulate necrosome-associated RIP1 ubiquitination. Cell Death Dis. 6, e1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Obeng E. A., Carlson L. M., Gutman D. M., Harrington W. J. Jr., Lee K. P., and Boise L. H. (2006) Proteasome inhibitors induce a terminal unfolded protein response in multiple myeloma cells. Blood 107, 4907–4916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hideshima T., Richardson P., Chauhan D., Palombella V. J., Elliott P. J., Adams J., and Anderson K. C. (2001) The proteasome inhibitor PS-341 inhibits growth, induces apoptosis, and overcomes drug resistance in human multiple myeloma cells. Cancer Res. 61, 3071–3076 [PubMed] [Google Scholar]
- 42. Palombella V. J., Conner E. M., Fuseler J. W., Destree A., Davis J. M., Laroux F. S., Wolf R. E., Huang J., Brand S., Elliott P. J., Lazarus D., McCormack T., Parent L., Stein R., Adams J., and Grisham M. B. (1998) Role of the proteasome and NF-kappaB in streptococcal cell wall-induced polyarthritis. Proc. Natl. Acad. Sci. U.S.A. 95, 15671–15676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Geserick P., Wang J., Schilling R., Horn S., Harris P. A., Bertin J., Gough P. J., Feoktistova M., and Leverkus M. (2015) Absence of RIPK3 predicts necroptosis resistance in malignant melanoma. Cell Death Dis. 6, e1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Feng X., Song Q., Yu A., Tang H., Peng Z., and Wang X. (2015) Receptor-interacting protein kinase 3 is a predictor of survival and plays a tumor suppressive role in colorectal cancer. Neoplasma 62, 592–601 [DOI] [PubMed] [Google Scholar]
- 45. Koo G. B., Morgan M. J., Lee D. G., Kim W. J., Yoon J. H., Koo J. S., Kim S. I., Kim S. J., Son M. K., Hong S. S., Levy J. M., Pollyea D. A., Jordan C. T., Yan P., Frankhouser D., Nicolet D., Maharry K., Marcucci G., Choi K. S., Cho H., Thorburn A., and Kim Y. S. (2015) Methylation-dependent loss of RIP3 expression in cancer represses programmed necrosis in response to chemotherapeutics. Cell Res. 25, 707–725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Nugues A. L., El Bouazzati H., Hétuin D., Berthon C., Loyens A., Bertrand E., Jouy N., Idziorek T., and Quesnel B. (2014) RIP3 is downregulated in human myeloid leukemia cells and modulates apoptosis and caspase-mediated p65/RelA cleavage. Cell Death Dis 5, e1384. [DOI] [PMC free article] [PubMed] [Google Scholar]