Abstract
The mechanical and microstructural bases of tendon fatigue, by which damage accumulates and contributes to degradation, are poorly understood. To investigate the tendon fatigue process, rat flexor digitorum longus tendons were cyclically loaded (1–16 N) until reaching one of three levels of fatigue damage, defined as peak clamp-to-clamp strain magnitudes representing key intervals in the fatigue life: i) Low (6.0%–7.0%); ii) Moderate (8.5%–9.5%); and iii) High (11.0%–12.0%). Stiffness, hysteresis, and clamp-to-clamp strain were assessed diagnostically (by cyclic loading at 1–8 N) before and after fatigue loading and following an unloaded recovery period to identify mechanical parameters as measures of damage. Results showed that tendon clamp-to-clamp strain increased from pre- to post-fatigue loading significantly and progressively with the fatigue damage level (p≤0.010). In contrast, changes in both stiffness and hysteresis were significant only at the High fatigue level (p≤0.043). Correlative microstructural analyses showed that Low level of fatigue was characterized by isolated, transverse patterns of kinked fiber deformations. At higher fatigue levels, tendons exhibited fiber dissociation and localized ruptures of the fibers. Histomorphometric analysis showed that damage area fraction increased significantly with fatigue level (p≤0.048). The current findings characterized the sequential, microstructural events that underlie the tendon fatigue process and indicate that tendon deformation can be used to accurately assess the progression of damage accumulation in tendons.
Keywords: tendon, fatigue, damage accumulation, biomechanics, morphology
Tendon pathology and rupture are a major cause of pain and disability, afflicting a wide spectrum of society, from young athletes to the elderly. However, clinical and scientific knowledge of the onset of low-level injury and progression of tendon degeneration to rupture in humans is limited. The early stages of tendon disease are typically treated nonoperatively, so that degenerate tissue available in biopsied specimens represent an end stage of injury from cumulative microtrauma.1 In a large-scale study on spontaneous tendon ruptures, Kannus and Jozsa2 found that 35% of nonruptured tendons from the control, healthy population showed histological evidence (such as reduced periodicity of collagen fibers and change in cellular morphology) of a degenerative response and microscopic injuries. That such microscopic-level changes were observed so frequently in “normal” tendons argues strongly for the occurrence of cumulative damage. Indeed, overuse and/or repetitive loading has been widely considered to be a major contributor to failure of tendons.1,3,4
The process by which tendon damage initiates and accumulates at the microstructural level is poorly understood. Previous studies focused primarily on examining the relationship between applied stress and time or number of cycles to failure in ex vivo tests,5–9 while few studies have explored subrupture behavior in tendons10–14 and ligaments.15 In vivo investigations of overuse have relied on experimental protocols that imposed loading to the tendon through muscle stimulation,12 treadmill running,14 or by performing reaching and grasping tasks16 to induce tendon damage. Despite these various approaches, the fatigue behavior of tendon and the structural bases of wear and tear failure remain obscure. It is therefore necessary to study the process of damage accumulation using a model system (i.e., an ex vivo model) in which the contribution of microstructural changes to fatigue loading response in tendons can be elucidated.
Previous studies7,8,17 have proposed stiffness loss as a marker of fatigue damage in tendon based on analogy to fatigue behavior of bone and other composite materials, in which stiffness loss from fatigue loading correlates to microscopic void formation (microdamage).18,19 Indeed, we postulated that, in tendons, rupture of fibers and dissociations among them contribute to stiffness loss. The goal of the current study was to quantify the changes in the mechanical properties and their morphological correlates at controlled levels of tendon fatigue, which reflect the sequential, microstructural events that constitute the tendon fatigue damage process. Findings in the ex vivo experiments will provide a foundation of understanding the process of tendon fatigue before studying a biologically complex, in vivo system model, in which the cellular/matrix response to fatigue can be evaluated.
MATERIALS AND METHODS
Rats were utilized for the present studies, as this species has been used widely in biomechanical, physiological, and molecular studies of tendon.20–23 The flexor digitorum longus (FDL) tendon was selected for these ex vivo studies because it is large relative to other rat tendons and has a grossly uniform geometric structure from the muscle attachment to its digital branches. Immediately following euthanasia, FDL tendons (n=35; one tendon from 7 rats and both tendons from 14 rats) were harvested from young adult, 17–19-week-old, female Sprague-Dawley rats (Charles River Laboratories, Wilmington, MA). The FDL was separated from the neighboring Achilles tendon, released from its surrounding retinaculum, and then removed by isolating the FDL muscle and severing its digital branches at their distal attachments. The FDL muscle fibers were gently removed with the blunt end of a scalpel handle, leaving the intramuscular tendinous fibers intact. Tendons were kept moist with phosphate buffered saline (PBS) solution during dissection and preparation for mechanical testing or histological processing. All protocols described were approved by our Institutional Animal Care and Use Committee.
Fatigue Loading
Mechanical testing was conducted using a servo-hydraulic materials testing system (Instron 8872, Canton, MA). Tendons underwent fatigue loading in a PBS bath maintained at 39°C (rat physiologic temperature). FDL tendon ends were gripped at a clamp-to-clamp gauge length of 12.5 mm using sandpaper-covered plates to minimize slippage during tensile loading. The upper and lower plates were secured to custom grips which were centered relative to the actuator via a custom-designed alignment apparatus. Calibration studies demonstrated that 92% of clamp-to-clamp displacement is translated to tendon deformation. Loads were measured using a 50-lb (222 N) load cell (Transducer Techniques, Temecula, CA) attached in series between the upper grips and actuator. Each sample was allowed to equilibrate to the saline bath temperature for 15 min followed by a 3 N preload for 3 min to establish its initial length, L0, and initial actuator position, d0. Time, actuator displacement (measured by the system LVDT), and load data were collected at 50 Hz using LabView 6.0 (National Instruments, Inc., Austin, TX). Tendons were loaded according to a load control-based, damage accumulation protocol adapted from that used in studies of fatigue damage in cortical bone.24 As a reference for the load levels used in the fatigue experiments, 16 FDL tendons from additional age-matched animals were loaded monotonically to failure to determine mean maximum tensile force (49±8 N) and mean maximum clamp-to-clamp strain (16.2%±2.5%). All failures occurred at the midsubstance via tensile pullout. Cross-sectional area of the FDL tendons also showed small variation (1.11±0.10 mm2). As such, load levels used in the present experiments were not adjusted based on cross-sectional area of the individual tendons.
In the first series of fatigue loading tests, tendons (n=8) were loaded to a subfailure endpoint determined based on stiffness loss (indicative of mechanical property degradation), which was proposed as a proxy for tendon damage based on standard engineering approaches demonstrated in previous fatigue studies of tendon,7,8,17 ligament,15,25 bone,26 and other composites.27 Each sample was initially preconditioned by cyclic loading between 1 and 8 N using a havertriangle waveform at 1 Hz for 6 min and then subjected to fatigue loading using a 16 N cyclic load, which was ~33% of the monotonic failure strength. This corresponded to ~15 MPa stress level and a cyclic clamp-to-clamp strain amplitude of ~3.1% at its initial cycles. Each sample was cycled between 1 N and 16 N at 0.75 Hz using a havertriangle waveform until reaching 20% loss of secant stiffness from the reference value. The reference stiffness was determined at cycle 15, which was the number of cycles required for the loading system feedback controller to consistently reach the designated load magnitudes at a high precision.
Based on our observations in the first series of loading tests (see Results), tendon stiffness did not degrade until reaching the late stages of the fatigue loading. In contrast, peak clamp-to-clamp strain, which was defined by (d–d0)/L0, where d is the actuator position at peak cyclic load, increased continuously throughout fatigue loading. To evaluate tendon behavior throughout the fatigue life, we conducted the second series of fatigue studies, in which loading (cyclically between 1–16 N at 0.75 Hz using a havertriangle waveform) was stopped at one of three key intervals during cyclic fatigue loading based on tendon strain behavior observed in the first series of loading tests (see Results). These intervals, which represent three progressive levels of fatigue, were defined at 6.0%–7.0% (Low level, n=9), 8.5%–9.5% (Moderate level, n=10), and 11.0%–12.0% (High level, n=8) of peak clamp-to-clamp strain. To determine the effects of fatigue loading on the tendon, Diagnostic tests 1 and 2, in which the tendon was loaded cyclically between 1 and 8 N at 1 Hz for 2 min (120 cycles) using a havertriangle waveform, were conducted to assess the mechanical properties of the tendon at pre-fatigue (i.e., undamaged state) and post-fatigue loading (damaged state) (Fig. 1A), respectively. The tendon was then completely unloaded to a slightly slackened state (in a zero stress configuration) and held in position control for 80 min in the Recovery period (approximately eight times the mean duration of fatigue loading for the High fatigue level) to assess whether transient effects were present in the immediate post-fatigue changes in the mechanical behavior.28 Following recovery, Diagnostic test 3 was conducted in identical fashion as the earlier diagnostic tests to characterize post-recovery mechanical behavior. At the diagnostic load level, load-displacement curves fell within the linear region of the monotonic loading curve, and exhibited a linear regression coefficient of r2>0.99 at the upper 60% force range. Pilot studies showed that tendon samples subjected to multiple cyclic loading periods at the diagnostic load level, interposed with unloaded recovery periods produced no histologic evidence of tendon damage, while cyclic mechanical properties consistently reached an asymptotic level within 120 cycles and were not degraded over the multiple loading periods.
Figure 1.
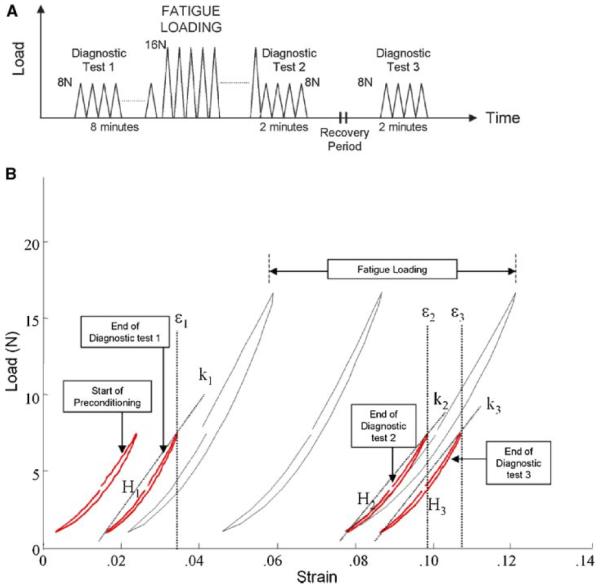
Tendon fatigue damage test protocol. Fatigue damage is induced by cyclically loading the tendon (to 16 N) until reaching one of three damage levels. Repeated measurements of stiffness, hysteresis, and clamp-to-clamp strain from Diagnostic tests 1, 2, and 3 are used to determine the relative contributions of transient and permanent damage effects arising from fatigue loading (A). Representative mechanical response during loading is shown in (B). Secant stiffness (slope of the loading curve; k1, k2, k3), hysteresis (area bounded by the loading and unloading curves; H1, H2, H3), and clamp-to-clamp strain (at peak cyclic load; ε1, ε2, ε3) were computed at Diagnostic tests 1, 2, and 3 to characterize the tendon's mechanical behavior at the pre-fatigue, post-fatigue, and post-recovery states, respectively. [Color scheme can be viewed in the online issue, which is available at http://www.interscience.wiley.com]
Assessment of Mechanical Properties
Mechanical behavior of the tendon was characterized by secant stiffness (k), hysteresis (H), and clamp-to-clamp strain (ε), which were determined at Diagnostic tests 1, 2, and 3, representing the pre-fatigue (k1, H1, ε1), post-fatigue (k2, H2, ε2) and post-recovery states (k3, H3, ε3) of the tendon, respectively. Secant stiffness for a particular loading cycle was computed as (Fmax–Fmin)/(dmax–dmin), where Fmax and dmax are the maximum values of the force and test actuator position, respectively, while Fmin and dmin represent the minimum values. Hysteresis for each cycle was defined by the area bounded between the loading and unloading curves and computed by the trapezoidal method using a custom MATLAB (Mathworks, Natick, MA) routine. Both stiffness and hysteresis were evaluated as the mean value of the respective data from the final five cycles at each diagnostic test. Tendon clamp-to-clamp strain was the cumulative increase in length of the tendon, computed by (d′ – d0)/L0, where d′ is the peak actuator position at the last cycle of loading of each diagnostic test (Fig. 1B). Mechanical degradation of the tendon was defined as the changes in each parameter at post-fatigue (Δk1→2, ΔH1→2, Δε1→2) and post-recovery (Δk1→3, ΔH1→3, Δε1→3) at each fatigue damage level, normalized by its corresponding values at pre-fatigue.
Microstructural Studies
After loading, tendons were processed for histological evaluation using a methyl methacrylate embedding protocol developed in our laboratory.29 Briefly, tendon samples were fixed in neutral buffered formalin for 24 h, with the ends of the tendon secured in a custom jig under ~2 N of tension. Based on our preliminary studies, the 2 N load sufficiently straightens the crimps in our control and fatigue loaded tendons. Following fixation, the tendons were isolated from the jig by removing the tendon ends and then dehydrated in ethylene glycol monoethyl ether followed by acetonitrile, cleared with methyl salicylate, and embedded in methyl methacrylate. For both control and fatigue loaded tendons, serial longitudinal sections were cut at 5 μm thickness and stained with Toluidine blue for assessment of damage under bright-field microscopy. In addition, to confirm that changes in tendon morphology observed in thin sections were not cutting artifacts, tendons prepared as thick sections (100 μm) were evaluated under confocal microscopy (Radiance MP 2000; Carl Zeiss, Inc., Germany). Whole tendons were bulk stained in Basic Fuchsin, which binds to proteoglycans, prior to embedding. Thick sections were cut using a diamond wafering saw (Isomet; Buehler, Ltd., Lake Bluff, IL) with a silicone-based lubricant (Eros; Megasol Cosmetic GmbH, Föhren, Germany) to prevent hydration of the embedded tissue from using a water-based lubricant, which causes artifactual fibril fraying during cutting.
Quantitative Morphologic Damage Assessment
Toluidine blue-stained sections were digitally photographed along their entire length in a piecewise fashion under a 40×magnification objective using Axiovision software v3.0 and an Axioplan microscope with an Axiocam HRc digital camera (Carl Zeiss, Inc, Jena, Germany). Three noncontiguous sections from each tendon were selected for histomorphometric analysis and the proximal, middle, and distal regions of the tendon section were analyzed using a 400 μm×1,000 μm grid spaced at 40 μm squares. The number of grid squares in which matrix deformation/disruption patterns (including distortion of fibers and/or discontinuity) appeared, regardless of the morphological nature of the pattern, was independently tabulated by three observers, who were blinded to the tendon loading history. Damage area fraction for each tendon was determined by the number of grid squares with tissue deformity and expressed as a percentage of the total number of grid squares among the three noncontiguous histologic sections.
Statistical Analyses
Changes in secant stiffness, hysteresis, and clamp-to-clamp strain were analyzed among the three levels of fatigue damage. First, changes in the parameters at post-fatigue (Δk1→2, ΔH1→2, Δε1→2) and post-recovery (Δk1→3, ΔH1→3, Δε1→3) within each fatigue damage level were individually analyzed using the Wilcoxon Rank Sum test for significant difference from zero (GraphPad v3.0, Prism Software, San Diego, CA). To evaluate differences in mechanical response among the Low, Moderate, and High fatigue damage levels, changes in the parameters at post-fatigue (Δk1→2, ΔH1→2, Δε1→2) and post-recovery (Δk1→3, ΔH1→3, Δε1→3) were compared using the Kruskal-Wallis test with Dunn's post-hoc test. To examine whether the detected changes at post-fatigue were transient in nature, changes in the parameters at post-fatigue were compared to the corresponding values at post-recovery (Δk1→2 vs. Δk1→3; ΔH1→2 vs. ΔH1→3; Δε1→2 vs. Δ ε1→3) within each fatigue damage level using the Wilcoxon Rank Sum test. Additionally, to determine quantitatively whether the morphological response varied among the different levels of fatigue damage, mean damage area fraction was compared among the Low, Moderate, and High damage groups and among the three observers using two-way analysis of variance (ANOVA). Significant relationships identified by two-way ANOVA were further analyzed using the Kruskal-Wallis test with Dunn's post-hoc test. Statistical significance was established at p<0.05.
RESULTS
In the first series of fatigue tests, in which loading was stopped upon reaching the endpoint of 20% stiffness loss, progression of stiffness during the course of fatigue loading followed a triphasic pattern, characterized by: 1) a slight stiffening in the initial phase of loading (approximately in the first 50–100 cycles); 2) a stable level of stiffness throughout the secondary phase for an extended period of time; 3) an accelerated decline in stiffness in the tertiary phase (Fig. 2). Endpoint was reached at 501±371 cycles of loading. Overall, stiffness was maintained at an increased level from that at the reference cycle for 70.3%±4.6% of the duration of fatigue loading, with the peak stiffness reaching 4.8%±3.6% beyond the reference stiffness (at cycle 15) within this loading period, before stiffness declined at an accelerated rate toward the endpoint. As such, stiffness loss was not observed until the late stages of fatigue loading.
Figure 2.
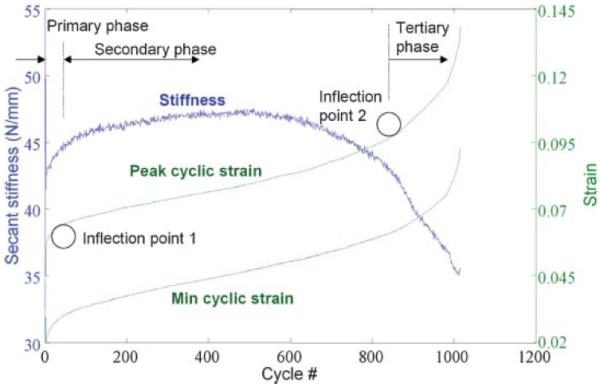
Representative load history during fatigue loading. Progression of cumulative clamp-to-clamp strain is characterized by a continuously increasing, triphasic pattern (denoted by Primary, Secondary, and Tertiary phases), with inflection points 1 and 2 that mark the transition from one phase to the next. Stiffness increases initially in early fatigue life, leading to a relatively stable level for an extended duration of loading, until reaching the end stages when stiffness declines at an accelerated rate. [Color scheme can be viewed in the online issue, which is available at http://www.interscience.wiley.com]
In contrast, peak clamp-to-clamp strain changed throughout the fatigue loading period, following a different triphasic pattern: 1) an initial rapid increase during the primary phase (approximately in the first 20–80 cycles); 2) a slow and steady increase in the secondary phase; 3) an accelerated increase in the tertiary phase (Fig. 2). Peak clamp-to-clamp strain increased at 6.8±3.5×10−3%/cycle in the secondary phase. Based on the characteristic changes in curvature in the triphasic pattern of peak clamp-to-clamp strain, inflection points were mathematically defined to mark the transitions from one phase to the next. The triphasic pattern was fitted using a high-order polynomial, defined by p(x)=p1xn+ p2xn−1+…+pnx + pn+1, where n is the degree and pn is row vector containing polynomial coefficients, and the radius of curvature, ρ, was derived by ρ=(1+[p′ (x)]2)3/2/p″ (x) along the triphasic curve using a custom MATLAB routine. Local minima in radius of curvature were determined to define the inflection points. The first occurred at 5.8%±1.0% and the second at 9.0%±1.5% peak clamp-to-clamp strain. Loading from the first to the second inflection point constituted 60.2%±6.8% of the duration of fatigue loading. Based on the peak clamp-to-clamp strain pattern, three levels of progressive fatigue damage were defined at 6.0%–7.0%, 8.5%–9.5%, and 11.0%–12.0% peak clamp-to-clamp strain to represent the onset of secondary phase (Low level), transition between the secondary and tertiary phase (Moderate level), and the tertiary phase (High level), respectively, in the second series of fatigue loading tests.
Mechanical Changes
Tendon samples reached the Low, Moderate, and High levels of damage at 164±128, 346±233, and 432±238 cycles of loading, respectively. Within any level of damage, tendon samples experienced a significant increase in clamp-to-clamp strain from pre- to post-fatigue (Δε1→2) (p≤0.014). Analyzed by damage level, tendon clamp-to-clamp strain from pre- to post-fatigue (Δε1→2) exhibited a dose-dependent response (Fig. 3); clamp-to-clamp strain increased progressively and significantly with the level of imposed fatigue damage (p ≤ 0.010). In contrast, significant changes in stiffness and hysteresis (i.e., Δk1→2, ΔH1→2) were not observed within or among damage groups until reaching the High level of fatigue damage (p ≤ 0.043); degradation of both stiffness and hysteresis was not significantly different between the Low and Moderate levels of fatigue damage (p ≥ 0.17). Further, degradation of these properties at post-recovery showed the same relationships as those at post-fatigue and was permanent (p ≥ 0.15). Tendon clamp-to-clamp strain did not demonstrate any recovery, and, in fact, continued to increase slightly (p = 0.057) following the unloading period at the High level of fatigue damage.
Figure 3.
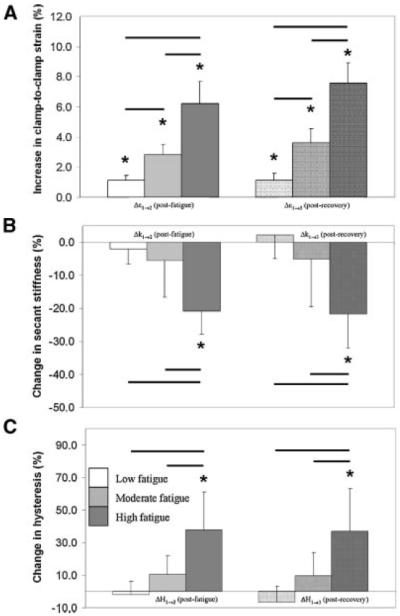
Changes in the mechanical behavior of the tendons resulting from fatigue loading and recovery period. Within any level of fatigue, tendon samples experienced a significant increase in clamp-to-clamp strain (A) following fatigue loading (*p<0.05), while significant changes in stiffness (B) and hysteresis (C) were observed only at the High fatigue level. Among the different fatigue groups, tendon clamp-to-clamp strain (A) increased significantly at post-fatigue at increasing levels of fatigue (−p<0.05). However, changes in secant stiffness (B) and hysteresis (C) were not significant between the Low and Moderate levels, but were significant at the High fatigue level. Changes in the parameters observed at post-fatigue were permanent; recovery of these changes was not evident at any fatigue level.
Microstructural Changes
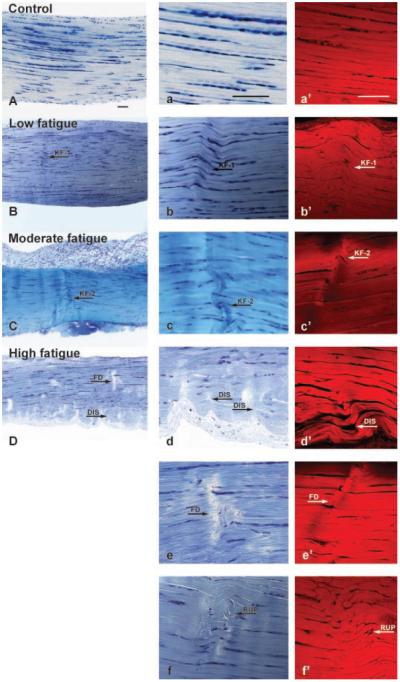
Under light microscopy, thin sections of nonloaded control tendons exhibited highly aligned, parallel collagen fibers (Figs. 4A and a). Tenocytes appeared in a columnar fashion between the collagen fibers. The same morphological features were also identified in thick sections of control tendons observed under confocal microscopy (Fig. 4a′). In contrast, fatigue-loaded tendons displayed isolated regions of disruption within an otherwise normal collagenous architecture with intact synovium at the tendon surface, which were observed under both bright-field and confocal microscopy. In the Low damage group, the predominant type of damage observed was a pattern of kinked fiber deformations that spanned transversely across several adjacent fibers, appearing as a ridge-like formation (Figs. 4B, b, and b′). Tenocyte arrangement appeared distorted within the damaged fibers. Fiber ruptures were not evident in the matrix. At the Moderate level, an increased number of local patterns of fiber disruption was observed in the matrix (Figs. 4C, c, and c′). Discontinuities were seen occasionally at the apex of the kinked fiber patterns, while few instances of isolated fibers tears were also observed. Tendons that were loaded to the High fatigue level demonstrated: i) dissociation among fibers (Figs. 4D, d, and d′); ii) transversely oriented fiber discontinuities (Figs. 4e, and e′); and iii) isolated rupture patterns with fibers that appeared distorted in alignment and deformed into a curled formation (Figs. 4f and f′). Damage area fraction was zero for the nonloaded, control group of tendons. In fatigue loaded tendons, damage area fraction increased consistently (p ≤ 0.048) with the level of fatigue damage (Fig. 5), with values of 5.6% ± 1.0% at Low, 8.3% ± 1.7% at Moderate, and 12.0% ± 2.0% at High level of fatigue damage. Interobserver variability and interaction between observer and fatigue damage level were not statistically significant (p ≥ 0.97).
Figure 4.
Morphology of nonloaded, control, and fatigue damaged tendons under bright-field (A–D in low magnification, a–f in high magnification; stained in Toluidine blue) and confocal (a′ –f′ bulk stained in basic Fuchsin) microscopy. Both bright-field (A, a) and confocal (a′) microscopy exhibited parallel fibers that are highly aligned in nonloaded, control tendons. Tendons at the Low fatigue damage level are characterized by kinked fiber deformation (KF-1 in B, b, b′). At the Moderate fatigue damage level, tendons showed the same type of kinked fiber deformation patterns with an increase in the amount of damage area fraction (KF-2 in C, c, c′). Microstructural changes at the High fatigue damage level are characterized by: i) dissociation among fibers (DIS in D, d, d′); ii) transversely-oriented fiber discontinuities (FD in D, e, e′); and iii) fiber rupture patterns (RUP in f, f′; not shown in D). Scale bar=100 μm.
Figure 5.
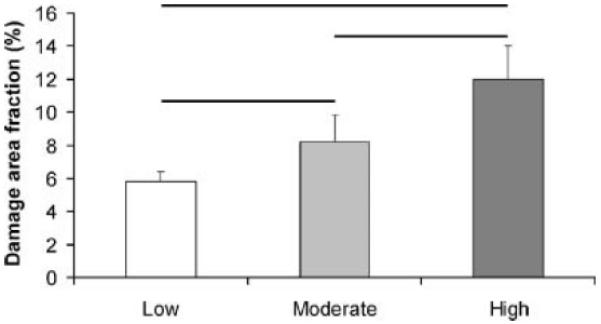
Morphometric measurement of damage in tendon. Damage area fraction, a measure of the amount of damage in the tendons, increased significantly at increasing levels of damage (−p<0.05).
DISCUSSION
The current study investigated the correlative changes in the mechanical behavior and morphology of subrupture, fatigue-loaded rat tendons. Our results obtained from increasing, controlled levels of imposed fatigue provide insights into the mechanical and microstructural mechanisms of the tendon fatigue process, by which damage accumulates and contributes to mechanical property degradation and rupture. During fatigue loading, tendon peak clamp-to-clamp strain increased continuously, following a repeatable pattern marked by inflection points. At increasing levels of fatigue damage, tendon clamp-to-clamp strain, which also may be interpreted as creep deformation, from pre- to post-fatigue, increased progressively and significantly (p ≤ 0.010). Assessment of tissue morphology clearly revealed the distinct types of microdamage in the tendon which were not observed in the nonloaded, control tendons (Fig. 4), and an increasing severity of microstructural damage and damage area fraction from early to late fatigue. Stiffness loss, which has been postulated previously as a marker of tendon damage, demonstrated high variability among samples and did not reveal statistically significant levels of changes until reaching late fatigue life. While the current study does not invalidate stiffness loss as an indicator of damage, our results do demonstrate that, with cyclic loading, tendon clamp-to-clamp strain is a useful and accurate marker of damage accumulation in the progression of tendon fatigue. Furthermore, the observation that tendon strain is nonrecoverable reflects that the damage which is manifested by the strain increase is structural and not associated with a transient response resulting from fatigue loading.
The current findings are consistent with previous studies on cyclic loading behavior of ligaments and tendons. Wren et al.9 showed in ex vivo loading of human Achilles tendons that initial strain correlates well with the time to failure during cyclic loading. Cyclic loading of the human inferior glenohumeral ligament to increasing magnitudes of subfailure strains resulted in a decrease in baseline load response and increase in residual length.30 Short-term cyclic loading of rabbit medial collateral ligaments ex vivo showed that elongation increased at successive loading cycles prior to the occurrence of microscopic rupture of fibers.15 In a study on the effects of subfailure stretch in the rat medial collateral ligament, in which loading was applied to the tissue monotonically in a single pull, damage is characterized by nonrecoverable increase in elongation.31 Furthermore, in an ex vivo study of the human extensor digitorum longus tendon,17 the authors discussed the possible role of a creep mechanism involved in the observed reduction in ultimate stress following partial fatigue.
Peak applied stress in the current study was approximately 15 MPa, which lies within the low range of load levels investigated in previous cyclic loading studies of tendons.5,6,8 Moreover, the clamp-to-clamp strain amplitude during our studies reached ~3%, which corresponds to submaximal habitual physiologic levels of strain observed in tendons and ligaments,32–34 and lies below the range generated by maximal muscle load (5%–6%).35 Our load-displacement curves during fatigue loading indicate that tendons have been stretched well into the elastic range, as the diagnostic loads, which were half of the fatigue load level, exhibited linearity (with a linear regression coefficient of r2 > 0.99) at the upper 60% force range. As such, the current study demonstrated that repetitive loads within the elastic, physiologic range can induce structural damage in tendons.
Examination of the fatigue damage morphology and mechanical behavior provide new insights into the failure mechanisms at the microstructural level. Early fatigue is characterized by the formation of local, transverse patterns of kinked fiber deformation, with no evidence of fiber ruptures (Fig. 4B). The population of fibers in a tendon likely has a distribution of variable lengths and material properties. We postulate that the early fatigue damage (kinked fiber) patterns result from stretching a small population of fibers into their plastic range of deformation (Fig. 6). They are then compressed and kinked as the elastically elongated fibers in parallel shorten upon unloading. Development of this type of microstructural damage allows increased elongation with subsequent cycles, reflecting the presence of a load sharing mechanism by which local stresses can be redistributed among the fibers of the tendon following local failure. Upon stretching the initially loaded fibers into their plastic range of deformation and rupture, loading is assumed by the surviving, intact fibers with longer lengths or of a higher fatigue quality. Additional loading contributes to accumulation of fiber deformation and rupture, while loads among the fibers would continue to be redistributed by means of the load sharing/transfer mechanism provided that surviving, lesser loaded fibers remain sufficiently available. This mechanism is likely associated with a transverse network of molecular and/or fibrillar linkages 36 that facilitate the load transfer to neighboring fibers. Presumably, the formation of fiber deformation patterns, which characteristically span across several adjacent fibers instead of appearing as a random distribution of fiber ruptures, resulted from a shear lag-type effect 37 that is facilitated by this transverse network. Nonetheless, these observations suggest that the transverse structural network plays a critical role in the mechanical and microstructural mechanisms of damage accumulation.
Figure 6.
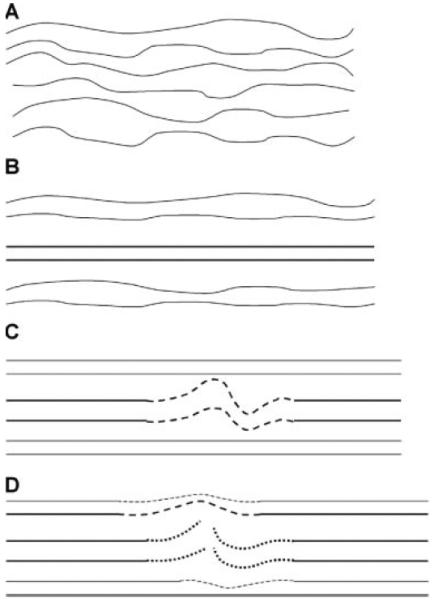
Schematic diagram of damage mechanism that underlies tendon fatigue. Initial fatigue loading lengthens the fibers from the crimped (A) to the uncrimped (B) state. Continued loading causes stretching of a local population of fibers into their plastic range of deformation (C, dashed line), resulting in the formation of kinked fiber deformation patterns. Further loading leads to rupture of the plastically deformed fibers (D, dotted line). Subsequently, loading is assumed by the surviving, intact fibers with longer lengths and/or higher fatigue quality (dashed line).
At late fatigue, damage area fraction continued to increase, and consisted microstructurally of ruptured fibers that appeared to have subsequently formed a curled pattern at the site of rupture (Figs. 4f and f′). The similar findings with bulk-stained, thick sections under confocal microscopy rule out histologic sectioning artifact as a factor in these findings. Mechanistically, the damage morphology reflects the reduction of the number of surviving, intact fibers that are available to support the imposed loading, leading to a reduction in stiffness. Evidence of shear interaction among the fibers could also be identified at the matrix interface between fibers, which have presumably been stretched differentially and slipped past one another. These observations are consistent with X-ray diffraction and confocal microscopic studies of individual tendon fascicles, which showed that tensile deformation of tendon consists of fibril stretching38,39 as well as sliding of adjacent fibrils which together generate nonuniform strain within the matrix.40,41
The damage morphology observed in the current study, such as kinked fiber deformation and dissociation among fibers, appear to parallel histologic findings of human tendon pathology and injury, including fibril dissociation, longitudinal splitting, fiber angulation, and fiber delaminations.2,38,42–44 In recent in vivo tendon injury studies,12,45 an increased rate of stimulation of the flexor digitorum profondus muscle in rabbits resulted in an increased amount of microtears in the attached tendon, supporting the notion that damage accumulation occurs in the living tendon with repetitive loading. Our study also demonstrates that morphologic damage accumulation increases with degradation of mechanical properties, which represents the mechanical state of a pathologic tendon. Hence, we postulate that the proposed mechanism by which microstructural damage forms and accumulates from repetitive loading underlies the fatigue process in living tendons. However, the precise cellular/matrix response invoked by the formation and accumulation of different types of microstructural damage, and other proposed pathologic processes, such as hypoxia46 or hyperthermia,47 leading to matrix degeneration, remain to be investigated in future studies.
The current loading protocol enabled the separation of changes in the mechanical properties into their transient and permanent components, which have been poorly accounted for in previous studies of fatigue loading of tendons or ligaments. Changes in clamp-to-clamp strain (as well as loss of stiffness and increases in hysteresis at High fatigue damage) were not recoverable, in contrast to loading-induced changes in bone.24,48 This finding reflects the plastic nature of the induced microstructural changes; formation of damage patterns of various types, including those that do not involve fiber rupture, is not reversible following the unloading period. In fact, at the High level of damage, the tendon clamp-to-clamp strain increased slightly following the recovery period. This small increase in clamp-to-clamp strain is not likely attributable to additional damage produced by loading a tendon that has been degraded by fatigue, since clamp-to-clamp strain accumulated during the course of Diagnostic test 3 loading is observed among all levels of induced damage and is comparable to that of Diagnostic test 1, which characterizes the mechanical state of the undamaged tendon.
We note several limitations in the current study. First, fatigue loading was conducted under ex vivo conditions, which does not allow cellular and physiologic processes that are present in the living tendon. Given the paucity of data on the process of tendon fatigue, we believe it is necessary to study the process thoroughly using an ex vivo model, by which we establish experimental methods to induce damage repeatably in the tendon and quantify damage at progressive levels of fatigue. These results will provide the foundation of understanding damage accumulation in tendons and identifying useful, in situ mechanical measures of damage, which would facilitate the development of an in vivo animal model. Nonetheless, the current experimental conditions ensured loading standardization across the tendons and measurability, which muscle stimulation may not achieve. While the tendons were isolated from age-matched animals, small, intrinsic variations in dimensions and material properties must be present due to variability among outbred animals. Further, tendon deformation was determined by clamp-to-clamp movements. While our calibration studies have shown that the clamps are highly efficient in translating deformation in the tendon, this mode of measurement is indeed necessitated by the fact that tendon deformation measurement is needed to control and monitor the fatigue experiments. Strain transducers instrumented on the tissue likely produce artifacts due to stress concentration at or surrounding the point of contact. Noncontact optical techniques cannot operate quickly enough to be used in the closed loop feedback control. Additionally, strain levels at which damage was characterized may be dependent on the loading protocol. Altering the load frequency or magnitude may change the strain profile during the progression of the fatigue life. However, the triphasic nature of strain increase, characteristic of fatigue behavior of biocomposite materials, is likely maintained and presents analogous intervals in the fatigue process that mark the occurrence of mechanistic change. We also note that fatigue is a highly variable phenomenon; it is well established that fatigue life duration varies by as much as 10-fold difference among samples of tendon,6 bone,49 engineered composite materials,27 and even metals.50 As such, it is not surprising that our tendon samples may sustain a similar number of loading cycles to reach different levels of fatigue damage, or vice versa, although the current study does show that tendons, on average, were loaded to an increasing number of cycles at increasing levels of fatigue damage.
In summary, we investigated the tendon fatigue process by characterizing changes in the mechanical properties and morphology at increasing levels of fatigue damage. Results showed that tendon clamp-to-clamp strain increased progressively and significantly with the increasing levels of fatigue damage, while changes in stiffness and hysteresis were not significant before reaching a stage in the fatigue life when degradation increases at an accelerated rate. Damage area fraction also increased consistently with the level of fatigue damage. As such, tendon clamp-to-clamp strain better reflects the progression of tendon fatigue at the early stages of fatigue life. The clamp-to-clamp strain increase in early fatigue was reflected primarily by localized plastic deformation of the fibers, while the stiffness loss at late fatigue life was attributed to dissociation and rupture of the fibers. Based on these results, the current study establishes a mechanistic basis of using a cumulative deformation-based criterion to define levels of fatigue damage; we are presently adapting our techniques to studies of fatigue of tendon–bone insertions in an in vivo tendon damage model to assess intrinsic tissue repair mechanisms.51 These data will further advance our understanding of the relationships between tendon fatigue, cell-matrix biological response, and microscopic damage accumulation, and will facilitate the identification of fundamental biological and biomechanical mechanisms related to tendon failure.
ACKNOWLEDGMENTS
We acknowledge grant support from the Aircast Foundation and the NIH (AR41210, AR44927, AR49967, and AR52743). Confocal laser scanning microscopy was performed at the Mount Sinai School of Medicine Microscopy Shared Resource Facility, supported with funding from NIH-NCI shared resources grant (R24 CA095823) and NSF Major Research Instrumentation grant (DBI-9724504).
REFERENCES
- 1.Jarvinen M, Jozsa L, Kannus P, et al. Histopathological findings in chronic tendon disorders. Scand J Med Sci Sports. 1997;7:86–95. doi: 10.1111/j.1600-0838.1997.tb00124.x. [DOI] [PubMed] [Google Scholar]
- 2.Kannus P, Jozsa L. Histopathological changes preceding spontaneous rupture of a tendon. A controlled study of 891 patients. J Bone Joint Surg [Am] 1991;73:1507–1525. [PubMed] [Google Scholar]
- 3.Kelly DW, Carter VS, Jobe FW, et al. Patellar and quadriceps tendon ruptures—jumper's knee. Am J Sports Med. 1984;12:375–380. doi: 10.1177/036354658401200508. [DOI] [PubMed] [Google Scholar]
- 4.Paavola M, Kannus P, Jarvinen TA, et al. Achilles tendinopathy. J Bone Joint Surg [Am] 2002;84-A:2062–2076. doi: 10.2106/00004623-200211000-00024. [DOI] [PubMed] [Google Scholar]
- 5.Pike AV, Ker RF, Alexander RM. The development of fatigue quality in high- and low-stressed tendons of sheep (Ovis aries) J Exp Biol. 2000;203(Pt 14):2187–2193. doi: 10.1242/jeb.203.14.2187. [DOI] [PubMed] [Google Scholar]
- 6.Schechtman H, Bader DL. In vitro fatigue of human tendons. J Biomech. 1997;30:829–835. doi: 10.1016/s0021-9290(97)00033-x. [DOI] [PubMed] [Google Scholar]
- 7.Wang XT, Ker RF. Creep rupture of wallaby tail tendons. J Exp Biol. 1995;198(Pt 3):831–845. doi: 10.1242/jeb.198.3.831. [DOI] [PubMed] [Google Scholar]
- 8.Wang XT, Ker RF, Alexander RM. Fatigue rupture of wallaby tail tendons. J Exp Biol. 1995;198:847–852. doi: 10.1242/jeb.198.3.847. [DOI] [PubMed] [Google Scholar]
- 9.Wren TA, Lindsey DP, Beaupre GS, et al. Effects of creep and cyclic loading on the mechanical properties and failure of human Achilles tendons. Ann Biomed Eng. 2003;31:710–717. doi: 10.1114/1.1569267. [DOI] [PubMed] [Google Scholar]
- 10.Archambault JM, Hart DA, Herzog W. Response of rabbit Achilles tendon to chronic repetitive loading. Connect Tissue Res. 2001;42:13–23. doi: 10.3109/03008200109014245. [DOI] [PubMed] [Google Scholar]
- 11.Backman C, Boquist L, Friden J, et al. Chronic Achilles paratenonitis with tendinosis: an experimental model in the rabbit. J Orthop Res. 1990;8:541–547. doi: 10.1002/jor.1100080410. [DOI] [PubMed] [Google Scholar]
- 12.Nakama LH, King KB, Abrahamsson S, et al. Evidence of tendon microtears due to cyclical loading in an in vivo tendinopathy model. J Orthop Res. 2005;23:1199–1205. doi: 10.1016/j.orthres.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 13.Scott A, Khan KM, Heer J, et al. High strain mechanical loading rapidly induces tendon apoptosis: an ex vivo rat tibialis anterior model. Br J Sports Med. 2005;39:e25. doi: 10.1136/bjsm.2004.015164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Soslowsky LJ, Thomopoulos S, Tun S, et al. Neer Award 1999.Overuse activity injures the supraspinatus tendon in an animal model: a histologic and biomechanical study. J Shoulder Elbow Surg. 2000;9:79–84. [PubMed] [Google Scholar]
- 15.Thornton GM, Shrive NG, Frank CB. Ligament creep recruits fibres at low stresses and can lead to modulus-reducing fibre damage at higher creep stresses: a study in rabbit medial collateral ligament model. J Orthop Res. 2002;20:967–974. doi: 10.1016/S0736-0266(02)00028-1. [DOI] [PubMed] [Google Scholar]
- 16.Barbe MF, Barr AE, Gorzelany I, et al. Chronic repetitive reaching and grasping results in decreased motor performance and widespread tissue responses in a rat model of MSD. J Orthop Res. 2003;21:167–176. doi: 10.1016/S0736-0266(02)00086-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schechtman H, Bader DL. Fatigue damage of human tendons. J Biomech. 2002;35:347–353. doi: 10.1016/s0021-9290(01)00177-4. [DOI] [PubMed] [Google Scholar]
- 18.Carter DR, Caler WE. A cumulative damage model for bone fracture. J Orthop Res. 1985;3:84–90. doi: 10.1002/jor.1100030110. [DOI] [PubMed] [Google Scholar]
- 19.Carter DR, Hayes WC. Compact bone fatigue damage—I. Residual strength and stiffness. J Biomech. 1977;10:325–337. doi: 10.1016/0021-9290(77)90005-7. [DOI] [PubMed] [Google Scholar]
- 20.Covizi DZ, Felisbino SL, Gomes L, et al. Regional adaptations in three rat tendons. Tissue Cell. 2001;33:483–490. doi: 10.1054/tice.2001.0202. [DOI] [PubMed] [Google Scholar]
- 21.Oshiro W, Lou J, Xing X, et al. Flexor tendon healing in the rat: a histologic and gene expression study. J Hand Surg [Am] 2003;28:814–823. doi: 10.1016/s0363-5023(03)00366-6. [DOI] [PubMed] [Google Scholar]
- 22.Kjaer M. Role of extracellular matrix in adaptation of tendon and skeletal muscle to mechanical loading. Physiol Rev. 2004;84:649–698. doi: 10.1152/physrev.00031.2003. [DOI] [PubMed] [Google Scholar]
- 23.Lin TW, Cardenas L, Soslowsky LJ. Biomechanics of tendon injury and repair. J Biomech. 2004;37:865–877. doi: 10.1016/j.jbiomech.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 24.Jepsen KJ, Davy DT. Comparison of damage accumulation measures in human cortical bone. J Biomech. 1997;30:891–894. doi: 10.1016/s0021-9290(97)00036-5. [DOI] [PubMed] [Google Scholar]
- 25.Thornton GM, Schwab TD, Oxland TR. Fatigue is more damaging than creep in ligament revealed by modulus reduction and residual strength. Ann Biomed Eng. 2007;35:1713–1721. doi: 10.1007/s10439-007-9349-z. [DOI] [PubMed] [Google Scholar]
- 26.Tami AE, Nasser P, Schaffler MB, et al. Noninvasive fatigue fracture model of the rat ulna. J Orthop Res. 2003;21:1018–1024. doi: 10.1016/S0736-0266(03)00099-8. [DOI] [PubMed] [Google Scholar]
- 27.Reifsnider KL, Pipes RB, editors. Fatigue of composite materials. Elsevier; New York: 1991. (Composite materials series, no. 4). [Google Scholar]
- 28.Flatow EL, Nasser P, Lee L, et al. Overestimation of the degradation state in fatigue loaded tendon due to transient effects. Trans Orthop Res Soc. 2002;27:621. [Google Scholar]
- 29.Laudier D, Schaffler MB, Flatow EL, et al. Novel procedure for high-fidelity tendon histology. J Orthop Res. 2007;25:390–395. doi: 10.1002/jor.20304. [DOI] [PubMed] [Google Scholar]
- 30.Pollock RG, Wang VM, Bucchieri JS, et al. Effects of repetitive subfailure strains on the mechanical behavior of the inferior glenohumeral ligament. J Shoulder Elbow Surg. 2000;9:427–435. doi: 10.1067/mse.2000.108388. [DOI] [PubMed] [Google Scholar]
- 31.Provenzano PP, Heisey D, Hayashi K, et al. Subfailure damage in ligament: a structural and cellular evaluation. J Appl Physiol. 2002;92:362–371. doi: 10.1152/jappl.2002.92.1.362. [DOI] [PubMed] [Google Scholar]
- 32.Fleming BC, Beynnon BD. In vivo measurement of ligament/tendon strains and forces: a review. Ann Biomed Eng. 2004;32:318–328. doi: 10.1023/b:abme.0000017542.75080.86. [DOI] [PubMed] [Google Scholar]
- 33.Maganaris CN, Paul JP. In vivo human tendon mechanical properties. J Physiol. 1999;521(Pt 1):307–313. doi: 10.1111/j.1469-7793.1999.00307.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Woo SL, Weiss JA, Gomez MA, et al. Measurement of changes in ligament tension with knee motion and skeletal maturation. J Biomech Eng. 1990;112:46–51. doi: 10.1115/1.2891125. [DOI] [PubMed] [Google Scholar]
- 35.Monti RJ, Roy RR, Zhong H, et al. Mechanical properties of rat soleus aponeurosis and tendon during variable recruitment in situ. J Exp Biol. 2003;206(Pt 19):3437–3445. doi: 10.1242/jeb.00550. [DOI] [PubMed] [Google Scholar]
- 36.Scott JE. Proteoglycan-fibrillar collagen interactions. Biochem J. 1988;252:313–323. doi: 10.1042/bj2520313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Goh KL, Meakin JR, Aspden RM, et al. Stress transfer in collagen fibrils reinforcing connective tissues: effects of collagen fibril slenderness and relative stiffness. J Theor Biol. 2007;245:305–311. doi: 10.1016/j.jtbi.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 38.Kastelic J, Baer E. Deformation in tendon collagen. Symp Soc Exp Biol. 1980;34:397–435. [PubMed] [Google Scholar]
- 39.Puxkandl R, Zizak I, Paris O, et al. Viscoelastic properties of collagen: synchrotron radiation investigations and structural model. Philos Trans R Soc Lond B Biol Sci. 2002;357:191–197. doi: 10.1098/rstb.2001.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bruehlmann SB, Kelly EJ, Duncan NA. In situ tendon damage occurs within the interfibril matrix. Trans Orthop Res Soc. 2005;30:389. [Google Scholar]
- 41.Bruehlmann SB, Matyas JR, Duncan NA. ISSLS prize winner: Collagen fibril sliding governs cell mechanics in the anulus fibrosus: an in situ confocal microscopy study of bovine discs. Spine. 2004;29:2612–2620. doi: 10.1097/01.brs.0000146465.05972.56. [DOI] [PubMed] [Google Scholar]
- 42.Jozsa L, Kannus P, Balint JB, et al. Three-dimensional ultrastructure of human tendons. Acta Anat (Basel) 1991;142:306–312. doi: 10.1159/000147207. [DOI] [PubMed] [Google Scholar]
- 43.Torp S, Baer E, Friedman B. Effects of age and of mechanical deformation on the ultrastructure of tendon. In: Atkins EDT, Keller A, editors. Structure of fibrous biopolymers. Butterworths; London: 1975. pp. 223–250. [Google Scholar]
- 44.Sonnabend DH, Yu Y, Howlett CR, et al. Laminated tears of the human rotator cuff: a histologic and immunochemical study. J Shoulder Elbow Surg. 2001;10:109–115. doi: 10.1067/mse.2001.112882. [DOI] [PubMed] [Google Scholar]
- 45.Nakama LH, King KB, Abrahamsson S, et al. Effect of repetition rate on the formation of microtears in tendon in an in vivo cyclical loading model. J Orthop Res. 2007;25:1176–1184. doi: 10.1002/jor.20408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Birch HL, Rutter GA, Goodship AE. Oxidative energy metabolism in equine tendon cells. Res Vet Sci. 1997;62:93–97. doi: 10.1016/s0034-5288(97)90127-2. [DOI] [PubMed] [Google Scholar]
- 47.Wilson AM, Goodship AE. Exercise-induced hyperthermia as a possible mechanism for tendon degeneration. J Biomech. 1994;27:899–905. doi: 10.1016/0021-9290(94)90262-3. [DOI] [PubMed] [Google Scholar]
- 48.Joo W, Jepsen KJ, Davy DT. The effect of recovery time and test conditions on viscoelastic measures of tensile damage in cortical bone. J Biomech. 2007;40:2731–2737. doi: 10.1016/j.jbiomech.2007.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pattin CA, Caler WE, Carter DR. Cyclic mechanical property degradation during fatigue loading of cortical bone. J Biomech. 1996;29:69–79. doi: 10.1016/0021-9290(94)00156-1. [DOI] [PubMed] [Google Scholar]
- 50.Johnson WS, Hillbery BM, editors. Probabilistic aspects of life prediction. ASTM International; West Conshohocken, PA: 2004. [Google Scholar]
- 51.Wang VM, Lee H, Rajan L, et al. A novel in vivo model of tendon fatigue damage accumulation. Paper presented at: Proceedings of the 5th International Symposium on Ligaments and Tendon; Washington, DC. Feb 19, 2005. 2005. [Google Scholar]