Abstract
It is crucial to reveal the regulatory mechanism of nitrification to understand nitrogen conversion in agricultural systems and wastewater treatment. In this study, the nwiI gene of Nitrobacter winogradskyi was confirmed to be a homoserine lactone synthase by heterologous expression in Escherichia coli that synthesized several acyl-homoserine lactone signals with 7 to 11 carbon acyl groups. A novel signal, 7, 8-trans-N-(decanoyl) homoserine lactone (C10:1-HSL), was identified in both N. winogradskyi and the recombined E. coli. Furthermore, this novel signal also triggered variances in the nitrification rate and the level of transcripts for the genes involved in the nitrification process. These results indicate that quorum sensing may have a potential role in regulating nitrogen metabolism.
Nitrification is a crucial component of the global nitrogen cycle and has an important role in the conversion of nitrogen in fertilizer in agricultural systems and nitrogen elimination in wastewater treatment1. Nitrification is a two-step process. Ammonia-oxidizing bacteria (AOB) and nitrite-oxidizing bacteria (NOB) are considered to be the most important contributors to nitrification and always appear to work together to convert ammonia into nitrate2,3. However, recent studies indicated that ammonia-oxidizing archaea (AOA) are also widely distributed in various types of ecosystems and play important roles in the global nitrogen biogeochemical cycle4,5. Nitrobacter winogradskyi is a key member of the NOB family that oxidizes nitrite into nitrate to obtain energy for growth6,7. Additionally, as a facultative chemolithoautotroph, N. winogradskyi can also utilize organic compounds as their sole carbon and energy sources1. During the microbial nitrification process, AOB, NOB and other bacteria could form a biofilm to efficiently oxidize ammonia and nitrite8,9. Because bacteria use small molecules to assess the density and identity of nearby organisms, some signal transduction mechanisms that act via bioactive molecules may be the strategy to regulate their metabolism and physiological behavior to adapt to various conditions. Biofilm formation is controlled by a cell density-dependent quorum sensing (QS) mechanism in many Gram-negative bacteria10. Recent studies have indicated that long-chain QS signals could increase the anoxic ammonium oxidation rate in the oxygen-limited autotrophic nitrification/denitrification (OLAND) process11, and NO3− reduction and N2 production were decreased after C4-HSL was added to QS mutants of Pseudomonas aeruginosa PAO1, which indicated that the denitrification activity was controlled by rhl-mediated QS in P. aeruginosa12. Increasing evidence indicates that QS is a global regulation system in some Proteobacteria strains13. These studies have raised questions of whether the QS system could have major consequences in regulating nitrogen metabolism.
QS is a cell-cell communication mechanism that employs autoinducers to regulate bioluminescence, biofilm formation, swarming, plasmid transfer and exoenzyme secretion14,15,16. QS bacteria can sense and respond to cell density variations to regulate gene expression. Acyl-homoserine lactone (AHL) molecules are primarily biosynthesized by the LuxI protein family, and then QS-relative genes can be activated in response to the interaction between the AHL molecules and the cognate LuxR regulator protein17,18,19. The environmental concentration of AHLs is the key factor that triggers the response20. Three AHL synthase families have been characterized: the LuxI family, the AinS family and the HdtS family21. Based on the homology of known QS system genes, the genome sequence of N. winogradskyi revealed that the nwi_0626 and nwi_0627 genes were predicted to encode a pair of LuxI and LuxR homologs that are responsible for the synthesis of AHLs as a QS signal and a responsive transcription factor, respectively1,22. A previous study isolated two AHLs from N. winogradskyi in response to cell density23. However, the authors were unable to clarify the structure of the presumed unsaturated acyl-HSL, and the function of the postulated signal synthase (nwi_0626) currently remains unclear. Although the AHL-producing activity was confirmed in N. winogradskyi, the AHL synthase, LuxI/R QS system and the postulated QS in N. winogradskyi have not been proven. We found that different AHL types were produced by this Nitrobacter in a recent preliminary study and examined the DNA sequence of this bacterium to identify luxI homologs in this species. The polypeptide encoded by nwi_0626 (NwiI) clusters with homologs of the LuxI subfamily, which could enzymatically synthesize a QS signal with a unique structure (Supplementary Figure S1 A). To investigate the mechanism by which this Nitrobacter produces different acyl-HSL quorum-sensing signals and the possibility that the QS system may serve to regulate the physiological functions of N. winogradskyi, we sought to identify the differences in AHL synthesis in various growth conditions and the products of the AHL synthase activity.
Here, we describe the potential AHL synthase (NwiI) of N. winogradskyi, which belongs to the LuxI family. When nwiI was introduced into Escherichia coli, various types of AHLs were identified in the extracts of the recombinant strain. Additionally, we discovered that N. winogradskyi produced different types of AHL signals under different culture conditions, including an acyl-homoserine lactone (HSL) with an unsaturated C10 acyl side chain that clearly demonstrates a specific configuration. The molecule also exhibits a different position for the double bond compared with the previously reported structure23. The addition of the C10:1-HSL extract to the culture medium revealed that it influenced the process of nitrification. These results demonstrate that the nitrite-oxidizing bacteria N. winogradskyi has an acyl homoserine lactone-based QS system that is responsible for the biosynthesis of various AHLs with a series of saturated and unsaturated acyl chains, and the predominant autoinducer C10:1-HSL has the capacity to regulate the nitrite oxidation process.
Results
Identification of the nwiI gene responsible for AHL production
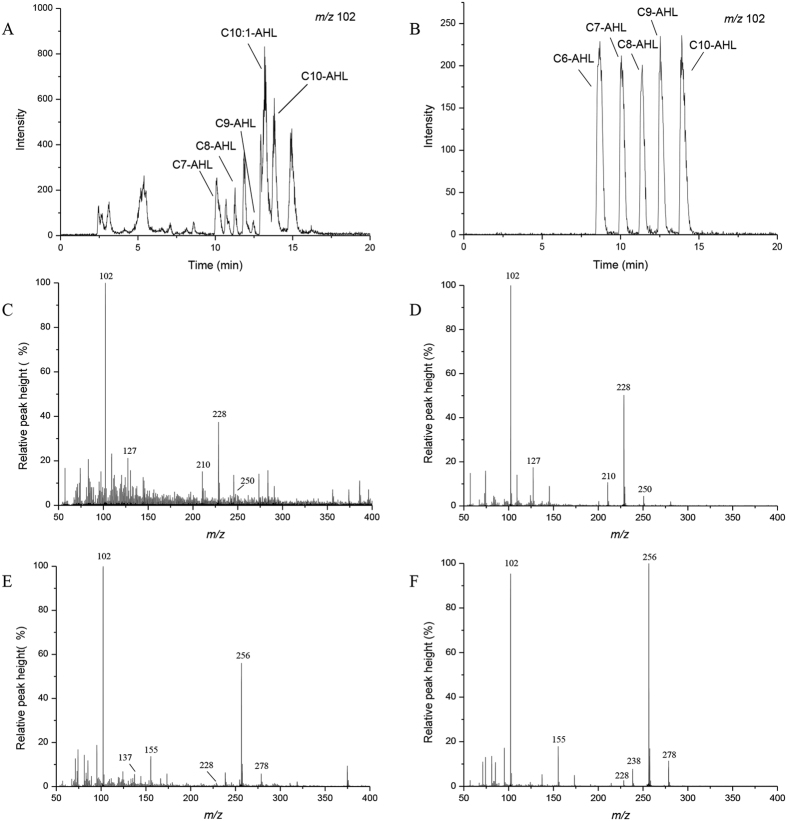
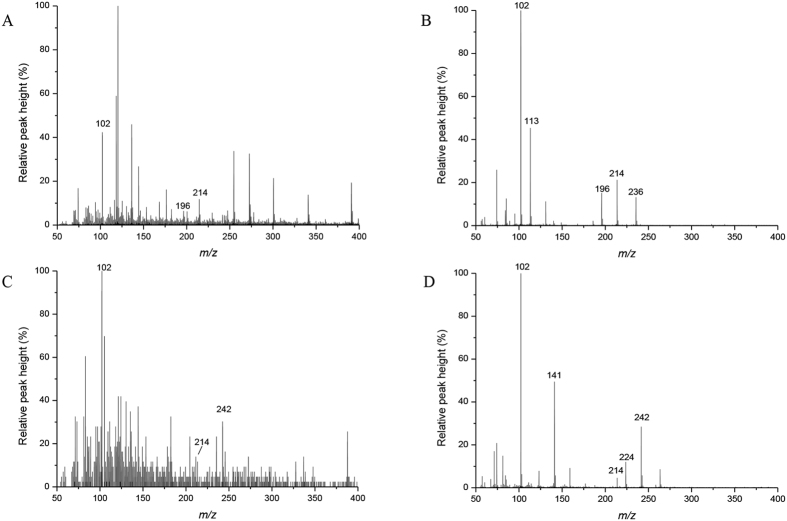
To investigate the potential AHL synthase from N. winogradskyi, it is necessary to compare, clone, and heterologously express the homologs from N. winogradskyi. The sequence for the putative QS signal synthase NwiI from N. winogradskyi was conserved among the group of AHL synthase proteins. According to the analysis of the 16S and 23S rRNA gene sequences, the alphaproteobacterium N. winogradskyi is closely related to phototrophic bacterium Rhodopseudomonas palustris CGA009 and the dinitrogen-fixing legume symbiont Bradyrhizobiaceae japonicum USDA1101. The phylogenetic tree of the LuxI and HdtS family members revealed that the NwiI polypeptide sequence was closely related to the AHL synthetases BraI, BjaI and RapI from Bradyrhizobium and Rhodopseudomonas (Supplementary Figure S2). The open reading frame (ORF) (nwi_0626) encoded a protein that was 39% and 25% similar to the BjaI protein from B. japonicum USDA110 and the LasI protein from Pseudomonas aeruginosa PAO1, respectively (Supplementary Figure S1 A). Moreover, the open reading frame (ORF) (nwi_0627) encoded a protein that was 33 and 31% similar to the BjaR protein from B. japonicum USDA110 and LasR protein from Pseudomonas aeruginosa PAO1, respectively (Supplementary Figure S1 B). Based on the structural similarities between NwiI and the autoinducer synthases employed by other bacteria, NwiI contained the same conserved amino acid residues that were important for the active sites as the proteins (BjaI and LasI) with known QS functions24,25. Next, nwiI was expressed in E. coli, which does not produce AHLs. The extracts of LB medium alone were also performed by the LC-MS, and no AHLs were detected (Supplementary Figure S3 A). The nwiI gene was cloned into the pGEX-4T-1 plasmid and transformed into the E. coli BL21 (DE3) strain. The AHL bioassay strain A. tumefaciens KYC55 uses the T7 expression system to strongly overexpress the regulator TraR. When AHL signals are present, TraR can activate a traI-lacZ reporter fusion protein, resulting in the production of β-galactosidase26. The signals obtained from the recombinant sample turned the biosensor supernatant solution yellow, and no activity was observed in the control E. coli strain carrying pGEX-4T-1 without the nwiI gene. The levels of lacZ induction by AHLs were then reported in Miller units (Fig. 1). This finding suggested that nwiI from N. winogradskyi encoded an AHL synthase when overexpressed in the heterologous host E. coli BL21(DE3). A positive result was obtained in the bioassays, and high-performance liquid chromatography-mass spectrometry (HPLC-MS) was used to identify the acyl chains of the AHLs. The retention time and mass spectra of the AHLs in the recombinant extract were compared with AHL standards. Here, we confirmed that four different acyl chains of AHLs were present in the recombinant extract. Two compounds with an even number of N-linked acyl chains were clearly identified with molecular ions [M+H]+ of m/z 228 and 256 that correspond to C8-HSL and C10-HSL, respectively (Fig. 2). These two AHLs were previously identified in a number of Gram-negative bacteria27,28. In addition, we also identified two AHLs with an odd number of carbons in the acyl chain with molecular ions [M+H]+ of m/z 214 and 242 that correspond to C7-HSL and C9-HSL, respectively (Fig. 3). The AHLs with an odd number of N-linked acyl chains used propionyl-CoA and malonyl-CoA as the respective chain starter and extender units via a pathway that produces odd chain fatty acids29. However, the odd-numbered AHLs were not as prevalent as the even-numbered AHLs.
Figure 1. AHL production (Miller units) from the recombinant extract and control E. coli extract in A. tumefaciens KYC55 (pJZ372) (pJZ384) (pJZ410) using the liquid assay.
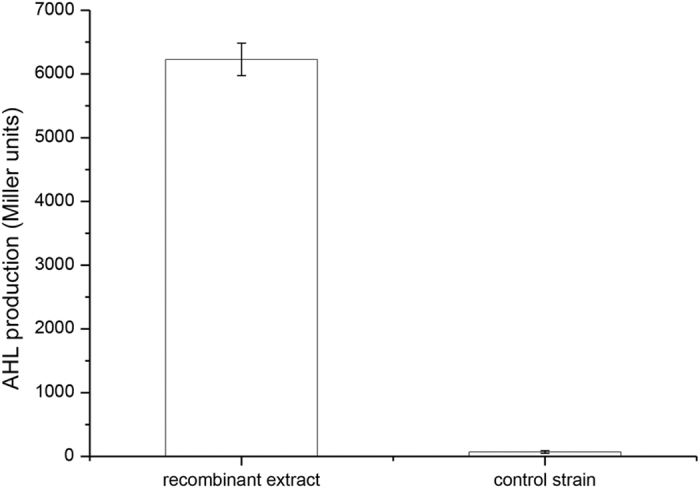
The values are the mean of three independent biological replicates. The error bars indicate mean ± standard deviation of three biological replicates determinations.
Figure 2. LC-MS chromatograms of the AHL molecules in E. coli containing pGEX-nwiI and the AHL standards.
(A) Chromatogram of the lactone moiety at m/z 102 from an extract of the recombinant E. coli strain. (B) Selected ion (m/z 102) chromatograms for the C6-HSL, C7-HSL, C8-HSL, C9-HSL and C10-HSL standards. (C,E) The mass spectra of the extracts from recombinant E. coli containing pGEX-nwiI reveal molecular ions [M+H] of m/z 228 and 256. The comparable fragmentation products are labeled with their respective m/z values. (D,F) The fragmentation patterns of the extract of the recombinant strain were consistent with those of the C8-HSL (D) and C10-HSL (F) standards.
Figure 3. LC-MS chromatograms of C7-HSL and C9-HSL in E. coli containing pGEX-nwiI.
(A,C) The mass spectra of extracts from recombinant E. coli containing pGEX-nwiI reveal molecular ions [M+H] of m/z 214 and 242. The comparable fragmentation products are labeled with their respective m/z values. (B,D) The fragmentation patterns of the extract from the recombinant strain were consistent with those of the C7-HSL (B) and C9-HSL (D) standards.
Structural analysis of a new AHL molecule with an unsaturated C10 acyl side chain
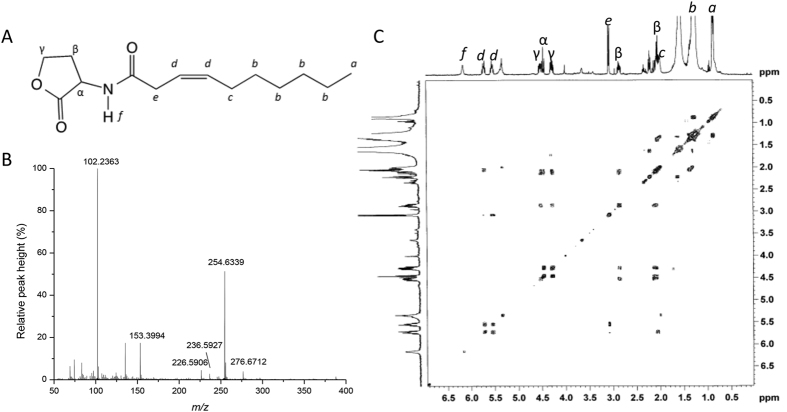
As described above, there were eight autoinducer molecules produced by E. coli containing pGEX-nwiI (Fig. 2A). Only four of the molecules were identified as standard AHLs (C7-HSL, C8-HSL, C9-HSL and C10-HSL). We purified the other four putative AHLs using preparative HPLC. The analysis of the highest mass spectrum peak revealed pseudomolecular ion peaks for [M + H]+ and [M + Na]+ at m/z 254 and m/z 276, respectively, with major fragmentation ions at m/z 102 and m/z 153 and minor fragmentation ions at m/z 226 ([M + H-CO]+) and 236 ([M + H-H2O]+) (Fig. 4B). This mass could not be attributed to any known AHL, and this putative AHL molecule might possess an unsaturated acyl chain compared with the mass spectrum of C10-HSL. The proton nuclear magnetic resonance (1H NMR) spectrum of the putative purified AHL was similar to the other AHLs, and all of the protons could be unambiguously assigned (Supplementary Figure S4)30,31. According to the 13C nuclear magnetic resonance (13C NMR) spectrum (Supplementary Figure S5), we predicted that this molecule contained a 10-carbon acyl group with one unsaturated carbon-carbon (trans configuration) bond. As determined by correlation spectroscopy (COSY), the double bond in the acyl chain was located between carbons 7 and 8 (Fig. 4). The HPLC-MS results revealed that the m/z of [M + H]+ was 254.6339. This value corresponded to a chemical composition of C14H23NO3, which was consistent with the structure presented in Fig. 4.
Figure 4.
Predicted structure (A), LC-MS (B), and proton NMR and COSY spectrum (C) purified from the extracts of the recombinant E. coli strain containing the nwiI gene. The protons are indicated by italicized letters and correspond to the following peaks in the 1H-NMR (CDCl3, 400 MHz) spectrum: δH 0.88 (3H, t, a,), 1.29 (8H, m, b), 2.01 (2H, m, c), 2.11 (1H, m, β), 2.86 (1H, m, β), 3.08 (2H, d, e), 4.28 (1H, m, γ), 4.47 (1H, t, α), 4.53 (1H, m, γ), 5.54 (1H, m, d), 5.73 (1H, m, d), and 6.17 (1H, m, f).
In addition to the five identified AHL molecules (C7-HSL, C8-HSL, C9-HSL, C10-HSL and C10:1-HSL), three probable AHL molecules were difficult to identify due to the lack of standards. In addition, the extremely low concentrations and interference from other similar compounds that were likely aromatics influenced the NMR spectrum (data not shown). Generally, using MS detection, the AHLs decompose into two main fragments, a [M + H]+ ion from neutral loss of homoserine lactone and the m/z 102 ion from protonation of the homoserine lactone. Furthermore, two other protonations of the HSLs lead to the [M + H-H2O]+ and [M + H-CO]+ ions32. Although the remaining components in the solution have not yet been purified, it could be hypothesized that the recombinant E. coli strain containing nwiI produced C8:1-HSL, C9:1-HSL and C11:1-HSL, according to the liquid chromatography-mass spectrometry (LC-MS) chromatograms (Supplementary Figure S6).
Characterization of the AHLs produced by N. winogradskyi under different growth conditions
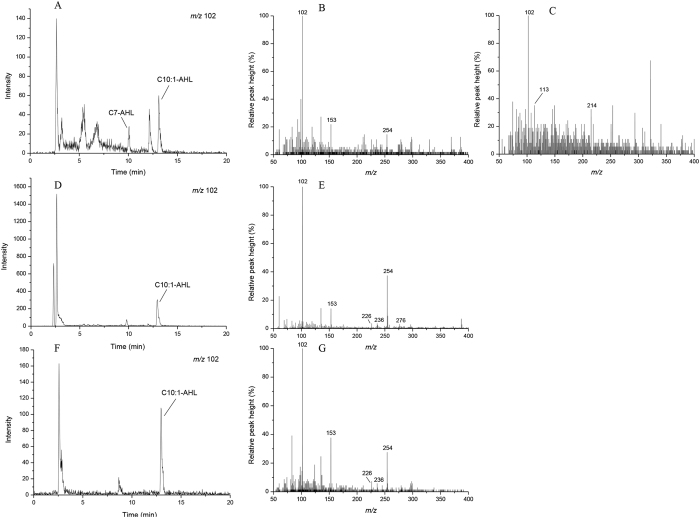
As a facultative chemolithoautotrophic bacterium, N. winogradskyi can grow in heterotrophic, mixotrophic and autotrophic culture conditions. Thus, we set out to examine these NOB AHLs in different culture conditions using the bioassay strain Agrobacterium tumefaciens KYC55. Positive results were obtained in the bioassay experiments using all three culture mediums. As shown in Fig. 5, in autotrophic medium, the AHL levels were obviously increased compared with the other culture conditions. The amount of acyl-HSL in the autotrophic substrate significantly accumulated and exhibited a five-fold increase in Miller units/OD600 compared with the heterotrophic culture. In addition, we also used LC-MS to confirm the AHLs that were present in the N. winogradskyi extract. Here, we confirmed that AHLs with two different acyl chains, C7-HSL and C10:1-HSL, were present in the extract of the cells cultured in the mixotrophic medium, whereas only C10:1-HSL was detected in the extracts of the cells cultured in the heterotrophic and autotrophic media (Fig. 6). The extract of culture medium alone were also performed by LC-MS, and no AHLs were detected (Supplementary Figure S3 B).
Figure 5. AHL production (Miller units/OD600) in N. winogradskyi in heterotrophic medium, autotrophic medium and mixotrophic medium by A. tumefaciens KYC55 (pJZ372) (pJZ384) (pJZ410) using the liquid assay.
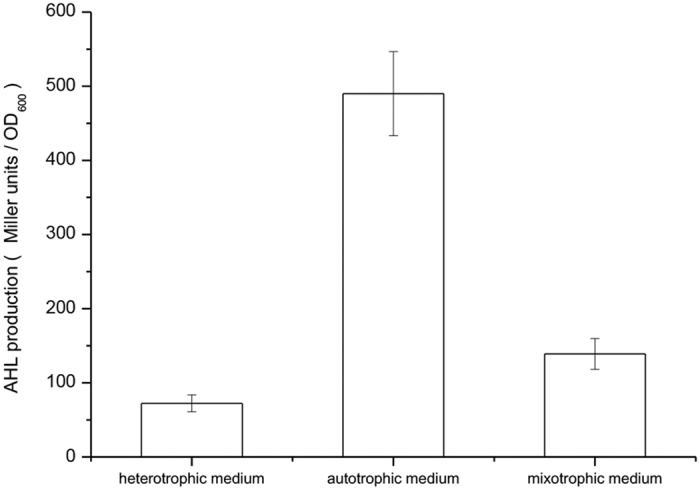
The values are the mean of three independent biological replicates. The error bars indicate mean ± standard deviation of three biological replicates determinations.
Figure 6. LC-MS chromatograms of the AHLs molecules in N. winogradskyi under different culture conditions.
(A) Chromatogram of the lactone moiety at m/z 102 from the N. winogradskyi mixotrophic culture extract. (B,C) The mass spectra of mixotrophic culture extracts reveal molecular ions [M + H] of m/z 214 and 254. (D) Chromatogram of the lactone moiety at m/z 102 from the N. winogradskyi autotrophic culture extract. (E) The mass spectra of the autotrophic culture extracts reveal molecular ions [M + H] of m/z 254. (F) Chromatogram of the lactone moiety at m/z 102 from the N. winogradskyi heterotrophic culture extract. (G) The mass spectra of the heterotrophic culture extracts reveal molecular ions [M + H] of m/z 254.
7,8-trans-N-(decanoyl) homoserine lactone affects the nitrification process
To further characterize the impact of AHLs on the nitrification process in N. winogradskyi, 2 μM C10:1-HSL that was purified from the recombinant E. coli extracts was added to the N. winogradskyi culture medium. The purity of the C10:1-HSL generated by preparative HPLC was confirmed by LC-MS (Supplementary Figure S7). During the logarithmic phase of N. winogradskyi growth, the nitrite oxidation rate followed Michaelis-Menten kinetics33. Michaelis-Menten kinetics are characterised by the hypothesis that the enzyme and substrate participate in equilibrium with the enzyme-substrate complex34. Although the experiments were not performed with purified enzymes, the term Km was used to determine nitrite oxidation kinetics, because the kinetics in short-term activity assays over a few hours, where growth can be neglected33. After the addition of C10:1-HSL, the half-saturation constant for nitrite oxidation (Km) was 138.06 ± 27.04 μM. The value for the control group was 143.63 ± 47.25 μM. Although the nitrite affinity in C10:1-HSL addition group was slightly higher than control, no significant differences in the Km values were noted (p ≥ 0.05). However, the half-lives, t1/2, of nitrite were 5.19 h and 4.81 h in the control and C10:1-HSL-treated groups, respectively (Supplementary Table 1). The result indicated that the utilization of nitrite was slightly increased after the addition of C10:1-HSL.
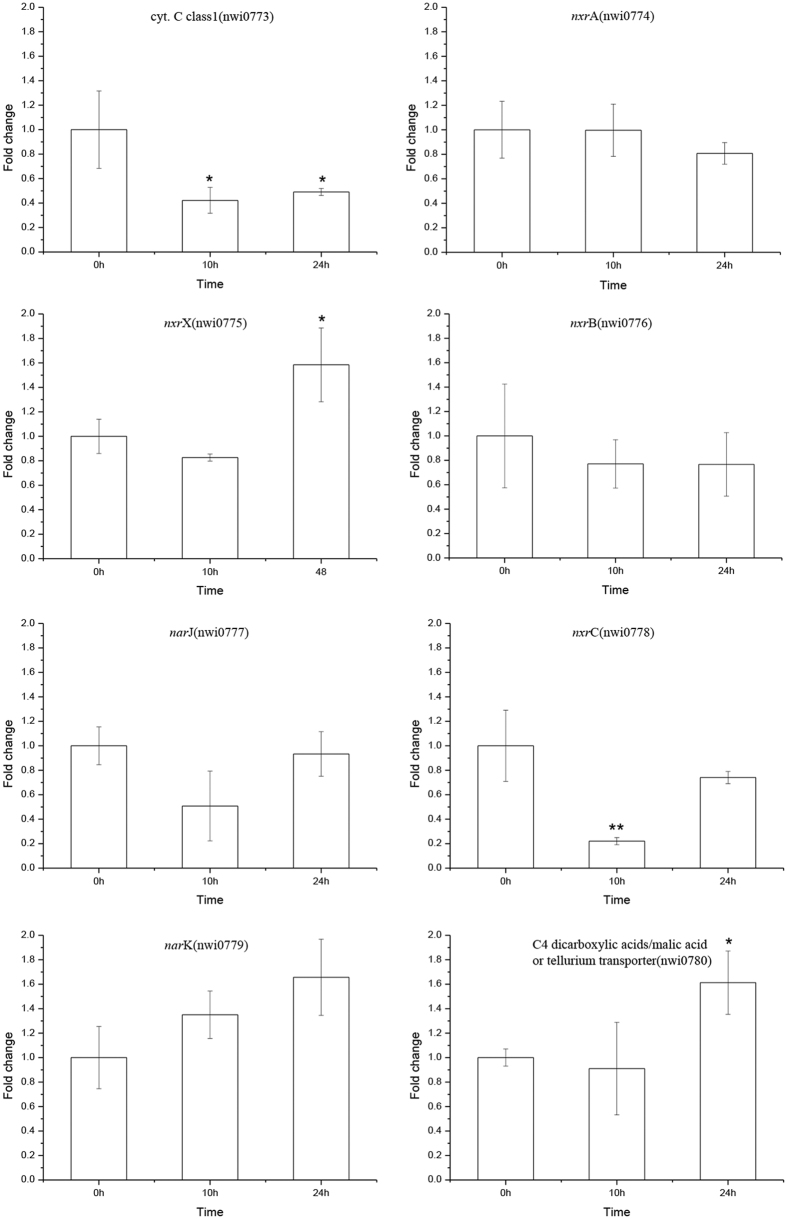
The expression of genes in the nitrite oxidoreductase (NXR) cluster, which contains a molybdopterin that converts nitrite to nitrate, was also investigated. NXR is a heterodimer that contains an α subunit (NxrA) and a β subunit (NxrB) and is highly similar to the membrane-bound dissimilatory nitrate reductase (Nar) of E. coli and many other bacteria35. Two copies of nxrA and nxrB were present in the N. winogradskyi genome, but only one central gene cluster (nwi_0773 to nwi_0780) encodes the accessory proteins of the NXR complex1. The nwi_0773 gene is predicted to encode a c-type cytochrome that is likely a part of the electron transport system that couples the oxidation and reduction of nitrite and is located upstream of nxrA1. The nxrX (nwi_0775) gene is a homolog of norX, which encodes a peptidyl-prolyl cis-trans isomerase to aid in the folding of NXR1. Moreover, two genes, narJ (nwi_0777) and nxrC (nwi_0778), which are downstream of nxrB, are predicted to encode homologs of NarJ and NxrC, respectively. NarJ is a chaperone required for insertion of the molybdenum cofactor in nitrate reductase A. And NxrC is a periplasmic cytochrome c that serves as the electron acceptor and donor36. In addition, a NarK-like nitrate/nitrite transporter (nwi_0779) and transporter for C4 dicarboxylic acids/malic acid or tellurium (nwi_0780) are also conserved in N. winogradskyi1. The oxidation of nitrite by NXR is reversible, and NXR can also catalyze the reduction of nitrate to nitrite, which is considered to be part of the denitrification pathway in N. winogradskyi37. Both strains that were treated with autoinducer and blank strains were evaluated for their ability to activate or inactivate the transcription of the NXR cluster genes in N. winogradskyi. The quantitative PCR results indicated that the expression levels of nxrA (nwi_0774) and nxrB (nwi_0776) were not obviously altered in the strain that was incubated with C10:1-HSL (Fig. 7). However, the expression levels of nxrX (nwi_0775), narK (nwi_0779) and a gene encoding for C4 dicarboxylate transporter (nwi_0780) were increased 24 h after C10:1-HSL was added compared with the samples without the novel signal (Fig. 7). Although nxrA and nxrB were not activated, transcriptional inhibition of c-type cytochrome (nwi_0773) (p value: 0.039), narJ (nwi_0777) and nxrC (nwi_0778) (p value: 0.0099) were observed after a 10 h incubation with 2 μM C10:1-HSL (Fig. 7).
Figure 7. Changes in gene expression after the addition of exogenous AHLs during the culture process.
The fold changes in gene expression were measured by qPCR. The stars indicate significant changes in gene expression compared with the samples at 0 h (*p ≤ 0.05, **p ≤ 0.01). Statistical analyses of the ΔCt values were analyzed by SPSS using the two-sample t-test for the means. Results were considered significant when p ≤ 0.05.
Discussion
To identify the signal types that were produced by the AHLs synthase in N. winogradskyi, we introduced nwiI into E. coli, which resulted in the synthesis of a series of AHLs with chain lengths ranging from C7 to C11. Five of the AHLs were identified as C7-AHL, C8-AHL, C9-AHL, C10-AHL and C10:1-AHL. However, three probable AHL molecules remain to be confirmed due to the lack of standards and the extremely low concentration of the samples. Despite the fact that the remaining components of the solution have not been purified, it could be hypothesized that the recombinant E. coli strain containing nwiI produced C8:1-AHL, C9:1-AHL and C11:1-AHL according to the LC-MS chromatograms. It is unexpected that one QS signal synthase gene was responsible for the biosynthesis of various AHLs with a series of saturated and unsaturated acyl chains ranging from 7 to 11 carbons in length. According to the MS, NMR and COSY analyses, a new AHL molecule with an unsaturated C10 acyl side chain was discovered. Although C10:1-HSL signaling molecules were analyzed in several reports, the physicochemical characterizations were often incomplete, and a definite structure was merely implied38,39,40. Mellbye et al.23 presumed that a monounsaturated acyl-HSL (C10:1-HSL) with the C=C bond at the end of the acyl chain was present in N. winogradskyi using UPLC-IDA-MS; however, the results were insufficient to clarify the isomeric form of the C=C bond. Our results clearly indicated that N. winogradskyi produced a novel type of acyl-HSL, 7,8-trans-N-(decanoyl) homoserine lactone, which differed from the C10:1-HSL structure, as mentioned above.
As a facultative chemolithoautotrophic bacterium, N. winogradskyi can grow in heterotrophic, mixotrophic and autotrophic culture conditions. Thus, we examined these NOB AHLs in different culture conditions. It is interesting that the AHL signals produced by N. winogradskyi were inconsistent with the recombinant E. coli strain. Schaefer et al.25 reported that the LuxI homolog of Rhodobacter capsulatus synthesized a different type of AHLs in E. coli instead of the native AHL signals. E. coli containing acyl-HSL synthase synthesized C14-HSL but did not produce the native C16-HSL of R. capsulatus. Our study obtained similar results, as many more AHLs were produced by the recombinant E. coli compared with N. winogradskyi. The biosynthesis of AHLs showed that S-adenosylmethionine (SAM) and acyl carrier protein (ACP) charged with the appropriate fatty acid as sources of the homoserine lactone and acyl chain, respectively41. The variety of the N-acyl chains represents a broad range of modifications that are generated in fatty acid biosynthesis. The LuxI homologue often shows a preference for one specific fatty acyl-ACP derivative leading to the formation of one major AHL product, but closely related AHLs with similar chain lengths frequently occur in the same bacterial species41. However, there was lack of mechanism to illuminate different production range of AHLs in heterologous expression strain. The main AHL product of one AHL synthase reflects its preferred substrate, and the metabolically versatile property of N. winogradsky probably resulted in these difference. Moreover, in the detection of the AHLs in the original N. winogradskyi strain, the amount of AHLs in the autotrophic medium was obviously increased compared with the other culture conditions. The C7-HSL and C10:1-HSL signals were confirmed in the mixotrophic medium extract, whereas only C10:1-HSL was detected in the heterotrophic and autotrophic medium extracts. A recent study has demonstrated that different signals are utilized when the cell densities were high or low, particularly when multiple signals were integrated40. The growth-dependent QS study in P. aeruginosa demonstrated that the signaling pathway performs differently when the cell densities were different. The integration of multiple signals with different chemical half-lives allowed P. aeruginosa to decode its physical and social environment with high resolution40. Additionally, it is worth noting that environmental stimuli, particularly the nature of the carbon sources, have a powerful influence on the type of autoinducer molecule produced42. N. winogradskyi was able to utilize nitrite, carbon dioxide and organic compounds in the absence of nitrate to support growth1. This metabolically versatile property likely resulted in the release of different AHLs from N. winogradskyi in response to various survival conditions and its own demands.
One interesting phenotype we measured was that the maximum accumulation of AHLs occurred in autotrophic cultures with the highest nitrite concentration (Fig. 5), suggesting a potential role for QS in preparing the organism for higher cell densities by controlling nitrogen metabolism in N. winogradskyi. From the results of the growth condition assays in N. winogradskyi, C10:1-HSL appeared to be a necessary autoinducer, as it was detected in all growth conditions (Fig. 6). To further characterize the impact of the AHLs on the nitrification process in N. winogradskyi, 2 μM purified C10:1-HSL was added to the N. winogradskyi culture medium. NxrA/B are the key catalytic subunits for nitrite oxidation in N. winogradskyi, as the expression levels of the nxrA/B did not respond to AHL. Transcriptional inhibition of c-type cytochrome (nwi_0773) and nxrC (nwi_0778) were observed in the presence of C10:1-HSL, indicated a reduced NXR activity. However, The Km results indicated that the affinities for the substrate nitrite were slightly increased by additional C10:1-HSL in N. winogradskyi; thus, other factors must regulate the increased nitrite oxidation activity. The results of Nowka et al.33 indicated the Nitrobacter affinities for nitrite substrate was related to the transporters which shuttled nitrite across the cytoplasmic membrane. The increased expression level of NarK-like nitrate/nitrite transporter (nwi_0779) after the addition of C10:1-HSL may lead to the results described above. Both the active uptake of nitrite or efflux of nitrate would be important for maintaining NXR activity in N. winogradskyi; therefore, the effect of adding exogenous C10:1-HSL to the culture medium appeared to activate the nitrification process. Interestingly, combined with the results of the different culture media on the AHL concentrations, these results suggest that the QS system was strongly induced in the nutrient-rich environment and was much more sensitive to nitrite. It is reasonable that the nitrite-rich culture could in fact trigger the production of a sufficient amount autoinducer to reach an effective concentration, and the increasing AHL concentration has a profound effect on accelerating nitrite absorption, which subsequently underlie these results.
In summary, a novel C10:1-HSL molecule, 7,8-trans-N-(decanoyl) homoserine lactone was generated by the N. winogradskyi autoinducer synthase, as confirmed by the MS, NMR and COSY analyses. We measured the effect of adding exogenous C10:1-HSL to the N. winogradskyi culture medium, resulting in slightly variance of the nitrification process. Moreover, we cannot exclude the possibility that the different types of AHLs produced by Nitrobacter provide an opportunity to study the combinatorial information processing that is regulated by the QS system. The discovery of the ability of Nitrobacter to synthesize AHLs opens up additional avenues of research to demonstrate the regulatory role of QS in the global nitrogen cycle. It remains to be investigated whether the QS effects confer a broad advantage to N. winogradskyi compared with other environmental nitrifying bacteria.
Methods
Bacterial strains and growth media
N. winogradskyi (ATCC 25391) was grown in heterotrophic nitrobacter medium 756 (http://www.dsmz.de/microorganisms/medium/pdf/DSMZ_Medium756.pdf), mixotrophic nitrobacter medium 756a (http://www.dsmz.de/microorganisms/medium/pdf/DSMZ_Medium756a.pdf) and autotrophic nitrobacter medium with 60 mM NaNO2 minimal salts as previously described43. All cultures were checked for contamination by spread plating on nutrient agar (incubated at 26 °C). If no growth on agar occurred after 5 days the cultures were assumed to be axenic. Only data from axenic cultures were used. And the purity of the nitrifying bacteria was confirmed by microscopical examination. E. coli BL21(DE3) and its derivatives were grown at 37 °C and 200 rpm in Luria-Bertani (LB) broth or on LB agar containing ampicillin (100 μg/ml), as required. A. tumefaciens KYC55 (pJZ372) (pJZ384) (pJZ410) was used as the AHL bioassay strain26. The strain was cultivated at 28 °C and 200 rpm in A. tumefaciens (AT) medium44 containing 100 μg/ml spectinomycin, 100 μg/ml gentamicin, and 5 μg/ml tetracycline. The AT medium contains AT salts with 0.5% glucose45.
Bioinformatic analyses
The GenBank database was searched using the BLAST program (http://blast.ncbi.nlm.nih.gov) to identify the genes encoding AHL synthases. The sequences were aligned and compared using the CLUSTAL W Server (http://www.ch.embnet.org/software/ClustalW.html).
DNA manipulations
N. winogradskyi DNA was prepared from cells grown in mixotrophic nitrobacter medium at 26 °C using a Genomic DNA Purification Kit (Thermo Fisher Scientific, Waltham, USA), according to the manufacturer’s instructions. The DNA concentrations were determined using a Nanodrop spectrophotometer (Nanodrop Technologies, Rockland, DE). DNA isolated from N. winogradskyi was used as the template to amplify the nwiI gene using the following primers: 5′- CAGGATCCATGATTCACATCGTAACGGC-3′ (nwiI-forward) and 5′- CCGCTCGAGCTAGGCACGAAGCCGC-3′ (nwiI-reverse). The nwiI-forward primer included a BamHI restriction site, and the nwiI-reverse primer included an XhoI restriction site (underlined). The PCR conditions were 2 min at 95 °C followed by 30 cycles of 30 s at 95 °C, 30 s at 50 °C, and 1 min at 72 °C with a final step of 10 min at 72 °C. The amplified nwiI gene was cloned into pGEX-4T-1 (GE), according to the manufacturer’s instructions. The recombinant pGEX-4T-1 plasmid containing nwiI was termed pGEX-nwiI. pGEX-nwiI was transformed into E. coli BL21(DE3), and the transformant was grown on LB medium containing ampicillin (100 μg/ml) at 37 °C. The detailed characteristics of the bacterial strains and plasmids used in this study are presented in Supplementary Table S2.
AHLs bioassays
AHL bioactivity was determined by measuring the β-galactosidase bioactivity of the ultrasensitive AHL biosensor strain A. tumefaciens KYC55 using a previously described method26. The cultures of recombinant E. coli and N. winogradskyi were centrifuged at 5,000 × g for 5 min to remove the cells. A 0.22-μm syringe filter was used to filter the assayed supernatants. The cell-free culture supernatants were stored at −20 °C until further examination. A. tumefaciens KYC55 (5 × 107) were inoculated into 2 ml of A. tumefaciens culture medium containing 200 μl of the supernatant of the recombinant E. coli45. The supernatant of an E. coli strain carrying pGEX-4T-1 without the nwiI gene was used as a negative control. For each sample, the absorbance at OD600 was recorded after approximately 10 h at 28 °C. Then, 200 μl of the supernatant from the AT culture medium was combined with 0.8 ml of Z buffer (60 mM Na2HPO4, 40 mM NaH2PO4, 10 mM KCl, 1 mM MgSO4, and 50 mM 2-mercaptoethanol, pH 7.0) in 2-ml microcentrifuge tubes. Then, 2 drops of a 0.05% SDS solution were added, and 3 drops of chloroform were added. The samples were vigorously vortexed for 10 s. Then, 0.1 ml of 4 mg/ml ortho-nitrophenyl-β-D-galactopyranoside (ONPG) was added, and the solutions were placed in a 30 °C water bath for 10 min. The reactions were stopped by the addition of 0.6 ml of 1 M Na2CO3. The cell debris were centrifuged at 16,000 × g for 3 min at room temperature, and the OD420 of the supernatant was measured. The β-galactosidase units were calculated as follows: Miller units = (1000 × OD420)/(OD600 × 10 × 0.2), where the OD420 was read from the supernatant of the reaction mixture. AHL production in N. winogradskyi in the heterotrophic, autotrophic and mixotrophic medium was calculated by Miller units per OD600 of culture.
Extraction, purification and detection of AHLs
The supernatants of the recombinant E. coli strain and N. winogradskyi were extracted two times with an equal volume of acidified ethyl acetate (EtAc) containing 0.2% glacial acetic acid and finally dried by a rotary evaporator. The extracts and AHL standards were reconstituted in HPLC-grade acetonitrile. LB and 756 medium alone were also extracted and detected using the same methods. The AHLs’ profiles were confirmed with an HPLC-MS system (Waters; USA) using a C18 reverse-phase column (5 μm by 250 mm by 4.6 mm) (Zorbax Eclipse XDB-C18; Agilent) coupled with positive-ion electrospray ionization (ESI) mass spectrometry32 and eluted with a linear gradient of acetonitrile in water (10–70%) at a flow rate of 1 ml/min from 0 to 10 min and a linear gradient of acetonitrile in water (70–10%) at a flow rate of 1 ml/min from 10 to 20 min. The retention times and spectral properties of the ESI spectra (m/z range, 50 to 400) containing a fragment product at m/z 102 were compared with the corresponding synthetic AHL standards46 (Sigma-Aldrich, USA). These extracts were applied to a C18 reverse-phase preparative HPLC column and eluted in 50% acetonitrile at a flow rate of 15 ml/min over a 2-h period by monitoring the spectra at 210 nm. The eluant was collected, and the active fractions were analyzed by LC-MS, 1H NMR, 13C NMR and COSY. 1H NMR and 13C NMR was recorded in CDCl3 with a Bruker 400 MHz (100 MHz for 13C) NMR spectrometer (Bruker Corporation, Germany).
Calculation of the oxidation kinetics and data analysis
Samples were prepared from cells grown in mixotrophic nitrobacter medium at 26 °C to measure the nitrite concentration. The sample times were 0, 2, 4, 6, 8, 12 and 24 h after treatment with or without 2 μM C10:1-HSL. LC-MS was used to detect the purity of the C10:1-HSL generated by preparative HPLC. The optical densities of the samples were analyzed for the presence of NO2− at 520 nm using sulfanilic acid and α-naphthylamine as reagents47. Nitrite oxidation is an enzymatic reaction that happens in the cytoplasm of the cell in Nitrobacter, so the characteristics of the nitrite oxidation kinetics were obtained by fitting the data to the Michaelis-Menten kinetics. The Michaelis–Menten constant was calculated using Lineweaver-Burk plots of the double reciprocal of the Michaelis-Menten equation: V = (Vmax∙[S])/(Km + [S]). Here, V is activity, Vmax is the maximum specific activity (μmol/ml/h), Km is the half-saturation constant for nitrite oxidation (μM), and [S] is the nitrite concentration (μM). The pharmacokinetic parameters were calculated using the decrease in the nitrite concentration. The decrease in the nitrite concentration appeared to follow a pseudo-first-order kinetic reaction. The nitrite concentrations (C) and the sampling time (t) were expressed as follows: lnC = −kt + b. The half-life t1/2 of nitrite was calculated as follows: t1/2 = 0.693/k.
RNA extraction and quantitative PCR (qPCR)
The total RNA was prepared from cells grown in mixotrophic nitrobacter medium supplemented with or without 2 μM C10:1-HSL at 26 °C at 0, 10 and 24 h using an RNeasy mini kit (Qiagen, Germantown, MD), according to the manufacturer’s recommendations. The RNA concentrations were determined using a Nanodrop spectrophotometer (Nanodrop Technologies, Rockland, DE). The reverse transcription reaction was performed using a PrimeScriptTM RT Master Mix (TaKaRa, Biotechnology, China). Real-time PCR was performed on a CFX96TM Real-Time PCR system (Bio-Rad Laboratories, USA) with SYBR Premix Ex TaqTM (TaKaRa Biotechnology, China). Each reaction was performed in a total reaction mixture of 25 μl containing 12.5 μl of SYBR Premix Ex TaqTM, 10 μM of each primer and 1 ng of DNA. The relevant primers are listed in Supplementary Table S3. The fold changes were calculated using the double ΔCT method (i.e., using the equation 2−ΔΔCt). The experiments were performed in triplicate. The ΔCt values were statistically analyzed with SPSS software (SPSS Inc., Chicago, USA) using the two-sample t-test to determine the differences between the mean values. The results were considered significant when p ≤ 0.05.
Additional Information
Accession codes: Sequence data from this study have been deposited in GenBank under accession number KR703654.
How to cite this article: Shen, Q. et al. A New Acyl-homoserine Lactone Molecule Generated by Nitrobacter winogradskyi. Sci. Rep. 6, 22903; doi: 10.1038/srep22903 (2016).
Supplementary Material
Acknowledgments
This work was supported by the Strategic Priority Research Program of the Chinese Academy of Sciences (XDB15030101) and the National Natural Science Foundation of China (No. 41371266, 41501250, 21377157 and 21177145). The authors wish to express their gratitude to Dr. Jun Zhu (University of Pennsylvania), Dr. Huiming Zheng (Nanjing Agricultural University) and Dr. Hui Wang (Nanjing Agricultural University) for kindly providing the AHL reporter strain.
Footnotes
Author Contributions Q.S. and J.G. conducted the experiments, analyzed the data, and wrote the manuscript. J.L. performed the analysis of NMR and COSY data. S.L. contributed to the interpretation of the data and critically read the manuscript. Z.L., Y.W. and B.G. performed the isolation of compounds. X.Z. and G.Z. were responsible for the design of experiments and modified this manuscript. All authors reviewed the manuscript.
References
- Starkenburg S. R. et al. Genome sequence of the chemolithoautotrophic nitrite-oxidizing bacterium Nitrobacter winogradskyi Nb-255. Appl. Environ. Microbiol. 72, 2050–2063 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa E., Pérez J. & Kreft J. U. Why is metabolic labour divided in nitrification? Trends Microbiol. 14, 213–219 (2006). [DOI] [PubMed] [Google Scholar]
- Starkenburg S. R., Arp D. J. & Bottomley P. J. Expression of a putative nitrite reductase and the reversible inhibition of nitrite-dependent respiration by nitric oxide in Nitrobacter winogradskyi Nb-255. Environ. Microbiol. 10, 3036–3042 (2008). [DOI] [PubMed] [Google Scholar]
- Nicol G. W. & Schleper C. Ammonia-oxidising Crenarchaeota: important players in the nitrogen cycle? Trends Microbiol. 14, 207–212 (2006). [DOI] [PubMed] [Google Scholar]
- Zhang L. M., Hu H. W., Shen J. P. & He J. Z. Ammonia-oxidizing archaea have more important role than ammonia-oxidizing bacteria in ammonia oxidation of strongly acidic soils. ISME J. 6, 1032–1045 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freitag A. & Bock E. Energy conservation in Nitrobacter. FEMS Microbiol. Lett. 66, 157–162 (1990). [Google Scholar]
- Chain P. et al. Genomics for key players in the N cycle from guinea pigs to the next frontier. Methods Enzymol. 496, 289–318 (2010). [DOI] [PubMed] [Google Scholar]
- Raj S. A. & Murthy D. Nitrification of synthetic wastewater in a cross flow medium trickling filter. Bioprocess Eng. 19, 149–154 (1998). [Google Scholar]
- Okabe S., Kindaichi T., Ito T. & Satoh H. Analysis of size distribution and areal cell density of ammonia-oxidizing bacterial microcolonies in relation to substrate microprofiles in biofilms. Biotechnol. Bioeng. 85, 86–95 (2004). [DOI] [PubMed] [Google Scholar]
- Davies D. G. et al. The involvement of cell-to-cell signals in the development of a bacterial biofilm. Science 280, 295–298 (1998). [DOI] [PubMed] [Google Scholar]
- De Clippeleir H. et al. Long-chain acylhomoserine lactones increase the anoxic ammonium oxidation rate in an OLAND biofilm. Appl. Microbiol. Biot. 90, 1511–1519 (2011). [DOI] [PubMed] [Google Scholar]
- Toyofuku M. et al. Quorum sensing regulates denitrification in Pseudomonas aeruginosa PAO1. J. Bacteriol. 189, 4969–4972 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuster M., Lostroh C. P., Ogi T. & Greenberg E. Identification, timing, and signal specificity of Pseudomonas aeruginosa quorum-controlled genes: a transcriptome analysis. J. Bacteriol. 185, 2066–2079 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuqua C., Parsek M. R. & Greenberg E. P. Regulation of gene expression by cell-to-cell communication: acyl-homoserine lactone quorum sensing. Annu. Rev. Genet. 35, 439–468 (2001). [DOI] [PubMed] [Google Scholar]
- Miller M. B. & Bassler B. L. Quorum sensing in bacteria. Annu. Rev. Microbiol. 55, 165–199 (2001). [DOI] [PubMed] [Google Scholar]
- Williams P. Quorum sensing, communication and cross-kingdom signalling in the bacterial world. Microbiology 153, 3923–3938 (2007). [DOI] [PubMed] [Google Scholar]
- Parsek M. R., Val D. L., Hanzelka B. L., Cronan J. E. & Greenberg E. Acyl homoserine-lactone quorum-sensing signal generation. Proc. Natl. Acad. Sci. USA 96, 4360–4365 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng W.-L. & Bassler B. L. Bacterial quorum-sensing network architectures. Annu. Rev. Genet. 43, 197–222 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehead N. A., Barnard A. M., Slater H., Simpson N. J. & Salmond G. P. Quorum-sensing in Gram-negative bacteria. FEMS Microbiol. Rev. 25, 365–404 (2001). [DOI] [PubMed] [Google Scholar]
- Ahlgren N. A., Harwood C. S., Schaefer A. L., Giraud E. & Greenberg E. P. Aryl-homoserine lactone quorum sensing in stem-nodulating photosynthetic bradyrhizobia. Proc. Natl. Acad. Sci. USA 108, 7183–7188 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasser W. & Reverchon S. New insights into the regulatory mechanisms of the LuxR family of quorum sensing regulators. Anal. Bioanal. Chem. 387, 381–390 (2007). [DOI] [PubMed] [Google Scholar]
- Case R. J., Labbate M. & Kjelleberg S. AHL-driven quorum-sensing circuits: their frequency and function among the Proteobacteria. ISME J. 2, 345 (2008). [DOI] [PubMed] [Google Scholar]
- Mellbye B. L., Bottomley P. J. & Sayavedra-Soto L. A. nitrite-oxidizing bacterium Nitrobacter winogradskyi produces N-acyl-homoserine lactone autoinducers. Appl. Environ. Microbiol. 81, 5917–5926 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson W. T., Minogue T. D., Val D. L., von Bodman S. B. & Churchill M. E. Structural basis and specificity of acyl-homoserine lactone signal production in bacterial quorum sensing. Mol. Cell 9, 685–694 (2002). [DOI] [PubMed] [Google Scholar]
- Schaefer A. L., Taylor T. A., Beatty J. T. & Greenberg E. Long-chain acyl-homoserine lactone quorum-sensing regulation of Rhodobacter capsulatus gene transfer agent production. J. Bacteriol. 184, 6515–6521 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J., Chai Y., Zhong Z., Li S. & Winans S. C. Agrobacterium bioassay strain for ultrasensitive detection of N-acylhomoserine lactone-type quorum-sensing molecules: detection of autoinducers in Mesorhizobium huakuii. Appl. Environ. Microbiol. 69, 6949–6953 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewenza S., Conway B., Greenberg E. & Sokol P. A. Quorum sensing in Burkholderia cepacia: identification of the LuxRI homologs CepRI. J. Bacteriol. 181, 748–756 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotschlich A. et al. Synthesis of multiple N-acylhomoserine lactones is wide-spread among the members of the Burkholderia cepacia complex. Syst. Appl. Microbiol. 24, 1–14 (2001). [DOI] [PubMed] [Google Scholar]
- Lithgow J. K. et al. The regulatory locus cinRI in Rhizobium leguminosarum controls a network of quorum-sensing loci. Mol. Microbiol. 37, 81–97 (2000). [DOI] [PubMed] [Google Scholar]
- Penalver C. G. N. et al. Methylobacterium extorquens AM1 produces a novel type of acyl-homoserine lactone with a double unsaturated side chain under methylotrophic growth conditions. FEBS Lett. 580, 561–567 (2006). [DOI] [PubMed] [Google Scholar]
- Puskas A., Greenberg E. á., Kaplan S. & Schaefer A. á. A quorum-sensing system in the free-living photosynthetic bacterium Rhodobacter sphaeroides. J. Bacteriol. 179, 7530–7537 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin D., Grasland B., Vallée-Réhel K., Dufau C. & Haras D. On-line high-performance liquid chromatography-mass spectrometric detection and quantification of N-acylhomoserine lactones, quorum sensing signal molecules, in the presence of biological matrices. J. Chromatogr. A 1002, 79–92 (2003). [DOI] [PubMed] [Google Scholar]
- Nowka B., Daims H. & Spieck E. Comparison of oxidation kinetics of nitrite-oxidizing bacteria: nitrite availability as a key factor in niche differentiation. Appl. Environ. Microbiol. 81, 745–753 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul F. C. Steady-State enzyme kinetics in Encyclopedia of biophysics (ed. Gordon C. R.) 2466–2470 (Springer: Berlin Heidelberg,, 2013).
- Spieck E. et al. Two-dimensional structure of membrane-bound nitrite oxidoreductase from Nitrobacter hamburgensis. J. Struct. Biol. 117, 117–123 (1996). [Google Scholar]
- Zumft W. G. & Körner H. Enzyme diversity and mosaic gene organization in denitrification. Antonie van Leeuwenhoek 71, 43–58 (1997). [DOI] [PubMed] [Google Scholar]
- Bock E., Koops H. P., Harms H. & Ahlers B. The biochemistry of nitrifying organisms in Variations in autotrophic life (eds. Shively J. M. & Barton L. L.) 171–200 (Academic Press, 1991).
- Fekete A. et al. Dynamic regulation of N-acyl-homoserine lactone production and degradation in Pseudomonas putida IsoF. FEMS Microbiol. Ecol. 72, 22–34 (2010). [DOI] [PubMed] [Google Scholar]
- Wang F. et al. Soil remediation with a microbial community established on a carrier: Strong hints for microbial communication during 1, 2, 4-Trichlorobenzene degradation. Chemosphere 92, 1403–1409 (2013). [DOI] [PubMed] [Google Scholar]
- Cornforth D. M. et al. Combinatorial quorum sensing allows bacteria to resolve their social and physical environment. Proc. Natl. Acad. Sci. USA 111, 4280–4284 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooley M., Chhabra S. R. & Williams P. N-Acylhomoserine lactone-mediated quorum sensing: a twist in the tail and a blow for host immunity. Chem. Biol. 15, 1141–1147 (2008). [DOI] [PubMed] [Google Scholar]
- Wang L., Hashimoto Y., Tsao C. Y., Valdes J. J. & Bentley W. E. Cyclic AMP (cAMP) and cAMP receptor protein influence both synthesis and uptake of extracellular autoinducer 2 in Escherichia coli. J. Bacteriol. 187, 2066–2076 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayavedra-Soto L. et al. Nitrobacter winogradskyi transcriptomic response to low and high ammonium concentrations. FEMS Microbiol. Lett. 362, 1–7 (2015). [DOI] [PubMed] [Google Scholar]
- Tempé J., Petit A., Holsters M., Van Montagu M. & Schell J. Thermosensitive step associated with transfer of the Ti plasmid during conjugation: possible relation to transformation in crown gall. Proc. Natl. Acad. Sci. USA 74, 2848–2849 (1977). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuqua W. C. & Winans S. C. A LuxR-LuxI type regulatory system activates Agrobacterium Ti plasmid conjugal transfer in the presence of a plant tumor metabolite. J. Bacteriol. 176, 2796–2806 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao J., Ma A., Zhuang X. & Zhuang G. An N-acyl homoserine lactone synthase in the ammonia-oxidizing bacterium Nitrosospira multiformis. Appl. Environ. Microbiol. 80, 951–958 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elstner E. F. & Heupel A. Inhibition of nitrite formation from hydroxylammoniumchloride: a simple assay for superoxide dismutase. Anal. Biochem. 70, 616–620 (1976). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.