Abstract
Microbiology of a hypersaline oil reservoir located in Central Africa was investigated with molecular and culture methods applied to preserved core samples. Here we show that the community structure was partially acquired during sedimentation, as many prokaryotic 16S rRNA gene sequences retrieved from the extracted DNA are phylogenetically related to actual Archaea inhabiting surface evaporitic environments, similar to the Cretaceous sediment paleoenvironment. Results are discussed in term of microorganisms and/or DNA preservation in such hypersaline and Mg-rich solutions. High salt concentrations together with anaerobic conditions could have preserved microbial/molecular diversity originating from the ancient sediment basin wherein organic matter was deposited.
Subsurface environments harbor 1/10 to 1/3 of global living biomass, thus playing an important role in biogeochemical cycling of elements1,2. Subsurface oil-bearing reservoir rocks have been extensively studied during the last century with respect to the critical economic value of hydrocarbons. The probable rarefaction of this fossil resource and the possibility to sequestrate CO2 within depleted oil fields3 stimulate multidisciplinary scientific investigations including microbiology. Whereas the presence of microorganisms in oil reservoirs was recognized earlier4, the reality of their effective in situ activity remains elusive1. Biodegradation of organic matter in sedimentary rocks contributes to the biogeochemical cycling of carbon1 and strongly impacts the quality and exploitation of hydrocarbons1. Oil reservoirs are nutrient-depleted environments, especially lacking of phosphorus and nitrogen. They contain an excess of reduced electron donors (hydrocarbons) but a shortage of electron acceptors (e.g., nitrate, oxygen…) and are considered as anaerobic environments5,6,7. We characterized the indigenous microbiota of a hypersaline oil reservoir by molecular and cultural analyzes of rock samples, rather than from fluid samples (e.g. production fluid from well heads of oil producing wells) possibly affected by greater exogenous microbial contamination8. The core was collected from an onshore oil reservoir in Central Africa, before oil exploitation and any fluid injection.
Results
Core chemistry and mineralogy
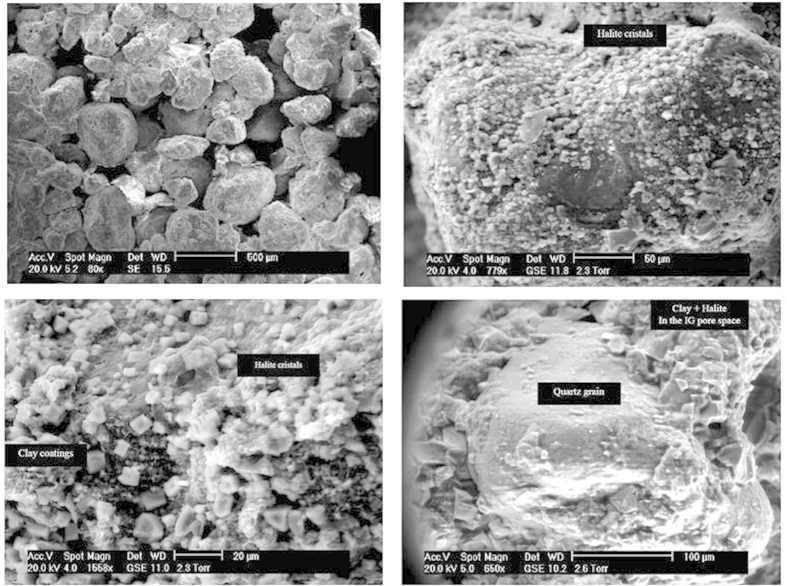
The core was sampled at 1153–1154 m depth in a lower Cretaceous sandstone, underlying the transgressive Gamba Formation and thick salt deposits9. Reservoir pressure and temperature were respectively 12 Mpa and 43 °C. These sediments are associated to the South Atlantic Aptian salt deposition contemporary of the basin creation resulting from the break-up between Africa and South America10. The unconsolidated sandstone consisted in quartz-felspar grains coated with green clays and salt crystals, without stratification. NaCl crystals were very abundant on the grain surfaces and within the clay coatings (Fig. 1). Intergranular cements, such as quartz, were absent. Most of the grains were coated by clays (smectite, chlorite, illite), which probably inhibited the development of quartz overgrowth in the intergranular pore space. Local chemical analyzes and X-ray diffraction on the bulk sample and on separated clays confirmed the nature of the minerals. Moreover, as chlorite minerals from the inner core were not oxidized, anaerobic conditions during coring, sample preservation (by aluminium barrel, freezing in liquid nitrogen), shipping to and transferring at the laboratory should have been preserved. In this respect, our sampling conditions should not have affected the composition of the existing microbial diversity of the inner section (6 cm) of the 10 cm core. Chemical analyzes are summarized in Table S1. Formation waters had salinity close to saturation and the core itself contained above 13 mg/g of Na+ (supplementary material, Table S1).
Figure 1. SEM image of the sand.
Quartz-felspar grains are coated with clays, salt (NaCl) crystal crystalized on the grain surfaces.
Bacterial and archaeal molecular diversities
16S rRNA gene surveys, including classical clone libraries analyses (case of Bacteria and Archaea) and high throughput sequencing methods (case of Bacteria) were conducted on DNA extracted directly from the core (Tables 1 and 2). The rarefaction curves from cloning (supplementary material Fig. S2) showed that the Rabi core harbored low archaeal diversity as saturation was obtained. But the Rabi core was inhabited by a more diverse bacterial community than the one estimated by the clone library as the rarefaction curve was not saturated, highlighting the need to perform deeper 16S rRNA gene sequencing to assess it.
Table 1. Diversity of Archaea and Bacteria retrieved in the core by 16S rRNA gene surveys.
| Clone | % in library | Closest related microorganism (% of similarity by blast) | Lineage | Main metabolic traits of the related microorganisms |
|---|---|---|---|---|
| Members of the Bacteria domain - Proteobacteria | ||||
| MIG-B2 | 50.0 | Ochrobactrum sp. (99) | Rhizobiales; Brucellaceae | Aerobic, mesophilic, possible degradation of crude oil by some Ochrobactrum spp. |
| MIG-B13 | 7.6 | Halomonas venusta (99) | Oceanospirillales; Halomonadaceae | Facultative anaerobic, halophilic, marine bacterium; possible degradation of crude oil by some Halomonas spp. |
| MIG-B23 | 4.3 | Halomonas meridiana (99) Halomonas aquamarina (99) | Oceanospirillales; Halomonadaceae | Facultative anaerobic, halophilic, marine bacterium; possible degradation of crude oil by some Halomonas spp. |
| MIG-B19 | 4.3 | Burkholderia ferrariae (99) Burkholderia silvatlantica (99) | Burkholderiales; Burkholderiaceae | Aerobic, mesophilic; possible degradation of alkanes and aromatic hydrocarbons by some Burkholderia spp. |
| MIG-B18 | 2.2 | Rhizobium loti (99) | Rhizobiales; Phyllobacteriaceae | Aerobic and mesophilic bacterium |
| MIG-B1 | 1.1 | Halomonas phoceae (97) Halomonas xinjiangensis (97) | Oceanospirillales; Halomonadaceae | Facultative anaerobic, halophilic, marine bacterium; possible degradation of crude oil by some Halomonas spp. |
| MIG-B9 | 1.1 | Chromohalobacter salexigens (99) | Oceanospirillales; Halomonadaceae | Aerobic, moderate halophilic, marine bacterium |
| Members of the Bacteria domain – Firmicutes, Actinobacteria, and Thermotogae | ||||
| MIG-B16 | 19.6 | Halolactibacillus halophilus (98) Halolactibacillus miuriensis (98) | Firmicute; Bacillales; Bacillaceae | Facultative anaerobic, halophilic and alkaliphilic marine lactic acid bacterium |
| MIG-B8 | 4.3 | Halanaerobium fermentans (99) | Firmicute; Halanaerobiales; Halanaerobiaceae | Anaerobic, halophilic fermentative bacterium |
| MIG-B25 | 2.2 | Orenia marismortui (95) | Firmicute; Halanaerobiales; Halobacteroidaceae | Anaerobic, halophilic fermentative bacterium |
| MIG-B42 | 1.1 | Orenia marismortui (95) | Firmicute; Halanaerobiales; Halobacteroidaceae | Anaerobic, halophilic fermentative bacterium |
| MIG-B15 | 1.1 | Microbacterium paraoxydans (99) | Actinobacteria Actinomycetales; Microbacteriaceae | Aerobic, mesophilic; possible degradation of crude oil by some Microbacterium spp. |
| MIG-B3 | 1.1 | Geotoga aestuarianus (99) | Thermotogales; Thermotogaceae | Anaerobic, thermophilic fermentative bacterium |
| Members of the Archaea domain - Euryarchaeota | ||||
| MIG-ARCH1 | 33.3 | Halococcus hamelinensis (99) | Halobacteriales | Aerobic, extreme halophile |
| MIG-ARCH6 | 30.6 | Haloplanus natans (98) | Halobacteriales | Aerobic, extreme halophile |
| MIG- ARCH2 | 16.7 | Halorhabdus tiamatea (97) Halorhabdus utahensis (97) | Halobacteriales | Facultative anaerobic (microaerobic) extreme halophile |
| MIG-ARCH27 | 16.7 | Halarchaeum acidiphilum (97) | Halobacteriales | Aerobic, extreme halophile |
| MIG-ARCH10 | 2.8 | Thermococcaceae (77) | Thermococcales | Anaerobic hyperthermophile |
Retrieved environmental 16S rRNA gene sequences are available under Genbank accession numbers JQ690672 to JQ690688.
Table 2. Diversity of Bacteria retrieved from the core using Illumina sequencing of the 16S rRNA gene.
| % in library | Closest relative* | Lineage | Main metabolic traits of the related microorganisms |
|---|---|---|---|
| 27.9 | Halomonas | Gammaproteobacteria; Oceanospirillales; Halomonadaceae | Facultative anaerobic, halophilic, marine bacterium; possible degradation of crude oil by some Halomonas spp. |
| 11.0 | Desulfonauticus | Deltaproteobacteria; Desulfovibrionales; Desulfohalobiaceae | Anaerobic, thermophilic sulfate-reducing bacteria isolated from oil-production water and deep-sea hydrothermal vents |
| 10.1 | Pseudomonas | Gammaproteobacteria; Pseudomonadales; Pseudomonadaceae | Aerobic, known to degrade aromatic hydrocarbons |
| 7.1 | Marinobacter | Gammaproteobacteria; Alteromonadales; Alteromonadaceae | Facultative anaerobic, halophilic, marine bacterium; possible degradation of crude oil by some Marinobacter spp. |
| 6.1 | n.d. | Gammaproteobacteria; Enterobacteriales; Enterobacteriaceae | Facultative anaerobic, many of them are nitrate reducers under anaerobic conditions |
| 5.7 | n.d. | Bacteroidia; Bacteroidales; | |
| 4.2 | n.d. | Clostridia; Clostridiales; Clostridiaceae | Primarily anaerobic; many of its genera are fermenters |
| 2.9 | n.d. | Deltaproteobacteria; Desulfovibrionales; | Obligatory anaerobic, mesophilic or moderately thermophilic sulfate reducers |
| 2.1 | n.d. | [Cloacamonae]; [Cloacamonales]; MSBL8 | Affiliated with Spirochaetes, anaerobic, halotolerant |
| 1.4 | Oceanobacillus | Bacilli; Bacillales; Bacillaceae | Halotolerant, alkaliphilic, mesophilic, facultative anaerobic or strictly aerobic bacterium |
| 1.2 | n.d. | Gammaproteobacteria; Oceanospirillales; Oceanospirillaceae | Most genera are halophilic, aerobic or facultative anaerobic, chemoorganotrophs |
| 1.1 | n.d. | Betaproteobacteria; Burkholderiales; Comamonadaceae | Aerobic organotrophs, anaerobic denitrifiers and Fe(III)-reducing bacteria, hydrogen oxidizers, photoautotrophic and photoheterotrophic bacteria, and fermentative bacteria, members are known to degrade hydrocarbons |
| 0.9 | Ralstonia | Betaproteobacteria; Burkholderiales; Oxalobacteraceae | Aerobic, can reduce nitrate anaerobically, found in both soil and water, used in bioremediation, chlorinated aromatic compounds degrader |
| 0.8 | Desulfovibrio | Deltaproteobacteria; Desulfovibrionales; Desulfovibrionaceae | Anaerobic, sulfate-reducer |
| 0.7 | Pelobacter | Deltaproteobacteria; Desulfuromonadales; Pelobacteraceae | Strictly anaerobic, Fe(III) or S(0) reducer |
| 0.6 | Halanaerobium | Clostridia; Halanaerobiales; Halanaerobiaceae | Anaerobic, halophilic fermentative bacterium |
| 0.4 | n.d. | Flavobacteria; Flavobacteriales; Flavobacteriaceae | |
| 0.4 | Thermovirga | Synergistia; Synergistales; Thermovirgaceae | Moderately thermophilic, anaerobic, sulfur reducing, amino-acid-degrading bacterium |
| 0.3 | Marinilactibacillus | Bacilli; Lactobacillales; Aerococcaceae | Facultative anaerobic, halophilic, alkaliphilic, marine, mesophilic, lactic acid producing bacterium |
| 6.2 | Unassigned | ||
| 9.0 | Other |
Sequences are available at BioProject ID PRJNA301727; n.d.: not determined.
Clone libraries analyses
Most of the bacterial OTUs (Operational Taxon Units) were related to aerobes and facultative anaerobes known to degrade oil compounds. From 92 clones analyzed, the library was dominated (50%) by OTU related to aerobic Ochrobactrum spp., of which some strains were found in crude oil or reported to degrade aliphatic and aromatic hydrocarbons11. Three OTUs (13% of the library) were related to facultative anaerobic, halophilic marine species of the Halomonas genus with H. shengliensis being reported to degrade crude oil12. A fourth Halomonadaceae OTU was related to the halophilic strain Chromohalobacter salexigens, formerly Halomonas elongata. As well, minor OTUs were related to aerobes of the genera Burkholderia (4.3% of the library) and Microbacterium (1.1% of the library) of which species are capable to aerobically degrade crude oil13,14.
Besides the Halomonas spp., other OTUs were also related to halophilic microbes, a physiological trait compatible with the in situ salinity. Indeed, the second most dominant OTU (19.6% of the library) was related to the marine genus Halolactibacillus recognized as facultative anaerobic halophiles15. We retrieved a member of the Firmicutes related to the Halanaerobium genus (4.3% of the library); members of this genus have been frequently recovered from oil reservoirs16,17 and some of them inhabit sebkhas similar to the paleoenvironment of Rabi sandstone18. We also report the occurrence of members of the genus Orenia, family Halobacteroidaceae. Interestingly, few representatives of this family have been retrieved by molecular approaches from oil reservoirs so far19. They include phylogenetic relatives of the genus Orenia recovered from hot reservoirs19 where their in situ activity is highly questionable. We found only one clone phylogenetically related to the anaerobic, thermophilic and halophilic genus Geotoga, order Thermotogales. Presence of members of this order is also recurrent in oil fields16.
Within the 36 archaeal clones analyzed, the large majority of OTUs belonged to the Halobacteriaceae family regrouping mainly extreme aerobic halophiles. Similar microorganisms have been already isolated from high saline reservoirs20. The library is dominated (>30%) by OTUs related to Halococcus hamelinensis and Haloplanus natans isolated from saline environments, respectively stromatolithes from Shark Bay, Australia21 and the Dead Sea22. Then, 16.7% of the OTUs belonged to the Halorhabdus genus; among the two described species within this genus, H. utahensis is a facultatively anaerobic, extremely halophilic archaeon isolated from the Great Salt Lake23, whereas H. tiamatea, isolated from a deep-sea anoxic basin of the Red Sea, grew under anaerobic or microaerobic conditions24. As well, 16.7% of the OTUs were related to the newly described aerobic and extreme halophilic archaeon Halarchaeum acidiphilum isolated from solar salt25. Beside mesophilic representatives, one OTU was related to the Thermococcaceae family only represented by hyperthermophilic microorganisms commonly found in hot and low saline ecosystems, including oil reservoirs16,19. The phylogenetic divergence (only 77% identity) with known Thermococcaceae members clearly showed that it represents a new lineage within this family. Very low diversity of archaeal sequences was assessed by the saturation of the rarefaction curve (Supplementary material Fig. S2).
Bacterial diversity estimated by high throughput sequencing
The most abundant OTU in the core (Table 2) was related to the marine, halophilic, facultative anaerobe Halomonas (27.9%). Some Halomonas spp. are reported to be involved in the degradation of crude oil12 and others are reported to originate from highly saline environments26. Besides Halomonas, a high proportion of OTUs was related to the thermophilic sulfate-reducing bacterium Desulfonauticus, family Desulfohalobiaceae (11%). Desulfonauticus spp. were previously isolated from oil production water and deep sea hydrothermal vents27,28. We have also identified a number of OTUs related to the genus Pseudomonas (10.1%) comprising species possibly degrading aromatic hydrocarbons both aerobically and anaerobically. Another genus that matched many of the produced OTUs was the facultative anaerobic, halophilic marine bacterium Marinobacter (7.1%) members of which are capable to also degrade petroleum hydrocarbons29.
In addition to Desulfonauticus mentioned above, we identified in moderate amounts some OTUs that are related to anaerobic mesophilic sulfate and/or sulfur reducers such as Desulfovibrio (0.8%), order Desulfovibrionales (2.9%), Pelobacter (0.7%), as well as to anaerobic thermophiles (e.g. Thermovirga, 0.4%). Members of these taxa have been already successfully isolated from petroleum hydrocarbon associated environments30,31,32. Additionally, fermentative bacteria such as those belonging to the genus Halanaerobium (0.6%), as already mentioned in the bacterial clone library, and the Clostridiaceae family (4.2%) were also found to have representative OTUs in the dataset. Halanaerobium has been previously found in formation water samples from an oil reservoir33 whereas members of the Clostridiaceae family are thought to be using hydrocarbon intermediates to generate organic acid precursors under sulfate, methanogenic or iron reducing conditions34,35,36. Other genera related to the produced OTUs include Oceanobacillus (1.4%), Ralstonia (0.9%), Marinilactibacillus (0.3%) as well as members of the families Enterobacteriaceae (6.1%), Clone MSBL8 (2.1%), Oceanospirillaceae (1.2%), Comamonadaceae (1.1%), Flavobacteriaceae (0.4%). Finally, OTUs related to members of the order Bacteroidales were also found in the sample at a relative high number (5.7%). The significance of many of the above taxa to the Rabi’s in situ reservoir conditions is still unknown as many of them are either still poorly characterized in the literature.
Culture experimentation
Neither sulfate reducers, nitrate reducers, sulfide oxidizers nor hydrogenotrophic and methylotrophic methanogens were encountered. Only fermentative Bacteria, and specially members of the phylum Firmicutes, representatives of the Bacillaceae and Halobacteroidaceae families (Table 3) were cultivated. Strains belonging to the genus Halanaerocella petrolearia of the family Halobacteroidaceae were isolated; this species is perfectly adapted to this environment as it grew anaerobically at high saline concentrations37. However, it was not recovered from the molecular surveys. Other isolates were also related to highly saline shallow or surface environments. It is the case of strains closely related to (i) Halobacillus trueperi commonly found in Tunisian sebkhas or chotts38, (ii) Orenia marismortui, which is described as adapted also to highly saline surface environments and degrading complex organic compounds39 and (iii) Thalassobacillus devorans, formerly isolated from hypersaline habitats and able to oxidize aromatic compounds40.
Table 3. Microbial diversity of isolates originating from the hypersaline oil reservoir and their condition of isolation.
| Isolates | Total | Closest phylogenetic relative | % blast similarity | Condition of isolation |
|---|---|---|---|---|
| RAH4 and FERM001 | 2 | Thalassobacillus devorans | 99–100 | 250 g.l−1 NaCl pH 8.8 anaerobiosis (Table S2) |
| FERM003 and AER001 | 2 | Bacillus circulans | 99–100 | 120 g.l−1 NaCl pH 8.8 anaerobiosis (Table S2) |
| 1D4 | 1 | Orenia marismortui | 99 | 250 g.l−1 NaCl pH 8.8 anaerobiosis (Table S2) |
| Delta1, Delta2 and Delta3 | 3 | Halanaerocella petrolearia (Strain S200T) | 100 | 250 g.l−1 NaCl pH 8.8 anaerobiosis (Tables S3 and S6) |
| Alpha | 1 | Halobacillus trueperi/dabanensis | 99 | 250 g.l−1 NaCl pH 7.5 anaerobiosis (Table S2) |
16S rRNA gene sequences of isolates are available under Genbank accession numbers JQ690689 to JQ690697.
Within archaeal clone library, mainly hyperhalophilic aerobic Archaea and facultative anaerobes appeared to be dominant from molecular studies, although the core was essentially anaerobic. However, despite repeated cultivation efforts, none of these archaeons were isolated thus suggesting that they are not metabolically active in the oil field, most probably because of strict anaerobic conditions prevailing in situ and limited access to organic matter restricted to hydrocarbons, together with the lack of suitable electron acceptors possibly used by some of these microorganisms (e.g. nitrate, fumarate).
Discussion
Clone library analysis and Illumina sequencing results gave two different pictures of molecular bacterial diversity. Expected strict anaerobes are found essentially from the Illumina sequencing where they account for a larger part of the diversity (27.6%) than in the clone library (8.7%). But the originality of both results is the abundance of aerobic and facultative anaerobic microorganisms in a strictly anaerobic environment. As expected when considering the number of reads, Illumina sequencing showed a greater diversity than the clone library. The applied protocol included a nested-PCR which, in addition to increase specificity, will also increase sensitivity. Sequences initially poorly amplified may be enriched after the nested-PCR step, resulting in a larger diversity retrieved by sequencing. As DNA used for high throughput sequencing came from the same core but after a longer storage time, we may also infer that Illumina sequencing platform, which amplify shorter fragments (440 bp) than one used for the construction of the clone library (1400 bp), has found target sequences on shorter genomic DNA fragments that have been degraded. A 2-step approach was however necessary to produce amplicons from the Rabi core DNA of sufficient yield and quality for Illumina sequencing. Even if acknowledging the introduction of biases, according to in-silico primer analysis, we estimated a limited reduction of coverage of the Bacteria lineages, especially if a one mismatch is allowed between primer and template (data not shown).
There is some recovery between molecular and cultural studies, namely the presence of fermentative Bacillaceae, Halanaerobiaceae and Halobacteroidaceae in the clone libraries (approximately 30% of the clone sequenced) and the effective culture of some representatives of these families. But we didn’t succeed in the cultivation of members of the Proteobacteria (about 70% of the clone library and 60% of the high throughput sequencing results), especially in the case of the Halomonas or Ochrobactrum genera. As the genus Ochrobactrum is solely detected in the clone library and not by the culture-dependent method or the Illumina sequencing, its presence may be due to an external contamination of the core, the genus Ochrobactrum being clearly retrieved from shallow environments like soils, followed by a PCR artefact. Furthermore, no Archaea was cultivated. Some studies reveal the successful isolation of Archaea from old and salty environments41,42. Isolates belonged to Halobacteriaceae and Haloferacaceae, as sequences retrieved from Rabi core. However, contrary to these environments, the Rabi core is strictly anaerobic and not suitable to sustain viability of these hyperhalophilic aerobic archaeons. It could also be due to the medium formulations or to specific niches being sampled. Discrepancies between culture-dependent and -independent methods targeting biological diversity are common in microbiological studies43,44. It could reflect our inability to mimic natural environmental conditions sufficiently to sustain growth, and/or our inability to extract and amplify correctly DNA from natural and industrial environments. The retrieved strict anaerobes as well as the facultative ones show traits compatible with the Rabi environment (ability to develop in the absence of oxygen and at high salinity) and have for some of them already been retrieved from oil reservoirs (e.g. Halanaerobium, Orenia, Geotoga, Desulfonauticus spp.). Concerning aerobes, there is increasing evidence of an aerobic community including hydrocarbon degraders being inhabitant of oil reservoirs and oil sand cores, one discussed reason would be the in situ availability of oxygen possibly originating from meteoric water45,46. As suggested in Head et al.45, a cryptic community using oxygen, possibly provided by water radiolysis may be present in some oil reservoirs. Despite, the in situ role of recovered aerobic microbes and especially the hyperhalophilic archaeons in the Rabi core is questionable; it is known that some of them are able to grow anaerobically using nitrate or fumarate as terminal electron acceptors. In this respect, unknown types of anaerobic metabolism to be performed by these microorganisms cannot be excluded.
Are they of ecological significance in this ecosystem or do they reflect the existing biodiversity during the sediment settling in the basin before the process of oil filling, resulting in a long-term dormancy? Two hypotheses could afford for the detection of aerobic microorganisms in an anaerobic, highly saline environment. First, microorganisms are not active and DNA has been preserved over millions of years47; second, microbial activity is scarce and community has been preserved in a slow-growing state for millions years48.
DNA preservation over geological times has been a matter of debate in the subsurface microbiology47,49,50. Although bacterial DNA recovery from historical samples can lead to full genome sequencing of ancient Bacteria51,52, there are nowadays few studies that report the effectiveness of very ancient (at least prior to the Holocene) microbial DNA recovery53,54. If fully hydrated DNA spontaneously decays over only hundreds of years55, extension of the half-life of intact DNA can be achieved by low temperature, high ionic strength, anoxic conditions and protection from enzymatic degradation56,57. High salt concentrations could thus be a great factor of biomolecule preservation. The salt-saturated solution filling the reservoir pores may explain the microbial diversity and the possible DNA preservation within the oil reservoir. Presence of physiological concentrations of K+ and Mg2+ strongly reduce thermal degradation of DNA56. Protective effect of salt on biological macromolecules such as tRNA is demonstrated at NaCl concentration between 0.5 and 3 M57. Effects of the extremely chaotropic and soluble MgCl2 salt have been studied in the deep sea hypersaline lake Discovery (eastern Mediterranean)58. MgCl2 concentration above 1.26 M inhibited the growth of all microorganisms taken from this environment. MgCl2 at high concentrations not only denatures macromolecules, but also preserves the more stable ones, like DNA58. Other results confirmed the preservation and the transforming power of DNA when solubilized in this type of brine59, thus being prone to PCR amplification and cloning. Highly saline and anaerobic solution like those of the studied reservoir (supplementary material, Table S1) can then be a potential reservoir for ancient DNA. Clays coating the grains could also favour the adsorption of DNA, thus preserving it from hydratation. Interestingly, archaeal isolates from Eocene rock salt were polyploid, with genome copy numbers of 11–14 genomes during exponential growth phase42. Abundance of DNA in single archaeal cells in Rabi paleoenvironment could explain preservation of archaeal DNA. This strong hypothesis should get more confirmation.
With regard to the second hypothesis, dormant or slow activity communities have been recovered from tenths to million years in a freshwater sediment60 or from a surface isolated lake in Antarctica48. Moreover, microbial survival related to fermentative activity rather than sporulation has been proven on an up to half a million years period due to fermentation processes in permafrost environment61. Both metabolic and phenotypic features should be taken into consideration to explain the presence of microorganisms retrieved by cultural and molecular methods in the reservoir (e.g. sporeformers of the order Bacillales). As fermentative Prokaryotes constitute the large majority of the microorganisms detected by each technique, and given the amount of reduced hydrocarbon present in the Rabi reservoir, this hypothesis can be viewed as probable as the first. The most important question in the case of the studied oil reservoir is whether the retrieved archaeal 16S rRNA genes belong to dormant Archaea that are part of the non-cultivated microorganisms, or are part to dead microorganisms which have no more chance to be cultivated. Presence of both possibly active microorganisms and microorganisms inherited from the time of the sediment deposition suggests that the microbial diversity integrates separate parts of the reservoir history.
Studies of microbial diversity from a core rather from production fluids (e.g. formation water) in oil fields are rare and may not be accurate for comparison with our study, due to the difference in salinity. However, there are few reports on the predominance of aerobic microorganisms in oil reservoirs that are generally inhabited by strict anaerobes including sulfate-reducers and methanogens6,7. The reservoir rock studied here belongs to a cretaceous formation dominated by evaporitic conditions9. Protective presence of salt, adsorption on clays and low water activity could have preserved macromolecules such as DNA56,57,59 and may therefore explain the repeated molecular detection of aerobic hyperhalophilic Archaea. In this respect, we believe that molecular analyzes in particular provide a frozen picture of the past microbial community existing in the saline sedimentary basin, which is no more active in the hypersaline reservoir studied. This hypothesis is strengthened by the presence of archaeal DNA sequences that are phylogenetically related to those retrieved from actual hypersaline ecosystems, but also from cultivated haloarchaea originating from similar extreme environments. In addition, the anaerobic halophilic bacterium that we have isolated pertains to the family Halobacteroidaceae (e.g. Halobacteroides spp.), which are common inhabitants of terrestrial saline ecosystems. In contrast, there are several examples of isolation from oil reservoirs in literature of bacteria pertaining to the family Halanaerobiaceae (e.g. Halanaerobium genus)7, which have been retrieved only by molecular approaches during the course of this study. In this respect, the novel isolated halophilic anaerobe37 might have been a microbial remnant of the original microbial community in the sedimentary basin. Finally, we demonstrate here that studying the microbiology of deep subsurface cores may be of geobiological significance by delivering important information on the existing microbial diversity at surface several millions years ago.
Methods
Core handling
A core was sampled within aluminum sleeves at 1153–1554 m depth below surface and immediately frozen (−80 °C) on the rigsite with liquid nitrogen. Reservoir pressure and temperature were respectively 12 Mpa and 43 °C. The core was defrosted in an anaerobic box glove chamber to ensure that no oxygen could impede development of anaerobic microorganisms. The chamber was decontaminated (bactericide and ethanol) and only sterilized materials or materials cleaned with a bactericide were introduced in order to preserve the core from any contamination during the sub-samples preparation. Only the inner part of the core (excluding ca. 2 cm in a 10 cm diameter core) was analyzed to avoid contamination by the drilling fluid. The full preservation of such unconsolidated sandstone witnesses the lack of drilling fluid invasion in the core.
Petrographical and chemical analyses
Samples were observed under binocular for macroscopic observation, and with SEM (Philips) for ultramicroscopic analysis. X-ray diffraction was performed on a Philips θ–2θ (PW1050/81, PW3710) diffractometer. Clays were separated from the bulk sample by ultrasonication after hydrogen peroxide oxidization and grains (quartz-felspar) sedimentation.
Porosity and permeability of the sandstone were respectively between 24% and 32% and 1 to 3.5 Darcy. The water content was 12.9%. The fluid had an alkaline pH value of 8.8. Organic carbon, measured on a Flash EA CHNS/O analyzer (Thermo-scientific), was 0.38%. Cations (Ca2+, Na+, K+, Mg2+) and metals (Fe, Mn, Co, Cu, Pb, Zn) were measured by ICP-AES, Jobin Yvon JY2000 Ultrace after mineralization of the sample. Anions (Cl−, NO3−, SO42−) were determined by ionic chromatography Dionex DX100. Phosphorus was measured by the Joret-Hébert method (NF X31 161), which consists of an extraction of phosphorus with oxalate before optical reading at 825 nm. Chemical analyses on the core material are given in the supplementary material Table S1.
Dna extraction
A portion of the sediment was washed with the aim to remove hydrocarbons and PCR inhibitory material and improve DNA recovery and efficiency of PCR amplification62. Washing was carried out by re-suspension in wash solution (2 mL per g of sample), vortexing for 2 min, followed by centrifugation at full speed for 5 min in a bench top microfuge (14,000 g). Three successive washes were performed in wash solution 1 (50 mM Tris–HCl pH 8.3, 200 mM NaCl, 5 mM Na2EDTA, 0.05% Triton X-100), then in wash solution 2 (50 mM Tris–HCl pH 8.3, 200 mM NaCl, 5 mM Na2EDTA), and finally in wash solution 3 (50 mM Tris–HCl pH 8.3, 0.1 mM Na2EDTA). Despite there can be some potential loss of cell during these washing steps, this procedure was performed as it provided greater DNA quantities (data not shown). Bacterial genomic DNA was extracted from the washed sediment with the Fast DNA Spin Kit for Soil (Bio101) and the UltraClean Mega Soil DNA isolation kit (MoBio). The manufacturers protocols were slightly modified: as samples contained a priori low biomass, Poly-dIdC (polydexoyinosinic-deoxycytidylic acid, Sigma), a synthetic nucleotide acting both as a blocking- and carrier-agent was added at the first step of the DNA extraction procedure63. DNA extracts were pooled and concentrated for subsequent PCR experiments.
For identifying members of the Bacteria domain, 16S rRNA genes were amplified with bacterial primer 8F (5′-AGAGTTTGATCMTGGCTCAG-3′) and universal primer 1406R (5′- GACGGGCGGTGTGTRCA-3′), 30 cycles and hybridization at 55 °C. For identifying Archaea members, domain specific primers Arch363F (5′-ACGGGGYGCAGCAGGCGCGA-3′) and Arch915R (5′-GTGCTCCCCCGCCAATTCCT-3′), 35 cycles and hybridization at 65 °C were used.
Clone libraries generation and screening
16S rRNA gene libraries were constructed from gel-purified (Nucleospin extract II, Macherey-Nagel) PCR products using the TOPO-TA Cloning Kit for Sequencing (Invitrogen). The gene libraries were screened for correct-length inserts by direct PCR amplification from a colony, using primers T3 and T7 targeting the plasmid. The V3 region of the insert of 92 Bacteria clones and 36 Archaea clones was then amplified respectively with primers w49/w34FAM or w36/w34FAM using the amplified inserts as templates, and grouped by identical CE-SSCP migration pattern, i.e. identical sequence. Selected plasmids were purified (Nucleospin Multi-8 Plus, Macherey-Nagel) and the insert was sequenced (Sanger sequencing) by Genome Express (France) with primers T7 and T3. OTU were determined by identical CE-SSCP pattern or 98% 16S rRNA gene sequence similarity.
Sequence analyses
Sequence manipulations, analyses and alignments were performed using the BioEdit program (http://www.mbio.ncsu.edu/BioEdit/bioedit.html). Similarities of concensus 16S rRNA gene nucleotide sequences with sequences available in the Genbank database were determined using the BLASTN program (www3.ncbi.nlm.nih.gov/BLAST).
Amplicon preparation for next-generation sequencing
Amplicons of the 16S rRNA gene suitable for sequencing on a MiSeq (Illumina) v3 kit (2 × 300bp) were produced using a nested PCR approach. During the first PCR reaction the nearly full length 16S RNA gene was amplified using the bacterial primers 8F (5′-AGAGTTTGATYMTGGCTCAG-3′) and 1492R (5′-TACCTTGTTAYGACTT-3′) for 30 cycles and hybridization at 52 °C. This step was then followed by a second PCR round using the universal primer pair 515F (5′-GTGNCAGCMGCCGCGGTAA-3′) and 926R (5′-CCGYCAATTYMTTTRAGTTT-3′) for 20 cycles and hybridization at 55 °C. The produced 440 bp long amplicons were then subjected to a third PCR amplification round using the 515MiSeq.F (5′-TCGTCGGCAGCGTCAGATGTGTATAAGAGACAGGTGNCAGCMGCCGCGGTAA-3′) and 926MiSeq.R (5′-GTCTCGTGGGCTCGGAGATGTGTATAAGAGACAGCCGYCAATTYMTTTRAGTTT-3′) primer pair which introduced Illumina’s overhang adaptors. Using Illumina’s Nextera Index kit, a final round of PCR amplification was then performed to introduce the indexes and remaining part of the Illumina’s adaptors according to the manufacturer’s recommendations.
The produced amplicons were cleaned up using the AMPure XP beads (Agencourt) and quantified on a Qubit fluorometer (Life Technologies) prior to pooling and sequencing on a MiSeq instrument (Illumina).
Sequence analysis of miseq reads
The produced 183,776 paired-end reads were quality filtered, pair-end joined, demultiplexed and analyzed using the QIIME 1.8.0 package64. OTUs were picked using the uclast method65 at the 97% threshold. Taxonomic assignments were performed against the Greengenes database (May 2013 version).
Cultivation
A basal medium was defined (see supplementary Table S4) and additives were added to isolate various physiological groups possibly thriving in such extreme habitats, e.g. yeast extract, biotrypcase and glucose for isolating fermentative microorganisms; Na2SO4 and H2/CO2 gas phase for isolating hydrogenotrophic sulfate reducers; Na2SO4 and lactate for isolating sulfate reducers oxidizing lactate; succinate and NO3− for isolating nitrate reducers; TMA (trimethylamine) or methanol for isolating methylotrophic methanogens. Media were also designed to isolate anaerobic sulfide-oxidizers and aerobic thiosulfate oxidizers. With the first results of molecular diversity becoming available, aerobic enrichments from core samples were also performed for isolation of aerobic and anaerobic halophilic microorganisms (see supplementary Tables S5–S7). Media sterilized by filtration, and not autoclaving were also used, to prevent Maillard reaction that could impede prokaryotic development (see supplementary Table S6). pH was fixed at 8.8 (pH measured from the sediment), but 7.5, a close neutral value, was also tested, as both alkaline pH and highly saline conditions may limit growth and even survival of anaerobic micro-organisms. Temperature was fixed at 43 °C (in situ temperature).
Exclusion of contamination
Several criteria can be used to evaluate if an isolate or a 16S rRNA gene sequence can be considered as native to the formation of interest or not. This includes the sampling technique as discussed above. For instance, production waters collected from separators and wellhead samples have a higher probability to contain exogenous contamination than core samples6. Coring was performed prior to any fluid injection in the oil field, which prevented contamination by any anthropogenic fluid. Specifically designed techniques for coring in soft materials and adapted to integrity preservation were used (aluminum sleeves, adequate drilling parameters and fluid pressure). Moreover, freezing of the core on the rigsite in liquid nitrogen will have prevented further chance of chemical and microbiological contamination. Microscopic and SEM observations of the core did not show any physical evidence (disruption, mineralogical contamination) of penetration of the drilling fluid into the core. As the clays from core samples were green and not oxidized, it seems that coring processes did not affect physico-chemical conditions of the reservoir. Only the inner part (6 cm) of the core (10 cm) was inoculated into culture media or submitted to DNA extraction. Community structures (molecular CE-SSCP fingerprints of 16S rRNA genes, data not shown) of inner and outer (2–3 cm) compartments were highly similar, supporting the absence of detectable exogenous microbe contamination by the drilling fluid. Furthermore, microbes retrieved from the molecular surveys were related to organisms showing physiological features compatible to in situ conditions. Another possibility to evaluate the contamination risk is to compare physiological adaptation of isolated microorganisms to the in situ physico-chemical conditions. The optimum temperature for growth of a microorganism isolated from an oil reservoir can be a good indicator of its physiological adaptation to the environment if it corresponds to the in situ temperature. In high saline reservoirs, comparison of salt tolerance or dependance of isolates can be compared to in situ water salinity as well. Culture optimal conditions (temperature 25–47 °C; NaCl 10–26%) of a strain isolated from the sediment and closely affiliated to the Halobacteroides genus were representative of the in situ conditions. All the halophilic microorganisms that we have isolated require subsequent concentrations of NaCl for growing, lowering the probability of microbial contamination during sample handling.
Additional Information
How to cite this article: Gales, G. et al. Preservation of ancestral Cretaceous microflora recovered from a hypersaline oil reservoir. Sci. Rep. 6, 22960; doi: 10.1038/srep22960 (2016).
Supplementary Material
Acknowledgments
We thank the petroleum company SHELL for its financial support.
Footnotes
Author Contributions C.J. and S.C. conducted the molecular analyses with support from G.G. and I.N. High throughput sequence analyses were performed by N.T. Under the supervision of B.O., D.A., G.G. and I.N performed the isolation of the strains. G.G. and J.B. conducted the petrophysical observations. B.L. provided the sample and data from the studied oil field. G.G., C.J. and B.O. wrote the manuscript with input from all of the authors. C.J., G.G., D.M. and J.B. initially built the research project financed by SHELL Company.
References
- Head I. M., Jones D. M. & Larter S. R. Biological activity in the deep subsurface and the origin of heavy oil. Nature 426, 344–352 (2003). [DOI] [PubMed] [Google Scholar]
- Whitman W. B., Coleman D. C. & Wiebe W. J. Prokaryotes: the unseen majority. Proc. Natl. Acad. Sci. USA 95, 6578–6583 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matter J. M. & Kelemen P. B. Permanent storage of carbon dioxide in geological reservoirs by mineral carbonation. Nature Geoscience 2, 837–841 (2009). [Google Scholar]
- Bastin E. S., Greer F. E., Merritt C. A. & Moulton G. The presence of sulphate reducing bacteria in oil fields waters. Science 63, 21–24 (1926). [DOI] [PubMed] [Google Scholar]
- Van Hamme J. D., Singh A. & Ward O. P. Recent advances in petroleum microbiology. Microbiol. Mol. Biol. Rev. 67, 503–49 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ollivier B. & Alazard D. The oil reservoir ecosystem. In Handbook of Hydrocarbon Microbiology. Part 4: Microbial Interactions with Hydrocarbons, Oils, Fats and Related Hydrophobic Substrates and Products (Timmis K.N. ed), 2259–2269 (Springer Verlag 2010). [Google Scholar]
- Ollivier B., Borgomano J. & Oger P. Petroleum: from formation to microbiology. In Microbial life of the deep biosphere (eds Kallmeyer J. & Wagner D.), Ch. 8, 161–185 (De Gruyter 2014). [Google Scholar]
- Basso O. et al. The effect of cleaning and disinfecting the sampling well on the microbial communities of deep subsurface water samples. Environ. Microbiol. 7, 13–21 (2005). [DOI] [PubMed] [Google Scholar]
- Brink A. H. Petroleum Geology of Gabon Basin. Am. Assoc. Pet. Geol. Bull. 58, 216–235 (1974). [Google Scholar]
- Burke K. & Engör C. Ten meters global sea-level change associated with South Atlantic Aptian salt deposition. Mar. Geol. 83, 309–312 (1988). [Google Scholar]
- Yoshida N. et al. Bacterial communities in petroleum oil in stockpiles. J. Biosci. Bioeng. 99, 143–149 (2005). [DOI] [PubMed] [Google Scholar]
- Wang Y.-N. et al. Halomonas shengliensis sp. nov., a moderately halophilic, denitrifying, crude-oil-utilizing bacterium. Intl. J. Syst. Evol. Microbiol. 57, 1222–1226 (2007). [DOI] [PubMed] [Google Scholar]
- Okoh A., Ajisebutu S., Babalola G. & Trejo-Hernandez M. R. Potential of Burkholderia cepacia RQ1 in the biodegradation of heavy crude oil. Int. Microbiol. 4, 83–87 (2001). [DOI] [PubMed] [Google Scholar]
- Schippers A. et al. Microbacterium oleivorans sp. nov. and Microbacterium hydrocarbonoxydans sp. nov., novel crude-oil-degrading Gram-positive bacteria. Intl. J. Syst. Evol. Microbiol. 55, 655–660 (2005). [DOI] [PubMed] [Google Scholar]
- Cao S. J., Qu J. H., Yang J. S., Sun Q. & Yuan H. L. Halolactibacillus alkaliphilus sp. nov., a moderately alkaliphilic and halophilic bacterium isolated from a soda lake in Inner Mongolia, China, and emended description of the genus Halolactibacillus. Int. J. Syst. Evol. Microbiol. 58, 2169–2173 (2008). [DOI] [PubMed] [Google Scholar]
- Ollivier B. & Cayol J.-L. The fermentative, iron-reducing, and nitrate-reducing microorganisms. In Petroleum Microbiology (eds Ollivier B. & Magot M.), Ch. 5, 71–88 (ASM press, 2005). [Google Scholar]
- Ravot G. et al. Haloanaerobium congolense sp. nov., an anaerobic, moderately halophilic, thiosulfate- and sulfur-reducing bacterium from an African oil field FEMS Microbiol. Lett. 147, 89–95 (1997). [DOI] [PubMed] [Google Scholar]
- Abdeljabbar H. et al. Halanaerobium sehlinense sp. nov., an extremely halophilic, fermentative, strictly anaerobic bacterium from sediments of the hypersaline lake Sehline Sebkha. Intl. J. Syst. Evol. Microbiol. 63, 2069–74 (2013). [DOI] [PubMed] [Google Scholar]
- Dahle H. et al. Microbial community structure analysis of produced water from a high-temperature North Sea oil-field. Antonie Van Leeuwenhoek 93, 37–49 (2008). [DOI] [PubMed] [Google Scholar]
- Zviagintseva I. S. et al. Halophilic archaebacteria from the Kalamkass oilfield. Mikrobiologiya 64, 83–7 (1995). [PubMed] [Google Scholar]
- Goh F. et al. Halococcus hamelinensis sp. nov., a novel halophilic archaeon isolated from stromatolites in Shark Bay, Australia. Intl. J. Syst. Evol. Microbiol. 56, 1323–1329 (2006). [DOI] [PubMed] [Google Scholar]
- Bardavid R. E., Mana L. & Oren A. Haloplanus natans gen. nov., sp. nov., an extremely halophilic, gas-vacuolate archaeon isolated from Dead Sea–Red Sea water mixtures in experimental outdoor ponds. Intl. J. Syst. Evol. Microbiol. 57, 780–783 (2007). [DOI] [PubMed] [Google Scholar]
- Waino M., Tindall B. J. & Ingvorsen K. Halorhabdus utahensis gen. nov., sp. nov., an aerobic, extremely halophilic member of the Archaea from Great Salt Lake, Uta. Intl. J. Syst. Evol. Microbiol. 50, 183–190 (2000). [DOI] [PubMed] [Google Scholar]
- Antunes A. et al. Halorhabdus tiamatea sp. nov., a non-pigmented, extremely halophilic archaeon from a deep-sea, hypersaline anoxic basin of the Red Sea, and emended description of the genus Halorhabdus. Int. J. Syst. Evol. Microbiol. 58, 215–220 (2008). [DOI] [PubMed] [Google Scholar]
- Minegishi H., Echigo A., Nagaoka S., Kamekura M. & Usami R. Halarchaeum acidiphilum gen. nov., sp. nov., a moderately acidophilic haloarchaeon isolated from commercial solar salt. Intl. J. Syst. Evol. Microbiol. 60, 2513–2516 (2010). [DOI] [PubMed] [Google Scholar]
- Quillaguaman J., Hatti-Kaul R., Mattiasson B., Alvarez M. T. & Delgado O. Halomonas boliviensis sp. nov., an alkalitolerant, moderate halophile isolated from soil around a Bolivian hypersaline lake. Intl. J. Syst. Evol. Microbiol. 54, 721–725 (2004). [DOI] [PubMed] [Google Scholar]
- Piceno Y. M. et al. Temperature and injection water source influence microbial community structure in four Alaskan North Slope hydrocarbon reservoirs. Front. Microbiol. 7, 405–409 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Audiffrin K. et al. Desulfonauticus submarinus gen. nov., sp. nov., a novel sulfate-reducing bacterium isolated from a deep-sea hydrothermal vent. Intl. J. Syst. Evol. Microbiol. 53, 1585–1590 (2003). [DOI] [PubMed] [Google Scholar]
- Fathepure B. Z. Recent studies in microbial degradation of petroleum hydrocarbons in hypersaline environments. Front Microbiol. 5, 173 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feio M. J. et al. Desulfovibrio alaskensis sp. nov., a sulphate-reducing bacterium from a soured oil reservoir. Intl. J. Syst. Evol. Microbiol. 54, 747–52 (2004). [DOI] [PubMed] [Google Scholar]
- Zapata-Penasco I. et al. Bisulfite reductase and nitrogenase genes retrieved from biocorrosive bacteria in saline produced waters of offshore oil recovery facilities. Int. Biodet. Biodeg. 81, 17–27 (2012). [Google Scholar]
- Dahle H. & Birkeland N. K. Thermovirga lienii gen. nov., sp. nov., a novel moderately thermophilic, anaerobic, amino-acid-degrading bacterium isolated from a North Sea oil well. Intl. J. Syst. Evol. Microbiol. 56, 1539–1545 (2006). [DOI] [PubMed] [Google Scholar]
- de Oliveira V. M., Sette L. D., Marques Simioni K. C. M & dos Santos Neto E. V. Bacterial diversity characterization in petroleum samples from Brazilian reservoirs. Braz. J. Microbiol. 39, 445–52 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherry A. et al. Anaerobic biodegradation of crude oil under sulphate-reducing conditions leads to only modest enrichment of recognized sulphate-reducing taxa. Int. Biodet. & Biodeg. 81, 105–113 (2013). [Google Scholar]
- Gieg L. M., Duncan K. E. & Suflita J. M. Bioenergy production via microbial conversion of residual oil to natural gas. Appl. Environ. Microbiol. 74, 3022–3029 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunapuli U., Lueders T. & Meckenstock R. U. The use of stable isotope probing to identify key iron-reducing microorganisms involved in anaerobic benzene degradation. ISME J. 1, 643–653. (2007). [DOI] [PubMed] [Google Scholar]
- Gales G. et al. Characterization of Halanaerocella petrolearia gen. nov., sp. nov., a new anaerobic moderately halophilic fermentative bacterium isolated from a deep subsurface hypersaline oil reservoir. Extremophiles 15, 565–571 (2011). [DOI] [PubMed] [Google Scholar]
- Guesmi et al. Uneven distribution of Halobacillus trueperi species in arid natural saline systems of Southern Tunisian Sahara. Microb. Ecol. 66, 831–839 (2013). [DOI] [PubMed] [Google Scholar]
- Oren A., Gurevich P. & Henis Y. Reduction of nitrosubstituted aromatic compounds by the halophilic anaerobic eubacteria Haloanaerobium praevalens and Sporohalobacter marismortui. Appl. Environ. Microbiol. 57, 3367–3370 (1991). [DOI] [PMC free article] [PubMed] [Google Scholar]
- García M. T., Gallego V., Ventosa A. & Mellado E. Thalassobacillus devorans gen. nov., sp. nov., a moderately halophilic, phenol-degrading, Gram-positive bacterium. Intl. J. Syst. Evol. Microbiol. 55, 1789–95 (2005). [DOI] [PubMed] [Google Scholar]
- Park J. S. Haloarchaeal diversity in 23, 121 and 419 MYA salts. Geobiology 7, 515–23 (2009). [DOI] [PubMed] [Google Scholar]
- Jaakkola S. T. et al. Halophilic archaea cultivated from surface sterilized middle-late eocene rock salt are polyploid. PLoS One 9, e110533 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabowski A., Nercessian O., Fayolle F., Blanchet D. & Jeanthon C. Microbial diversity in production waters of a low-temperature biodegraded oil reservoir. FEMS Microbiol. Ecol. 54, 427–443 (2005). [DOI] [PubMed] [Google Scholar]
- Kaster K. M., Bonaunet K., Berland H., Kjeilen-Eilertsen G. & Brakstad O. G. Characterization of culture-independent and -dependent microbial communities in a high-temperature offshore chalk petroleum reservoir. Antonie Van Leeuwenhoek 96, 423–439 (2009). [DOI] [PubMed] [Google Scholar]
- Head I. M., Gray N. D. & Larter S. R. Life in the slow lane; biogeochemistry of biodegraded petroleum containing reservoirs and implications for energy recovery and carbon management. Frontiers in Microbiology 5, 566. 10.3389/fmicb.2014.00566 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- An D. et al. Metagenomics of hydrocarbon resource environments indicates aerobic taxa and genes to be unexpectedly common. Environmental Science and Technology 47, 10708–10717 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inagaki F., Okada H., Tsapin A. I. & Nealson K. H. Microbial survival: the paleome: a sedimentary genetic record of past microbial communities. Astrobiology 5, 141–53 (2005). [DOI] [PubMed] [Google Scholar]
- Mikucki J. A. et al. A contemporary microbially maintained subglacial ferrous “ocean”. Science 324, 397–400 (2009). [DOI] [PubMed] [Google Scholar]
- Hoehler T. M. Cretaceous Park? A commentary on microbial paleomics. Astrobiology 5, 141–153 (2005). [DOI] [PubMed] [Google Scholar]
- Damsté J. S. & Coolen M. J. Fossil DNA in cretaceous black shales: myth or reality? Astrobiology 6, 299–302. (2006). [DOI] [PubMed] [Google Scholar]
- Bos K. I. et al. A draft genome of Yersinia pestis from victims of the Black Death. Nature 478, 506–510 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuenemann V. J. et al. Genome-Wide Comparison of Medieval and Modern Mycobacterium leprae. Science 341, 179–183 (2013). [DOI] [PubMed] [Google Scholar]
- Coolen M. J. & Overmann J. 217 000-year-old DNA sequences of green sulfur bacteria in Mediterranean sapropels and their implications for the reconstruction of the paleoenvironment. Environ. Microbiol. 9, 238–249 (2007). [DOI] [PubMed] [Google Scholar]
- Panieri G. et al. Ribosomal RNA gene fragments from fossilized cyanobacteria identified in primary gypsum from the late Miocene, Italy. Geobiology 8, 101–111 (2010). [DOI] [PubMed] [Google Scholar]
- Hofreiter M., Serre D., Poinar H. N., Kuch M. & Pääbo S. Ancient DNA. Nat. Rev. Genet. 2, 353–9 (2001). [DOI] [PubMed] [Google Scholar]
- Marguet E. & Forterre P. Protection of DNA by salts against thermodegradation at temperatures typical for hyperthermophiles. Extremophiles 2, 115–122 (1998). [DOI] [PubMed] [Google Scholar]
- Tehei M. et al. The search for traces of life: the protective effect of salt on biological macromolecules. Extremophiles 6, 427–430 (2002). [DOI] [PubMed] [Google Scholar]
- Hallsworth J. E. et al. Limits of life in MgCl2-containing environments: chaotropicity defines the window. Environ. Microbiol. 9, 801–813 (2007). [DOI] [PubMed] [Google Scholar]
- Borin S. et al. DNA is preserved and maintains transforming potential after contact with brines of the deep anoxic hypersaline lakes of the Eastern Mediterranean Sea. Saline Syst. 4, 10 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jørgensen K. S. & Tiedje J. M. Survival of denitrifiers in nitrate-free, anaerobic environments. Appl. Environ. Microbiol. 59, 3297–3305 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson S. S. et al. Ancient bacteria show evidence of DNA repair. Proc. Natl. Acad. Sci. USA 104, 14401–14405 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortin N. et al. Soil washing improves the recovery of total community DNA from polluted and high organic content sediments. J. Microbiol. Met. 56, 181–191 (2004). [DOI] [PubMed] [Google Scholar]
- Barton H. A. et al. DNA extraction from low-biomass carbonate rock: an improved method with reduced contamination and the low-biomass contaminant database. J. Microbiol. Met. 66, 21–31 (2006). [DOI] [PubMed] [Google Scholar]
- Caporaso J. G. et al. QIIME allows analysis of high-throughput community sequencing data. Nature Methods 7, 335–336 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar R. C. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 26, 2460–2461 (2010). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.