SUMMARY
Baylisascaris procyonis, the raccoon roundworm, infects a wide range of vertebrate animals, including humans, in which it causes a particularly severe type of larva migrans. It is an important cause of severe neurologic disease (neural larva migrans [NLM]) but also causes ocular disease (OLM; diffuse unilateral subacute neuroretinitis [DUSN]), visceral larva migrans (VLM), and covert/asymptomatic infections. B. procyonis is common and widespread in raccoons, and there is increasing recognition of human disease, making a clinical consideration of baylisascariasis important. This review provides an update for this disease, especially its clinical relevance and diagnosis, and summarizes the clinical cases of human NLM and VLM known to date. Most diagnosed patients have been young children less than 2 years of age, although the number of older patients diagnosed in recent years has been increasing. The recent development of recombinant antigen-based serodiagnostic assays has aided greatly in the early diagnosis of this infection. Patients recovering with fewer severe sequelae have been reported in recent years, reinforcing the current recommendation that early treatment with albendazole and corticosteroids should be initiated at the earliest suspicion of baylisascariasis. Considering the seriousness of this zoonotic infection, greater public and medical awareness is critical for the prevention and early treatment of human cases.
INTRODUCTION
Baylisascariasis is a zoonotic infection caused by larvae of the raccoon roundworm, Baylisascaris procyonis (1, 2, 3). This parasite has gained increasing attention as an important cause of larva migrans disease in humans and animals, with a steady increase in recognized human cases (4–8). The severity of reported human infections, and the importance of B. procyonis as a pathogen of wildlife and domestic-animal species (1, 9), makes the study of baylisascariasis a priority for public health, veterinary, and wildlife management officials.
A wide range of vertebrate orders have been reported to host B. procyonis in the larval stage, and it is currently considered to be a significant cause of neural larva migrans (NLM) and ocular larva migrans (OLM) in animals and humans (1, 2, 6–9). The most important etiologic agents of visceral larva migrans (VLM) and OLM in humans are the Toxocara species, especially T. canis (10, 11). However, the involvement of other ascarid worms was hypothesized first by Beaver (12) and later by Anderson et al. in the first report of a probable case of human neurologic baylisascariasis (13). One key difference is that toxocariasis usually does not manifest as overtly severe central nervous system (CNS) disease (10), although there is mounting evidence that CNS Toxocara infection may result in various neuropsychiatric and neurodegenerative disorders in humans; see the excellent review by Fan et al. (11). Several of these clinical problems have also been seen in cases of CNS baylisascariasis, and the entire area of progression to neurodegeneration subsequent to brain injury from these parasites demands greater attention, especially considering their ubiquity near humans (2, 6, 9, 11).
In recent years, data regarding the epidemiology, transmission, pathogenesis, diagnosis, and treatment of baylisascariasis have been rapidly accumulating. Here, we provide an update on the most relevant aspects of this disease and provide an overview and summary of the human neurologic and visceral infections in the United States and Canada that have been recorded since 1975.
BIOLOGY, LIFE CYCLE
B. procyonis is a large, tan-colored roundworm with small cervical alae. Adult females are 20 to 22 cm long, and males are 9 to 11 cm long and have distinct pericloacal roughened areas. Ellipsoidal eggs produced by the females measure 63 to 88 μm by 50 to 70 μm. The eggs are thick shelled and golden brown and have a distinct surface morphology, i.e., a finely granular protein coat (1) (see image gallery at www.cdc.gov/dpdx/baylisascariasis/gallery.html). Baylisascaris is one of several genera (e.g., Ascaris, Parascaris, Toxascaris, Toxocara) that belong to the family Ascarididae, order Ascaridida, phylum Nematoda. There are several Baylisascaris species of potential importance to human and animal health as causes of clinical larva migrans, but the most prevalent and pathogenic is B. procyonis, which primarily infects raccoons (1). However, Baylisascaris species infecting skunks, badgers, and other animals are also potential causes of larva migrans in humans and animals (1, 14).
There are two types of life cycles for B. procyonis, direct and indirect, and they have complementary roles in maintaining the parasite in nature (1). The direct (monoxenous) life cycle involves only raccoons (Procyon lotor) as well-adapted definitive hosts, and infection is achieved via the ingestion of embryonated eggs from the environment. During the indirect (heteroxenous) life cycle, infective eggs are ingested by paratenic hosts and the invading larvae migrate to various organs. Raccoons then acquire the infection through predation of paratenic hosts (1).
In raccoons, adult worms live in the small intestine. When eggs laid by the female worms are passed in feces and reach the ground, under ideal conditions of humidity and temperature it takes a minimum of about 2 weeks for the first-stage larvae (L1) to form and develop into 300-μm-long infective third-stage larvae (L3) (2, 15). Upon ingestion by young raccoons, the latter invade the intestinal wall and develop there for several weeks, and then preadult stage larvae return to the intestinal lumen to mature into male and female worms, mate, and start oviposition 50 to 76 days after infection (1, 16). Juvenile raccoons typically become infected via the accidental ingestion of embryonated eggs that are present in the environment (1, 16, 17).
When B. procyonis eggs are ingested by a paratenic host, such as a mouse or human, and the L3 larvae are released, the larvae penetrate the intestinal wall and migrate through the bloodstream to reach other organs and tissues, including the CNS and eyes (1, 18, 19). Two to 4 weeks after infection, the larvae grow in their third stage of development to achieve lengths of 1,300 to 1,900 μm, while actively migrating (20, 21, 22). They then settle and become encapsulated in granulomas in various organs and tissues (1). Larvae remain dormant in granulomas in paratenic hosts until ingestion by predatory raccoons, whereupon they molt and continue their development in the intestinal lumen and mature into adult worms, reaching patency earlier (32 to 38 days) than in the direct life cycle described above (1, 16, 17). In transmission studies, 100% of raccoons that ingested an infected rodent under experimental conditions became infected (16, 23). Interestingly, widespread larval migration to other tissues does not appear to occur in raccoons (1).
Raccoons are well-adapted hosts for B. procyonis—they exhibit a high susceptibility to infection, and they release a significant number of eggs into the environment each day, typically at latrines (communal areas of raccoon defecation) (1, 2, 9). Female worms can produce between 115,000 and 179,000 eggs per day per worm; therefore, infected raccoons can shed millions of eggs per day in their feces (1, 2, 4–7). The distribution of eggs in raccoon feces has been observed to be fairly homogeneous, with no differences noted in the mean numbers of eggs present in anterior, central, and posterior fecal sections (23). However, variations have been observed in the number of eggs eliminated each day, and, occasionally, an absence of eggs has been observed. Correspondingly, it was reported that 27% and 66% of infected raccoons had negative stool results (23, 24). The thick-walled eggs of B. procyonis may remain viable in the environment for several years (25), even when frozen at −15°C for 6 months. However, the eggs do not remain viable when they are heated to 62°C or when they are desiccated for 7 months (26). A seasonal peak in egg production has also been observed in fall months (September to November), with an apparent self-cure occurring in the winter and early spring in northern temperate regions (1, 27).
B. procyonis is very nonspecific regarding its infection of paratenic hosts, and there are more than 150 species of rodents, lagomorphs, primates (including humans), carnivores, and birds that have been affected with B. procyonis neural larva migrans (1, 2, 9; K. R. Kazacos, unpublished data). Susceptibility varies across species lines, but most paratenic hosts are highly susceptible to B. procyonis infection and suffer clinical effects of NLM (1). In a survey in a fragmented agricultural landscape, Page et al. (28) found a large proportion (28%) of white-footed mice (Peromyscus leucopus) to be infected with B. procyonis larvae, and this species appears to represent a common and well-adapted paratenic host. Experimentally, most P. leucopus mice became infected at a lower level and had a longer average onset of CNS disease than laboratory mice (Mus musculus), but the majority still developed clinical signs (19). Even though they were deemed less susceptible to infection, the fact that a single larva in the brain of a small animal is usually fatal (1, 29) indicates that they would still serve as important paratenic hosts via predation and scavenging. The fact that paratenic hosts develop CNS disease and have altered behavior increases the risk of predation by raccoons and further increases the risk of transmission near human dwellings when those raccoons shed eggs (1, 9, 25, 30).
B. procyonis is not a neurotropic nematode; only a low percentage of larvae (on the order of 5% to 7%) migrate into the CNS of paratenic hosts (1, 18, 25). Migration of B. procyonis to brain and eye tissues is considered to be accidental, a consequence of somatic migration and dissemination (1, 25, 31). However, this is the most important biological aspect of clinical infections in humans and animals. It has long been known that the presence of larvae in the CNS promotes various degrees of morbidity, thereby affecting the behavior of paratenic hosts and increasing the chance for transmission by predation (1, 18, 25). Unfortunately, the larvae behave in humans much as they do in any other paratenic host, with the potential impact of very serious CNS and eye disease.
EPIDEMIOLOGY
Human Infection
The prevalence of B. procyonis in humans is currently unknown. Moreover, it is likely much higher than what is estimated from the few reported symptomatic patients with neurological disease due to the potential for a larger number of covert and asymptomatic infections to exist undetected (2, 3, 7, 8). This hypothesis is supported by data from serology examinations performed on individuals and in population-based studies (32, 33) (K. R. Kazacos, unpublished data; C. A. Hall, unpublished data) and by blood cell counts of family members used to detect eosinophilia (13, 33).
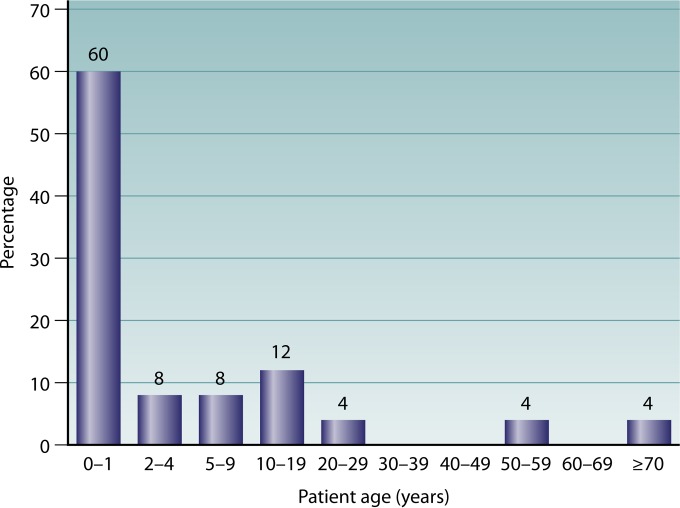
To date, there have been 25 cases of human neural and visceral larva migrans due to B. procyonis reported (Table 1) (5–8, 13, 33–52). Of the reported cases, pica and/or geophagia was described or suspected in 16/25 cases (64%) (Table 1). In 10 cases (40%), abnormal development and behaviors, including Down syndrome, developmental delays or disabilities, mental retardation, and autism, were also noted (Table 1). In general, baylisascariasis has been found to mostly affect young (<10-year-old) male children (64%), with 75% of these patients aged <2 years and 25% aged <1 year (average, 13.1 months); in addition, 3 cases were seen in female children aged <2 years (Fig. 1). However, several patients who have been diagnosed more recently have been older, including two >50 years of age (Table 1). For adults, those in occupations at higher risk of baylisascariasis include zoo and wildlife workers (including scientific personnel and wildlife rehabilitators), animal damage control and remediation workers, agricultural workers, trappers, and hunters (33, 53, 54; C. A. Hall, unpublished data). Furthermore, certain behavioral or mental problems related to developmental delay, drug abuse, or dementia are risk factors because they favor ingestion of contaminated material from outdoor transmission sites (2, 7, 33, 41, 45, 51). Children are particularly at risk for becoming infected with B. procyonis due to their curiosity, their time playing outdoors, and their habit of frequently putting their hands in their mouth (1, 4, 30, 55). All of these are common and expected behaviors for younger children, but they increase the risk of exposure to eggs via pica and/or geophagia (2, 4, 7, 25, 55).
TABLE 1.
Summary of 25 reported cases of Baylisascaris procyonis neural and visceral larva migrans in humans, 1973 to 2012a
| Patient no./yrb/sex/age/location | Developmental disability | Probable infection source(s) | Symptoms/clinical manifestations/Dx | Risk factor(s) and note(s) | Treatment | Outcome | Reference(s) |
|---|---|---|---|---|---|---|---|
| 1/1973/F/18 mos/Missouri | None | Soil | Irritability, right-sided hemiplegia; Dx, eosinophilic meningoencephalitis | Frequent geophagia; patient lived on a farm | Piperazine citrate (65 mg/kg/day × 2 days) | Weakness, spasticity | 5, 7, 8, 13 |
| 2/1980/M/10 mos/Pennsylvania | None | Fireplaces, firewood, raccoon feces | Decreased head control, lethargy, irritability, obtundation, loss of movement, coma; Dx, eosinophilic meningoencephalitis | Frequent pica; raccoons in chimneys | None | Death | 5–8, 34 |
| 3/1984/M/18 mos/Illinois | Down syndrome | Firewood bark, wood chips | Low-grade fever, lethargy, stupor, hypertonicity of right arm, vertical nystagmus, mild hepatomegaly progressing to coma, diffuse hypotonia; Dx, eosinophilic meningoencephalitis | Pica; contaminated firewood positive for B. procyonis eggs | Thiabendazole (50 mg/kg/day) | Death | 5–8, 35 |
| 4/∼1985/M/21 yrs/Oregon | Autism, chronic mental retardation | Soil | Seizures, left-sided hemiplegia, no speech, encephalopathy; Dx, meningoencephalitis | Pica, geophagia | Craniotomy and craniectomy | Persistent CNS deficits, seizures, hemiplegia | 5, 6, 7, 33; K. D. Thomson, unpublished data and written communication to K. R. Kazacos (2005, 2010) |
| 5/1990/M/13 mos/New York | None | Soil | Refusal to walk, right torticollis, right-sided gaze preference, marked nuchal rigidity, hepatomegaly, cortical blindness; Dx, eosinophilic meningoencephalitis | Pica, geophagia near raccoon latrine on farm positive for B. procyonis eggs; raccoons common | Thiabendazole (50 mg/kg/day), prednisone, and ivermectin (175 μg/kg) | Severe CNS impairment and developmental delay, wheelchair-bound status, cortical blindness, hemiparesis, brain atrophy | 5–8, 33 |
| 6/1993/M/13 mos/California | None | Soil, play area, sandbox, or woodpile | Listlessness, lethargy, right and then bilateral esotropia, speech and fine motor control deterioration, leg hypotonia; Dx, eosinophilic meningoencephalitis | None noted but geophagia likely; raccoons common in neighborhood and yard | Methylprednisolone and prednisone | Severe CNS deficits, wheelchair-bound status, seizures, cortical blindness, incontinence, brain atrophy | 5–8, 36 |
| 7/1993/M/9 mos/Michigan | None | Soil, raccoon feces | Low-grade fever, acute somnolence, decreased awareness, unresponsive and noninteractive behavior, loss of visual tracking, nuchal rigidity, hyperextension, seizures, meningismus; Dx, eosinophilic meningitis and undetermined encephalitis | Contact with dirt, probable pica; raccoons common in area | Phenobarbital, dexamethasone, prednisolone, antibiotics | Severe global CNS deficits, cortical blindness, hearing loss, seizures, brain atrophy | 5, 6, 8; J. M. Proos and C. Gushurst, unpublished data (1993) and written communication to K. R. Kazacos (1993–1994) |
| 8/∼1994/M/10 yrs/Massachusetts | Hyperkinetic disorder (minimal brain dysfunction syndrome) | Unknown | Recurrent abdominal pain, unresponsiveness, eosinophilia, sudden death | Probable geophagia due to hyperkinetic syndrome; raccoons common in yard | ND | Death due to cardiac pseudotumor | 5, 7, 8, 37 |
| 9/1996/M/6 yrs/Illinois | Developmental delay, mental retardation | Soil, raccoon feces | Ataxia, progressive spastic quadriparesis, unable to sit or stand unaided, progressive encephalopathy | Frequent pica and geophagia; contact with raccoon feces | ND until post-3.5 yrs, then albendazole (20 mg/kg/day) and prednisone | Severe CNS impairment and developmental delay, brain atrophy, wheelchair-bound status, seizures, incontinence, absence of speech, feeding by tube | 5–8, 38 |
| 10/1996/M/13 mos/Minnesota | None | Soil, raccoon feces | Irritability, ataxia, weakness, inability to cruise, sit up or walk, dysmetria, hypertonia, hyperreflexia, CNS dysfunction, decreased awareness; Dx, eosinophilic meningoencephalitis | None noted; exposed to pet and wild raccoons and their feces | Methylprednisolone, vincristine, thioguanine, and prednisone | Death | 5–8, 39 |
| 11/1997/M/19 mos/Minnesota | Developmental delay; Klinefelter syndrome | Soil, wood chips in yard | Severe ataxia, unresponsiveness, hypertonia; Dx, eosinophilic meningoencephalitis | None noted; probable geophagia | Prednisone, vincristine, and thioguanine | Death | 5–8, 39 |
| 12/1998/M/11 mos/California | None | Soil in play area, raccoon feces | Irritability, behavioral regression, decreased activity, right eye deviation, progression to lethargy, decreased interaction, hypertonia, extensor posturing; Dx, eosinophilic meningoencephalitis | Pica and geophagia; many raccoons and latrines on property | Albendazole (40 mg/kg/day × 4 weeks) and methylprednisolone | Severe CNS impairment, encephalopathy, seizures, profound visual impairment | 5–8, 40 |
| 13/2000/M/2.5 yrs/Illinois | None | Soil, raccoon feces | Fever and mild cough, lethargy, somnolence, irritability, confusion, ataxia, followed later by coma, decerebrate posturing, hypertonicity; Dx, progressive encephalopathy | Pica and geophagia, including from raccoon latrine source positive for B. procyonis eggs | Albendazole (30 mg/kg/day) and methylprednisolone | Severe CNS impairment and developmental delay, semicomatose status, spastic quadriparesis, cortical blindness, brain atrophy | 5–8, 38, 41 |
| 14/2000/M/17 yrs/California | Severe | Soil, raccoon feces | Low-grade fever, drowsiness, incoordination, altered mental status progressing to unconsciousness, hypertonia, hyperreflexia, coma; Dx, eosinophilic meningoencephalitis | Marked geophagia from a contaminated sandbox; raccoons common on property | Albendazole (500 mg b.i.d. × 7 mos) and dexamethasone | Death | 5–8, 41; W. A. Kennedy, unpublished data (2001) and written communication to K. R. Kazacos (2001) |
| 15/2002/M/11 mos/California | None | Soil, raccoon feces | Irritability, decreased activity, somnolence progressing to incoordination, loss of speech and vision, and ability to sit up; Dx, eosinophilic meningoencephalitis | Pica, geophagia from contaminated area at child care facility; raccoon latrines on roof | Albendazole (40 mg/kg/day × 6 mos) and dexamethasone | Severe CNS deficits, seizures, cortical blindness; gradual improvement but with lingering CNS and visual deficits | 5–8; K. R. Kazacos, unpublished data (2002–2004); D. Paul, unpublished data (2003) and written communication to K. R. Kazacos (2003) |
| 16/2004/M/4 yrs/Louisiana | None | Soil, raccoon feces | Dysmetria, ataxia | None noted; raccoons in neighborhood | Albendazole (10 mg/kg b.i.d. × 5 days) and dexamethasone | Mild dysmetria and then full recovery | 42 |
| 17/2004/F/15 mos/New York | None | Soil, raccoon feces | Irritability, tremors, altered mental status, sudden loss of developmental skills, apathy, hypertonia, marked eosinophilia, encephalopathy; Dx, eosinophilic meningoencephalitis | Exposure to soil in front yard; raccoons common, latrine and feces on roof | Albendazole (× 10 days) and prednisone | Severe CNS impairment with cognitive deficits, seizures, brain atrophy | 43; C. J. Crosley, unpublished data (2004) and written communication to K. R. Kazacos (2004) |
| 18/2005/M/7 yrs/Ontario | Autism, ADHD | Soil, raccoon feces | Drowsiness, lethargy, anorexia, abdominal pain, decorticate posturing, hyperreflexia, right-sided gaze preference; Dx, eosinophilic meningoencephalitis | Geophagia; contact with B. procyonis-positive latrines and sandbox; puts hands in mouth | Albendazole (25 mg/kg/day × 5 days) and methylprednisolone | Improvement with treatment, but seizures, cortical visual impairment, absence of speech, brain atrophy | 8, 44 |
| 19/2007/M/17 yrs/Oregon | Neuropsychiatric issues; drug abuse | Soil, raccoon feces | Altered mental status, confusion, slurred speech, fatigue, disorientation; Dx, eosinophilic meningoencephalitis | Camping along river, known site of latrines | Methylprednisolone | Gradual improvement but with severe cognitive deficits in memory, processing, communication | 45 |
| 20/2007/F/18 mos/Missouri | None | Soil | Altered mental status, nuchal rigidity, disorientation, lethargy, tremors, torticollis, leftward conjugate gaze and inability to track, torticollis, opisthotonos, hypertonia; Dx, eosinophilic meningoencephalitis | Geophagia, contact with contaminated soil in park where raccoons are common | Albendazole (× 28 days) and steroids | Severe CNS impairment, seizures, partial paralysis, cortical blindness, brain atrophy, feeding by tube | 46; M. A. Jackson, unpublished data (2007) and written communication to K. R. Kazacos (2007) |
| 21/2008/M/12 mos/New York | None | Soil, raccoon feces | Irritability, progressive weakness, head lag, hypotonia, vacant stare, inability to crawl or stand, lack of response to stimuli; Dx, eosinophilic meningoencephalitis | None noted; raccoons known in neighborhood | Albendazole (20 mg/kg/day increased to 40 mg/kg/day on day 33), methylprednisolone, and prednisone | Severe CNS impairment, cortical blindness, lack of cognitive function, spastic paralysis, brain atrophy | 47 |
| 22/2008/M/14 mos/Massachusetts | None | Soil | Fever, progressive motor weakness, lethargy, gait deterioration, decreased attention and eye contact, irritability, meningismus, loss of speech, inability to sit or stand; Dx, eosinophilic meningoencephalitis | Frequent pica and geophagia; oral contact with shoes; raccoons common, soil positive for B. procyonis eggs | Albendazole (40 mg/kg/day × 25 days) and methylprednisolone | Improvement following treatment but with residual paraparesis, speech development delay, mild brain atrophy | 48, 49 |
| 23/2008/M/14 mos/Ontario | None | Soil, raccoon feces | Fever, speech regression, inability to bear wt, vision loss with no eye tracking; Dx, eosinophilic meningoencephalitis | Puts hands in mouth; numerous raccoons in backyard, positive for B. procyonis eggs | Albendazole (200 mg t.i.d. × 28 days) and prednisone | Severe CNS deficits, cortical blindness, inability to stand, epilepsy | 50 |
| 24/2009/M/54 yrs/Missouri | Mental retardation | Contaminated food or other material | Staggering gait, difficulty moving legs, incontinence progressing to lethargy, unresponsiveness, catatonia, spastic quadriparesis; Dx, encephalopathy | Eats food scraps from garbage cans, smokes cigarette butts from ground | ND | Death | 51; M. Cohen, unpublished data (2009) and written communication to K. R. Kazacos (2011) |
| 25/NR/F/73 yrs/British Columbia | Alzheimer's dementia | Soil | Unrelated sudden death | Predisposed to accidental infection via geophagia | ND | Unrelated death; incidental finding at autopsy | 52 |
Based on tables by Murray and Kazacos (5), Gavin et al. (6), Shafir et al. (8), and K. R. Kazacos (unpublished data). F, female; M, male; Dx, diagnosis; kg, kilograms of body weight; NR, not reported; CNS, central nervous system; ND, not done; ADHD, attention deficit hyperactivity disorder; b.i.d., two times a day; t.i.d., three times a day.
yr, year of onset or presentation.
FIG 1.
Age distribution of 25 patients with baylisascariasis (from Table 1).
Pica is defined by the American Psychiatric Association as “a persistent eating of nonnutritive substances that is inappropriate to development level, [it] occurs outside culturally sanctioned practice and, if observed during the course of another mental disorder, is sufficiently severe to warrant independent attention” (56). Geophagia is more specific and involves the deliberate consumption of earth, soil, or clay (57, 58), which, depending on the level, in some cultures and age groups is considered normal (58). Therefore, geophagia could be considered a category of pica, an expected and universal behavior among very young children and not necessarily a pathological behavior. There may be various benefits of geophagy to young children (related to exposure to antigens [Ag] and proper immune system development), but eating the wrong soil can have dire consequences, as clearly exemplified by cases of Baylisascaris infection (55, 58). Taking this into consideration, the most important risk factors for B. procyonis infection are as follows: (i) children less than 2 years of age with frequent mouth-soil contact, with more males involved than females; (ii) altered behavior and pica associated with mental dysfunction in any age group; (iii) either altered behavior or pica in children in contact with potentially contaminated areas, especially raccoon latrines. Of course, accidental infection may occur through any number of normal activities if they involve such contact and a failure to take adequate precautions (1, 4–8, 30).
Contact with contaminated sites may also result in a clustering phenotype. For example, the brother and sister in the first probable case of baylisascariasis were asymptomatic and yet exhibited high levels of blood eosinophilia (15% and 58%, respectively) (13). In a more recent report, the parents of a B. procyonis-positive child were also reported to have positive serology results (33). This clustered distribution of infections may be explained by the focused nature of the usual transmission sources (raccoon latrines), with shared or common exposure (1, 9, 59, 60).
Raccoons as Source of Infection
Raccoon latrines are the most important source of infection with B. procyonis, and they are routinely visited by a variety of animals, especially small rodents and birds which forage for seeds among raccoon feces (59, 60, 61). Raccoons and other animals may also share feeding areas, thereby providing additional opportunities for transmission (62). Raccoons defecate at the base of trees, in the raised crotches of trees, and on large logs, stumps, rocks, tree limbs, and debris piles in the wild. However, raccoons also defecate in garages and barn lofts and on woodpiles, decks, roofs, and other peridomestic sites (1, 9, 62, 63). Typically, groups of raccoons (ranging from 2 to 7 raccoons) visit the same latrine sites, and individual raccoons may visit as many as six sites within a 14-day period. Based on continued contamination, this pattern of latrine visitation greatly increases the chances for B. procyonis transmission, especially for paratenic hosts, including humans (60, 61), since B. procyonis eggs accumulate in latrines and can remain viable over an extended period of time (1, 17, 54, 61). Because of all the seeds, berries, invertebrates, small plants, and other objects present in raccoon latrines, young children are fascinated by these areas, investigate and play in these sites, and knowingly or unwittingly ingest material from raccoon latrines, potentially ingesting large numbers of B. procyonis eggs that may also be present (1, 4, 30, 55).
The prevalence of B. procyonis in raccoon populations is generally quite high, especially in the northeastern, midwestern, and West Coast regions of the United States, where it has usually varied from 66% to over 90% in individual studies (1). Wise et al. (7) and Shafir et al. (8) did a breakdown of prevalence data presented elsewhere (1) and found that 58% of 3,967 raccoons examined in the midwestern United States were infected, 64% of 476 in the northeastern/mid-Atlantic region were infected, 4% of 1,868 in the Southeast region were infected, and 49% in the West/Southwest were infected. Most studies have reported a higher prevalence of the parasite in juvenile raccoons than in adult raccoons (1, 9, 64), although there are also reports where the ages (or genders) of infected animals did not significantly differ (65, 66).
Land use, fragmentation of forest areas, and urbanization may significantly affect the transmission of B. procyonis, especially in paratenic hosts, including humans (9). For example, in a study of a fragmented forest along a gradient of urbanization (in Chicago, IL), the prevalence of B. procyonis in Peromyscus spp. was found to increase as human population density increased (67). Even though the opposite relationship was observed for raccoons, because of much higher densities of raccoons in urban environments, overall loads of infective B. procyonis eggs would still be very high and present a danger of transmission (67). Raccoon populations have increasingly been detected in densely populated and urbanized areas, such as Brooklyn, NY (31, 68), Atlanta, GA, Orange County, CA, Portland, OR, Chicago, IL, and Toronto, Ontario, Canada (61, 69, 70, 71), showing the great adaptability of this species. Despite raccoons having large populations in urban settings, this has not necessarily correlated with higher prevalences of baylisascariasis in paratenic hosts. This is probably due to modifications in the foraging behavior of raccoons that live in areas with abundant human-associated food sources, resulting in a reduced incidence of predation on small mammals (9, 72). Despite this, however, the widespread occurrence of raccoon latrines in urban and suburban areas ensures that the risk of human infection from raccoons in these areas remains high (9, 73).
The natural movement of raccoon populations and their dynamic ability to adapt to human-altered landscapes has created opportunities for human infections to occur far from the wild. B. procyonis infections have been documented based on examinations of raccoon feces or soil found in the immediate surroundings of infected individuals, including their neighborhoods, backyards, porches, rooftops, gardens, firewood piles, and childrens' play areas and sandboxes (40, 41, 44, 49, 50, 63). The availability of pet food and other food sources in proximity to human dwellings has also favored the proximity of raccoons to humans and has encouraged the raccoons to establish latrines nearby. Further increasing the risk for human infection is the keeping of raccoons as pets or obtaining them for zoos or wildlife parks or for other reasons. This has led to at least two cases of ocular larva migrans (74, 75) and the introduction of B. procyonis into new areas, including Europe, Japan, and, more recently, China (76, 77, 78). Dogs and exotic carnivores (e.g., kinkajous [Potos flavus] and coatis [Nasua spp.]) are also commonly kept as pets and represent additional hosts of B. procyonis (dogs), B. potosis (kinkajous), or undetermined Baylisascaris spp. (coatis) that are able to eliminate eggs in their feces, potentially posing a risk of infection for humans (1, 79, 80, 81). In contrast, B. procyonis is so extensively adapted to raccoons that they will continue to have the central role in the transmission of this parasite (1).
As the range and prevalence of B. procyonis expand along with its raccoon host, it may have far-reaching effects on other animal species besides humans. Because of its pathogenicity as well as the behavior of paratenic hosts, B. procyonis appears to have played an important role in the decline of certain wildlife species. An important example of this is the Allegheny woodrat (Neotoma magister), an endangered species that is in serious decline or gone from several areas of its former range (1, 9). It is now known that, in addition to other factors, this species is being killed off by B. procyonis, due to its behavior of caching raccoon feces in its living area as a source of seeds or other items (1, 9). Thus, wildlife management, particularly in areas with endangered species, may require additional vigilance in monitoring the presence of B. procyonis, especially considering the increasing number of raccoons being observed in many locales (9, 82). In addition, the levels of infection of and effects on local wildlife may be important indicators of possible infection of humans in an area. For a complete and detailed review of baylisascariasis as a problem for wildlife, see Kazacos (1) and Page (9).
Geographic Occurrence
Sixty-eight percent of the reported B. procyonis-infected patients (Table 1) come from regions broadly surrounding the Great Lakes, including the midwestern region of the United States (36%), the northeastern region of the United States (24%), and Ontario Province, Canada (8%). The other 28% of B. procyonis patients live in the Western regions of the United States and Canada (British Columbia), with one patient living in the southeastern region of the United States (Louisiana). Therefore, most of these patients with B. procyonis are from areas with the highest prevalences of the parasite in raccoons (1, 2, 7, 8). Although the regional or large-scale prevalences of B. procyonis in raccoons are clear, within these and other areas, the parasite can have a patchy distribution with focal areas of high prevalence, as demonstrated in various epidemiological studies, including one by Kresta et al. (83). There are also some pockets within regions that used to be considered free of the parasite, such as in the southeastern region of the United States, where infections have now been found (53, 69, 84, 85). The observed expansion of B. procyonis into new areas may be due to the natural dispersion of infected raccoons or paratenic hosts (such as migratory birds), as well as to translocated wild or domestic animals (9, 69, 83).
Raccoons are native to North and Central America, with a geographical distribution extending from Canada to Panama (9, 86). Both raccoons and B. procyonis have been introduced into other parts of the world, including Europe and parts of Asia, where cases of neural larva migrans have also been seen in animals or humans (1, 9, 75, 77). Early raccoon introductions into Germany and the former Soviet Union for hunting and the fur trade led to a gradual spread into other areas, and now these animals are present in at least 20 European countries (9, 87). In Germany alone, over 100,000 raccoons exist in the wild, with a prevalence of B. procyonis of 71% (1, 88). Starting in 1977, an astonishing number of more than 20,000 raccoons were introduced as pets into Japan (77, 89); many of these animals escaped, and raccoons became established in the wild. Following past importations and spread within the system, infected raccoons were recently documented throughout the wildlife parks of China (78). It is obvious that greater regulations need to be put in place governing the translocation of “exotic” animals like raccoons, in order to prevent the introduction and spread of parasites like B. procyonis. Thus far, only the United States, Canada, Germany, and Austria have reported human infections, although animal cases of NLM are also known in Europe and Japan (1). Due to the spread of raccoons and B. procyonis, additional cases in both humans and animals are to be expected. For detailed and updated reviews of baylisascariasis, see Kazacos (1), Page (9), and Kazacos et al. (2).
PATHOGENESIS
Invasion and mechanical damage by larvae are key pathogenic factors and often result in the characteristic pathological finding of necrotic “track-like” linear areas (malacic tracks) in affected CNS tissues (1, 90, 91). Larvae also represent large organisms that grow while migrating, increasing substantially in size in the CNS and other tissues (25, 91). The invasion and migration ability of larvae is illustrated by the finding of numerous malacic tracks, associated with migrating larvae, in the brains of experimentally infected monkeys (90) and by the finding of larvae within the lumen of a small pulmonary vein (92) and the wall of a medium-sized CNS artery (35), related to their hematogenous dissemination.
Larvae, migratory lesions, and granulomas have been documented in numerous organs and tissues in a wide variety of species (1, 25, 35, 90, 91). In the CNS of mice experimentally infected with B. transfuga or B. columnaris, well-developed granulomas have been found to surround larvae and may explain their exhibiting lower pathogenicity for paratenic hosts than B. procyonis, which migrates more aggressively for an extended period (1, 25). These reactions also occur in the brain with B. procyonis, but they are generally slow to develop, resulting in more-extensive CNS migration and clinical disease (1, 18, 25, 91). Thus, CNS granulomas with B. procyonis are typically seen in longer-standing or chronic clinical cases or in situations of low-level infection with few larvae in the CNS, where humans or animals are usually asymptomatic or affected only mildly. Walling off B. procyonis larvae in the CNS has been seen in humans (34, 36, 52) and in various nonhuman primates (93–96) as well as occasionally in rodents (19). In the CNS, a granulomatous reaction takes time to fully develop, and gliosis, rather than fibrosis, predominates as the reaction type, in contrast to what occurs in other tissues (1, 91).
Mechanical damage alone is not responsible for the lesions created by B. procyonis. It is well known that migrating larvae produce abundant excretory-secretory antigens (ES-Ag) as they migrate (97). This consists of a potent mixture of migratory enzymes, shed cuticular proteins, and metabolic waste products, which are both highly antigenic and stimulatory of host inflammatory reactions, especially eosinophilic and granulomatous responses (2, 11, 91, 98). Toxic substances can also be generated by the inflammatory response, including various eosinophil products from degranulation of these cells in lesions and areas affected by larvae (98). Correspondingly, in a report of two children affected by B. procyonis, highly elevated levels of eosinophil-derived neurotoxin and major basic protein were detected in the cerebrospinal fluid (CSF) and were felt to contribute in part to the neuropathology and clinical signs in these infections (39). Larvae can also be present in tissues without signs of inflammation or degeneration (90, 91, 99), indicating that larvae are actively migrating and that inflammatory lesions occur in response to the substances (ES-Ag) generated by live larvae, as well as in response to the substances released by degenerating parasites (35, 91, 100). The active production of excretory-secretory antigens appears to have an important role in the pathogenesis of B. procyonis similar to that observed for other tissue-dwelling parasites (101).
An exciting area beginning to be investigated in detail involves the relationship of neurologic infection with nematode larvae and the development and progression of neuropsychiatric and neurodegenerative disorders, as elegantly presented by Fan et al. (11). Much of what is being found for neurotoxocariasis may also apply to baylisascariasis, as the mechanisms of brain injury would likely be similar. Positive associations between T. canis seropositivity and epilepsy (seizures), schizophrenia, and cognitive deficits have been shown, and evidence from human cases and animal models indicates a possible relationship between brain injury due to neurotoxocariasis and progression to neurodegeneration associated with dementia. Mice infected with T. canis eggs express various biomarkers associated with brain injury that are also seen in Alzheimer's disease (11). Various patients with neurobaylisascariasis have also had similar clinical signs (seizures, cognitive deficits, brain atrophy, etc.), and similar types of brain injury would be expected in NLM due to B. procyonis.
CLINICAL SYNDROMES
There are three main clinical syndromes associated with human infection by B. procyonis: neural larva migrans (NLM), visceral larva migrans (VLM), and ocular larva migrans (OLM). Similarly to toxocariasis, VLM is typically related to heavy or repeated infection, with larval migration into and through the viscera (54, 91). When larvae extend their migration into the CNS, NLM ensues and becomes clinically relevant based on larval load and migration damage (54, 55, 91). It is understood that when the burden of larvae is low, i.e., when few larvae have entered the CNS, clinical NLM does not manifest or is covert (1, 2, 6, 7, 55). In contrast, clinical OLM can occur when only a single larva migrates into the eye, based on the sensitivity of that organ (2, 31, 91). The majority of B. procyonis infections involve only a few larvae and remain neurologically asymptomatic (2, 6, 7). More-severe neurological lesions are associated with the ingestion of large numbers of embryonated eggs, usually as a result of pica behavior or of the ingestion of contaminated soil or raccoon feces by small children (2, 6, 55).
Sequelae showing various levels of severity have been observed in the reported cases of baylisascariasis (Table 1). Neurologic sequelae, often severe (16/25, 64%), or death (7/25, 28%) was the most common outcome of baylisascariasis, although there are other reports that suggest that less-severe morbidity or improvement may be seen in some cases. Approximately one-third (5/16, 31%) of the reports published to date have described less-severe sequelae and/or some improvement in symptoms, and most of these reports were published between 2004 and 2012. It is possible that these more recent results are due to early suspicion and/or diagnosis of baylisascariasis, followed by prompt administration of therapy (42, 44, 45, 49); however, they may also be due to lower levels of CNS infection with less-pronounced damage (1). In some cases, once severe clinical disease has been brought under control with aggressive treatment, very young children may gradually or partially improve due to their continued brain development and “rewiring” of neural pathways (C. J. Crosley, personal communication, 2004). Several cases showed improvement in the response to clinical disease following treatment. A 4-year-old boy from New Orleans was reported to have fully recovered without sequelae after presenting with eosinophilic meningitis (42). A 7-year-old boy in Ontario improved significantly with treatment, regaining baseline levels of function, but was still left without speech and with a cortical visual impairment and seizure disorder (44). The commonality of subclinical infection is suggested by epidemiological studies conducted in Chicago and Georgia, where samples from 30/389 (7.7%) asymptomatic children and 7/43 (16.3%) nonclinical raccoon trappers, respectively, were seropositive for baylisascariasis (32; C. A. Hall, unpublished data), as were numerous other samples examined serologically (K. R. Kazacos, unpublished). Apart from clinical disease, the majority of infections with B. procyonis are believed to be subclinical or covert and related to low-level, incidental infection (2, 6, 7).
VLM
Visceral larva migrans (VLM) was defined by Beaver (12) as the prolonged migration and long persistence of larval parasites such as would occur in their normal intermediate or paratenic hosts. The classic example of this is Toxocara canis, whose larvae migrate extensively and last for years walled off in the tissues of humans and other hosts (10, 11, 54, 91). Clinically, VLM typically involves signs referable to the viscera, usually the intestinal tract, liver, lungs, heart, and contiguous tissues (10, 54, 55). With heavy or repeated infection with T. canis, and trapping of larvae primarily in the liver and lungs, children develop a characteristic clinical picture of leukocytosis with high and persistent levels of eosinophilia, hepatomegaly, pneumonitis, and hypergammaglobulinemia (10, 54, 91). This is often accompanied by abdominal pain, sleep disturbances, and other signs (10, 54). In severe cases, there may be CNS invasion and neurologic disease (11, 102). In contrast to Toxocara, Baylisascaris larvae more aggressively migrate through the liver and lungs to somatic tissues and the CNS, and most clinical cases are characterized by neural larva migrans (2, 6, 54). However, although neurologic disease is the overwhelming manifestation, patients may also have clinical signs indicative of visceral involvement and VLM (2, 54, 55). In addition to the CNS, larvae have been found in various internal organs and tissues, including the heart, lung, mesentery, ileocecal wall, and soft tissues, in both human and experimental infections (1, 2, 34, 35, 90, 103).
In the present group of reviewed clinical reports (Table 1), migrating B. procyonis larvae were clinically suspected or confirmed in several tissues, but the patients did not present with classic VLM other than high levels of eosinophilia. In the case of the first patient to be subjected to autopsy, migration was extensive and granulomas were numerous in the mesentery, heart, and brain but were also noted in other tissues (34). In another case of high infection, the brain was markedly necrotic and numerous granulomas were observed in both the pleural and mesentery serosas, the myocardium, and epicardium (35). In a third case, an intracardiac eosinophilic mass with a degenerating nematode larva amid a granulomatous reaction was accompanied by marked eosinophilic infiltration, and this resulted in the sudden death of a previously healthy 10-year-old boy who presented with enlarged mesenteric lymph nodes (37). In two patients who presented with NLM, mild hepatomegaly was described (33, 35). However, an ultrasonography scan of the 13-month-old patient was negative for liver or splenic lesions and results of chest radiographs performed on both patients were normal (33, 35). At autopsy, the liver did not contain larvae or granulomas (35). Abdominal disease was also not manifested in the other patients, with negative imaging analyses obtained for two of them (39, 45).
It is interesting that transient respiratory signs have been observed at between 2 and 5 days after the experimental inoculation of rodents and primates with B. procyonis (1, 90); in the primates, respiratory signs were still present but were less severe 8 days postinfection (p.i.) (90). Larvae and hemorrhagic migratory lesions have been found in the lungs of experimentally infected mice and jirds within the first 1 to 3 days of infection (1, 91, 103). Bronchoalveolar lavage demonstrated a marked increase in red blood cells in the lungs of mice on day 1 p.i., peaking on days 2 to 3, followed by a large influx of leukocytes, including eosinophils (104). Early migration of larvae through the lungs may explain the respiratory symptoms present in five patients who exhibited the following respiratory manifestations just prior to the onset of CNS disease: a “mild respiratory infection” several days before becoming increasingly lethargic and developing CNS signs (35); a “brief, flu-like, febrile illness” 2 weeks before developing lethargy, decreased head control, loss of ability to crawl, obtundation, and then loss of most spontaneous movement (34); a mild cough and fever in a 2.5-year-old boy 8 days before admission and 5 days before becoming lethargic, somnolent, confused, and ataxic (38); rhinorrhea, cough, and nasal congestion for a few days followed by the sudden onset of confusion, slurred speech, fatigue, and disorientation (45); and “symptoms of upper respiratory tract infection” for several days before presentation with torticollis, refusal to walk, and a right-sided gaze preference (33). Pulmonary infiltrates (mild bihilar and right middle lobe; mild right perihilar) were detected in chest radiographs performed on two patients 3 days (13) and about 5 days (36) after the onset of increasing lethargy or progressive limb weakness (13, 36).
Therefore, concerning B. procyonis and VLM, larvae apparently migrate quickly through the liver to reach the lungs, where they may produce clinical signs related to early migratory damage (hemorrhage) and inflammation (1, 104). The classic trapping of larvae in the liver seen with toxocariasis (10, 54, 55, 91) is not present, and except for mild hepatomegaly in some cases, early respiratory signs accompany high levels of eosinophilia and subsequent CNS disease. From a diagnostic standpoint, the problem is that these respiratory signs are nonspecific and are typically attributed to other etiologies (viral pneumonitis, etc.) on initial presentation, rather than VLM and especially baylisascariasis. For early consideration of the latter, these signs must quickly be combined with the other signs of high levels of eosinophilia and the development of behavioral and motor abnormalities related to ensuing meningoencephalitis. This should also prompt a thorough exposure history, including possible contact with raccoons, their latrines, or other contaminated materials, as well as pica or geophagia.
NLM
Neural larva migrans (NLM) is related to invasion of the central nervous system by helminth larvae, especially by nematodes like Baylisascaris (2, 6, 54, 91). CNS invasion takes place as part of somatic migration, and in the case of B. procyonis, a low proportion (usually 5% to 7%) of invading larvae actually enter the CNS (1, 2, 19, 29). Therefore, clinical NLM is a dose-dependent disease and its severity ranges from mild to severe based on the level of initial infection, how many larvae enter the CNS, the location of these migrating larvae, and the extent of damage, including the intensity of the inflammatory reaction (1, 2, 5–8, 54, 55). Typically, the brain is affected the worst, although involvement of the cervical and thoracic spinal cord in a 14-month-old boy has also been described (48, 49). Cervical spinal cord lesions were also seen in experimentally infected primates (90), and larvae were recovered from the spinal cords of experimentally infected mice and other animals (19; K. R. Kazacos, unpublished).
It is well known that there is a lag time in the development of CNS disease in experimentally infected animals following infection with B. procyonis; the earliest that CNS signs have been seen in mice is about 8 to 10 days p.i. (19). Eight infected primates of two species first developed CNS signs at 10 to 15 days p.i. and 9 to 19 days p.i., respectively, and then rapidly succumbed to CNS disease (90, 99; K. R. Kazacos, unpublished). At low dosages, signs may be slow to develop and may take several weeks to manifest (1). Despite the greater size of the human brain, given a significant dose, it is believed that CNS signs may develop as early as 1.5 to 2 weeks p.i. At high doses, NLM caused by B. procyonis often results in acute disease, and the symptomatic period before the first evaluation in the reported cases (Table 1) ranged from 1 to 21 days (mean, 7 days).
Signs in heavily infected children include sudden lethargy or irritability, weakness, nuchal rigidity, torticollis, opisthotonos, ataxia, decreased head control, loss of fine motor skills, and the inability to sit, stand, or walk without assistance. These may be accompanied by cranial nerve dysfunction or impaired vision or speech, and patients may become stuporous, lapse into a coma, or succumb. Children who survive may be profoundly neurologically impaired, suffering from blindness, seizures, incontinence, and partial paralysis (2, 6, 55). The blindness may be related to destruction of the visual cortex, rather than to actual OLM or DUSN (5, 6, 33, 38, 50) (Table 1). In one case, a 2.5-year-old Chicago boy experienced progressive developmental delay and mental retardation for 3.5 years and was evaluated (38). He had become increasingly ataxic with worsening spastic quadriparesis, and by 6 years of age, he could not sit or stand without assistance. It was not until he was referred for an ophthalmology consultation that Baylisascaris infection was considered, based on the presence of a syndrome called “diffuse unilateral subacute neuroretinitis” (DUSN) (105). Based on his known pica and geophagia and exposure to raccoons and raccoon feces, he was believed to have been infected several years earlier and to have undergone slow, progressive CNS deterioration (38).
Subtle, low-level infection with mild CNS disease has been documented in natural infections in nonhuman primates (1), is also likely in many human cases, and would be hard to accurately diagnose (2, 7). Such cases may have mild, nonspecific signs with or without concomitant eosinophilia similar to what occurs in covert toxocariasis (10, 11). It appears that motor deficits are the most frequent type of clinical manifestations and have affected the majority of reported patients (Table 1). Also common are disturbances in levels of consciousness, behavioral alterations, coordination deficits, and loss of vision and/or speech, followed by meningismus, early onset coma, and other signs (Table 1). Fever has been reported as a presenting characteristic in only 6 of the 25 patients (Table 1).
Most patients with B. procyonis NLM are admitted with significantly elevated eosinophil levels, both in CSF and peripheral blood (2, 5, 6, 8, 54, 55) (Table 2). The proportions of eosinophils detected in initial blood and CSF samples obtained from reported patients infected with B. procyonis ranged from 8% to 45% and from 4% to 68%, respectively, and are shown in Tables 2, 3, and 4. The initial proportion of eosinophils was greater than 4% in both the blood and CSF samples of all patients and averaged 26% in the blood and 37% in CSF, often increasing significantly within 2 to 7 or more days. Although these cases deal with B. procyonis NLM, there are other possible causes of eosinophilic meningoencephalitis that must be considered, depending on exposure history, etc. However, given the geographic location of these cases, the common parasitic etiologies are limited, and in North America, B. procyonis would be at or near the top of the list of suspected causes (36, 54). A differential diagnosis of eosinophilic meningoencephalitis has been reviewed elsewhere (106).
TABLE 2.
Diagnostic and laboratory findings for 25 reported cases of Baylisascaris procyonis neural and visceral larva migransa
| Patient no./yrb/sex/age/location | Funduscopic exam result | Blood eosinophilia (%) | CSF eosinophilia (%) | Serology for B. procyonisc | Chest radiography result | Reference(s) |
|---|---|---|---|---|---|---|
| 1/1973/F/18 mos/Missouri | NR | 30 | 49, 80, 34, 100 | Positive for Ascaris by bentonite flocculation, S = 1:160, C = 1:10 | Mild right perihilar infiltrate | 5, 7, 8, 13 |
| 2/1980/M/10 mos/Pennsylvania | Optic atrophy later | 27 | 68 | S = strong positive by IFA | NR | 5–8, 34 |
| 3/1984/M/18 mos/Illinois | Normal | 37, 34 | NR, 50, 80 | S = positive by IFA and later by ELISA, S = 1.635d | Normal | 5–8, 35 |
| 4/∼1985/M/21 yrs/Oregon | NR | NR | NR | S = positive by ELISA, 1.800 | NR | 5, 6, 7, 33; K. D. Thomson, unpublished data and written communication to K. R. Kazacos (2005, 2010) |
| 5/1990/M/13 mos/New York | Normal | 39 | 60, 52 | Positive by EIA (Western blotting; S = >1:10, 240, C = 1:40–80) and later by ELISA (S = 2.600–2.961, C = 1.655–1.689) | Normal | 5–8, 33 |
| 6/1993/M/13 mos/California | R, diffusely mottled retina, reddish macular lesion, optic pallor | 45 | Prominent eosinophilia | S = positive by IFA (1:1,024) and ELISA (0.809, 1:102, 400) | Mild bihilar and right middle lobe infiltrates | 5–8, 36 |
| 7/1993/M/9 mos/Michigan | Normal | 29, 33, 43, 40 | many eosinophils, 85, 89, 62, 71 | S = positive by ELISA, 3.113 | Normal | 5, 6, 8; J. M. Proos and C. Gushurst, unpublished data (1993) and written communication to K. R. Kazacos (1993–1994) |
| 8/∼1994/M/10 yrs/Massachusetts | ND | 15 | ND | ND | NR | 5, 7, 8, 37 |
| 9/1996/M/6 yrs/Illinois | L, DUSN | 5 (post-3.5 yrs) | 6 (post-3.5 yrs) | Positive later by IFA (S = 1:4,096, C = 1:64) and by ELISA (S = 2.230, C = 1.114) | NR | 5–8, 38 |
| 10/1996/M/13 mos/Minnesota | Normal | 35, 14, 12 | 54, 73, 4, 6 | Positive by IFA (S = 1:1,024–4,096; C = 1:64) and by ELISA (S = 1.361, C = 0.438, 1.333) | NR | 5–8, 39 |
| 11/1997/M/19 mos/Minnesota | Normal | 18, 22, 22, 26 | 4, 19, 13, 5, 67 | Positive by IFA (S = 1:4,096, C = 1:64–256) and by ELISA (S = 2.316, C = 0.289–0.795) | NR; CT normal | 5–8, 39 |
| 12/1998/M/11 mos/California | R, DUSN, with chorioretinal scarring, linear track lesions, optic atrophy | 17, 17 | 6, 7 | Positive by IFA (S = 1:64–1,024, C = 1:64) and by ELISA (S = 1.334, 2.574, C = 0.128) | NR | 5–8, 40 |
| 13/2000/M/2.5 yrs/Illinois | R, Multifocal choroiditis with infiltrates | 28, 6 | 32, 26 | Positive by IFA (S = 1:1,024–4,096, C = 1:1,024) and by ELISA (S = 3.132–3.218, C = 1.888, 2.021) | Normal | 5–8, 38, 41 |
| 14/2000/M/17 yrs/California | ND | 15 | 37, 42 | Positive by IFA (S = 1:4,096, C = 1:256) and by ELISA (S = 3.025, 3.035, C = 1.746, 1.765) | ND | 5–8, 41; W. A. Kennedy, unpublished data (2001) and written communication to K. R. Kazacos (2001) |
| 15/2002/M/11 mos/California | Normal | 30, 30 | 23, 54, 33 | Positive by ELISA, S = 1.869, 2.451, C = 0.624–1.017 | ND | 5–8; K. R. Kazacos, unpublished data (2002–2004); D. Paul, unpublished data (2003) and written communication to K. R. Kazacos (2003) |
| 16/2004/M/4 yrs/Louisiana | NR | 12 | 55 | Positive by ELISA, S = 0.547, 0.976 | NR | 42 |
| 17/2004/F/15 mos/New York | NR | Marked eosinophilia | Eosinophilia | Positive by ELISA, S = 1.921–2.556, C = 0.175, 0.347 | NR | 43; C. J. Crosley, unpublished data (2004) and written communication to K. R. Kazacos (2004) |
| 18/2005/M/7 yrs/Ontario | Bilateral patchy subretinal infiltrates | 24, 21, 8 | 30, 7 | Positive by ELISA (S = 1.813, C = 0.186, 0.723) and by Western blotting | NR | 8, 44 |
| 19/2007/M/17 yrs/Oregon | Bilateral subtle edema of optic discs | 8, 21 | 49, 39 | Positive by ELISA (S = 2.376, C = 0.600) and by Western blotting | Normal | 45 |
| 20/2007/F/18 mos/Missouri | NR | Profound eosinophilia | 40 | Positive by ELISA (S = 2.130, 2.164, C = 1.919) and by Western blotting | NR | 46; M. A. Jackson, unpublished data (2007) and written communication to K. R. Kazacos (2007) |
| 21/2008/M/12 mos/New York | Normal | 30, 24 | 29, 46, 19 | Positive by ELISA, S = 1.385, C = 1.453 | Normal | 47 |
| 22/2008/M/14 mos/Massachusetts | Normal | 24, 10, 4 | 24 | S = Positive by ELISA with rising titer (0.243–0.500) and by Western blotting | NR | 48, 49 |
| 23/2008/M/14 mos/Ontario | Normal | 32 | NR | Positive by ELISA (S = 0.744, C = neg) and by Western blotting | NR | 50 |
| 24/2009/M/54 yrs/Missouri | NR | NR | Normal | ND | NR | 51; M. Cohen, unpublished data (2009) and written communication to K. R. Kazacos (2011) |
| 25/NR/F/73 yrs/British Columbia | NR | NR | NR | ND | NR | 52 |
CSF; cerebrospinal fluid; R, right eye; L, left eye; NR, not reported; ND, not done; IFA, indirect immunofluorescence assay; ELISA, enzyme-linked immunosorbent assay; DUSN, diffuse unilateral subacute neuroretinitis; EIA, enzyme immunoassay; CT, computed tomography; neg, negative.
yr, year of onset or presentation.
Data include unpublished ELISA results from K. R. Kazacos. S, serum; C, CSF.
For ELISA, the value is the optical density (OD) reading, where an OD of >0.250 is considered positive for serum.
TABLE 3.
Percentage of eosinophils in peripheral blood samples of 20 patients with baylisascariasis
| Patient no.a | % eosinophils on indicated day after initial evaluationb |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 10 | 14 | 18 | 30 | 37 | 57 | 104 | 106 | 122 | 180 | |
| 1 | 30 | ||||||||||||||
| 2 | 27 | ||||||||||||||
| 3 | 37 | 34 | |||||||||||||
| 5 | 39 | ||||||||||||||
| 6 | 45 | ||||||||||||||
| 7 | 29 | 33 | 43 | 40 | |||||||||||
| 8 | 15 | ||||||||||||||
| 9 | 5c | ||||||||||||||
| 10 | 35 | 14 | 12 | ||||||||||||
| 11 | 18 | 22 | 22 | 26 | |||||||||||
| 12 | 17 | 17 | |||||||||||||
| 13 | 28 | 6 | |||||||||||||
| 14 | 15 | ||||||||||||||
| 15 | 30 | 30 | |||||||||||||
| 16 | 12 | ||||||||||||||
| 18 | 24 | 21 | 8 | ||||||||||||
| 19 | 8 | 21 | |||||||||||||
| 21 | 30 | 24 | |||||||||||||
| 22 | 24 | 10 | 4 | ||||||||||||
| 23 | 32 | ||||||||||||||
See Table 1 for patient characteristics.
Day 0, initial evaluation.
3.5 years after initial signs.
TABLE 4.
Percentage of eosinophils in cerebrospinal fluid (CSF) samples of 18 patients with baylisascariasisa
| Patient no.b | % eosinophils on indicated day after initial evaluationc |
||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 5 | 6 | 7 | 10 | 14 | 15 | 17 | 29 | 30 | 37 | 57 | 65 | 76 | 106 | 118 | |
| 1 | 49 | 80 | 34 | 100 | |||||||||||||||
| 2 | 68 | ||||||||||||||||||
| 3 | NR | 50 | 80 | ||||||||||||||||
| 5 | 60 | 52 | |||||||||||||||||
| 7 | High | 85 | 89 | 62 | 71 | ||||||||||||||
| 9 | 6d | ||||||||||||||||||
| 10 | 54 | 73 | 4 | 6 | |||||||||||||||
| 11 | 4 | 19 | 13 | 5 | 67 | ||||||||||||||
| 12 | 6 | 7 | |||||||||||||||||
| 13 | 32 | 26 | |||||||||||||||||
| 14 | 37 | 42 | |||||||||||||||||
| 15 | 23 | 54 | 33 | ||||||||||||||||
| 16 | 55 | ||||||||||||||||||
| 18 | 30 | 7 | |||||||||||||||||
| 19 | 49 | 39 | |||||||||||||||||
| 20 | 40 | ||||||||||||||||||
| 21 | 29 | 46 | 19 | ||||||||||||||||
| 22 | 24 | ||||||||||||||||||
NR, not reported.
See Table 1 for patient characteristics.
Day 0, initial evaluation.
3.5 years after initial signs.
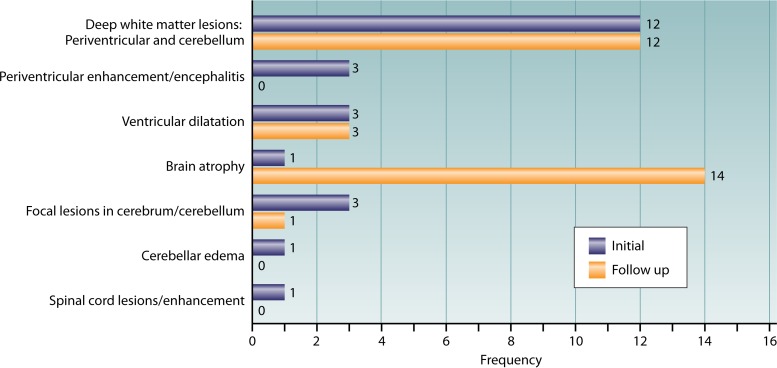
Magnetic resonance imaging of the CNS of B. procyonis NLM patients may reveal findings consistent with acute disseminated encephalomyelitis, as evidenced by diffuse, deep white matter abnormalities that exhibit a periventricular and cerebellar distribution (2, 36). In the later stages of baylisascariasis, white matter lesions become more widespread and patients may develop generalized brain atrophy (2, 36) (Table 5 and Fig. 2). However, ventricular dilation, cerebellar edema, and spinal enhancement have also been observed (35, 36, 49). Although possible in human infections, single focal lesions have not been detected, but a single focal lesion was reported in a white-handed gibbon (Hylobates sp.) with B. procyonis NLM (93). An infected orangutan (Pongo pygmaeus) had lesions similar to those seen in human cases, with hyperintense regions in the thalami and central white matter (95).
TABLE 5.
Central nervous system electroencephalogram, imaging, and biopsy/autopsy results for 25 reported cases of Baylisascaris procyonis neural and visceral larva migransa
| Patient no./yrb/sex/age/location | EEG result | CNS imaging result | CNS imaging follow-up result (time interval) | Biopsy/autopsy result | Larva identified (size) | Reference(s) |
|---|---|---|---|---|---|---|
| 1/1973/F/18 mos/Missouri | Normal | Radionuclide brain scans normal | NR | ND | ND | 5, 7, 8, 13 |
| 2/1980/M/10 mos/Pennsylvania | NR | CT: severe periventricular encephalitis, acute hydrocephalus | CT: cerebral atrophy, resolution of periventricular enhancement | Autopsy: systemic larva migrans with severe brain involvement | B. procyonis, 1 mm × 60-μm diam | 5–8, 34 |
| 3/1984/M/18 mos/Illinois | Abnormal—diffuse slowing | CT: marked periventricular enhancement and ventricular dilatation | NR | Autopsy: systemic larva migrans with severe brain involvement; 3 larvae/g of brain | B. procyonis, 1.5–1.9 mm × 60–74-μm diam | 5–8, 35 |
| 4/∼1985/M/21 yrs/Oregon | NR | Large right frontoparietal lesion | CT, MRI (20 yrs): diffuse cerebral and cerebellar atrophy, mixed porencephaly and gliotic changes in cerebrum, and right ventricular dilatation | Biopsy: characteristic malacic and inflammatory lesions present | NR | 5, 6, 7, 33; K. D. Thomson, unpublished data and written communication to K. R. Kazacos (2005, 2010) |
| 5/1990/M/13 mos/New York | Normal (2 occasions) | CT, MRI normal | MRI (6 mos): diffuse brain atrophy with selective loss of white matter and progressive CSF space enlargement | ND | ND | 5–8, 33 |
| 6/1993/M/13 mos/California | Normal | MRI: bilateral, patchy T2 hyperintensity of white matter in periventricular regions and deep cerebellum | MRI (6 weeks): progressive, increasingly confluent white matter changes throughout supratentorial regions and tegmentum of brain stem, with diffuse atrophy | Biopsy: positive; subcortical white matter granuloma with larva | B. procyonis, 36–40-μm diam, posterior intestinal region | 5–8, 36 |
| 7/1993/M/9 mos/Michigan | Abnormal—diffuse slowing | CT, MRI normal | MRI (17 days): Increased T2 signal bilaterally in deep cerebral white matter, especially corona radiata, focally in cerebellum; increased prominence of ventricles and sulci; minimal cerebral atrophy | ND | ND | 5, 6, 8: J. M. Proos and C. Gushurst, unpublished data (1993) and written communication to K. R. Kazacos (1993–1994) |
| 8/∼1994/M/10 yrs/Massachusetts | NR | NR | NR | Autopsy: large nodular eosinophilic cardiac pseudotumor with larva in left ventricle | B. procyonis, 60–70-μm diam | 5, 7, 8, 37 |
| 9/1996/M/6 yrs/Illinois | NR | ND initially (3.5 yrs prior) | MRI (post-3.5 yrs): active demyelination in deep periventricular white matter, with diffuse cortical atrophy | ND | ND | 5–8, 38 |
| 10/1996/M/13 mos/Minnesota | Abnormal—diffuse slowing | CT: normal; MRI: iron deposition and early white matter changes | MRI (4 weeks): progressive abnormalities with severe cortical atrophy and diffuse white matter degeneration | ND | ND | 5–8, 39 |
| 11/1997/M/19 mos/Minnesota | NR | MRI: minor white matter changes | MRI (60 days): ongoing severe white matter loss and cortical atrophy | ND | ND | 5–8, 39 |
| 12/1998/M/11 mos/California | Abnormal—diffuse slowing | MRI: small foci of enhancement in left temporal and periventricular regions and overall patchy white matter abnormalities and demyelination | MRI (10 days): marked progression of white matter abnormalities | ND | ND | 5–8, 40 |
| 13/2000/M/2.5 yrs/Illinois | Abnormal—diffuse slow waves | CT: initially normal and then increased signal involving both cerebellar hemispheres | MRI (2 mos): progressive cerebellar and supratentorial white matter demyelination and diffuse cortical atrophy | ND | ND | 5–8, 38, 41 |
| 14/2000/M/17 yrs/California | Abnormal—diffuse encephalopathy | CT: initially normal; MRI: deep white matter abnormalities in dentate nuclei of cerebellum bilaterally | MRI (11 days): additional lesions in bifrontal, left temporal, and parietal white matter | Biopsy: focal patchy necrosis in gray matter with perivascular infiltrates of macrophages, plasma cells, and eosinophils; positive for larva | B. procyonis | 5–8, 41; W. A. Kennedy, unpublished data (2001) and written communication to K. R. Kazacos (2001) |
| 15/2002/M/11 mos/California | Normal | CT and MRI: ventricular dilatation | MRI (14 days): diffuse cerebral atrophy and T2 signal enhancement, marked volume loss, eventually stabilizing with a decrease in inflammation | ND | ND | 5–8; K. R. Kazacos, unpublished data (2002–2004); D. Paul, unpublished data (2003) and written communication to K. R. Kazacos (2003) |
| 16/2004/M/4 yrs/Louisiana | NR | MRI: cerebellar edema | MRI (12 days): improvement of cerebellar edema | ND | ND | 42 |
| 17/2004/F/15 mos/New York | NR | MRI: multiple scattered foci of hyperintensity and edema along cortex-white matter junction | MRI (9 weeks): extensive periventricular and cerebellar white matter hypersignal intensity and significantly increased volume loss of white matter | ND | ND | 43; C. J. Crosley, unpublished data (2004) and written communication to K. R. Kazacos (2004) |
| 18/2005/M/7 yrs/Ontario | NR | MRI: patchy areas of abnormal enhancement of the cerebellar gray-white junction and small foci of nodular enhancement bilaterally in the cerebral cortex | MRI (3 mos): postinflammatory atrophy with ventricular enlargement, diffuse volume loss, and white matter gliosis | ND | ND | 8, 44 |
| 19/2007/M/17 yrs/Oregon | NR | MRI: bilateral diffuse patchy T2 hyperintense lesions in periventricular and juxtacortical white matter, cerebellum, medulla, pons, and midbrain | NR | ND | ND | 45 |
| 20/2007/F/18 mos/Missouri | NR | CT: scattered hypoattenuation in periventricular white matter; MRI: multifocal T2 hyperintense lesions throughout white matter | MRI (2 and 6 weeks): global atrophy and worsening hyperintense lesions throughout white matter | ND | ND | 46; M. A. Jackson, unpublished data (2007) and written communication to K. R. Kazacos (2007) |
| 21/2008/M/12 mos/New York | NR | MRI: acute demyelinating encephalomyelitis | MRI (8 days): increased demyelination progressing to severe atrophy | ND | ND | 47 |
| 22/2008/M/14 mos/Massachusetts | NR | MRI: patchy T2 hyperintensity bilaterally in periventricular and deep cerebellar white matter; two areas of hyperintensity in cervical and thoracic spinal cord | NR | ND | ND | 48, 49 |
| 23/2008/M/14 mos/Ontario | NR | MRI: diffuse, scattered subcortical, periventricular, and deep white matter lesions bilaterally | NR | ND | ND | 50 |
| 24/2009/M/54 yrs/Missouri | Isoelectric (very low voltage) | MRI: cortical and central brain atrophy | MRI: scattered T2 hyperintense foci in corona radiata, subcortical white matter, and thalami | Biopsy: right frontal lobe showing marked nonspecific gliosis; autopsy: necrotizing eosinophilic meningoencephalitis with larvae | B. procyonis, 52-μm diam | 51; M. Cohen, unpublished data (2009) and written communication to K. R. Kazacos (2011) |
| 25/NR/F/73 yrs/British Columbia | NR | NR | NR | Autopsy: diffuse cerebral atrophy, probably unrelated; larvae in focal reactive and inflammatory nodules in deep white matter | B. procyonis, 65-μm diam | 52 |
EEG, electroencephalogram; CNS, central nervous system; NR, not reported; ND, not done; CT, computed tomography; MRI, magnetic resonance imaging.
yr, year of onset or presentation.
FIG 2.
Frequency of lesions detected using CNS imaging (computed tomography [CT] and magnetic resonance imaging [MRI]) on patients with baylisascariasis (see Table 3); the same patients were not necessarily seen on initial and follow-up evaluations, and individual patients may have presented with several lesions.
OLM
Ocular larva migrans (OLM) is caused by the migration of helminth larvae in eye tissues, and is usually due to nematodes, particularly Toxocara spp. and Baylisascaris (10, 11, 54, 91). Larvae gain access to the eyes via the retinal arteries, as a result of somatic migration and dissemination (1, 54, 91). OLM is typically characterized by retinochoroiditis, vitritis, and the formation of intraocular granulomas (as larvae become walled off), and on occasion there are more-severe sequelae, including retinal detachment and/or panophthalmitis (10, 54, 55, 91). Inflammation in the eye appears to be due to the same pathogenetic factors as in cases of VLM and NLM, i.e., physical damage by the larvae, production of ES-Ag, and an influx of inflammatory cells, especially eosinophils, with subsequent degranulation and damage to sensitive ocular tissues (2, 11, 54, 91, 107). Importantly, OLM is usually a stand-alone disease, resulting from chance migration of a single larva into the eye, and patients typically develop ocular problems without any indication of VLM or NLM (2, 6, 54, 91). Clinically, OLM is usually unilateral and involves sudden blurry or decreased vision, photophobia, and other ocular signs; depending on severity, it may progress to blindness in the affected eye (10, 54).
OLM can manifest as diffuse unilateral subacute neuroretinitis (DUSN) (108). DUSN is characterized by vison loss, papillitis, vitritis, and recurrent crops of gray-white retinal lesions. Later, progressive vison loss, optic nerve atrophy, retinal vessel narrowing, and diffuse retinal pigment epithelial degeneration develop (2, 22, 31, 75, 108). Other than the characteristic lesions, a diagnosis is established by direct visualization of the intraocular parasites, and one key aspect is their size. Nematode larvae that cause DUSN are typically separated into two size ranges, “small” (500 to 700 μm in length) and “large” (1,500 to 2,000 μm in length), and Baylisascaris is considered the primary etiological agent of the latter (1, 2, 22, 31). In many of the cases, a large (∼1,600-to-1,850-μm) moving larva was observed (22, 31, 74, 75, 109–112), matching those seen in primates experimentally infected with B. procyonis (99, 113). Based on dissemination of larvae, a number of patients suffering from Baylisascaris NLM also have had DUSN due to concomitant invasion of the eye (2, 36, 38, 40, 44).
There have been over two dozen cases of B. procyonis OLM-DUSN identified and/or reported (see references 22, 31, 74, 75, 105, 109, 110, 111, and 112) (K. R. Kazacos, unpublished). They have involved severe ocular disease and were identified based on the lesions of DUSN and by visualizing the characteristically large larvae in the retina or vitreous. Most patients were from North America and had direct contact with raccoons or with raccoon latrines or other contaminated materials. Two patients developed OLM-DUSN shortly after they obtained pet raccoons (74, 75). Cases have occurred in residents or hunters in rural areas (22, 109, 112) and in suburban areas (36, 38, 40, 105) as well as in a highly urban area (New York City) (31, 114). The latter case is unique in that the patient did not present with typical DUSN lesions but instead presented with advanced granulomatous OLM with retinal detachment similar to that seen in toxocariasis. She was also seropositive for Baylisascaris in both serum and vitreous fluid (114). Other cases of Baylisascaris OLM or OLM-DUSN with granuloma formation are expected to occur, since granulomas, along with lesions of DUSN, were also noted in nonhuman primates and rodents experimentally infected with B. procyonis (99, 113).
Fundoscopic examinations were performed for 16/25 (64%) of the reported NLM patients (Table 2), and ocular lesions were detected in 7 of these patients. In particular, inflammatory infiltrates were found in association with the linear track-like lesions and degenerative changes in the retina and associated vessels that are characteristic of DUSN.
In contrast with VLM and NLM, both of which tend to affect infant children (<2 years of age) (Fig. 1), isolated OLM usually affects older children and adults (2, 10, 11, 22, 54). In 27 cases of OLM-DUSN due to B. procyonis, the average patient age was 25 years (range, 11 months to 65 years) (22, 36, 38, 40, 44, 74, 75, 105, 109–112). Opportunities for the ingestion of large numbers of eggs are less frequent for adults than for small children, helping to explain the age-related distribution observed for NLM versus OLM. Similarly to the situation for subclinical or covert VLM and NLM (2, 6, 7, 11), it is also possible that a certain number of ocular cases may occur and go undiagnosed due to minor visual dysfunctions, especially in the very young. Thus, future population-based seroepidemiological investigations should also include fundoscopic evaluations.
DIAGNOSIS
Diagnosis of baylisascariasis is dependent upon a compatible history of exposure, clinical symptoms and signs, and the results of diagnostic tests, including what can be learned from biopsies and at autopsy. Clinical testing usually includes a complete blood count with differential, a lumbar tap and CSF cytologic evaluation, serology, identification of larvae recovered from the tissues (by biopsy or autopsy) or visualized in the eye, and imaging of the CNS for characteristic changes (1, 2, 6, 7, 8, 55). Although recovery and identification of larvae are confirmatory (5, 7), this approach is not always possible or practical, so clinical diagnosis with probable cause rests heavily on serologic testing (2, 6, 7, 8). Because of the potential seriousness of baylisascariasis and of the limitations of effective therapy, early consideration and diagnosis of this condition become very important. Damage to the brain can occur rapidly, and treatment should be started without delay (2, 6, 55).
Pathology
The principal lesions of Baylisascaris larva migrans involve eosinophilic and granulomatous inflammation, which occurs in a variety of organs and tissues, particularly the CNS (2, 34, 35, 90, 91). Larvae migrating through the body leave a trail of hemorrhage, necrosis, and edema and engender a cellular infiltrate consisting of eosinophils and other leukocytes (91). Following this migration, larvae become walled off in well-circumscribed granulomas that are usually 1 to 3 mm in diameter but are occasionally larger, and the brain undergoes postinflammatory degenerative changes (1, 2, 6, 91). Only a few patients with baylisascariasis have undergone biopsy (36, 41, 51) or were examined at autopsy (34, 35, 37, 51, 52). In two patients who were subjected to autopsy, mild to severe brain atrophy was observed. In addition, thick and gray meninges (especially at the base) were present (34, 52), along with softening and deep laminar cavitation in the cortex and a dilated ventricular system, and the cerebral and cerebellar white matter was reduced in size, pale, and hard (34). Another patient had marked brain swelling and softening, cerebellar herniation, congested and opacified leptomeninges, and severe periventricular necrosis with numerous “track-like” spaces, probably representing large malacic areas (35). A third patient had numerous partially confluent areas of yellow-tan to red-tan discoloration in the deep cerebral cortex and subcortical white matter (51). A fourth case of a patient examined at autopsy involved an older woman who was affected by Alzheimer's disease and died due to pulmonary thrombosis (52). The only gross brain abnormality was mild, diffuse cerebral atrophy, and only a small number of lesions, consisting of larvae walled off in granulomas, were detected in the deep white matter of her frontal lobe. The small number and localization of lesions in the frontal lobe, especially in a patient with dementia secondary to an Alzheimer's condition, may explain the asymptomatic baylisascariasis that was detected. It was felt that these parasite-related lesions were incidental and unlikely to have been contributors to her condition and that her confusion and altered behavior probably led to accidental infection through geophagia (52). What this case and the others indicate is that if a brain biopsy is elected for diagnosis, a neurosurgeon should sample the deep white matter, since this appears to be the area predominantly affected by lesions and the presence of larvae (6, 36). However, it is doubtful such approaches would be justified in most cases in light of the present availability of good serologic methods (1, 4, 6, 7).
Pathology studies of experimental animal models and natural cases have shown that the cerebrum, cerebellum, brain stem, and spinal cord can all be affected by the migration of larvae (1, 89, 90, 100). Hemorrhagic and malacic migration tracks were seen throughout the brains of primates experimentally infected with B. procyonis and were directly associated with migrating larvae (90). As larvae migrate further, early hemorrhagic lesions quickly became infiltrated with macrophages and other inflammatory cells, and hemorrhagic migration tracks could be traced for over a centimeter in coronal slices of brain (90). With extensive brain involvement, there is histopathologic evidence of widespread necrotizing meningoencephalitis and neurodegeneration, sometimes progressing to nonspecific gliosis and sclerosis (1, 34, 35, 51, 91; K. R. Kazacos, unpublished). The distribution of lesions and B. procyonis larvae in the CNS was depicted by Sato et al. in a rodent model (103) and was compared to the distribution of lesions associated with B. transfuga and T. canis. B. procyonis invasion and distribution were much more extensive than those seen with the other species and resulted in more-serious clinical disease despite dosages that were 10 to 50 times lower than those used with the other species (103).
Gross pathological changes related to visceral migration are rarely noted in clinically affected humans or animals with baylisascariasis, except in cases in which they were examined within several days of actual infection. Then, pulmonary hemorrhages and brown discoloration of the lungs (hemosiderosis) may be seen (1, 91). However, early histopathologic evidence of visceral migration, consisting of hemorrhagic and inflammatory migration tracks and associated tissue inflammation in the heart, musculature, etc., has been noted in various animals (1, 90, 115, 116). Usually, visceral lesions are limited to the finding of larval granulomas in various tissues (1, 6, 90, 91, 115).
Histopathologic lesions of Baylisascaris OLM-DUSN are known only from experimental animals (as human eyes have not yet been examined to this effect), but the findings are dramatic. In nonhuman primates and rodents, Baylisascaris OLM-DUSN results in extensive retinal and choroidal disruption, pyknosis of outer and inner nuclear layer nuclei, hyperplasia of the pigment epithelium, and eosinophilic retinitis, choroiditis, vitritis, and optic neuritis (1, 2, 91, 99, 113). In areas of migration tracks, there is total retinal disruption and necrosis with extensive vasculitis (99, 113). Eventually, larvae are walled off in retinochoroidal granulomas such as occurs in other tissues (99, 113).
Walling off larvae in granulomas is a typical end-stage reaction for long-term survival of larvae in paratenic hosts and also occurs in humans, in the viscera, musculature, and, eventually, in the CNS (1, 2, 91). In mammals, including primates, granulomas may be numerous on the large and small intestinal serosas, in the mesentery, on or within the heart, lungs, thoracic vessels, and perihilar tissues, and throughout the musculature, favoring an anterior distribution (1, 90, 91). Similarly, in four patients with baylisascariasis, well-circumscribed larval granulomas (sometimes fibrotic or calcified) were described in various tissues, including the CNS (34–37). In the CNS, granulomas characteristically involve gliosis rather than fibrosis (1, 91). If a patient goes to autopsy, it is important for the pathologist to look for larval granulomas in other sites besides the CNS, since living larvae may be expressed and identified from these lesions, corroborating a Baylisascaris infection even before histopathologic evaluation is undertaken (1).
Eosinophils are typically observed in combination with macrophages, lymphocytes, and plasma cells in early and more-extensive migratory lesions but may dissipate in later CNS reactions as larvae become encapsulated and the brain becomes more sclerotic (34). However, their degranulation products (major basic protein) are still able to be detected in migration tracks and malacic areas as well as granulomas (91, 98). In one patient who died of a cardiac pseudotumor, there was extensive eosinophilic infiltration of the heart that was especially associated with a large polypoid mass containing a degenerated larva (37).
Unless they are present in large numbers or multiple sections are examined, finding larvae in histopathological sections may be difficult (1, 91). For this reason, larval isolation methods are recommended at autopsy, if possible, in addition to routine pathology methods (1). When Baylisascaris larvae are found in the tissues, they have features that facilitate a histopathological diagnosis distinguishing such infections from those by other tissue-dwelling nematodes that affect humans and other animals. Their best identification features are seen in cross-sections through the midbody region (1, 91). Baylisascaris larvae typically measure 60 to 70 μm in maximum diameter and are 1,500 to 2,000 μm in length. Their diameter helps to differentiate them from other common nematode larvae with single lateral alae, including Toxocara canis (14 to 20 μm in diameter), Toxocara cati (12 to 16 μm), Toxascaris leonina (25 to 28 μm), and Ascaris lumbricoides (35 to 45 μm) (1, 34, 91). Baylisascaris larvae have large, paired lateral alae and a large, centrally located intestine. The intestine is lined with columnar cells that contain cytoplasmic granules and have microvilli at their cell surface. The intestine is compressed by paired lateral cords that support lateral excretory columns which are smaller than the intestine, roughly triangular in shape, and slightly dissimilar in size and have prominent canaliculi. There are 4 to 8 large muscle cells per quadrant that extend into the pseudocoelom (polymyarian-coelomyarian type). Three hypodermal nuclei are usually visible in the lateral chords, just below the cuticle (1, 91).
Parasitological Diagnosis
When larvae are present in a tissue, a parasitological diagnosis is obtained when the larvae are recovered and identified directly, as was done at autopsy in one case (35). Larvae may be isolated by several methods, including a modified Baermann technique, artificial digestion, or brain squash techniques (1). Larvae may also be detected and identified in tissue sections, as described in the Pathology section above. For 7/25 of the cases reviewed here, a parasitological diagnosis was confirmed (34–37, 41, 51, 52).
In cases of OLM and DUSN, larvae can sometimes be observed in the retina during fundoscopic examinations. They may be hard to detect, and it might take repeated examinations before they are seen. In one case, the parasite was not appreciated ophthalmoscopically but was subsequently seen in fundus photographs (31). The larvae are large (1,500 to 2,000 long by 60 to 70 μm in diameter) compared with the other common types of nematode larvae that may occur in the eye, including Toxocara spp. (350 to 450 by 16 to 19 μm) and Ancylostoma spp. (575 to 700 by 22 μm) (22, 31, 74, 75, 110). For several patients with OLM-DUSN, a history of exposure to raccoons and raccoon feces in the surroundings of the patient's residence or neighborhood or through their activities was noted (22, 31, 109, 112, 114), and two patients had pet raccoons (74, 75). This is consistent with the finding of a large nematode larva in the patient's eye, further supporting a diagnosis of baylisascariasis, even though serology results were indeterminate. This is important, since most cases of OLM and DUSN represent a lower larval burden, often resulting in lower levels of peripheral antibodies and less-intense blood eosinophilia (1, 2, 31).
Epidemiological investigations have the potential to reveal the presence of Baylisascaris in the environment surrounding a patient's home, and they represent a significant step in the diagnostic sequence recommended by Rowley et al. (36). Soil samples and raccoon scats can be examined for eggs using any one of several fecal sedimentation and/or flotation concentration methods employing zinc sulfate (1.18 specific gravity [SG]), Sheather's sugar solution (1.25 to 1.27 SG), other flotation solutions, or a formalin ethyl-acetate procedure. These methods are routinely used for detecting Baylisascaris in the environment and in raccoon feces (23, 24, 69, 117, 118). Because false-negative stool examination results can also occur (23, 24), despite the large number of eggs that are eliminated, it is recommended that three daily fecal samples be examined before a raccoon is judged to be negative for Baylisascaris (23).
Molecular Diagnosis (Antibodies and DNA Detection)
Immunoglobulin G (IgG) antibodies can be detected in serum, CSF, and ocular fluids, preferably by enzyme-linked immunosorbent assay (ELISA) or immunoblotting but also by immunofluorescence staining of frozen sections of third-stage larvae (2, 6, 39) (Table 2). Both ELISA and immunoblotting methods are quite sensitive, since patients with clinical CNS disease usually have strongly positive immunological results from both serum and CSF samples (119, 120, 121) (Table 2). Brain biopsies or analyses of autopsy material have also confirmed a diagnosis of baylisascariasis in several of these patients (34, 35, 36, 41). Also, using the B. procyonis ES-Ag ELISA, 76 of 259 (29.3%) nonhuman primates tested from six major zoos were seropositive, with 15 high-positive reactors, further indicating just how common and widespread exposure to this parasite is (122).
Excretory-secretory antigens (ES-Ag) have been used in ELISA and Western blot assays, and the fraction with a molecular mass of 30 to 45 kDa appears to be a good target for the specific detection of anti-Baylisascaris antibodies (97, 119). There are epitopes that exhibit cross-reactivity between B. procyonis antigens and other parasites, including Toxocara canis, but nonspecific reactivity can be removed by carbohydrate oxidation, leading to the selection of the most specific antigenic components in the 30-to-45-kDa fraction (33, 97).
The Veterinary Parasitology Laboratory at Purdue University College of Veterinary Medicine has made significant contributions to the knowledge currently available regarding baylisascariasis and was the reference laboratory for serology testing, parasitological studies, and the development of molecular tests. Human serum samples were collected and tested for over 22 years, and these have been used to evaluate the capacity for ELISA and Western blot assays to confirm the diagnostic value of 30-to-45-kDa antigenic components (119). In the series of patients reviewed here, only one was also positive for toxocariasis serology and was considered to harbor a dual infection (45, 119).
When a cDNA library was generated from third-stage larvae and was screened, repeated antigen 1 (RAG1) was identified. RAG1 is an ES-Ag protein with 12 tandem repetitions of a 12-amino-acid sequence. Following expression of RAG1 in a prokaryotic system (123), recombinant B. procyonis RAG1 (rBpRAG1) was subsequently tested in ELISA against 384 human serum samples. The estimated sensitivity and specificity of the rBpRAG1 ELISA were 85% and 86.9%, respectively, compared to only 39.4% specificity for the B. procyonis ES-Ag ELISA (120), but cross-reactivity was still detected in 25% of the samples containing Toxocara-positive serum (120). The rBpRAG1 antigen was donated to the Centers for Disease Control and Prevention (CDC), Atlanta, GA, USA, and the National Reference Centre for Parasitology, Canada (NRCP-CA), whose researchers worked jointly to develop a Western blotting assay for the diagnosis of human baylisascariasis (121). In Western blot assays of 680 samples, detection by rBpRAG1 had an overall sensitivity of 88% and a specificity of 98% (121). Moreover, no (0/66) cross-reactivity with Toxocara-positive serum samples was observed, although some (8/280) reactivity was detected with the sera obtained from noninfected patients and with sera from patients with strongyloidiasis (1/8), malaria (3/10), and filariasis (1/7). However, it was pointed out that if the full infection history of these test sera was not known, e.g., if possible cross-reactors might also be infected with Baylisascaris as covert dual infections, the actual levels of sensitivity and specificity of the assay might even be higher than reported (121). This Western blot assay employing rBpRAG1 is currently considered the “gold standard” for serodiagnosis of baylisascariasis and is being performed by the CDC and the NRCP-CA (121).
Rapid advances are being made in the molecular characterization and phylogenetic comparison of Baylisascaris species (76, 124–127). The complete mitochondrial genomes of B. procyonis (124), B. schroederi, B. ailuri, and B. transfuga (125) have been published, and mitochondrial and nuclear markers have been developed for B. columnaris (76). Molecular tests have also been developed for environmental investigations and for the diagnostic identification of Baylisascaris DNA in definitive host fecal samples and tissue samples from biopsies and autopsies performed in clinical cases of NLM (128–131). Specialists are usually required for larval identification, although even their experience is not always sufficient to differentiate B. procyonis from other types of larvae, particularly within the genus Baylisascaris. Ideally, molecular tests would provide a rapid identification of B. procyonis, separating it from closely related species, including B. columnaris (76), and would also serve as a tool for epidemiologic studies and sanitary management, considering the close association between raccoons and humans that can lead to the contamination of soil, water, and food with eggs of Baylisascaris (128, 129). These tests are also very important for host-based or environmental diagnosis in cases in which captive animals may be at risk (130).
The cytochrome c oxidase subunit 2 (cox2) gene is the most-studied gene for the detection of Baylisascaris species and indeed appears to more specifically differentiate between this genus and other ascarids (128, 129). Two types of PCR assays targeting the cox2 gene of B. procyonis were able to detect single larvae and as few as 20 unembryonated eggs per gram of canine feces (128) or as few as 25 eggs in soil and 5 eggs in water samples (129). Both assays were specific for Baylisascaris spp., and one of the two could separate B. procyonis and B. columnaris from B. transfuga (128); however, it could not discriminate between B. procyonis and B. columnaris, because the cox2 sequences of these two species differ by only two bases (128). Other examples of cross-reactivity within the ascarid group have also been observed at the molecular level, especially in cases in which certain mitochondrial markers were compared (127) or very closely related species like B. procyonis and B. columnaris were examined (128, 129). However, because of several polymorphisms in both mitochondrial and nuclear markers of B. columnaris, it was hypothesized that these could potentially be used to differentiate between these two species in molecular diagnostic assays, including by PCR (76). This information is relevant to epidemiological investigations and to the development of control measures and yet is not as important for clinical management of cases, since the treatments for the two infections are similar (1).
As part of their studies, Dangoudoubiyam et al. (128) used their real-time PCR assay to identify Baylisascaris DNA in formalin-fixed tissues. It worked well for identifying Baylisascaris in tissue sections of brain from a porcupine naturally infected with NLM. This is an important diagnostic tool because, even when larvae are found in a histopathological section, confirmation of larval identity is difficult unless typical morphological features are present, and even then a level of expertise is needed. No case reports of studies that have used this new method have been published to date; however, it would have definite usefulness in molecularly identifying Baylisascaris larvae seen in histopathologic sections from clinical cases to the genus level and at some point may be able to identify which Baylisascaris species is actually involved. Additional investigations, using a larger number and array of samples, are needed to assess the wider utility of this assay in the diagnosis of this parasite.
TREATMENT
Early diagnosis and treatment of baylisascariasis are critical if one is to have hope of improvement and favorable outcome (2, 6, 55). Because of the lag time in the development of clinical CNS signs (1), by the time such signs are evident, CNS damage is already taking place and may worsen quickly (1, 2). Therefore, the prognosis for CNS baylisascariasis, especially in cases of heavy infections, is often poor with or without treatment (2, 6, 25, 54, 55). Despite this, the goal of effective treatment is to kill off inciting larvae and control the accompanying inflammatory response without making the condition of the patient worse. Of the drugs that exhibited a protective effect in experimental infections, albendazole is currently considered the drug of choice for treating B. procyonis infections in humans. It is well absorbed, achieves good tissue levels, crosses the blood-brain barrier well, and is well tolerated (2, 5, 6, 7).
Mice treated in experiments performed with administration of oral albendazole at 25 to 50 mg/kg of body weight on days 1 to 10 p.i. were protected 100% from developing CNS disease, which killed all untreated mice (132, 133). Unfortunately, extrapolation of these dosages to humans, especially children, is difficult and current recommendations are based mainly on results of empirical treatment of human cases, using the regimens described above as guidelines. The usual dosages of albendazole administered for treating human patients are 20 to 40 mg/kg/day or 400 mg twice a day (b.i.d.) for a minimum of 10 days and often for 3 to 4 weeks (38, 40, 42, 44, 49) (Table 1). Ivermectin is also larvicidal in somatic tissues, but it does not cross the blood-brain barrier very well, thereby lowering its efficacy against larvae in the CNS (1, 134) and preventing its effective use for the treatment of NLM (1). In numerous clinical cases of B. procyonis NLM in animals and in a child treated with ivermectin, the subjects failed to improve and continued to deteriorate, and living larvae were later recovered from the brain (1). Current recommendations are to start albendazole as quickly as possible in probable or suspected cases (CNS disease with CSF eosinophilia and likely exposure) while serology and other diagnostic testing are being pursued (2, 6).
Although concomitant corticosteroid treatment is recommended and commonly used (2, 6), data from the small number of clinical observations prevent any conclusive remarks. Experimental data are also inconclusive (1, 133), and currently, the administration of anthelmintics alone, or in combination with corticosteroids, remains an empirical choice. However, eosinophilic inflammation of the CNS is a major problem in baylisascariasis and likely contributes to both the pathology and the clinical signs (37, 39, 55, 91). Control of inflammation is the goal of anti-inflammatory drug treatments for baylisascariasis, similarly to the treatment of other helminthic infections affecting the CNS (10, 102, 135). There is also the concern regarding the potentially deleterious effects of liberating antigens when the larvae are killed, thus possibly increasing CNS inflammation to even greater levels (1, 7, 91, 136).
There are several indications that early treatment of baylisascariasis is very important. Early and aggressive intervention with albendazole and steroids in clinical cases appears to have contributed to the improvement or recovery of infected human patients by obviating more-severe damage (Table 1) (5, 7, 42, 44, 49). Thus, it is important to avoid any delay in the start of treatment as soon as baylisascariasis is suspected, even if the diagnosis at the time is based only on clinical presentation and/or epidemiological history and awaits serological confirmation. The empirical use of albendazole (administered for 10 days at the dose cited above) is also recommended for the treatment of known or probable exposures and suspected cases, for example, when a child is known to have or suspected of having ingested material from a raccoon latrine (5, 6, 25, 54, 55). This “preventive” treatment after a known exposure is especially important given the potentially devastating effects that B. procyonis can have on the CNS as well as the small window of opportunity available to stop the larvae before they reach the brain (1, 2, 6, 55).
Laser photocoagulation of the parasite is recommended for the treatment of OLM-DUSN when larvae are seen by fundoscopy and are away from critical areas (1, 2, 6, 54, 55). This procedure has been shown to be successful in killing larvae (22, 31, 109, 110) and is recommended especially during the early stages of infection if possible. Generally, corticosteroids are indicated in OLM to reduce the inflammatory reaction associated with the killing of worms but do not appear to be necessary when photocoagulation is used. In contrast, anthelmintics are not considered universally effective and are not recommended, except in cases in which OLM exists concomitantly with NLM or VLM or in which additional parasites are suspected (31).
PREVENTION AND CONTROL
Since baylisascariasis is a condition characterized by severe damage to the CNS tissues and is associated with problems involving early diagnosis and effective treatment, preventive measures are of utmost importance. Those designing prevention and control programs need to consider (i) how to reduce environmental contamination with infective eggs, (ii) how to limit or prevent contact with contaminated areas or materials, and (iii) how to educate the public and medical community about the dangers posed by this parasite (for a comprehensive review, see Kazacos [1]).
Reducing environmental contamination with eggs is best approached by treatment, removal, and/or translocation of infected raccoons in an area. Treatment of pet animals is easily accomplished (1), but treatment of wild populations takes novel approaches, such as anthelmintic baiting (discussed below). Because of the potential dangers, keeping raccoons as pets, particularly in households with young children, should be discouraged (1, 25, 55). To limit or prevent contact with contaminated areas or materials, people have to learn of the danger and how to identify the raccoon feces and latrines that they may encounter in their environment so that they can keep children away from these sites. In addition, contaminated areas, especially those near peoples' homes or businesses, should be decontaminated and remediated to remove or kill any infective eggs that may be present (1, 25, 55).
On a community level, identification and evaluation of contaminated areas, i.e., raccoon latrines in neighborhoods, are essential for the optimization of control measures that are needed to address the potential risk of transmission in those environments and where dangerous areas are located (9, 63, 73). Using these approaches, Roussere et al. (63) mapped the locations and assessed the infectivity (B. procyonis eggs) of raccoon latrines in two contiguous Northern California communities; one (Pacific Grove) that had instituted a raccoon control program (because of a case of NLM in a child as well as resident complaints) and one (Carmel) that did not. They clearly showed the benefits of control by finding a much lower density of latrines in Pacific Grove (8.7/hectare) than in Carmel (21.7/hectare) following raccoon trapping and removal. Page et al. (73) mapped raccoon latrines in suburban Chicago, IL, and examined attributes of yards that might attract or deter raccoons. They found a positive association between the presence of raccoon latrines and the availability of food sources such as bird feed and that the presence of an outdoor pet seemed to be a deterrent. Other studies related to landscape attributes, such as land use, forest fragmentation, and urbanization and how these affect raccoons, paratenic hosts, and the transmission dynamics of B. procyonis in human-dominated landscapes, are being done in urban and suburban areas; for a detailed review, see Page (9). These detailed epidemiological studies of transmission variables are pivotal to establish predictions in these areas with regard to latrine occurrence and potential risk to humans (9, 63, 73, 83). The regions and areas that are more prone to transmission can be better predicted by using mathematical modeling and geospatial risk maps so that precautions and/or remediation can be undertaken.
Environmental risk evaluation is thus assessed based on (i) necropsies of raccoons (trapped or road kill), to directly assess prevalence of infection; (ii) parasitological examinations of feces collected from live trapped raccoons, to assess egg shedding, prevalence of patency, etc.; and (iii) performance of raccoon latrine surveys as an indicator of contamination with infective eggs and thus of direct risk (24, 118). Raccoon latrine surveys have usually been performed by pooling several scats for analysis by homogenization and by the use of a fecal flotation method to recover the eggs present (118). Only the latrine surveys represent a direct approach for establishing the risk of transmission to humans and other animals (9, 24, 118). Both the latrine size and the number of scats per latrine are confounders for estimating the risk of transmission, and assessment of individual scats, rather than assessment of a pooling of scats from within a latrine, has provided a more accurate method for evaluation of risk (118).
In addition to removal and translocation of raccoons, anthelmintic baiting of raccoons is gaining increased attention as an effective measure for lowering the risk of transmission (9). Page et al. (137, 138) and Smyser et al. (139) demonstrated that this type of control measure can reduce both the amount of eggs in an environment and the prevalence of larvae in paratenic hosts. For example, Page (137) saw a >3-fold decline in numbers of eggs in latrines in treatment patches versus controls, and 1 year later there was a significant reduction in numbers of B. procyonis larvae in mice from treated versus control patches, which has direct relevance to human infections. Progress is currently being made in the development of acceptable baits and delivery systems as well as their utilization in the control of B. procyonis (140). Bait deworming of raccoons should prove to be not only a useful method for reducing latrine contamination in zoos or the environment of endangered species (9) but also very important for decreasing possible transmission to humans in areas where raccoons are common. Rather than using the approach of trapping and removing raccoons, which would create niche voids filled by others, from a public health standpoint it may be wiser to maintain stable local raccoon populations but to decrease the prevalence of B. procyonis by bait deworming and latrine cleanup/decontamination (1, 9).
When people encounter raccoon latrines around their homes, whether on a deck or patio, on a backyard woodpile, or at the base of a tree, these areas need to be treated with caution, especially if there are young children or pets in the home (1, 9, 55). People also need to know that raccoons establish latrines in elevated locations such as on the rooftops of homes and garages and that these also pose a risk due to contamination of the areas below (1, 63). These latrines all need to be avoided and, if possible, decontaminated, and raccoons need to be discouraged from reusing them. B. procyonis eggs are surprisingly resistant to damage and long-lasting in the environment (1, 25), and it takes special procedures to deal with them (1). Contaminated material, including raccoon feces, leaves and other debris, and soil can be carefully removed and discarded or burned and the area then treated with some form of heat to kill residual eggs (1).
B. procyonis eggs have been found to be resistant to 90 min of treatment in undiluted household bleach and to survive at least 6 months at −15°C (26). Thus, for swimming pools that may be contaminated by raccoons, complete filtering or draining is indicated, since chlorination may not guarantee the killing of embryonated eggs (26). The application of some form of heat, especially by the use of a steam-producing device or a propane flame gun, has been found to be the most effective decontamination method (1, 2, 6, 25). The thermal death point of embryonated B. procyonis eggs is actually relatively low, and they are rendered inviable by exposure to a temperature of 62°C for less than 1 min (26, 141). This temperature also inactivates the eggs of other ascarids, including A. suum and A. lumbricoides (142). This temperature is easily achievable by several means, including the use of regular and point-of-use household water heaters and portable steam cleaners as well as of water heated on stoves (for example, in a large teapot, etc.) (1). For additional details on decontamination, see Kazacos (1).
Considering the potential seriousness of baylisascariasis, education of the public about B. procyonis and its transmission is clearly warranted in order to promote behavioral changes for both adults and children that would prevent infection (1, 2, 5–8). In particular, common-sense measures of personal hygiene (e.g., handwashing) and avoidance of contaminated areas where raccoons and their latrines are prevalent are simple and cost-free. Children need to be taught to recognize and avoid raccoon latrines and to wash their hands after playing outside or with animals (2, 4, 6, 7, 25, 55). Not providing anthropogenic food sources (such as pet food or garbage) at or near homes is an important preventive measure, since evidence indicates that it serves as an attractant and that raccoons often establish latrines nearby (1, 4, 9, 30). In a study in Southern California, pet food was found in the stomachs of 43% of raccoons examined (143). The feeding and keeping of wild animals as pets, especially raccoons, should also be strongly discouraged (1, 2, 4, 6, 7, 25, 55).
Currently, there is no vaccine to limit the susceptibility of hosts to B. procyonis infection or to modulate morbidity. Inorganic pyrophosphatases (PPases) are pivotal in the development of the larval stages of several nematodes, and one homologue of PPases (Bsc-PYP-1) from B. schroederi has been identified. Its antigenicity has been demonstrated, and it may be considered a vaccine candidate against baylisascariasis (144). Considering the demonstrated homology and potential cross-antigenicity of PPases from other ascarid species, it is possible that this or other PPases will be identified as candidates for the development of vaccines against B. procyonis (144).
CONCLUDING REMARKS
This review summarizes several perspectives regarding baylisascariasis as a zoonotic infection, focusing on the clinical and diagnostic aspects of human cases. Much has been learned over the past 35 or so years about B. procyonis and the diseases that it causes, indicating that it is a common and widespread parasite of raccoons with the potential of causing very serious neurologic and ocular disease in humans accidentally infected with the larvae. Because of its commonness and the close association of raccoons with humans, there are increasing data on the risks of human infection, and B. procyonis should be given increased consideration by physicians dealing with clinical cases, especially those involving eosinophilic meningoencephalitis, ocular larva migrans, and DUSN.
However, despite the studies that have been conducted and the advances made in our knowledge of this disease, there remains a need for (i) seroepidemiological population-based surveys to better determine the occurrence of asymptomatic covert infections and to promote a better understanding of transmission dynamics; (ii) assessment of better strategies of prevention and control, including more-effective methods for dealing with the parasite in raccoon populations, e.g., using anthelmintic baiting and other means; (iii) the identification of additional recombinant antigens in order to further improve our ability to achieve accurate immunodiagnosis in clinical cases; (iv) extended evaluations of DNA detection methods in tissue, serum, and CSF samples; (v) further drug and vaccine development and testing; and (vi) improved educational strategies to better induce behavioral changes in human populations aimed at prevention.
Whether there are more-appropriate combinations of anthelmintics and anti-inflammatory drugs for the treatment of baylisascariasis remains to be evaluated. An important endpoint for experimental models would be to find drugs or drug combinations that kill larvae effectively in the CNS without exacerbating inflammatory reactions resulting from larval death. It may be possible to use substances that modulate the parasite's pathogenesis by interfering with its production of ES-Ag or that inhibit the host's reaction to these antigens. The most current and effective recommendation for treatment is that albendazole and corticosteroids should be administered as soon as possible once a suspicion of baylisascariasis is established (2, 5–8, 41). However, dosages are empirically determined at this point, and identification of minimally effective doses, especially of albendazole, should be sought for use in human cases. The identification and monitoring of areas of endemicity, particularly on a local level, in combination with having well-informed public health workers are also essential to face the ongoing challenges of prevention of baylisascariasis in humans.
ACKNOWLEDGMENTS
We have no conflict of interests.
C. Graeff-Teixeira is the recipient of a CNPq-Brazil research fellowship (Bolsa de Produtividade em Pesquisa) and grant 307005/2014-3. A. L. Morassutti has been awarded a CAPES Edital 32 (Parasitologia Básica) fellowship and grant. K. R. Kazacos has received numerous grants and awards for research on Baylisascaris from the United States Department of Agriculture, Indiana Department of Natural Resources, Purdue University Agricultural Experiment Station, Purdue School of Veterinary Medicine, and Memphis Zoo Foundation.
Biographies

Carlos Graeff-Teixeira, M.D., Ph.D., graduated at the Medical School, University of Passo Fundo, and was a clinical fellow, Internal Medicine at Hospital das Clinicas, UFRGS, Porto Alegre, Brazil (1980 to 1982). He got a M.Sc. degree on Infectious Diseases at UFRJ, Rio de Janeiro (1986), and Ph.D. in Tropical Medicine at Instituto Oswaldo Cruz, FIOCRUZ, Rio de Janeiro (1991). He was a research fellow at the National Institute for Medical Research, London (1990), at R. M. E. Parkhouse's laboratory. He is a past president of the Brazilian Society for Parasitology (2000 to 2004). Since 1992 he has been Professor of Parasitology at the Ecology and Diversity Department, Faculdade de Biociencias, Pontifical Catholic University of Rio Grande do Sul (PUCRS), Porto Alegre, Brazil. He is also head of the Molecular Parasitology Laboratory at the Instituto de Pesquisas Biomedicas at the same university, a reference laboratory for diagnosis of eosinophilic meningoencephalitis. He has been studying the diagnosis and epidemiology of Angiostrongylus costaricensis since 1978 and of A. cantonensis since 2000.

Alessandra Loureiro Morassutti, M.Sc., Ph.D., graduated at the Biological School, Pontifical Catholic University of Rio Grande do Sul (PUCRS) (1999). She got her M.Sc. degree in molecular and cellular biology at Federal University of Rio Grande do Sul (2004) and Ph.D. in zoology at PUCRS (2011). She was a research fellow at the Division of Parasitic Diseases and Malaria, Centers for Disease Control and Prevention (CDC), Atlanta, GA, USA (2010 and 2011), and a postdoctoral fellow (2012) at School of Biology, Parasitology Group, Nottingham University, Nottingham, United Kingdom. In 2014, she became Associate Professor of Parasitology at the Medical School and the Biosciences School at PUCRS. Her main research interests have been in genomics and proteomics of parasitic helminths and both molecular and morphological diagnosis of parasitic infections.

Kevin R. Kazacos, D.V.M., Ph.D., Dipl.A.C.V.M., is Professor Emeritus of Veterinary Parasitology and former Director of Clinical Parasitology at the College of Veterinary Medicine, Purdue University, where he was on the faculty from 1979 to 2014. He is a Charter Diplomate of the American College of Veterinary Microbiologists (Parasitology) and a founding member and past president of the Companion Animal Parasite Council. Dr. Kazacos has done extensive research on zoonotic helminths, particularly those that cause visceral, ocular, and neural larva migrans. He is best known for his comprehensive studies on the raccoon ascarid, Baylisascaris procyonis, as a cause of animal and human disease. Dr. Kazacos is the author or coauthor of 143 refereed journal articles, 12 book chapters or monographs, and 184 research abstracts and proceedings and has given over 580 presentations at scientific and professional meetings.
REFERENCES
- 1.Kazacos KR. 2001. Baylisascaris procyonis and related species, p 301–341. In Samuel WM, Kocan AA, Pybus MJ (ed), Parasitic diseases of wild mammals, 2nd ed Iowa State University Press, Ames, IA. doi: 10.1002/9780470377000. [DOI] [Google Scholar]
- 2.Kazacos KR, Jelicks LA, Tanowitz HB. 2013. Baylisascaris larva migrans, p 251–262. In Garcia HH, Tanowitz HB, Del Brutto OH (ed), Handbook of clinical neurology 114 (3rd series). Neuroparasitology and tropical neurology. Elsevier B.V., Amsterdam, the Netherlands. [DOI] [PubMed] [Google Scholar]
- 3.Sorvillo F, Ash LR, Berlin OGW, Yatabe J, Degiorgio C, Morse SA. 2002. Baylisascaris procyonis: an emerging helminthic zoonosis. Emerg Infect Dis 8:355–359. doi: 10.3201/eid0804.010273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Murray WJ. 2004. Raccoon roundworm infection (baylisascariasis): a zoonosis of pediatric and public health concern, p 159–175. In Scheld W, Murray B, Hughes J (ed), Emerging infections 6. ASM Press, Washington, DC. [Google Scholar]
- 5.Murray WJ, Kazacos KR. 2004. Raccoon roundworm encephalitis. Clin Infect Dis 39:1484–1492. doi: 10.1086/425364. [DOI] [PubMed] [Google Scholar]
- 6.Gavin PJ, Kazacos KR, Shulman ST. 2005. Baylisascariasis. Clin Microbiol Rev 18:703–718. doi: 10.1128/CMR.18.4.703-718.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wise ME, Sorvillo FJ, Shafir SC, Ash LR, Berlin OG. 2005. Severe and fatal central nervous system disease in humans caused by Baylisascaris procyonis, the common roundworm of raccoons: a review of current literature. Microbes Infect 7:317–323. doi: 10.1016/j.micinf.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 8.Shafir SC, Wise ME, Sorvillo FJ, Ash LR. 2006. Central nervous system and eye manifestations of infection with Baylisascaris procyonis. Curr Infect Dis Rep 8:307–813. doi: 10.1007/s11908-006-0076-7. [DOI] [PubMed] [Google Scholar]
- 9.Page LK. 2013. Parasites and the conservation of small populations: the case of Baylisascaris procyonis. Int J Parasitol Parasites Wildl 2:203–210. doi: 10.1016/j.ijppaw.2013.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Magnaval J-F, Glickman LT, Dorchies P, Morassin B. 2001. Highlights of human toxocariasis. Korean J Parasitol 39:1–11. doi: 10.3347/kjp.2001.39.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fan CK, Holland CV, Loxton K, Barghouth U. 2015. Cerebral toxocariasis: silent progression to neurodegenerative disorders? Clin Microbiol Rev 28:663–686. doi: 10.1128/CMR.00106-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beaver PC. 1969. The nature of visceral larva migrans. J Parasitol 55:3–12. [PubMed] [Google Scholar]
- 13.Anderson DC, Greenwood R, Fishman M, Kagan IF. 1975. Acute infantile hemiplegia with cerebrospinal fluid eosinophilic pleocytosis: an unusual case of visceral larva migrans. J Pediatr 86:247–249. doi: 10.1016/S0022-3476(75)80481-1. [DOI] [PubMed] [Google Scholar]
- 14.Bauer C. 2013. Baylisascariosis—infections of animals and humans with ‘unusual’ roundworms. Vet Parasitol 193:404–412. doi: 10.1016/j.vetpar.2012.12.036. [DOI] [PubMed] [Google Scholar]
- 15.Sakla AA, Donnelly JJ, Khatami M, Rockey JH. 1989. Baylisascaris procyonis (Stefanski and Zarnowski, 1951) Ascarididae: Nematoda. I. Embryonic development and morphogenesis of second stage larvae. Assiut Vet Med J 21:68–76. [Google Scholar]
- 16.Kazacos KR. 1983. Life cycle studies on Baylisascaris procyonis in raccoons. Abstr Proc 64th Conf Res Work Anim Dis, abstr 130. [Google Scholar]
- 17.Kazacos KR. 1983. Raccoon roundworms (Baylisascaris procyonis). A cause of animal and human disease. Bulletin no. 422 Purdue University Agricultural Experiment Station, West Lafayette, IN. [Google Scholar]
- 18.Tiner JD. 1953. The migration, distribution in the brain, and growth of ascarid larvae in rodents. J Infect Dis 92:105–113. doi: 10.1093/infdis/92.2.105. [DOI] [PubMed] [Google Scholar]
- 19.Sheppard CH, Kazacos KR. 1997. Susceptibility of Peromyscus leucopus and Mus musculus to infection with Baylisascaris procyonis. J Parasitol 83:1104–1111. doi: 10.2307/3284370. [DOI] [PubMed] [Google Scholar]
- 20.Bowman DD. 1987. Diagnostic morphology of four larval ascaridoid nematodes that may cause visceral larva migrans: Toxascaris leonina, Baylisascaris procyonis, Lagochilascaris sprenti, and Hexametra leidyi. J Parasitol 73:1198–1215. doi: 10.2307/3282306. [DOI] [PubMed] [Google Scholar]
- 21.Donnelly JJ, Sakla AA, Khatami M, Rockey JH. 1989. Baylisascaris procyonis (Stefanski and Zarnowski, 1951) Ascarididae: Nematoda. II. Third stage larvae, morphogenesis and migratory behaviour. Assiut Vet Med J 21:77–91. [Google Scholar]
- 22.Goldberg MA, Kazacos KR, Boyce WM, Ai E, Katz B. 1993. Diffuse unilateral subacute neuroretinitis. Morphometric, serologic, and epidemiologic support for Baylisascaris as a causative agent. Ophthalmology 100:1695–1701. [DOI] [PubMed] [Google Scholar]
- 23.Reed C, Henke SE, Kresta AE. 2012. Frequency of deposition and location of Baylisascaris procyonis eggs in raccoon feces. J Wildl Dis 48:190–194. doi: 10.7589/0090-3558-48.1.190. [DOI] [PubMed] [Google Scholar]
- 24.Page LK, Gehrt SD, Titcombe KK, Robinson NP. 2005. Measuring prevalence of raccoon roundworm (Baylisascaris procyonis): a comparison of common techniques. Wildl Soc Bull 33:1406–1412. doi: 10.2193/0091-7648(2005)33[1406:MPORRB]2.0.CO;2. [DOI] [Google Scholar]
- 25.Kazacos KR, Boyce WM. 1989. Baylisascaris larva migrans. J Am Vet Med Assoc 195:894–903. [PubMed] [Google Scholar]
- 26.Shafir SC, Sorvillo FJ, Sorvillo T, Eberhard ML. 2011. Viability of Baylisascaris procyonis eggs. Emerg Infect Dis 17:1293–1295. doi: 10.3201/eid1707.101774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Page LK, Delzell DAP, Gehrt SD, Harrell ED, Hiben M, Walter E, Anchor C, Kazacos KR. The structure and seasonality of Baylisascaris procyonis populations in raccoons. J Wildl Dis, in press . [DOI] [PubMed] [Google Scholar]
- 28.Page LK, Swihart RK, Kazacos KR. 2001. Changes in transmission of Baylisascaris procyonis to intermediate hosts as a function of spatial scale. Oikos 93:213–220. doi: 10.1034/j.1600-0706.2001.930205.x. [DOI] [Google Scholar]
- 29.Tiner JD. 1953. Fatalities in rodents caused by larval Ascaris in the central nervous system. J Mammalogy 34:153–167. doi: 10.2307/1375616. [DOI] [Google Scholar]
- 30.Murray WJ. 2002. Human infections caused by the raccoon roundworm, Baylisascaris procyonis. Clin Microbiol Newsl 24:1–7. doi: 10.1016/S0196-4399(02)80001-0. [DOI] [Google Scholar]
- 31.Saffra NA, Perlman JE, Desai RU, Kazacos KR, Coyle CM, Machado FS, Kedhar SR, Engelbert M, Tanowitz HB. 2010. Baylisascaris procyonis induced diffuse unilateral subacute neuroretinitis in New York City. J Neuroparasitol 1:N100401. doi: 10.4304/jnp/N100401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brinkman WB, Kazacos KR, Gavin PJ, Binns HJ, Robichaud JD, O'Gorman M, 2003. Seroprevalence of Baylisascaris procyonis (raccoon roundworm) in Chicago area children. Abstr 2003 Annu Meet Pediatr Acad Soc, Seattle, WA, 3–6 May 2003, abstr 1872 http://www.abstracts2view.com/pasall/authorindex.php Accessed 10 January 2015. [Google Scholar]
- 33.Cunningham CK, Kazacos KR, McMillan JA, Lucas JA, McAuley JB, Wozniak EJ, Weiner LB. 1994. Diagnosis and management of Baylisascaris procyonis infection in an infant with nonfatal meningoencephalitis. Clin Infect Dis 18:868–872. [DOI] [PubMed] [Google Scholar]
- 34.Huff DS, Neafie RC, Binder MJ, De León GA, Brown LW, Kazacos KR. 1984. Case 4. The first fatal Baylisascaris infection in humans: an infant with eosinophilic meningoencephalitis. Pediatr Pathol 2:345–352. [DOI] [PubMed] [Google Scholar]
- 35.Fox AS, Kazacos KR, Gould NS, Heydemann PT, Thomas C, Boyer KM. 1985. Fatal eosinophilic meningoencephalitis and visceral larva migrans caused by the raccoon ascarid Baylisascaris procyonis. N Engl J Med 312:1619–1623. doi: 10.1056/NEJM198506203122507. [DOI] [PubMed] [Google Scholar]
- 36.Rowley HA, Uht RM, Kazacos KR, Sakanari J, Wheaton WV, Barkovich AJ, Bollen AW. 2000. Radiologic-pathologic findings in raccoon roundworm (Baylisascaris procyonis) encephalitis. Am J Neuroradiol 21:415–420. [PMC free article] [PubMed] [Google Scholar]
- 37.Boschetti A, Kasznica J. 1995. Visceral larva migrans induced eosinophilic cardiac pseudotumor: a cause of sudden death in a child. J Forensic Sci 40:1097–1099. [PubMed] [Google Scholar]
- 38.Gavin PJ, Kazacos KR, Tan TQ, Brinkman WB, Byrd SE, Davis AT, Mets MB, Shulman ST. 2002. Neural larva migrans caused by the raccoon roundworm Baylisascaris procyonis. Pediatr Infect Dis J 21:971–975. doi: 10.1097/00006454-200210000-00017. [DOI] [PubMed] [Google Scholar]
- 39.Moertel CL, Kazacos KR, Butterfield JH, Kita H, Watterson J, Gleich GJ. 2001. Eosinophil-associated inflammation and elaboration of eosinophil-derived proteins in 2 children with raccoon roundworm (Baylisascaris procyonis) encephalitis. Pediatrics 108:e93. doi: 10.1542/peds.108.5.e93. [DOI] [PubMed] [Google Scholar]
- 40.Park SY, Glaser C, Murray WJ, Kazacos KR, Rowley HA, Fredrick DR, Bass N. 2000. Raccoon roundworm (Baylisascaris procyonis) encephalitis: case report and field investigation. Pediatrics 106:e56. doi: 10.1542/peds.106.4.e56. [DOI] [PubMed] [Google Scholar]
- 41.CDC. 2002. Raccoon roundworm encephalitis—Chicago, Illinois, and Los Angeles, California, 2000. JAMA 287:580–581. doi: 10.1001/jama.287.5.580-JWR0206-3-1. [DOI] [PubMed] [Google Scholar]
- 42.Pai PJ, Blackburn BG, Kazacos KR, Warrier RP, Bégué RE. 2007. Full recovery from Baylisascaris procyonis eosinophilic meningitis. Emerg Infect Dis 13:928–930. doi: 10.3201/eid1306.061541. [DOI] [PubMed] [Google Scholar]
- 43.Crosley CJ, Kazacos KR. 2005. Baylisascaris meningoencephalitis in three infants. Ann Neurol 58(Suppl 9):S114. [Google Scholar]
- 44.Hajek J, Yau Y, Kertes P, Soman T, Laughlin S, Kanani R, Kazacos K, Dangoudoubiyam S, Opavsky MA. 2009. A child with raccoon roundworm meningoencephalitis: a pathogen emerging in your own backyard? Can J Infect Dis Med Microbiol 20:e177–e180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chun CS, Kazacos KR, Glaser C, Bardo D, Dangoudoubiyam S, Nash R. 2009. Global neurologic deficits with baylisascaris encephalitis in a previously healthy teenager. Pediatr Infect Dis J 28:925–927. doi: 10.1097/INF.0b013e3181a648f1. [DOI] [PubMed] [Google Scholar]
- 46.Mehta P, Boyd Z, Cully B. 2010. Raccoon roundworm encephalitis. Pediatr Radiol 40:1834–1836. doi: 10.1007/s00247-010-1625-7. [DOI] [PubMed] [Google Scholar]
- 47.Perlman JE, Kazacos KR, Imperato GH, Desai RU, Schulman SK, Edwards J, Pontrelli LR, Machado FS, Tanowitz HB, Saffra NA. 2010. Baylisascaris procyonis neural larva migrans in an infant in New York City. J Neuroparasitol 1:10.4303/jnp/N100502. doi: 10.4303/jnp/N100502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kelly TG, Madhavan VL, Peters JM, Kazacos KR, Silvera VM. 2012. Spinal cord involvement in a child with raccoon roundworm (Baylisascaris procyonis) meningoencephalitis. Pediatr Radiol 42:369–373. doi: 10.1007/s00247-011-2151-y. [DOI] [PubMed] [Google Scholar]
- 49.Peters JM, Madhavan VL, Kazacos KR, Husson RN, Dangoudoubiyam S, Soul JS. 2012. Good outcome with early empiric treatment of neural larva migrans due to Baylisascaris procyonis. Pediatrics 129:e806–e811. doi: 10.1542/peds.2011-2078. [DOI] [PubMed] [Google Scholar]
- 50.Haider S, Khairnar K, Martin DS, Yang J, Ralevski F, Kazacos KR, Pillai DR. 2012. Possible pet-associated baylisascariasis in child, Canada. Emerg Infect Dis 18:347–349. doi: 10.3201/eid1802.110674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ciarlini P, Gutierrez Y, Gambetti P, Cohen M. 2011. Necrotizing eosinophilic meningoencephalitis. Abstr 52nd Annu Diagn Slide Session, 87th Annu Meet Am Assoc Neuropathologists, case 2011-06 http://neuro.pathology.pitt.edu/DSSFiles/DSSCases2011.htm. [Google Scholar]
- 52.Hung T, Neafie RC, Mackenzie IR. 2012. Baylisascaris procyonis infection in elderly person, British Columbia, Canada. Emerg Infect Dis 18:341–342. doi: 10.3201/eid1802.111046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Blizzard EL, Yabsley MJ, Beck MF, Harsch S. 2010. Geographic expansion of Baylisascaris procyonis roundworms, Florida, USA. Emerg Infect Dis 16:1803–1804. doi: 10.3201/eid1611.100549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kazacos KR. 1991. Visceral and ocular larva migrans. Semin Vet Med Surg (Small Anim) 6:227–235. [PubMed] [Google Scholar]
- 55.Kazacos KR. 2000. Protecting children from helminthic zoonoses. Contemp Pediatr 17(Suppl):1–24. [Google Scholar]
- 56.American Psychiatric Association. 1994. Diagnostic and statistical manual of mental disorder, 4th ed American Psychiatric Association, Washington, DC. [Google Scholar]
- 57.Hawass NED, Alnozha MM, Kolawole T. 1987. Adult geophagia—report of three cases with review of the literature. Trop Geogr Med 39:191–195. [PubMed] [Google Scholar]
- 58.Callahan GN. 2003. Eating dirt. Emerg Infect Dis 9:1016–1021. doi: 10.3201/eid0908.AD0908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Page LK, Swihart RK, Kazacos KR. 1999. Implications of raccoon latrines in the epizootiology of baylisascariasis. J Wildl Dis 35:474–480. doi: 10.7589/0090-3558-35.3.474. [DOI] [PubMed] [Google Scholar]
- 60.Hirsch BT, Prange S, Hauver SA, Gehrt SD. 2014. Patterns of latrine use by raccoons (Procyon lotor) and implication for Baylisascaris procyonis transmission. J Wildl Dis 50:243–249. doi: 10.7589/2013-09-251. [DOI] [PubMed] [Google Scholar]
- 61.Evans RH. 2002. Baylisascaris procyonis (Nematoda: Ascaridoidea) eggs in raccoon (Procyon lotor) latrine scats in Orange County, California. J Parasitol 88:189–190. doi: 10.1645/0022-3395(2002)088[0189:BPNAEI]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 62.Page LK, Swihart RK, Kazacos KR. 1998. Raccoon latrine structure and its potential role in transmission of Baylisascaris procyonis to vertebrates. Am Midl Nat 140:180–185. doi: 10.1674/0003-0031(1998)140[0180:RLSAIP]2.0.CO;2. [DOI] [Google Scholar]
- 63.Roussere GP, Murray WJ, Raudenbush CB, Kutilek MJ, Levee DJ, Kazacos KR. 2003. Raccoon roundworm eggs near homes and risk for larva migrans disease, California communities. Emerg Infect Dis 9:1516–1522. doi: 10.3201/eid0912.030039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Snyder DE, Fitzgerald PR. 1985. The relationship of Baylisascaris procyonis to Illinois raccoons (Procyon lotor). J Parasitol 71:596–598. doi: 10.2307/3281430. [DOI] [PubMed] [Google Scholar]
- 65.Chavez DJ, LeVan IK, Miller MW, Ballweber LR. 2012. Baylisascaris procyonis in raccoons (Procyon lotor) from eastern Colorado, an area of undefined prevalence. Vet Parasitol 185:330–334. doi: 10.1016/j.vetpar.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 66.Pipas MJ, Page LK, Kazacos KR. 2014. Surveillance for Baylisascaris procyonis in raccoons (Procyon lotor) from Wyoming, USA. J Wildl Dis 50:777–783. doi: 10.7589/2013-10-263. [DOI] [PubMed] [Google Scholar]
- 67.Kellner KF, Page LK, Downey M, McCord SE. 2012. Effects of urbanization on prevalence of Baylisascaris procyonis in intermediate host populations. J Wildl Dis 48:1083–1087. doi: 10.7589/2011-09-267. [DOI] [PubMed] [Google Scholar]
- 68.Slavinski S, Fine A, 2009. Baylisascaris (raccoon roundworm) infection with severe outcome identified in two New York City children. Health Advisory #08; New York City Department of Health and Mental Hygiene: http://www.nyc.gov/html/doh/downloads/pdf/cd/2009/09md08.pdf Accessed April 2015. [Google Scholar]
- 69.Eberhard ML, Nace EK, Won KY, Punkosdy GA, Bishop HS, Johnston SP. 2003. Baylisascaris procyonis in the metropolitan Atlanta area. Emerg Infect Dis 9:1636–1637. doi: 10.3201/eid0912.020795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Page LK, Gehrt SD, Cascione A, Kellner KF. 2009. The relationship between Baylisascaris procyonis prevalence and raccoon population structure. J Parasitol 95:1314–1320. doi: 10.1645/GE-1998.1. [DOI] [PubMed] [Google Scholar]
- 71.Yeitz JL, Gillin CM, Bildfell RJ, Debess EE. 2009. Prevalence of Baylisascaris procyonis in raccoons (Procyon lotor) in Portland, Oregon, USA. J Wildl Dis 45:14–18. doi: 10.7589/0090-3558-45.1.14. [DOI] [PubMed] [Google Scholar]
- 72.Page LK, Gehrt SD, Robinson NP. 2008. Land-use effects on prevalence of raccoon roundworm (Baylisascaris procyonis). J Wildl Dis 44:594–599. doi: 10.7589/0090-3558-44.3.594. [DOI] [PubMed] [Google Scholar]
- 73.Page LK, Anchor C, Luy E, Kron S, Larson G, Madsen L, Kellner K, Smyser TJ. 2009. Backyard raccoon latrines and risk for Baylisascaris procyonis transmission to humans. Emerg Infect Dis 15:1530–1531. doi: 10.3201/eid1509.090128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Raymond LA, Gutierrez Y, Strong LE, Wander AH, Buten R, Cordan D. 1978. Living retinal nematode (filarial-like) destroyed with photocoagulation. Ophthalmology 85:944–949. doi: 10.1016/S0161-6420(78)35596-2. [DOI] [PubMed] [Google Scholar]
- 75.Küchle M, Knorr HLJ, Medenblik-Frysch S, Weber A, Bauer C, Naumann GOH. 1993. Diffuse unilateral subacute neuroretinitis syndrome in a German most likely caused by the raccoon roundworm, Baylisascaris procyonis. Graefes Arch Clin Exp Ophthalmol 231:48–51. doi: 10.1007/BF01681701. [DOI] [PubMed] [Google Scholar]
- 76.Franssen F, Xie K, Sprong H, van der Giessen J. 2013. Molecular analysis of Baylisascaris columnaris revealed mitochondrial and nuclear polymorphisms. Parasit Vectors 6:124. doi: 10.1186/1756-3305-6-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sato H, Une Y, Kawakami S, Saito E, Kamiya H, Akao N, Furuoka H. 2005. Fatal Baylisascaris larva migrans in a colony of Japanese macaques kept by a safari-style zoo in Japan. J Parasitol 91:716–719. doi: 10.1645/GE-3374RN. [DOI] [PubMed] [Google Scholar]
- 78.Xie Y, Zhou X, Li M, Liu T, Gu X, Wang T, Lai W, Peng X, Yang G. 2014. Zoonotic Baylisascaris procyonis roundworms in raccoons, China. Emerg Infect Dis 20:2170–2172. doi: 10.3201/eid2012.140970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tokiwa T, Nakamura S, Taira K, Une Y. 2014. Baylisascaris potosis n. sp., a new ascarid nematode isolated from captive kinkajou, Potos flavus, from the Cooperative Republic of Guyana. Parasitol Int 63:591–596. doi: 10.1016/j.parint.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 80.CDC. 2011. Raccoon roundworms in pet Kinkajous—three states, 1999 and 2010. MMWR Morb Mortal Wkly Rep 60:303–305. [PubMed] [Google Scholar]
- 81.Parkanzky MC. 2015. Baylisascaris spp. in non-raccoon procyonid hosts and assessment of potential risk of human exposure. M.S. thesis. Purdue University, West Lafayette, IN. [Google Scholar]
- 82.McCleery RA, Foster GW, Lopez RR, Peterson MJ, Forrester DJ, Silvy NJ. 2005. Survey of raccoons on Key Largo, Florida, USA, for Baylisascaris procyonis. J Wildl Dis 41:250–252. doi: 10.7589/0090-3558-41.1.250. [DOI] [PubMed] [Google Scholar]
- 83.Kresta AE, Henke SE, Pence DB. 2010. Baylisascaris procyonis in raccoons in Texas and its relationship to habitat characteristics. J Wildl Dis 46:843–853. doi: 10.7589/0090-3558-46.3.843. [DOI] [PubMed] [Google Scholar]
- 84.Kerr CL, Henke SE, Pence DB. 1997. Baylisascariasis in raccoons from southern coastal Texas. J Wildl Dis 33:653–655. doi: 10.7589/0090-3558-33.3.653. [DOI] [PubMed] [Google Scholar]
- 85.Hernandez SM, Galbreath B, Riddle DF, Moore AP, Palamar MB, Levy MG, DePerno CS, Correa MT, Yabsley MJ. 2013. Baylisascaris procyonis in raccoons (Procyon lotor) from North Carolina and current status of the parasite in the USA. Parasitol Res 112:693–698. doi: 10.1007/s00436-012-3186-1. [DOI] [PubMed] [Google Scholar]
- 86.Lotze J-H, Anderson S. 1979. Procyon lotor. Mamm Species 119:1–8. [Google Scholar]
- 87.Beltrán-Beck B, Garcia FJ, Gortazar C. 2012. Raccoons in Europe: disease hazards due to the establishment of an invasive species. Eur J Wildl Res 58:5–15. doi: 10.1007/s10344-011-0600-4. [DOI] [Google Scholar]
- 88.Gey AB. 1998. Synopsis der Parasitenfauna des Waschbären (Procyon lotor) unter Berücksichtigung von Befunden aus Hessen. Dissertation. Fachbereich Veterinärmedizin, Justus Liebig Universität, Giesen, Germany: (In German.) [Google Scholar]
- 89.Furuoka H, Sato H, Kubo M, Owaki S, Kobayashi Y, Matsui T, Kamiya H. 2003. Neuropathological observation of rabbits (Oryctolagus cuniculus) affected with raccoon roundworm (Baylisascaris procyonis) larva migrans in Japan. J Vet Med Sci 65:695–699. doi: 10.1292/jvms.65.695. [DOI] [PubMed] [Google Scholar]
- 90.Kazacos KR, Wirtz WL, Burger PP, Christmas CS. 1981. Raccoon ascarid larvae as a cause of fatal central nervous system disease in subhuman primates. J Am Vet Med Assoc 179:1089–1094. [PubMed] [Google Scholar]
- 91.Kazacos KR. 1997. Visceral, ocular, and neural larva migrans, p 1459–1473. In Connor DH, Chandler FW, Schwartz DA, Manz HJ, Lack EE (ed), Pathology of infectious diseases, vol II. Appleton & Lange, Stamford, CT. [Google Scholar]
- 92.Dixon D, Reinhard GR, Kazacos KR, Arriaga C. 1988. Cerebrospinal nematodiasis in prairie dogs from a research facility. J Am Vet Med Assoc 193:251–253. [PubMed] [Google Scholar]
- 93.Ball RL, Dryden M, Wilson S, Veatch J. 1998. Cerebrospinal nematodiasis in a white-handed gibbon (Hylobates lar) due to Baylisascaris sp. J Zoo Wildl Med 29:221–224. [PubMed] [Google Scholar]
- 94.Garlick DS, Marcus LC, Pokras M, Schelling SH. 1996. Baylisascaris larva migrans in a spider monkey (Ateles sp.). J Med Primatol 25:133–136. doi: 10.1111/j.1600-0684.1996.tb00204.x. [DOI] [PubMed] [Google Scholar]
- 95.Hanley CS, Simmons HA, Wallace RS, Clyde VL. 2006. Visceral and presumptive neural baylisascariasis in an orangutan (Pongo pygmaeus). J Zoo Wildl Med 37:553–557. doi: 10.1638/06-036.1. [DOI] [PubMed] [Google Scholar]
- 96.Gozalo AS, Cheng LI, Maximova O, St Claire M, Montali RJ, Ward JM, Elkins WR, Kazacos KR. 2008. Visceral and neural larva migrans in Rhesus macaques. J Am Assoc Lab Anim Sci 47:64–67. [PMC free article] [PubMed] [Google Scholar]
- 97.Boyce WM, Asai DJ, Wilder JK, Kazacos KR. 1989. Physicochemical characterization and monoclonal and polyclonal antibody recognition of Baylisascaris procyonis larval excretory-secretory antigens. J Parasitol 75:540–548. doi: 10.2307/3282903. [DOI] [PubMed] [Google Scholar]
- 98.Hamann KJ, Kephart GM, Kazacos KR, Gleich GJ. 1989. Immunofluorescent localization of eosinophil granule major basic protein in fatal human cases of Baylisascaris procyonis infection. Am J Trop Med Hyg 40:291–297. [DOI] [PubMed] [Google Scholar]
- 99.Kazacos KR, Vestre WA, Kazacos EA. 1984. Raccoon ascarid larvae (Baylisascaris procyonis) as a cause of ocular larva migrans. Invest Ophthalmol Vis Sci 25:1177–1183. [PubMed] [Google Scholar]
- 100.Evans RH. 2002. Baylisascaris procyonis (Nematoda: Ascarididae) larva migrans in free-ranging wildlife in Orange County, California. J Parasitol 88:299–301. doi: 10.1645/0022-3395(2002)088[0299:BPNALM]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 101.Brindley PJ, Gam AA, McKerrow JH, Neva FA. 1995. Ss40: the zinc endopeptidase secreted by infective larvae of Strongyloides stercoralis. Exp Parasitol 80:1–7. doi: 10.1006/expr.1995.1001. [DOI] [PubMed] [Google Scholar]
- 102.Nicoletti A. 2013. Toxocariasis, p 217–228. In Garcia HH, Tanowitz HB, Del Brutto OH (ed), Handbook of clinical neurology 114 (3rd series), Neuroparasitology and tropical neurology. Elsevier B.V., Amsterdam, the Netherlands. [Google Scholar]
- 103.Sato H, Matsuo K, Osanai A, Kamiya H, Akao N, Owaki S, Furuoka H. 2004. Larva migrans by Baylisascaris transfuga: fatal neurological diseases in mongolian jirds, but not in mice. J Parasitol 90:774–781. doi: 10.1645/GE-3330. [DOI] [PubMed] [Google Scholar]
- 104.Wyand-Ouellette WK, Kazacos KR, DeNicola DB. 1983. Assessment of pulmonary migration of ascarids (Baylisascaris, Ascaris) by bronchopulmonary lavage. Abstr Proc 28th Ann Meet Am Assoc Vet Parasitologists, abstr 42. [Google Scholar]
- 105.Mets MB, Noble AG, Bast S, Gavin P, Davis AT, Shulman ST, Kazacos KR. 2003. Eye findings of diffuse unilateral subacute neuroretinitis and multiple choroidal infiltrates associated with neural larva migrans due to Baylisascaris procyonis. Am J Ophthalmol 135:888–890. doi: 10.1016/S0002-9394(02)01539-8. [DOI] [PubMed] [Google Scholar]
- 106.Graeff-Teixeira C, da Silva AC, Yoshimura K. 2009. Update on eosinophilic meningoencephalitis and its clinical relevance. Clin Microbiol Rev 22:322–348. doi: 10.1128/CMR.00044-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kazacos KR. 1996. Baylisascaris procyonis and other helminth larvae as causes of ocular larva migrans and DUSN. Abstr Proc Int Soc Ophthalmic Pathol Am Assoc Ophthalmic Pathol, Chicago IL, p 20. [Google Scholar]
- 108.Gass JDM, Braunstein RA. 1983. Further observations concerning the diffuse unilateral subacute neuroretinitis syndrome. Arch Ophthalmol 101:1689–1697. doi: 10.1001/archopht.1983.01040020691004. [DOI] [PubMed] [Google Scholar]
- 109.Williams GA, Aaberg TM, Dudley SS. 1988. Perimacular photocoagulation of presumed Baylisascaris procyonis in diffuse unilateral subacute neuroretinitis, p 275–280. In Gitter KA, Schatz H, Yannuzzi LA, McDonald HR (ed), Laser photocoagulation of retinal disease. Pacific Medical Press, San Francisco, CA. [Google Scholar]
- 110.Sivalingam A, Goldberg RE, Augsburger J, Frank P. 1991. Diffuse unilateral subacute neuroretinitis. Arch Ophthalmol 109:1028. doi: 10.1001/archopht.1991.01080070140052. [DOI] [PubMed] [Google Scholar]
- 111.Brasil OF, Lewis H, Lowder CY. 2006. Migration of Baylisascaris procyonis into the vitreous. Br J Ophthalmol 90:1203–1204. doi: 10.1136/bjo.2006.095323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Tarantola RM, Elkins KA, Kay CN, Folk JC. 2011. Photoreceptor recovery following laser photocoagulation and albendazole in diffuse unilateral subacute neuroretinitis. Arch Ophthalmol 129:669–671. [DOI] [PubMed] [Google Scholar]
- 113.Kazacos KR, Raymond LA, Kazacos EA, Vestre WA. 1985. The raccoon ascarid. A probable cause of human ocular larva migrans. Ophthalmology 92:1735–1743. [DOI] [PubMed] [Google Scholar]
- 114.Liu G, Fennelly G, Kazacos KR, Grose C, Dobroszycki J, Saffra N, Coyle CM, Weiss LM, Szlechter MM, Tanowitz HB. 2015. Case report: Baylisascaris procyonis and herpes simplex virus 2 co-infection presenting as ocular larva migrans with granuloma formation in a child. Am J Trop Med Hyg 93:612–614. doi: 10.4269/ajtmh.15-0272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kazacos KR, Reed WM, Kazacos EA, Thacker HL. 1983. Fatal cerebrospinal disease caused by Baylisascaris procyonis in domestic rabbits. J Am Vet Med Assoc 183:967–971. [PubMed] [Google Scholar]
- 116.Craig SJ, Conboy GA, Hanna PE. 1995. Baylisascaris sp. infection in a guinea pig. Lab Anim Sci 45:312–314. [PubMed] [Google Scholar]
- 117.Samson A, Dubay SA, Huspeni TC, Cyr A. 2012. Influence of environmental variables on Baylisascaris procyonis infection in raccoons. J Parasitol 98:1279–1282. doi: 10.1645/GE-3073.1. [DOI] [PubMed] [Google Scholar]
- 118.Smyser TJ, Page LK, Rhodes OE. 2010. Optimization of raccoon latrine surveys for quantifying exposure to Baylisascaris procyonis. J Wildl Dis 46:929–933. doi: 10.7589/0090-3558-46.3.929. [DOI] [PubMed] [Google Scholar]
- 119.Dangoudoubiyam S, Kazacos KR. 2009. Differentiation of larva migrans caused by Baylisascaris procyonis and Toxocara species by Western blotting. Clin Vaccine Immunol 16:1563–1568. doi: 10.1128/CVI.00251-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Dangoudoubiyam S, Vemulapalli R, Ndao M, Kazacos KR. 2011. Recombinant antigen-based enzyme-linked immunosorbent assay for diagnosis of Baylisascaris procyonis larva migrans. Clin Vaccine Immunol 18:1650–1655. doi: 10.1128/CVI.00083-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Rascoe LN, Santamaria C, Handali S, Dangoudoubiyam S, Kazacos KR, Wilkins PP, Ndar M. 2013. Interlaboratory optimization and evaluation of a serological assay for diagnosis of human baylisascariasis. Clin Vaccine Immunol 20:1758–1763. doi: 10.1128/CVI.00387-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zimmerman D, Dangoudoubiyam S, Kazacos KR. 2010. Human serological testing for Baylisascaris procyonis in nonhuman primates. Abstr Proc Joint Conf Am Assoc Zoo Veterinarians Am Assoc Wildl Veterinarians, p 124. [DOI] [PubMed] [Google Scholar]
- 123.Dangoudoubiyam S, Vemulapalli R, Hancock K, Kazacos KR. 2010. Molecular cloning of an immunogenic protein of Baylisascaris procyonis and expression in Escherichia coli for use in developing improved serodiagnostic assays. Clin Vaccine Immunol 17:1933–1939. doi: 10.1128/CVI.00404-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Xie Y, Zhang Z, Niu L, Wang Q, Wang C, Lan J, Deng J, Fu Y, Nie H, Yan N, Yang D, Hao G, Gu X, Wang S, Peng X, Yang G. 2011. The mitochondrial genome of Baylisascaris procyonis. PLoS One 6:e27066. doi: 10.1371/journal.pone.0027066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Xie Y, Zhang Z, Wang C, Lan J, Li Y, Chen Z, Fu Y, Nie H, Yan N, Gu X, Wang S, Peng X, Yang G. 2011. Complete mitochondrial genomes of Baylisascaris schroederi, Baylisascaris ailuri and Baylisascaris transfuga from giant panda, red panda and polar bear. Gene 482:59–67. doi: 10.1016/j.gene.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 126.Testini G, Papini R, Lia RP, Parisi A, Dantas-Torres F, Traversa D, Otranto D. 2011. New insights into the morphology, molecular characterization and identification of Baylisascaris transfuga (Ascaridida, Ascarididae). Vet Parasitol 175:97–102. doi: 10.1016/j.vetpar.2010.09.017. [DOI] [PubMed] [Google Scholar]
- 127.Chang QC, Gao JF, Sheng ZH, Lou Y, Zheng X, Wang CR. 2015. Sequence variability in three mitochondrial genes among four roundworm species from wild animals in China. Mitochondrial DNA 26:75–78. doi: 10.3109/19401736.2013.823171. [DOI] [PubMed] [Google Scholar]
- 128.Dangoudoubiyam S, Vemulapalli R, Kazacos KR. 2009. PCR assays for detection of Baylisascaris procyonis eggs and larvae. J Parasitol 95:571–577. doi: 10.1645/GE-1905.1. [DOI] [PubMed] [Google Scholar]
- 129.Gatcombe RR, Jothikumar N, Dangoudoubiyam S, Kazacos KR, Hill VR. 2010. Evaluation of a molecular beacon real-time PCR assay for detection of Baylisascaris procyonis in different soil types and water samples. Parasitol Res 106:499–504. doi: 10.1007/s00436-009-1692-6. [DOI] [PubMed] [Google Scholar]
- 130.Zhang W, Yie S, Yue B, Zhou J, An R, Yang J, Chen W, Wang C, Zhang L, Shen F, Yang G, Hou R, Zhang Z. 2012. Determination of Baylisascaris schroederi infection in wild giant pandas by an accurate and sensitive PCR/CE-SSCP method. PLoS One 7:e41995. doi: 10.1371/journal.pone.0041995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Zhou X, Yu H, Wang N, Xie Y, Liang YN, Li DS, Wang CD, Chen SJ, Yan YB, Gu XB, Wang SX, Peng XR, Yang GY. 2013. Molecular diagnosis of Baylisascaris schroederi infections in giant panda (Ailuropoda melanoleuca) feces using PCR. J Wildl Dis 49:1052–1055. doi: 10.7589/2012-07-175. [DOI] [PubMed] [Google Scholar]
- 132.Miyashita M. 1993. Prevalence of Baylisascaris procyonis in raccoons in Japan and experimental infections of the worm to laboratory animals. Seikatsu Eisei (J Urban Living Hlth Assoc) 37:137–151. (In Japanese with English summary.) [Google Scholar]
- 133.Garrison RD. 1996. Evaluation of anthelmintic and corticosteroid treatment in protecting mice (Mus musculus) from neural larva migrans due to Baylisascaris procyonis. M.S. thesis. Purdue University, West Lafayette, IN. [Google Scholar]
- 134.Fu Y, Nie HM, Niu LL, Xie Y, Deng JB, Wang Q, Yang GY, Gu XB, Wang SX. 2011. Comparative efficacy of ivermectin and levamisole for reduction of migrating and encapsulated larvae of Baylisascaris transfuga in mice. Korean J Parasitol 49:145–151. doi: 10.3347/kjp.2011.49.2.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Sawanyawisuth K. 2008. Treatment of angiostrongyliasis. Trans R Soc Trop Med Hyg 102:990–996. doi: 10.1016/j.trstmh.2008.04.021. [DOI] [PubMed] [Google Scholar]
- 136.Prociv P, Spratt DM, Carlisle MS. 2000. Neuro-angiostrongyliasis: unresolved issues. Int J Parasitol 30:1295–1303. doi: 10.1016/S0020-7519(00)00133-8. [DOI] [PubMed] [Google Scholar]
- 137.Page K, Beasley JC, Olson ZH, Smyser TJ, Downey M, Kellner KF, McCord SE, Egan TS II, Rhodes OE Jr. 2011. Reducing Baylisascaris procyonis roundworm larvae in raccoon latrines. Emerg Infect Dis 17:90–93. doi: 10.3201/eid1701.100876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Page K, Smyser TJ, Dunkerton E, Gavard E, Larkin B, Gehrt S. 2014. Reduction of Baylisascaris procyonis eggs in raccoon latrines, suburban Chicago, Illinois, USA. Emerg Infect Dis 20:2137–2140. doi: 10.3201/eid2012.140977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Smyser TJ, Page LK, Johnson SA, Hudson CM, Kellner KF, Swihart RK, Rhodes OE Jr. 2013. Management of raccoon roundworm in free-ranging raccoon populations via anthelmintic baiting. J Wildl Mgt 77:1372–1379. doi: 10.1002/jwmg.585. [DOI] [Google Scholar]
- 140.Smyser TJ, Johnson SR, Stallard MD, McGrew AK, Page LK, Crider N, Ballwebe LR, Swihart RK, VerCauteren KC. 2015. Evaluation of anthelmintic fishmeal polymer baits for the control of Baylisascaris procyonis in free-ranging raccoons (Procyon lotor). J Wildl Dis 51:640–650. doi: 10.7589/2014-09-236. [DOI] [PubMed] [Google Scholar]
- 141.Shafir SC, Wang W, Sorvillo FJ, Wise ME, Moore L, Sorvillo T, Eberhard MT. 2007. Thermal death point of Baylisascaris procyonis eggs. Emerg Infect Dis 13:172–173. doi: 10.3201/eid1301.060966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Feachem R, Bradley DJ, Garelick H, Mara DD. 1983. Ascaris and ascariasis, p 391 In Sanitation and disease: health aspects of excreta and wastewater management. World Bank, Washington, DC. [Google Scholar]
- 143.Moore L, Ash L, Sorvillo F, Berlin OG. 2004. Baylisascaris procyonis in California. Emerg Infect Dis 10:1693–1694. doi: 10.3201/eid1009.040034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Xie Y, Chen S, Yan Y, Zhang Z, Li D, Yu H, Wang C, Nong X, Zhou X, Gu X, Wang S, Peng X, Yang G. 2013. Potential of recombinant inorganic pyrophosphatase antigen as a new vaccine candidate against Baylisascaris schroederi in mice. Vet Res 44:90. doi: 10.1186/1297-9716-44-90. [DOI] [PMC free article] [PubMed] [Google Scholar]