SUMMARY
Bacterial vaginosis (BV) is the most commonly reported microbiological syndrome among women of childbearing age. BV is characterized by a shift in the vaginal flora from the dominant Lactobacillus to a polymicrobial flora. BV has been associated with a wide array of health issues, including preterm births, pelvic inflammatory disease, increased susceptibility to HIV infection, and other chronic health problems. A number of potential microbial pathogens, singly and in combinations, have been implicated in the disease process. The list of possible agents continues to expand and includes members of a number of genera, including Gardnerella, Atopobium, Prevotella, Peptostreptococcus, Mobiluncus, Sneathia, Leptotrichia, Mycoplasma, and BV-associated bacterium 1 (BVAB1) to BVAB3. Efforts to characterize BV using epidemiological, microscopic, microbiological culture, and sequenced-based methods have all failed to reveal an etiology that can be consistently documented in all women with BV. A careful analysis of the available data suggests that what we term BV is, in fact, a set of common clinical signs and symptoms that can be provoked by a plethora of bacterial species with proinflammatory characteristics, coupled to an immune response driven by variability in host immune function.
INTRODUCTION
The clinical syndrome that is currently known as bacterial vaginosis (BV) has been studied extensively for over 60 years. The originally described syndrome included various symptoms of mucosal inflammation, including vaginal discharge, itching, and burning, but was associated with a lack of leukocytic exudate, redness, and swelling; therefore, to be distinguished from classic vaginitis, it was termed vaginosis. This syndrome has been associated with a wide array of health issues over the ensuing decades, including preterm births, pelvic inflammatory disease, increased susceptibility to HIV infection, and other chronic health problems. Taken together, these complications represent an extraordinarily common complaint among women of childbearing age, with preterm birth alone affecting over 10% of all pregnancies (1), and an expensive medical issue for both the women affected and babies born prematurely to mothers with BV. According to a report from the Institute of Medicine, preterm birth in the United States alone cost at least $26.2 billion in 2005, or an average of $51,600 per infant (2). A number of potential microbial pathogens, singly and in combinations, have been implicated in the disease process. The list of possible agents continues to expand and includes members of a number of genera, including Gardnerella, Atopobium, Prevotella, Bacteroides, Peptostreptococcus, Mobiluncus, Sneathia, Leptotrichia, Mycoplasma, and recently genetically characterized organisms of the order Clostridiales (BV-associated bacterium 1 [BVAB1] to BVAB3). A great deal of research has resulted in many excellent reviews of BV describing both the clinical manifestations and the potentially associated microbiological agents, with most pointing toward a complex microbe-host interaction. Despite these efforts, a specific etiology remains elusive. Of particular public health concern is the observation that BV is the most commonly reported microbiological syndrome among women of childbearing age. Extensive research has documented the association of BV as a risk factor for preterm births, which has been a major reason for pursuing a specific etiology that can be identified during pregnancy and treated to prevent preterm births and the subsequent complications and costs associated with such births.
This review is directed at examining previous clinical, epidemiological, and microbiological definitions of BV as well as the host immune response, with the intent of determining whether there really is a clinical syndrome identifiable as BV that can be associated with a specific community or cluster of bacterial species. At the outset, the possibility that what we call BV may, in fact, be a limited repertoire of common clinical symptoms resulting from a variety of biological processes caused by a wide array of microorganisms in various combinations cannot be ruled out. It is entirely possible that what we have labeled BV is really a spectrum of microbiological perturbations whose only common features are some of the associated clinical symptoms that can be present or absent in women depending on a variety of host factors, including genetic predisposition to certain types of vaginal colonization and the interplay of environmental factors with host genome-microbiome encounters, as conceptualized in Fig. 1.
FIG 1.
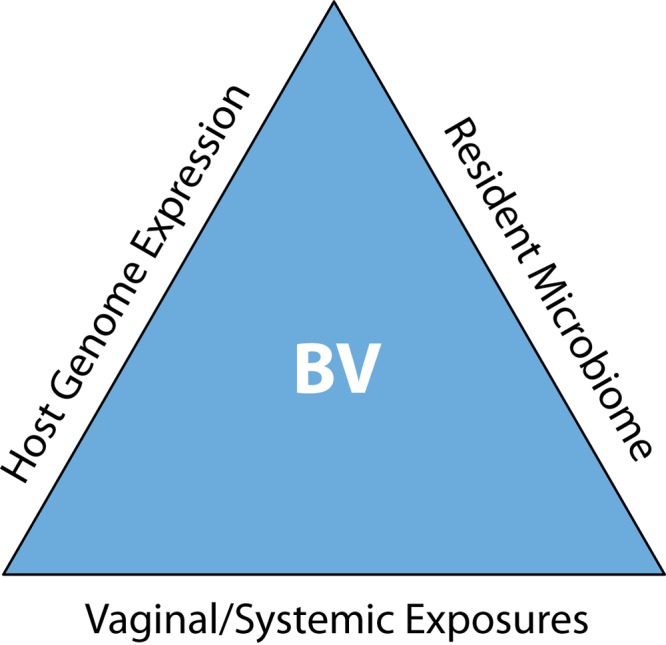
Schematic framework for syndromatic bacterial vaginosis (BV) presentation. The resident microbiome is shaped by host genetics, and in turn, the microbiome regulates host gene expression, while both vaginal and systemic exposures influence the vaginal microbiome-human genome encounter. The result of these interactions determines symptom severity and disparities within the syndrome known as BV.
The human vagina of menarcheal women is a metabolically and microbiologically complex environment. Microbiological descriptions of the vaginal vault of overtly healthy women almost always include the presence of an abundance of Gram-positive rods, usually members of the genus Lactobacillus, a low pH (<4.5), and the absence of both facultative and obligately anaerobic Gram-negative rods. The low pH is presumed to be due to the breakdown of glycogen present in the vaginal epithelium of menarchal women, followed by fermentation of carbohydrate, with the formation of lactic acid being responsible for the low pH values. In turn, the low pH tends to suppress the growth of many facultative and obligately anaerobic organisms with pathogenic potential that may be transiently isolated from the vaginal vault. Culture-based studies have shown that predictable quantitative and qualitative changes in the microbiome occur during the menstrual cycle in overtly healthy women monitored for multiple menstrual cycles, regardless of the type of catamenial protection used (3–6). During menstrual flow, the pH rises, and the numbers of lactobacilli decrease, with a concomitant increase in the numbers of other facultative and anaerobic species normally present as part of the vaginal microbiome. Following the cessation of menstrual flow, the pH decreases, and the numbers of lactobacilli increase, with a concomitant reduction in the numbers of other facultative and obligately anaerobic organisms present. Longitudinal studies have shown that these quantitative changes in the microbiome during the menstrual cycle are consistent from cycle to cycle for the same subjects as well as for all overtly healthy menarchal women, although there is considerable qualitative subject-to-subject variability in the composition of the vaginal bacterial community. Qualitative studies using conventional culture methods have characterized over 100 separate phenotypes that can be isolated from the vaginal microbiome, with some phenotypes always being present in high numbers when assessed longitudinally and some phenotypes being present only sporadically, while other phenotypes are consistently present in low numbers (3–5). It has been shown that certain species of Lactobacillus (Lactobacillus crispatus, L. jensenii, L. gasseri, and L. iners) are more common than others in overtly healthy women, while species of obligately anaerobic rods of the genera Atopobium, Prevotella, Mobiluncus, and Sneathia are more commonly present in women with symptoms of bacterial vaginosis (7–13). More recent studies using both molecular and advanced culture methods (see below) suggest that what can be considered normal or abnormal occurs on a complex biological spectrum that indicates a greater microbiological diversity for BV than previously thought. Recently described species, such as BVAB1, BVAB2, and BVAB3, have also been added to the milieu (14) of potential pathogens associated with symptomatic disease. This has made the search for the etiology of BV more difficult and perhaps suggests that BV is not a specific microbiological process but rather a spectrum of changes within the bacterial community making up the vaginal microbiome that result in a limited number of common clinical symptoms. Equally germane is the knowledge that genetic attributes of the host and the response provided by the host immune system may be important factors in how the vaginal microbiome contributes to both health and disease. This review is not meant to simply reiterate what has already been reported but is meant to integrate the information already available from over a decade of molecular analysis of the vaginal microbiome (10, 12, 15–22).
Definitions of BV
Inflammation of the vagina usually presents with a limited repertoire of symptoms and may be associated with pain, itching, a burning sensation, discomfort, and vaginal discharge with or without demonstrable inflammatory mediators. These generalized symptoms are associated with many different biological markers, including alterations of hormonal levels or infection with specific microorganisms such as Neisseria gonorrhoeae, Trichomonas vaginalis, and Candida sp. Culture-based assessment of symptomatic women has commonly been employed for the detection of vaginitis caused by these agents; however, recent studies have shown that the use of taxon-specific molecular probes for known etiological agents in consort with clinical symptoms is a more sensitive and accurate method for the diagnosis of vaginitis (23). When a specific pathogen is present, the vaginitis is usually named after the infectious agent that has been documented to cause the symptoms (i.e., gonorrhea, trichomoniasis, or vaginal candidiasis). However, when nonspecific changes in the vaginal microbiome are associated with this constellation of symptoms, it is more difficult to provide a meaningful descriptive terminology.
There are many different ways to label the clinical syndrome associated with nonspecific changes in the vaginal microbiome, starting with that put forward by Gardner and Dukes, who observed a large quantity of Haemophilus vaginalis (renamed Gardnerella vaginalis) bacteria in subjects presenting with the symptoms noted above in the absence of any known pathogens (24). They named this particular form of vaginal inflammation H. vaginalis vaginitis. Over the last several decades, the phylogenetic characterization of the suspect organism has been altered, and refinements to this terminology have occurred to reflect the presence of clinical symptoms (nonspecific vaginitis) not associated with a known pathogen; the term BV came into use in the early 1980s and in general reflects the understanding that BV, in contrast to other forms of vaginitis caused by specific microorganisms, often presents without the hallmarks of an acute infectious inflammatory process that includes high levels of polymorphonuclear cells as part of the vaginal discharge (15).
Epidemiological Characteristics of BV
Epidemiological studies of the clinical syndrome called BV have been performed over several decades (13, 18, 19, 25). A great deal of information regarding possible risk factors has been obtained from these studies, including population prevalence, socioeconomic and racial characteristics, and other behavioral or physical risk factors such as smoking, the presence of sexually transmitted diseases, and underlying immune deficiencies such as those associated with HIV infection. One of the major concerns for epidemiological studies has been the imprecise ways in which the diagnosis of BV is made. Methods such as the use of clinical criteria, Gram staining and other microscopic methods, culture and related phenotyping methods, and molecular diagnostic methods have all failed to provide a precise definition of BV that can be uniformly applied for epidemiological purposes (18). Not surprisingly, the data generated from epidemiological studies assessing the risk factors associated with a diagnosis of BV are often flawed from the outset due to the fact that there is no single uniformly accepted definition of BV. There is often population bias inherent in these studies and a lack of recognition that many of the identified markers may be only surrogates for other risk factors (18).
In one review of reports on the prevalence of BV, based only on clinical criteria, the prevalence of BV ranged from 4%, as reported for asymptomatic college women, to 61%, in women attending a sexually transmitted disease (STD) clinic (18). Differences in prevalence were clearly related to the populations sampled during these studies as well as ethnicity, socioeconomic status, and sexual activity. In another review of BV on a global basis using data compiled from peer-reviewed literature from studies performed in multiple countries where the definition of BV was based on Nugent criteria (semiquantitative assessment of various bacterial morphologies and concentrations present in Gram-stained vaginal smears), prevalence was highest in parts of Africa and lowest in Asia and Europe, with the caveat that certain defined populations in Africa may have very low rates and some European and Asian populations may have very high rates depending on geographic location and population characteristics such as age (19). When stratified by ethnic group, it was noted that black and Hispanic women tended to have the highest prevalence of BV, regardless of geographic location. The extensive analysis of these compiled studies points out that ethnicity and geography, while important risk factors, are clearly not the only predisposing risk factors that account for the elevated prevalence of BV compared to that in appropriate cohorts within the same geographic boundaries.
Other risk factors implicated in BV include smoking, low socioeconomic status, douching, recent antibiotic use, and the number and frequency of sexual contacts. However, it is unknown whether these described risk factors are causally related to BV or serve only as surrogate markers for other contributory factors (26). A recent review and discussion of the etiology of BV that included data from epidemiological, microbiological, clinical, and immunological studies suggested that BV is a “multidimensional process without a single unifying explanation” (10).
Epidemiological studies have recently taken a new approach to BV based on the use of molecular sequencing of the bacterial communities present. Based on the phylogenetic characteristics of the 5 to 8 described communities thought to make up the vaginal microbiome, the data suggest that women with BV are prone to harboring a specific cluster of microorganisms, while women at higher risk due to other established risk factors may harbor other BV-associated clusters as well (25). It was further hypothesized that biofilm-producing communities of G. vaginalis may play a role in the initiation and perpetuation of symptoms (25). While these recent observations add additional evidence supporting the complexity of our definition of BV, they have not clarified the underlying etiology responsible for the symptoms noted to occur in women diagnosed with BV by clinical, microscopic, or microbiological assessment methods.
Definitions of BV Based on Clinical and Microscopic Criteria
A review of the clinical literature documents that a variety of clinical criteria have been reported for BV (10, 15, 20); the most widely adopted criteria currently used for the diagnosis of BV are those proposed by Amsel et al. (27). According to these investigators, the criteria include at least three of the following four characteristics: vaginal discharge with a pH of >4.5, presence of a homogeneous discharge, a fishy volatile amine odor when the discharge is treated with a potassium hydroxide solution, and presence of squamous epithelial cells coated with bacteria (clue cells) when the discharge is examined microscopically. Of interest is the fact that three of the four criteria can be evaluated in clinical microbiology laboratories (pH, fishy odor, and clue cells) by using standardized methods. However, even these markers are not consistent in all subjects suspected of having BV, and a revision of the original criteria to include only two of the three laboratory-measured markers along with the presence of clinical symptoms was thought to be an acceptable basis for making a diagnosis of BV (27). The importance of each of these clinical markers for the diagnosis of BV has been discussed extensively in other reviews (15, 16), and while not every case of suspected BV conforms to the clinical criteria presented by Amsel et al., they still retain a reputation for being the most objective approach to the clinical diagnosis of BV despite the lack of sensitivity compared to other laboratory-based methods.
Since the putative cause of BV is thought to be microbiological, it is not surprising that Gram staining has also been widely employed as a method for evaluating vaginal health. This technique for diagnosing BV was first employed to detect the presence of tiny Gram-negative rods in symptomatic women (28). Variations and expansion of this methodology led to studies using a more complete evaluation of the vaginal microbiome, including the presence or absence of Gram-positive, Gram-variable, Gram-negative, and curved Gram-negative rods as markers for BV (29). One of the problems with the use of Gram staining as an assessment tool for BV was the variability of results based on the skill and experience of the person reading the smear. This issue was resolved in 1991 when a standardized evaluation method using a 0-to-10 scoring system (Nugent score) was reported (30), which provided a grading system for the semiquantitative assessment of numbers of Gram-positive rods (Nugent scores, 0 to 4+), small Gram-variable and Gram-negative rods (0 to 4+), and curved, “Mobiluncus-like” rods (0 to 2+) that were present in Gram-stained smears obtained from the vaginal vault. Using this system, subjects with clinical symptoms of BV generally had scores of 7 to 10, while subjects considered “normal” had scores of 0 to 3, and scores of 4 to 6 were considered indeterminate.
Definitions Determined by Using Culture-Based Microbiological Criteria
Microbiological studies of the vaginal vault date to the original description of “Doderlein's bacillus” in 1894 as a significant part of the normal vaginal microbiome (31). Although a universally accepted taxonomic system for bacteria, particularly obligate anaerobes, was not generally employed during the ensuing 50 years, reports in the literature related to vaginitis include those describing pigmented, anaerobic, Gram-negative rods (Prevotella and Porphyromonas); curved anaerobic rods (Mobiluncus); anaerobic cocci; Mycoplasma; and a variety of other potential pathogens (16). It was generally recognized that the vaginal microbiome in women with symptoms of BV consisted of a consortium of potentially pathogenic species, including a variety of obligate anaerobes, and that often the dominant Lactobacillus species noted to be present in overtly healthy women either were not present or were present at greatly reduced numbers (a detailed description of the various species thought to be associated with BV is provided below).
Starting with the observations of Gardner and Dukes (24), more definitive studies of the vaginal microbiome during BV were initiated by using better techniques and a more uniform taxonomic system that allowed clinical scientists throughout the world to apply the same phenotyping methods and nomenclature for the identification of putative pathogens. While the presence of the bacillus noted by Gardner and Dukes, now known as G. vaginalis, continued to be found as part of the microbiome present during BV, other organisms, such as anaerobic Gram-negative bacilli, Gram-positive cocci, and Mycoplasma species, were also identified as potential pathogens (32, 33). While the notion that BV was due to a specific microbial species continued to provoke searches for a common microbial agent that would fulfill Koch's postulates, it was becoming clear by the early 1990s that BV was associated with a constellation of microbial species with potentially pathogenic characteristics and that no single agent or combination of agents was detected in all cases of BV (17).
Early microbiological studies of women with BV included listings of the prevalence of specific species thought to be associated with symptomatic disease compared to their prevalence in overtly healthy subjects. These organisms included G. vaginalis, anaerobic Gram-negative rods, anaerobic cocci, and Lactobacillus sp. (34–36). While culture methods and identification techniques varied from study to study, it was noted that species such as G. vaginalis were present in >90% of symptomatic subjects but were present in <45% of normal subjects. Lactobacillus sp., on the other hand, was present in >70% of overtly healthy subjects and was isolated in <40% of symptomatic subjects. More detailed quantitative microbiological culture information demonstrated that, when present, G. vaginalis, anaerobic Gram-negative rods, and anaerobic Gram-positive cocci were all found at high concentrations, while the population of Lactobacillus sp. was no longer numerically dominant and showed greatly reduced numbers compared to the populations measured in healthy subjects. Organisms such as Mobiluncus, commonly thought to be associated with BV, have been reported to be present in 40 to 60% of BV subjects (17) at a very high concentration; however, more recent non-culture-based data suggest that these descriptions probably included phylotypes of curved Gram-negative rods representing multiple species (see below).
In addition to the symptoms in nonpregnant women with BV, studies of women during pregnancy revealed that BV was associated with increased rates of both preterm rupture of membranes and preterm births (37–39). A number of quantitative and qualitative studies of pregnant women documented that the rates of preterm births were higher among those with BV, as assessed by culture, Amsel criteria, and Nugent scores. Mathematical modeling of the microbiological data indicated that changes in the composition and numbers of organisms present could be used to predict pregnancy outcomes (37, 40, 41). Unfortunately, clinical trials that have used various antibiotic regimens to suppress potentially pathogenic microorganisms during pregnancy have had little effect on pregnancy outcomes (42).
Non-Culture-Based Methods for Diagnosis of BV
Applications of molecular methods to studies of the vaginal microbiome have not been limited to studies of microbially diverse populations and community composition alone but have also been applied as part of the diagnostic process for BV. Based on findings from previous culture-based and sequence-based assessments of women with a clinical diagnosis of BV as determined by Amsel criteria and Nugent scores, quantitative PCR (qPCR) assays for specific organisms, including G. vaginalis, Mycoplasma hominis, and Lactobacillus sp., were performed on cervical vaginal lavage samples from 200 women with BV with Nugent scores of 0 to 3 and 200 women with Nugent scores of 7 to 10. A comparison of the three methods indicated that women with a Nugent score of 7 to 10 met the clinical criteria proposed by Amsel et al. less than 50% of the time; however, the qPCR results for these same women showed increases in the concentrations of G. vaginalis and M. hominis according to predetermined threshold values over 80% of the time. Interestingly, women with a Nugent score of 0 to 3 exceeded the qPCR thresholds only 30% of the time for the same organisms. The qPCR results for lactobacilli overlapped between the two groups. It was concluded that the Nugent score and qPCR for G. vaginalis and M. hominis were significantly better than Amsel criteria for diagnosis of BV (43).
In another study, samples categorized by the Nugent scoring system were evaluated by using qPCR assays directed at sequences of the 16S rRNA genes of eight different bacterial species. It was shown that the qPCR results for Atopobium vaginae and G. vaginalis provided the highest predictive values for BV diagnosed by the Nugent scoring system, with a sensitivity of 95% and a specificity of 99%. Although other organisms, including Mobiluncus, Mycoplasma, and Lactobacillus sp., were targeted, the combination of results for A. vaginae and G. vaginalis provided the most predictive information (44). Alternatively, studies of the intravaginal microflora obtained from vaginal fluid of women by using 16S rRNA gene sequencing and clone library analysis have also been employed. Principal-component analysis suggested that there was a unique relative ratio of L. iners, A. vaginae, and obligate anaerobes in samples from women with BV. However, this study also relied on culture-based criteria as well as clone library analysis (45). Because 16S rRNA gene sequencing is not a sensitive method for detecting organisms that may be present in low numbers, investigators have also applied taxon-directed PCR assays for more sensitive detection of putative pathogens that may be associated with the presence of symptoms of BV. One study using Amsel criteria, Nugent scoring, and targeted PCR assays indicated that PCR detection of one or more fastidious species is more reliable as an indicator of BV than culture detection of G. vaginalis alone (46). In particular, the possible role of BVAB1 to -3 was pointed out in this study. Other investigators have used DNA hybridization studies to document the presence of various pathogens in women with Nugent scores indicative of BV (47). Although hybridization studies have targeted only a few well-known pathogens, the results are consistent with those of other studies that showed that the presence of one or more specific pathogens correlates well with a diagnosis of BV. The search for molecular tools for the diagnosis of BV has also included the use of microarray technology (48), although the utility of such assays for routine diagnostic purposes remains to be proven. Commercially available, FDA-approved assays that utilize molecular methods of detection are usually not directed at BV alone but include probes for other known causes of vaginitis, such as N. gonorrhoeae, Trichomonas vaginalis, Chlamydia trachomatis, and G. vaginalis.
Although many different molecular methods have been used in attempts to provide more definitive diagnostic information about BV, it is clear from the plethora of combinations and permutations of possible pathogens analyzed that no one organism or cluster of organisms can identify all cases of BV. Indeed, a careful analysis of the organisms identified by molecular methods as part of BV diagnosed by using Nugent criteria may be a self-fulfilling prophesy, because the scoring system was developed to specifically identify women with low numbers of lactobacilli and high numbers of small Gram-variable and anaerobic Gram-negative rods. Imagine that the clinical symptoms associated with BV may occur in the absence of the changes identified by Nugent scoring, such as aerobic vaginitis, as described by Donders (49). In this scenario, the numbers of lactobacilli are also decreased but are replaced by aerobic organisms such as Staphylococcus aureus, Escherichia coli, and streptococci. By using Nugent criteria, scores would likely fall in the indeterminate range, and culture or molecular detection results for putative pathogens such as G. vaginalis, M. hominis, A. vaginae, Sneathia, Mobiluncus, and BVAB1 to -3 would be negative, yet symptoms would still be present. It is the inability to satisfy Koch's postulates by either culture-based or molecular methods that continues to challenge our ability to provide a clear definition of BV (20).
INDIVIDUAL MICROBIAL SPECIES AS POSSIBLE MEDIATORS OF BV AND HOST IMMUNITY
Studies continue to seek a unified explanation for the clinical signs and symptoms that we identify as BV. Over the last several decades, by using culture-based and molecular methods, a number of suspect bacterial species have been identified. Each of these species has unique characteristics that need to be integrated into our evaluation of the human vaginal microbiome and the interactions between the microbe(s) and host that occur on an ongoing basis. Since it is clear from historical studies of BV that no single bacterial species is present during all cases of BV by any definition, the interactions between organisms acting in consort on the human host need to be considered in detail (8). Although numerous studies have revealed an association between BV and the presence of a number of bacterial genera and species, the role of these bacteria in the etiology and pathology of the disease remains unclear. The complexity and variability of the vaginal microflora do not allow a simple and straightforward determination of which organisms are truly pathogenic. The combination of molecular and culture techniques applied to complex clinical probes and the establishment of physiological models that dissect host immune responses to individual microbes (Fig. 2) have led to significant new information on key attributes for individual bacterial species of interest to BV.
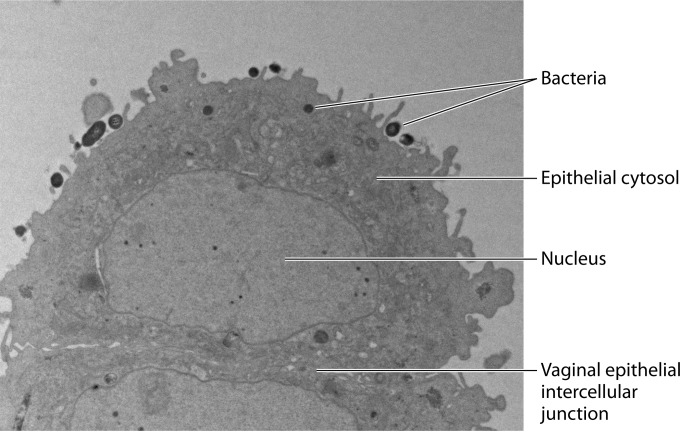
FIG 2.
Physiological in vitro model for the study of host-microbiome interactions in BV. Transmission electron microscopy illustrates human vaginal epithelial cells colonized with one of the signature bacterial species of BV, Atopobium vaginae. Epithelial cells show no signs of apoptosis. Bacteria appear as dense, dark, round bodies, intimately attached to the epithelial surface or taken up inside the epithelial cell cytosol. (Reprinted from reference 150 with permission of the publisher.)
Gardnerella vaginalis
Gardnerella vaginalis was first isolated by Leopold in 1953 (50). A year later, it was associated with nonspecific bacterial vaginitis by Gardner and Dukes and named Haemophilus vaginalis (51). It was later classified in the genus Corynebacterium based on metabolic requirements and Gram stain reactions. Following additional analyses, later supported by DNA-DNA hybridization, it was placed into its own genus, Gardnerella (52). Gardnerella is in the family Bifidobacteriaceae. The cells are small, nonmotile, nonencapsulated, non-spore-forming, pleomorphic rods with average dimensions of 0.4 by 1.0 to 1.5 μm. Upon Gram staining, they can appear Gram variable due to a thin peptidoglycan layer. G. vaginalis is a fastidious organism, requires complex medium for growth, and grows best in the presence of carbon dioxide. Biochemical tests revealed that G. vaginalis is catalase, oxidase, and β-glucosidase negative. It can ferment starch, dextrin, sucrose, glucose, fructose, ribose, maltose, and raffinose but not rhamnose, melibiose, mannitol, and sorbitol. Some strains can also ferment xylose and trehalose (53). Additionally, G. vaginalis can hydrolyze hippurate but not gelatin or esculin. This microorganism is also positive for α-glucosidase activity and for beta-hemolysis on human blood but not sheep's blood (10). The cellular surface of G. vaginalis is covered with fimbriae, which are responsible for the attachment of G. vaginalis to vaginal epithelial cells.
G. vaginalis has shown high sensitivity (100%) but low specificity (49%) for BV. It is often detected in the absence of BV as well as in both sexually inexperienced and experienced women (54, 55). Numerous studies have confirmed the presence of G. vaginalis not only in women with BV but also in women without BV, via both conventional culture techniques and molecular-based studies (11, 56). Nevertheless, the overall abundance of G. vaginalis DNA or the actual bacterial concentration increases with higher Nugent scores and BV-positive status. G. vaginalis has been associated with three of the four Amsel criteria, including amine odor, elevated pH, and the presence of clue cells (57). Importantly, G. vaginalis has been detected in male partners of women with BV (58), and biofilm-forming adherent types of G. vaginalis were particularly linked between sexual partners, suggesting the possibility of sexual transmission (59).
Much attention has been focused on possible virulence factors that could elucidate the pathological potential and possible role of G. vaginalis or specific G. vaginalis biotypes in BV. G. vaginalis possesses a number of molecular characteristics that can lead to the development of disease. Putative virulence factors have been identified in the G. vaginalis genome. One virulence factor in particular is similar to adhesins produced by species of Mycoplasma that are involved in adherence to human tissue (60). Other virulence factors produced by G. vaginalis are cytolysins that cause cell death by activating the protein kinase pathway in human epithelial cells. Among the best-studied cytolysins is vaginolysin, a member of the cholesterol-dependent family of pore-forming toxins that lyses human red blood cells and vaginal epithelial cells (61). In vivo, the cytolytic activity of vaginolysin is thought to increase nutrient availability for G. vaginalis. IgA antibodies against vaginolysin have been detected and linked to the mucosal immune response during BV. G. vaginalis also produces sialidase, prolidase, and putrescine, which may play a role in degrading mucosal protective factors such as mucins and may contribute to the exfoliation of vaginal epithelial cells (62).
The ability of G. vaginalis to adhere to vaginal epithelial cells provides the scaffold for biofilm formation and for other BV signature bacteria such as Atopobium vaginae to become established in this biofilm. G. vaginalis and A. vaginae have been detected together in vaginal biofilms and in association with the presence of clue cells. Biofilm formation is key for the development of disease since it confers heightened antibiotic tolerance and resistance to host immune defenses, making diseases chronic and/or relapsing. Swidsinski et al. showed by fluorescence in situ hybridization (FISH) that a characteristic dense biofilm made up of confluent or patchy layers was attached to at least 50% of the intact epithelial surface in 90% of vaginal biopsy specimens of patients with BV, compared to 10% in healthy controls (63). G. vaginalis was the predominant bacterium in these biofilms, followed by A. vaginae, which was present in 80% of the biofilms and contributed up to 40% of the biofilm mass. The G. vaginalis biofilms detected in women with BV tolerated higher concentrations of hydrogen peroxide and lactic acid (7). The resistance of Atopobium to metronidazole and its association with G. vaginalis biofilms may explain the high rates of recurrence of BV. The production of amines results in an increased pH and favors the growth of other anaerobes associated with BV. Finally, the G. vaginalis peptidases can act on the protein-rich vaginal environment to release peptides and amino acids, which in turn stimulate bacterial growth and provide the nutrients necessary to facilitate the growth and codependency of other BV-related organisms. Interestingly, viable G. vaginalis bacteria can be taken up by vaginal epithelial cells with the participation of active epithelial cytoskeleton reorganization, and this uptake upregulates factors facilitating the adherence of other pathogenic bacteria, e.g., E. coli (64). Thus, multiple qualities of G. vaginalis, including biofilm formation, metabolic activities, epithelial cell uptake, and altered host immunity, as described below, may contribute to the diversity and survival of the BV-associated microbiota and its resilience to therapy. The resilience of BV to therapy has been linked to higher levels of G. vaginalis following a standard 7-day metronidazole regime (65).
Biodiversity within the G. vaginalis species may explain the variability in the epidemiological association of this organism with BV. G. vaginalis isolates associated with BV tend to produce more biofilm growth through enhanced aggregation and adherence and are more cytotoxic than non-BV-associated G. vaginalis isolates (53). Numerous research groups have attempted to identify specific virulent subtypes/biotypes of G. vaginalis. So far, biotypes have been grouped according to lipase, hippurate hydrolysis, and β-galactosidase reactions as well as the fermentation of arabinose, galactose, and xylose (10). Various conflicting results have been reported, and it remains unclear whether any of the biochemical characteristics examined for biotyping G. vaginalis are linked to the virulence of this microorganism.
In vitro studies have shown that G. vaginalis can weaken epithelial barrier function via direct tissue damage and inflammation. A study by Patterson et al. reported that G. vaginalis adherence to a cervical squamous cell carcinoma cell line induced rounding and lysis of these cells (66). A more physiological model applying nontransformed immortalized human vaginal and cervical epithelial cells that maintain the morphological characteristics of their primary tissues of origin (67, 68) provided additional insights into host interactions with G. vaginalis (64, 69, 70). Eade et al. used an ATCC strain of G. vaginalis and showed upregulation of the proinflammatory chemokine interleukin-8 (IL-8) and a more inconsistent effect on IL-6 production (69). Fichorova et al. used a primary G. vaginalis isolate obtained from a woman with BV and showed that it caused upregulation of IL-8, RANTES, and soluble leukocyte protease inhibitor (SLPI) (70). The upregulation of RANTES is of particular significance since increased cervical levels of RANTES have been identified as the single most predictive marker of increased risk of HIV seroconversion in the sub-Saharan epicenter of the AIDS epidemics (71). Importantly, the proinflammatory activity of G. vaginalis was synergistically enhanced by coinfection with the protozoan parasite T. vaginalis, the most frequent companion of BV, and especially in the presence of Trichomonas vaginalis virus 1–4 species of the genus Trichomonasvirus, which are carried by this parasite (70).
Clinical studies have confirmed the significant association of G. vaginalis with a perturbed vaginal immune environment. Hedges and colleagues (72) found that women with the highest numbers of Gardnerella or Prevotella (described below) morphotypes present in vaginal smears (>30 per high-power microscopy field) had higher vaginal levels of IL-1β. Using culture techniques, Anderson and colleagues (73) associated the presence of G. vaginalis in vaginal swabs from low-risk pregnant women with higher cervical levels of IL-1β, interferon gamma (IFN-γ), tumor necrosis factor alpha (TNF-α), and granulocyte-macrophage colony-stimulating factor (GM-CSF).
Similarly, genomic PCR analysis of vaginal swabs implicated G. vaginalis in increased cervicovaginal levels of IL-1β and other proinflammatory cytokines, e.g., IL-1α, IL-6, IL-8, and IL-12p70, even though some immunosuppressive effects, e.g., reduced levels of interferon gamma-induced protein 10 (IP-10) and SLPI, were also observed (74). G. vaginalis was also one of the six dominant species identified by 16S rRNA gene sequencing in a microbiota community type distinguished by higher cervicovaginal levels of IL-1α, IL-β, IL-8, TNF-α, IFN-γ, and IL-10 in young South Africans at risk of HIV (75). A recent 16S rRNA gene analysis confirmed that a high abundance of Gardnerella in particular, combined with a low abundance of Lactobacillus, may contribute to the increased risk of preterm birth (76). Thus, strong evidence supports the role of G. vaginalis in modifying host immunity and BV pathogenesis.
Atopobium vaginae
A. vaginae was first associated with BV in 2004 (77). A. vaginae bacteria are anaerobic, small, elongated, Gram-positive cocci that occur singly, in pairs, or in short chains. This variable cell morphology can explain how A. vaginae can be camouflaged by other organisms and overlooked upon diagnostic Gram staining. On blood agar, A. vaginae forms tiny pinpoint colonies (78). Its fastidious nature and slow growth can elucidate why it is not readily detected in culture studies. The major metabolic product is lactic acid. Susceptibility to metronidazole is variable, with the MIC ranging from 8 to 256 μg/ml; however, A. vaginae is susceptible to clindamycin and nifuratel (79).
A. vaginae has been detected by PCR in 96% of women with BV and in only 12 to 19% of those without BV (14, 80, 81). However, Menard et al. detected A. vaginae in 69% of samples from women without BV, suggesting that the mere detection of A. vaginae has a poor predictive value for BV. Nevertheless, their results showed that quantification of A. vaginae bacteria is a good predictor, since higher levels were detected in BV-positive samples (44). A. vaginae has been associated with three out of four Amsel clinical criteria, including vaginal discharge, elevated pH, and the presence of clue cells (57). A. vaginae and G. vaginalis have been shown to be present in 78% to 96% of BV samples, in contrast to 5% to 10% of normal flora samples (44, 82). Analysis of the composition and structural organization of the biofilm adherent to the vaginal mucosa in subjects with BV showed that in 70% of the samples, A. vaginae was present and accounted for 1 to 40% of the film mass (63). The association of A. vaginae with biofilm formation along with resistance to metronidazole can explain therapeutic failures and recurrences of BV.
Along with G. vaginalis and Prevotella bivia (described below), A. vaginae has emerged as a strong trigger of inflammation and vaginal epithelial innate immune responses (70, 83–85). It activates the major proinflammatory transcription factor NF-κB in cervicovaginal epithelial cells (84). Although inconsistent effects on cytokines, e.g., IL-6 and TNF-α, have been reported (69, 85), all studies reported so far have shown that A. vaginae significantly boosts the expression of chemokines in vaginal and/or cervical epithelial cells, including IL-8 (69, 70, 83, 84), MIP-3α (CCL20) (85), and RANTES (CCL5) (70, 84). Fichorova et al. showed that similarly to G. vaginalis, A. vaginae synergizes with Trichomonas vaginalis virus and can be taken up by vaginal epithelial cells, where it maintains viability and, after antibiotic treatment, perhaps finds protection from competition with the protozoan parasite or other vaginal organisms (70). In clinical studies, the detection of A. vaginae in vaginal swabs was correlated with higher levels of the same inflammatory mediators that were associated with G. vaginalis (74), and Atopobium was among the taxa most abundant in microbiota community types distinguished by the highest levels of cervicovaginal inflammatory mediators in a study of South African women (75).
Prevotella and Porphyromonas
Prevotella and Porphyromonas are anaerobic, Gram-negative, pleomorphic, nonmotile rods that were previously classified as Bacteroides. These genera include both nonpigmented (Prevotella) and black-pigmented (Prevotella and Porphyromonas) species. Numerous studies have detected these bacteria by Gram stain, culture, and/or molecular techniques in virtually all women with and without BV (9, 82, 86, 87). Research has shown that Prevotella and Porphyromonas make up the “Bacteroides morphotype” used to determine Nugent scores and that species of the genus Bacteroides are rare (9, 86). Prevotella bivia and black-pigmented Prevotella species are significantly associated with BV (9). Zozaya-Hinchliffe et al., using quantitative PCR to assess the load and presence of various species associated with BV, found that Prevotella was present in every case regardless of Nugent score and that it represented a high percentage of total species in BV specimens (82). Similar results were reported by Delaney and Onderdonk in a culture-based study. They reported that bacterial concentrations of Prevotella increased significantly as the total Nugent score increased, with the mean concentration of Prevotella being close to 4 logs higher in the BV group than in the group with Nugent scores of 0 to 3 (normal) (8.42 log10 CFU/g of vaginal secretions versus 4.73 log10 CFU/g, respectively) (87). Prevotella was associated with a positive whiff test, one of the clinical criteria comprising the Amsel test (57). A positive whiff test is attributed to the production of polyamines, including putrescine, cadaverine, and trimethylamine. Prevotella species are capable of producing polyamines during normal metabolic activity. These amines can increase the vaginal pH, which in turn may enhance the growth of other anaerobes associated with BV. The production of ammonia by Prevotella was demonstrated to enhance the growth of G. vaginalis, which in turn produced amino acids that were utilized by Prevotella in a synergistic relationship (88). An additional symbiotic relationship between Prevotella and Peptostreptococcus anaerobius was reported, where amino acids produced by Prevotella enhanced the growth of P. anaerobius (89). In addition to these polyamines, Prevotella species such as P. bivia and P. disiens produce collagenase and fibrinolysins, which can degrade the mucosal surface and promote the detachment of vaginal epithelial cells, and sialidase and prolidases, which lead to vaginal sloughing (54).
Immunologically, P. bivia appears to be a prominent modifier of the cervicovaginal mucosal environment. In their in vitro epithelial cell model, Fichorova et al. showed that even though P. bivia efficiently activates the proinflammatory transcription factor NF-κB and the production of MIP-3α, RANTES, and IL-8 (70, 84), it can suppress these inflammatory responses to T. vaginalis (70). Later, Doerflinger et al. confirmed that P. bivia increased the levels of MIP-3α and also IL-1β in vitro (85). Clinically, P. bivia was associated with higher cervicovaginal levels of IL-1β (72, 74) and IL-8 (74) and with the most proinflammatory (highest levels of IL-1α, IL-1β, and TNF-α) cervical microbiota community type in African women (75).
Anaerobic Cocci
Anaerobic Gram-positive cocci are common inhabitants of the skin and mucosal surfaces. Up until the last decade, anaerobic cocci were classified as either Peptostreptococcus or Peptococcus. However, because of the genetic and phenotypic heterogeneity of Peptostreptococcus, a number of new genera have been proposed, including Finegoldia, Parvimonas, Gallicola, Peptoniphilus, and Anaerococcus. These taxonomic changes must be kept in mind when reviewing previous research.
Both culture- and DNA-based studies have detected these genera in both women with and those without BV (4, 9, 14, 20, 54, 57, 82, 87). Delaney and Onderdonk reported that the bacterial concentration of Peptostreptococcus increased significantly as the total Nugent score increased. The mean bacterial concentration for Peptostreptococcus increased from 4.86 log10 CFU/g in the normal group to 7.62 log10 CFU/g in the BV group (87). Peptoniphilus, which includes butyrate-producing species, is associated with persistent cases of BV. Peptoniphilus was detected in 36% of women experiencing BV treatment failure (90). This genus has been reported to adhere to vaginal epithelial cells, while Peptostreptococcus does not (66, 90). These genera produce a number of virulence factors and have shown variable antibiotic resistance patterns toward penicillins, clindamycin, and metronidazole (91).
A number of molecular-based studies employing various methods, including PCR amplification of 16S rRNA genes with clone analysis, bacterium-specific PCR assays of 16S rRNA genes, quantitative PCR, pyrosequencing, and fluorescence in situ hybridization, have detected two uncultivated Megasphaera-like phylotypes, which have been termed Megasphaera types 1 and 2. These phylotypes have been detected in both women with and those without BV, with significantly higher concentrations in women with BV (14, 20, 92).
The role of vaginal anaerobic cocci in vaginal mucosal immunity remains to be studied. Imai et al. reported that butyric acid produced by Peptoniphilus and a number of vaginal Anaerococcus bacteria activates latent HIV-1 via chromatin remodeling, but no other aspects of the immune barrier were investigated in that study (93).
Sneathia (Leptotrichia)
Sneathia bacteria are long, Gram-negative, nonmotile rods that can exhibit bulbous protrusions. These bacteria were originally designated Leptotrichia, but based on phenotypic and phylogenetic evidence, the strains were reclassified into the genus Sneathia in 2012. These organisms are fastidious and require serum or blood for growth. Colonies on blood agar plates after 72 h are flat, are ∼1 mm in diameter, and exhibit alpha-hemolytic activity. To date, there are two species, S. sanguinegens and S. amnii. They are strict anaerobes, although S. amnii can tolerate transient exposure to air and is positive for superoxide dismutase activity (94, 95). S. amnii is extremely fastidious, which can explain why it has not been detected by conventional culture-based microbiological techniques.
By use of molecular methods, Sneathia has been detected in vaginal samples from women with and those without BV. S. amnii was detected in 40% of midvaginal samples from 736 women participating in the Human Microbiome Project. These subjects attended urban outpatient clinics for reasons including annual examination, vaginal discharge, sexually transmitted infection (STI) screening, and pregnancy, etc. In this same population, S. amnii and S. sanguinegens often cooccurred, with 70% of women with one Sneathia species also having the other (94). S. amnii was present in 78% of women attending an urban STD clinic but was more common in women with BV than in those without BV (57). Fethers et al. reported that Sneathia and Leptotrichia species had similar prevalences in women with and those without BV (73% and 74%, respectively), and this prevalence was strongly related with increasing sexual activity, a finding that was not observed for other bacteria associated with BV. Since the prevalence of Sneathia and Leptotrichia increased with increased sexual activity and not with the incidence of BV, those authors concluded that these genera are epidemiologically associated with BV rather than being involved in the development of BV (55). Nevertheless, Sneathia and Leptotrichia should not be excluded from possibly playing a role in the symptom and disease pathogeneses of BV. In fact, a study by Srinivasan et al. (57) examined the associations of Amsel criteria with bacterial taxa. Their results indicated that S. amnii was positively associated with all four clinical criteria, including vaginal discharge, amine odor, elevated pH, and the presence of clue cells. Its association with clue cells can be related to the fact that Sneathia bacteria produce collagenase and fibrinolysins that can degrade the mucosal barrier and promote the detachment of vaginal epithelial cells (54).
Sneathia strains tend to be susceptible to many antimicrobials, including metronidazole, but can be resistant to erythromycin, kanamycin, vancomycin, aminoglycosides, and fluoroquinolones (96). Studies by Harwich et al. indicated that S. amnii was more sensitive than G. vaginalis to metronidazole; however, it was resistant to tetracycline and ciprofloxacin (94). In addition, S. amnii was vancomycin sensitive, suggesting that the cell envelope differs from that of typical Gram-negative bacteria. Analysis of the genome identified genes that encoded a number of a potential invasins and adhesins as well as a protein with 63% identity and 77% similarity to an O-sialoglycoprotein endopeptidase that could be involved in the degradation of sialylated proteins. S. amnii was cytotoxic to ME-180 human cervical cancer cells and exhibited adherence to these cells. Analysis of the genome sequence revealed a hemolysin that could produce this cytotoxic activity (94). In the vaginal in vitro model, S. amnii and S. sanguinegens produced significant upregulation of IL-1α, IL-β, TNF-α, and IL-8 (75). Clinically, Sneathia was among the 6 taxa most prevalent in the proinflammatory microbiota community types and was most strongly correlated with proinflammatory cytokines (75). These finding illustrate the pathogenic potential of this organism and its possible role in BV.
Mobiluncus
Mobiluncus bacteria are motile, anaerobic, slow-growing, fastidious organisms that require an enriched medium containing rabbit or horse serum or fermentable carbohydrates such as maltose or glycogen for growth. The genus contains two species, M. curtisii, comprising Gram-variable or Gram-negative short, curved rods, and M. mulieris, comprising Gram-negative long, straight, or slightly curved rods. M. curtisii is divided into two subspecies, M. curtisii subsp. curtisii and M. curtisii subsp. holmesii (15). Mobiluncus bacteria are susceptible to penicillin, clindamycin, and vancomycin and resistant to colistin and nalidixic acid. Of the two species, M. curtisii is resistant to metronidazole (97). Mobiluncus bacteria produce malic acid and trimethylamine, which has been associated with vaginal irritation and malodor associated with BV (54).
Despite their fastidious nature, Mobiluncus bacteria were detected in 65 to 85% of women with BV and in <5% of healthy women by culture-based studies that employed stringent anaerobic techniques (98). These culture findings corresponded with microscopy data that showed the prevalence of Mobiluncus to be as high as 77% (15). In 1991, Hillier et al. reported that the sensitivity of detection of Mobiluncus in Gram-stained vaginal smears compared with culture and whole-chromosome DNA probes was 84% (99). They reported that Mobiluncus was detected in 53% of women with BV and in 45% of those without BV by visualization of Gram-stained vaginal smears (99). A more recent study using PCR to detect Mobiluncus in vaginal specimens reported that Mobiluncus was detected in 38% of women without BV and in 84.5% of women with BV. M. mulieris was the predominant species in normal women, while M. curtisii was detected in 65.3% of BV cases. The predominance of M. curtisii and its persistence were associated with treatment failure (97, 100).
The recent discovery of BVAB1 has turned researchers' attention toward identifying the curved rods that are associated with Nugent scores of 9 to 10. BVAB1 as well as Mobiluncus have curved morphologies. Srinivasan et al. used qPCR and FISH to determine the concentrations of these bacteria in vaginal smears. BVAB1 DNA was detected in all women with Nugent scores of 9 to 10, while Mobiluncus DNA was detected in 76% of these women. The mean concentration of BVAB1 was significantly higher, by ∼3 logs, than the mean concentration of Mobiluncus (86). This finding, along with the FISH findings that BVAB1 was more abundant than Mobiluncus in vaginal fluid of women with Nugent scores of 10, led those authors to conclude that the curved-rod morphotypes seen upon Gram staining of samples of women with Nugent scores of 9 to 10 were more likely to be BVAB1 and not Mobiluncus. However, the exact number of 16S rRNA gene copies per genome for BVAB1 is not currently known, while it has been determined that M. curtisii has two 16S rRNA copies per genome. Since the copy number per genome is not known for BVAB1, it is difficult to correlate the 16S rRNA gene copy numbers to absolute quantities of bacteria, especially since bacteria with high copy numbers may be overrepresented in studies using molecular techniques.
A strong association has been found between Mobiluncus morphotypes found on Nugent smears and elevated cervicovaginal levels of sialidase but not IL-1β and IL-8 (72, 101). However, a recent study of South African women that utilized 16S rRNA genotyping of cervical microbiota identified Mobiluncus among the species most strongly correlated with higher cervicovaginal levels of proinflammatory cytokines, and when tested in vitro, M. mulieris significantly increased vaginal epithelial cell production of IL-1α, IL-1β, TNF-α, and IL-8 (75). The fact that cervical but not vaginal Mobiluncus was associated with inflammation could be explained by the higher virulence of biotypes capable of ascending and colonizing the upper genital tract.
Mycoplasma and Ureaplasma
Mycoplasma and Ureaplasma are in the Mollicutes class. They are facultative anaerobes and replicate in a parasitic manner. Since they lack a cell wall, they cannot be visualized via Gram staining and are therefore missed if detection is based solely on Gram staining. Two of the seven species of Mycoplasma, M. hominis and M. genitalium, have been isolated from the genital tract of women. M. genitalium has been found to have a role in causing symptomatic cervicitis or urethritis, whereas M. hominis is associated with the presence of BV (102). By using culture methods, M. hominis was detected in 24 to 75% of BV cases and in 13 to 22% of women without BV (15). Detection of M. hominis using PCR yielded similar results. Keane et al. reported a 53% carriage rate of M. hominis in women with BV and zero detection in normal women (103). Similarly, a study by Taylor-Robinson and Rosenstein reported that M. hominis was present in low numbers in the healthy vagina but that the concentration of M. hominis was increased by a factor of 10,000 in women with BV (104). Furthermore, studies using quantitative PCR have shown that women with BV have larger quantities of M. hominis and that these levels correlate with Gram stain criteria for BV (43). A decrease in Mycoplasma colonization in women with BV following treatment with either topical metronidazole or clindamycin was reported by Austin et al. (105).
Contradicting results on the association of Ureaplasma urealyticum with BV have been reported. Hillier et al. reported that the prevalence of U. urealyticum was 78% in pregnant women with normal flora and was significantly higher, at 92%, in pregnant women with BV (9). Results reported by Keane et al. were similar in that women with BV had a higher U. urealyticum carriage rate, at 65%, than did normal women, who had a carriage rate of 48%; however, the difference was not significant (103). A review by Patel and Nyirjesy reveals that U. urealyticum is detected in women with and without BV and that there does not appear to be an association between this particular species and BV (102). Nevertheless, U. urealyticum expresses hemolytic activity and secretes enzymes, including elastase and IgA protease, which reduce mucosal immunity, as well as phospholipase C and urease, which hydrolyze urea to cytotoxic ammonia (54), all of which can precipitate the symptoms and pathogenesis of BV.
U. urealyticum has been found to induce proinflammatory cytokine production in cervical and vaginal epithelial cells (106) and in macrophages (107), suggesting the potential of this organism to contribute to the altered vaginal immune environment of BV. Significant evidence from both clinical and experimental studies supports the role of Mycoplasma and M. hominis in the pathogenesis of BV-associated immune dysregulation (108).
BVAB1 to BVAB3
In 2005, Fredricks et al. described three novel bacteria that were detected in vaginal fluid of women with BV by PCR and confirmed via FISH (14). Their 16S rRNA gene sequences were not closely related to those of any known bacteria and were termed bacterial vaginosis-associated bacteria (BVAB). To date, there are three groups of these bacteria, designated BVAB1, BVAB2, and BVAB3, all in the order Clostridiales and, more specifically, in the phylum Clostridium. These bacteria have been observed microscopically in vaginal smears of women with BV to be attached to vaginal epithelial cells, resembling the clue cells that are characteristic of BV. Visualization of vaginal smears using FISH revealed that BVAB1 cells were thin curved rods; BVAB2 cells were shorter, fatter rods; and BVAB3 cells were long, lancet-shaped rods. Further examination of BVAB1 using electron microscopy illustrated that this bacterium was much shorter and thinner than M. curtisii, which has similar curved-shaped rods (14, 20). Although Mobiluncus is morphologically similar to BVAB1, their 16S rRNA genes share only 80% nucleotide identity. Molecular-based studies using species-specific quantitative PCR, pyrosequencing, and FISH showed that BVAB1, and not Mobiluncus, was the dominant curved, rod-shaped bacterium visualized in Gram-stained slides with Nugent scores of 9 to 10 (20, 109). In addition, the mean concentration of BVAB1 DNA was 2 logs higher than that of Mobiluncus DNA in these women. BVAB1 has been detected in women with Nugent scores of 9 to 10 at rates ranging from 89 to 100%, while BVAB2 has been detected in 89% of subjects with BV (14, 86). Zozaya-Hinchliffe et al. found no association between the presence of BVAB1 and a clinical diagnosis of BV; however, the concentration of BVAB1 was significantly higher in BV specimens (82). BVAB3 along with S. sanguinegens were detected in >90% of BV cases diagnosed by Nugent scoring but in only a few women with normal Nugent scores (110).
BVAB1 is associated with a positive whiff test, possibly indicating the production of polyamines by this bacterium (57). These amines can include putrescine, cadaverine, and trimethylamine and can increase vaginal pH and promote the growth of other anaerobic bacteria. BVAB1 is strongly associated with BVAB3 and Prevotella, indicating that BVAB1 may require particular metabolites that are produced by these taxa to facilitate growth or vice versa, representing a codependency or synergistic relationship among these bacteria.
BVAB1 and -2 have not been isolated by culture; however, BVAB3 was recently grown by using conventional culture methods and characterized biochemically. Mageeibacillus indolicus has been proposed as the name for BVAB3. This organism is described as an obligate anaerobe; it is a slow-growing, nonmotile, non-spore-forming, Gram-positive rod. M. indolicus is asaccharolytic, indole positive, urease negative, and negative for esculin and gelatin hydrolysis as well as lecithinase and lipase activity (8, 111).
The role of BVAB1, BVAB2, and M. indolicus in modulating host immunity has not been sufficiently investigated yet. The presence of the Clostridiales taxon, identified by sequencing of variable region 4 of the bacterial 16S rRNA gene in cervical swabs, was not correlated with cervical inflammation assessed by cytokines in young South African women (75). One study showed an association of M. indolicus with friable (easily bleeding on touch) cervix but not with other classic symptoms of cervicitis, e.g., mucopus and increased numbers of polymorphonuclear leukocytes in the cervix (112).
LINKING IMMUNITY TO BV AND MICROBIAL GROUP PATTERNS
Innate Immunity and Cytokines in BV
Classification of the vaginal microbiota by Nugent criteria as well as by 16S rRNA taxonomic features linked microbial patterns to significant variations in the cervicovaginal immune response, including cytokines, antimicrobial proteins, and immune cell populations. A significant body of literature compares cervicovaginal levels of cytokines in women with vaginal microbiota classified by Nugent scores (113). A number of studies have reported higher levels of IL-1β and more variable but higher levels of IL-8 in women with BV than in controls with normal Nugent scores (65, 72, 114–116). Significant cross-sectional differences in IL-1β and IL-1α levels and, more consistently, high levels of IL-8 were also found for women with normal Nugent scores (≤3) compared to those with intermediate Nugent scores (4 to 6) (72, 116), suggesting that intermediate scores may represent an independent pathological condition or a transition to BV. Moreover, elevated cervicovaginal levels were correlated with inflammation at the tissue and cellular levels (117) and related medical risks such as HIV acquisition and pathological pregnancy, as described in more detail below, but not with symptoms of inflammation (118), supporting the conclusion that asymptomatic BV should not be regarded as a harmless condition. The host reacts to the increased diversity and abundance of pathogenic vaginal organisms by not only resetting the cytokine network but also increasing vaginal levels of antimicrobial effectors produced by leukocytes, e.g., nitric oxide (NO) (119) and heat shock protein 70 (hsp70) (120). However, BV has been associated with divergent effects on immune cell populations in the vaginal mucosa, ranging from suppressed numbers of leukocytes (identified by the panleukocyte marker CD45) to increased numbers of CD4+ versus CD8+ cells compared to controls (65). Women with BV had lower levels of neutrophils than did women with vulvovaginal candidiasis, the latter presenting with classic vaginitis (121, 122). In women with aerobic vaginitis, the number of vaginal leukocytes on wet mounts positively correlated with vaginal levels of IL-1β (123), suggesting that higher levels of IL-1β in women with BV are not sufficient to drive the same leukocyte response as that in women with aerobic vaginitis or that BV organisms reduce leukocyte recruitment or half-life by mechanisms that are yet to be defined. BV has also been associated with a significant reduction in vaginal levels of the secretory leukocyte protease inhibitor (SLPI), which is produced by both epithelial and immune cells (74, 124–126).
Inflammatory Changes and Preterm Birth
The unwanted clinical consequences of the immunoinflammatory changes associated with BV are of significant public health concern. There is evidence that immune responses to BV bacteria ascending into the upper reproductive tract and colonizing the placenta can cause inflammation, with an impact on newborn health. Using culture-based techniques, Onderdonk et al. showed that vaginal bacteria can ascend and frequently colonize the preterm placenta during pregnancy (127). Others have confirmed the presence of bacteria in the placenta by using histology and molecular techniques (128, 129). Fichorova et al. (130) showed that BV organisms prevalent in the placenta of very preterm neonates, e.g., Prevotella, Gardnerella, anaerobic streptococci, peptostreptococci, and genital mycoplasmas, were each associated with a different pattern of upregulation of systemic inflammatory mediators in newborns. In contrast, Lactobacillus colonization of the placenta was associated with low odds of an inflammatory response in newborns. In turn, the patterns of maternal microbe-dependent systemic inflammation were associated with developmental delay in psychomotor function, brain damage, and a myriad of inflammation-driven complications in very-early-gestational-age children studied up to 9 years of age (131–137).
HIV Infection and Vaginal Immunity
Another significant consequence of BV-altered immunity is the increased risk of HIV acquisition and transmission. The proinflammatory cytokines and chemokines, whose levels are increased by BV-associated bacteria in vitro, and which are associated with BV in vivo, e.g., IL-1β and IL-8, enhance the risk of HIV transmission by directly stimulating HIV replication in latent viral reservoirs and by facilitating the trafficking and activation of CD4-positive host cells, which are normally sparse in the cervicovaginal mucosa (138). The reduction in the levels of antiviral factors by BV, e.g., SLPI, has also been associated with a higher risk of HIV acquisition (71), and women with BV have shown lower innate anti-HIV activity of their cervicovaginal secretions than controls (65).
Host Factors Associated with Vaginal Immune Function
Interpretations of cervicovaginal immunity fluctuations and their covariation or dependence on BV have been hampered by the limited understanding of the nonmicrobial factors that govern the set point of vaginal mucosal homeostasis. Age, ethnicity, genetic factors, reproductive hormones, nutrition, socioeconomic and psychosomatic stress, physical fitness and exercise, and diurnal variations have all been suspected to be factors in the regulation of cytokine networks but have been only sporadically studied and have not been systematically characterized as immunity modifiers in the female genital tract (116, 139). Ethnic variations in vaginal cytokines and microbiota have been observed (140), which could in part be explained by genetic polymorphisms in cytokines and innate immunity genes (141) and need to be addressed by more studies. Cytokine polymorphisms can affect vaginal levels of cytokines such as TNF-α and IL-1 and predispose women to higher-level proinflammatory responses to BV (142, 143). Not only did Toll-like receptor 4 (TLR4) polymorphism affect colonization by BV bacteria, but a particular TLR4 genotype also amplified the IL-1β responses to G. vaginalis and anaerobic Gram-negative rods (144).
Endogenous reproductive hormones as well as exogenous synthetic progestogens and estrogens have a profound effect on cervicovaginal immunity. Studies of microbiota-host interactions have to control for menstrual cycle phase, which affects both cytokine levels (145, 146) and microbial composition (147, 148). Cervical ectopy (columnar epithelium replacing the squamous multilayer epithelium of the ectocervix), which is hormone dependent and especially common in adolescents, is also characterized by higher levels of proinflammatory mediators (146) and a shift to BV microbiota (149). Analysis of cervical cytokines in ∼900 Ugandan and Zimbabwean women showed age-related and geographic differences as well as significant modifying effects of pregnancy, breastfeeding, and the use of hormonal contraceptives (71). The presence of BV and altered microbiota defined by intermediate Nugent scores significantly modified the immunoregulatory effects on hormonal contraceptives and especially the changes associated with an increased risk of HIV compared to controls with normal Nugent scores (118), suggesting that unwanted side effects of hormonal contraceptives may be reduced by prevention and treatment of BV as well as the abnormal intermediate vaginal microbiota.
The defining line between healthy variation in vaginal microbiome composition and the pathogenic deviations that require clinical interventions has yet to be elucidated; however, the overwhelming majority of reported studies support the role of BV as defined by Nugent scores in reducing the chances of a healthy reproductive outcome and increasing susceptibility to HIV. Conversely, regardless of geographic areas or populations, the presence of Lactobacillus species, e.g., L. crispatus, L. jensenii, and L. gasseri, is associated with a lower-level proinflammatory state in the cervicovaginal mucosa. Moreover, placental colonization with BV-associated bacteria conveys an increased risk of systemic inflammation in newborns, which can have long-term adverse effects on child development and mental health. Therefore, the development of a multifactorial approach and design for preventative interventions targeting vaginal microbes and the human host to maintain a Lactobacillus-dominant vaginal microbiota appears to be a reasonable goal for future translational research.
CONCLUSION
It is clear from the literature that what we call bacterial vaginosis cannot be easily characterized by any of the methods that have been utilized to date. While the clinical symptoms associated with this disease are relatively uncomplicated and easily measured, the fact that not all symptoms occur in all women diagnosed with BV remains problematic. Similarly, efforts to characterize BV using epidemiological, microscopic, microbiological culture, and sequence-based methods have all failed to reveal an etiology that can be consistently documented for all women with BV. This is not surprising given the complexity of the vaginal microbiome, host immunity, and the variability in individual responses to potentially inflammatory mediators produced by an array of microorganisms. A careful analysis of the available data suggests that what we term BV is, in fact, a set of common clinical signs and symptoms that can be provoked by a plethora of bacterial species with proinflammatory characteristics, coupled to an immune response driven by individual variability in host immune function. This is not a medical revelation because there are many other clinical syndromes where the symptoms are common among individuals but the underlying infectious agent(s) is not. Examples might include intra-abdominal sepsis, which can include a variety of bacterial species; viral pharyngitis caused by a number of different viral agents; and wound infections generated from penetrating trauma, which also include many different microbial species. In each case, the clinical symptoms and inflammatory processes among affected patients are similar, but the underlying microbial etiology can vary. Based on the available evidence, BV most closely fits the definition of a polymicrobial infectious process involving multiple bacterial species that may vary from patient to patient. Perhaps, a better term for BV is polymicrobial vaginosis, since this may reflect what is actually happening during the inflammatory process and does not connote a common etiology.
Biographies

Andrew B. Onderdonk, Ph.D., is the Director of Clinical Microbiology at the Brigham and Women's Hospital and a Professor of Pathology at Harvard Medical School. His research encompasses several areas related to human microbial flora and its role in health and disease. These interests include the pathogenesis of obligately anaerobic microorganisms, the in vivo and in vitro modeling of both normal microflora and pathogenic microorganisms, and the evaluation and development of new therapeutic agents, including probiotics, antibiotics, and immunomodulators.

Mary L. Delaney is a Research Associate in the Anaerobe Research Laboratory at the Brigham and Women's Hospital. She received her master's degree in biology from Harvard University. She has over 30 years of experience as a research microbiologist in areas related to human microbial flora and its role in health and disease. She has participated in research evaluating the role of vaginal microflora in preterm labor and has extensive experience in the cultivation and identification of obligately anaerobic microorganisms.

Raina N. Fichorova, M.D., Ph.D., is the Director of Genital Tract Biology at the Brigham and Women's Hospital and Professor of Obstetrics, Gynecology and Reproductive Biology, Harvard Medical School. Her research has spanned more than 25 years with a focus is on immunity and inflammation at the intersection of reproduction and infectious disease. She has made contributions to understanding the fundamental pathways of host-pathogen-microbiota interactions in the female reproductive tract and their role in pregnancy and infant health.
REFERENCES
- 1.Chang HH, Larson J, Blencowe H, Spong CY, Howson CP, Cairns-Smith S, Lackritz EM, Lee SK, Mason E, Serazin AC, Walani S, Simpson JL, Lawn JE. 2013. Preventing preterm births: analysis of trends and potential reductions with interventions in 39 countries with very high human development index. Lancet 381:223–234. doi: 10.1016/S0140-6736(12)61856-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Behrman RE, Butler AS. 2007. Preterm birth: causes, consequences, and prevention. National Academy of Sciences, Washington, DC. doi: 10.17226/11622. [DOI] [PubMed] [Google Scholar]
- 3.Onderdonk AB, Delaney ML, Zamarchi GR, Hirsch ML, Munoz A, Kass EH. 1989. Normal vaginal microflora during use of various forms of catamenial protection. Rev Infect Dis 11(Suppl 1):S61–S67. [DOI] [PubMed] [Google Scholar]
- 4.Onderdonk AB, Zamarchi GR, Rodriguez ML, Hirsch ML, Munoz A, Kass EH. 1987. Qualitative assessment of vaginal microflora during use of tampons of various compositions. Appl Environ Microbiol 53:2779–2784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Onderdonk AB, Zamarchi GR, Rodriguez ML, Hirsch ML, Munoz A, Kass EH. 1987. Quantitative assessment of vaginal microflora during use of tampons of various compositions. Appl Environ Microbiol 53:2774–2778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Onderdonk AB, Zamarchi GR, Walsh JA, Mellor RD, Munoz A, Kass EH. 1986. Methods for quantitative and qualitative evaluation of vaginal microflora during menstruation. Appl Environ Microbiol 51:333–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Danielsson D, Teigen PK, Moi H. 2011. The genital econiche: focus on microbiota and bacterial vaginosis. Ann N Y Acad Sci 1230:48–58. doi: 10.1111/j.1749-6632.2011.06041.x. [DOI] [PubMed] [Google Scholar]
- 8.Datcu R. 2014. Characterization of the vaginal microflora in health and disease. Dan Med J 61:B4830. [PubMed] [Google Scholar]
- 9.Hillier SL, Krohn MA, Rabe LK, Klebanoff SJ, Eschenbach DA. 1993. The normal vaginal flora, H2O2-producing lactobacilli, and bacterial vaginosis in pregnant women. Clin Infect Dis 16(Suppl 4):S273–S281. [DOI] [PubMed] [Google Scholar]
- 10.Turovskiy Y, Sutyak Noll K, Chikindas ML. 2011. The aetiology of bacterial vaginosis. J Appl Microbiol 110:1105–1128. doi: 10.1111/j.1365-2672.2011.04977.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van de Wijgert JH, Borgdorff H, Verhelst R, Crucitti T, Francis S, Verstraelen H, Jespers V. 2014. The vaginal microbiota: what have we learned after a decade of molecular characterization? PLoS One 9:e105998. doi: 10.1371/journal.pone.0105998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ravel J, Gajer P, Abdo Z, Schneider GM, Koenig SS, McCulle SL, Karlebach S, Gorle R, Russell J, Tacket CO, Brotman RM, Davis CC, Ault K, Peralta L, Forney LJ. 2011. Vaginal microbiome of reproductive-age women. Proc Natl Acad Sci U S A 108(Suppl 1):4680–4687. doi: 10.1073/pnas.1002611107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hickey RJ, Zhou X, Pierson JD, Ravel J, Forney LJ. 2012. Understanding vaginal microbiome complexity from an ecological perspective. Transl Res 160:267–282. doi: 10.1016/j.trsl.2012.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fredricks DN, Fiedler TL, Marrazzo JM. 2005. Molecular identification of bacteria associated with bacterial vaginosis. N Engl J Med 353:1899–1911. doi: 10.1056/NEJMoa043802. [DOI] [PubMed] [Google Scholar]
- 15.Spiegel CA. 1991. Bacterial vaginosis. Clin Microbiol Rev 4:485–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eschenbach DA. 1993. History and review of bacterial vaginosis. Am J Obstet Gynecol 169:441–445. doi: 10.1016/0002-9378(93)90337-I. [DOI] [PubMed] [Google Scholar]
- 17.Hill GB. 1993. The microbiology of bacterial vaginosis. Am J Obstet Gynecol 169:450–454. doi: 10.1016/0002-9378(93)90339-K. [DOI] [PubMed] [Google Scholar]
- 18.Mead PB. 1993. Epidemiology of bacterial vaginosis. Am J Obstet Gynecol 169:446–449. doi: 10.1016/0002-9378(93)90338-J. [DOI] [PubMed] [Google Scholar]
- 19.Kenyon C, Colebunders R, Crucitti T. 2013. The global epidemiology of bacterial vaginosis: a systematic review. Am J Obstet Gynecol 209:505–523. doi: 10.1016/j.ajog.2013.05.006. [DOI] [PubMed] [Google Scholar]
- 20.Srinivasan S, Fredricks DN. 2008. The human vaginal bacterial biota and bacterial vaginosis. Interdiscip Perspect Infect Dis 2008:750479. doi: 10.1155/2008/750479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ravel J, Brotman RM, Gajer P, Ma B, Nandy M, Fadrosh DW, Sakamoto J, Koenig SS, Fu L, Zhou X, Hickey RJ, Schwebke JR, Forney LJ. 2013. Daily temporal dynamics of vaginal microbiota before, during and after episodes of bacterial vaginosis. Microbiome 1:29. doi: 10.1186/2049-2618-1-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Srinivasan S, Liu C, Mitchell CM, Fiedler TL, Thomas KK, Agnew KJ, Marrazzo JM, Fredricks DN. 2010. Temporal variability of human vaginal bacteria and relationship with bacterial vaginosis. PLoS One 5:e10197. doi: 10.1371/journal.pone.0010197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lowe NK, Neal JL, Ryan-Wenger NA. 2009. Accuracy of the clinical diagnosis of vaginitis compared with a DNA probe laboratory standard. Obstet Gynecol 113:89–95. doi: 10.1097/AOG.0b013e3181909f63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gardner HL, Dukes CD. 1955. Haemophilus vaginalis vaginitis: a newly defined specific infection previously classified non-specific vaginitis. Am J Obstet Gynecol 69:962–976. [PubMed] [Google Scholar]
- 25.Kenyon CR, Osbak K. 2014. Recent progress in understanding the epidemiology of bacterial vaginosis. Curr Opin Obstet Gynecol 26:448–454. doi: 10.1097/GCO.0000000000000112. [DOI] [PubMed] [Google Scholar]
- 26.Cherpes TL, Hillier SL, Meyn LA, Busch JL, Krohn MA. 2008. A delicate balance: risk factors for acquisition of bacterial vaginosis include sexual activity, absence of hydrogen peroxide-producing lactobacilli, black race, and positive herpes simplex virus type 2 serology. Sex Transm Dis 35:78–83. doi: 10.1097/OLQ.0b013e318156a5d0. [DOI] [PubMed] [Google Scholar]
- 27.Amsel R, Totten PA, Spiegel CA, Chen KC, Eschenbach D, Holmes KK. 1983. Nonspecific vaginitis. Diagnostic criteria and microbial and epidemiologic associations. Am J Med 74:14–22. [DOI] [PubMed] [Google Scholar]
- 28.Dunkelberg WE., Jr 1965. Diagnosis of Hemophilus vaginalis vaginitis by Gram-stained smears. Am J Obstet Gynecol 91:998–1000. [DOI] [PubMed] [Google Scholar]
- 29.Spiegel CA, Amsel R, Holmes KK. 1983. Diagnosis of bacterial vaginosis by direct Gram stain of vaginal fluid. J Clin Microbiol 18:170–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nugent RP, Krohn MA, Hillier SL. 1991. Reliability of diagnosing bacterial vaginosis is improved by a standardized method of Gram stain interpretation. J Clin Microbiol 29:297–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dodelein A. 1894. Die scheidensekretuntersuchugen. Zentralb Gynakol 18:10–14. [Google Scholar]
- 32.Spiegel CA, Amsel R, Eschenbach D, Schoenknecht F, Holmes KK. 1980. Anaerobic bacteria in nonspecific vaginitis. N Engl J Med 303:601–607. doi: 10.1056/NEJM198009113031102. [DOI] [PubMed] [Google Scholar]
- 33.Holst E. 1990. Reservoir of four organisms associated with bacterial vaginosis suggests lack of sexual transmission. J Clin Microbiol 28:2035–2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Spiegel CA, Davick P, Totten PA, Chen KC, Eschenbach DA, Amsel R, Holmes KK. 1983. Gardnerella vaginalis and anaerobic bacteria in the etiology of bacterial (nonspecific) vaginosis. Scand J Infect Dis Suppl 40:41–46. [PubMed] [Google Scholar]
- 35.Blackwell AL, Fox AR, Phillips I, Barlow D. 1983. Anaerobic vaginosis (non-specific vaginitis): clinical, microbiological, and therapeutic findings. Lancet ii:1379–1382. [DOI] [PubMed] [Google Scholar]
- 36.Masfari AN, Duerden BI, Kinghorn GR. 1986. Quantitative studies of vaginal bacteria. Genitourin Med 62:256–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Onderdonk AB, Lee ML, Lieberman E, Delaney ML, Tuomala RE. 2003. Quantitative microbiologic models for preterm delivery. J Clin Microbiol 41:1073–1079. doi: 10.1128/JCM.41.3.1073-1079.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hillier SL, Nugent RP, Eschenbach DA, Krohn MA, Gibbs RS, Martin DH, Cotch MF, Edelman R, Pastorek JG II, Rao AV, McNellis D, Regan JA, Carey JC, Klebanoff MA. 1995. Association between bacterial vaginosis and preterm delivery of a low-birth-weight infant. The Vaginal Infections and Prematurity Study Group. N Engl J Med 333:1737–1742. [DOI] [PubMed] [Google Scholar]
- 39.Martius J, Krohn MA, Hillier SL, Stamm WE, Holmes KK, Eschenbach DA. 1988. Relationships of vaginal Lactobacillus species, cervical Chlamydia trachomatis, and bacterial vaginosis to preterm birth. Obstet Gynecol 71:89–95. [PubMed] [Google Scholar]
- 40.Lee ML, Ross RA, Delaney ML, Onderdonk AB. 1994. Predicting abnormal microbial population levels in the vaginal ecosystem. Microb Ecol Health Dis 7:235–240. doi: 10.3109/08910609409141360. [DOI] [Google Scholar]
- 41.Lee ML, Ross RA, Delaney ML, Onderdonk AB. 1995. Mathematical modeling of the vaginal microflora. Microecol Ther 23:8–15. [Google Scholar]
- 42.Lamont RF, Nhan-Chang CL, Sobel JD, Workowski K, Conde-Agudelo A, Romero R. 2011. Treatment of abnormal vaginal flora in early pregnancy with clindamycin for the prevention of spontaneous preterm birth: a systematic review and metaanalysis. Am J Obstet Gynecol 205:177–190. doi: 10.1016/j.ajog.2011.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sha BE, Chen HY, Wang QJ, Zariffard MR, Cohen MH, Spear GT. 2005. Utility of Amsel criteria, Nugent score, and quantitative PCR for Gardnerella vaginalis, Mycoplasma hominis, and Lactobacillus spp. for diagnosis of bacterial vaginosis in human immunodeficiency virus-infected women. J Clin Microbiol 43:4607–4612. doi: 10.1128/JCM.43.9.4607-4612.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Menard JP, Fenollar F, Henry M, Bretelle F, Raoult D. 2008. Molecular quantification of Gardnerella vaginalis and Atopobium vaginae loads to predict bacterial vaginosis. Clin Infect Dis 47:33–43. doi: 10.1086/588661. [DOI] [PubMed] [Google Scholar]
- 45.Yoshimura K, Morotomi N, Fukuda K, Nakano M, Kashimura M, Hachisuga T, Taniguchi H. 2011. Intravaginal microbial flora by the 16S rRNA gene sequencing. Am J Obstet Gynecol 205:235.e1–235.e9. doi: 10.1016/j.ajog.2011.04.018. [DOI] [PubMed] [Google Scholar]
- 46.Fredricks DN, Fiedler TL, Thomas KK, Oakley BB, Marrazzo JM. 2007. Targeted PCR for detection of vaginal bacteria associated with bacterial vaginosis. J Clin Microbiol 45:3270–3276. doi: 10.1128/JCM.01272-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Boggess KA, Trevett TN, Madianos PN, Rabe L, Hillier SL, Beck J, Offenbacher S. 2005. Use of DNA hybridization to detect vaginal pathogens associated with bacterial vaginosis among asymptomatic pregnant women. Am J Obstet Gynecol 193:752–756. doi: 10.1016/j.ajog.2005.01.068. [DOI] [PubMed] [Google Scholar]
- 48.Dols JA, Smit PW, Kort R, Reid G, Schuren FH, Tempelman H, Bontekoe TR, Korporaal H, Boon ME. 2011. Microarray-based identification of clinically relevant vaginal bacteria in relation to bacterial vaginosis. Am J Obstet Gynecol 204:305.e1–305.e7. doi: 10.1016/j.ajog.2010.11.012. [DOI] [PubMed] [Google Scholar]
- 49.Donders G. 2010. Diagnosis and management of bacterial vaginosis and other types of abnormal vaginal bacterial flora: a review. Obstet Gynecol Surv 65:462–473. doi: 10.1097/OGX.0b013e3181e09621. [DOI] [PubMed] [Google Scholar]
- 50.Leopold S. 1953. Heretofore undescribed organism isolated from the genitourinary system. U S Armed Forces Med J 4:263–266. [PubMed] [Google Scholar]
- 51.Gardner HL, Dukes CD. 1954. New etiologic agent in nonspecific bacterial vaginitis. Science 120:853. doi: 10.1126/science.120.3125.853. [DOI] [PubMed] [Google Scholar]
- 52.Piot P, van Dyck E, Goodfellow M, Falkow S. 1980. A taxonomic study of Gardnerella vaginalis (Haemophilus vaginalis) Gardner and Dukes 1955. J Gen Microbiol 119:373–396. [DOI] [PubMed] [Google Scholar]
- 53.Harwich MD Jr, Alves JM, Buck GA, Strauss JF III, Patterson JL, Oki AT, Girerd PH, Jefferson KK. 2010. Drawing the line between commensal and pathogenic Gardnerella vaginalis through genome analysis and virulence studies. BMC Genomics 11:375. doi: 10.1186/1471-2164-11-375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Africa CW, Nel J, Stemmet M. 2014. Anaerobes and bacterial vaginosis in pregnancy: virulence factors contributing to vaginal colonisation. Int J Environ Res Public Health 11:6979–7000. doi: 10.3390/ijerph110706979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fethers K, Twin J, Fairley CK, Fowkes FJ, Garland SM, Fehler G, Morton AM, Hocking JS, Tabrizi SN, Bradshaw CS. 2012. Bacterial vaginosis (BV) candidate bacteria: associations with BV and behavioural practices in sexually-experienced and inexperienced women. PLoS One 7:e30633. doi: 10.1371/journal.pone.0030633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schwebke JR, Flynn MS, Rivers CA. 2014. Prevalence of Gardnerella vaginalis among women with lactobacillus-predominant vaginal flora. Sex Transm Infect 90:61–63. doi: 10.1136/sextrans-2013-051232. [DOI] [PubMed] [Google Scholar]
- 57.Srinivasan S, Hoffman NG, Morgan MT, Matsen FA, Fiedler TL, Hall RW, Ross FJ, McCoy CO, Bumgarner R, Marrazzo JM, Fredricks DN. 2012. Bacterial communities in women with bacterial vaginosis: high resolution phylogenetic analyses reveal relationships of microbiota to clinical criteria. PLoS One 7:e37818. doi: 10.1371/journal.pone.0037818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schwebke JR, Muzny CA, Josey WE. 2014. Role of Gardnerella vaginalis in the pathogenesis of bacterial vaginosis: a conceptual model. J Infect Dis 210:338–343. doi: 10.1093/infdis/jiu089. [DOI] [PubMed] [Google Scholar]
- 59.Swidsinski A, Doerffel Y, Loening-Baucke V, Swidsinski S, Verstraelen H, Vaneechoutte M, Lemm V, Schilling J, Mendling W. 2010. Gardnerella biofilm involves females and males and is transmitted sexually. Gynecol Obstet Invest 70:256–263. doi: 10.1159/000314015. [DOI] [PubMed] [Google Scholar]
- 60.Reddy SP, Rasmussen WG, Baseman JB. 1995. Molecular cloning and characterization of an adherence-related operon of Mycoplasma genitalium. J Bacteriol 177:5943–5951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gelber SE, Aguilar JL, Lewis KL, Ratner AJ. 2008. Functional and phylogenetic characterization of vaginolysin, the human-specific cytolysin from Gardnerella vaginalis. J Bacteriol 190:3896–3903. doi: 10.1128/JB.01965-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cauci S, Culhane JF, Di Santolo M, McCollum K. 2008. Among pregnant women with bacterial vaginosis, the hydrolytic enzymes sialidase and prolidase are positively associated with interleukin-1beta. Am J Obstet Gynecol 198:132.e1–132.e7. [DOI] [PubMed] [Google Scholar]
- 63.Swidsinski A, Mendling W, Loening-Baucke V, Swidsinski S, Dorffel Y, Scholze J, Lochs H, Verstraelen H. 2008. An adherent Gardnerella vaginalis biofilm persists on the vaginal epithelium after standard therapy with oral metronidazole. Am J Obstet Gynecol 198:97.e1–97.e6. [DOI] [PubMed] [Google Scholar]
- 64.Marrs CN, Knobel SM, Zhu WQ, Sweet SD, Chaudhry AR, Alcendor DJ. 2012. Evidence for Gardnerella vaginalis uptake and internalization by squamous vaginal epithelial cells: implications for the pathogenesis of bacterial vaginosis. Microbes Infect 14:500–508. doi: 10.1016/j.micinf.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Thurman AR, Kimble T, Herold B, Mesquita PM, Fichorova RN, Dawood HY, Fashemi T, Chandra N, Rabe L, Cunningham TD, Anderson S, Schwartz J, Doncel G. 2015. Bacterial vaginosis and subclinical markers of genital tract inflammation and mucosal immunity. AIDS Res Hum Retroviruses 31:1139–1152. doi: 10.1089/aid.2015.0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Patterson JL, Stull-Lane A, Girerd PH, Jefferson KK. 2010. Analysis of adherence, biofilm formation and cytotoxicity suggests a greater virulence potential of Gardnerella vaginalis relative to other bacterial-vaginosis-associated anaerobes. Microbiology 156:392–399. doi: 10.1099/mic.0.034280-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fichorova RN, Rheinwald JG, Anderson DJ. 1997. Generation of papillomavirus-immortalized cell lines from normal human ectocervical, endocervical, and vaginal epithelium that maintain expression of tissue-specific differentiation proteins. Biol Reprod 57:847–855. doi: 10.1095/biolreprod57.4.847. [DOI] [PubMed] [Google Scholar]
- 68.Fichorova RN, Anderson DJ. 1999. Differential expression of immunobiological mediators by immortalized human cervical and vaginal epithelial cells. Biol Reprod 60:508–514. doi: 10.1095/biolreprod60.2.508. [DOI] [PubMed] [Google Scholar]
- 69.Eade CR, Diaz C, Wood MP, Anastos K, Patterson BK, Gupta P, Cole AL, Cole AM. 2012. Identification and characterization of bacterial vaginosis-associated pathogens using a comprehensive cervical-vaginal epithelial coculture assay. PLoS One 7:e50106. doi: 10.1371/journal.pone.0050106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fichorova RN, Buck OR, Yamamoto HS, Fashemi T, Dawood HY, Fashemi B, Hayes GR, Beach DH, Takagi Y, Delaney ML, Nibert ML, Singh BN, Onderdonk AB. 2013. The villain team-up or how Trichomonas vaginalis and bacterial vaginosis alter innate immunity in concert. Sex Transm Infect 89:460–466. doi: 10.1136/sextrans-2013-051052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Morrison C, Fichorova R, Mauck C, Chen P, Kwok C, Chipato T, Salata R, Doncel GF. 2014. Cervical inflammation and immunity associated with hormonal contraception, pregnancy and HIV-1 seroconversion. J Acquir Immune Defic Syndr 66:109–117. doi: 10.1097/QAI.0000000000000103. [DOI] [PubMed] [Google Scholar]
- 72.Hedges SR, Barrientes F, Desmond RA, Schwebke JR. 2006. Local and systemic cytokine levels in relation to changes in vaginal flora. J Infect Dis 193:556–562. doi: 10.1086/499824. [DOI] [PubMed] [Google Scholar]
- 73.Anderson BL, Cu-Uvin S, Raker CA, Fitzsimmons C, Hillier SL. 2011. Subtle perturbations of genital microflora alter mucosal immunity among low-risk pregnant women. Acta Obstet Gynecol Scand 90:510–515. doi: 10.1111/j.1600-0412.2011.01082.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kyongo JK, Crucitti T, Menten J, Hardy L, Cools P, Michiels J, Delany-Moretlwe S, Mwaura M, Ndayisaba G, Joseph S, Fichorova R, van de Wijgert J, Vanham G, Arien KK, Jespers V. 2015. Cross-sectional analysis of selected genital tract immunological markers and molecular vaginal microbiota in sub-Saharan African women, with relevance to HIV risk and prevention. Clin Vaccine Immunol 22:526–538. doi: 10.1128/CVI.00762-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Anahtar MN, Byrne EH, Doherty KE, Bowman BA, Yamamoto HS, Soumillon M, Padavattan N, Ismail N, Moodley A, Sabatini ME, Ghebremichael MS, Nusbaum C, Huttenhower C, Virgin HW, Ndung'u T, Dong KL, Walker BD, Fichorova RN, Kwon DS. 2015. Cervicovaginal bacteria are a major modulator of host inflammatory responses in the female genital tract. Immunity 42:965–976. doi: 10.1016/j.immuni.2015.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.DiGiulio DB, Callahan BJ, McMurdie PJ, Costello EK, Lyell DJ, Robaczewska A, Sun CL, Goltsman DS, Wong RJ, Shaw G, Stevenson DK, Holmes SP, Relman DA. 2015. Temporal and spatial variation of the human microbiota during pregnancy. Proc Natl Acad Sci U S A 112:11060–11065. doi: 10.1073/pnas.1502875112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Burton JP, Devillard E, Cadieux PA, Hammond JA, Reid G. 2004. Detection of Atopobium vaginae in postmenopausal women by cultivation-independent methods warrants further investigation. J Clin Microbiol 42:1829–1831. doi: 10.1128/JCM.42.4.1829-1831.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rodriguez Jovita M, Collins MD, Sjoden B, Falsen E. 1999. Characterization of a novel Atopobium isolate from the human vagina: description of Atopobium vaginae sp. nov. Int J Syst Bacteriol 49(Part 4):1573–1576. [DOI] [PubMed] [Google Scholar]
- 79.Polatti F. 2012. Bacterial vaginosis, Atopobium vaginae and nifuratel. Curr Clin Pharmacol 7:36–40. doi: 10.2174/157488412799218824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Verhelst R, Verstraelen H, Claeys G, Verschraegen G, Delanghe J, Van Simaey L, De Ganck C, Temmerman M, Vaneechoutte M. 2004. Cloning of 16S rRNA genes amplified from normal and disturbed vaginal microflora suggests a strong association between Atopobium vaginae, Gardnerella vaginalis and bacterial vaginosis. BMC Microbiol 4:16. doi: 10.1186/1471-2180-4-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bradshaw CS, Tabrizi SN, Fairley CK, Morton AN, Rudland E, Garland SM. 2006. The association of Atopobium vaginae and Gardnerella vaginalis with bacterial vaginosis and recurrence after oral metronidazole therapy. J Infect Dis 194:828–836. doi: 10.1086/506621. [DOI] [PubMed] [Google Scholar]
- 82.Zozaya-Hinchliffe M, Lillis R, Martin DH, Ferris MJ. 2010. Quantitative PCR assessments of bacterial species in women with and without bacterial vaginosis. J Clin Microbiol 48:1812–1819. doi: 10.1128/JCM.00851-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Libby EK, Pascal KE, Mordechai E, Adelson ME, Trama JP. 2008. Atopobium vaginae triggers an innate immune response in an in vitro model of bacterial vaginosis. Microbes Infect 10:439–446. doi: 10.1016/j.micinf.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 84.Fichorova RN, Yamamoto HS, Delaney ML, Onderdonk AB, Doncel GF. 2011. Novel vaginal microflora colonization model providing new insight into microbicide mechanism of action. mBio 2:e00168-11. doi: 10.1128/mBio.00168-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Doerflinger SY, Throop AL, Herbst-Kralovetz MM. 2014. Bacteria in the vaginal microbiome alter the innate immune response and barrier properties of the human vaginal epithelia in a species-specific manner. J Infect Dis 209:1989–1999. doi: 10.1093/infdis/jiu004. [DOI] [PubMed] [Google Scholar]
- 86.Srinivasan S, Morgan MT, Liu C, Matsen FA, Hoffman NG, Fiedler TL, Agnew KJ, Marrazzo JM, Fredricks DN. 2013. More than meets the eye: associations of vaginal bacteria with Gram stain morphotypes using molecular phylogenetic analysis. PLoS One 8:e78633. doi: 10.1371/journal.pone.0078633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Delaney ML, Onderdonk AB. 2001. Nugent score related to vaginal culture in pregnant women. Obstet Gynecol 98:79–84. doi: 10.1016/S0029-7844(01)01402-8. [DOI] [PubMed] [Google Scholar]
- 88.Pybus V, Onderdonk AB. 1997. Evidence for a commensal, symbiotic relationship between Gardnerella vaginalis and Prevotella bivia involving ammonia: potential significance for bacterial vaginosis. J Infect Dis 175:406–413. doi: 10.1093/infdis/175.2.406. [DOI] [PubMed] [Google Scholar]
- 89.Pybus V, Onderdonk AB. 1998. A commensal symbiosis between Prevotella bivia and Peptostreptococcus anaerobius involves amino acids: potential significance to the pathogenesis of bacterial vaginosis. FEMS Immunol Med Microbiol 22:317–327. doi: 10.1111/j.1574-695X.1998.tb01221.x. [DOI] [PubMed] [Google Scholar]
- 90.Marrazzo JM, Thomas KK, Fiedler TL, Ringwood K, Fredricks DN. 2008. Relationship of specific vaginal bacteria and bacterial vaginosis treatment failure in women who have sex with women. Ann Intern Med 149:20–28. doi: 10.7326/0003-4819-149-1-200807010-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Murphy EC, Frick IM. 2013. Gram-positive anaerobic cocci—commensals and opportunistic pathogens. FEMS Microbiol Rev 37:520–553. doi: 10.1111/1574-6976.12005. [DOI] [PubMed] [Google Scholar]
- 92.Zozaya-Hinchliffe M, Martin DH, Ferris MJ. 2008. Prevalence and abundance of uncultivated Megasphaera-like bacteria in the human vaginal environment. Appl Environ Microbiol 74:1656–1659. doi: 10.1128/AEM.02127-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Imai K, Yamada K, Tamura M, Ochiai K, Okamoto T. 2012. Reactivation of latent HIV-1 by a wide variety of butyric acid-producing bacteria. Cell Mol Life Sci 69:2583–2592. doi: 10.1007/s00018-012-0936-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Harwich MD Jr, Serrano MG, Fettweis JM, Alves JM, Reimers MA, Buck GA, Jefferson KK. 2012. Genomic sequence analysis and characterization of Sneathia amnii sp. nov. BMC Genomics 13(Suppl 8):S4. doi: 10.1186/1471-2164-13-S8-S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Collins MD, Hoyles L, Tornqvist E, von Essen R, Falsen E. 2001. Characterization of some strains from human clinical sources which resemble “Leptotrichia sanguinegens”: description of Sneathia sanguinegens sp. nov., gen. nov. Syst Appl Microbiol 24:358–361. doi: 10.1078/0723-2020-00047. [DOI] [PubMed] [Google Scholar]
- 96.Gharbia SE, Shah HN, Bernard K. 2012. International Committe on Systematics of Prokaryotes, Subcommittee on the Taxonomy of Gram-Negative Anaerobic Rods. Int J Syst Evol Microbiol 62:467–471. doi: 10.1099/ijs.0.039677-0. [DOI] [Google Scholar]
- 97.Meltzer MC, Desmond RA, Schwebke JR. 2008. Association of Mobiluncus curtisii with recurrence of bacterial vaginosis. Sex Transm Dis 35:611–613. doi: 10.1097/OLQ.0b013e318167b105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Roberts MC, Hillier SL, Schoenknecht FD, Holmes KK. 1985. Comparison of Gram stain, DNA probe, and culture for the identification of species of Mobiluncus in female genital specimens. J Infect Dis 152:74–77. doi: 10.1093/infdis/152.1.74. [DOI] [PubMed] [Google Scholar]
- 99.Hillier SL, Critchlow CW, Stevens CE, Roberts MC, Wolner-Hanssen P, Eschenbach DA, Holmes KK. 1991. Microbiological, epidemiological and clinical correlates of vaginal colonisation by Mobiluncus species. Genitourin Med 67:26–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Schwebke JR, Lawing LF. 2001. Prevalence of Mobiluncus spp among women with and without bacterial vaginosis as detected by polymerase chain reaction. Sex Transm Dis 28:195–199. doi: 10.1097/00007435-200104000-00002. [DOI] [PubMed] [Google Scholar]
- 101.Culhane JF, Nyirjesy P, McCollum K, Goldenberg RL, Gelber SE, Cauci S. 2006. Variation in vaginal immune parameters and microbial hydrolytic enzymes in bacterial vaginosis positive pregnant women with and without Mobiluncus species. Am J Obstet Gynecol 195:516–521. doi: 10.1016/j.ajog.2006.02.036. [DOI] [PubMed] [Google Scholar]
- 102.Patel MA, Nyirjesy P. 2010. Role of Mycoplasma and Ureaplasma species in female lower genital tract infections. Curr Infect Dis Rep 12:417–422. doi: 10.1007/s11908-010-0136-x. [DOI] [PubMed] [Google Scholar]
- 103.Keane FE, Thomas BJ, Gilroy CB, Renton A, Taylor-Robinson D. 2000. The association of Mycoplasma hominis, Ureaplasma urealyticum and Mycoplasma genitalium with bacterial vaginosis: observations on heterosexual women and their male partners. Int J STD AIDS 11:356–360. doi: 10.1258/0956462001916056. [DOI] [PubMed] [Google Scholar]
- 104.Taylor-Robinson D, Rosenstein IJ. 2001. Is Mycoplasma hominis a vaginal pathogen? Sex Transm Infect 77:302. doi: 10.1136/sti.77.4.302-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Austin MN, Beigi RH, Meyn LA, Hillier SL. 2005. Microbiologic response to treatment of bacterial vaginosis with topical clindamycin or metronidazole. J Clin Microbiol 43:4492–4497. doi: 10.1128/JCM.43.9.4492-4497.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Peltier MR, Berlin Y, Tee SC, Smulian JC. 2009. Does progesterone inhibit bacteria-stimulated interleukin-8 production by lower genital tract epithelial cells? J Perinat Med 37:328–333. doi: 10.1515/JPM.2009.064. [DOI] [PubMed] [Google Scholar]
- 107.Li Y-H, Brauner A, Jonsson B, van der Ploeg I, Soder O, Holst M, Jensen JS, Lagercrantz H, Tullus K. 2000. Ureaplasma urealyticum-induced production of proinflammatory cytokines by macrophages. Pediatr Res 48:114–119. doi: 10.1203/00006450-200007000-00020. [DOI] [PubMed] [Google Scholar]
- 108.Hasebe A, Mu H-H, Cole BC. 2014. A potential pathogenic factor from Mycoplasma hominis is a TLR2-dependent, macrophage-activating, P50-related adhesin. Am J Reprod Immunol 72:285–295. doi: 10.1111/aji.12279. [DOI] [PubMed] [Google Scholar]
- 109.Muzny CA, Sunesara IR, Griswold ME, Kumar R, Lefkowitz EJ, Mena LA, Schwebke JR, Martin DH, Swiatlo E. 2014. Association between BVAB1 and high Nugent scores among women with bacterial vaginosis. Diagn Microbiol Infect Dis 80:321–323. doi: 10.1016/j.diagmicrobio.2014.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Martin DH. 2012. The microbiota of the vagina and its influence on women's health and disease. Am J Med Sci 343:2–9. doi: 10.1097/MAJ.0b013e31823ea228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Austin MN, Rabe LK, Srinivasan S, Fredricks DN, Wiesenfeld HC, Hillier SL. 2015. Mageeibacillus indolicus gen. nov., sp. nov.: a novel bacterium isolated from the female genital tract. Anaerobe 32:37–42. doi: 10.1016/j.anaerobe.2014.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Gorgos LM, Sycuro LK, Srinivasan S, Fiedler TL, Morgan MT, Balkus JE, McClelland SR, Fredricks DN, Marrazzo JM. 2015. Relationship of specific bacteria in the cervical and vaginal microbiotas with cervicitis. Sex Transm Dis 42:475–481. doi: 10.1097/OLQ.0000000000000318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Mitchell C, Marrazzo J. 2014. Bacterial vaginosis and the cervicovaginal immune response. Am J Reprod Immunol 71:555–563. doi: 10.1111/aji.12264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Spandorfer SD, Neuer A, Giraldo PC, Rosenwaks Z, Witkin SS. 2001. Relationship of abnormal vaginal flora, proinflammatory cytokines and idiopathic infertility in women undergoing IVF. J Reprod Med 46:806–810. [PubMed] [Google Scholar]
- 115.Cauci S, Guaschino S, de Aloysio D, Driussi S, De Santo D, Penacchioni P, Quadrifoglio F. 2003. Interrelationships of interleukin-8 with interleukin-1beta and neutrophils in vaginal fluid of healthy and bacterial vaginosis positive women. Mol Hum Reprod 9:53–58. doi: 10.1093/molehr/gag003. [DOI] [PubMed] [Google Scholar]
- 116.Fichorova RN, Lai JJ, Schwartz JL, Weiner DH, Mauck CK, Callahan MM. 2011. Baseline variation and associations between subject characteristics and five cytokine biomarkers of vaginal safety among healthy non-pregnant women in microbicide trials. Cytokine 55:134–140. doi: 10.1016/j.cyto.2011.03.016. [DOI] [PubMed] [Google Scholar]
- 117.Mauck CK, Lai JJ, Weiner DH, Chandra N, Fichorova RN, Dezzutti CS, Hillier SL, Archer DF, Creinin MD, Schwartz JL, Callahan MM, Doncel GF. 2013. Toward early safety alert endpoints: exploring biomarkers suggestive of microbicide failure. AIDS Res Hum Retroviruses 29:1475–1486. doi: 10.1089/aid.2012.0345. [DOI] [PubMed] [Google Scholar]
- 118.Fichorova RN, Chen PL, Morrison CS, Doncel GF, Mendonca K, Kwok C, Chipato T, Salata R, Mauck C. 2015. The contribution of cervicovaginal infections to the immunomodulatory effects of hormonal contraception. mBio 6:e00221-15. doi: 10.1128/mBio.00221-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Genc MR, Delaney ML, Onderdonk AB, Witkin SS, Microbiology and Prematurity Study Group. 2006. Vaginal nitric oxide in pregnant women with bacterial vaginosis. Am J Reprod Immunol 56:86–90. doi: 10.1111/j.1600-0897.2006.00388.x. [DOI] [PubMed] [Google Scholar]
- 120.Sugiyama R, Abe M, Nishitsuji H, Murakami Y, Takeuchi H, Takaku H. 2013. Induction of heat-shock protein 70 by prostaglandin A1 inhibits HIV-1 Vif-mediated degradation of APOBEC3G. Antiviral Res 99:307–311. doi: 10.1016/j.antiviral.2013.06.017. [DOI] [PubMed] [Google Scholar]
- 121.Giraldo PC, de Carvalho JB, do Amaral RL, da Silveira Goncalves AK, Eleuterio J Jr, Guimaraes F. 2012. Identification of immune cells by flow cytometry in vaginal lavages from women with vulvovaginitis and normal microflora. Am J Reprod Immunol 67:198–205. doi: 10.1111/j.1600-0897.2011.01093.x. [DOI] [PubMed] [Google Scholar]
- 122.Sobel JD. 2007. Vulvovaginal candidosis. Lancet 369:1961–1971. doi: 10.1016/S0140-6736(07)60917-9. [DOI] [PubMed] [Google Scholar]
- 123.Donders GG, Bosmans E, Dekeersmaecker A, Vereecken A, Van Bulck B, Spitz B. 2000. Pathogenesis of abnormal vaginal bacterial flora. Am J Obstet Gynecol 182:872–878. doi: 10.1016/S0002-9378(00)70338-3. [DOI] [PubMed] [Google Scholar]
- 124.Valore EV, Wiley DJ, Ganz T. 2006. Reversible deficiency of antimicrobial polypeptides in bacterial vaginosis. Infect Immun 74:5693–5702. doi: 10.1128/IAI.00524-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Novak RM, Donoval BA, Graham PJ, Boksa LA, Spear G, Hershow RC, Chen HY, Landay A. 2007. Cervicovaginal levels of lactoferrin, secretory leukocyte protease inhibitor, and RANTES and the effects of coexisting vaginoses in human immunodeficiency virus (HIV)-seronegative women with a high risk of heterosexual acquisition of HIV infection. Clin Vaccine Immunol 14:1102–1107. doi: 10.1128/CVI.00386-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Huppert JS, Huang B, Chen C, Dawood HY, Fichorova RN. 2013. Clinical evidence for the role of Trichomonas vaginalis in regulation of secretory leukocyte protease inhibitor in the female genital tract. J Infect Dis 207:1462–1470. doi: 10.1093/infdis/jit039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Onderdonk AB, Hecht JL, McElrath TF, Delaney ML, Allred EN, Leviton A. 2008. Colonization of second-trimester placenta parenchyma. Am J Obstet Gynecol 199:52.e1–52.e10. doi: 10.1016/j.ajog.2007.11.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Stout MJ, Conlon B, Landeau M, Lee I, Bower C, Zhao Q, Roehl KA, Nelson DM, Macones GA, Mysorekar IU. 2013. Identification of intracellular bacteria in the basal plate of the human placenta in term and preterm gestations. Am J Obstet Gynecol 208:226.e1–226.e7. doi: 10.1016/j.ajog.2013.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Cao B, Stout MJ, Lee I, Mysorekar IU. 2014. Placental microbiome and its role in preterm birth. Neoreviews 15:e537–e545. doi: 10.1542/neo.15-12-e537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Fichorova RN, Onderdonk AB, Yamamoto H, Delaney ML, DuBois AM, Allred E, Leviton A. 2011. Maternal microbe-specific modulation of inflammatory response in extremely low-gestational-age newborns. mBio 2:e00280-10. doi: 10.1128/mBio.00280-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Leviton A, Fichorova RN, O'Shea TM, Kuban K, Paneth N, Dammann O, Allred EN. 2013. Two-hit model of brain damage in the very preterm newborn: small for gestational age and postnatal systemic inflammation. Pediatr Res 73:362–370. doi: 10.1038/pr.2012.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Leviton A, Kuban K, O'Shea TM, Paneth N, Fichorova R, Allred EN, Dammann O. 2011. The relationship between early concentrations of 25 blood proteins and cerebral white matter injury in preterm newborns: the ELGAN study. J Pediatr 158:897–903. doi: 10.1016/j.jpeds.2010.11.059. [DOI] [PubMed] [Google Scholar]
- 133.Leviton A, Kuban KC, Allred EN, Fichorova RN, O'Shea TM, Paneth N. 2011. Early postnatal blood concentrations of inflammation-related proteins and microcephaly two years later in infants born before the 28th post-menstrual week. Early Hum Dev 87:325–330. doi: 10.1016/j.earlhumdev.2011.01.043. [DOI] [PubMed] [Google Scholar]
- 134.O'Shea TM, Joseph RM, Kuban KCK, Allred EN, Ware J, Coster T, Fichorova RN, Dammann O, Leviton A, ELGAN Study Investigators. 2014. Elevated blood levels of inflammation-related proteins are associated with an attention problem at age 24 mo in extremely preterm infants. Pediatr Res 75:781–787. doi: 10.1038/pr.2014.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.O'Shea TM, Allred EN, Kuban KC, Dammann O, Paneth N, Fichorova R, Hirtz D, Leviton A, Extremely Low Gestational Age Newborn Study Investigators. 2012. Elevated concentrations of inflammation-related proteins in postnatal blood predict severe developmental delay at 2 years of age in extremely preterm infants. J Pediatr 160:395–401. doi: 10.1016/j.jpeds.2011.08.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.O'Shea TM, Shah B, Allred EN, Fichorova RN, Kuban KC, Dammann O, Leviton A, ELGAN Study Investigators. 2013. Inflammation-initiating illnesses, inflammation-related proteins, and cognitive impairment in extremely preterm infants. Brain Behav Immun 29:104–112. doi: 10.1016/j.bbi.2012.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kuban KC, O'Shea TM, Allred EN, Fichorova RN, Heeren T, Paneth N, Hirtz D, Dammann O, Leviton A, ELGAN Study Investigators. 2015. The breadth and type of systemic inflammation and the risk of adverse neurological outcomes in extremely low gestation newborns. Pediatr Neurol 52:42–48. doi: 10.1016/j.pediatrneurol.2014.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Buve A, Jespers V, Crucitti T, Fichorova RN. 2014. The vaginal microbiota and susceptibility to HIV. AIDS 28:2333–2344. doi: 10.1097/QAD.0000000000000432. [DOI] [PubMed] [Google Scholar]
- 139.Fichorova RN. 2004. Guiding the vaginal microbicide trials with biomarkers of inflammation. J Acquir Immune Defic Syndr 37(Suppl 3):S184–S193. [PMC free article] [PubMed] [Google Scholar]
- 140.Dutt R, Raker C, Anderson BL. 2015. Ethnic variations in cervical cytokine concentrations and vaginal flora during pregnancy. Am J Reprod Immunol 73:141–150. doi: 10.1111/aji.12291. [DOI] [PubMed] [Google Scholar]
- 141.Nguyen DP, Genc M, Vardhana S, Babula O, Onderdonk A, Witkin SS. 2004. Ethnic differences of polymorphisms in cytokine and innate immune system genes in pregnant women. Obstet Gynecol 104:293–300. doi: 10.1097/01.AOG.0000133486.85400.5e. [DOI] [PubMed] [Google Scholar]
- 142.Genc MR, Vardhana S, Delaney ML, Witkin SS, Onderdonk AB, MAP Study Group. 2007. TNFA-308G>A polymorphism influences the TNF-alpha response to altered vaginal flora. Eur J Obstet Gynecol Reprod Biol 134:188–191. doi: 10.1016/j.ejogrb.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 143.Genc MR, Onderdonk AB, Vardhana S, Delaney ML, Norwitz ER, Tuomala RE, Paraskevas LR, Witkin SS, MAP Study Group. 2004. Polymorphism in intron 2 of the interleukin-1 receptor antagonist gene, local midtrimester cytokine response to vaginal flora, and subsequent preterm birth. Am J Obstet Gynecol 191:1324–1330. doi: 10.1016/j.ajog.2004.05.074. [DOI] [PubMed] [Google Scholar]
- 144.Genc MR, Vardhana S, Delaney ML, Onderdonk A, Tuomala R, Norwitz E, Witkin SS, MAP Study Group. 2004. Relationship between a Toll-like receptor-4 gene polymorphism, bacterial vaginosis-related flora and vaginal cytokine responses in pregnant women. Eur J Obstet Gynecol Reprod Biol 116:152–156. doi: 10.1016/j.ejogrb.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 145.Gargiulo AR, Fichorova RN, Politch JA, Hill JA, Anderson DJ. 2004. Detection of implantation-related cytokines in cervicovaginal secretions and peripheral blood of fertile women during ovulatory menstrual cycles. Fertil Steril 82(Suppl 3):1226–1234. doi: 10.1016/j.fertnstert.2004.03.039. [DOI] [PubMed] [Google Scholar]
- 146.Kyongo JK, Jespers V, Goovaerts O, Michiels J, Menten J, Fichorova RN, Crucitti T, Vanham G, Arien KK. 2012. Searching for lower female genital tract soluble and cellular biomarkers: defining levels and predictors in a cohort of healthy Caucasian women. PLoS One 7:e43951. doi: 10.1371/journal.pone.0043951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Chaban B, Links MG, Jayaprakash TP, Wagner EC, Bourque DK, Lohn Z, Albert AY, van Schalkwyk J, Reid G, Hemmingsen SM, Hill JE, Money DM. 2014. Characterization of the vaginal microbiota of healthy Canadian women through the menstrual cycle. Microbiome 2:23. doi: 10.1186/2049-2618-2-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Gajer P, Brotman RM, Bai G, Sakamoto J, Schutte UM, Zhong X, Koenig SS, Fu L, Ma ZS, Zhou X, Abdo Z, Forney LJ, Ravel J. 2012. Temporal dynamics of the human vaginal microbiota. Sci Transl Med 4:132ra52. doi: 10.1126/scitranslmed.3003605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Junior JE, Giraldo PC, Goncalves AK, do Amaral RL, Linhares IM. 2014. Uterine cervical ectopy during reproductive age: cytological and microbiological findings. Diagn Cytopathol 42:401–404. doi: 10.1002/dc.23053. [DOI] [PubMed] [Google Scholar]
- 150.Fichorova R. 2014. Safety aspects of topical anti-HIV microbicides, p 117–150. In Das Neves J, Sarmento B (ed), Drug delivery and development of anti-HIV microbicides. Pan Stanford Publishing, Boca Raton, FL. [Google Scholar]